Abstract
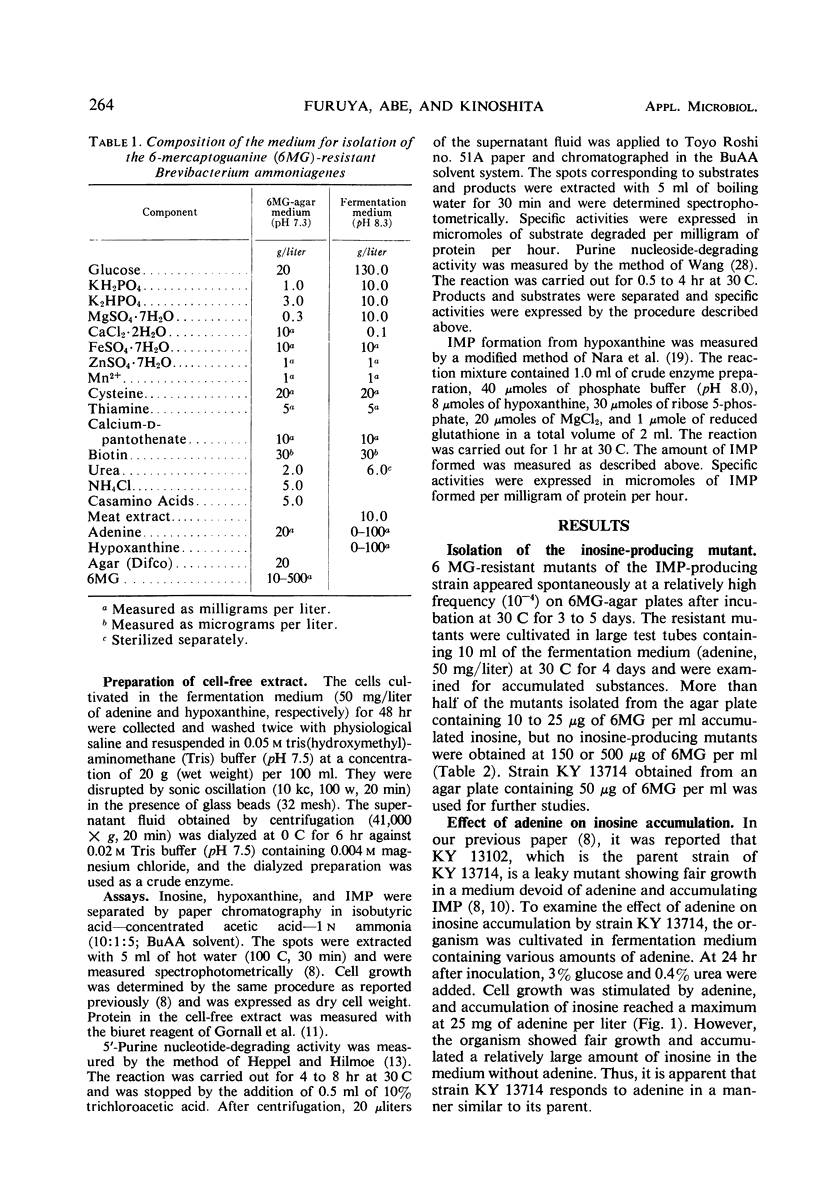
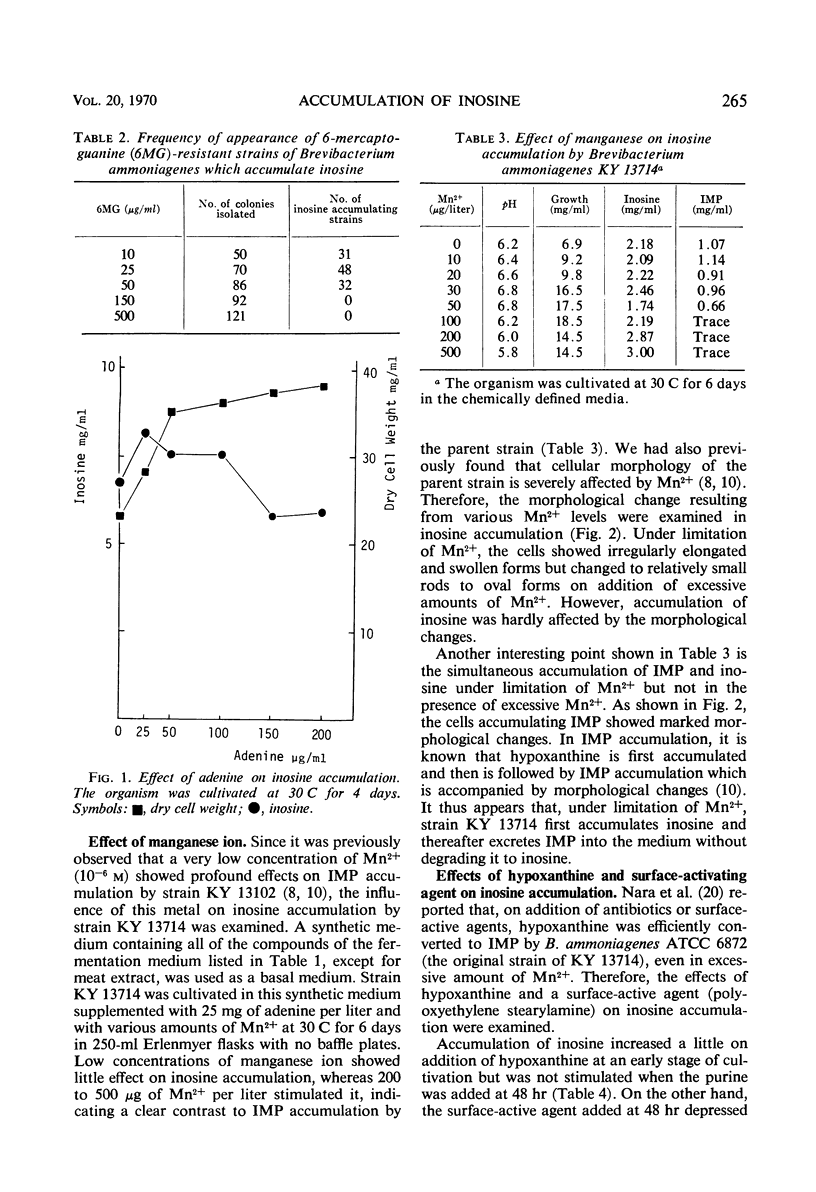
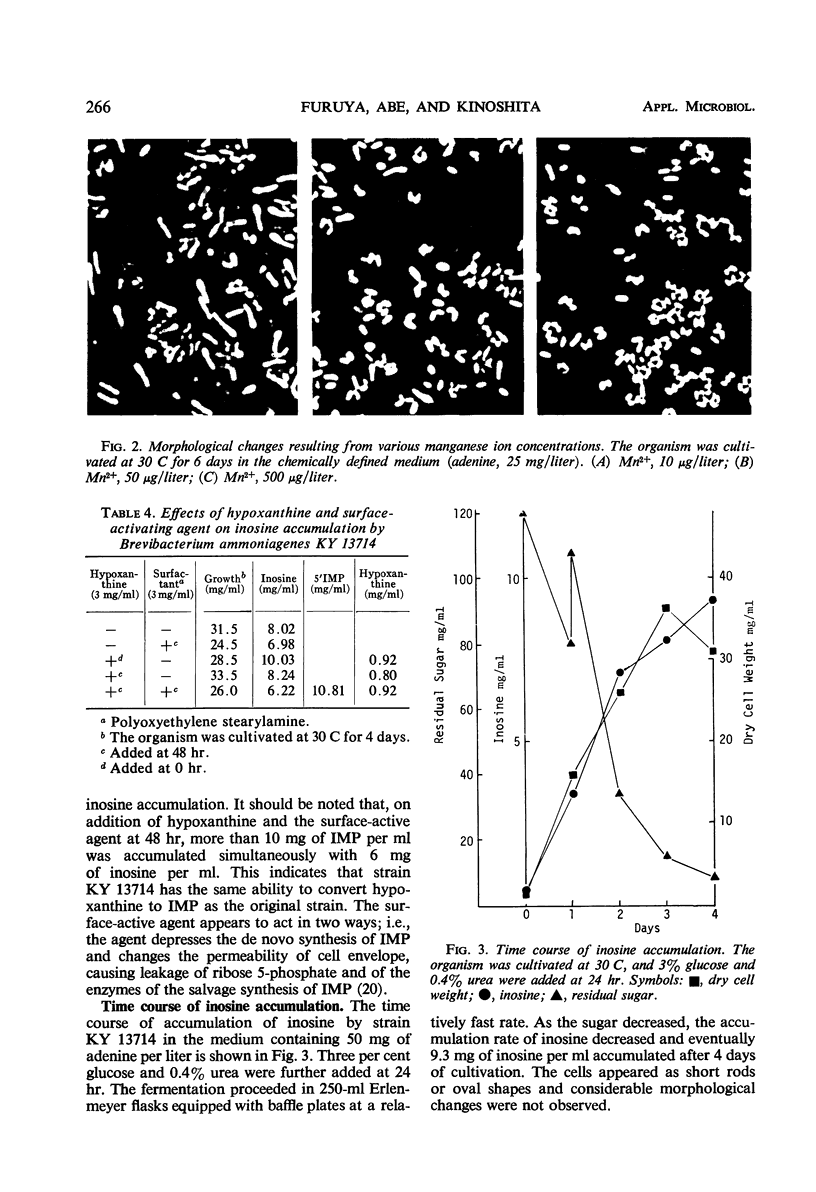
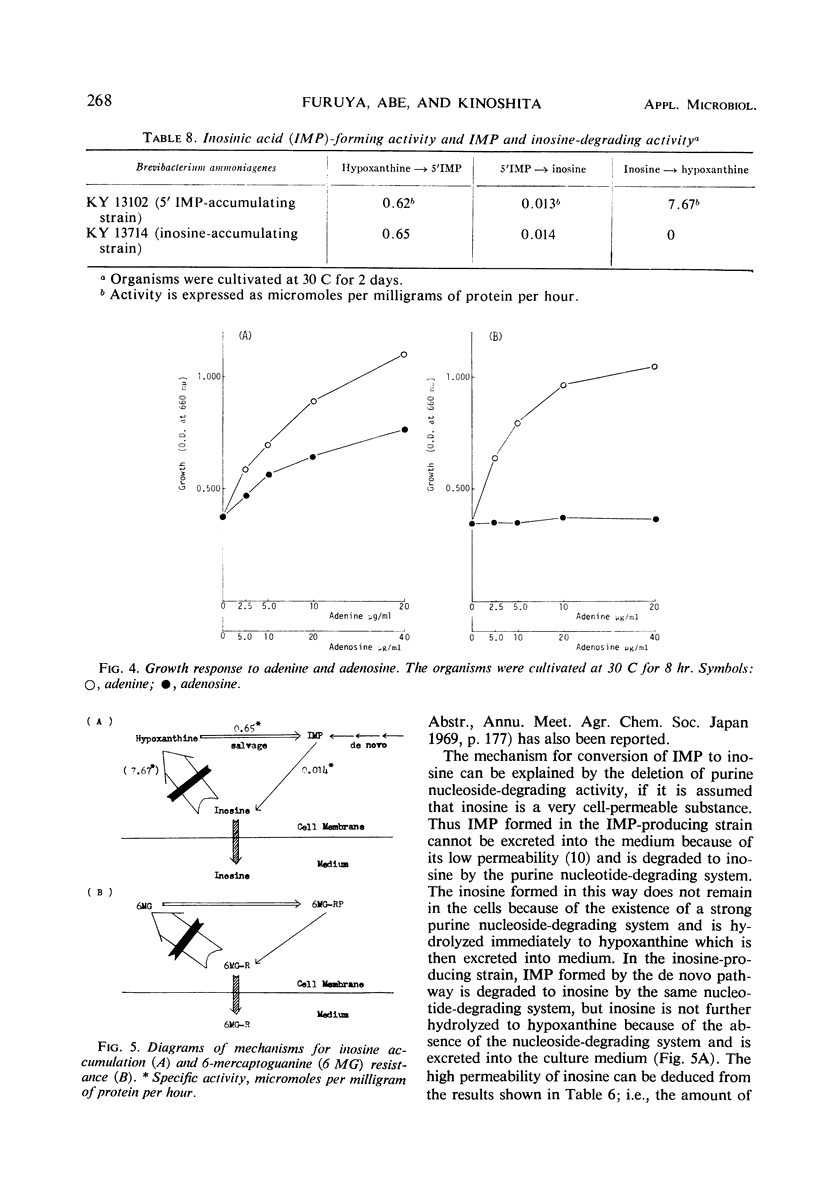
Inosine-producing cultures were found among mutants resistant to 6-mercaptoguanine (6MG) derived from a 5′-inosinic acid (IMP)-producing strain, KY 13102, of Brevibacterium ammoniagenes. Inosine-producing ability was very frequent among the mutants resistant to a low concentration (10 to 50 μg/ml) of 6MG. The accumulation of inosine by strain KY 13714 was stimulated by a low concentration of adenine (25 mg/liter) but was depressed by high levels of adenine. The accumulation by strain KY 13714 was not inhibited by manganese ion but instead was stimulated by its excess, in contrast to IMP accumulation by KY 13102. Addition of hypoxanthine at an early stage of cultivation accelerated inosine accumulation. Furthermore, on addition of hypoxanthine and of a surface-activating agent after 48 hr of cultivation, the simultaneous accumulation of IMP and inosine was observed. A 9.3-mg amount of inosine per ml accumulated after 4 days of cultivation at 30 C. The inosine-producing mutant did not differ from the IMP-producing strain either in 5′ purine nucleotide degradation or in IMP formation from hypoxanthine. However, it was found to be completely devoid of purine nucleoside-degrading activity. The conversion of IMP accumulation to inosine can be explained by the lack of nucleosidedegrading activity. The relationship between deficiency of nucleoside-degrading activity and resistance to low levels of 6MG is discussed, and a new mechanism for 6MG resistance is presented.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROCKMAN R. W., ANDERSON E. P. BIOCHEMISTRY OF CANCER (METABOLIC ASPECTS). Annu Rev Biochem. 1963;32:463–512. doi: 10.1146/annurev.bi.32.070163.002335. [DOI] [PubMed] [Google Scholar]
- Demain A. L., Jackson M., Viatali R. A., Hendlin D., Jacob T. A. Production of xanthosine-5'-monophosphate and inosine-5'-monophosphate by auxotrophic mutants of a coryneform bacterium. Appl Microbiol. 1965 Sep;13(5):757–761. doi: 10.1128/am.13.5.757-761.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber E., Shull K. H., McConomy J. M., Castillo A. E. The effects of inosine and aminoimidazole carboxamide upon the ethionine fatty liver. Biochem Pharmacol. 1965 May;14(5):761–767. doi: 10.1016/0006-2952(65)90094-8. [DOI] [PubMed] [Google Scholar]
- Furuya A., Abe S., Kinoshita S. Production of nucleic acid-related substances by fermentative processes. 28. Accumulation of 5' inosinic acid by a manganese-insensitive mutant of Brevibacterium ammoniagenes. Appl Microbiol. 1969 Dec;18(6):977–984. doi: 10.1128/am.18.6.977-984.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya A., Abe S., Kinoshita S. Production of nucleic acid-related substances by fermentative processes. XIX. Accumulation of 5'-inosinic acid by a mutant of Brevibacterium ammoniagenes. Appl Microbiol. 1968 Jul;16(7):981–987. doi: 10.1128/am.16.7.981-987.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTS J. S., GOLLUB E. G. Purine analogs as feedback inhibitors. Proc Soc Exp Biol Med. 1959 Aug-Sep;101:641–643. doi: 10.3181/00379727-101-25045. [DOI] [PubMed] [Google Scholar]
- MCCOLLISTER R. J., GILBERT W. R., Jr, ASHTON D. M., WYNGAARDEN J. B. PSEUDOFEEDBACK INHIBITION OF PURINE SYNTHESIS BY 6-MERCAPTOPURINE RIBONUCLEOTIDE AND OTHER PURINE ANALOGUES. J Biol Chem. 1964 May;239:1560–1563. [PubMed] [Google Scholar]
- SARTORELLI A. C., LEPAGE G. A., MOORE E. C. Metabolic effects of 6-thioguanine. I. Studies on thioguanine-resistant and-sensitive Ehrlich ascites cells. Cancer Res. 1958 Nov;18(10):1232–1239. [PubMed] [Google Scholar]
- STUTTS P., BROCKMAN R. W. A biochemical basis for resistance of L1210 mouse leukemia to 6-thioguanine. Biochem Pharmacol. 1963 Feb;12:97–104. doi: 10.1016/0006-2952(63)90175-8. [DOI] [PubMed] [Google Scholar]




