Abstract
The philosophy behind personalized medicine is that each patient has a unique biologic profile that should guide the choice of therapy, resulting in an improved treatment outcome, ideally with reduced toxicity. Thus, there has been increasing interest in identifying genetic variations that are predictive of a drug’s efficacy or toxicity. Although it is one of the most effective drugs for treating breast cancer, tamoxifen is not effective in all estrogen receptor (ER)-positive breast cancer patients, and it is frequently associated with side effects, such as hot flashes. Relative resistance to tamoxifen treatment may be a result, in part, from impaired drug activation by cytochrome P450 2D6 (CYP2D6). Indeed, recent studies have identified allelic variations in CYP2D6 to be an important determinant of tamoxifen’s activity (and toxicity). This article will summarize the current information regarding the influence of the major genotypes and CYP2D6 inhibitors on tamoxifen metabolism, with a focus on its clinical utility and the current level of evidence for CYP2D6 genotyping of patients who are candidates for tamoxifen treatment.
The philosophy behind personalized medicine is that every patient has a unique biologic profile that should guide the choice of therapy, resulting in an improved treatment outcome, ideally with less treatment-related toxicity. In this regard, the presence or absence of estrogen receptor (ER) has been one of the oldest examples of how a biologic marker can guide therapy in patients with breast cancer. The development of the first targeted therapy for breast cancer, namely, the selective ER modulator tamoxifen, was in the forefront of personalized medicine in the late 1970s.1 Since then, tamoxifen has been the most effective and available therapy for the treatment of ER-positive breast cancer, being used in the neoadjuvant, adjuvant, and palliative settings, as well as more recently in the chemoprevention of ER-positive breast cancer. Using tamoxifen as an adjuvant therapy for 5 years after surgery almost halves the annual recurrence rate and reduces the breast cancer mortality rate by one third in both pre- and postmenopausal women with ER-positive breast cancer.2
More recently, advances in large genome-scale sequencing, including greater availability of less costly methods, and improvements in bioinformatic tools have led to significant developments in the fields of pharmacogenetics and pharmacogenomics.3 With the goal of improving the risk/benefit profile of pharmaceuticals based on an individual’s genotype, there has been an increasing interest in identifying genetic variations that are predictive of a drug’s efficacy or toxicity. Although it is one of the most effective drugs for treating breast cancer, tamoxifen is not effective in all ER-positive breast cancer patients, and it is frequently associated with side effects, such as hot flashes. Several culprits have been related to tamoxifen resistance, and identifying tumor and host characteristics remains the main challenge to effective treatment with tamoxifen. Estrogen hypersensitivity associated with increased transcriptional activity of ER, estrogen super-sensitivity, and estrogen independence, among others, are important tumor factors. Impaired drug activation by cytochrome P450 2D6 (CYP2D6) is an important host factor that also has been associated with tamoxifen resistance. Indeed, studies have identified allelic variations in CYP2D6 to be an important determinant of tamoxifen’s activity (and toxicity). Evidence obtained over the past few years suggests that CYP2D6 genotype is associated with the production of the tamoxifen active metabolite endoxifen, which in turn may relate to clinical efficacy. In practice, women who are poor metabolizers (ie, poor activators) of tamoxifen may be inadequately exposed to endoxifen, and thus may be better served by being placed on an aromatase inhibitor (AI). Conversely, women who are extensive metabolizers may have more endoxifen exposure and better outcomes, potentially at the expense of more adverse events. Among the common adverse effects of tamoxifen are hot flashes, which are frequently treated with antidepressants. Some drugs in this class also are metabolized by CYP2D6 and thus the potential exists for significant drug interactions to occur. By associating genetic variations in the CYP2D6 gene with the extent of individual drug metabolism, thus predicting who is more likely to benefit from tamoxifen therapy and/or experience side effects, CYP2D6 genotyping holds the promise of again placing tamoxifen in the forefront of personalized medicine.
Recently, the Pharmaceutical Science Clinical Pharmacology Subcommittee of the US Food and Drug Administration (FDA) recommended including information on CYP2D6 genotypes and their potential effect on patient outcomes in the label for tamoxifen, but a consensus on whether genotyping should be required or considered optional was not reached. This article will summarize the current translational and clinical data regarding the influence of the major CYP2D6 genotypes and inhibitors on tamoxifen metabolism, with a focus on the clinical utility and the current level of evidence for CYP2D6 genotyping.
TAMOXIFEN METABOLISM
Tamoxifen is a classic pro-drug. With a weak ER affinity itself, tamoxifen requires metabolic conversion to its active metabolites, endoxifen and 4-hydroxy-tamoxifen (4-OH-TAM), to exert its anti-tumor effects. Several cytochrome P450 isoforms play a role in tamoxifen biotransformation. CYP3A4 and CYP3A5 are the major enzymes responsible for N-demethylation, whereas 4-hydroxylation is predominantly mediated by CYP2D6.4-7 Once tamoxifen is absorbed, CYP3A4/5 converts it to N-desmethyl-tamoxifen, a weak anti-estrogen that is quantitatively the most abundant tamoxifen metabolite in the plasma, accounting for 92% of primary tamoxifen oxidation.8
N-desmethyl-tamoxifen is subsequently converted to 4-hydroxy-N-desmethyl-tamoxifen (endoxifen) by CYP2D6. CYP2D6 also is involved in the primary metabolism, converting tamoxifen to 4-OH-TAM, which is then converted by CYP3A4/5 to endoxifen. The anti-estrogen activities of endoxifen and 4-hydroxytamoxifen are similar in terms of their binding affinities to ERα and ERβ, suppression of ER-dependent proliferation of breast cancer cells, and modulation of ER-mediated global gene expression.9-11 Furthermore, they both have at least a 10-fold higher affinity12 and are 30-to 100-fold more potent blockers of ER than tamoxifen. However, endoxifen reaches higher plasma concentrations than 4-OH-TAM, and it has been shown recently that patients on chronic tamoxifen therapy have a sixfold to 12-fold higher concentration of endoxifen than 4-OH-TAM,11,13 providing strong evidence that endoxifen is likely the most important active tamoxifen metabolite.
As shown in Figure 1, CYP2D6 is the leading enzyme involved in endoxifen production, participating in the primary and secondary phases of tamoxifen metabolism. It is also an important phase 1 drug-metabolizing enzyme involved in the processing of a myriad of other substrates that range from β-blockers to antidepressants, including drugs to treat depression and hot flashes that are commonly used by patients with breast cancer.
Figure 1.
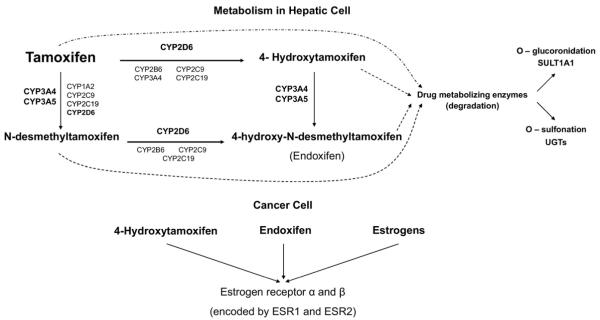
Tamoxifen metabolism in the hepatic cell, where it is converted to its active metabolites, 4-hydroxytamoxifen and endoxifen, mainly through CYP2D6. Phase II conjugation or inactivation occurs via conjugation by sulfotransferases such as sulfotransferase1A1 (SULT1A1), or glucuronidation by the UDP-glucuronosyltransferases (UGT), including UGT1A8, UGT1A10, UGT2B7, UGT2B15, and UGT2B17. In the tumor cells, tamoxifen metabolites bind to estrogen receptors, and polymorphisms in their genes have also been suggested to contribute to interindividual variability to tamoxifen benefits.
Natural genetic variation in alleles for CYP2D6 can lead to more or less active enzymatic function, with implications for drug–drug as well as in tumor–drug interactions. More than 100 genetic variants of CYP2D6 have been described (available at www.cypalleles.ki.se/cyp2d6.htm) and can be classified as nonfunctional alleles, reduced function alleles, and wild-type alleles, the latter with a normal enzymatic activity. Based on allele combinations and metabolic ratio (concentration of unchanged drug/concentration drug metabolite), patients can be classified as poor metabolizers (PM), intermediate metabolizers (IM), normal or extensive metabolizers (EM), and ultrarapid metabolizers (UM). Intuitively, patients who are homozygous for inactive alleles are PM; those who are heterozygous are mainly IM, and those homozygous for wild-type alleles are EM. Those carrying more than two CYP2D6 copies in their genome are UM.
The most important null alleles responsible for a PM phenotype are CYP2D6*4 (splice defect) and CYP2D6*5 (gene deletion), whereas the most common alleles with severely reduced activity are CYP2D6*10 and CYP2D6*17, found in IM patients. The CYP2D6 alleles also are subject to significant interethnic differences, as shown in Table 1 by CYP2D6 haplotype frequencies in geographically defined groups of populations.14 Furthermore, CYP2D6*4 is most prevalent in Caucasians, and 5% to 10% of Caucasians are PM,15 whereas less than 1% of East Asians are PM. Conversely, CYP2D6*10 is common in East Asians, and the IM phenotype is common in Asia,14 while only 10% to 15% of Caucasians are IM. The UM carry gene duplications of functional alleles, which leads to higher CYP2D6 enzymatic activity, with relatively low frequency observed in Caucasians and Asians, but being the second most common group of metabolizers in North Africa, the Middle East, and Oceania.14 This heterogeneity underscores the need to analyze comprehensively all relevant genetic variants, including common PM alleles (*3, *4, and *5), and IM alleles, depending on the patient’s ethnicity.
Table 1.
Frequencies (%) of Different CYP2D6 Alleles Within Populations
| Functional |
Nonfunctional |
Reduced |
Duplications |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Population | *1 | *2 | *3 | *4 | *5 | *6 | *9 | *10 | *17 | *41 | *1×2 | *2×2 | *4×2 |
| America | 60 | 30 | – | 3 | 1 | – | – | – | 1 | – | 2 | 3 | – |
| Europe | 34 | 29 | – | 17 | 3 | 1 | 3 | 3 | – | 7 | 7 | 1 | 1 |
| East Asia | 31 | 16 | – | 3 | 6 | – | – | 40 | – | 2 | – | 1 | – |
| Central/South Asia | 43 | 29 | – | 8 | 4 | – | – | 4 | – | 11 | 1 | 1 | – |
| North Africa | 12 | 28 | – | 12 | 3 | – | – | – | 8 | 8 | – | 28 | – |
| Subsaharan Africa | 24 | 32 | – | 3 | 6 | – | – | 4 | 12 | 3 | 2 | 1 | 4 |
| Middle East | 35 | 25 | – | 7 | 4 | 1 | – | 1 | 2 | 17 | 4 | 3 | – |
| Oceania | 72 | – | – | – | 1 | – | – | 3 | – | 1 | 12 | – | – |
Adapted with permission of Wolters Kluwer Health from Sistonen J, et al.14
IMPACT OF CYP2D6 GENOTYPE ON TAMOXIFEN EFFICACY AND PATIENT OUTCOMES
The clinical validity of a test can be established when the test actually identifies a biologic difference that may or may not be clinically useful. In the CYP2D6 genotyping case, this can be translated to whether there is association of CYP2D6 genotype with the plasma levels of active tamoxifen metabolites (ie, endoxifen), and association of in vivo endoxifen levels with clinical outcomes. Jin et al16 measured the tamoxifen and endoxifen concentrations in plasma following initiation of adjuvant tamoxifen therapy. After 4 months of therapy, patients who were homozygous for the *4/*4 genotype (PM) had mean endoxifen concentrations between fourfold and twofold lower than patients who were EM and IM, respectively, suggesting a gene–dose effect. The association between endoxifen levels and patient outcomes is yet to be fully determined.
Several studies have been conducted to investigate the impact of the CYP2D6 genotypes and phenotypes on outcomes for breast cancer patients on tamoxifen. The underlying hypothesis is that given endoxifen’s high anti-estrogen activity and the influence of CYP2D6 activity on endoxifen levels, women with a reduced CYP2D6 activity (and thus decreased endoxifen levels) would have worse outcomes.
The first evidence of a relationship between CYP2D6 variants and treatment response was reported by the collaboration of investigators from the Consortium of Breast Cancer Pharmacogenetics (COBRA) and the North Central Cancer Treatment Group (NCCTG)/Mayo Clinic.17 CYP2D6 genotype was determined by extraction of DNA from paraffin archival tissue from postmenopausal women randomly assigned to 5-year adjuvant tamoxifen (20 mg/d), without chemotherapy in a prospective phase III trial that included mainly European descendant women. Of the 190 patients for whom analysis of the most common allele associated with the CYP2D6 PM phenotype, CYP2D6 *4, was possible, 137 (72.1%) had wt/wt, 40 (21.1%) wt/*4, and 13 (6.8%) *4/*4 genotype. In multivariate analysis, women who were homozygous for this nonfunctional allele (*4/*4) tended to have shorter relapse-free times (hazard ratio [HR] 1.85, P = .176) and worse relapse-free survival (HR 1.86, P = .089) than heterozygous women (*4/wt) or women without this allele (wt/wt). These findings were confirmed in a further study with the same group of patients, where the concomitant prescription of CYP2D6 inhibitors was an independent predictor of worse outcome.18,19
This study population was further expanded and analyzed in combination with a German breast cancer cohort. With a longer median follow-up of 6.3 years, and expansion of the genotyping to include the non-functional alleles *3, *4, and *5, and the reduced function alleles *10 and *41, a total of 1,325 patients (95.4% postmenopausal), who were treated only with adjuvant tamoxifen for early-stage, hormone receptor–positive breast cancer, were analyzed.20 Patient were classified as CYP2D6 EM (46% of patients), IM (heterozygous EM/IM) (48%), or PM (6%). Patients with genotypes corresponding to EM achieved a longer time to recurrence (P <.001), and better event-free survival (P <.003) and disease-free survival (P <.005) than those with reduced or absent CYP2D6 activity. Although the recurrence rates at 9 years for patients with extensive, reduced, and absent enzyme activity were 14.9%, 20.9%, and 29.0%, respectively, no differences in overall survival were detected between groups. Similarly smaller trials in Asian populations,21-24 where the reduced activity *10 allele is common, showed similar findings.
These results contrast to several published studies in which no association of CYP2D6 genotype and outcome after tamoxifen treatment was found. Nowell et al25 reported a nonsignificant trend toward better overall survival (OS; HR 0.77; 95% confidence interval [CI], 0.32–1.81) in a cohort of adjuvant tamoxifen-treated breast cancer patients with at least one nonfunctional allele (*4), an opposite finding to what was previously expected. Wegman et al26 reported a decrease in the number of recurrences in patients who were treated with 40 mg/d of adjuvant tamoxifen for 2 years and carried the CYP2D6 *4/*4 genotype (odds ratio [OR] 0.28; 95% CI, 0.11– 0.74; P = .0089).
The same investigators performed a larger retrospective tumor tissue analysis of 677 postmenopausal women treated with either 20 or 40 mg of adjuvant tamoxifen therapy for 2 or 5 years.27 Patients homozygous for CYP2D6*4 had a significantly better disease-free survival compared to patients homozygous or heterozygous for the *1 allele (P = .05 and P = .04, respectively). However, this effect was not significant in a multivariate analysis (P = .055). Similarly, Okishiro et al28 reported that among 173 Asian women receiving adjuvant tamoxifen, reduced metabolism was not associated with recurrence-free survival. In the largest report so far, preliminary data from the International Tamoxifen Phamacogenomics Consortium,29 which included 2,880 patients from 12 different sites in the United States, Europe, and Asia, demonstrated no association of CYP2D6 genotype with disease-free or overall survival in women receiving adjuvant tamoxifen. However, a comprehensive analysis was not performed since there was incomplete allele coverage, with some sites only providing information on the *4 allele, and data on CYP2D6-inhibiting drugs were not available in most of the patients. In addition, there was heterogeneity in dose and duration of tamoxifen treatment among the sites, with only 65% of the cohort receiving 20 mg/d for the intended 5 years. In another study of 747 postmenopausal ER-positive patients who were randomized to receive tamoxifen followed by exemestane after 2.5 to 3 years within the Tamoxifen Exemestane Adjuvant Multinational (TEAM) trial, no association was found between CYP2D6 phenotype (based on variants *3, *4, *6, *14, and *41 and concomitant CYP2D6 inhibitor use)30 and disease-free survival. Patients were censored at the time of switch to exemestane, and with a median follow-up of 2.5 years, only early relapses were likely detected in this report. Most recently, two retrospective analyses of large prospective trials, the Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial, comparing tamoxifen and anastrozole, and the Breast International Group (BIG) 1–98, comparing tamoxifen and letrozole, have been reported. In the ATAC analysis, 588 of 3,116 women (19%) who participated in the trial were genotyped and classified as PM, IM, and EM based on a previously described CYP2D6 scoring system that assigns predicted allele activities from 0 (no activity) to 2 (high activity).31 There was no associations between any of the CYP2D6 scores and rates of recurrence in tamoxifen-treated patients (PM v EM, HR 1.06; 95% CI, 0.51–2.22, P = .873).32 Similarly, in the BIG 1–98 analysis, 1,243 postmenopausal women were genotyped (48% of trial participants), and no difference was found among the different metabolizers groups and breast cancer–free survival in the tamoxifen group (PM v EM, HR 0.58; 95% CI, 0.28 –1.21, P = .35).33 A summary of all of these studies can be found in Table 2.
Table 2.
Summary of Trials Evaluating the Association Between CYP2D6 Genotype and Response to Tamoxifen Therapy
|
Study |
Type of Study |
Disease Setting/Population |
N |
Confounding Factors Controlled for in the Study |
Adjustment for CYP2D6 Inhibitors |
Outcome (compared to EM) |
|---|---|---|---|---|---|---|
| Studies that did not find a positive association between reduced CYP2D6 metabolizers and outcomes | ||||||
| Nowell et al, 200525 | Retrospective cohort study | Adjuvant Pre- and postmenopausal |
162 | Age, stage, race, ER/PR status | No | PM/IM: no difference in PFS |
| Wegman et al, 200526 | Retrospective review of a prospective trial |
Adjuvant Postmenopausal |
76 | Age, tumor size, node status | No | PM: lower recurrence risk |
| Wegman et al, 200727 | Retrospective regional registry |
Adjuvant Postmenopausal |
677 | Tumor size, node status | No | PM: better RFS |
| Newman et al, 200871 | Retrospective—registry of BRCA1/2 carriers |
Adjuvant Pre- and postmenopausal |
115 | Node status | Included in group definition | PM: no difference in RFS |
| Okishiro et al, 200928 | Retrospective cohort study in Japanese patients |
Adjuvant Pre- and postmenopausal |
173 | Adjuvant therapy | Patients taking paroxetine were excluded |
IM: no difference in RFS |
| Toyama et al, 200972 | Retrospective cohort study in Japanese patients |
Adjuvant Pre- and postmenopausal |
154 | Tumor size, stage, HER2, Ki-67 | No | IM: no difference in DFS and OS |
| Goetz et al, 200929 | Retrospective cohort study in Europe and Asia |
Adjuvant Pre- and postmenopausal |
2,880 | NA | No | PM: No difference in DFS or OS |
| Dezentje et al, 201030 | Retrospective review of a prospective trial |
Adjuvant Postmenopausal |
747 | NA | Included in group definition | PM/IM: no difference in DFS |
| Rae et al, 201032 | Retrospective review of a prospective trial |
Adjuvant Postmenopausal |
588 | Adjuvant therapy, ER/PR status | Included in group definition (scoring system) |
PM/IM: no difference in recurrence rate. |
| Leyland-Jones et al, 201033 | Retrospective review of a prospective trial |
Adjuvant Postmenopausal |
1,243 | Tumor size, node status, grade, HER2, Ki-67, race, local therapy. |
No | PM/IM: no difference in DFS |
| Studies suggesting worse outcomes for reduced CYP2D6 metabolizers | ||||||
| Goetz et al, 200718,19 | Retrospective review of a prospective trial |
Adjuvant Postmenopausal |
190 | Tumor size, node status | Included in group definition | PM: worse TTR and DFS |
| Schroth et al, 200773 | Retrospective cohort study | Adjuvant Pre- and postmenopausal |
206 | Tumor size, node status | No | PM/IM: worse RFS and EFS |
| Lim et al, 200723 | Prospective cohort study in Korean patients |
Metastatic | 21 | Age, ER/PR status, number of disease sites, organ of disease sites, and prior use of aromatase inhibitor |
Patients taking SSRI were excluded |
IM: worse TTP |
| Ramon y Cajal et al, 200974 | Retrospective cohort study | Adjuvant Pre- and postmenopausal |
91 | No | No | PM/IM: worse DFS |
| Bijl et al, 200975 | Retrospective—regional registry |
NA | 85 | Age, tamoxifen duration | Yes | PM: worse breast cancer survival |
| Xu et al, 200824 | Mostly retrospective, some prospective cohort study |
Adjuvant Pre- and postmenopausal |
152 | Age, tumor size, node status, adjuvant therapy, surgery, ER/PR status, HER2 status |
No | IM: worse DFS |
| Schroth et al, 200920 | Mostly retrospective, some prospective cohort study |
Adjuvant 95% Postmenopausal |
1,325 | Tumor size, node status, histological grade, menopause status, retrospective recruitment |
No | IM/PM: worse RFS |
| Kiyotani et al, 201021,22 | Retrospective cohort study | Adjuvant Pre- and postmenopausal |
282 | Age, menopausal status, tumor size, node status, nuclear grade, ER/PR status, HER2 status |
Patients taking SSRI were excluded |
IM: worse RFS |
Abbreviations: PM, poor metabolizers; IM, intermediate metabolizers; EM, extensive metabolizers; RFS, relapse-free survival; PFS, progression-free survival; DFS, disease-free survival; OS, overall survival; TTR, time-to-relapse; EFS, event-free survival; TTP, time-to-progression; ER, estrogen receptor; PR, progesterone receptor; NA, not available; SSRI, selective serotonin reuptake inhibitor.
As shown in Table 3, the inconsistencies and discrepancies among studies can be explained by several factors, including selection bias, the inclusion of ineligible person-time bias,34 uncontrolled confounders, and misclassification of patients. For example, the retrospective cohort design of most of the trials may have biased towards survivor patients by selecting through availability of tumor samples. Also, uncontrolled confounders, such as patient adherence and duration of tamoxifen therapy, use of chemotherapeutic agents before or after tamoxifen, prognostic factors, or the concomitant use of CYP2D6 inhibitors, have not been accounted for comprehensively in any of the studies. Notably, Lash et al recently argued that uncontrolled confounders are unlikely to explain the large heterogeneity in the studies.35
Table 3.
Limitations and Source of Heterogeneity in Studies
| Retrospective nature of most studies, with heterogeneous populations |
| Misclassification of patients’ hormonal status |
| Many studies are underpowered to detect a significant difference |
| Not all relevant genetic variants and enzymes included |
| Potential uncontrolled confounders in the analysis: prognostic markers, CYP2D6- inhibiting medications, tamoxifen adherence and dose |
CYP2D6 INHIBITORS
Some selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine and paroxetine, and selective noradrenaline-dopamine reuptake inhibitors, such as bupropion, are strong CYP2D6 inhibitors, and are commonly administered with tamoxifen to treat depression or alleviate hot flashes, common and undesirable side effects of tamoxifen.36 The effect of CYP2D6 inhibitors was first evaluated in a pilot pharmacokinetic study of 12 women with breast cancer who were taking adjuvant tamoxifen.11 The coadministration of paroxetine was associated with a 56% reduction in plasma levels of endoxifen. In a follow-up study including 80 newly diagnosed patients receiving tamoxifen, the coadministration of fluoxetine and/or paroxetine could convert a CYP2D6 EM/EM genotype to a phenotypic PM, as shown by reductions in endoxifen to a PM level.16 Further studies, with conflicting results, have addressed the impact of inhibitor coprescription on tamoxifen-associated outcomes. Using a case control design, one study reported 28 patients without recurrences of breast cancer (controls) matched to an equal number of cases (recurrences), by cancer stage and year of diagnosis.37 No significant difference was found for CYP2D6 inhibitor or substrate exposure between cases and controls. A follow-up study using a registry database covering all pharmacies in Denmark also reported no association between breast cancer recurrence and 15 different medications.38 The impact of CYP2D6 inhibition in a large sample of 1,962 stage I–III breast cancer patients, among whom 150 (7.6%) used a moderate or strong inhibitor during tamoxifen treatment, also was reported.39 In this study, patients on an inhibitor had the same event-free survival (distant metastases, locoregional recurrences, and second primary breast cancers) as patients who were not taking an inhibitor. The three negative studies were retrospective in nature and had several limitations, including a relatively small number of patients on inhibitors, and no control for confounding factors.
Two other studies provided different results and suggested a significant drug interaction between tamoxifen and CYP2D6 inhibitors. In a population-based registry study that included 2,430 women treated with tamoxifen and a single SSRI in Ontario, Canada, paroxetine was found to increase the risk of death from breast cancer.40 In contrast, other SSRI antidepressants, including fluoxetine, which is also a strong CYP2D6 inhibitor, were not associated with an increased risk. The investigators suggested that this was due to the small number of women exposed to fluoxetine in the study. Finally, another retrospective analysis of a US drug provider’s database evaluated 1,300 women who were prescribed tamoxifen and followed for at least 2 years. At 2 years, the patients on a moderate to potent CYP2D6 inhibitor had a significantly higher risk of breast cancer recurrence (13.9% v 7.5%; P <.001) than women receiving tamoxifen alone.41
In practice, clinicians should be aware of drug interactions, and although no prospective study has evaluated this important issue, strong CYP2D6 inhibitors should be avoided or used for the shortest period of time and an alternative agent chosen, if feasible, in women receiving tamoxifen.42 If treatment of hot flashes is considered, a SSRI such as citalopram (weak inhibitor), or a serotonin–norepinephrine reuptake inhibitor, such as venlafaxine (not an inhibitor), can be used as an alternative.16
RELEVANCE OF CYP2D6 GENOTYPING TO CLINICAL PRACTICE
Placing the data in context, evidence for clinical utility of a test is established when the results of the test lead to a clinical decision that has been shown, with high level of evidence, to improve patient outcomes: in this case, by showing that the use of CYP2D6 genotype to select an endocrine therapy regimen improves recurrence and survival outcomes in women with ER-positive breast cancer.
In the prevention setting, CYP2D6 genotyping data are very scarce. The two FDA-approved agents, tamoxifen and raloxifen, have different profiles and, in theory, CYP2D6 genotyping could be beneficial in the decision-making process and possibly increase the low chemoprevention initiation rate of about 15% in high-risk women.43 While tamoxifen has been shown to be slightly better in terms of breast cancer recurrence events, raloxifen has a better toxicity profile.44,45 In this regard, an analysis of 47 women with breast cancer and 135 controls from the Italian Tamoxifen Trial46 suggested that women with a CYP2D6*4/*4 genotype may be less likely to benefit from tamoxifen as a chemopreventive agent. Given the small sample size and limited evidence in the chemopreventive setting, experts have not recommended CYP2D6 genotyping in this setting.47
In the adjuvant setting, long-term data demonstrate that the use of tamoxifen reduces recurrence and mortality by more than 30%. More recently, AIs, such as anastrozole or exemestane, have been proven to be effective or superior to tamoxifen in the postmenopausal setting,48 but because of differing side effect profiles, tamoxifen remains the treatment of choice for a large percentage of women. In the premenopausal adjuvant setting, data are limited also because most of the trials assessing CYP2D6 excluded this patient population. The lack of definitive evidence for alternatives to tamoxifen in the premenopausal setting renders the test less useful in this setting. Pharmocogenomics studies incorporated into larger trials evaluating the alternative treatments, such as the ongoing Suppression of Ovarian Function Trial (SOFT),49 which investigates whether adding ovarian suppression to tamoxifen or to AI provides superior reduction in risk of recurrence of early-stage premenopausal breast cancer compared with tamoxifen alone, and the Tamoxifen and Exemestane Trial (TEXT),50 will provide further evidence in this regard.
In the postmenopausal setting, unlike in the premenopausal setting, AIs are generally the preferred agents, as they are more effective in preventing breast cancer recurrence in the first 2 years after surgery.48 However, tamoxifen is still widely used in Asia and in developing countries. In this scenario, one can potentially harm the patient by offering tamoxifen instead of AIs to those PM patients unlikely to benefit from the drug. To corroborate this hypothesis, in a modeling analysis that estimated 5-year progression-free survival rates for PM, IM, and EM patients following treatment with AIs versus tamoxifen in the BIG 1–98 and NCCTG studies, tamoxifen-treated EM patients had outcomes similar to genotypically unselected patients treated with AIs.51 However, given the conflicting results of the several retrospective studies assessing postmenopausal women and the lack of prospective data, there is still much controversy regarding whether CYP2D6 testing should be performed in routine clinical practice. While the American Society of Clinical Oncology Clinical (ASCO) Practice guideline,52 National Comprehensive Cancer Network (NCCN),53 and the St. Gallen’s expert consensus54 do not endorse its routine use, some experts suggest that CYP2D6 genotyping may be appropriate in selected cases, such as patients who are not tolerating the AIs55 or when there is a contraindication for AI therapy and tamoxifen is the preferred alternative.56
In the metastatic setting, the only data come from a small prospective study of 21 pre- and postmenopausal Korean patients with breast cancer who were taking tamoxifen.23 Patients with reduced CYP2D6 functioning (IM/IM genotype), common in this ethnic group, had a shorter time to disease progression than other patients. An Eastern Cooperative Oncology Group (ECOG) phase II prospective study correlating CYP2D6 activity in patients with metastatic or recurrent breast cancer treated with tamoxifen with progression-free survival is currently enrolling patients, and will provide further evidence in this regard (ECOG-E3108/NCT01124695).
FUTURE DIRECTIONS
The clinical utility of CYP2D6 genotyping is an unfinished story and several questions and venues for further research exist. For example, the overall correlation between CYP2D6 genotype and endoxifen levels is relatively poor and a wide interindividual variation exists in steady-state levels of tamoxifen and its metabolites, not explained by genotype variations.57 Also, the efficacy of tamoxifen is not thought to be dose-dependent as higher doses of tamoxifen have not been associated with improved outcomes,12,58-60 and receptor binding studies have suggested that tamoxifen metabolites may reach levels adequate for full therapeutic effect irrespective of CYP2D6 genotype.35
In addition, by considering CYP2D6 genotyping the sole method of predicting response, we are accepting the concept that a single metabolic enzyme on a single gene is completely responsible for therapeutic outcome. Instead, pharmacogenetic variation in genes that do not predict endoxifen levels but are involved in drug elimination and transport, such as ABCC2, SULTA1A, and UGT, also may impact tamoxifen response.21 Finally, genetic variants in ERα and ERβ genes may be associated with tamoxifen-induced lipid changes, further contributing to interindividual variability to tamoxifen benefits.61,62
Ongoing trials are exploring the possibility of administering pure endoxifen, thereby bypassing the need for CYP2D6 activation.63 Preclinical studies demonstrated high oral bioavailability and substantially higher concentrations in comparison to a similar dose of tamoxifen.64 If future endoxifen trials are positive, there will no longer be any need for genotyping of CYP2D6 to guide tamoxifen treatment.
CONCLUSION
The quest for personalized medicine holds the promise of matching treatment to tumor, with benefits measured in increased efficacy and less toxicity, and potentially lower costs. Inconsistencies in the data across studies, as described previously, originate from selection, information and data bias. Retrospective pharmacogenetic analyses of larger adjuvant tamoxifen trials may shed some light on this topic, but due to the same intrinsic limitations of retrospective analyses, they will likely not provide definitive evidence.
Finally, the financial and public health impacts of adopting CYP2D6 genotyping in practice will be large. Given the added costs of genotyping and the lack of strong definitive evidence, it just may not be conceivable to test all eligible breast cancer patients. The same argument applies to choosing AIs over tamoxifen based on CYP2D6 genotyping with the current level of evidence, when tamoxifen is a cheaper drug with similar efficacy. In fact, a greater challenge for patients and doctors is how to address the adherence and persistence to adjuvant hormonal therapy, when the discontinuation rate is approximately 7% to 10% per year for tamoxifen and AIs,65-68 and even higher in socioeconomically disadvantaged women.69 In this regard, although the barriers to adherence are many, patients with higher out-of-pocket costs for the AIs are more likely to be nonadherent,70 and advocating switching to AIs based on controversial data may not be beneficial to the patient.
Furthermore, while greater individualization of treatment should be further promoted, current data do not support routine CYP2D6 genotyping in clinical practice. Data from adequately powered randomized prospective trials, comparing outcomes of patients who had their tamoxifen utilization based on comprehensive genotyping to those who did not, should be the gold standard evidence in deciding whether or not CYP2D6 genotyping should be adopted in routine practice.
REFERENCES
- 1.Jordan VC. The 38th David A. Karnofsky lecture: the paradoxical actions of estrogen in breast cancer-survival or death? J Clin Oncol. 2008;26:3073–82. doi: 10.1200/JCO.2008.17.5190. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 3.Meyer UA. Pharmacogenetics—five decades of therapeutic lessons from genetic diversity. Nat Rev Genet. 2004;5:669–76. doi: 10.1038/nrg1428. [DOI] [PubMed] [Google Scholar]
- 4.Coller JK, Krebsfaenger N, Klein K, et al. The influence of CYP2B6, CYP2C9 and CYP2D6 genotypes on the formation of the potent antioestrogen Z-4-hydroxy-tamoxifen in human liver. Br J Clin Pharmacol. 2002;54:157–67. doi: 10.1046/j.1365-2125.2002.01614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crewe HK, Ellis SW, Lennard MS, Tucker GT. Variable contribution of cytochromes P450 2D6, 2C9 and 3A4 to the 4-hydroxylation of tamoxifen by human liver microsomes. Biochem Pharmacol. 1997;53:171–8. doi: 10.1016/s0006-2952(96)00650-8. [DOI] [PubMed] [Google Scholar]
- 6.Dehal SS, Kupfer D. CYP2D6 catalyzes tamoxifen 4-hydroxylation in human liver. Cancer Res. 1997;57:3402–6. [PubMed] [Google Scholar]
- 7.Jacolot F, Simon I, Dreano Y, Beaune P, Riche C, Berthou F. Identification of the cytochrome P450 IIIA family as the enzymes involved in the N-demethylation of tamoxifen in human liver microsomes. Biochem Pharmacol. 1991;41:1911–9. doi: 10.1016/0006-2952(91)90131-n. [DOI] [PubMed] [Google Scholar]
- 8.Desta Z, Ward BA, Soukhova NV, Flockhart DA. Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther. 2004;310:1062–75. doi: 10.1124/jpet.104.065607. [DOI] [PubMed] [Google Scholar]
- 9.Johnson M, Zuo H, Lee K-H, et al. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat. 2004;85:151–9. doi: 10.1023/B:BREA.0000025406.31193.e8. [DOI] [PubMed] [Google Scholar]
- 10.Lim YC, Li L, Desta Z, et al. Endoxifen, a secondary metabolite of tamoxifen, and 4-OH-tamoxifen induce similar changes in global gene expression patterns in MCF-7 breast cancer cells. J Pharmacol Exp Ther. 2006;318:503–12. doi: 10.1124/jpet.105.100511. [DOI] [PubMed] [Google Scholar]
- 11.Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, Bhargava P. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95:1758–64. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]
- 12.Fabian C, Tilzer L, Sternson L. Comparative binding affinities of tamoxifen, 4-hydroxytamoxifen, and desmethyltamoxifen for estrogen receptors isolated from human breast carcinoma: correlation with blood levels in patients with metastatic breast cancer. Biopharmaceut Drug Disposition. 1981;2:381–90. doi: 10.1002/bdd.2510020407. [DOI] [PubMed] [Google Scholar]
- 13.Wu X, Hawse JR, Subramaniam M, Goetz MP, Ingle JN, Spelsberg TC. The tamoxifen metabolite, endoxifen, is a potent antiestrogen that targets estrogen receptor α for degradation in breast cancer cells. Cancer Res. 2009;69:1722–7. doi: 10.1158/0008-5472.CAN-08-3933. [DOI] [PubMed] [Google Scholar]
- 14.Sistonen J, Sajantila A, Lao O, Corander J, Barbujani G, Fuselli S. CYP2D6 worldwide genetic variation shows high frequency of altered activity variants and no continental structure. Pharmacogenet Genomics. 2007;17:93–101. doi: 10.1097/01.fpc.0000239974.69464.f2. [DOI] [PubMed] [Google Scholar]
- 15.Ingelman-Sundberg M. Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J. 2004;5:6–13. doi: 10.1038/sj.tpj.6500285. [DOI] [PubMed] [Google Scholar]
- 16.Jin Y, Desta Z, Stearns V, et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst. 2005;97:30–9. doi: 10.1093/jnci/dji005. [DOI] [PubMed] [Google Scholar]
- 17.Goetz MP, Rae JM, Suman VJ, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol. 2005;23:9312–8. doi: 10.1200/JCO.2005.03.3266. [DOI] [PubMed] [Google Scholar]
- 18.Goetz M, Suman V, Ames M, et al. Tamoxifen pharmacogenetics of CYP2D6, CYP2C19, and SULT1A1: long term follow-up of the North Central Cancer Treatment Group 89 –30-52 adjuvant trial. Cancer Res. 2009;69:6037. [Google Scholar]
- 19.Goetz MP, Knox SK, Suman VJ, et al. The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res Treat. 2007;101:113–21. doi: 10.1007/s10549-006-9428-0. [DOI] [PubMed] [Google Scholar]
- 20.Schroth W, Goetz MP, Hamann U, et al. Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA. 2009;302:1429–36. doi: 10.1001/jama.2009.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiyotani K, Mushiroda T, Imamura CK, et al. Significant effect of polymorphisms in CYP2D6 and ABCC2 on clinical outcomes of adjuvant tamoxifen therapy for breast cancer patients. J Clin Oncol. 2010;28:1287–93. doi: 10.1200/JCO.2009.25.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiyotani K, Mushiroda T, Sasa M, et al. Impact of CYP2D6*10 on recurrence-free survival in breast cancer patients receiving adjuvant tamoxifen therapy. Cancer Sci. 2008;99:995–9. doi: 10.1111/j.1349-7006.2008.00780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim H-S, Ju Lee H, Seok Lee K, Sook Lee E, Jang I-J, Ro J. Clinical implications of CYP2D6 genotypes predictive of tamoxifen pharmacokinetics in metastatic breast cancer. J Clin Oncol. 2007;25:3837–45. doi: 10.1200/JCO.2007.11.4850. [DOI] [PubMed] [Google Scholar]
- 24.Xu Y, Sun Y, Yao L, et al. Association between CYP2D6 *10 genotype and survival of breast cancer patients receiving tamoxifen treatment. Ann Oncol. 2008;19:1423–9. doi: 10.1093/annonc/mdn155. [DOI] [PubMed] [Google Scholar]
- 25.Nowell SA, Ahn J, Rae JM, et al. Association of genetic variation in tamoxifen-metabolizing enzymes with overall survival and recurrence of disease in breast cancer patients. Breast Cancer Res Treat. 2005;91:249–58. doi: 10.1007/s10549-004-7751-x. [DOI] [PubMed] [Google Scholar]
- 26.Wegman P, Vainikka L, Stal O, et al. Genotype of metabolic enzymes and the benefit of tamoxifen in postmenopausal breast cancer patients. Breast Cancer Res. 2005;7:R284–90. doi: 10.1186/bcr993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wegman P, Elingarami S, Carstensen J, Stal O, Nordenskjold B, Wingren S. Genetic variants of CYP3A5, CYP2D6, SULT1A1, UGT2B15 and tamoxifen response in postmenopausal patients with breast cancer. Breast Cancer Res. 2007;9:R7. doi: 10.1186/bcr1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okishiro M, Taguchi T, Jin Kim S, Shimazu K, Tamaki Y, Noguchi S. Genetic polymorphisms of CYP2D6 10 and CYP2C19 2, 3 are not associated with prognosis, endometrial thickness, or bone mineral density in Japanese breast cancer patients treated with adjuvant tamoxifen. Cancer. 2009;115:952–61. doi: 10.1002/cncr.24111. [DOI] [PubMed] [Google Scholar]
- 29.Goetz M, Berry D, Klein T, International Tamoxifen Pharmacogenomics Consortium Adjuvant tamoxifen treatment outcome according to cytochrome P450 2D6 (CYP2D6) phenotype in early stage breast cancer: findings from the International Tamoxifen Pharmacogenomics Consortium. Cancer Res. 2010;69:33. [Google Scholar]
- 30.Dezentje VO, Van Schaik RH, Vletter-Bogaartz JM, et al. Pharmacogenetics of tamoxifen in relation to disease-free survival in a Dutch cohort of the Tamoxifen Exemestane Adjuvant Multinational (TEAM) trial. J Clin Oncol (Meeting Abstracts) 2010;28:510. [Google Scholar]
- 31.Borges S, Desta Z, Jin Y, et al. Composite functional genetic and comedication CYP2D6 activity score in predicting tamoxifen drug exposure among breast cancer patients. J Clin Pharmacol. 2010;50:450–8. doi: 10.1177/0091270009359182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rae JM, Drury S, Hayes DF, et al. [S1–7] Lack of correlation between gene variants in tamoxifen metabolizing enymes with primary endpoints in the ATAC trial; Abstract presented at: 33rd Annual San Antonio Breast Cancer Symposium; San Antonio, TX. December 9, 2010. [Google Scholar]
- 33.Leyland-Jones B, Regan MM, Bouzyk M, et al. [S1– 8] outcome according to CYP2D6 genotype among postmenopausal women with endocrine-responsive early in-vasive breast cancer randomized in the BIG 1–98 trial; Abstract presented at: 33rd Annual San Antonio Breast Cancer Symposium; San Antonio, TX. December 9, 2010. [Google Scholar]
- 34.Lash TL, Cole SR. Immortal person-time in studies of cancer outcomes. J Clin Oncol. 2009;27:e55–6. doi: 10.1200/JCO.2009.24.1877. [DOI] [PubMed] [Google Scholar]
- 35.Lash TL, Lien EA, Sorensen HT, Hamilton-Dutoit S. Genotype-guided tamoxifen therapy: time to pause for reflection? Lancet Oncol. 2009;10:825–33. doi: 10.1016/S1470-2045(09)70030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alfaro CL, Lam YW, Simpson J, Ereshefsky L. CYP2D6 inhibition by fluoxetine, paroxetine, sertraline, and venlafaxine in a crossover study: intraindividual variability and plasma concentration correlations. J Clin Pharmacol. 2000;40:58–66. doi: 10.1177/00912700022008702. [DOI] [PubMed] [Google Scholar]
- 37.Lehmann D, Nelsen J, Ramanath V, Newman N, Duggan D, Smith A. Lack of attenuation in the antitumor effect of tamoxifen by chronic CYP isoform inhibition. J Clin Pharmacol. 2004;44:861–5. doi: 10.1177/0091270004266618. [DOI] [PubMed] [Google Scholar]
- 38.Ahern TP, Pedersen L, Cronin-Fenton DP, Sorensen HT, Lash TL. No increase in breast cancer recurrence with concurrent use of tamoxifen and some CYP2D6-inhibiting medications. Cancer Epidemiol Biomarkers Prev. 2009;18:2562–4. doi: 10.1158/1055-9965.EPI-09-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dezentje VO, van Blijderveen NJ, Gelderblom H, et al. Effect of concomitant CYP2D6 inhibitor use and tamoxifen adherence on breast cancer recurrence in early-stage breast cancer. J Clin Oncol. 2010;28:2423–9. doi: 10.1200/JCO.2009.25.0894. [DOI] [PubMed] [Google Scholar]
- 40.Kelly CM, Juurlink DN, Gomes T, et al. Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: a population based cohort study. BMJ. 2010;340:c693. doi: 10.1136/bmj.c693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aubert RE, Stanek EJ, Yao J, et al. Risk of breast cancer recurrence in women initiating tamoxifen with CYP2D6 inhibitors. J Clin Oncol (Meeting Abstracts) 2009;27:CRA508. [Google Scholar]
- 42.Sideras K, Ingle JN, Ames MM, et al. Coprescription of tamoxifen and medications that inhibit CYP2D6. J Clin Oncol. 2010;28:2768–76. doi: 10.1200/JCO.2009.23.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol. 2010;28:3090–5. doi: 10.1200/JCO.2009.27.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serrano D, Lazzeroni M, Zambon CF, et al. Efficacy of tamoxifen based on cytochrome P450 2D6, CYP2C19 and SULT1A1 genotype in the Italian Tamoxifen Prevention Trial. Pharmacogenomics J. doi: 10.1038/tpj.2010.17. Epub 2010 March 23, 10.1038/tpj.2010.17. Available from: www.nature.com/tpj. [DOI] [PubMed] [Google Scholar]
- 45.Vogel VG, Costantino JP, Wickerham DL, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing Breast Cancer. Cancer Prev Res. 2010;3:696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonanni B, Macis D, Maisonneuve P, et al. Polymorphism in the CYP2D6 tamoxifen-metabolizing gene influences clinical effect but not hot flashes: data from the Italian Tamoxifen Trial. J Clin Oncol. 2006;24:3708–9. doi: 10.1200/JCO.2006.06.8072. [DOI] [PubMed] [Google Scholar]
- 47.Visvanathan K, Chlebowski RT, Hurley P, et al. American Society of Clinical Oncology clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. J Clin Oncol. 2009;27:3235–58. doi: 10.1200/JCO.2008.20.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor–positive breast cancer: status report 2004. J Clin Oncol. 2005;23:619–29. doi: 10.1200/JCO.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 49.IBCSG 24 – 02/BIG 2– 02 (SOFT) A phase III trial evaluating the role of ovarian function suppression and the role of exemestane as adjuvant therapies for premenopausal women with endocrine responsive breast cancer. Available from: http://www.ibcsg.org/Public/PatientsandPublic/Clinical_Trials/Open_Trials/IBCSG_24–02/Pages/IBCSG24–02BIG2–02(SOFT).aspx.
- 50.A phase III trial evaluating the role of exemestane plus GnRH analogue as adjuvant therapy for premenopausal women with endocrine responsive breast cancer (TEXT) Available from: http://www.ibcsg.org/Public/Health_Professionals/Open_Trials/IBCSG_25–02/Pages/IBCSG25–02BIG3–02(TEXT).aspx.
- 51.Punglia RS, Burstein HJ, Winer EP, Weeks JC. Pharmacogenomic variation of CYP2D6 and the choice of optimal adjuvant endocrine therapy for postmenopausal breast cancer: a modeling analysis. J Natl Cancer Inst. 2008;100:642–8. doi: 10.1093/jnci/djn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784–96. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.National Comprehensive Cancer Network Practice Guidelines in Oncology: Breast Cancer-v. 1.2011. Available from: http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf.
- 54.Goldhirsch A, Ingle JN, Gelber RD, et al. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2009. Ann Oncol. 2009;20:1319–29. doi: 10.1093/annonc/mdp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goetz MP. Tamoxifen, endoxifen, and CYP2D6: the rules for evaluating a predictive factor. Oncology (Williston Park) 2009;23:1233–4. [PubMed] [Google Scholar]
- 56.Higgins M, Stearns V. CYP2D6 polymorphisms and tamoxifen metabolism: clinical relevance. Curr Oncol Rep. 2010;12:7–15. doi: 10.1007/s11912-009-0076-5. [DOI] [PubMed] [Google Scholar]
- 57.Langan-Fahey SM, Tormey DC, Jordan VC. Tamoxifen metabolites in patients on long-term adjuvant therapy for breast cancer. Eur J Cancer. 1990;26:883–8. doi: 10.1016/0277-5379(90)90191-u. [DOI] [PubMed] [Google Scholar]
- 58.Bratherton DG, Brown CH, Buchanan R, et al. A comparison of two doses of tamoxifen (Nolvadex) in postmenopausal women with advanced breast cancer: 10 mg bd versus 20 mg bd. Br J Cancer. 1984;50:199–205. doi: 10.1038/bjc.1984.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Decensi A, Robertson C, Viale G, et al. A randomized trial of low-dose tamoxifen on breast cancer proliferation and blood estrogenic biomarkers. J Natl Cancer Inst. 2003;95:779–90. doi: 10.1093/jnci/95.11.779. [DOI] [PubMed] [Google Scholar]
- 60.Kisanga ER, Gjerde J, Guerrieri-Gonzaga A, et al. Tamoxifen and metabolite concentrations in serum and breast cancer tissue during three dose regimens in a randomized preoperative trial. Clin Cancer Res. 2004;10:2336–43. doi: 10.1158/1078-0432.ccr-03-0538. [DOI] [PubMed] [Google Scholar]
- 61.Jin Y, Hayes DF, Li L, et al. Estrogen receptor genotypes influence hot flash prevalence and composite score before and after tamoxifen therapy. J Clin Oncol. 2008;26:5849–54. doi: 10.1200/JCO.2008.16.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ntukidem NI, Nguyen AT, Stearns V, et al. Estrogen receptor genotypes, menopausal status, and the lipid effects of tamoxifen. Clin Pharmacol Ther. 2008;83:702–10. doi: 10.1038/sj.clpt.6100343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahmad A, Ali S, Ahmad M, Sheikh S, Ahmad I. Orally administered endoxifen is a new therapeutic agent for breast cancer. Breast Cancer Res Treat. 2010;122:579–84. doi: 10.1007/s10549-009-0704-7. [DOI] [PubMed] [Google Scholar]
- 64.Reid JM, Buhrow SA, Safgren SL, Jia L. Endoxifen pharmacokinetics and bioavailability of in female rats and dogs [abstract]; Proceedings of the 101st Annual Meeting of the American Association for Cancer Research; Washington, DC. Philadelphia (PA): AACR. Apr 17–21, 2010; 2010. p. 25. Abstract no. 2607. [Google Scholar]
- 65.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–8. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA. Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat. 2006;99:215–20. doi: 10.1007/s10549-006-9193-0. [DOI] [PubMed] [Google Scholar]
- 67.Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26:556–62. doi: 10.1200/JCO.2007.11.5451. [DOI] [PubMed] [Google Scholar]
- 68.Ziller V, Kalder M, Albert US, et al. Adherence to adjuvant endocrine therapy in postmenopausal women with breast cancer. Ann Oncol. 2009;20:431–6. doi: 10.1093/annonc/mdn646. [DOI] [PubMed] [Google Scholar]
- 69.Kimmick G, Anderson R, Camacho F, Bhosle M, Hwang W, Balkrishnan R. Adjuvant hormonal therapy use among insured, low-income women with breast cancer. J Clin Oncol. 2009;27:3445–51. doi: 10.1200/JCO.2008.19.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sedjo RL, Devine S. Predictors of non-adherence to aromatase inhibitors among commercially insured women with breast cancer. Breast Cancer Res Treat. 2010;125:191–200. doi: 10.1007/s10549-010-0952-6. [DOI] [PubMed] [Google Scholar]
- 71.Newman WG, Hadfield KD, Latif A, et al. Impaired tamoxifen metabolism reduces survival in familial breast cancer patients. Clin Cancer Res. 2008;14:5913–8. doi: 10.1158/1078-0432.CCR-07-5235. [DOI] [PubMed] [Google Scholar]
- 72.Toyama T, Yamashita H, Sugiura H, Kondo N, Iwase H, Fujii Y. No association between CYP2D6*10 genotype and survival of node-negative Japanese breast cancer patients receiving adjuvant tamoxifen treatment. Jpn J Clin Oncol. 2009;39:651–6. doi: 10.1093/jjco/hyp076. [DOI] [PubMed] [Google Scholar]
- 73.Schroth W, Antoniadou L, Fritz P, et al. Breast cancer treatment outcome with adjuvant tamoxifen relative to patient CYP2D6 and CYP2C19 genotypes. J Clin Oncol. 2007;25:5187–93. doi: 10.1200/JCO.2007.12.2705. [DOI] [PubMed] [Google Scholar]
- 74.Ramon y, Cajal T, Altes A, Pare L, et al. Impact of CYP2D6 polymorphisms in tamoxifen adjuvant breast cancer treatment. Breast Cancer Res Treat. 2010;119:33–8. doi: 10.1007/s10549-009-0328-y. [DOI] [PubMed] [Google Scholar]
- 75.Bijl MJ, van Schaik RH, Lammers LA, et al. The CYP2D6*4 polymorphism affects breast cancer survival in tamoxifen users. Breast Cancer Res Treat. 2009;118:125–30. doi: 10.1007/s10549-008-0272-2. [DOI] [PubMed] [Google Scholar]


