Abstract
Although the murine late pregnant (LP) heart is speculated to be a better functioning heart during physiological conditions, the susceptibility of LP hearts to I/R injury is still unknown. The aims of this study were to investigate the cardiac vulnerability of LP rodents to ischemia/reperfusion (I/R) injury and to explore its underlying mechanisms. In-vivo female rat hearts (non-pregnant (NP) or LP) or ex-vivo Langendorff-perfused mouse hearts were subjected to I/R.. The infarct size was ~4-fold larger in LP animals compared to NP both in-vivo and ex-vivo. The heart functional recovery was extremely poor in LP mice compared to NP (~10% recovery in LP vs. 80% recovery in NP at the end of reperfusion, P < 0.01). Interestingly, the poor functional recovery and the larger infarct size in LP were partially restored one day post-partum and almost fully restored one week post-partum to their corresponding NP levels. Mitochondrial respiratory function and the threshold for opening of the mitochondrial permeability transition pore were significantly lower in LP compared to NP when they both were subjected to myocardial I/R injury (Respiratory control ratio=1.9±0.1 vs. 4.0±0.5 in NP, P<0.05; calcium retention capacity(CRC)=167±10 vs. 233±18 nmol/mg protein in NP, P<0.01). Cardiac ROS generation, as well mitochondrial superoxide production, were ~2-fold higher in LP compared to NP following I/R. The phosphorylation levels of Akt, ERK1/2 and STAT3, but not GSK3β, were significantly reduced in the hearts from LP subjected to I/R. In conclusion, increased mitochondrial ROS generation, decreased CRC as well as impaired activation of Akt/ERK/STAT3 at reperfusion are the possible underlying mechanisms for higher vulnerability of LP hearts to I/R.
Keywords: Pregnancy, ischemia/reperfusion, heart hypertrophy, mPTP, ROS
INTRODUCTION
Ischemic heart disease in pregnancy is not very common. However, increased maternal age together with changes in women’ lifestyle patterns (stress, smoking, diabetes, hypercholesterolemia, chronic hypertension and alcohol, together with physical inactivity) has dramatically increased the incidence of ischemic heart disease in pregnant women older than 33 years of age over the past decade [32;42;57;58]. Due to increased cardiac workload and greater myocardial oxygen demand, most myocardial infarctions occur during the third trimester, which is associated with significant maternal mortality and morbidity that can jeopardize the fetus’ life. Acute coronary artery dissection and thrombosis in the setting of angiographically with normal coronary arteries may also lead to myocardial infarction in pregnancy [33]. The best hope of salvaging viable myocardium after a coronary occlusion is by rapid reperfusion [14]. Although reperfusion restores blood flow, oxygen, and nutrients to the cardiac muscle, it also has the potential to induce reperfusion injury.
The mPTP is a large non-selective conductance pore located in the inner membrane of mitochondria. The mPTP remains closed during ischemia, but opens during the reperfusion period [24;44]. The opening of the mPTP during reperfusion has been implicated in cell death [16;26;28;72]. Ca2+ accumulation and overproduction of ROS during reperfusion are the two major triggers of the opening of the mPTP. Several signaling pathways in preconditioning and postconditioning converge on the mitochondria to protect the heart against reperfusion injury. Among them Reperfusion Injury Salvage Kinases (RISK) pathway, which involves the kinases Akt/ERK1/PI3 as well as more recently recognized Survivor Activating Factor Enhancement (SAFE) pathway which involves TNFa/JAK/STAT3 have received a lot of attention [3;25;29;30;37;63].
Although the incidence of ischemic heart disease is increasing in pregnant women, there is still a paucity of experimental data on the mechanisms responsible for these events. Here we compared the postischemic cardiac function and infarct size of late pregnant (LP) rodents to ischemia/reperfusion (I/R) injury with non-pregnant animals both in-vivo to take into account the complex physiology of pregnancy, as well as in ex-vivo Langendorff-perfused mouse hearts. We found that in the in-vivo rat model of I/R injury, the cardiac infarct size of LP rats was ~4 fold larger compared to NP rats. Consistent with much larger infarct size during late pregnancy, the heart functional recovery was extremely poor in LP ex-vivo animal hearts. The higher susceptibility of LP hearts to I/R injury was associated with a lower threshold for triggering the mitochondrial permeability transition pore (mPTP) opening in response to Ca2+ overload which may at least be in part due to higher ROS generation and downregulation of pro-survival signaling.
METHODS
Animals
Female mice (C57BL/6, 2–3 months old) non-pregnant at diestrus stage (NP), late pregnant (LP, day 19–20 of pregnancy), one day and seven days post-partum (PP1 and PP7) and female rats (Sprague-Dawley, 2–3 months old) NP (at diestrus stage) and LP were used. Protocols received institutional approval.
Coronary artery occlusion and measurement of infarct size
Female rats were anesthetized with ketamine (80 mg/kg i.p.) and xylazine (8 mg/kg i.p.). The coronary artery ligation procedures as well as measurements of infarct size are described in detail in the supplementary material.
Langendorff preparation
The hearts were perfused in the Langendorff mode-constant flow (3ml/min) as described in our previous publication [54]. After equilibration, hearts were subjected to 20min of global normothermic (37°C) ischemia, followed by reperfusion (40 min for function and infract size, 10min for CRC and Western blot analysis, and 5min for ROS generation) with Krebs Henseleit (KH). See supplementary materials for more details.
Ca2+-induced mitochondrial permeability transition
Mitochondria were isolated as previously described [54]. The mPTP opening was assessed following in-vitro Ca2+ overload [54]. External mitochondrial Ca2+ concentration was recorded with 0.5µM calcium green-5N (Invitrogen) using excitation and emission wavelengths set at 500 and 530nm, respectively. CaCl2 pulses (20nmol/mg of mitochondrial protein) were applied every 60sec in the spectrofluorometer and CRC was defined as the amount of Ca2+ required to trigger the massive Ca2+ release. See supplementary materials for more details.
Measurement of Mitochondrial Respiration
Mitochondrial respiration in state 3 (stimulated by 0.2 mM ADP) and state 4 (ADP-limited) was measured in freshly isolated mitochondria using a fiber optic oxygen sensor FOXY-AL300 (Ocean Optics) at 25°C as reported [39]. Substrates combinations of 1.6 mmol/L pyruvate plus 1.6 mmol/L L-malate and 1.6 mmol/L glutamate were added to the mitochondria to measure complex I. The Respiratory Control Ratio (RCR) was measured by dividing the respiratory values in state 3 to state 4 and were normalized to mg of mitochondrial protein.
Measurement of cardiac function
A catheter (1.4F Millar SPR-671) connected to a pressure transducer (Power Lab, AD Instruments) was directly inserted into the left ventricle (LV) to measure left ventricular systolic pressure (LVSP), left ventricular end-diastolic pressure (LVEDP) and heart rate (HR). The left ventricular developed pressure (LVDP) was calculated as LVDP =LVSP−LVEDP and the rate pressure product (RPP) as RPP=HR×LVDP. The maximum rate of rise of LV pressure (dP/dtmax) and the maximum isovolumetric rate of relaxation (−dP/dtmin) were directly calculated from the recordings.
Myocardial necrosis
At the end of reperfusion, the hearts were cut into slices and myocardial necrosis was assessed by measurement of the infarct size using triphenyltetrazolium chloride (TTC) staining [6]. The slices were fixed in 4% paraformaldehyde. The area of necrosis was quantified by Adobe Photoshop and expressed as the percentage of total ventricular (LV) area.
Dihydroethidium (DHE) staining and electron spin resonance for measurements of ROS production
At the end of reperfusion, freshly processed frozen cardiac tissue sections were used to measure ROS production using dihydroethidium (DHE) method. Images were obtained with a laser scanning confocal microscope (Olympus) at identical laser settings. Image J was then used to quantify DHE fluorescence intensity. Using electron spin resonance (ESR), superoxide production were also measured by mixing freshly isolated mitochondria with the O2•−-specific spin probe in the presence or absence of 100 U/ml of SOD as previously reported [12;49;69;70]. The SOD-inhibitable O2•− signals at 10 min time point, normalized by protein concentrations, were compared among different experimental groups.
Western Blot analysis
Standard Western Blot analysis was performed using ex-vivo whole heart lysates. The details of the Western blot analysis and the antibodies used are given in the supplementary materials.
Statistical Analysis
For the in-vivo study which has only 2 groups, means were compared using the t-test. For the ex vivo studies, mean profiles over time were compared across groups using the repeated measure analysis of variance (ANOVA) method. For cardiac infarct size in ex vivo, mitochondrial calcium retention capacity, mitochondrial respiration and ROS production, means were compared between groups using one-way ANOVA. When significant differences were detected, individual mean values were compared by a post hoc test, which allowed for multiple comparisons. SPSS, version 13.0, (SPSS Inc, Chicago Ill) was used to carry out the computations. As all outcomes were continuous, results were summarized with Means ± standard errors of the mean (SEM). P < 0.05 was considered statistically significant.
RESULTS
The in-vivo cardiac infarct size is larger in LP rats subjected to ischemia/reperfusion injury
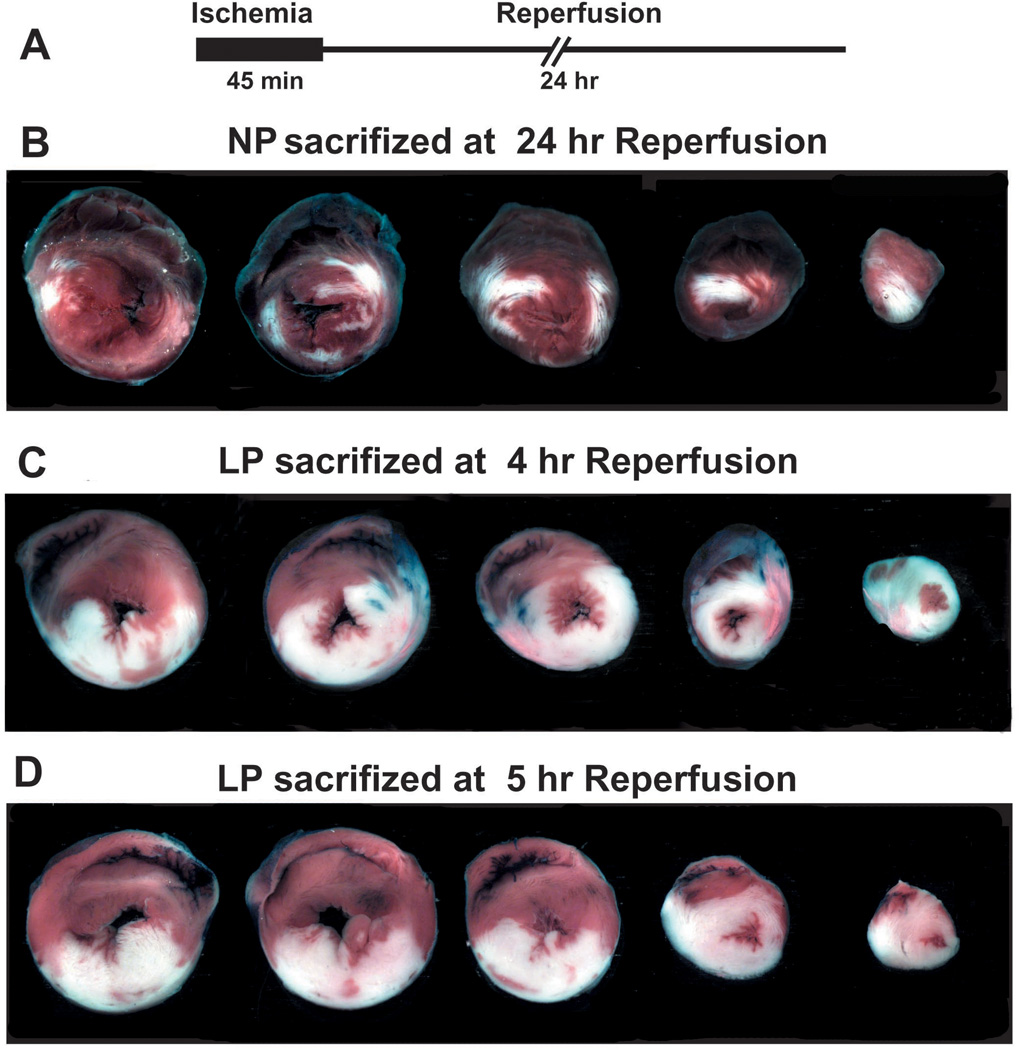
Here we first compared the susceptibility of LP hearts to ischemia/reperfusion injury with NP hearts in the in-vivo rat model. The left coronary artery was occluded for 45 min followed by 3 hrs of reperfusion (Fig. 1A). Representative cross sections of NP and LP hearts are shown in Fig. 1B. Although the two groups were subjected to a comparable degree of ischemic risk (the area at risk (AAR)/LV=58.6±3.3% in LP vs. 62.8±1.8% in NP) (Fig. 1C), the infarct size was significantly larger in the LP group compared to NP. The ratio of infarct size to AAR was 53.0±4.2% in LP vs. 12.5±1.4% in NP and the ratio of infarct size to LV was 31.8±4.1% in LP vs. 7.8±0.8% in NP (P<0.01) (Fig. 1 D, E). When rats were subjected to 45 min ischemia followed by 24hr reperfusion in NP and LP groups, unfortunately none of the LP rats survived 24 hr reperfusion. Unlike LP rats, all NP rats survived 24 hr reperfusion, the infarct size in NP subjected to 24 hr reperfusion (IS/AAR=35.3±4.8) was significantly larger than that in NP subjected to 3hr reperfusion (Fig. S). These data clearly demonstrate that LP hearts have much higher vulnerability to ischemia/reperfusion injury than NP.
Figure 1. The infarcts size is larger in LP rats subjected to ischemia/reperfusion injury than NP.
A. experimental protocol, the LAD was occluded in NP and LP rats for 45minutes followed by 3 hr of reperfusion. B. Representative heart cross sections of NP and LP rats. Percentage of area at risk (AAR) divided by LV (C), infarct size (IS) divided by AAR (D) and infarct size (IS) divided by LV (E). **p<0.01 vs. LP (n = 4–6).
Poor post-ischemic functional recovery and larger infarct size of LP mouse hearts subjected to ischemia/reperfusion injury
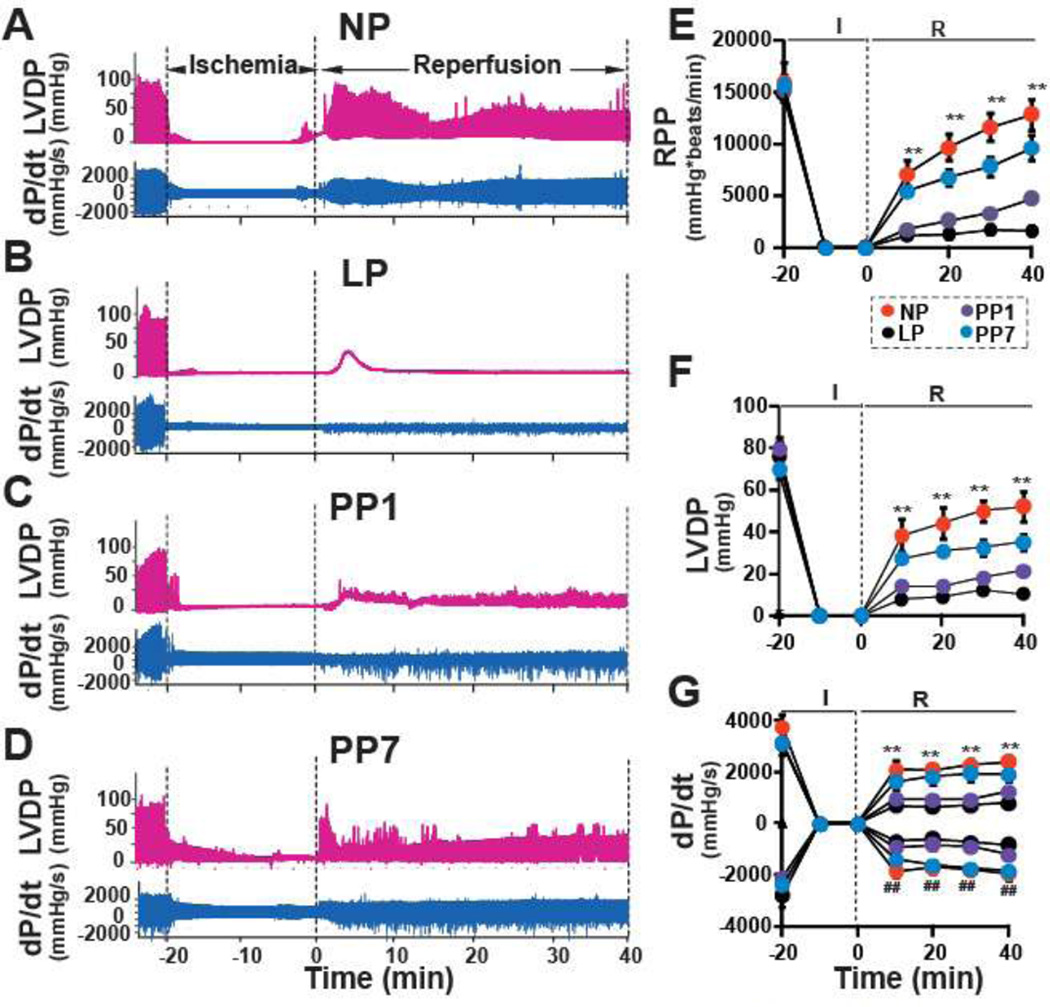
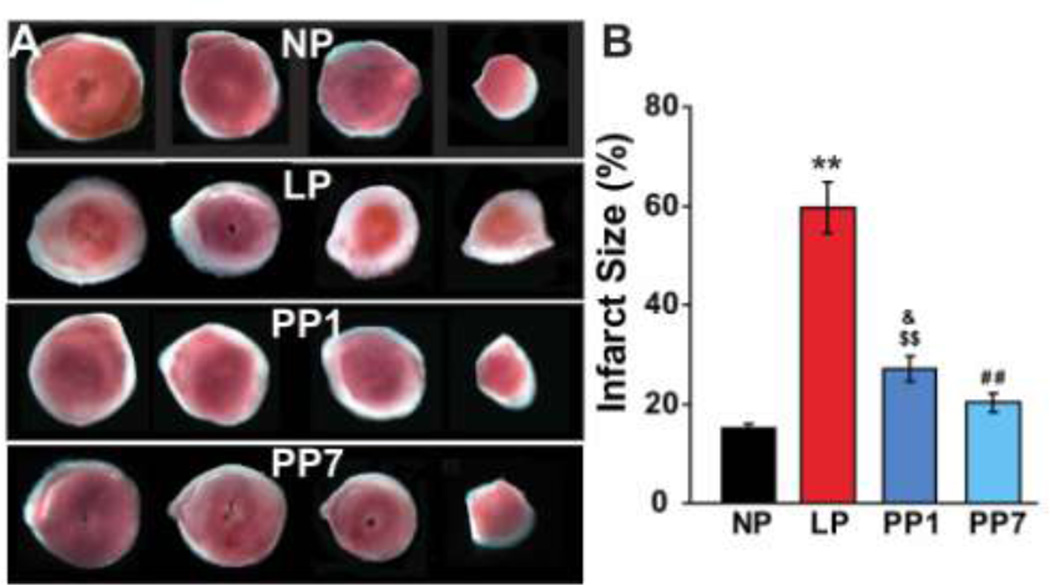
To determine whether the high vulnerability of the LP hearts to ischemia/reperfusion (I/R) injury is due to intrinsic characteristics of the heart independent of the complex physiology of pregnancy, Langendorff-perfused isolated mouse hearts were subjected to I/R injury using the well-established protocol in mice [6;34;54]. The hemodynamic parameters were similar between NP and LP before ischemia, as the rate pressure product (RPP) in LP (14751±771 mmHg*beats/min) was not significantly different from NP (15971±1539 mmHg*beats/min, Fig. 2A,B). However, after ischemia, the functional recovery was extremely poor in LP mice compared to NP mice (~10% recovery in LP vs. 80% recovery in NP at the end of reperfusion, p < 0.01). RPP was reduced from 12818±1213 mmHg*beats/min at the end of 40 min of reperfusion in NP to 1617±287 mmHg*beats/min in LP mice. LP group also showed a significantly reduced LVDP as well as LV dP/dtmax and LV dP/dtmin compared to NP hearts (Fig. 2C,D). Interestingly, all of the hemodynamic parameters recovered partially in hearts obtained from one day post-partum mice (PP1) and further improvements were observed in hearts seven days (PP7) post-partum (e.g. RPP=4715±479mmHg*beats/min in PP1 and 9604±1215 mmHg*beats/min in PP7). Consistent with the poor functional recovery in LP, the infarct size was also markedly larger in LP (57.4±5.2%) compared with NP (16.3±1.3%, Fig 3A,B) mice. The infarct size was partially restored in the PP1 group (28.5± 2.9%) and reached similar values as NP hearts one week post-partum (20.8±2.0%). These data demonstrate that pregnancy increases the intrinsic vulnerability of the murine heart to ischemia/reperfusion injury. Interestingly, the higher susceptibility to reperfusion injury is partially restored one day after partum and almost fully eliminated one week post-partum, suggesting that acquiring such vulnerability is a highly dynamic and reversible process.
Figure 2. Poor heart functional recovery of LP mice subjected to I/R injury restored 7 days postpartum.
Representatives of the left ventricular developed pressure (LVDP) and dP/dtMax and dP/dtMin as a function of time in NP (A), LP(B), PP1(C) and PP7(D). Rate Pressure Product (RPP)(E), LVDP (F) and dP/dtMax and dP/dtMin (G) as a function of time in NP(squares), LP(triangles), PP1(filled circles) and PP7(open circles). ** p < 0.01 vs. LP or PP1 in RPP,LVDP and dP/dtMax, # # p < 0.01 vs LP or PP1 in dP/dtMin (n = 6).
Figure 3. Larger infarct size of isolated LP hearts subjected ischemia/reperfusion injury was fully reversed one week postpartum in mice.
A. Four slices of the same heart in each group after TTC staining. The white area represents the infarct zone and the red shows the viable area. B. The area of necrosis as the percentage of total ventricular area, **p <0.01 LP vs. NP; ##p<0.01 PP1 vs. LP; $$p<0.01 PP1 vs. LP; &p<0.05 PP1 vs. NP; ^ p<0.05 PP7 vs. PP1; p >0.05 NP vs. PP7(n=6).
Lower mitochondrial respiratory function in LP hearts subjected to ischemia/reperfusion injury
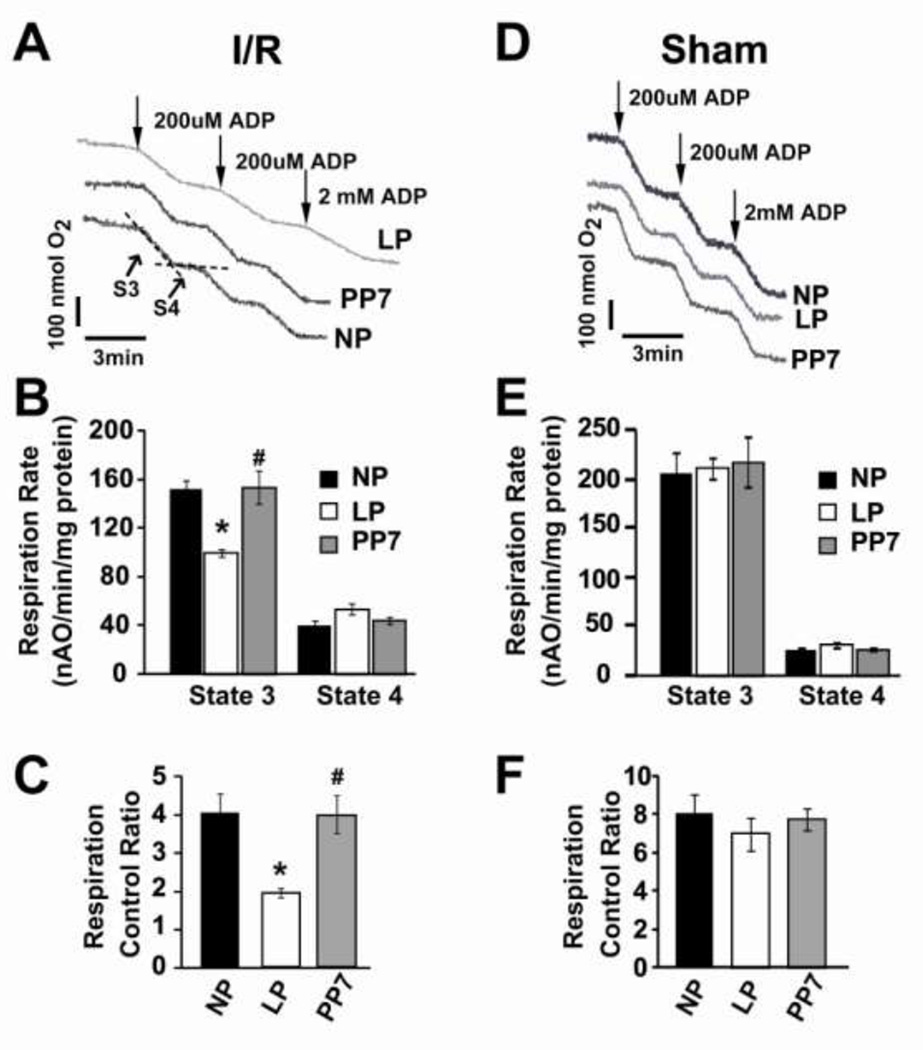
We compared the mitochondrial respiratory function isolated from the hearts of NP, LP and PP7 following ischemia/reperfusion injury. The visual inspection of oxygen electrode traces in isolated cardiac mitochondria revealed a shallower slope of state 3 (the ADP-stimulated rate) in LP compared to NP and post-partum (Fig. 4A). The significantly lower state 3 oxidative rates (ADP-stimulated), but not state 4 (resting state, ADP-limited, Fig. 4B) in LP resulted in significantly lower respiratory control ratio compared to NP and PP7 (RCI=1.9±0.1 in LP, 4.0±0.5 in NP and 3.9±0.5in PP7, P<0.05 LP vs. NP and PP7, Fig 4C). Interestingly, if the LP hearts were not subjected to ischemia/reperfusion injury (sham), the oxidative rates and respiration control index in LP group was not significantly different than NP and PP7 groups (RCI=8.0±0.9 in NP; 7.0±0.8 in LP and 7.7±0.5 in PP7, Fig. 4D–F).
Figure 4. Mitochondrial respiration decreased in LP heart subjected to ischemia reperfusion injury.
A, D. Typical oxygen electrode traces showing the respiration in state 3 stimulated by 0.2 mM ADP (S3) and in resting state 4 (ADP-limited, S4) of complex-I in isolated mitochondria from NP, LP and PP7 subjected ischemia/reperfusion injury (A), or not subjected to ischemia/reperfusion injury (sham, D). B, E. Respiration rate of state 3 and state 4. and C, F. Respiratory control ratio (respiration rate of state 3/state 4) in NP, LP and PP7, **p <0.05 LP vs. NP; #p<0.05 PP7 vs. LP; p >0.05 NP vs. PP7(n=4).
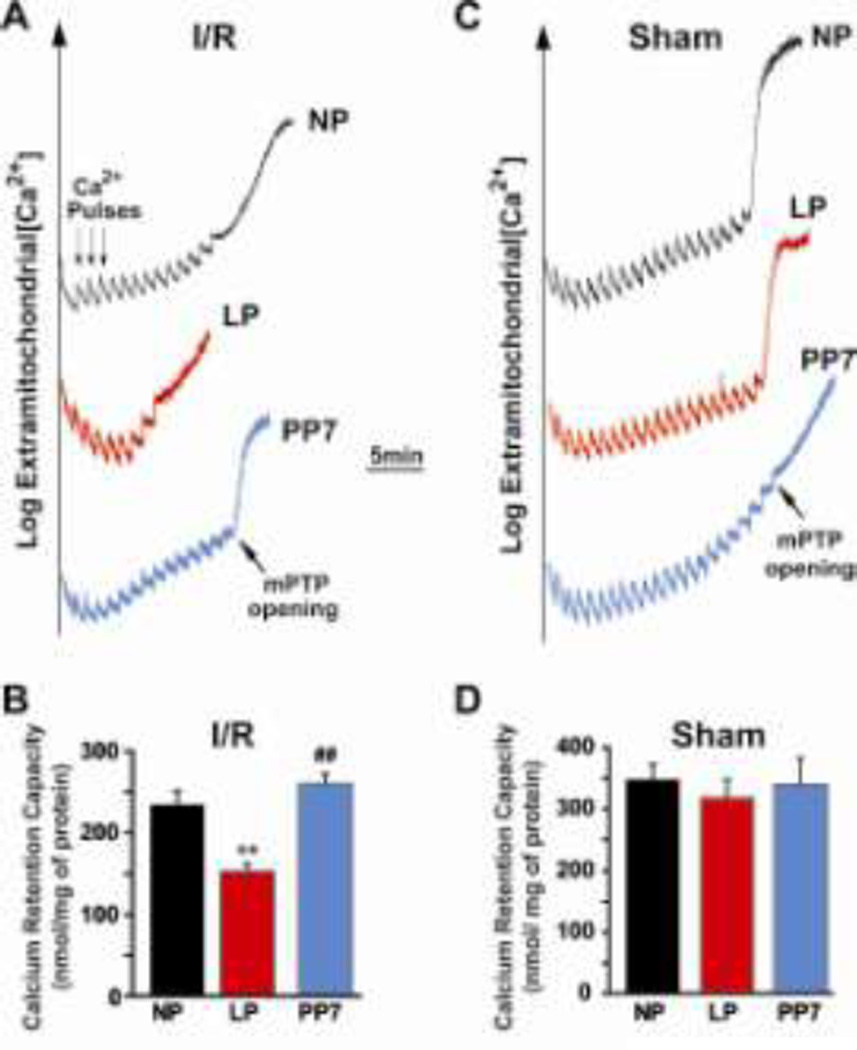
Lower threshold for triggering the opening of the mPTP to Ca2+ overload in LP hearts subjected to ischemia/reperfusion injury
Since the opening of the mPTP at the onset of reperfusion has been implicated in cell death, we investigated whether higher susceptibility of LP hearts to I/R injury is associated with less resistance of the mPTP opening in response to Ca2+ overload. We compared the threshold for mPTP opening in response to calcium overload using purified mitochondria prepared from NP, LP and PP7 hearts following I/R. The representative data showing the change of Ca2+ concentration in the mitochondrial external medium after calcium pulses are shown in Fig. 5A. The average number of calcium pulses to open the mPTP was 12 in NP, while 7 pulses of Ca2+ were sufficient in the LP group to trigger the opening of the mPTP. Interestingly, the reduced calcium retention capacity (CRC) in LP hearts subjected to I/R was fully restored to NP levels 7 days after delivery. The bar plot in Fig. 5B summarizes the CRC. CRC in LP hearts (167±10 nmol/mg-mitochondrial protein) was significantly lower compared with NP (233±18 nmol/mg-mitochondrial protein) and PP7 (260±12 nmol/mg-mitochondrial protein, p<0.01). To examine whether the lower CRC in LP mice is an intrinsic property of pregnancy and is independent of I/R, we compared CRC in isolated mitochondria from NP and LP hearts which were not subjected to I/R (sham). Interestingly, the number of calcium pulses required to trigger the opening of the mPTP were similar in NP and LP sham hearts (~20 pulses), which resulted in similar CRC values (318±30 nmol/mg-mitochondrial protein in sham LP hearts vs. 348±26 in NP; 340± 42 nmol/mg-mitochondrial protein in PP7, Fig. 5C,D). In summary, the higher vulnerability of LP hearts to I/R injury is associated with lower threshold for triggering mPTP opening in these hearts. However, late pregnancy alone is not sufficient to trigger this change.
Fig. 5. Lower threshold for triggering mPTP opening in response to calcium overload in LP compared to NP and PP7.
A. Typical recordings of the mPTP opening in isolated mitochondria from NP, LP and PP7 groups subjected to 20min of global ischemia followed by 10min of reperfusion. Twelve pulses (arrows) of 20 nmol calcium were required to trigger the opening of mPTP in NP compared to 7 pulses in LP and 14 pulses in PP7. B. Calcium retention capacity (CRC) in NP (white bar), LP (black bar) and PP7 (gray bar) subjected to ischemia/reperfusion injury. C. Typical recordings of the mPTP opening in isolated mitochondria from NP, LP and PP7 groups not subjected to ischemia/reperfusion injury. D. Calcium retention capacity (CRC) in NP (white bar), LP (black bar) and PP7 (gray bar) in mice not subjected to ischemia/reperfusion injury, **p <0.01 LP vs. NP; ##p<0.01 PP7 vs. LP; p >0.05 NP vs. PP7(n=6).
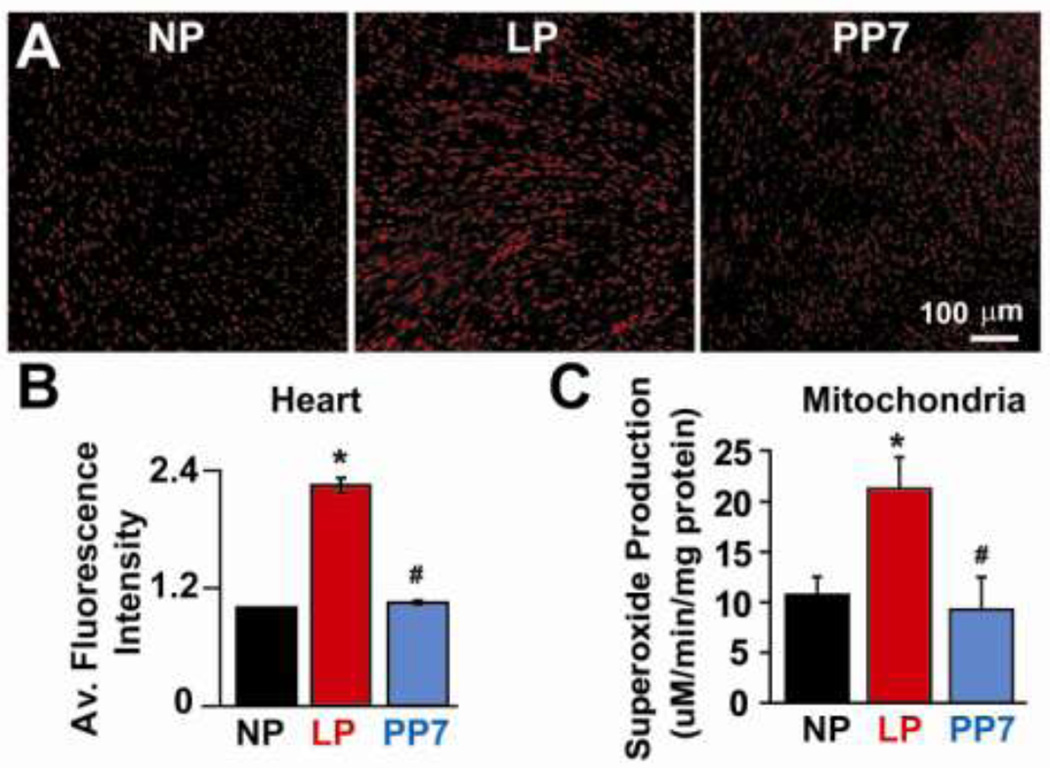
Cardiac ROS generation as well as mitochondrial superoxide production are increased in late pregnant hearts subjected to ischemia/reperfusion injury
Free radicals are known to contribute significantly to the ischemic pathophysiology. ROS is one of the major contributors to the opening of mPTP during reperfusion. We therefore investigated whether the lower mitochondrial threshold for opening of the mPTP in LP hearts could be at least in part due to increased cardiac ROS generation and/or superoxide production in isolated cardiac mitochondria in LP hearts subjected to ischemia/reperfusion injury. Oxyethidium signal in the DHE-stained cardiac sections was significantly higher in LP hearts subjected to ischemia/reperfusion injury and returned to almost NP levels one week after partum (normalized to NP: 2.25±0.07 in LP and 1.05±0.02 in PP7, p<.05 LP vs NP and PP7. Fig. 6A,B). Since the mitochondria may represent the major sources of ROS production after I/R, we performed electron spin resonance (ESR) to quantify the superoxide production in isolated mitochondria. The superoxide production was also significantly higher in isolated cardiac mitochondria from LP hearts subjected to ischemia/reperfusion injury (10.7±1.7µM/min/mg protein in NP; 21.3±3.1µM/min/mg protein in LP and 9.3±3.3µM/min/mg protein in PP7; p<.05 LP vs. NP and PP7. Fig. 6C). Therefore, lower mitochondrial threshold for calcium overload in pregnancy may in part be due to increased cardiac ROS production as well as mitochondrial superoxide generation.
Figure 6. Increased cardiac ROS generation as well as mitochondrial superoxide production in late pregnant hearts subjected to ischemia/reperfusion.
A. Representative images of DHE staining of cardiac sections of NP, LP and PP7. B. Average fluorescence intensity in NP (black bar), LP (red bar) and PP7 (gray bar). C. Superoxide production in isolated mitochondria from NP, LP and PP7 using electron spin resonance, *p <0.05 LP vs. NP; #p<0.05 PP7 vs. LP; p >0.05 NP vs. PP7(n=4–5).
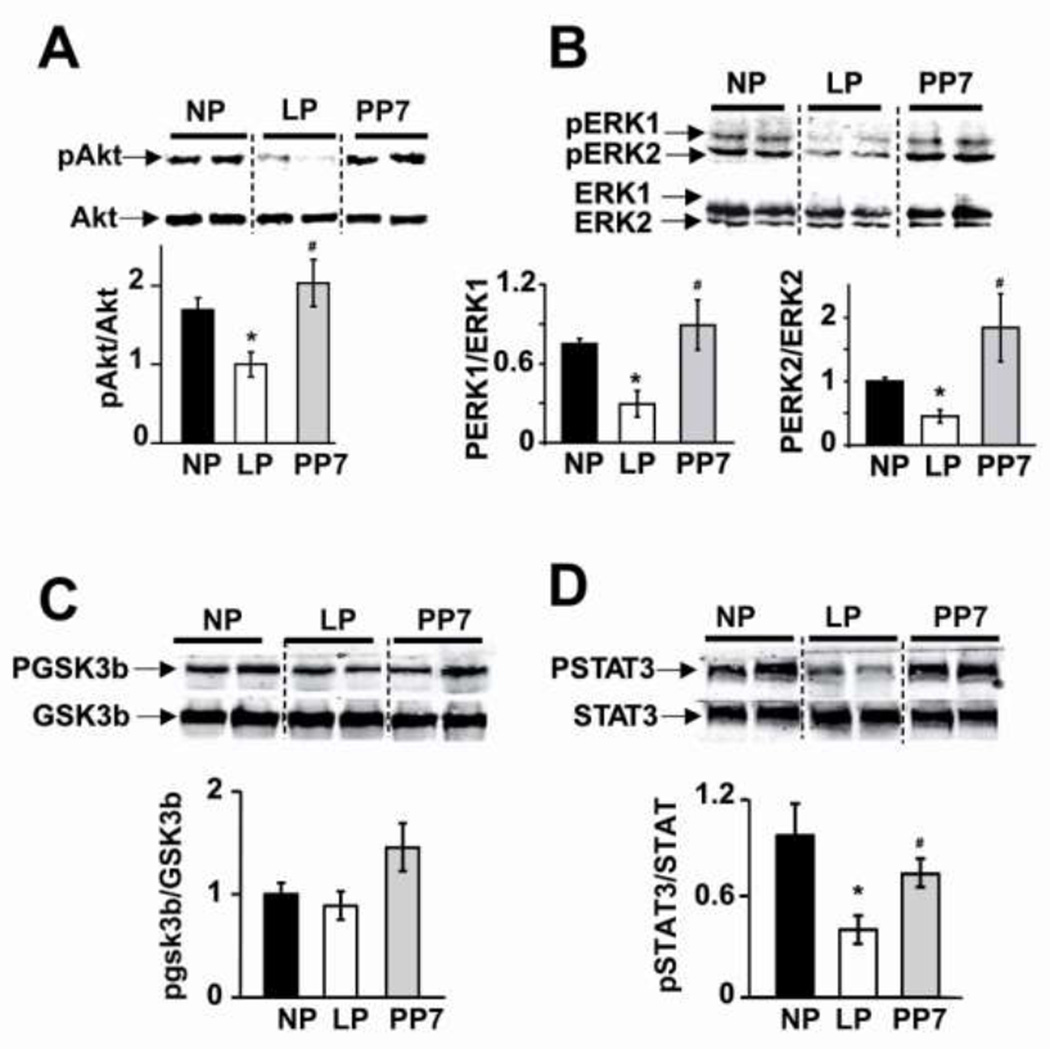
Reduced phosphorylation levels of Akt, ERK1/2 and STAT3, but not GSK3β, in LP hearts subjected to ischemia/reperfusion injury
We performed western blot analysis to explore whether the higher susceptibility of LP hearts to I/R injury are associated with downregulation/inactivation of the two well established pro-survival signaling pathways of Reperfusion Injury Salvage Kinase (RISK) and survivor activating factor enhancement (SAFE) pathway in the context of I/R injury. We also examined the involvement of GSK3β as many signaling pathways converge at GSK to either inhibit or trigger the opening of mPTP [22;65]. Phosphorylation levels of Akt and ERK1/2 from the RISK pathway were significantly downregulated in the hearts of LP subjected to ischemia/reperfusion injury and were all fully reversed one week after delivery (normalized to NP: pAkt/Akt: 0.59±0.15 in LP and 1.19±0.29 in PP7; pERK1/ERK1: 0.39±1.3 in LP, 1.1±0.25 in PP7; pERK2/ERK2: 0.45±0.09 in LP, 1.8±0.5 in PP7, P<0.05 LP vs. NP and PP7 Fig. 7). Pregnancy was also associated with decreased phosphorylation levels of STAT3 from the SAFE pathway (normalized to NP: pSTAT3/STAT3: 0.41±0.08 in LP, 0.76±0.08 in PP7; P<0.05 LP vs. NP and PP7). There was no significant difference in the phosphorylation level of GSK3β in NP, LP and PP7 subjected to I/R injury. These data support the view that deactivation of Akt/ERK and STAT3 signaling pathways in LP confers greater susceptibility to ischemia.
Figure 7. Reduced phosphorylation levels of Akt, ERK1/2 and STAT3 in LP hearts subjected to ischemia/reperfusion injury.
Representative immunoblots and Western Blot analysis of A. pAkt/Akt, B. pERK1/2/ERK1/2, C. pGSK3β/GSK3β and D. pSTAT3/STAT3 in heart homogenates subjected to ischemia/reperfusion from NP, LP and PP7, *p <0.05 LP vs. NP; #p<0.05 PP7 vs. LP; p >0.05 NP vs. PP7(n=4–7).
DISCUSSION
Characterization of physiological heart hypertrophy during pregnancy [18] led us to speculate that the pregnant heart is “a better functioning heart,” as cardiac pumping capacity is enhanced in response to increased demand, making the heart mechanically more efficient [19]. However, the late pregnant heart had an increased susceptibility to cell stress as the infarct size was about 4 fold higher than non pregnant hearts when the heart was subjected to ischemia/reperfusion injury (Fig. 1, 3). The left ventricular functional recovery was also extremely poor (recovery of about 10%) in LP hearts (Fig. 2). Interestingly, the poor functional recovery and the larger infarct size in late pregnancy were partially restored one day post-partum and almost fully restored one week post-partum to their corresponding levels in NP hearts. The higher susceptibility of LP hearts to I/R injury was associated with lower mitochondrial respiratory function as well as lower threshold for triggering the mPTP opening in response to Ca2+ overload. Increased mitochondrial ROS production together with the inhibition of the two well known prosurvival pathways RISK and SAFE in LP are the potential mechanisms triggering the opening of the mPTP and thus leading to a larger infarct size. Consistent with the functional and morphology restoration in PP7, all molecular abnormalities in LP such as decreased mitochondrial respiration and calcium retention capacity, increased ROS production, as well as the deactivation of RISK and SAFE signaling pathway were all fully reversed one week after partum to their corresponding levels in NP suggesting that acquiring such vulnerability in LP is a highly dynamic and reversible process. In our study, we demonstrated this phenomenon in two different rodent species. The strength of our study is the use of in vivo rat model followed up by isolated mouse heart perfusion studies to eliminate the confounding factor in the in vivo work of the extracardiac hemodynamics of the pregnant state.
Mitochondrial dysfunction in late pregnant hearts subjected to I/R injury
Mitochondria play a key role in protection against cell injury [59;60]. Our data demonstrate that the higher susceptibility of LP hearts to I/R injury is associated with lower mitochondrial respiratory function, calcium retention capacity and increased mitochondrial ROS production (Figs. 4–6). It is well accepted that Ca2+ accumulation and overproduction of ROS during reperfusion are the two major triggers of the mPTP opening [15;38;45;67]. These free radicals in the heart are usually produced during the first few minutes of reperfusion [38;45]. The mitochondria are considered to be an important locus of ROS production, mainly at the level of complexes I and III of the respiratory chain and hence potential contributors to cardiac reperfusion injury [36;40]. As a major source of ROS production, mitochondria also could be major targets of injury caused by ROS [23]. Due to the role played by ROS in the opening of the mPTP, it is possible that a higher ROS generation in late pregnancy during I/R may promote the opening of the mPTP, and this may also contribute to aggravating mitochondrial dysfunction and cardiac damage. Our results are consistent with this view, showing both increased ROS generation and decreased CRC which can contribute to the opening of the mPTP. However, further experimentation is required to clarify whether ROS or Ca2+ is the most critical factor responsible for mPTP induction in the LP heart.
Cardiac function in late pregnancy
Physiological heart hypertrophy which occurs during pregnancy differs from pathological hypertrophy, as in pregnancy, the pumping capacity of the heart is enhanced in response to increased demand, making the heart mechanically more efficient [18;19]. Here we report that the LV function before ischemia is similar between NP and LP (Fig. 2A). The threshold for opening of the mPTP, as well as the mitochondrial respiratory function are also not significantly different between mitochondria isolated from NP and LP sham hearts that were not subjected to I/R injury (Figs. 4,5). However, when these hearts are subjected to I/R injury, the functional recovery is extremely poor and the infarct size is about 4 times larger in LP compared to NP rodents. Therefore, although the function of the late pregnant heart seems to be preserved under physiological conditions at the baseline, these hearts are very sensitive to ischemia/reperfusion injury. Whether the enhanced susceptibility of late pregnancy to ischemia-reperfusion injury makes the heart more susceptible to other forms of cell stress in the late pregnancy is not clear and needs to be investigated elsewhere.
What makes the late pregnant heart more prone to ischemia/reperfusion injury?
The major hemodynamic changes induced by pregnancy include an increase in cardiac output, sodium and water retention leading to volume overload, and reductions in systemic vascular resistance and systemic blood pressure. These changes begin early in pregnancy, reach their peak during the second trimester, and then remain relatively constant until delivery [13]. These drastic hemodynamic changes during pregnancy may contribute to higher vulnerability of these hearts to additional insult. These cardiovascular physiologic changes resolve slowly after delivery. A study that evaluated cardiac output and stroke volume in 15 healthy nonlaboring patients at 38 weeks of gestation, and again at 2, 6, 12, and 24 weeks postpartum demonstrated a gradual diminution in cardiac output from 7.42 L/min at 38 weeks gestation to 4.96 L/min at 24 weeks postpartum [56]. Here we found that the heart functional recovery one week after partum is fully restored to NP values. The heart also develops physiological hypertrophy during pregnancy. Physiological heart hypertrophy which occurs during pregnancy in response to volume overload and hormonal stimuli enables the heart to fulfill its function without significant long-term detrimental effects on cardiac function [9;11;17;53;62]. Similar to pregnancy, in exercise-induced hypertrophy myocardial function has been demonstrated to be increased in rats [52] as well as in dogs [55]. However, it is still not known whether these hearts are better protected against ischemia/reperfusion injury compared to non-exercised animals. The pregnancy-induced heart hypertrophy however differs from exercise-induced hypertrophy, as it is also associated with drastic hormonal changes. Both estrogen and testosterone steadily increase and reach their maximum levels at the end of pregnancy. Our group previously reported that E2 treatment of OVX mice prolongs left ventricular (LV) action potential duration leading to longer QT interval as was observed in LP mice [18;61]. In the context of I/R injury, estrogen treatment of ovariectomized (OVX) mice resulted in smaller infarct size [35]. Reports regarding the role of testosterone on the cardiac functional recovery or infarct size have been conflicting, and testosterone therapy of gonadectomized female or male rats has also been shown to have no deleterious effects on myocardial function or infarct size after ischemia [47;48]. Callies et al. reported that testosterone therapy reduces the susceptibility of male rats to myocardial ischemia [8]. Therefore, the higher vulnerability of LP hearts to ischemia/reperfusion injury can not be due to the high levels of circulating estrogen or testosterone.
It has been shown that in healthy pregnant women, both oxidative stress and antioxidant capacity increase as pregnancy advances [66]. Therefore, under physiological conditions, the pregnant woman has sufficient antioxidant reserves to cope with the production of free radicals. However, ischemia/reperfusion injury may disrupt the delicate balance between ROS production and antioxidative activity leading to accumulation of ROS in the myocardium and mitochondria, as we observed in LP mice (Fig. 6), therefore deteriorating the heart function.
Activation of pro-survival kinases PI3K/Akt/ERK from the RISK pathway has been demonstrated to protect cardiomyocytes against various injury [10;20;27]. GSK3β phosphorylation has also emerged as an end effecter step where multiple protective signaling pathways converge resulting in mPTP inhibition [2;2;7;21;46;68]. Here we report that the phosphorylation levels of Akt and ERK are significantly reduced in LP hearts at reperfusion (Fig. 7). Impaired activation of Akt and ERK1/2 in LP heart at reperfusion could promote the opening of mPTP. However, since the phosphorylation levels of GSK3β, a downstream target of both Akt and ERK1/2 was not altered at reperfusion in LP hearts, the triggering of the pore opening does not seem to be mediated through GSK3β. Unaltered GSK phosphorylation in LP may suggest that alteration in Akt phosphorylation is a less likely modulation of susceptibility to mitochondrial permeability transition by late pregnancy. However, impaired activation of Akt may modulate intracellular calcium handling by decreasing sarcoplasmic reticulum calcium uptake [1] and/or changing mitochondrial morphology in a way which makes the mPTP more prone to open [50].
The activation of the Survivor Activating Factor Enhancement (SAFE) pathway, which includes the activation of the cytokine tumor necrosis factor alpha (TNFα) and the transcription factor signal transducer and activator of transcription-3 (STAT3), is recently recognized as a ‘RISK-free’ pathway that can also confer cardioprotection [4;5;43;51;64]. The role of STAT3 has been highlighted in cardioprotection as ischemic postconditioning failed to protect STAT3-deficient mice [41]. Here we found that the phosphorylation of pSTAT3 was downregulated during reperfusion in LP hearts. Reduced pSTAT3 levels will contribute to earlier opening of mPTP, as recently shown [3]. STAT3 has also been shown to be necessary for protecting mice against peripartum cardiomyopathy, as female mice with a cardiomyocyte-specific deletion of STAT3 develop postpartum cardiomyopathy with a very high mortality rate. STAT3 protein levels have also been shown to be reduced in peripartum cardiomyopathy (PPCM) patients [31]. It is not clear what leads to reduced pSTAT3 levels in ischemic LP. Reduced phosphorylation of STAT3 as well as Akt also mediates reduced protection by ischemic postconditioning in depressed rats induced by chronic mild stress [71]. It has been claimed that these two apparently distinct TNF/JAK/STAT3 and Akt pathways interact with each other to induce cardioprotective effects. Incapability of LP hearts to phosphorylate these key kinases Akt/ERK/STAT3 at the time of reperfusion could be the major defect contributing to the lower tolerance of these hearts to reperfusion injury.
In conclusion, higher vulnerability of late pregnant hearts to ischemia/reperfusion injury is associated with increased ROS generation and decreased threshold for triggering the opening of the mPTP in response to Ca2+ overload. Impaired activation of the key kinases Akt/ERK/STAT3 of the two well established prosurvival signaling pathways RISK and SAFE are the underlying mechanisms for higher vulnerability of LP hearts to I/R injury. Inhibiting the opening of the mPTP could serve as a novel therapeutic approach for pregnant women with cardiovascular complications.
Supplementary Material
Acknowledgments
Funding Sources
Supported by NIH grants HL089876 (M.E.), HL089876S1 (M.E.), HL077440 (H.C.) and HL088975 (H.C.).
Footnotes
Disclosures
None.
Reference List
- 1.Abdallah Y, Gkatzoflia A, Gligorievski D, et al. Insulin protects cardiomyocytes against reoxygenation-induced hypercontracture by a survival pathway targeting SR Ca2+ storage. Cardiovasc Res. 2006 May 1;70(2):346–353. doi: 10.1016/j.cardiores.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 2.Birnbaum Y, Long B, Qian J, Perez-Polo JR, Ye Y. Pioglitazone limits myocardial infarct size, activates Akt, and upregulates cPLA2 and COX-2 in a PPAR-gamma-independent manner. Basic Res Cardiol. 2011 May;106(3):431–446. doi: 10.1007/s00395-011-0162-3. [DOI] [PubMed] [Google Scholar]
- 3.Boengler K, Buechert A, Heinen Y, et al. Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice. Circ Res. 2008 Jan 4;102(1):131–135. doi: 10.1161/CIRCRESAHA.107.164699. [DOI] [PubMed] [Google Scholar]
- 4.Boengler K, Hilfiker-Kleiner D, Drexler H, Heusch G, Schulz R. The myocardial JAK/STAT pathway: from protection to failure. Pharmacol Ther. 2008 Nov;120(2):172–185. doi: 10.1016/j.pharmthera.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Boengler K, Hilfiker-Kleiner D, Heusch G, Schulz R. Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res Cardiol. 2010 Nov;105(6):771–785. doi: 10.1007/s00395-010-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bopassa JC, Eghbali M, Toro L, Stefani E. A novel estrogen receptor GPER inhibits mitochondria permeability transition pore opening and protects the heart against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2010 Jan;298(1):H16–H23. doi: 10.1152/ajpheart.00588.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breivik L, Helgeland E, Aarnes EK, Mrdalj J, Jonassen AK. Remote postconditioning by humoral factors in effluent from ischemic preconditioned rat hearts is mediated via PI3K/Akt-dependent cell-survival signaling at reperfusion. Basic Res Cardiol. 2011 Jan;106(1):135–145. doi: 10.1007/s00395-010-0133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callies F, Stromer H, Schwinger RH, et al. Administration of testosterone is associated with a reduced susceptibility to myocardial ischemia. Endocrinology. 2003 Oct;144(10):4478–4483. doi: 10.1210/en.2003-0058. [DOI] [PubMed] [Google Scholar]
- 9.Cantor EJ, Babick AP, Vasanji Z, Dhalla NS, Netticadan T. A comparative serial echocardiographic analysis of cardiac structure and function in rats subjected to pressure or volume overload. J Mol Cell Cardiol. 2005 May;38(5):777–786. doi: 10.1016/j.yjmcc.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Cao W, Xie YH, Li XQ, et al. Burn-induced apoptosis of cardiomyocytes is survivin dependent and regulated by PI3K/Akt, p38 MAPK and ERK pathways. Basic Res Cardiol. 2011 Nov;106(6):1207–1220. doi: 10.1007/s00395-011-0199-3. [DOI] [PubMed] [Google Scholar]
- 11.Carabello BA. Concentric versus eccentric remodeling. J Card Fail. 2002 Dec;8(6 Suppl):S258–S263. doi: 10.1054/jcaf.2002.129250. [DOI] [PubMed] [Google Scholar]
- 12.Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2005 Jun 21;102(25):9056–9061. doi: 10.1073/pnas.0409594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapman AB, Abraham WT, Zamudio S, et al. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int. 1998 Dec;54(6):2056–2063. doi: 10.1046/j.1523-1755.1998.00217.x. [DOI] [PubMed] [Google Scholar]
- 14.Christian TF, Schwartz RS, Gibbons RJ. Determinants of infarct size in reperfusion therapy for acute myocardial infarction. Circulation. 1992 Jul;86(1):81–90. doi: 10.1161/01.cir.86.1.81. [DOI] [PubMed] [Google Scholar]
- 15.Cohen MV, Yang XM, Downey JM. Acidosis, oxygen, and interference with mitochondrial permeability transition pore formation in the early minutes of reperfusion are critical to postconditioning's success. Basic Res Cardiol. 2008 Sep;103(5):464–471. doi: 10.1007/s00395-008-0737-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999 Jul 15;341(Pt 2):233–249. [PMC free article] [PubMed] [Google Scholar]
- 17.Daniels SR, Meyer RA, Liang YC, Bove KE. Echocardiographically determined left ventricular mass index in normal children, adolescents and young adults. J Am Coll Cardiol. 1988 Sep;12(3):703–708. doi: 10.1016/s0735-1097(88)80060-3. [DOI] [PubMed] [Google Scholar]
- 18.Eghbali M, Deva R, Alioua A, et al. Molecular and functional signature of heart hypertrophy during pregnancy. Circ Res. 2005 Jun 10;96(11):1208–1216. doi: 10.1161/01.RES.0000170652.71414.16. [DOI] [PubMed] [Google Scholar]
- 19.Eghbali M, Wang Y, Toro L, Stefani E. Heart hypertrophy during pregnancy: a better functioning heart? Trends Cardiovasc Med. 2006 Nov;16(8):285–291. doi: 10.1016/j.tcm.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000 Feb 15;101(6):660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghaboura N, Tamareille S, Ducluzeau PH, et al. Diabetes mellitus abrogates erythropoietin-induced cardioprotection against ischemic-reperfusion injury by alteration of the RISK/GSK-3beta signaling. Basic Res Cardiol. 2011 Jan;106(1):147–162. doi: 10.1007/s00395-010-0130-3. [DOI] [PubMed] [Google Scholar]
- 22.Gomez L, Paillard M, Thibault H, Derumeaux G, Ovize M. Inhibition of GSK3beta by postconditioning is required to prevent opening of the mitochondrial permeability transition pore during reperfusion. Circulation. 2008 May 27;117(21):2761–2768. doi: 10.1161/CIRCULATIONAHA.107.755066. [DOI] [PubMed] [Google Scholar]
- 23.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion–a target for cardioprotection. Cardiovasc Res. 2004 Feb 15;61(3):372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 24.Halestrap AP, Kerr PM, Javadov S, Woodfield KY. Elucidating the molecular mechanism of the permeability transition pore and its role in reperfusion injury of the heart. Biochim Biophys Acta. 1998 Aug 10;1366(1–2):79–94. doi: 10.1016/s0005-2728(98)00122-4. [DOI] [PubMed] [Google Scholar]
- 25.Hausenloy DJ, Lecour S, Yellon DM. Reperfusion injury salvage kinase and survivor activating factor enhancement prosurvival signaling pathways in ischemic postconditioning: two sides of the same coin. Antioxid Redox Signal. 2011 Mar 1;14(5):893–907. doi: 10.1089/ars.2010.3360. [DOI] [PubMed] [Google Scholar]
- 26.Hausenloy DJ, Ong SB, Yellon DM. The mitochondrial permeability transition pore as a target for preconditioning and postconditioning. Basic Res Cardiol. 2009 Mar;104(2):189–202. doi: 10.1007/s00395-009-0010-x. [DOI] [PubMed] [Google Scholar]
- 27.Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005 Feb;288(2):H971–H976. doi: 10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- 28.Heusch G, Boengler K, Schulz R. Inhibition of mitochondrial permeability transition pore opening: the Holy Grail of cardioprotection. Basic Res Cardiol. 2010 Mar;105(2):151–154. doi: 10.1007/s00395-009-0080-9. [DOI] [PubMed] [Google Scholar]
- 29.Heusch G, Boengler K, Schulz R. Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation. 2008 Nov 4;118(19):1915–1919. doi: 10.1161/CIRCULATIONAHA.108.805242. [DOI] [PubMed] [Google Scholar]
- 30.Heusch G, Musiolik J, Gedik N, Skyschally A. Mitochondrial STAT3 activation and cardioprotection by ischemic postconditioning in pigs with regional myocardial ischemia/reperfusion. Circ Res. 2011 Nov 11;109(11):1302–1308. doi: 10.1161/CIRCRESAHA.111.255604. [DOI] [PubMed] [Google Scholar]
- 31.Hilfiker-Kleiner D, Kaminski K, Podewski E, et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007 Feb 9;128(3):589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 32.James AH, Jamison MG, Biswas MS, Brancazio LR, Swamy GK, Myers ER. Acute myocardial infarction in pregnancy: a United States population-based study. Circulation. 2006 Mar 28;113(12):1564–1571. doi: 10.1161/CIRCULATIONAHA.105.576751. [DOI] [PubMed] [Google Scholar]
- 33.James PR. Drugs in pregnancy. Cardiovascular disease. Best Pract Res Clin Obstet Gynaecol. 2001 Dec;15(6):903–911. doi: 10.1053/beog.2001.0237. [DOI] [PubMed] [Google Scholar]
- 34.Jin ZQ, Goetzl EJ, Karliner JS. Sphingosine kinase activation mediates ischemic preconditioning in murine heart. Circulation. 2004 Oct 5;110(14):1980–1989. doi: 10.1161/01.CIR.0000143632.06471.93. [DOI] [PubMed] [Google Scholar]
- 35.Kam KW, Qi JS, Chen M, Wong TM. Estrogen reduces cardiac injury and expression of beta1-adrenoceptor upon ischemic insult in the rat heart. J Pharmacol Exp Ther. 2004 Apr;309(1):8–15. doi: 10.1124/jpet.103.058339. [DOI] [PubMed] [Google Scholar]
- 36.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003 Jan 4;361(9351):13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 37.Kelly RF, Lamont KT, Somers S, et al. Ethanolamine is a novel STAT-3 dependent cardioprotective agent. Basic Res Cardiol. 2010 Nov;105(6):763–770. doi: 10.1007/s00395-010-0125-0. [DOI] [PubMed] [Google Scholar]
- 38.Kevin LG, Novalija E, Stowe DF. Reactive oxygen species as mediators of cardiac injury and protection: the relevance to anesthesia practice. Anesth Analg. 2005 Nov;101(5):1275–1287. doi: 10.1213/01.ANE.0000180999.81013.D0. [DOI] [PubMed] [Google Scholar]
- 39.Korge P, Ping P, Weiss JN. Reactive oxygen species production in energized cardiac mitochondria during hypoxia/reoxygenation: modulation by nitric oxide. Circ Res. 2008 Oct 10;103(8):873–880. doi: 10.1161/CIRCRESAHA.108.180869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuch M, Janiszewski M, Mamcarz A, Cudnoch-Jedrzejewska A, Dluzniewski M. Major adverse cardiac event predictors in survivors of myocardial infarction with asymptomatic left ventricular dysfunction or chronic heart failure. Med Sci Monit. 2009 Jun;15(6):H40–H48. [PubMed] [Google Scholar]
- 41.Lacerda L, Somers S, Opie LH, Lecour S. Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res. 2009 Nov 1;84(2):201–208. doi: 10.1093/cvr/cvp274. [DOI] [PubMed] [Google Scholar]
- 42.Ladner HE, Danielsen B, Gilbert WM. Acute myocardial infarction in pregnancy and the puerperium: a population-based study. Obstet Gynecol. 2005 Mar;105(3):480–484. doi: 10.1097/01.AOG.0000151998.50852.31. [DOI] [PubMed] [Google Scholar]
- 43.Lecour S. Multiple protective pathways against reperfusion injury: a SAFE path without Aktion? J Mol Cell Cardiol. 2009 May;46(5):607–609. doi: 10.1016/j.yjmcc.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Lemasters JJ, Qian T, He L, et al. Role of mitochondrial inner membrane permeabilization in necrotic cell death, apoptosis, and autophagy. Antioxid Redox Signal. 2002 Oct;4(5):769–781. doi: 10.1089/152308602760598918. [DOI] [PubMed] [Google Scholar]
- 45.Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res. 2007 Mar 2;100(4):460–473. doi: 10.1161/01.RES.0000258450.44413.96. [DOI] [PubMed] [Google Scholar]
- 46.Matsui T, Tao J, del MF, et al. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001 Jul 17;104(3):330–335. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- 47.Nahrendorf M, Frantz S, Hu K, et al. Effect of testosterone on post-myocardial infarction remodeling and function. Cardiovasc Res. 2003 Feb;57(2):370–378. doi: 10.1016/s0008-6363(02)00701-0. [DOI] [PubMed] [Google Scholar]
- 48.Nam UH, Wang M, Crisostomo PR, et al. The effect of chronic exogenous androgen on myocardial function following acute ischemia-reperfusion in hosts with different baseline levels of sex steroids. J Surg Res. 2007 Sep;142(1):113–118. doi: 10.1016/j.jss.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 49.Oak JH, Cai H. Attenuation of angiotensin II signaling recouples eNOS and inhibits nonendothelial NOX activity in diabetic mice. Diabetes. 2007 Jan;56(1):118–126. doi: 10.2337/db06-0288. [DOI] [PubMed] [Google Scholar]
- 50.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010 May 11;121(18):2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 51.Pedretti S, Raddatz E. STAT3alpha interacts with nuclear GSK3beta and cytoplasmic RISK pathway and stabilizes rhythm in the anoxic-reoxygenated embryonic heart. Basic Res Cardiol. 2011 May;106(3):355–369. doi: 10.1007/s00395-011-0152-5. [DOI] [PubMed] [Google Scholar]
- 52.Penpargkul S, Scheuer J. The effect of physical training upon the mechanical and metabolic performance of the rat heart. J Clin Invest. 1970 Oct;49(10):1859–1868. doi: 10.1172/JCI106404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pluim BM, Zwinderman AH, van der LA, van der Wall EE. The athlete's heart. A meta-analysis of cardiac structure and function. Circulation. 2000 Jan 25;101(3):336–344. doi: 10.1161/01.cir.101.3.336. [DOI] [PubMed] [Google Scholar]
- 54.Rahman S, Li J, Bopassa JC, et al. Phosphorylation of GSK-3beta Mediates Intralipid-induced Cardioprotection against Ischemia/Reperfusion Injury. Anesthesiology. 2011 Aug;115(2):242–253. doi: 10.1097/ALN.0b013e318223b8b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riedhammer HH, Rafflenbeul W, Weihe WH, Krayenbuhl HP. Left ventricle contractile function in trained dogs with cardial hypertrophy. Basic Res Cardiol. 1976 May;71(3):297–308. doi: 10.1007/BF01906455. [DOI] [PubMed] [Google Scholar]
- 56.Robson SC, Dunlop W, Moore M, Hunter S. Combined Doppler and echocardiographic measurement of cardiac output: theory and application in pregnancy. Br J Obstet Gynaecol. 1987 Nov;94(11):1014–1027. doi: 10.1111/j.1471-0528.1987.tb02285.x. [DOI] [PubMed] [Google Scholar]
- 57.Roth A, Elkayam U. Acute myocardial infarction associated with pregnancy. Ann Intern Med. 1996 Nov 1;125(9):751–762. doi: 10.7326/0003-4819-125-9-199611010-00009. [DOI] [PubMed] [Google Scholar]
- 58.Roth A, Elkayam U. Acute myocardial infarction associated with pregnancy. J Am Coll Cardiol. 2008 Jul 15;52(3):171–180. doi: 10.1016/j.jacc.2008.03.049. [DOI] [PubMed] [Google Scholar]
- 59.Rottlaender D, Boengler K, Wolny M, et al. Connexin 43 acts as a cytoprotective mediator of signal transduction by stimulating mitochondrial KATP channels in mouse cardiomyocytes. J Clin Invest. 2010 May;120(5):1441–1453. doi: 10.1172/JCI40927. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Rottlaender D, Boengler K, Wolny M, et al. Glycogen synthase kinase 3beta transfers cytoprotective signaling through connexin 43 onto mitochondrial ATP-sensitive K+ channels. Proc Natl Acad Sci U S A. 2012 Jan 31;109(5):E242–E251. doi: 10.1073/pnas.1107479109. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Saito T, Ciobotaru A, Bopassa JC, Toro L, Stefani E, Eghbali M. Estrogen contributes to gender differences in mouse ventricular repolarization. Circ Res. 2009 Aug 14;105(4):343–352. doi: 10.1161/CIRCRESAHA.108.190041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schannwell CM, Zimmermann T, Schneppenheim M, Plehn G, Marx R, Strauer BE. Left ventricular hypertrophy and diastolic dysfunction in healthy pregnant women. Cardiology. 2002;97(2):73–78. doi: 10.1159/000057675. [DOI] [PubMed] [Google Scholar]
- 63.Skyschally A, van CP, Boengler K, et al. Ischemic postconditioning in pigs: no causal role for RISK activation. Circ Res. 2009 Jan 2;104(1):15–18. doi: 10.1161/CIRCRESAHA.108.186429. [DOI] [PubMed] [Google Scholar]
- 64.Suleman N, Somers S, Smith R, Opie LH, Lecour SC. Dual activation of STAT-3 and Akt is required during the trigger phase of ischaemic preconditioning. Cardiovasc Res. 2008 Jul 1;79(1):127–133. doi: 10.1093/cvr/cvn067. [DOI] [PubMed] [Google Scholar]
- 65.Tamareille S, Ghaboura N, Treguer F, et al. Myocardial reperfusion injury management: erythropoietin compared with postconditioning. Am J Physiol Heart Circ Physiol. 2009 Dec;297(6):H2035–H2043. doi: 10.1152/ajpheart.00472.2009. [DOI] [PubMed] [Google Scholar]
- 66.Toescu V, Nuttall SL, Martin U, Kendall MJ, Dunne F. Oxidative stress and normal pregnancy. Clin Endocrinol (Oxf) 2002 Nov;57(5):609–613. doi: 10.1046/j.1365-2265.2002.01638.x. [DOI] [PubMed] [Google Scholar]
- 67.Tuteja N, Ahmad P, Panda BB, Tuteja R. Genotoxic stress in plants: shedding light on DNA damage, repair and DNA repair helicases. Mutat Res. 2009 Mar;681(2–3):134–149. doi: 10.1016/j.mrrev.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 68.Yang XM, Krieg T, Cui L, Downey JM, Cohen MV. NECA and bradykinin at reperfusion reduce infarction in rabbit hearts by signaling through PI3K, ERK, and NO. J Mol Cell Cardiol. 2004 Mar;36(3):411–421. doi: 10.1016/j.yjmcc.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 69.Youn JY, Wang T, Cai H. An ezrin/calpain/PI3K/AMPK/eNOSs1179 signaling cascade mediating VEGF-dependent endothelial nitric oxide production. Circ Res. 2009 Jan 2;104(1):50–59. doi: 10.1161/CIRCRESAHA.108.178467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang J, Cai H. Netrin-1 prevents ischemia/reperfusion-induced myocardial infarction via a DCC/ERK1/2/eNOS s1177/NO/DCC feed-forward mechanism. J Mol Cell Cardiol. 2010 Jun;48(6):1060–1070. doi: 10.1016/j.yjmcc.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhuo C, Wang Y, Wang X, Wang Y, Chen Y. Cardioprotection by ischemic postconditioning is abolished in depressed rats: role of Akt and signal transducer and activator of transcription-3. Mol Cell Biochem. 2011 Jan;346(1–2):39–47. doi: 10.1007/s11010-010-0589-0. [DOI] [PubMed] [Google Scholar]
- 72.Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim Biophys Acta. 1995 Jul 17;1241(2):139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.