Abstract
Conflict of interest: none declared.
Introduction
COPD (chronic obstructive pulmonary disease) is characterized by airflow limitation that is not fully reversible. OBJECTIVE: to show the changes of pulmonary function in COPD during the 4 -year evolution of illness.
Material and Methods
The research was done on patients suffering from COPD treated at the Clinic “Podhrastovi” during 2006 and 2007. The tested parameters were examined from the date of receiving patient with COPD to hospital treatment in 2006 and 2007 and then followed prospectively until 2010 or 2011 (the follow-up period was 4 years). There were total 199 treated patients who were chosen at random and regularly attended the control examinations. The study was conducted on adult patients of both sexes, different age group. In each patient the duration of illness was recorded so is sex, age, data of smoking habits, information about the regularity of taking bronchodilator therapy during remissions of disease, about the treatment of disease exacerbations, results of pulmonary functional tests as follows: FVC (forced vital capacity), FEV1 (forced expiratory volume in one second) and bronchodilator reversibility testing. All these parameters were measured at the beginning and at the end of each hospital treatment on the apparatuses of Clinic “Podhrastovi”. We took in elaboration those data obtained in the beginning of the first hospitalization and at the end of the last hospitalization or at the last control in outpatient department when patient was in stable state. Patients were divided into three groups according to the number of exacerbations per year.
Results
airflow limitation in COPD is progressive; both FVC and FEV1 shows the statistically significant decrease during follow-up period of 4 years (p values / for both parameters/ =0.05) . But in patients regularly treated in phases of remission and exacerbations of illness the course of illness is slower. The fall of FVC and FEV1 is statistically significantly smaller in those received regular treatment in phases of remissions and exacerbations of illness (p values / for both parameters/ =0.01). The number of patients responding properly to bronchodilators decreased statistically significantly in patients with COPD during follow-up period (p=0.05).
Conclusion
COPD is characterized with airflow limitation which is progressive in the course of illness, but that course may be made slower using appropriate treatment during remission and exacerbations of diseases.
Key words: COPD, FVC, FEV1, airflow limitation, treatment, bronchodilator response
1. INTRODUCTION
COPD is one of the major causes of chronic morbidity and mortality worldwide. It is the fourth leading cause of death in the world (1), and further increases in its prevalence and mortality can be predicted in the coming decades (2, 3).
COPD is a pulmonary disease with significant extrapulmonary effects that may contribute to the severity of illness. Its pulmonary component is characterized by airflow limitation that is not fully reversible (4) and usually is progressive (1).
Systemic manifestations and comorbidities in COPD are body weight loss, skeletal muscle wasting, cachexia, osteoporosis, right heart failure, cardiac ischemia, cardiac arrhythmias, anaemia, hypoalbuminaemia, diabetes, cognitive deficits, depression (5, 6). Comorbidities are common for people with COPD because organ systems work differently when they do not receive enough oxygen.
Cigarette smoking is the most common risk factor for COPD, although in many countries, various kinds of air pollution have also been identified as COPD risk factors (1, 4, 7).
1.1. Airflow limitation in COPD
The chronic airflow limitation of COPD is caused by a mixture of small airway disease (obstructive bronchiolitis) and parenchymal destruction (emphysema), the relative contributions of which vary from person to person (1). Chronic inflammation causes structural changes and narrowing of small airways. Destruction of the lung parenchyma leads to the loss of alveolar attachments to the small airways and decreases lung elastic recoil; these changes diminish the ability of the airways to remain open during expiration (8, 9, 10, 11). So in COPD inflammation causes small airway disease (airway inflammation, airway remodelling) and parenchymal destruction that all lead to airflow limitation (9, 11, 12).
1.2. Measurement of Airflow Limitation (Spirometry)
should be done in all patients who may have COPD. It is needed to make a confident diagnosis of COPD and to exclude other diagnoses with similar symptoms. Spirometry remains the gold standard for diagnosing COPD and monitoring its progression. It is the best standardized, most reproducible, and most objective measurement of airflow limitation available (1).
Spirometry measures the volume of air forcibly exhaled from the point of maximal inspiration (forced vital capacity- FVC) and the volume of air exhaled during the first second of this maneuver (forced expiratory volume in one second- FEV1) (figure 1), and the ratio of these two measurements (FEV1/FVC) should be calculated. Spirometry measurements are evaluated by comparison with reference values based on age, height, weight, sex, and race (13).
Figure 1.
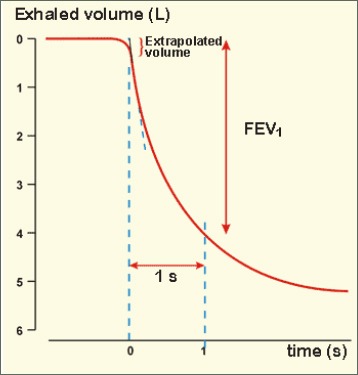
Forced expiratory volume in one second- FEV1
In doing spirometric tests each spirographic apparatus gives normal (predicted) values for that person according to sex, age, height, weight, race, realized values by that patient and shows the per cent values (realized as a per cent of normal- predicted values) (14).
The presence of post- bronchodilator FEV1 < 80% of predicted together with an FEV1/FVC < 0.70 confirms the presence of airflow limitation that is not fully reversible (13).
Volume of air forcibly exhaled from the point of maximal inspiration (FVC) and the volume of air exhaled during the first second of this maneuver (FEV1)-
It is also important to do- Bronchodilator reversibility testing that indicates the patient’s response to treatment (bronchodilator response). In this test the FEV1 should be measured before and after a bronchodilator is given. Possible dosage protocols are 400 μg β2-agonist, up to μg 160 anticholinergic, or the two combined (13). FEV1 should be measured again 10-15 minutes after a short-acting bronchodilator is given; 30-45 minutes after the combination. An increase in FEV1 that is both greater than 200 ml and 12% above the pre- bronchodilator FEV1 is considered significant (13).
Patients with COPD typically show a decrease in both FEV1 and FVC and also the decrease in bronchodilator response. The degree of spirometric abnormality reflects the severity of COPD.
1.3. Treatment of COPD
Although COPD is progressive illness it has periods or phases of remission and exacerbations. An exacerbation of COPD is defined as an event in the natural course of the disease characterized by a change in the patient’s baseline dyspnoea, cough, and/or sputum production that is beyond normal day-to-day variations, that is acute in onset, and may warrant a change in regular medication (1).
The treatment of COPD shows some differences in phases of remission and exacerbation. Bronchodilator medications are central to symptom management in COPD. Inhaled therapy is preferred (1, 14).
1.3.1 Bronchodilators in Stable COPD
The choice between β2-agonist, anticholinergics, theophylline, or combination therapy depends on availability and individual response in terms of symptom relief and side effects. Bronchodilators are prescribed on an as-needed or on a regular basis to prevent or reduce symptoms. Long-acting inhaled bronchodilators are more effective and convenient than treatment with short-acting bronchodilators. Combining bronchodilators may improve efficacy and decrease the risk of side effects compared to increasing the dose of a single bronchodilator (1). Today the combined inhaled bronchodilators (glucocorticosteroids and laba- long acting β2-agonist) are preferred (1, 14).
1.3.2. Treatment of COPD exacerbations
It is needed to: assess severity of symptoms, blood gases, chest X-ray. It is also needed to administering controlled oxygen therapy and repeating arterial blood gas measurement after 30-60 minutes. As to use of bronchodilators: – increase doses and/or frequency is needed, and so combination β2-agonists and anticholinergics, using spacers or air-driven nebulizers, adding intravenous methylxanthines, if needed, adding oral or intravenous glucocorticosteroids. Antibiotics (oral or intravenous) are administrated when signs of bacterial infection are presented. Non-invasive mechanical ventilation (NIMV) in some patients according to level of blood gases. At all time –monitor fluid balance and nutrition, considering subcutaneous heparin, identifying and treatment of associated conditions (e.g.heart failure, arrhythmias) (1).
2. OBJECTIVE
To show the changes in pulmonary function in COPD during the four–year evolution of illness.
3. MATERIAL AND METHODS
The research was done prospectively on patients suffering from COPD treated at the Clinic “Podhrastovi” during 2006 and 2007. The tested parameters were examined from the date of receiving a patient with COPD for a hospital treatment in 2006 and 2007 and then followed prospectively until 2010 or 2011 (the follow-up period was 4 years). There were total 199 treated patients who were chosen at random and regularly attended the control examinations. The study was conducted on adult patients of both sexes, different age group suffering from COPD.
In each patient the duration of illness was recorded so was the age, sex, data of smoking habits, information about the regularity of taking bronchodilatatory therapy during remissions of disease, data about the treatment of disease exacerbations, results of pulmonary functional tests as follows: FVC, FEV1, bronchodilator reversibility testing. All these parameters were measured at the beginning and at the end of each hospital treatment on the apparatuses of Clinic “Podhrastovi”. We took in elaboration those data obtained in the beginning of the first hospitalization and at the end of the last hospitalization or at the last control in outpatient department when patient was in stable state.
Patients were divided into three groups according to the number of exacerbations per year: 1st group made of 65 patients with one or less exacerbation per year, 2nd group made of 65 patients with 2-3 exacerbations per year, 3rd group made of 69 patients with more than 3 exacerbations of disease per year.
Smoking habits imply the duration of smoking in years, the number of cigarettes smoked per a day. The complete desobstructive therapy involves antibiotics, combined spray therapy (inhaled corticosteroids and long acting β-agonists), eventually anticholinergic, corticosteroids and methylxanthines (both orally or intravenously), Data were collected from patients’ hospital charts in phases of exacerbations and data during remissions were taken from the competent pulmonologist of Houses of Health or Counselling Department of Clinic “Podhrastovi”.
4. RESULTS
The average age of patients in 1st group is 57.36 years, in 2nd group 61.57 and in 3rd group 61.58 years. Patients with 1 or less exacerbations per year are statistically significantly younger than patients with 2-3 (p=0.05) and patients with more than 3 exacerbations per year (p=0.05).There is no statistic significant difference between two last groups.
There is 149 (74.87%) males and 50 (25.13%) females: in the 1st group is 44(67.69%) males and 21 (32.31%) females, in 2nd group 51 (78.46%) males and 14 (21.54%) females, in the 3rd group is 54 (78.26%) males and 15(21.74%) females. The number of male patients is statistically significantly bigger than females (p= 0.01). There are not statistically significant differences in sex according to the number of exacerbations of diseases per year. Chi-squared tests are respectively: 2.716 and 1.218, 2 degrees of freedom.
There is 157 (78.89%) smokers and 42 (21.11%) non –smokers .In the 1st group there is 42 (64.62%), in the 2nd group 54 (83.08%) and in the 3rd group 61 (88.41%) of smokers. There is high statistically significant difference in the number of smokers and non-smokers. The greatest number of non-smokers is in patients with the least number of exacerbations. Chi-squared test=12.367, the level of significance: p=0.01, 2 degrees of freedom. It is significant that 91 .05% of smokers have been smoking more than 20 years.
The average duration of illness (at the last examination) is 10.51 years. In the 1st group is 8 years, the 2nd group 10.82 and in the 3rd group 12.70 years .The number of exacerbations is statistically significantly bigger in patients with the longer duration of illness. The levels of significance are: between first and second group p=0.05, between first and third group p=0.05, between second and third group p=0.10 (Figure 2).
Figure 2.
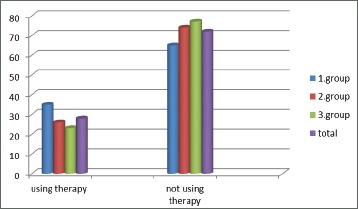
Using therapy in phases of COPD –remissions in groups of patients expressed as percentage of examined patients
The number of patients using regular therapy in the phases of remission of illness is statistically significantly smaller than number of patients not using therapy (p=0.05).
There are not statistically significant differences in receiving therapy in the phases of remission of illness according to the number of exacerbations of illness per year. Chi-squared test= 3.124; 2 degrees of freedom) (Figure 3).
Figure 3.
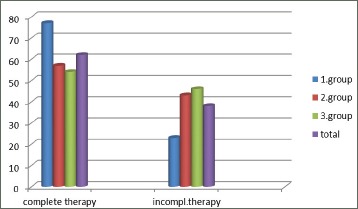
Using therapy in phases of COPD –exacerbations in groups of patients expressed as percentage of examined patients
In patients with bigger number of exacerbations there is less number of them who received complete therapy in phases of illness exacerbations. Chi- squared test = 9.307, level of significance: p=0.01, 2 degrees of freedom (Table 1).
Table 1.
The average measured values of FVC expressed as a per cent of average of normal values for that group of patients at first and last examination according to the number of exacerbations of illness per year
| Average number of exacerbationsper year | Firstexam. | Lastexam. | Difference | Significance | |
|---|---|---|---|---|---|
| 1exacerbation or less | _X | 60.91 | 63.25 | +2.34 | non.signif. |
| S.D. | 16.09 | 15.55 | 17.37 | ||
| 2-3 exacerbations | _X | 56.93 | 52.08 | -4.85 | p=0.05 |
| S.D. | 18.63 | 14.96 | 18.43 | ||
| More than 3 exacerbation | _X | 56.24 | 50.01 | -6.23 | p=0.01 |
| S.D. | 14.44 | 14.42 | 15.98 | ||
| All patients | _X | 57.97 | 54.95 | -3.02 | p=0.05 |
| S.D. | 16.58 | 16.08 | 17.74 |
*exam. =examination
In all patients, in second and third group FVC decreased statistically significantly in the follow-up period. In the second and third group decrease of FVC is statistically significantly bigger than in first group. P values are respectively: p=0.05 and p=0.01 (Table 2).
Table 2.
The average measured values of FVC expressed as a per cent of average of normal values for that group of patients at first and last examination according to the receiving therapy in phases of illness remissions
| All patients | Firstexam. | Lastexam. | Difference | |
|---|---|---|---|---|
| Received therapy | _X | 60.58 | 62.90 | +2.32 |
| S.D. | 17.51 | 13.58 | 16.65 | |
| Not received therapy | _X | 56.43 | 51.75 | -4.68 |
| S.D. | 14.86 | 15.90 | 17.06 |
In patients who received therapy during the remissions of illness FVC increased, in those not received therapy FVC decreased during the follow-up period. The difference of FVC between these two groups is statistically significant: p=0.01 (Table 3.)
Table 3.
The average measured values of FVC expressed as a per cent of average of normal values for that group of patients at first and last examination according to the using therapy in phases of illness exacerbations
| Received complete therapy | _X | 59.08 | 57.84 | -1.24 |
| S.D. | 16.35 | 15.92 | 18.04 | |
| Not received complete therapy | _X | 56.13 | 50.19 | -5.94 |
| S.D. | 16.78 | 15.18 | 16.84 |
In all patients with COPD FVC decreased during the follow-up period. That decrease is statistically significantly bigger in those who not received complete therapy in phases of illness exacerbations: p=0.01 (Table 4).
Table 4.
The average measured values of FEV1 expressed as a per cent of average of normal values for that group of patients at first and last examination according to the number of exacerbations of illness per year
| Average number of exacerbations per year | Firstexam. | Lastexam. | Difference | Significance | |
|---|---|---|---|---|---|
| 1exacerbation or less | _X | 43.91 | 44.05 | +0.14 | non signif. |
| S.D. | 13.83 | 17.05 | 16.08 | ||
| 2-3 exacerbations | _X | 36.99 | 33.22 | -3.77 | p=0.05 |
| S.D. | 11.36 | 13.48 | 14.69 | ||
| More than 3 exacerbations | _X | 33.89 | 28.30 | -5.59 | p=0.01 |
| S.D. | 10.95 | 11.14 | 11.99 | ||
| All patients | X | 38.26 | 35.20 | -3.06 | p=0.05 |
| S.D. | 12.78 | 14.61 | 14.42 |
In all patients, in second and third group FEV1 decreased statistically significantly in the follow-up period. In the second and third group decrease of FEV1 is statistically significantly bigger than in first group. P values are respectively: p=0.05 and p=0.01 (Table 5).
Table 5.
The average measured values of FEV1 expressed as a per cent of average of normal values for that group of patients at first and last examination according to the using therapy in phases of illness remissions
| All patients | Firstexam. | Lastexam. | Difference | |
|---|---|---|---|---|
| Received therapy | _X | 40.15 | 42.86 | +2.71 |
| S.D. | 13.32 | 14.32 | 15.06 | |
| Not received therapy | X | 37.08 | 32.66 | -4.42 |
| S.D. | 12.09 | 12.69 | 12.45 |
In patients who received therapy during the remissions of illness FEV1 increased, in those not received therapy FEV1 decreased during the follow-up period. Difference of FEV1 between these two groups is statistically significant: p=0.01 (Table 6).
Table 6.
The average measured values of FEV1 expressed as a per cent of average of normal values for that group of patients at first and last examination according to the using therapy in phases of illness exacerbations
| All patients | Firstexam. | Lastexam. | Difference | |
|---|---|---|---|---|
| Received complete therapy | _X | 39.36 | 38.04 | -1.32 |
| S.D. | 12.61 | 15.05 | 14.88 | |
| Not received completetherapy | _X | 36.13 | 29.99 | -6.14 |
| S.D. | 12.79 | 10.98 | 12.29 |
In all patients with COPD FEV1 decreased during the follow-up period. That decrease is statistically significantly bigger in those who not received complete therapy in phases of illness exacerbations: p=0.01 (Figure 4).
Figure 4.
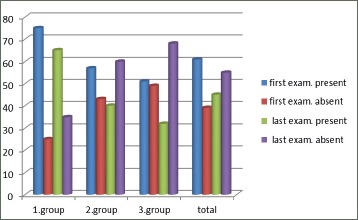
Bronchodilator reversibility tests–the response to bronchodilator therapy in phases of COPD exacerbations according to the number of exacerbations of illness per year
Bronchodilator effects of therapy are significantly much more present in patients with less number of exacerbations per year–Chi-squared test = 6.305, level of significance p=0.05, 1 degree of freedom. There is not difference between second and third group–Chi-squared test =0.0139.
5. DISCUSSION
COPD is one of the major causes of chronic morbidity and mortality worldwide. It is the fourth leading cause of death in the world (1).
COPD is a pulmonary disease with significant extrapulmonary effects. Its pulmonary component is characterized by airflow limitation that is not fully reversible (4), and is usually progressive (1). Cigarette smoking is the most common risk factor for COPD.
The real role of smoking in accelerating the course of COPD could not be determined in this study because of large number of smokers in this sample (78.89% of patients were smokers) and 91.05% of them have been smoking more than 20 years. The biggest number of non-smokers was in patients with one or fewer exacerbations per year; and knowing that a bigger number of exacerbations leads to faster damage of lung function in the course of COPD, these data indirectly indicate that cigarette smoking leads to faster damage of lung function.
In the phases of illness- remissions therapy (the maintenance therapy) is most oft en taken by those with one or less disease exacerbation per year, and most rarely taken by patients with more than three exacerbations per year which indicate the importance of taking therapy continuously that influence bronchial inflammation and so response to environmental factors –such as viruses, bacterias, air-pollution (1, 14).
Number of exacerbations per year and taking full therapy in these phases are close connected. The number of exacerbations per year is statistically significantly bigger in patients not taking full therapy. It implicate the importance of treating of each exacerbation that leads to less sequels on bronchial mucosa (airway re- modulation), because on more damaged bronchial mucosa it is easier for disease to exacerbate by doing of various environmental factors (1, 14).We considered why patients have not been given full therapy in phases of exacerbations of illness although all of them have been treated in hospital . That is because we didn’t have apparatuses for non-invasive ventilation until 2010, that we were not in the habit to give anticholinergics, that many of patients had diabetes that is the contraindication for glucocorticosteroids, that many of them couldn’t stand 24-hour oxygen therapy, that some of patients had allergy to proper antibiotics, and that we had no always full monitoring of using spray bronchodilators in conscious patients.
In all patients with COPD there is the decrease of FEV1 and FVC during follow-up period that indicates that airflow limitation is progressive but in patients taking regular therapy treatment during remissions and exacerbations of illness both FVC and FEV1 are statistically significantly bigger in comparison with patents not taking full therapy. These results indicate the importance of rapid- in right time inclusion of bronchodilator therapy to slow the progression of disease and preventing or postponing the development of irreversible airflow obstruction.
Effects of bronchodilator therapy –bronchodilator responses were statistically significantly more present in patients with fewer exacerbations per year compared to patients with a greater number of exacerbations. This phenomenon can be explained by the fact that these patients were statistically significantly younger, that there was statistically significantly lower number of smokers among them, that the duration of disease was statistically significantly less, that they statistically significantly have taken regular therapy during remissions and exacerbations of illness, which all leads to lessen of bronchial inflammation.
6. CONCLUSION
The basic characteristic of COPD is airflow limitation. The course of COPD is progressive, but the course of disease may be made slower with appropriate continuous therapy using in phases of remissions and appropriate treatment of exacerbations of illness . The regular using of therapy during the remissions of illness prevents or delays the occurrence of exacerbations of disease. There is the importance of vigorous treatment of each disease exacerbation because of better recovery of the inflamed bronchial mucosa because it leads to remaining of less number of mucosal changes –sequels. On more damaged bronchial mucosa various environmental factors cause more easily development of exacerbations, and by bigger number of exacerbations the prognosis of these patients worsens. In irregularly and improperly treated patients with COPD during the time it comes to reduction of response to bronchodilators due to the development of irreversible airflow obstruction factors.
There is the importance of ceasing smoking, and avoidance of other environmental factors causing the development of COPD. Spirometry is a very simple and available method in early diagnostic and monitoring progression of COPD.
REFERENCES
- 1.GOLD (Global Initiative for Chronic Obstructive Lung Disease)– global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease-revised 2006. Http//www.goldcopd.org:1-100 [Google Scholar]
- 2.World Health Report. Geneva: World Health Organization; Available from URL: http://www.who.int/whr/2000/en/statistics.htm; 2000 [Google Scholar]
- 3.Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL., et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006; 27(2): 397-412 [DOI] [PubMed] [Google Scholar]
- 4.Soriano JB, Visick GT, Muellerova H, Payvandi N, Hansell AL.Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest. 2005; 128(4): 2099-2107 [DOI] [PubMed] [Google Scholar]
- 5.Agusti AG.Systemic effects of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005; 2(4): 367-370 [DOI] [PubMed] [Google Scholar]
- 6.Van Weel C, Schellevis FG.Comorbidity and guidelines: conflicting interests. Lancet. 2006; 367(9510): 550-551 [DOI] [PubMed] [Google Scholar]
- 7.Eisner MD, Balmes J, Katz BP, Trupin L, Yelin E, Blanc P.Lifetime environmental tobacco smoke exposure and the risk of chronic obstructive pulmonary disease. Environ Health Perspect. 2005; 4: 7-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnes PJ, Shapiro SD, Pauwels RA.Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003; 22(4): 672-688 [DOI] [PubMed] [Google Scholar]
- 9.Hogg JC.Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004; 364(9435): 709-721 [DOI] [PubMed] [Google Scholar]
- 10.Saetta M, Turato G, Maestrelli P, Mapp CE, Fabbri LM.Cellular and structural bases of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001; 163 (6): 1304-1309 [DOI] [PubMed] [Google Scholar]
- 11.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM., et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004; 350(26): 2645-2653 [DOI] [PubMed] [Google Scholar]
- 12.Cosio MG, Majo J.Inflammation of the airways and lung parenchyma in COPD: role of T cells. Chest. 2002; 121(5 Suppl): 160S-165S [DOI] [PubMed] [Google Scholar]
- 13.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005; 26(5): 948-968 [DOI] [PubMed] [Google Scholar]
- 14.Cukic V, Lovre V, Dragisic D, Ustamujić A.Asthma and chronic obstructive pulmonary disease (COPD)-differences and similarities. Mat Soc Med. 2012. Jun; 24(2): 100-105 [DOI] [PMC free article] [PubMed] [Google Scholar]


