Summary
Saturated free fatty acid (FFA) is implicated in the metabolic response to obesity. In vitro studies indicate that FFA signaling may be mediated by the mixed-lineage protein kinase (MLK) pathway that activates cJun NH2-terminal kinase (JNK). Here we examined the role of the MLK pathway in vivo using a mouse model of diet-induced obesity. The ubiquitously expressed MLK2 and MLK3 protein kinases have partially redundant functions. We therefore compared wild-type and compound mutant mice that lack expression of MLK2 plus MLK3. MLK-deficiency protected mice against high fat diet-induced insulin resistance and obesity. Reduced JNK activation and increased energy expenditure contribute to the metabolic effects of MLK-deficiency. These data confirm that the MLK pathway plays a critical role in the metabolic response to obesity.
Introduction
Obesity represents a major risk factor for the development of type 2 diabetes. Metabolic stress caused by saturated free fatty acids (FFA) may mediate this effect of obesity (Kahn et al., 2006). Indeed, increased amounts of FFA in blood cause insulin resistance, a hallmark of metabolic syndrome (pre-diabetes). FFA can therefore cause hyperinsulinemia and can lead to β cell failure and diabetes. An understanding of the mechanisms that mediate FFA signaling is therefore important for the prevention and treatment of diabetes.
The cJun NH2-terminal kinase signal transduction pathway plays a key role in the development of insulin resistance (Sabio and Davis, 2010). Indeed, feeding a high fat diet (HFD) causes increased JNK activity in insulin target tissues (Hirosumi et al., 2002). JNK is also activated by the exposure of cells to saturated FFA (Jaeschke and Davis, 2007). The mechanism of saturated FFA-stimulated JNK activation is mediated by the MAP kinase kinases (MAP2K) MKK4 and MKK7 (Jaeschke and Davis, 2007). Many different MAP kinase kinase kinases (MAP3K) can activate the JNK pathway (Davis, 2000), but saturated FFA-stimulated JNK activation in vitro is mediated by the mixed-lineage protein kinase (MLK) sub-group of MAP3K (Jaeschke and Davis, 2007). Upstream components of this FFA signaling pathway may include Rac/Cdc42 (Sharma et al., 2011), Src (Holzer et al., 2011), and PKC (Jaeschke and Davis, 2007). Indeed, FFA-stimulated MLK activation could be mediated by a Src pathway that activates Rac/Cdc42 (Kant et al., 2011). This pathway may be initiated by receptor (Talukdar et al., 2011) or non-receptor (Holzer et al., 2011; Montell et al., 2001; Schmitz-Peiffer et al., 1999) mediated mechanisms.
Although these in vitro data implicate the MLK signal transduction pathway in the response to FFA-induced metabolic stress, the role of this pathway in vivo has not been established. The purpose of this study was to test whether the MLK pathway is relevant to the metabolic stress response to obesity. We report that the MLK pathway is critically required for diet-induced insulin resistance and obesity. This analysis indicates that the MLK pathway represents a potential target for the development of drugs that may be therapeutically beneficial for the treatment of obesity-related metabolic stress.
Results
MLK is required for diet-induced obesity
The MLK group of MAP3K is encoded by four genes (Gallo and Johnson, 2002) that have partially redundant functions (Kant et al., 2011). Gene expression analysis indicated that the genes that encode MLK2 (Map3k10) and MLK3 (Map3k11) (but not MLK1 (Map3k9) and MLK4 (Bc021891)) are highly expressed in peripheral insulin-responsive tissues, including white and brown adipose tissue, liver, and muscle (Figure S1). We therefore established compound mutant Map3k10−/− Map3k11−/− mice to study the role of MLK in insulin-responsive tissues.
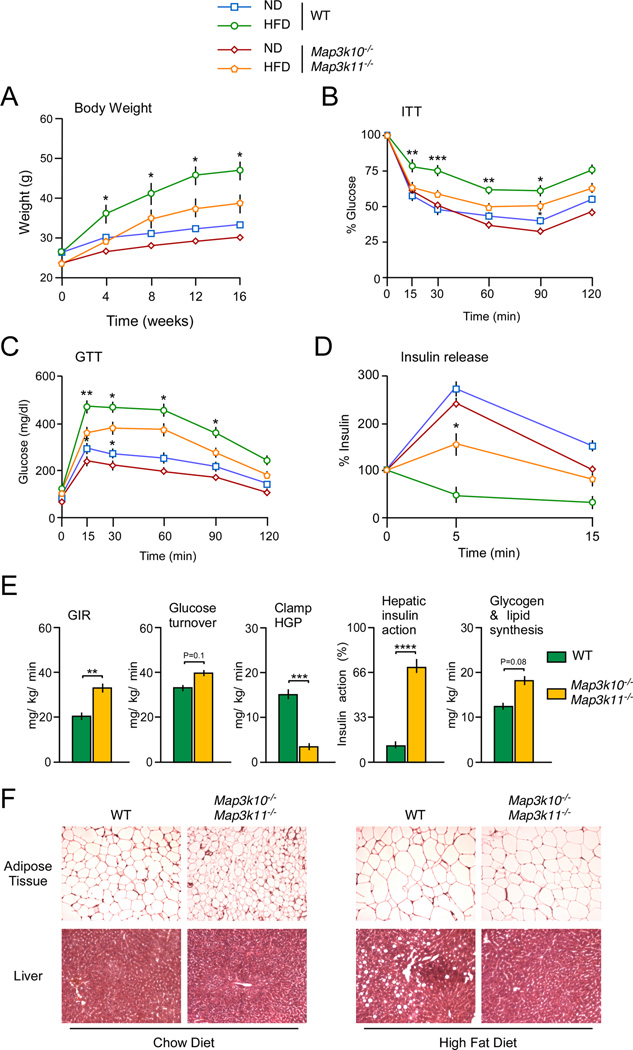
We examined diet-induced obesity in wild-type (WT) and Map3k10−/− Map3k11−/− mice. Control studies using chow-fed mice demonstrated that MLK-deficient mice were slightly smaller than WT mice, but no differences in the rate of weight gain between WT and mutant mice were detected (Figure 1A). In contrast, the Map3k10−/− Map3k11−/− mice exhibited partial resistance to HFD-induced obesity compared with WT mice (Figure 1A). Measurement of organ weight at necropsy demonstrated that the increased liver and adipose tissue mass detected in HFD-fed WT mice was reduced in HFD-fed Map3k10−/− Map3k11−/− mice (Figure S2). Analysis of liver sections demonstrated that HFD-induced hepatic steatosis in WT mice was absent in MLK-deficient mice (Figure 1F). Moreover, WT adipocytes were larger than adipocytes in Map3k10−/− Map3k11−/− mice (Figure 1F). Together, these data demonstrate that MLK-deficiency protects mice against HFD-induced obesity.
Figure 1. MLK-deficiency protects mice against the HFD-induced obesity and insulin resistance.
(A) WT and MLK-deficient male mice (8–10 wk old) were fed either a chow diet (ND) or a HFD (16 wk). The weight of the mice was measured (mean ± SE; n = 10).
(B) Insulin tolerance tests (ITT) on MLK-deficient and WT mice fed either a chow diet (ND) or a HFD (16 wks.) were performed by intraperitoneal injection of insulin (0.75 U/kg body mass). The concentration of blood glucose was measured (mean ± SE; n = 10).
(C) Glucose tolerance tests (GTT) on chow-fed (ND) and HFD-fed WT and MLK-deficient mice were performed by measurement of blood glucose concentration in animals following intraperitoneal injection of glucose (2 g/kg). The data presented represent the mean ± SE (n = 10).
(D) Glucose-induced insulin release. The effect of administration of glucose (2 g/kg body mass) by intraperitoneal injection on blood insulin concentration was examined (mean ± SE; n = 10).
(E) Insulin sensitivity was measured using a hyperinsulinemic-euglycemic clamp in conscious HFD-fed (3 wks) WT and MLK-deficient mice. The steady state glucose infusion rate (GIR) during the clamp, whole body glucose turnover, hepatic glucose production (HGP) during the clamp, and the hepatic insulin action (expressed as insulin-mediated percent suppression of basal HGP), and whole body glycogen plus lipid synthesis are presented (mean ± SE; n= 7~8).
(F) Sections prepared from epididymal adipose tissue and liver of chow-fed and HFD-fed WT and MLK-deficient mice were stained with hematoxylin and eosin. The panels are representative of 5 mice per group.
Statistically significant differences between WT and MLK-deficient mice are indicated (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
See also Figure S2.
MLK is required for diet-induced insulin resistance
Feeding a HFD to WT mice caused reduced responsiveness in insulin tolerance tests (Figure 1B) and glucose intolerance (Figure 1C). In contrast, HFD-fed Map3k10−/− Map3k11−/− mice showed improved insulin sensitivity in an insulin tolerance test (Figure 1B), improved glucose tolerance (Figure 1C), and improved glucose-induced insulin secretion (Figure 1D) compared with HFD-fed WT mice. These data indicate that Map3k10−/− Map3k11−/− mice are protected against HFD-induced insulin resistance.
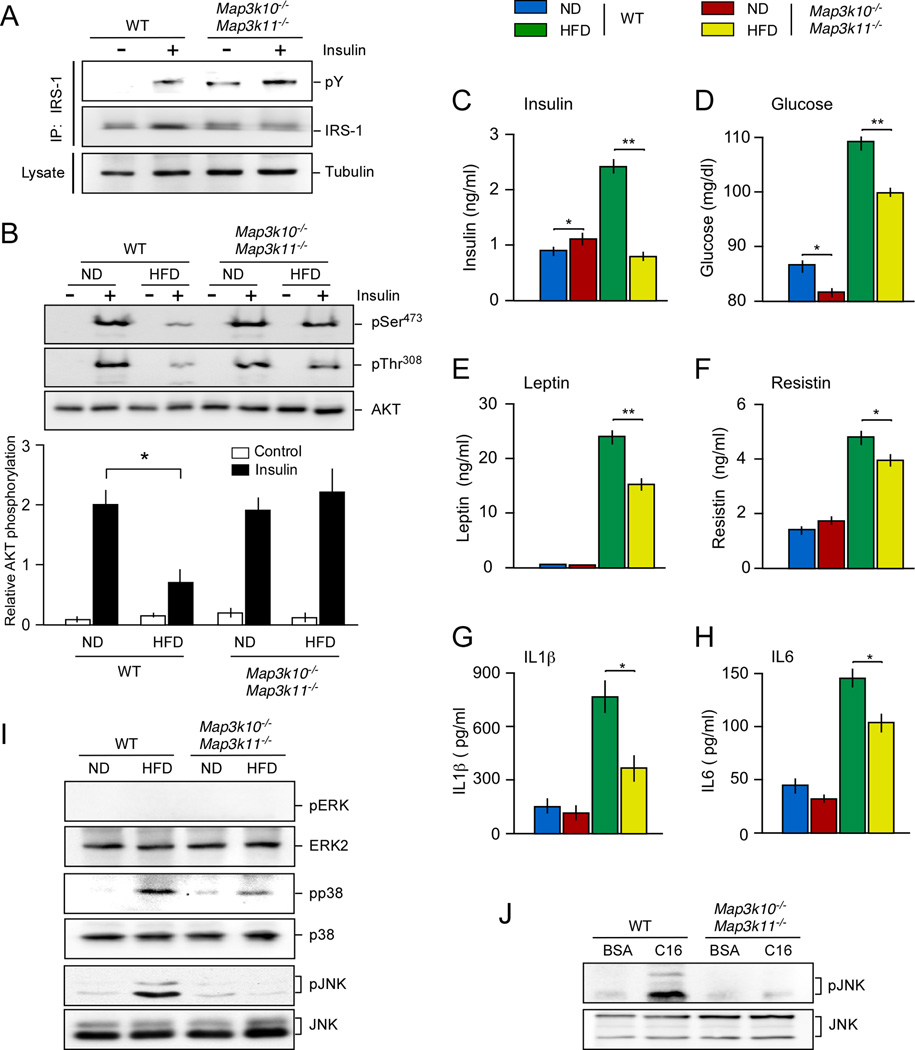
We performed a hyperinsulinemic-euglycemic clamp study to test whether the insulin sensitivity of HFD-fed Map3k10−/− Map3k11−/− mice was greater than HFD-fed WT mice. This analysis demonstrated that the glucose infusion rate during the clamp in the MLK-deficient mice was significantly greater than HFD-fed WT mice (Figure 1E), indicating that the Map3k10−/− Map3k11−/− mice exhibited increased insulin sensitivity compared to WT mice. This increased insulin sensitivity of Map3k10−/− Map3k11−/− mice was associated with reduced hepatic glucose production during the clamp and improved hepatic insulin action compared with HFD-fed WT mice (Figure 1E). Together, these data indicate that HFD-fed Map3k10−/− Map3k11−/− mice exhibit increased insulin sensitivity compared with HFD-fed WT mice. This conclusion was confirmed by biochemical measurement of insulin-stimulated tyrosine phosphorylation of the scaffold protein IRS-1 (Fig. 2A) and activation of the AKT signaling pathway (Fig. 2B). HFD-fed MLK-deficient mice exhibited decreased HFD-induced hyperinsulinemia (Figure 2C) and hyperglycemia (Figure 2D) compared with HFD-fed WT mice. Furthermore, the blood concentration of adipokines (leptin and resistin) and inflammatory cytokines (IL1β and IL6) was reduced in HFD-fed MLK-deficient mice compared with HFD-fed WT mice (Figure 2E–H).
Figure 2. MLK-deficiency reduces HFD-induced JNK activation and suppression of AKT.
(A,B) Chow-fed (ND) and HFD-fed WT and MLK-deficient mice (16 weeks) were fasted overnight and then treated by intraperitoneal injection of saline (Control) or 1.5 U/kg insulin (15 min.). Extracts prepared from epididymal fat pads were examined by (A) immunoprecipitation with an antibody to IRS-1 and immunoblot analysis with antibodies to IRS-1 / phosphotyrosine and (B) immunoblot analysis using antibodies to pThr308-AKT, pSer473-AKT, and AKT (upper panel) and by multiplexed ELISA (lower panel). Relative AKT phosphorylation (calculated as the ratio pSer473-AKT/AKT) is presented (mean ± SE; n =6; *, P < 0.05).
(C–H) WT and MLK-deficient mice were fed a chow diet (ND) or a HFD (16 wks.) and starved overnight. The blood concentration of insulin (E), glucose (F), leptin (G), resistin (H), IL1β (I), and IL6 (J) is presented (mean ± SE; n = 10). Statistically significant differences between MLK-deficient and WT mice are indicated (*, P < 0.05; **, P < 0.01).
(I) MAPK activation in epididymal adipose tissue of WT and MLK-deficient and mice was measured by immunoblot analysis by probing with antibodies to MAPK and phospho-MAPK.
(J) WT and MLK-deficient primary embryo fibroblasts were treated with 0.5% (w.v) bovine serum albumin (BSA) or 0.75 mM palmitate / 0.5%BSA (16 h). Cell lysates were examined by immunoblot analysis with antibodies to pJNK and JNK.
The MLK signaling pathway is implicated in the regulation of MAP kinases. However, no defects in ERK signaling were detected in MLK-deficient mice (Figure 2I). However, MLK-deficiency reduced the activation of p38 MAP kinase and prevented the activation of JNK caused by feeding a HFD (Figure 2I). MLK-deficiency also prevented the activation of JNK caused by FFA (Figure 2J). These data suggest that the metabolic phenotype of MLK-deficient mice may result from defects in stress-activated MAP kinase signaling, including p38 and JNK.
MLK-deficiency causes increased energy expenditure
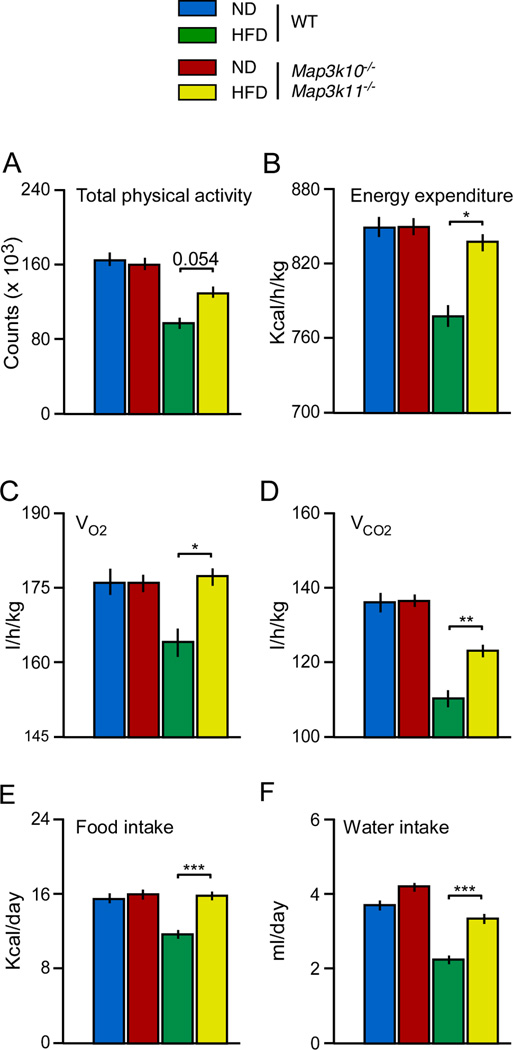
The resistance of MLK-deficient mice to HFD-induced obesity (Figure 1A) may represent a primary metabolic phenotype in these mice. Indeed, the reduced obesity of MLK-deficient mice compared with WT mice (Figure 1A) may contribute to the improved insulin sensitivity of MLK-deficient mice (Figure 1B). We therefore examined the mechanistic basis for the obesity phenotype of HFD-fed MLK-deficient mice. Studies using metabolic cages indicated that the food and water intake of chow-fed WT and MLK-deficient mice was similar (Figure 3). However, the MLK-deficient mice consumed significantly more food and water than WT mice when fed a HFD (Figure 3). These data do not provide an explanation for the reduced obesity of HFD-fed MLK-deficient mice compared with HFD-fed WT mice. Increased energy expenditure could contribute to the lean phenotype of HFD-fed MLK-deficient mice. Indeed, measurement of gas exchange demonstrated that VO2 and VCO2 were significantly increased in HFD-fed Map3k10−/− Map3k11−/− mice compared with HFD-fed WT mice (Figure 3). These changes were associated with increased physical activity (Figure 3). Collectively, these data indicate that MLK-deficient mice are resistant to HFD-induced obesity because of increased energy expenditure (Figure 3).
Figure 3. MLK-deficiency causes increased energy expenditure.
Food and water consumption, gas exchange (VO2 and VCO2), energy expenditure, and physical activity were measured using metabolic cages (mean ± SE, n = 6). Statistically significant differences between WT and MLK-deficient mice are indicated (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
MLK-deficiency and the hypothalamic-pituitary-thyroid axis
JNK is a target of the MLK signal transduction pathway (Gallo and Johnson, 2002). JNK1-deficient mice, like MLK-deficient mice (Figure 1), are resistant to HFD-induced obesity (Hirosumi et al., 2002). Tissue-specific Jnk1 gene ablation studies have established that central actions of JNK1 in the brain are responsible for this obesity phenotype (Sabio and Davis, 2010). Specifically, neuronal JNK1-deficiency acts through the hypothalamic-pituitary-thyroid axis to increase energy expenditure and suppress HFD-induced obesity (Sabio and Davis, 2010). This mechanism could account for the phenotype of MLK-deficient mice. Neuronal JNK1-deficiency causes central hyperthyroidism by increasing the expression of thyrotropin-releasing hormone (TRH) in the hypothalamus and thyroid stimulating hormone (TSH) in the anterior pituitary gland (Sabio et al., 2010). In contrast, we found that MLK-deficiency caused reduced TRH and TSH expression (Figure S3). These data indicate that the central hyperthyroidism of JNK1-deficient mice was not detected in MLK-deficient mice.
It is established that thyroid hormone causes feed-back inhibition of TRH and TSH expression (Chiamolera and Wondisford, 2009). The decreased TRH and TSH expression in MLK-deficient mice may therefore result from increased thyroid hormone signaling. To test this hypothesis, we examined the expression of thyroid hormone responsive genes (Ldhb, Ucp1, Pck1, Slc2A4, and Thrsp) in the brown fat of WT and Map3k10−/− Map3k11−/− mice. We found that MLK-deficiency increased thyroid hormone responsive gene expression in brown fat (Figure S4A). To test whether this increased thyroid hormone responsiveness might contribute to the metabolic phenotype of MLK-deficient mice, we treated WT and MLK-deficient mice with propylthiouracil (PTU), a drug that inhibits thyroperoxidase and prevents T4 production by the thyroid gland (Björkman and Ekholm, 2000). We found no significant differences in obesity, insulin tolerance, and blood concentrations of glucose and insulin between PTU-treated HFD-fed WT and MLK-deficient mice (Figure S4B–G). These data indicate that thyroid hormone contributes to the metabolic phenotype of MLK-deficient mice. Nevertheless, the MLK-deficient mice, unlike JNK1-deficient mice, do not exhibit central hyperthyroidism.
MLK-deficiency and the autonomic nervous system
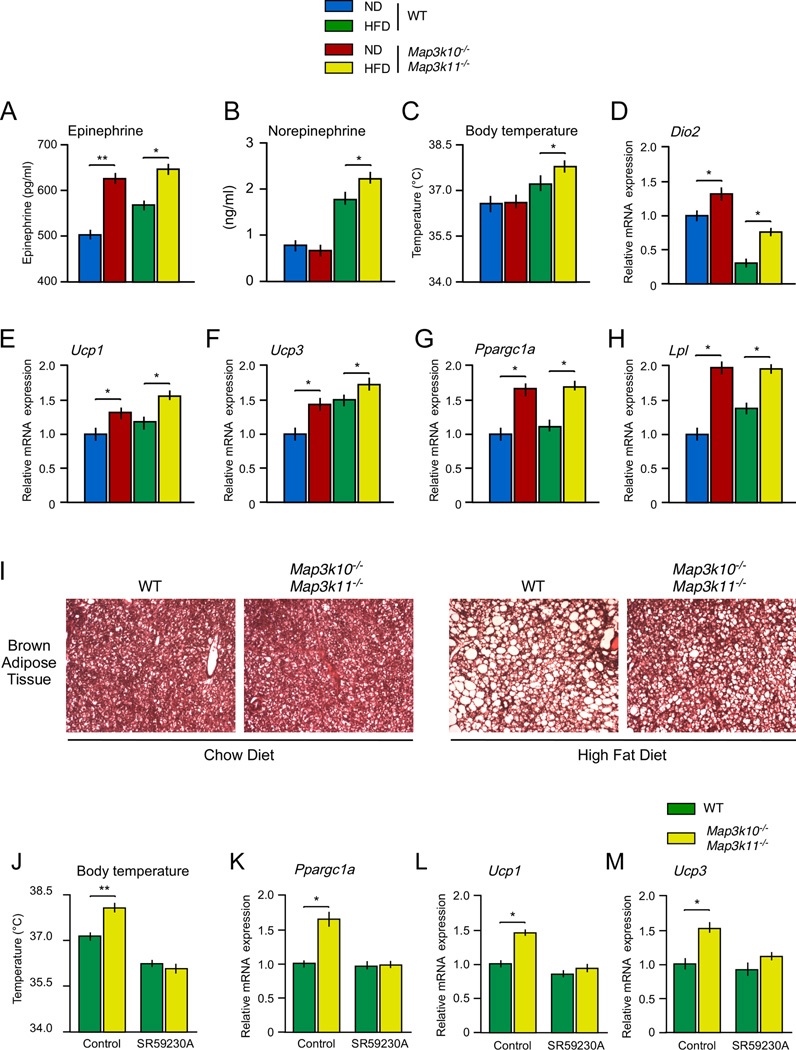
The hypothalamic-pituitary-thyroid axis may not represent a direct target of MLK signaling (Figure S3). Nevertheless, the hypothalamic-pituitary-thyroid axis may be indirectly targeted by MLK-deficiency through the autonomic nervous system. Indeed, it is established that the sympathetic nervous system, including the sympathoadrenal system, collaborates with the hypothalamic-pituitary-thyroid axis to regulate energy expenditure (Silva and Bianco, 2008). To test whether this is relevant to the phenotype of MLK-deficient mice, we examined brown fat in chow-fed and HFD-fed Map3k10−/− Map3k11−/− mice. Control studies using WT mice demonstrated that feeding a HFD caused an increase in interscapular brown fat mass (Figure S2). This increase in brown fat mass was suppressed in HFD-fed Map3k10−/− Map3k11−/− mice (Figure S2). Sections prepared from interscapular brown fat demonstrated that HFD-induced hypertrophy of brown adipocytes in WT mice was reduced in Map3k10−/− Map3k11−/− mice (Figure 4I). These data are consistent with increased sympathetic neuronal stimulation of brown fat metabolic activity in MLK-deficient mice.
Figure 4. The sympathoadrenal system causes increased thermogenesis in Mlk2−/− Mlk3−/− mice.
(A–B) WT and MLK-deficient mice were fed a chow diet (ND) or a HFD (16 wks.). The blood concentration of epinephrine and norepinephrine was measured (mean ± SE; n = 8).
(C) The body temperature of the mice was measured (mean ± SE; n = 8).
(D–H) The expression of Dio2 (D), Ucp1 (E), Ucp3 (F), Ppargc1a (G), and Lpl (H) mRNA expression in interscapular brown fat was measured by quantitative RT-PCR and was normalized to the amount of Gapdh mRNA in each sample (mean ± SE, n=8).
(I) Sections prepared from interscapular brown fat of chow-fed (ND) and HFD-fed WT and MLK-deficient mice were stained with hematoxylin and eosin. The panels presented are representative of 5 mice per group.
(J) The body temperature of WT and MLK-deficient mice was measured (mean ± SE; n = 10). The effect of treatment of the mice with solvent (Control) or the β3 adrenergic receptor antagonist SR59230A was examined.
(K–M) The expression of Ppargc1a (K), Ucp1 (L), and Ucp3 (M) mRNA expression in brown fat was measured by quantitative RT-PCR and was normalized to the amount of Gapdh mRNA in each sample (mean ± SE, n=10).
Statistically significant differences between WT and MLK-deficient mice are indicated (*, P < 0.05; **, P<0.01).
We found increased amounts of epinephrine and norepinephrine in the blood (Figure 4A,B) and increased expression of the adrenergic target genes Lpl (Carneheim et al., 1984), Dio2 (Silva and Larsen, 1983) and Ppargc1a (Puigserver et al., 1998) in brown fat of MLK-deficient mice compared with WT mice (Figure 4D,G,H). Dio2 expression causes increased intercellular conversion of T4 to T3 and occupancy of the thyroid hormone receptor (Bianco et al., 2002), while Ppargc1a expression increases PPARγ transcriptional activity (Handschin and Spiegelman, 2006). The thyroid hormone receptor, PPARγ, and CREB represent mediators of thermogenic gene expression in brown fat (Cannon and Nedergaard, 2004). Map3k10−/− Map3k11−/− mice exhibit increased expression of Ucp1 and Ucp3 in brown fat (Figure 4E,F) and increased body temperature (Figure 4C) compared with WT mice. Together, these data indicate that brown fat thermogenesis is increased in Map3k10−/− Map3k11−/− mice compared with WT mice.
The β3 adrenergic receptor can mediate effects of the sympathetic nervous system and circulating epinephrine on brown fat (Cannon and Nedergaard, 2004). To test the role of adrenergic regulation of brown fat in MLK-deficient mice, we treated mice with the selective β3 receptor antagonist SR59230A (Nisoli et al., 1996). This analysis demonstrated that β3 receptor blockade prevented the increased body temperature (Figure 4J) and the increased expression of Ppargc1a, Ucp1, and Ucp3 in brown fat (Figure 4K–M) caused by MLK-deficiency. Together, these data demonstrate that the sympathoadrenal system contributes to the increased energy expenditure detected in MLK-deficient mice.
Discussion
The MLK pathway is implicated as a mediator of saturated FFA signaling that causes JNK activation and insulin resistance (Jaeschke and Davis, 2007). Biochemical studies using cultured primary cells provide strong support for this conclusion (Jaeschke and Davis, 2007). However, the role of the MLK pathway in vivo has not been established. In this study, we tested whether the MLK pathway contributes to insulin resistance and obesity caused by feeding a HFD.
Four members of the MLK family have been identified (Gallo and Johnson, 2002). We found that two MLK isoforms (MLK2 and MLK3) are expressed ubiquitously. In contrast, all four MLK isoforms are detected in the brain. These MLK isoforms serve partially redundant functions (Kant et al., 2011). We therefore examined the effect of compound mutation of the MLK isoforms that are expressed in insulin target tissues. The HFD-fed MLK-deficient mice exhibited reduced obesity and improved hyperinsulinemia and hyperglycemia compared with HFD-fed WT mice. Moreover, these MLK-deficient mice have improved insulin sensitivity, improved glucose tolerance, and improved glucose-induced insulin secretion compared with WT mice when fed a HFD. Collectively, these data demonstrate that the MLK pathway plays an essential role in the metabolic stress response to feeding a HFD.
The effect of MLK-deficiency to protect against HFD-induced insulin resistance may be mediated by reduced JNK activation (Sabio and Davis, 2010). Indeed, MLK-deficiency suppressed HFD-induced p38 MAPK activation and prevented HFD-induced JNK activation. These defects in stress-activated MAPK regulation may contribute to the metabolic phenotype of MLK-deficient mice. However, the lean phenotype of HFD-fed MLK-deficient mice may also contribute to improved insulin sensitivity. Metabolic cage studies demonstrated that this lean phenotype was not caused by a reduction in food intake, but was associated with a large increase in energy expenditure. Previous studies have implicated the hypothalamic-pituitary-thyroid axis in JNK-mediated regulation of energy expenditure (Belgardt et al., 2010; Sabio et al., 2010). These studies have identified a role for JNK in the negative regulation of the hypothalamic-pituitary-thyroid axis by thyroid hormone (Sabio and Davis, 2010). However, the increased expression of TRH and TSH reported for JNK-deficient mice was not detected in MLK-deficient mice. It is possible that redundant functions of MLK1 and MLK4 may compensate for the deficiency of MLK2 plus MLK3 in the brain of these mice.
Although MLK-deficient mice did not exhibit the changes in TRH and TSH expression that are detected in JNK-deficient mice, increased expression of thyroid hormone-dependent gene expression was detected in the brown fat of mice with MLK2 plus MLK3-deficiency compared to WT mice. It is established that intracellular conversion of T4 to T3 is required for brown fat responsiveness to thyroid hormone (Bianco and Silva, 1987). This conversion is mediated by type 2 iodothyronine deiodinase (DIO2), a target of the sympathoadrenal system (Bianco et al., 2002). Indeed, increased expression of Dio2 was detected in the brown fat of MLK-deficient mice compared with WT mice. Together, these data indicate that MLK-deficiency may increase sympathetic regulation of brown fat, including norepinephrine release by nerves and epinephrine release by the adrenal gland. Moreover, we found increased epinephrine in the blood, increased expression of thermogenic genes, and increased body temperature of MLK-deficient mice. These changes were blocked when MLK-deficient mice were treated with a β3 adrenergic receptor antagonist. The sympathoadrenal system in MLK-deficient mice therefore causes increased energy expenditure that contributes to the protection against HFD-induced obesity. This mechanism may also contribute to the lean phenotype of HFD-fed JNK-deficient mice (Sabio and Davis, 2010).
It is established that the autonomic nervous system plays a key role in the regulation of glucose homeostasis and obesity (Kahn and Flier, 2000; Marino et al., 2011). The role of the sympathetic nervous system in the phenotype of MLK-deficient mice provides another example of the importance of metabolic regulation by the autonomic nervous system. This mechanism contributes to the increased energy expenditure and protection against obesity detected in HFD-fed MLK-deficient mice. Moreover, both the lean phenotype of HFD-fed MLK-deficient mice and the requirement of the MLK pathway for HFD-induced JNK activation contribute to the protection of MLK-deficient mice against HFD-induced insulin resistance. Collectively, these data identify the MLK pathway as a key mechanism that mediates metabolic stress responses to obesity. Moreover, these data identify MLK as a possible drug target for the treatment of obesity-related insulin resistance.
Experimental Procedures
Mice
Map3k10−/− (Kant et al., 2011), Map3k11−/− mice (Brancho et al., 2005) and compound mutant Map3k10−/− Map3k11−/− mice (Kant et al., 2011) were described previously. These mice were maintained on a C57BL/6J strain background (The Jackson Laboratories). Male mice (8–10 wks old) were fed a chow diet (Iso Pro 3000, Purina) or a HFD (F3282, Bioserve) for 16 wk. Treatment with the β3 antagonist SR59230A (2mg/kg; Tocris Bioscience) was performed by sub-cutaneous injection once each day (3 days). Mice were treated with PTU in the drinking water (cherry-flavored Kool-Aid without or with 1.2 mM PTU (Sigma)). Body temperature was measured using a Microthermia 2 Type “T” Thermometer (Braintree Scientific Inc.). The mice were housed in a facility accredited by the American Association for Laboratory Animal Care. The animal studies were approved by the Institutional Animal Care and Use Committee, University of Massachusetts Medical School.
Glucose and insulin tolerance tests
Glucose and insulin tolerance tests were performed (Mora et al., 2005) by intraperitoneal injection of mice starved overnight with glucose (2 mg/g of body mass) and intraperitoneal injection of mice fed ad-libitum with human insulin (0.75 mU/g of body mass).
Analysis of blood
Blood glucose was measured with an Ascensia Breeze 2 glucometer (Bayer). Plasma adipokines, cytokines, and insulin were measured by ELISA (Luminex 200; Millipore). Plasma epinephrine and norepinephrine was measured using the catecholamine assay kit Bi-CAT EIA (17-EA613-192; Alpco Diagnostics) by following the manufacturer’s instructions.
Statistical analysis
Differences between groups were examined for statistical significance using the Student’s test or analysis of variance (ANOVA) with the Fisher’s test.
For additional details regarding the materials and methods used in this work, see Extended Experimental Procedures.
Supplementary Material
Highlights.
Mixed-lineage protein kinases (MLK) 2/3 are expressed by insulin-responsive tissues.
MLK2/3 are required for HFD-induced JNK activation and insulin resistance.
MLK2/3 contribute to HFD-induced obesity by regulating energy expenditure.
The sympathoadrenal system contributes to metabolic regulation by MLK2/3.
Acknowledgments
We thank N. Kennedy, C. Morel, G. Sabio, and C. Standen for expert advice; V. Benoit, J-H Liu, and J. Reilly for expert technical assistance; and K. Gemme for administrative assistance. These studies were supported by a NIH grant DK090963. The Mouse Metabolic Phenotyping Center at the University of Massachusetts was supported by grant DK093000. R.J.D. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Information
Supplemental information includes four figures and Extended Experimental Procedures and can be found with this article on line at http://
References
- Belgardt BF, Mauer J, Wunderlich FT, Ernst MB, Pal M, Spohn G, Bronneke HS, Brodesser S, Hampel B, Schauss AC, et al. Hypothalamic and pituitary c-Jun N-terminal kinase 1 signaling coordinately regulates glucose metabolism. Proc Natl Acad Sci U S A. 2010;107:6028–6033. doi: 10.1073/pnas.1001796107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- Bianco AC, Silva JE. Intracellular conversion of thyroxine to triiodothyronine is required for the optimal thermogenic function of brown adipose tissue. J Clin Invest. 1987;79:295–300. doi: 10.1172/JCI112798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkman U, Ekholm R. Biochemistry of thyroid hormone formation and secretion. In: Greer MA, editor. The Thyroid Gland. New York: Raven Press; 2000. pp. 83–125. [Google Scholar]
- Brancho D, Ventura JJ, Jaeschke A, Doran B, Flavell RA, Davis RJ. Role of MLK3 in the regulation of mitogen-activated protein kinase signaling cascades. Mol Cell Biol. 2005;25:3670–3681. doi: 10.1128/MCB.25.9.3670-3681.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Carneheim C, Nedergaard J, Cannon B. Beta-adrenergic stimulation of lipoprotein lipase in rat brown adipose tissue during acclimation to cold. Am J Physiol. 1984;246:E327–E333. doi: 10.1152/ajpendo.1984.246.4.E327. [DOI] [PubMed] [Google Scholar]
- Chiamolera MI, Wondisford FE. Minireview: Thyrotropin-releasing hormone and the thyroid hormone feedback mechanism. Endocrinology. 2009;150:1091–1096. doi: 10.1210/en.2008-1795. [DOI] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Gallo KA, Johnson GL. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol. 2002;3:663–672. doi: 10.1038/nrm906. [DOI] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- Holzer RG, Park EJ, Li N, Tran H, Chen M, Choi C, Solinas G, Karin M. Saturated Fatty Acids Induce c-Src Clustering within Membrane Subdomains, Leading to JNK Activation. Cell. 2011;147:173–184. doi: 10.1016/j.cell.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke A, Davis RJ. Metabolic stress signaling mediated by mixed-lineage kinases. Mol Cell. 2007;27:498–508. doi: 10.1016/j.molcel.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- Kant S, Swat W, Zhang S, Zhang ZY, Neel BG, Flavell RA, Davis RJ. TNF-stimulated MAP kinase activation mediated by a Rho family GTPase signaling pathway. Genes Dev. 2011;25:2069–2078. doi: 10.1101/gad.17224711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino JS, Xu Y, Hill JW. Central insulin and leptin-mediated autonomic control of glucose homeostasis. Trends Endocrinol Metab. 2011;22:275–285. doi: 10.1016/j.tem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell E, Turini M, Marotta M, Roberts M, Noe V, Ciudad CJ, Mace K, Gomez-Foix AM. DAG accumulation from saturated fatty acids desensitizes insulin stimulation of glucose uptake in muscle cells. Am J Physiol Endocrinol Metab. 2001;280:E229–E237. doi: 10.1152/ajpendo.2001.280.2.E229. [DOI] [PubMed] [Google Scholar]
- Mora A, Lipina C, Tronche F, Sutherland C, Alessi DR. Deficiency of PDK1 in liver results in glucose intolerance, impairment of insulin-regulated gene expression and liver failure. Biochem J. 2005;385:639–648. doi: 10.1042/BJ20041782. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nisoli E, Tonello C, Landi M, Carruba MO. Functional studies of the first selective beta 3-adrenergic receptor antagonist SR 59230A in rat brown adipocytes. Mol Pharmacol. 1996;49:7–14. [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Sabio G, Cavanagh-Kyros J, Barrett T, Jung DY, Ko HJ, Ong H, Morel C, Mora A, Reilly J, Kim JK, et al. Role of the hypothalamic-pituitary-thyroid axis in metabolic regulation by JNK1. Genes Dev. 2010;24:256–264. doi: 10.1101/gad.1878510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabio G, Davis RJ. cJun NH2-terminal kinase 1 (JNK1): roles in metabolic regulation of insulin resistance. Trends Biochem Sci. 2010;35:490–496. doi: 10.1016/j.tibs.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem. 1999;274:24202–24210. doi: 10.1074/jbc.274.34.24202. [DOI] [PubMed] [Google Scholar]
- Sharma M, Urano F, Jaeschke A. Cdc42 and Rac1 are major contributors to the saturated fatty acid-stimulated JNK pathway in hepatocytes. J Hepatol. 2011 doi: 10.1016/j.jhep.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JE, Bianco SD. Thyroid-adrenergic interactions: physiological and clinical implications. Thyroid. 2008;18:157–165. doi: 10.1089/thy.2007.0252. [DOI] [PubMed] [Google Scholar]
- Silva JE, Larsen PR. Adrenergic activation of triiodothyronine production in brown adipose tissue. Nature. 1983;305:712–713. doi: 10.1038/305712a0. [DOI] [PubMed] [Google Scholar]
- Talukdar S, Olefsky JM, Osborn O. Targeting GPR120 and other fatty acid-sensing GPCRs ameliorates insulin resistance and inflammatory diseases. Trends Pharmacol Sci. 2011;32:543–550. doi: 10.1016/j.tips.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.