Abstract
Objective
This study was conducted to determine the impact of an 8-week intradialytic exercise program (consisting of 15 minutes low-intensity exercise during the first 2 hours of dialysis) on dialysis efficacy.
Methods
In an open randomized controlled trial, a total of 50 clinically stable hemodialysis patients were enrolled into the study and randomly allocated into two groups: the aerobic exercise group (n=25) and the control group (n=25). Aerobic exercises were done in the intervention group for 15 min/day, three times a week for 2 months. The dialysis efficacy was assessed prior to and at the end of each month of the program.
Results
The efficacy of dialysis increased at the end of the first month and remained elevated for the duration of the program in the exercise group (p<0.05).
Conclusion
A simplified aerobic exercise program has increased the efficacy of dialysis and may be considered as a safe, complementary and effective modality for hemodialysis patients.
Keywords: Exercise, Dialysis, Kidney Failure, Chronic
Introduction
Chronic kidney disease (CKD) is an important epidemic and public health problem,1 which occurs in many countries with an increasing prevalence. Over 50 million people throughout the world are known to have CKD and of these, more than 1 million require renal replacement therapies such as dialysis and renal transplantation. In recent years, the rising incidence of diabetes and hypertension, the most common two causes of CKD, have caused an increase in the prevalence of CKD.2 End-stage renal disease (ESRD) is a costly and disabling condition with a high mortality rate. In 2006, ESRD costs reached nearly $23 billion, >6% of the Medicare budget, and mortality rates were approximately eight times greater among individuals aged 20-64 years with ESRD treated by dialysis than among those in the general population of similar age.3 According to the United States Renal Data System (USRDS), the incidence of ESRD continues to increase each year.4 The intervention is typically prescribed 3 times per week, 3-6 hours per session, and remains ongoing for the lifetime of the patient or until successful kidney transplantation.5
In Iran, with over 13 thousand dialysis patients, approximately 150 thousand dialysis sessions are performed per month.6 Complications associated with the hemodialysis procedure are significant and account for many of the associated costs of dialysis.7 A central issue in the management of patients undergoing maintenance hemodialysis is the assessment of the adequacy of dialysis. Simply following the blood urea nitrogen (BUN) is insufficient because a low BUN can reflect inadequate nutrition rather than sufficient dialytic urea removal. Monitoring the patient's symptoms alone is also insufficient since the combination of dialysis plus erythropoietin to correct anemia can eliminate most uremic symptoms although the patient may be under-dialyzed. Thus, in addition to symptoms, patient nutrition and survival appear to best reflect the adequacy of dialysis.8 Adequacy of hemodialysis can be assessed by the Kt/V and urea reduction ratio (URR).
The National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative has set the minimum goal Kt/V at greater than 1.2 per treatment and the URR at greater than 65%. We approached intradialytic exercise from a physiologic perspective with the hypothesis that the increased muscle blood flow and greater amount of open capillary surface area in working muscles will result in a greater flux of urea and associated toxins from the tissue to the vascular compartment for subsequent removal at the dialyzer.9,10 Considering the importance of the adequacy of dialysis and its effect on hemodialysis complications and to note that inadequacy of dialysis is one of the determinants of disability and mortality for dialysis patients, therefore, increasing the adequacy of dialysis is very effective for improving the prognosis of dialysis patients.11
The purpose of the current study was to determine the impact of an 8-week intradialytic exercise program (consisting of 15 minutes of cumulative duration, exercise based on the patients capacity during the first 2 hours of dialysis) on the efficacy of dialysis in hemodialysis patients.
Methods
An open randomized controlled trial was conducted on a total of 50 hemodialysis patients at the dialysis center of Imam Khomeini hospital in Sari, Iran. This study was granted Institutional Review Board (IRB) approval by Mazandaran University of Medical Sciences and received ethical approval from the Research Ethics Committee at the university and registered in clinical trials database (IRCT138904084268N1, http//:www.irct.ir). All subjects were provided a copy of the written informed consent prior to the intervention and assured of their anonymity and confidentiality of data obtained.
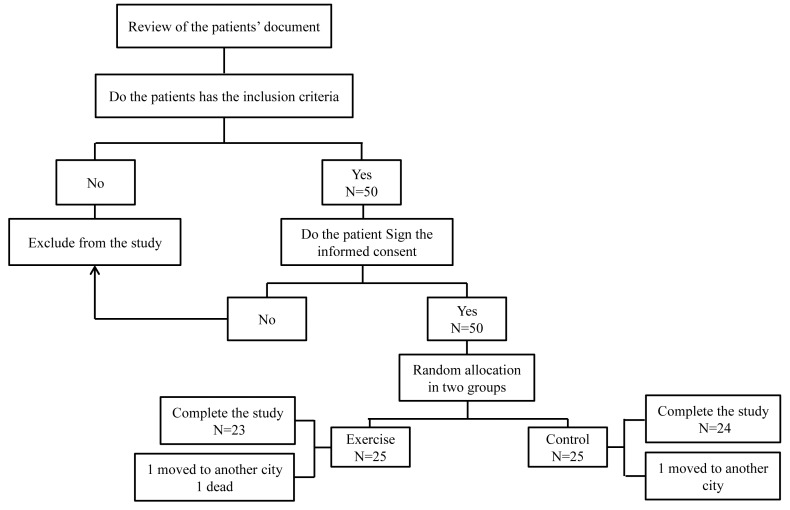
Fifty ESRD patients of age ranging from 18 to 76 years accepted to participate in this study. The inclusion criteria were: being under hemodialysis for more than 3 months, receiving hemodialysis 3 times per week, for 3 or 4 hr (180 min or 240 min) per treatment, having no problems in arteriovenous (AV) fistulas (based on the physician's diagnosis), and using bicarbonate solution for hemodialysis (because of the side effects of acetate solution such as hypotension during dialysis, cardiovascular problems, disequilibrium syndrome, nausea, vomiting, fatigue, headache, hypoxia, and hypoxemia).12 Each patient’s physician completed a medical screening form in order to permit the patients to participate. Exclusion criteria included the following: blood coagulation on dialysis filter (during hemodialysis), not adhering to the exercise program, instability in hemodynamic parameters before and during the exercises, having a history of angina pectoris in the last three months, and having any contraindication to exercise (based on the physician’s diagnosis). A list of random numbers generated by the random-number table was employed to allocate eligible patients into either the intervention and the control groups that were scheduled to have dialysis every odd (control group) and even (intervention group) days. Therefore, patients in the intervention and control groups could not see each other and they did not know which group they were in.
The study was conducted over a 2 months period (from June to July 2010), and aerobic exercise movements were performed for 15 min/day, three times a week, during dialysis sessions for the intervention group. Before the intervention and after sufficient explanation of the method of exercise, it was practiced for 2-3 training sessions. The patients in the intervention group also received the training documents including the description of the exercise movement. The protocol for exercise was as follow: after connecting the patient to the hemodialysis machine and turning off all of the alarms associated with connecting the patient, aerobic movement exercise of range of motion (ROM) was done for a 15 minutes period, during the first 2 hours of the dialysis session (based on the patients’ capacity) and no exercise was prescribed during the second half of the session.
The prescribed exercises included rotating the wrist as follows: 20 rounds per minute (RPM) clockwise, 20 RPM counter-clockwise, 20 times full flexion and extension of the wrist, 20 times full flexion and extension of the elbow joint, 20 RPM of rotating the ankles clockwise, 20 RPM of rotating the ankles counter-clockwise, 20 times full flexion and extension of the ankles. The first researcher supervised patients during the exercise. The limb that was connected to the hemodialysis machine was not exercised, and attention was also paid towards not disrupting the needles and catheters during the exercise. In addition, the patients were taught to stop the exercise and to notify the researcher if they felt any dizziness, headache, palpitations, nausea, anxiety, exhaustion or any other adverse effects. The patients’ vital signs were examined at least once during exercise and more for patients with hemodynamic disorders.
The patients were frequently questioned whether they were experiencing problems such as chest pain, dyspnea, nausea, vomiting, fatigue and headache during the exercise, but none complained of such problems. The patients’ heart rates, blood pressure and respiratory rates were also measured at the beginning, after 10 minutes and at the end of exercise and no instability in vital signs were observed. On average, heart rate increased by 10 beats per minute across all subjects. Exercise was performed for 15 minutes and for those patients who completed the movements in less than 15 mins, the movements were continued until the required 15 minutes were completed. The urea clearance was determined at baseline and on a monthly basis during the exercise program. Pre-and post-dialysis (immediately at the end of dialysis), blood samples were drawn by a nephrology nursing staff to measure the serum urea concentrations and calculate the urea kinetics (spKt/V). To prevent potential errors, all the blood samples were taken by the same person. Also, none of the laboratory staff were aware of the patients’ group assignments.
The single pool model was used as defined by Jindal et al. to calculate the dialysis efficacy: Dialysis efficacy (spKt⁄V= [0.04 (Co Ct⁄Co) (100) -1.2]), where Co and Ct are the initial and end dialysis serum urea concentrations (in mmol/L), respectively.13,14 The minimum target dose (in spKt/V) recommended by the Kidney Disease Quality Outcomes Initiative is 1.2.14 We also calculated urea reduction ratio (URR) because another measure of delivered dialysis dose is the URR. Direct measurement of URR has been proposed to be a simpler method of calculating dialysis dose and it is expressed as follows: URR = (BUNpre -BUNpost)/BUNpre, where BUNpre is pre-dialysis urea concentration and BUNpost is post-dialysis urea concentration. By convention, the value is multiplied by 100 and expressed as a percentage. It is also termed percentage reduction in urea (PRU).15 As a result, some experts recommend a minimum URR of 65%. The control group did not received any additional intervention, but their dialysis efficacy was checked only at the end of each month. High flux dialyzer membranes were used for all patients.
Chi-square test was used to compare the group’s characteristics (Table 1). Data analysis was performed using the SPSS 16 for windows. Descriptive statistics (mean, SD) were calculated for age, sex, time on HD (month), dialysis efficacy (Kt/V), duration of HD (hours) and co-morbidities. ANOVA test was used to compare serum urea clearance and Kt/V in intervention and control groups (Table 2). Statistical significance was set at 0.05 or less.
Table 1. Comparison of demographic and HD characteristics of the intervention and control groups.
| Variable | Intervention N (%) |
Control N (%) |
p value | |
|---|---|---|---|---|
| Sex | Male | 17 (74) | 13 (54) | 0.159 |
| Female | 6 (26) | 11 (46) | ||
| Co-morbidities | Diabetes mellitus | 5 (22) | 5 (21) | 0.671 |
| Hypertension | 6 (26) | 8 (33) | ||
| Others (Glomerular, Polycystic kidney, urine stone) |
5 (22) | 3 (13) | ||
| DM & HTN | 4 (17) | 7 (29) | ||
| Unknown | 3 (13) | 1 (4) | ||
| Age (yrs) | 53 ± 14 | 56 ± 11 | 0.546 | |
| Time on HD (month) (Mean±SD) | 26 ± 11 | 24 ± 14 | 0.561 | |
| Dialysis efficacy (Mean±SD) | 0.9 ± 0.2 | 0.9 ± 0.3 | 0.91 | |
| Duration of HD (hours) (Mean±SD) | 4 ± 0.4 | 4 ± 0.4 | 0.764 | |
Table 2. Comparison of spKt/v and URR in the intervention and control groups at first, fourth and eighth weeks of the study.
| Variables | Intervention | Control | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | End of 4th week | End of 8th week | p value | Baseline | End of 4th week | End of 8th week |
p value ANOVA |
|
| Kt/v Mean±SD | 0.9 ± 0.2 | 1.3 ± 0.6 | 1.2 ± 0.4 | 0.001 | 0.9 ± 0.3 | 0.99 ± 0.4 | 0.95 ± 0.2 | 0.73 |
| URR Mean±SD | 0.4 ± 0.1 | 0.6 ± 0.2 | 0.6 ± 0.1 | 0.003 | 0.5 ± 0.1 | 0.5 ± 0.2 | 0.5 ± 0.1 | 0.86 |
Figure 1.
Flow chart of trial.
Result
Baseline demographic data and the clinical characteristics of the participants are presented in Table 1. No significant differences were observed among the two groups for age, sex, time on HD (month), dialysis efficacy (spKt/V), duration of HD (hours) or co-morbidities. The HD patients were stable on medical therapy for the study duration, and new medications were not started during the study period. The baseline values of the variables increased by 11% in URR (p=0.003) and 38% in spKt/V (p=0.001) at the 8th week post-treatment in the intervention group. (Table 2)
Discussion
The purpose of the study was to examine the effect of Intradialytic aerobic exercise on dialysis efficacy in HD patients. As shown in Table 2, there was no significant difference in baseline URR and spKt/V among the two study groups. The present study demonstrates that aerobic exercise can improve dialysis efficacy. This improvement may be due to the direct beneficial effects of aerobic exercise or the general effects of regular exercise. These are points for further discussion. The primary findings of the current study were an overall 11% increase in URR and 38% improvement in spKt/V after 8 weeks of intradialytic exercise program.
The intradialytic aerobic exercise program resulted in a substantial increase in dialysis efficacy. It seems that during dialysis exercise increases the muscle blood flow and opens the capillary surface area which subsequently increases the flux of urea from the tissue to the vascular compartment.12 Such an increase would result in an increase in serum urea clearance and improvement in the dialysis efficacy. The Jindal equation was used to determine spKt/V, and thus significant changes in spKt/V were observed towards the end of the exercise program. These findings clearly indicate that exercise can be used as an adjunctive therapy to enhance dialysis efficacy.
This study demonstrates that a structured, 15-minute regular exercise program during dialysis based on the patients capacity can improve dialysis efficacy. To date, few studies have measured the acute or long-term effects of intradialytic exercise on urea removal and dialysis efficacy. The results of our study are in agreement with the findings of Parsons et al. who examined Kt/V responses to 20-week intradialytic exercise program and observed that spKt/V increased 11% at the end of the first month of the program and remained elevated for the duration of the program.12 Also, our results support the findings of Sun et al. who reported that exercise can improve spKt/V in HD patients.10 However, the results are in disagreement with those of Vaithilingam et al. who found no changes in equilibrated spKt/V or URR in 12 HD patients who performed cycle exercise on average of 69_16 min/wk or about 13 min/dialysis session.16 One possible explanation for this discrepancy could be that exercise should be done two months or more during HD in order to enhance urea removal and therefore improve dialysis efficacy. However, little information was provided by the above studies with respect to the timing of the exercise during the dialysis sessions and the exercise intensity, making it difficult to interpret the results in terms of a standardized exercise protocol.
A study conducted by Afshar et al. showed that aerobic exercise with moderate intensity during the first two hours of a dialysis session significantly improve sleep quality and decrease serum leptin and C-reactive protein (CRP) levels in hemodialysis patients.17 In another study by Afshar et al. on hemodialysis patients, they found that aerobic and resistance exercises were significantly correlated with a reduction of serum creatinine and hs-CRP levels, thus aerobic exercise induced more reduction; however, the exercise had no influence on weight, Kt/V values, serum urea, albumin, hemoglobin, or lipid levels.18 It has also been shown that 4 months of intradialytic endurance exercise improved physical performance and reduced serum oxidative stress (TBARS), serum alkaline phosphatase (ALP), and epicardial fat levels in hemodialysis patients.19 In addition, it has been demonstrated that in hemodialysis patients, serum phosphate and potassium levels significantly decreased after an 8-week intradialytic aerobic exercise.20 The results of a study by Van Vilsteren et al. which was conducted on hemodialysis patients has shown the beneficial effects of cycling during dialysis together with a pre-dialysis strength training program on behavioral changes, physical fitness, physiological conditions, and health-related quality of life.21 Another study by Johansen et al. showed that lower extremity resistance exercise training for 12 weeks during hemodialysis sessions three times per week using ankle weights in patients who were receiving maintenance hemodialysis, increased quadriceps muscle cross-sectional area and also improved self-reported physical functioning.22
Moreover, Cheema et al. suggested that patients with ESRD could improve skeletal muscle quality and derive other health-related adaptations solely by engaging in a 12-week high-intensity PRT regimen during routine hemodialysis treatment sessions.23 Exercise can be performed either as ‘intradialytic’ or ‘interdialytic,’ and both exercise programs have pros and cons. Although debate still exists, intradialytic exercise programs are superior to interdialytic exercise programs in terms of a lower dropout rate.24
The present study presented some limitations; one limitation being the use of three different types of dialysis machines. We recommend further study using one type of dialysis machine for all patients. We conducted the study at one dialysis center which was the only center for chronic hemodialysis in the city, therefore a limited number of hemodialysis patients were available and the sample size may be small for a definite conclusion. Therefore a larger, multicenter study may be suggested. Also, the intensity of exercise is usually controlled with a cycle ergometer; however, we used the patients’ heart rate as a measure of intensity of exercise. Another limitation was the fact that based on the type of intervention in this study (exercise), it was very difficult to have a good control group, and hence confounders might play a role here. Moreover, based on the type of intervention, blinding was not feasible and physicians were not blinded to the study groups.
Conclusion
In conclusion, the findings of the current study show that the prescribed intradialytic aerobic exercise program resulted in significant improvement in urea clearance. However, further investigations with larger samples may be required for the intervention to be prescribed as an adjunctive therapy to HD. The consequences of improved dialysis efficacy with intradialytic physiologic manifestations of the uremic syndrome (i.e., uremic myopathy, uremic neuropathy) have yet to be identified.
Acknowledgements
The authors wish to acknowledgment the efforts of the research deputy at Mazandaran University of Medical Sciences, also the head and staff at the dialysis department of Imam Khomeini Hospital in Sari for cooperating in the present study.
References
- 1.Mula-Abed WA, Al Rasadi K, Al-Riyami D. Estimated Glomerular Filtration Rate (eGFR): A Serum Creatinine-Based Test for the Detection of Chronic Kidney Disease and its Impact on Clinical Practice. Oman Med J 2012. Mar;27(2):108-113 10.5001/omj.2012.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang QL, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health 2008;8:117 10.1186/1471-2458-8-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burrows NR, Li Y, Geiss LS. Incidence of treatment for end-stage renal disease among individuals with diabetes in the U.S. continues to decline. Diabetes Care 2010. Jan;33(1):73-77 10.2337/dc09-0343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foley RN, Collins AJ. End-stage renal disease in the United States: an update from the United States Renal Data System. J Am Soc Nephrol 2007. Oct;18(10):2644-2648 10.1681/ASN.2007020220 [DOI] [PubMed] [Google Scholar]
- 5.Cheema BS, Singh MA. Exercise training in patients receiving maintenance hemodialysis: a systematic review of clinical trials. Am J Nephrol 2005. Jul-Aug;25(4):352-364 10.1159/000087184 [DOI] [PubMed] [Google Scholar]
- 6.Rambod H. Chronic renal failure. Sci Dial Patient Nurs Phys 2008;3(36):1-2 [Google Scholar]
- 7.Checheriţă IA, Turcu F, Dragomirescu RF, Ciocâlteu A. Chronic complications in hemodialysis: correlations with primary renal disease. Rom J Morphol Embryol 2010;51(1):21-26 [PubMed] [Google Scholar]
- 8.Cronin RE, Henrich WL. Kt/V and the adequacy of hemodialysis. Available from: http://www.uptodate.com/contents/kt-v-and-the-adequacy-of-hemodialysis (Accessed date: 19 Jan 2013).
- 9.Kong CH, Tattersall JE, Greenwood RN, Farrington K. The effect of exercise during haemodialysis on solute removal. Nephrol Dial Transplant 1999. Dec;14(12):2927-2931 10.1093/ndt/14.12.2927 [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, Chen B, Jia Q, Wang J. [The effect of exercise during hemodialysis on adequacy of dialysis]. Zhonghua Nei Ke Za Zhi 2002. Feb;41(2):79-81 [PubMed] [Google Scholar]
- 11.Borzo R, Galyaf M, Amin R. Assessment of velocity of blood flow affect on adequacy of dialysis in haemodialysis patients. Shahrekord Univ Med Sci J 2006; 8(2):60-6.10.
- 12.Parsons TL, Toffelmire EB, King-VanVlack CE. The effect of an exercise program during hemodialysis on dialysis efficacy, blood pressure and quality of life in end-stage renal disease (ESRD) patients. Clin Nephrol 2004. Apr;61(4):261-274 [DOI] [PubMed] [Google Scholar]
- 13.Parsons TL, Toffelmire EB, King-VanVlack CE. Exercise training during hemodialysis improves dialysis efficacy and physical performance. Arch Phys Med Rehabil 2006. May;87(5):680-687 10.1016/j.apmr.2005.12.044 [DOI] [PubMed] [Google Scholar]
- 14.National Kidney Foundation. Kidney Dialysis Outcome Quality Initiative (K/DOQI). Clinical practice guidelines for hemodialysis adequacy: update 2000. Available from: http://www.kidney.org/professionals/kdoqi/guidelines_updates/doqi_uptoc.html#hd (Accessed date: 11 February 2013).
- 15.Mehta AN, Fenves AZ. Hemodialysis Adequacy: A Review. Dial Transplant 2010;39(1):20-22 . 10.1002/dat.20392 [DOI] [Google Scholar]
- 16.Vaithilingam I, Polkinghorne KR, Atkins RC, Kerr PG. Time and exercise improve phosphate removal in hemodialysis patients. Am J Kidney Dis 2004. Jan;43(1):85-89 10.1053/j.ajkd.2003.09.016 [DOI] [PubMed] [Google Scholar]
- 17.Afshar R, Emany A, Saremi A, Shavandi N, Sanavi S. Effects of intradialytic aerobic training on sleep quality in hemodialysis patients. Iran J Kidney Dis 2011. Mar;5(2):119-123 [PubMed] [Google Scholar]
- 18.Afshar R, Shegarfy L, Shavandi N, Sanavi S. Effects of aerobic exercise and resistance training on lipid profiles and inflammation status in patients on maintenance hemodialysis. Indian J Nephrol 2010. Oct;20(4):185-189 10.4103/0971-4065.73442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilund KR, Tomayko EJ, Wu PT, Ryong Chung H, Vallurupalli S, Lakshminarayanan B, et al. Intradialytic exercise training reduces oxidative stress and epicardial fat: a pilot study. Nephrol Dial Transplant 2010. Aug;25(8):2695-2701 10.1093/ndt/gfq106 [DOI] [PubMed] [Google Scholar]
- 20.Makhlough A, Ilali E, Mohseni R, Shahmohammadi S. Effect of intradialytic aerobic exercise on serum electrolytes levels in hemodialysis patients. Iran J Kidney Dis 2012. Mar;6(2):119-123 [PubMed] [Google Scholar]
- 21.van Vilsteren MC, de Greef MH, Huisman RM. The effects of a low-to-moderate intensity pre-conditioning exercise programme linked with exercise counselling for sedentary haemodialysis patients in The Netherlands: results of a randomized clinical trial. Nephrol Dial Transplant 2005. Jan;20(1):141-146 10.1093/ndt/gfh560 [DOI] [PubMed] [Google Scholar]
- 22.Johansen KL, Painter PL, Sakkas GK, Gordon P, Doyle J, Shubert T. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: A randomized, controlled trial. J Am Soc Nephrol 2006. Aug;17(8):2307-2314 10.1681/ASN.2006010034 [DOI] [PubMed] [Google Scholar]
- 23.Cheema B, Abas H, Smith B, O’Sullivan A, Chan M, Patwardhan A, et al. Progressive exercise for anabolism in kidney disease (PEAK): a randomized, controlled trial of resistance training during hemodialysis. J Am Soc Nephrol 2007. May;18(5):1594-1601 10.1681/ASN.2006121329 [DOI] [PubMed] [Google Scholar]
- 24.Jung TD, Park SH. Intradialytic exercise programs for hemodialysis patients. Chonnam Med J 2011. Aug;47(2):61-65 10.4068/cmj.2011.47.2.61 [DOI] [PMC free article] [PubMed] [Google Scholar]