Abstract
The organization of the embryonic neural plate requires coordination of multiple signal transduction pathways, including fibroblast growth factors (FGFs), bone morphogenetic proteins (BMPs), and WNTs. Many studies have suggested that a critical component of this process is the patterning of posterior neural tissues by an FGF-caudal signaling cascade. Here, we have identified a novel player, Dazap2, and show that it is required in vivo for posterior neural fate. Loss of Dazap2 in embryos resulted in diminished expression of hoxb9 with a concurrent increase in the anterior marker otx2. Furthermore, we found that Dazap2 is required for FGF dependent posterior patterning; surprisingly, this is independent of Cdx activity. Furthermore, in contrast to FGF activity, Dazap2 induction of hoxb9 is not blocked by loss of canonical Wnt signaling. Functionally, we found that increasing Dazap2 levels alters neural patterning and induces posterior neural markers. This activity overcomes the anteriorizing effects of noggin, and is downstream of FGF receptor activation. Our results strongly suggest that Dazap2 is a novel and essential branch of FGF-induced neural patterning.
Keywords: Dazap2, Prtb, Posterior neural patterning, FGF, Cdx4, Noggin, Wnt Short title: Dazap2 in FGF-mediated neural patterning
INTRODUCTION
After its initial induction from the ectoderm, the developing neural tissue must be patterned along the anterior-posterior axes. Early models proposed that this occurred in a two-step process, where an initial inductive signal – the “activating” signal – instructs naïve ectoderm to adopt an anterior neural fate. Some of this induced neural tissue is then “transformed” into more posterior fates, such as hindbrain and spinal cord, by a second signal (Nieuwkoop, 1952; Harland and Gerhart, 1997). FGFs, as well as Wnts, retinoids and insulin-like growth factors may all be important for this regionalization of the neural plate (Pownall et al., 1996; Blumberg et al., 1997; McGrew et al., 1997; Shiotsugu et al., 2004). However, all of these factors have widespread biological activities, so it has been difficult to uncouple early requirements for these pathways from specific roles in posterior neural patterning. To further complicate matters, it appears that active repression of these pathways may be required for proper formation of the anterior brain, suggesting that endogenous “posterior” signals are present throughout the neural plate (Koide et al., 2001; Niehrs, 2004; Onai et al., 2004).
Of these candidate signals, fibroblast growth factors have been shown to induce posterior neural tissue directly from naïve ectoderm (Cox and Hemmati-Brivanlou, 1995; Kengaku and Okamoto, 1995; Lamb and Harland, 1995; Delaune et al., 2005). Consistent with these data, several studies using dominant-negative FGF receptors suggested that FGF signaling is required for the induction of posterior neural tissue (McGrew et al., 1997; Pownall et al., 1998; Holowacz and Sokol, 1999; Ribisi et al., 2000) as well as forebrain fates (Hongo et al., 1999). FGF8a has also been shown to induce neuronal markers, including neuronal β-tubulin, as well as an array of neural and neural crest markers (Hardcastle and Papalopulu, 2000; Monsoro-Burq et al., 2003; Fletcher et al., 2006). Thus, unlike Noggin, which can only induce anterior neural tissue, FGFs are able to induce and pattern the neurectoderm. This suggests a dual role for FGFs, where several aspects of posterior neural induction and differentiation could occur as the result of a single molecular cue.
In Xenopus, during patterning of the posterior neural plate, a caudal homologue, cdx4 (previously known as xcad3), has been shown to be a direct transcriptional target of FGF’s posteriorizing activity (Isaacs et al., 1998; Haremaki et al., 2003). Cdx proteins, in turn, can bind to and activate the promoters of posterior hox genes (Shimizu et al., 2006). In addition, inhibition of Cdx4 activity using antimorphic repressor mutants or loss of cdx genes in zebrafish has shown that this activity is required for posterior neural gene expression (Isaacs et al., 1998; Pownall et al., 1998; Skromne et al., 2007). These data are consistent with the model that FGF signaling is necessary for the establishment of posterior neural fate and that this activity requires at least two steps: immediate early induction of cdx4 followed by subsequent activation of posterior Hox targets. In this context, Cdx4 may be a direct “transforming” effector.
In this paper, we set out to identify additional molecules that might confer regional identity. We found dazap2 in an expression screen based on its ability to cause anterior truncations as well as to induce the spinal cord marker hoxb9 (Grammer, Liu et al., 2000). Subsequent examination showed that in vivo overexpression of dazap2 could expand the expression of the pan-neural marker nrp1 (Grammer, Liu et al., 2000). Thus, we set out to define its role in neural patterning further.
Although dazap2 is evolutionarily well conserved, with homologues throughout the vertebrate lineages, little is known about the activity of this protein family. Thus, the identification of the potent bioactivity of dazap2 provided us with an opportunity to study the function and requirements for this molecule in vivo. Here, we demonstrate that dazap2 is necessary and sufficient for the induction of posterior neural fates in Xenopus. We show that modulating dazap2 levels shifts the anterior-posterior boundaries within the neural plate. We then show that unlike FGF-mediated inductions, posterior fates mediated by Dazap2 action are independent of cdx4 and independent of Wnt/β-catenin signaling. Most interestingly, we find that dazap2 is required for FGF activity. Taken together, this suggests that dazap2 reveals a novel mechanism for patterning the posterior neural plate.
MATERIALS AND METHODS
Embryo manipulations and dissection
Xenopus laevis eggs were collected and fertilized using standard protocols (Sive, 2000). Embryos were grown in 1/3x MMR and staged according to Nieuwkoop and Faber (Nieuwkoop, 1994). For blocking FGF signalling in vivo, we used the chemical inhibitor SU5402 which inhibits FGFR kinase activity (Mohammadi et al., 1997). A concentration of 50–80μMM SU5402 was shown to inhibit most neural induction when applied at midblastula stages (Delaune et al., 2005). Thus, for each experiment, the efficiency of the SU5402 treatment was validated by the analysis of sibling embryos soaked in the inhibitor solution prior to and during neural induction (stages 7–18) and stained for sox2. In this regimen, neural induction was strongly impaired (100%, n=21, not shown). For animal cap experiments, the vitelline membrane of stage 9 embryos was removed and the ectoderm of the blastocoel roof dissected. The explants were aged in 3/4x NAM until the sibling controls reached the indicated stages. Then both explants and embryos were collected for RT-PCR (Sive, 2000).
RNA synthesis and antisense morpholino RNA (MO) microinjection
Synthetic messenger RNA was made using the in vitro SP6 mMessage mMachine kit (Ambion). RNA was stored at −80°C until thawed on ice prior to injection. The following plasmids were used to produce mRNA: dazap2 (cs107-dazap2; Genbank Accession BG354661; FGF8a (cs107-fgf8) (Monsoro-Burq et al., 2003); noggin (cs2+-noggin) (Mariani and Harland, 1998); cdx4, cdx-VP16 and cdx-EnR (Isaacs et al., 1998); and nuclear beta-galactosidase (cs2+-nlacZ) (Rupp et al., 1994). Morpholino oligonucleotides (MO) (Gene Tools) were designed to bind either the translation initiation region (DZ2-MO-CTTTATTATTCATAGCTCCGGGTTC) or the splice acceptor (SP-MO – GGTACTGTCCTGTAGAAAGGAAAGC) on the second exon of the dazap2 gene (Suppl. Fig. 2). Additionally, a commonly used Xenopus laevis β-catenin MO-(TTTCAACCGTTTCCAAAGAACCAGG) was acquired from Gene Tools (Heasman et al., 2000). Targeted mRNA or MO injections were performed in a 10nl volume into one blastomere at the 2-cell or 4-cell stage, with nlacZ mRNA co-injected for lineage tracing. Embryos were grown in 1/3x MMR until appropriate stages and then fixed with 3.7% formaldehyde in PBS for 20 minutes. Red-gal (Research Organics) was used to reveal β-galactosidase activity to trace the cells that inherited injected mRNA (Sive, 2000; Monsoro-Burq, 2007). Embryos were then fixed for an additional 2 hours at room temperature or overnight at 4°C then dehydrated in several washes of ethanol and stored at −20°C until ISH.
Whole-mount in situ hybridization (ISH)
The embryos were gently rehydrated with decreasing amounts of ethanol (mixed with PBS + 0.1% Tween-20). The ISH procedure used a multi-basket system (Sive, 2000), and a fast protocol optimized for ectoderm and neural tissue (Monsoro-Burq, 2007). Embryos were then thoroughly washed and AP activity was visualized with BCIP and NBT (Roche). Digoxigenin-labeled antisense RNA probes were made from the following plasmids: myoD (Hopwood et al., 1989); sox2 (Grammer et al., 2000); nrp1 (Knecht et al., 1995); otx2 (Lamb et al., 1993); krox20 (Bolce et al., 1992); hoxb9 (Sharpe et al., 1987); cpl1 (Knecht et al., 1995); sox11; and pax6 (Hirsch and Harris, 1997). Some embryos were cleared using benzyl benzoate and benzyl alcohol (2:1) (Sive, 1999).
RT-PCR
Proteinase K lysis buffer was used to isolate RNA from embryos and explants for reverse-transcriptase polymerase chain reaction (RT-PCR) (Wilson and Melton, 1994). A control embryo, not treated with reverse transcriptase, was used to assay the complete degradation of genomic DNA (lane 2 in RT-PCR gels). All samples used either ef1α or ornithine decarboxylase (ODC) to normalize the efficiency of the RT reaction and the loading on the gel. STORM phosphoimager analysis software was used to quantify gel signals. The primer sets are described in Supplementary Table 1.
RESULTS
Identification of Dazap2, a novel molecule involved in neural patterning
To identify novel genes important in patterning the neural plate, we screened pools of cDNAs for molecules that perturbed expression of an array of neural markers (Mariani and Harland, 1998; Grammer et al., 2000). One of the clones we identified encoded a homolog of human KIAA0058, also called dazap2 (Genbank accession D31767) and intriguingly, shifted the anterior-posterior identity of the neural plate (described below and reported as 81E10 in (Grammer et al., 2000)). Sequence comparisons revealed extensive conservation of this protein among deuterostomes (Supplementary Figure 1) while pilot experiments showed that human and mouse dazap2 mRNA had similar activities in our assays (data not shown).
The open reading frame of Xenopus dazap2 encodes a protein of 168 amino acids which includes several conserved SH2 and SH3 consensus target sequences, as well as a polyproline rich region (Supplementary Figure 1A–B and Genbank accession BG354661). While such domains suggest that Dazap2 may serve a scaffolding function to bring other proteins together, they do not address what pathways Dazap2 interacts with. Several reports have suggested varying activities for Dazap2, ranging from a role in the inner ear (Yang and Mansour, 1999), yeast two-hybrid interaction with Daz (Tsui et al., 2000) and Sox6 (Cohen-Barak et al., 2003), a role in cell adhesion (Sommerfeldt et al., 2002) to a critical role in stress granule formation and translational control (Kim et al., 2008). While these data are intriguing, the cellular function of Dazap2 requires further exploration. The identification of Dazap2 in our functional assay provided us with some insight into the in vivo roles of Dazap2.
Expression of dazap2 in Xenopus embryos
By RT-PCR, dazap2 expression is readily detected at mid-late blastula stage (Nieuwkoop and Faber stages 8–9) and is maintained throughout early development (Fig. 1A). By whole mount hybridization, dazap2 staining is seen throughout the animal hemisphere during blastula stages (not shown). Later, dazap2 expression appears to be moderately enriched in the neural plate, along the entire length of the anteroposterior axis (Fig. 1B–F, cleared embryos show enhanced neural staining at stage 19, Fig. 1E–F). Robust dazap2 expression is observed in the anterior part of the neural tube at late neurula and tailbud stages, encompassing the eyes, posterior brain, the anterior spinal cord and the branchial arches (Fig. 1G, G’). A fainter expression is observed in the pronephros and the dorsal fin (Fig. 1G). Cleared feeding tadpoles showed enhanced expression in the forebrain, the pronephros and the posterior digestive tract (Fig. 1H, I). These results suggest that Dazap2, a novel small molecule that displays protein interaction motifs conserved in vertebrates (Shi et al., 2007), and that has been isolated for its ability to alter anteroposterior patterning of the embryo (Grammer et al., 2000) is expressed in the right place and time to play a role in early neural patterning.
Figure 1. Temporal and spatial expression of dazap2 mRNA.
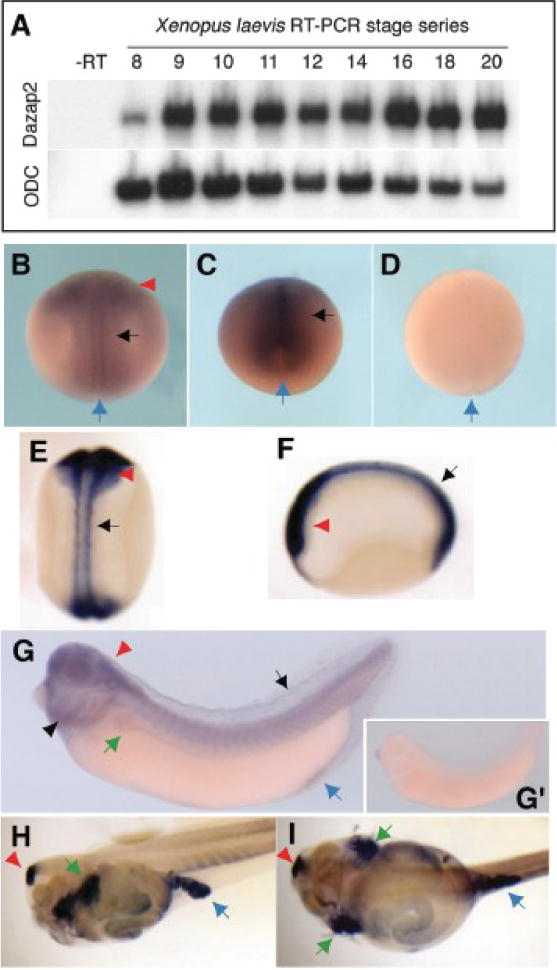
(A) RT-PCR using dazap2 primers shows that dazap2 is expressed during blastula to neurula stages. (B–I)Dazap2 spatial expression pattern was analyzed by whole-mount ISH, using either antisense (B–C, E–G and H–I) or sense (D and G’) full-length xl-dazap2 probe. Embryos are seen in dorsal view with anterior to top (B and D–E), posterior view with dorsal on top (C), lateral view with head to the left (F, G, G’ and H) or ventral view (I). The blue arrow indicates the blastopore. Embryos hybridized with the sense probe in the same ISH conditions show no staining at all stages tested (D and G’). (B) At midneurula stage 16 (B) and late neural stage 18 (C, posterior view), dazap2 is present in the head folds (red arrowhead) and throughout the neural plate (black arrow). The staining is enhanced when neurulae are cleared (stage 19, E and F). By stage 32 (G, G’), tailbud embryos express dazap2 in trunk and head region, including the brain (red arrowhead) and spinal cord, the eye, the branchial arches (arrowhead) and the pronephros (green arrow). A faint staining is found in the dorsal fin (arrow) and the proctodeum (blue arrow). Cleared stage 44 tadpoles (H, I) express dazap2 in the brain (red arrowhead), the pronephros (green arrows) and caecum (blue arrow).
Dazap2 is essential for patterning the spinal cord
To assess the in vivo requirements of Dazap2, we used targeted injections of antisense morpholino oligonucleotides (MO) to block translation of endogenous Dazap2. We used two different MOs, one targeting the translational start site (TR-MO), and the second blocking splicing of the first intron (SP-MO), resulting in degradation of the unspliced pre-mRNA (supplementary Fig. 2A). Analysis of dazap2 mRNA levels after injection of SP-MO showed a dose-dependent reduction in endogenous transcripts (up to 60%) (Supplementary Fig. 2B–C). Using these morpholino oligonucleotides allowed us to knockdown dazap2; effects of the MOs could be rescued by injection of intact dazap2 mRNA (Fig. 2I) indicating the specificity of the MOs. In subsequent experiments, an optimized dose of SP-MO (20ng per cell) was co-injected in one blastomere of 4-cell stage embryos along with lacZ mRNA, unless otherwise noted.
Figure 2. Dazap2 silencing results in a loss of hoxb9 with concomitant posterior shift of anterior neural markers.
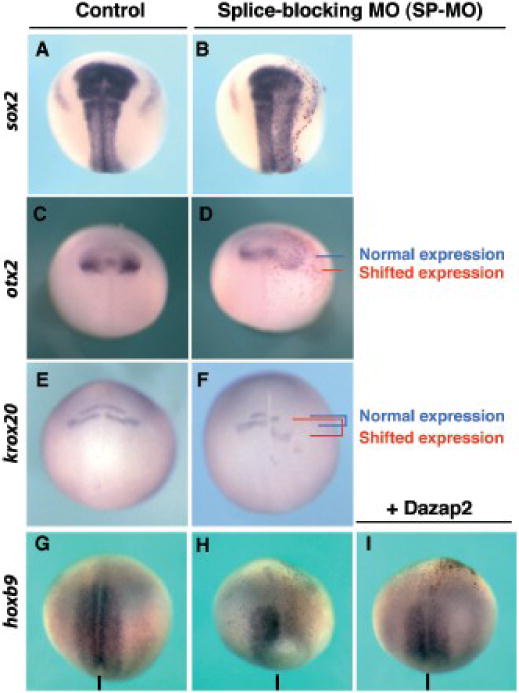
Embryos co-injected with Dazap2 SP-MO and lacZ mRNA were analyzed at stage 18 (shown in dorsal views, with anterior at the top). (A–B) Sox2 expression domain was only marginally affected. (C–D)Otx2 domain was expanded and shifted posteriorly within the neural plate (blue and red lines indicate normal and shifted posterior limits respectively). (E–F)Krox20 expression (rhombomeres 3 and 5) also shows a posterior shift in SP-MO injected embryos (blue and red lines indicate normal and posteriorized rhombomeres 3 and 5 respectively). Expression of hoxb9 is decreased/lost in injected cells (G–H) and can be rescued when dazap2 mRNA (250pg) is co-injected with SP-MO (I). The line indicates the sagittal midline.
Because we were interested in the patterning of the neural plate, particularly in the anterior-posterior axis, we examined the requirements for dazap2 using a selection of neural markers. We found that unilateral MO injections resulted in a slightly misshapen neural plate, based on sox2 expression, but the domain of expression was essentially normal, suggesting that loss of Dazap2 did not result in defective neural induction (75%, n=32, Fig. 2A–B). However, we found expression of the spinal cord marker hoxb9 strongly reduced (SP-MO 82%, n=71, Fig. 2G–H and TR-MO 75%, n=18). Concurrently, we found that in these injections, the anterior marker otx2 was expanded, with a posterior shift compared to the control side (83%, n=12, Fig. 2C–D). Similarly, rhombomeres 3 and 5, marked by krox20, also exhibited a posterior shift (51%, n=52, Fig. 2E–F). Of the embryos that did not exhibit a shift in krox20, many showed a complete loss in krox20 on the injected side (not shown, 25%, n=52). These phenotypes could be rescued by co-injection of properly spliced dazap2 mRNA with SP-MO (250pg of dazap2 mRNA and 20ng SP-MO, embryos with rescued hoxb9 expression: 73%, n=66, Fig. 2I, compared to 82% loss with SP-MO alone). We have next analyzed the induction of hoxb9 in whole embryos injected with SP-MO by RT-PCR. Global loss of dazap2 led to a reduction in hoxb9 expression, while sox2 expression was unaffected (Fig. 3E). Taken together, these data show that Dazap2 is required for the specification of posterior neural fates in the embryo as well as demarcating the anterior domains.
Figure 3. FGF8a posteriorizing activity requires Dazap2.
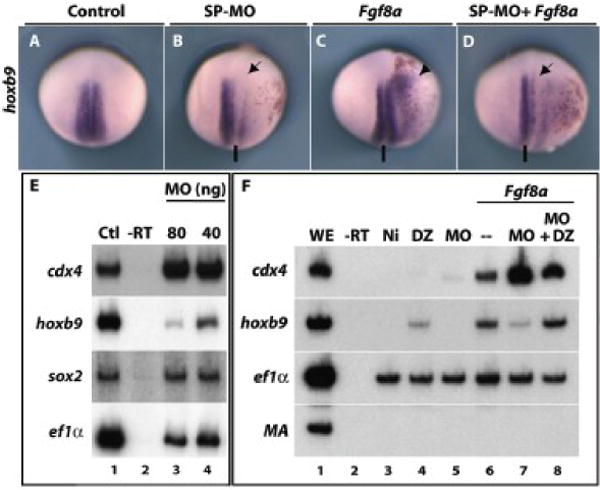
Embryos were co-injected with lacZ mRNA and either dazap2 SP-MO (20 ng, B), fgf8a (10pg, C) or a combination of SP-MO and fgf8a (20ng and 10pg, respectively, D) into one cell at the 4-cell stage. Stage 18 embryos are shown in dorsal views, with anterior to the top (the line indicates the sagittal plane). Compared to control embryos (A), the SP-MO blocks hoxb9 expression on the injected side (B, arrow) while Fgf8a induces ectopic hoxb9 expression (C, arrowhead). When fgf8a and SP-MO are combined, hoxb9 expression is blocked and no ectopic expression observed (D, arrow). (E) RT-PCR was performed on whole embryos injected with SP-MO (either 40ng/cell or 20ng/cell) into two cells at the 2-cell stage, then aged until stage 20 (Ctl, control sibling controls; -RT, no reverse transcriptase control). Hoxb9 expression is strongly decreased (lane 3, 4), especially at the highest concentration of SP-MO, while cdx4 and sox2 expression remain unaffected. (F) Animal caps were dissected at stage 9 from embryos injected with either dazap2 mRNA (250pg/embryo, lane 4), SP-MO (40ng/embryo, lane 5), fgf8a (200pg/embryo, lane 6), a combination of SP-MO and fgf8a (40ng/embryo + 200pg/embryo, lane 7) or a combination of fgf8a, SP-MO and dazap2 mRNA (40ng+200pg+250pg/embryo, lane 8) at the 2-cell stage (Ni: non injected caps). At stage 18, hoxb9 is induced by fgf8a (lane 6) but this induction is blocked in the presence of SP-MO (lane 7). This loss is rescued by addition of dazap2 mRNA (lane 8). In all combinations with Fgf8a, cdx4 is robustly induced.
Dazap2 is required for FGF posteriorizing activity
Loss of function experiments have shown that FGF signalling is required in the neural plate (Pownall et al., 1996; Isaacs et al., 1998; Fletcher et al., 2006 Ribisi). This suggested to us that loss of Dazap2 might perturb FGF pathway activity. We therefore used several approaches to explore the relationships between FGFs and Dazap2. First, we activated FGF in vivo while attenuating Dazap2 function using our MOs. As previously reported (Fletcher et al., 2006) injection of fgf8a mRNA (10pg) resulted in the anterior expansion of hoxb9 in vivo (81%, n=27. Fig. 3C) while dazap2 knockdown reduced the hoxb9 domain (82%, n=71, Fig. 3B). Supporting our hypothesis, injection of dazap2 SP-MO reversed the fgf8a overexpression phenotype, resulting in the reduction of hoxb9 expression in the injected area (61%, n=18, Fig. 3D). Thus, in vivo, the ability of FGF to increase hoxb9 expression requires full Dazap2 activity.
Second, in parallel experiments, RT-PCR analysis of FGF injected ectodermal explants confirmed that SP-MO strongly affected hoxb9 induction by FGF8a (Fig. 3F, compare lane 7 to lane 6). Again, these effects could be reversed by co-injection of dazap2 mRNA (Fig. 3F, lane 8). To our surprise, we found that this marked reduction in hoxb9 was consistently accompanied with increased levels of cdx4 expression (Fig. 3F, lanes 6, 7). However, this observation in explants was not reproduced in vivo by in situ hybridization (data not shown). Because adding back dazap2 mRNA efficiently restores hoxb9 despite the presence of fgf8a mRNA and SP-MO (lane 8), we are confident that loss of Dazap2 activity was indeed responsible for the lack of hoxb9 induction by Fgf8a. Hoxb9 has been shown to be a target of the FGF-cdx4 transcriptional cascade (Isaacs et al., 1998). Thus, these results suggest the intriguing possibility that Dazap2 activity, though essential for FGF signals to promote hoxb9 expression, is acting downstream of or in parallel to the known FGF-Cdx4 signalling cascade.
Cdx4 induction of hoxb9 is Dazap2 independent
To explore this interaction further, we hypothesized that Dazap2 might act in concert with or be required for Cdx4 induction of hoxb9. FGF induction of posterior fates has been shown to require the activation domain of Cdx (Isaacs et al., 1998); cdx genes are thought to act directly on hox genes (Shimizu et al., 2006). Therefore, we chose to deplete Dazap2 activity in the context of Cdx activity. As previously shown by Isaacs and colleagues (Isaacs et al., 1998), cdx4 can ectopically induce hoxb9 in the anterior neural plate (71%, n=7 Fig. 4C); as before, SP-MO reduces expression of endogenous hoxb9 (80%, n=60 Fig. 4B). The combination of Cdx4 gain of function and SP-MO still produces ectopic induction of hoxb9 (64%, n=33 Fig. 4D), indicating that Cdx4 activity does not rely on Dazap2. Similar results were obtained when Dazap2 SP-MO was co-injected with an activated cdx mRNA (cdx-VP16, cdx4 homeobox fused to VP16 activator domain, (Isaacs et al., 1998, data not shown). We further substantiated this finding in an explant assay, by co-injecting combinations of cdx4 and SP-MO (Fig. 4E). Similar to our in vivo observations, we found that Cdx4 robustly induced hoxb9 in noggin-neuralized explants (Fig. 4E, lane 7) and this did not require full levels of Dazap2 (Fig. 4E, lane 8), again suggesting that Cdx4 acts in parallel or downstream of Dazap2.
Figure 4. Cdx4 induction of hoxb9 does not require dazap2.
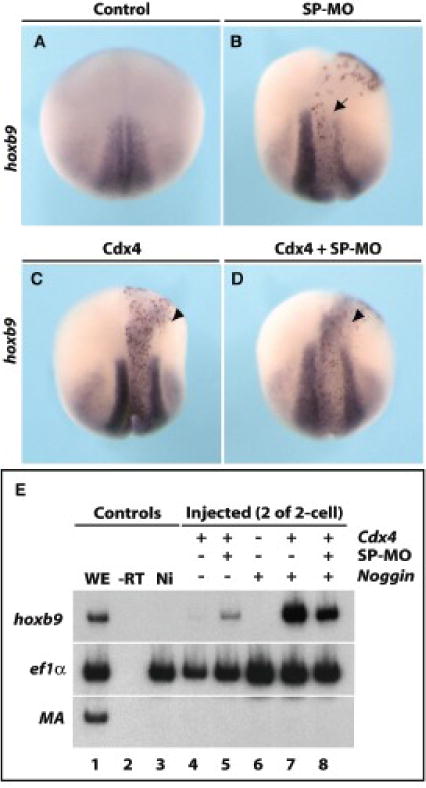
Embryos were injected with either SP-MO (20 ng, B), cdx4 (250pg, C), a combination of SP-MO and cdx4 (20ng and 250pg, respectively, D) into one cell of 4-cell stages (all were co-injected with lacZ mRNA). Stage 18 embryos are shown in dorsal views, with anterior at the top. As previously stated, SP-MO blocks the endogenous expression of hoxb9 (B, arrow), but has no effect on cdx4-induced ectopic expression of hoxb9 (C and D, arrowheads). (E) Ectodermal explants show that cdx4 induces hoxb9 in Noggin-neuralized caps (lane 7) and this is not affected by the silencing of dazap2 (lane 8). WE: whole embryo, -RT: no reverse transcriptase, Ni: not injected.
Dazap2 alters neural plate morphology at the expense of anterior tissues
Based on the requirements of Dazap2 seen above, as well as the initial expression screening phenotype (Grammer, Liu et al., 2000), we set out to determine the functional capacities of Dazap2. To do this, we injected dazap2 mRNA (250pg) into one blastomere of four-cell stage embryos; all injections were lineage traced by co-injection of lacZ mRNA (again, shown in red). When injected dorsally, within or adjacent to the neural plate, Dazap2 overexpression induced a marked lateral expansion of the pan-neural markers nrp1 (83%, n=6 Fig. 5A–A’), sox2 (75%, n=24 Fig. 5B–B’), and sox11 (78%, n=9 Fig. 5C–C’). This effect was limited to the dorsal side of the embryo, since injections targeted outside the neural plate, on the lateral or ventral side of the embryo, did not induce ectopic neural tissue (100%, n=18, Fig. 5A”–C”). This expansion was accompanied by delayed neural fold elevation. To rule out the possibility that Dazap2 was acting via the underlying paraxial mesoderm (Mariani et al., 2001), we also examined myoD expression in these embryos and found no change (stage 14, Fig. 5D–D”). Therefore, the increase observed in the size of the neural plate results from Dazap2 directly affecting neural development.
Figure 5. Dazap2 gain-of-function expands the neural plate and induces a posterior identity at the expense of anterior fates.
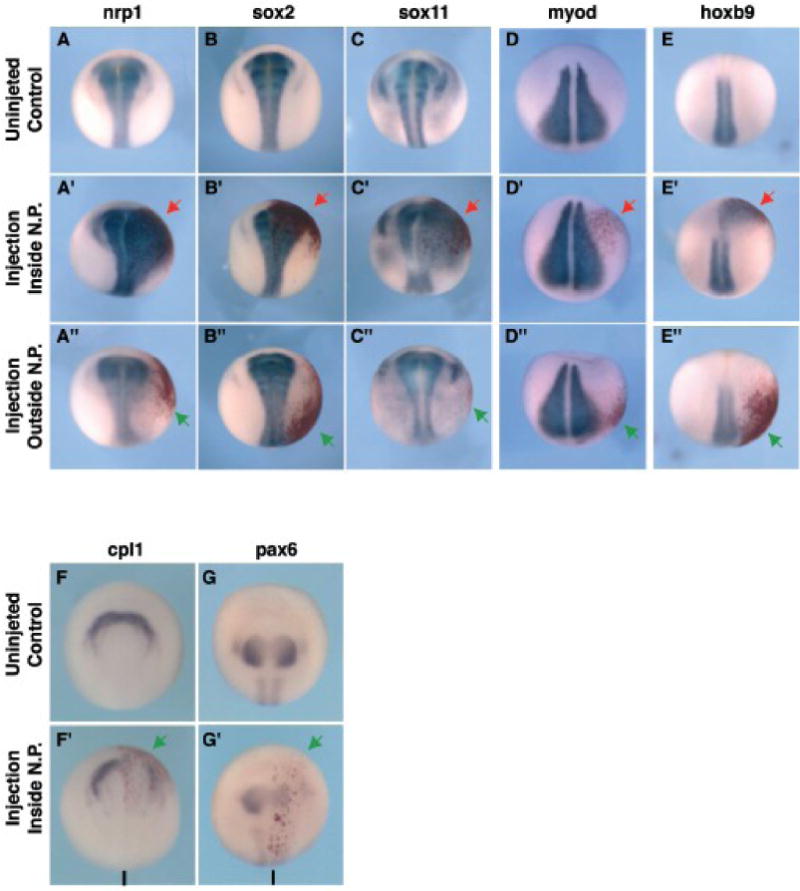
Injections of dazap2 mRNA were targeted either dorsally (A’-H’, red arrow) or ventrally (A”-E”, green arrow). Embryos were analyzed at stage 16–18 (shown in dorsal views with anterior at the top (A–E”), or frontal views (F–H’)). The injected side is on the right, injected cells are marked by the red nuclear stain). General neural markers nrp1 (A–A”), sox2 (B–B”) and sox11 (C–C”) are expanded when Dazap2 is expressed in or around the neural plate but not when dazap2 is injected on the ventral side of the embryo. MyoD (D–D”) expression is not effected by either dorsal or ventral injections. Hoxb9 (E–E”) is strongly ectopically expressed in dorsal but not ventral targeted injections. The expression of anterior neural markers cpl1 (F, F’) and pax6 (G, G’) is decreased when dazap2 is targeted to the anterior neural plate (arrows, bar indicates the midline).
We next examined the anteroposterior identity of the expanded neural region. When the injection was targeted dorsally, this region expressed the spinal cord marker hoxb9 (75%, n=32, Fig. 5E–E’), suggesting that the expanded neural tissue had acquired a posterior identity. No ectopic expression was observed when the mRNA was targeted to more ventral or lateral areas (Fig. 2E”). To bolster this observation, we also examined anterior markers. Increased dazap2 mRNA greatly reduced cpl1 (anterior neural fold/forebrain, 75%, n=8, Fig. 5G–G’), otx2 (forebrain, 65%, n=20, see Fig. 6G) and pax6 (forebrain, 100%, n=11, Fig. 5H–H’). Together, these data show that Dazap2 can expand the neural plate and promote posterior fates at the expense of the anterior neural tissue.
Figure 6. Dazap2 has potent posteriorizing activities in neuralized tissues.
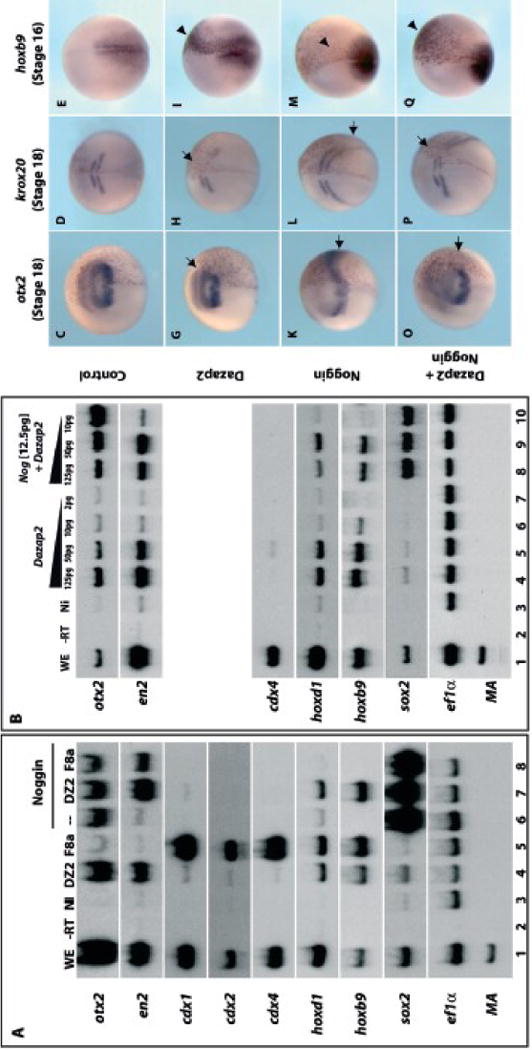
(A) Both cells of 2-cell stage embryos were injected with the following mRNAs: NI, non-injected; dazap2 (DZ2, 250pg/embryo); fgf8a (F8a, 100pg/embryo); noggin (12.5pg/embryo); or combination as indicated above lanes. RT-PCR analysis shows expression of a complete range of anterior and posterior markers in whole embryo (WE, line 1) but none in the NI caps (lane 3). Dazap2 and fgf8a both induce posterior genes hoxd1 and hoxb9, but Dazap2 also activates otx2 and en2 while fgf8 activates cdx1, 2 and 4 (lanes 4, 5). Upon neuralization by noggin (indicated by sox2 expression), animal caps express anterior markers only (otx2, lane 6). Dazap2 and noggin co-injections (lane 7) induce a complete range of anterior and posterior markers (but not cdx1, 2 or 4), while Fgf8 and Noggin co-injections only partially posteriorize the explants (en2 induction, lane 8). (B) A range of dazap2 mRNA (125pg/cell, 50pg/cell, 10pg/cell or 2pg/cell) alone or in combination with noggin (12.5pg/cell) was injected into both cells of 2-cell stage embryos. Explants were dissected at stage 9 from these embryos and aged until stage 18. Decreasing levels of dazap2, alone or in combination, shows a decrease level of induction of en2, hoxd1 or hoxb9. This is the opposite effect for otx2, which shows a decrease of induction alone but an increase induction in combination with noggin. Additionally, sox2 induction requires noggin. For in vivo analysis, embryos were injected at 4-cell stage with lacZ (100 pg/cell, C–E), dazap2 (250pg) and lacZ(G–I), noggin (25pg) and lacZ(K–M) or a combination of dazap2, noggin and lacZ(O–Q). Dazap2 injected embryos show a reduction of otx2 and krox20 expression (arrow) and ectopic expression of hoxb9 (arrowhead) in the anterior neural plate at stage 16–18 (G–I). Noggin injected embryos show an increase in both otx2 and krox20 (arrow), and decrease in hoxb9 expression (arrowhead) (K–M). The combination of dazap2 and noggin results in rescued otx2 and krox20 expression in injected cells (arrows) and hoxb9 expression is activated ectopically in the anterior neural plate (arrowhead) (O–Q).
Dazap2 has potent posteriorizing activity in neural tissues
These observations suggest that elevated dazap2 levels can confer posterior identity on the embryo. We decided to explore this further in a sensitized assay, where overexpressed BMP antagonists, such as Noggin or Chordin, potently induce anterior fates. In vivo, this results in tadpoles with enlarged head structures and truncated posterior axes (Smith et al., 1993; Sasai et al., 1995). In ectodermal explants, Noggin induces neural tissue of an anterior character (otx2) but not more posterior markers ((Lamb et al., 1993), Fig. 6A, lane 6). As expected, Noggin activated the pan-neural marker sox2 as well as the anterior marker otx2, but not more posterior markers (midbrain: en2, spinal cord: hoxd1 and hoxb9) (Fig 6A, lane 6). In contrast, Dazap2 induced the full array of anterior-posterior markers including otx2, en2, hoxd1 and hoxb9 (Fig. 6A, lane 4), while it only marginally activated sox2. The effect on hoxd1 and hoxb9 is comparable to FGF8a activity in animal caps (lane 5), but Dazap2 does not activate cdx1,2 or 4 genes like FGF8a. The combination of dazap2 and Noggin injection resulted in the co-expression of forebrain (otx2), midbrain (en2) and spinal cord (hoxd1 and hoxb9) markers in sox2-positive animal caps (Fig. 6A, lane 7). Again, none of the caudal related genes (cdx1, cdx2 and cdx4) were activated by Dazap/Noggin, suggesting that induction of the spinal cord markers by Dazap is independent of FGF-cdx activity.
In vivo, Dazap2 induces posterior tissues (hoxb9), at the expanse of anterior fates (cpl1, pax6). Surprisingly, in addition to the induction of posterior markers (hoxb1, hoxb9, en2) in the animal cap explants, we have consistently obtained induction of otx2, a forebrain marker. Moreover, this induction is dose-dependent and parallels hoxb9 induction (Fig 6B, lanes 4–6). However, in animal cap explants neuralized by noggin, we observed two groups of genes with distinct responses to increasing doses of dazap2. The posterior markers (en2, hoxb1, hoxb9) were induced robustly when Dazap2 activity increases (Fig. 6B, lanes 8–10). In marked contrast, otx2 induction is attenuated when Noggin is combined with increasing doses of Dazap2, indicating that Dazap2 interferes with the anteriorizing noggin action in the neural context (Fig6B, lanes 8–10).
Figure 8. Dazap2 activity is Cdx4 independent.
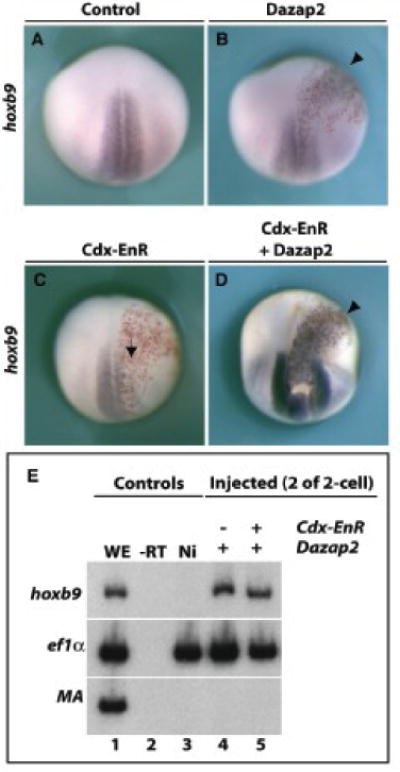
mRNAs for dazap2 (250pg, B), cdx-EnR (250pg, C) or a combination of dazap2 and cdx-EnR (250pg each, D) were injected into one cell of 4-cell stage embryos (all were co-injected with lacZ mRNA). Stage 18 embryos are shown in dorsal views, with anterior at the top. Dazap2 injections induce ectopic hoxb9 expression (B, arrowhead) while cdx4-EnR overexpression blocks hoxb9 induction (C, arrow). Combined Dazap2 and cdx-EnR injection results in robust hoxb9 induction (D, arrowhead). Parallel experiments in explants (E) show that Dazap2 induces hoxb9 either alone (lane 4) or in the presence of Cdx-EnR (lane 5).
We also found that Dazap2 could modulate the response to Noggin activity in whole embryos. Embryos were injected into the animal hemisphere by targeting a dorsal blastomere at 4-cell stage, with lacZ alone (100pg, Fig. 6C–E), dazap2 mRNA (250pg, Fig. 6G–I), noggin mRNA (25pg Fig. 6K–M) or a combination of dazap2 and noggin mRNA (Fig. 6O–Q). Dazap2 injection resulted in reduced otx2 and krox20 expression in neurula embryos (65%, n=34 and 100%, n=52 respectively, Fig. 6G–H). This suggests that, although Dazap2 is sufficient to induce both anterior and posterior neural markers in the explant assay, additional cues, such as neuralization, modulate this activity in the embryo or in neuralized explants and favor the posteriorizing effects. Hence, the induction of hoxb9 in the explant assay, both alone and in combination with Noggin (Fig. 6A, lane 4 and 6), can be related to the ectopic expression in the anterior neural plate (75%, n=32, Fig. 6 G). Excess Noggin results in enlarged heads and a reduced trunk ((Smith and Harland, 1992); 86% n=15, data not shown) as well as expanded otx2 and krox20 domains (3/4 and 5/6 respectively, Fig. 6K–L), along with reduced hoxb9 expression (4/4, Fig. 6M). When injection of noggin and dazap2 are combined, it appears that Dazap2 activity is antagonistic to and overcomes that of Noggin, with otx2 and krox20 expression levels comparable to dazap2 injected embryos while hoxb9 is ectopically induced (Fig. 6O–Q, n=3/4, n=5/5, and n=5/5, respectively). This is clearest in cells located in the lacZ-traced area as the effects on otx2 and krox20 outside of this area are due to the diffusible activity of Noggin. These data suggest that Noggin anteriorizing activity is superseded in the cells that overexpress Dazap2, and result in reversal of the Noggin phenotype.
Since we had not observed Dazap2 induction of neural markers outside the neural plate in the whole embryo (Fig. 5), we conclude that Dazap2 effects seen in ectodermal explants are modulated by additional cues in vivo. Thus, the effects relevant to neural patterning are well described by the neuralized animal explants and by in vivo injections. In both these situations, Dazap2 antagonized Noggin anteriorization of neural cells and favours posterior fates.
Dazap2 and FGF8a have different posteriorizing activities
Because of the requirement for Dazap2 in FGF posterior activity (Figure 3), we also compared FGF8a and Dazap2 activities in explant assays (Fig. 6A, lanes 4 and 5). fgf8a alone robustly induced posterior markers cdx1, cdx2 and cdx4 and spinal cord markers hoxd1 and hoxb9 but not the more anterior markers (Fig. 6A, lane 5). However, in combination with Noggin (Fig. 6A, lane 8), fgf8a failed to induce posterior markers. Instead, the midbrain marker en2 was induced, indicating the mild posteriorizing effect of FGF8a in the presence of Noggin. This differed greatly from our results on combined Dazap2/Noggin treatment (Fig. 6A, lane 7). Again, we found amongst the posterior markers, these differences appear to be at the level of cdx1, 2 and 4, which are robustly induced by fgf8a but not by dazap2 (compare Fig. 6A, lanes 4 and 5). This suggested to us that Dazap2 has a potent cdx-independent posteriorizing activity.
Loss of FGF signaling in the neural plate is rescued by Dazap2 activity
Because of the loss-of-function and gain-of-function interactions described above, we hypothesized that Dazap2 activity would be downstream of FGF and in parallel to or downstream of cdx4. If this were the case, dazap2 overexpression should bypass loss of FGF signalling in the neural plate. To test this, we used the chemical inhibitor SU5402 to block FGF signal transduction (Mizuseki et al., 1998; Delaune et al., 2005). Prior studies had shown that SU5402 treatment of Xenopus embryos during early stages disrupted mesodermal and neural development (Delaune et al., 2005). We used these data to ensure the potency of our SU5402, by treating sibling controls at stage 8 (data not shown). For our experiments, we bypassed these early effects by treating embryos at the end of gastrulation (stage 12), which did not affect neural induction (assayed by sox2 expression, Fig. 7A, B). We thus focus specifically on the role of FGF in posterior neural plate patterning. After verifying that dazap2 gain of function does not activate hoxb9 precociously (not shown) we challenged the ability of Dazap2 to promote posterior fates in the absence of FGF signalling. Embryos were injected with dazap2 mRNA then treated with either SU5402 or DMSO (vehicle) starting at stage 12. At stage 18 both SU5402-treated and control sibling embryos exhibited normal sox2 domains (94%, n=16, Fig. 7A–B) while dazap2 injected embryos showed enlarged neural plates both in the presence (Fig. 7A’) or absence of FGF signalling (Fig. 7B’). Although SU5402 treatment at these stages resulted in an almost complete loss of hoxb9 expression (59%, n=22, Fig. 7D), in this context, dazap2 still induced ectopic hoxb9, on the injected side, thus bypassing a requirement for FGF receptor signal during neural plate posterior patterning (100%, n=12, Fig. 7D’).
Figure 7. Loss of FGF receptor signaling in the neural plate is rescued by dazap2.
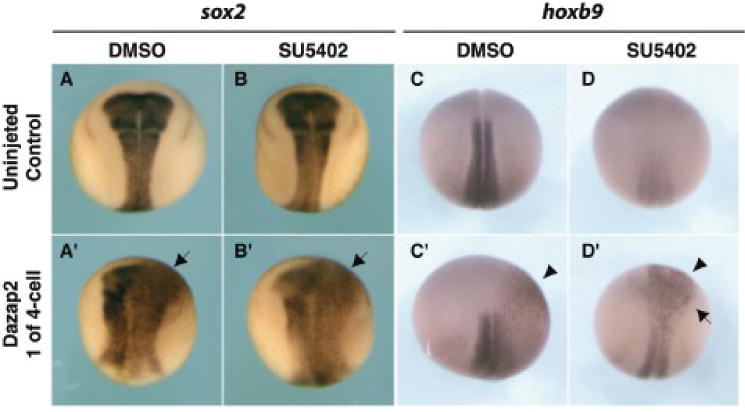
Uninjected control embryos (A–D) or embryos injected with dazap2 mRNA (250pg, one cell at 4-cell stage) (co-injected with lacZ mRNA, A’-D’) were treated with either DMSO (1%, carrier control) or SU5402 (80μM) at stage 12. Stage 18 embryos are shown in dorsal views, with anterior at the top. Uninjected embryos show no change in sox2 expression after SU5402 treatment (B), in contrast, hoxb9 expression was strongly reduced (D) in treated embryos, when compared to sibling controls (A and C, respectively). Dazap2 mRNA injected embryos show both an expansion (arrow) of sox2 expression (A’ and B’) and ectopic (arrowhead) expression hoxb9 (C’ and D’) when treated with either DMSO or SU5402.
Dazap2 activity is cdx4 independent
FGF induction of hoxb9 is thought to be a direct consequence of Cdx4 activation (Shimizu et al., 2006). Since we have shown that Dazap2 does not induce cdx1, 2 or 4 (Fig. 6), we tested the assumption that cdx4 would be dispensable for Dazap2 activity. To do this, we blocked hoxb9 activation using the antimorphic construct cdx-EnR (Isaacs et al., 1998) (57%, n=7 Fig. 8C), which fuses the cdx4 homeodomain with a repressor domain from engrailed and blocks cdx targets. Reinforcing our hypotheses, Dazap2 still efficiently increased hoxb9 expression, indicating that Dazap2 activity does not require a Cdx activating function (89%, n=9 Fig. 8B, D). RT-PCR experiments confirmed these results (Fig. 8E, lanes 4 and 5). This ruled out the possibility that Cdx4 might be downstream of Dazap2 (as suggested above, Fig 4). Since Cdx and Dazap2 are not reciprocally required for hoxb9 induction, we conclude that they are acting independently, and in parallel, downstream of FGF.
Together, our data suggest that FGF induction of posterior neural tissues can occur via two distinct pathways, one mediated by the caudal-like transcription factors and the other by Dazap2.
Dazap2 induces hoxb9 in a Wnt/β-catenin independent manner
FGF activity on induction of spinal cord marker depends on active Wnt signaling. In the presence of a dominant negative form of Wnt8, hoxb9 induction by bFGF is lost in animal cap explants. Moreover, analysis of the cdx4 gene regulatory sequences shows conserved LEF/TCF elements that are essential for the response to FGF. We thus tested whether Dazap2 activity on hoxb9 is also dependent on canonical Wnt signalling. We co-injected either fgf8a mRNA and β-catenin morpholino (20ng/cell) or dazap2 mRNA and β-catenin morpholino in whole embryos from which animal caps were dissected. The development of sibling embryos confirmed that the β-catenin MO was fully effective at this dose. While the presence of the β-catenin MO blocked FGF8a induction of cdx4 (not shown) and hoxb9 (Fig. 9A), Dazap2 still robustly induced hoxb9 in the absence of β-catenin-dependent signalling (Fig. 9B). These findings confirm that while FGF function depends on both Wnts and Dazap2, Dazap2 acts independently of both cdx genes and canonical Wnt activation.
Figure 9. Dazap2 activity does not depend on canonical WNT signaling.
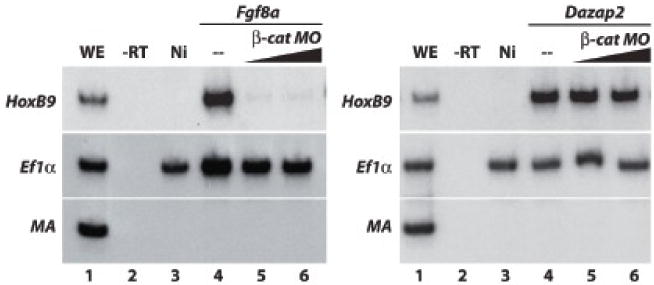
(A) RT-PCR was performed using whole embryos injected with fgf8a (100pg/cell) either alone or in combination with β-catenin MO (either 40ng/cell or 60ng/cell) into two cells at the 2-cell stage, animal caps were dissected and then aged until stage 19 (WE, whole embryo sibling controls; -RT, no reverse transcriptase control; Ni, non-injected explant control). Hoxb9 expression is lost at both concentration of β-cat MO (lane 5,6) when compared to Fgf8a alone (lane 4). (B) When the experiment is repeated using Dazap2 (125pg/cell), β-catenin MO is unable to block Dazap2 induction of HoxB9 expression (compare lane 4, with co-injection lane 5 and 6).
Dazap2 sensitizes cells to FGF signaling
Finally, we addressed how Dazap2, which is ubiquitously expressed in the neural plate during neural patterning (Fig. 1), could promote posterior fates (Fig. 5–6). We reasoned that Dazap2 provides some signal amplification, by promoting posterior fates in response to low FGF signal levels. We tested this hypothesis by injecting very low doses of FGF in animal cap explants, in combination with increasing levels of dazap2 mRNA (Fig. 10). We found that a minimum dose of 10pg/cell of fgf8a mRNA is needed to elicit hoxb9 expression in animal caps (lanes 4–6). A very low dazap2 mRNA dose (2pg/cell) does not change this response (lanes 7–9). However, when dazap2 mRNA is injected at a slightly higher dose (10pg/cell), which alone does not induce hoxb9, the cells potently respond to sub-threshold doses of fgf8a (2pg/cell and 0.1pg/cell respectively, lanes 10–12). As a positive control, injections of dazap2 mRNA (50pg/cell), which induces hoxb9 alone, show potent cooperation with fgf8a (10pg, lanes 13–15). We conclude that Dazap2 promotes sensitivity of the cells to FGF signals, and thus could participate in interpreting the posterior FGF gradient present in vivo.
Figure 10. Dazap2 activity potentiates induction of posterior fate by FGF8a.
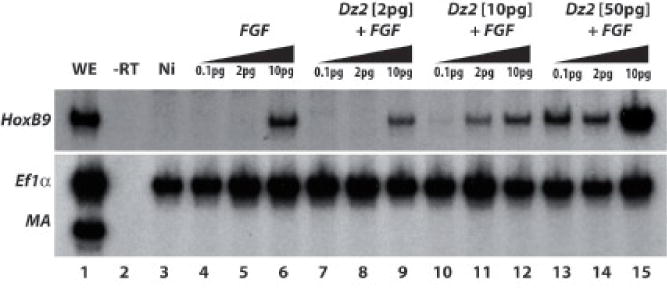
RT-PCR was performed using whole embryos injected with a range of fgf8a (0.1pg/cell, 2pg/cell and 10pg/cell) alone or in combination with dazap2 (either 2pg/cell, 10pg/cell or 60pg/cell) into two cells at the 2-cell stage, animal caps were dissected and then aged until stage 18 (WE, whole embryo control sibling; -RT, no reverse transcriptase control; Ni, non-injected explant control). Hoxb9 expression is observed only at the highest concentration of fgf8a alone (lane 6) or in combination with 2pg/cell dazap2 (lane 9). When fgf8a is combined with 10pg/cell or 50pg/cell of dazap2, lower levels are required to induce hoxb9 (lane 11 and lanes 13,14, respectively).
DISCUSSION
Dazap2 is a novel molecule in posterior patterning
Setting up regional territories in the embryo is critically important for proper development of an animal. In this report, we identify and characterize Dazap2, which plays an integral role in establishment of anterior-posterior boundaries within the neural plate. Many studies have identified signalling pathways that are important in regional specification of the neural plate. However, fewer studies have implicated direct positive regulators of spinal cord identity. This report identifies a new “transforming” factor that directly induces spinal cord markers; in the absence of Dazap2, posterior markers are lost while anterior markers are expanded (Fig. 2). Increasing the levels of Dazap2 in vivo results in a reciprocal increase in spinal cord at the expense of anterior markers (Fig. 5). Our data suggest that Dazap2 plays a role not only in specification of the spinal cord, but also in protecting caudal tissues from anteriorizing signals.
To address this potential protective activity, we assessed the ability of Dazap2 to re-pattern strongly anteriorized neurectoderm. We found that Dazap2 neutralizes the excess anterior domain observed in Noggin-injected embryos: Dazap2 can block the Noggin-induced expansion of otx2 and krox20 expression while promoting hoxb9 expression in vivo; this activity is cell-autonomous and also observed in neuralized explants (Fig. 6). Thus, we conclude that Dazap2 exerts a potent posteriorizing influence in vivo and this activity can protect against excess anteriorizing signals.
Dazap2 is required for FGF mediated posterior patterning
Many studies have enriched our knowledge of how anterior-posterior patterning of the neural plate is achieved: BMP, WNT and Nodal inhibitors secreted from the Spemann organizer establish the rostral neural fates during gastrulation while FGF, WNT and retinoids cooperate to pattern more caudal fates of the hindbrain and spinal cord (for review see (Niehrs, 2004)). In particular, FGF signalling is essential for spinal cord formation, and acts via caudal homologs to activate posterior hox genes (Pownall et al., 1996; Isaacs et al., 1998). In loss-of-function analyses, both in vitro and in vivo, we demonstrate that Dazap2 function is essential for FGF pathway induction of posterior hox genes in a caudal-independent mechanism (Fig. 3). We observed that while Dazap2 was required for activation of hoxb9, it was not required for induction of the FGF target cdx4 (Fig. 3F). This raised the possibility that loss of dazap2 does not block the FGF signalling itself but instead changes the Hox responsiveness (to hoxd1 and hoxb9) of the affected tissues, either downstream or in parallel to Cdx4.
To test the relationship between Cdx4 and Dazap2, we used Cdx4 gain-of-function experiments to induce hoxb9 expression. In contrast to ectopic hoxb9 induced by FGF, Cdx4 induced hoxb9 could not be blocked by Dazap2 SP-MO (Fig. 4). Conversely, blocking Cdx4 transcriptional activation results in hoxb9 loss; this loss is efficiently rescued by increasing levels of Dazap2 (Fig. 8). These results suggested that Dazap2 and Cdx4 exert parallel and independent posteriorizing activities. Thus, in the normal embryo, levels of Dazap2 and Cdx4 create a critical threshold for FGF responsiveness and activation of posterior hox genes. Increase of either Dazap2 or Cdx4 overrides the requirement for the other, while loss of either then results in a loss of hoxb9 expression. Taken together, our data provide evidence that Dazap2 is an essential component of FGF-induced neural patterning and acts in parallel to Cdx4. Moreover, dose-response experiments demonstrate that Dazap2 sensitizes the cells to low levels of FGF signalling (Fig. 10), suggesting that Dazap2 participates in fine-tuning the interpretation of the posteriorizing FGF gradient during neural patterning.
Dazap2 is sufficient to induce posterior markers in the absence of FGF receptor activation
Our analyses also suggested that Dazap2 could mimic FGF function in neural posteriorization. The data above implied that Dazap2 acts downstream of FGFs, in parallel to the caudal genes. However, we wanted to exclude the possibility of reciprocal requirements for FGF in Dazap2 activity. To do this, we blocked expression of hoxb9 using a chemical inhibitor of FGF receptors; we found that in this context, Dazap2 was sufficient to rescue expression of hoxb9 (Fig. 7). Thus, we conclude that Dazap2 is a required component of FGF posteriorizing activity, acting downstream of FGFR activation.
Finally, in order to investigate whether this novel parallel pathway can interact with other posteriorizing signals, we have addressed whether canonical Wnt signalling is required for Dazap2 activity. Such a requirement was previously shown to be essential for FGF activity on hoxb9 (Kudoh et al., 2002). Here we show that blocking the canonical Wnt pathway at the level of β–catenin does not affect the potent induction of hoxb9 by Dazap2 (Fig. 9). These observations show that the mechanisms mediated by FGF and Wnt in neural posteriorization are distinct from those of Dazap2 and suggest that as yet uncharacterized molecular mechanisms activated by Dazap2 confer posterior identity.
In summary, using gain and loss of function strategies, we showed that Dazap2 levels control the balance between anterior and posterior neural fates. Since dazap2 expression is not restricted to the posterior part of the embryo at the time of neural patterning, it is unlikely that Dazap2 works directly as a posteriorizing signal; instead, our data shows that Dazap2 activity is important to adapt cellular responses to positional cues. Elevated Dazap2 levels increase the domain responding to posterior signals, resulting in expansion of spinal cord fate, while loss of Dazap2 restricts the posterior domain, resulting in an expansion of anterior brain structures at the expense of the spinal cord and hindbrain domains. These findings shed light on a novel mechanism for modulating FGF responsiveness in the neural plate and raise the intriguing possibility that Dazap2 provides a permissive environment posterior Hox gene expression.
Supplementary Material
Supplemental Figure 1 – Dazap2 is conserved in the deuterostome clade.
(A)Dazap2 encodes a 168 amino acid protein that contains several conserved SH2 (YxxΦ, indicated by light grey boxes) and SH3 (PxxP, indicated by dark blue boxes) consensus target sequences, as well as a polyproline rich region (red box). (B) The alignment of fourteen Dazap2 proteins found throughout the deuterostome clade shows a high conservation (Metazome.net). The conserved domains are highlighted in red, with the annotation based on the Xenopus laevis protein sequence. (Hsa, Homo sapiens; Cfa, Canis familiaris; Rno, Rattus norvegicus; Mmu, Mus musculus; Xtr, Xenopus tropicalis; Xla, Xenopus laevis; Dre, Danio rerio; Gac, Gasterosteus aculeatus; Tru, Takifugu rubripes; Ola, Oryzias latipes; Csa, Ciona savignyi; Cin, Ciona intestinalis; Bfl, Branchiostoma floridae; Spu, Strongylocentrotus purpuratus.). (C) A phylogenic tree was generated to demonstrate the relative distances of proteins using a UPGMA best tree method (MacVector).
Supplemental Figure 2 – Dazap2 splice-blocking and translation blocking morpholinos: design and activity.
(A) Two antisense morpholino oligonucleotides were designed to reduce the level of endogenous Dazap2 protein. The scheme represents the Xenopus dazap2 gene, with light blue indicating UTRs and dark blue indicating coding sequence (arrow above the line is the translation start site). The translation blocking oligonucleotide (DZ2-MO) disrupts the binding of translation machinery to the spliced dazap2 mRNA. The splice blocking oligonucleotide (SP-MO) was used to block the second exon’s splice acceptor site, thus preventing proper splicing and ultimately leading to degradation of the pre-mRNA. (B) Semi-quantitative RT-PCR was used to monitor levels of endogenous dazap2 mRNA in vivo after SP-MO injections. A range of SP-MO was injected into both cells at the 2-cell stage, embryos were aged until stage 21 when RT-PCR was performed (total amount of SP-MO injected is indicated (ng) above each lane of the PCR radiograph, EF1a was used as a loading control). Quantification was acquired using the Typhoon 8600 system (Molecular Dynamics) and ratios spotted on the graph (C).
Acknowledgments
The authors are grateful to Dr Harvey Isaacs, for his generous gifts of constructs used in this study, and to Dr L. Kodjabachian for help with SU5402 protocol. We thank Dr. Timothy Grammer and Dr. Francesca Mariani as well as members of the Monsoro-Burq and Harland Labs for their support. We thank Marc Dionne for critical reading of the manuscript. This work was supported by grants from CNRS (ATIP Program), ARC (grant # 3821), Ligue contre le Cancer (Grant #1FI4117FRDZ), Fondation de France (Programme Tumeurs) to A. H. M-B.; grants from NIH (grant GM42341) to R. M. H.; grants from the BBSRC and Wellcome Trust to K. J. L. ; D. D. R. is supported by postdoctoral fellowships from Fondation de France and Institut Curie and Region Ile de France.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blumberg B, Bolado J, Jr, Moreno TA, Kintner C, Evans RM, Papalopulu N. An essential role for retinoid signaling in anteroposterior neural patterning. Development. 1997;124:373–9. doi: 10.1242/dev.124.2.373. [DOI] [PubMed] [Google Scholar]
- Bolce ME, Hemmati-Brivanlou A, Kushner PD, Harland RM. Ventral ectoderm of Xenopus forms neural tissue, including hindbrain, in response to activin. Development. 1992;115:681–8. doi: 10.1242/dev.115.3.681. [DOI] [PubMed] [Google Scholar]
- Cohen-Barak O, Yi Z, Hagiwara N, Monzen K, Komuro I, Brilliant MH. Sox6 regulation of cardiac myocyte development. Nucleic Acids Res. 2003;31:5941–8. doi: 10.1093/nar/gkg807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox WG, Hemmati-Brivanlou A. Caudalization of neural fate by tissue recombination and bFGF. Development. 1995;121:4349–58. doi: 10.1242/dev.121.12.4349. [DOI] [PubMed] [Google Scholar]
- Delaune E, Lemaire P, Kodjabachian L. Neural induction in Xenopus requires early FGF signalling in addition to BMP inhibition. Development. 2005;132:299–310. doi: 10.1242/dev.01582. [DOI] [PubMed] [Google Scholar]
- Fletcher RB, Baker JC, Harland RM. FGF8 spliceforms mediate early mesoderm and posterior neural tissue formation in Xenopus. Development. 2006;133:1703–14. doi: 10.1242/dev.02342. [DOI] [PubMed] [Google Scholar]
- Grammer TC, Liu KJ, Mariani FV, Harland RM. Use of large-scale expression cloning screens in the Xenopus laevis tadpole to identify gene function. Dev Biol. 2000;228:197–210. doi: 10.1006/dbio.2000.9945. [DOI] [PubMed] [Google Scholar]
- Hardcastle Z, Papalopulu N. Distinct effects of XBF-1 in regulating the cell cycle inhibitor p27(XIC1) and imparting a neural fate. Development. 2000;127:1303–14. doi: 10.1242/dev.127.6.1303. [DOI] [PubMed] [Google Scholar]
- Haremaki T, Tanaka Y, Hongo I, Yuge M, Okamoto H. Integration of multiple signal transducing pathways on Fgf response elements of the Xenopus caudal homologue Xcad3. Development. 2003;130:4907–17. doi: 10.1242/dev.00718. [DOI] [PubMed] [Google Scholar]
- Harland R, Gerhart J. Formation and function of Spemann’s organizer. Annu Rev Cell Dev Biol. 1997;13:611–67. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- Heasman J, Kofron M, Wylie C. Beta-catenin signaling activity dissected in the early Xenopus embryo: a novel antisense approach. Dev Biol. 2000;222:124–34. doi: 10.1006/dbio.2000.9720. [DOI] [PubMed] [Google Scholar]
- Hirsch N, Harris WA. Xenopus Pax-6 and retinal development. J Neurobiol. 1997;32:45–61. [PubMed] [Google Scholar]
- Holowacz T, Sokol S. FGF is required for posterior neural patterning but not for neural induction. Dev Biol. 1999;205:296–308. doi: 10.1006/dbio.1998.9108. [DOI] [PubMed] [Google Scholar]
- Hongo I, Kengaku M, Okamoto H. FGF signaling and the anterior neural induction in Xenopus. Dev Biol. 1999;216:561–81. doi: 10.1006/dbio.1999.9515. [DOI] [PubMed] [Google Scholar]
- Hopwood ND, Pluck A, Gurdon JB. A Xenopus mRNA related to Drosophila twist is expressed in response to induction in the mesoderm and the neural crest. Cell. 1989;59:893–903. doi: 10.1016/0092-8674(89)90612-0. [DOI] [PubMed] [Google Scholar]
- Isaacs HV, Pownall ME, Slack JM. Regulation of Hox gene expression and posterior development by the Xenopus caudal homologue Xcad3. Embo J. 1998;17:3413–27. doi: 10.1093/emboj/17.12.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kengaku M, Okamoto H. bFGF as a possible morphogen for the anteroposterior axis of the central nervous system in Xenopus. Development. 1995;121:3121–30. doi: 10.1242/dev.121.9.3121. [DOI] [PubMed] [Google Scholar]
- Kim JE, Ryu I, Kim WJ, Song OK, Ryu J, Kwon MY, Kim JH, Jang SK. Proline-rich transcript in brain protein induces stress granule formation. Mol Cell Biol. 2008;28:803–13. doi: 10.1128/MCB.01226-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht AK, Good PJ, Dawid IB, Harland RM. Dorsal-ventral patterning and differentiation of noggin-induced neural tissue in the absence of mesoderm. Development. 1995;121:1927–35. doi: 10.1242/dev.121.6.1927. [DOI] [PubMed] [Google Scholar]
- Koide T, Downes M, Chandraratna RA, Blumberg B, Umesono K. Active repression of RAR signaling is required for head formation. Genes Dev. 2001;15:2111–21. doi: 10.1101/gad.908801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudoh T, Wilson SW, Dawid IB. Distinct roles for Fgf, Wnt and retinoic acid in posteriorizing the neural ectoderm. Development. 2002;129:4335–46. doi: 10.1242/dev.129.18.4335. [DOI] [PubMed] [Google Scholar]
- Lamb TM, Harland RM. Fibroblast growth factor is a direct neural inducer, which combined with noggin generates anterior-posterior neural pattern. Development. 1995;121:3627–36. doi: 10.1242/dev.121.11.3627. [DOI] [PubMed] [Google Scholar]
- Lamb TM, Knecht AK, Smith WC, Stachel SE, Economides AN, Stahl N, Yancopolous GD, Harland RM. Neural induction by the secreted polypeptide noggin. Science. 1993;262:713–8. doi: 10.1126/science.8235591. [DOI] [PubMed] [Google Scholar]
- Mariani FV, Harland RM. XBF-2 is a transcriptional repressor that converts ectoderm into neural tissue. Development. 1998;125:5019–31. doi: 10.1242/dev.125.24.5019. [DOI] [PubMed] [Google Scholar]
- Mariani FV, Choi GB, Harland RM. The neural plate specifies somite size in the Xenopus laevis gastrula. Dev Cell. 2001;1:115–26. doi: 10.1016/s1534-5807(01)00018-1. [DOI] [PubMed] [Google Scholar]
- McGrew LL, Hoppler S, Moon RT. Wnt and FGF pathways cooperatively pattern anteroposterior neural ectoderm in Xenopus. Mech Dev. 1997;69:105–14. doi: 10.1016/s0925-4773(97)00160-3. [DOI] [PubMed] [Google Scholar]
- Mizuseki K, Kishi M, Matsui M, Nakanishi S, Sasai Y. Xenopus Zic-related-1 and Sox-2, two factors induced by chordin, have distinct activities in the initiation of neural induction. Development. 1998;125:579–87. doi: 10.1242/dev.125.4.579. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, McMahon G, Sun L, Tang C, Hirth P, Yeh BK, Hubbard SR, Schlessinger J. Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science. 1997;276:955–60. doi: 10.1126/science.276.5314.955. [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq AH. CSH Protocols. 2007. A Rapid Protocol for Whole-Mount In Situ Hybridization on Xenopus Embryos. [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq AH, Fletcher RB, Harland RM. Neural crest induction by paraxial mesoderm in Xenopus embryos requires FGF signals. Development. 2003;130:3111–24. doi: 10.1242/dev.00531. [DOI] [PubMed] [Google Scholar]
- Niehrs C. Regionally specific induction by the Spemann-Mangold organizer. Nat Rev Genet. 2004;5:425–34. doi: 10.1038/nrg1347. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD. Activation and organization of the central nervous system in amphibians: synthesis of a new working hypothesis. Journal of experimental zoology. 1952;120:83–108. [Google Scholar]
- Nieuwkoop PD, Nieuwkoop FJ. Normal Table of Xenopus laevis (Daudin) New York: Garland; 1994. [Google Scholar]
- Onai T, Sasai N, Matsui M, Sasai Y. Xenopus XsalF: anterior neuroectodermal specification by attenuating cellular responsiveness to Wnt signaling. Dev Cell. 2004;7:95–106. doi: 10.1016/j.devcel.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Pandur PD, Dirksen ML, Moore KB, Moody SA. Xenopus flotillin1, a novel gene highly expressed in the dorsal nervous system. Dev Dyn. 2004;231:881–7. doi: 10.1002/dvdy.20191. [DOI] [PubMed] [Google Scholar]
- Pownall ME, Isaacs HV, Slack JM. Two phases of Hox gene regulation during early Xenopus development. Curr Biol. 1998;8:673–6. doi: 10.1016/s0960-9822(98)70257-x. [DOI] [PubMed] [Google Scholar]
- Pownall ME, Tucker AS, Slack JM, Isaacs HV. eFGF, Xcad3 and Hox genes form a molecular pathway that establishes the anteroposterior axis in Xenopus. Development. 1996;122:3881–92. doi: 10.1242/dev.122.12.3881. [DOI] [PubMed] [Google Scholar]
- Ribisi S, Jr, Mariani FV, Aamar E, Lamb TM, Frank D, Harland RM. Ras-mediated FGF signaling is required for the formation of posterior but not anterior neural tissue in Xenopus laevis. Dev Biol. 2000;227:183–96. doi: 10.1006/dbio.2000.9889. [DOI] [PubMed] [Google Scholar]
- Rupp RA, Snider L, Weintraub H. Xenopus embryos regulate the nuclear localization of XMyoD. Genes Dev. 1994;8:1311–23. doi: 10.1101/gad.8.11.1311. [DOI] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, De Robertis EM. Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature. 1995;377:757. doi: 10.1038/377757a0. [DOI] [PubMed] [Google Scholar]
- Sharpe CR, Fritz A, De Robertis EM, Gurdon JB. A homeobox-containing marker of posterior neural differentiation shows the importance of predetermination in neural induction. Cell. 1987;50:749–58. doi: 10.1016/0092-8674(87)90333-3. [DOI] [PubMed] [Google Scholar]
- Shi YW, Shen R, Ren W, Tang LJ, Tan DR, Hu WX. Molecular features and expression of DAZAP2 in human multiple myeloma. Chin Med J (Engl) 2007;120:1659–65. [PubMed] [Google Scholar]
- Shimizu T, Bae YK, Hibi M. Cdx-Hox code controls competence for responding to Fgfs and retinoic acid in zebrafish neural tissue. Development. 2006;133:4709–19. doi: 10.1242/dev.02660. [DOI] [PubMed] [Google Scholar]
- Shiotsugu J, Katsuyama Y, Arima K, Baxter A, Koide T, Song J, Chandraratna RA, Blumberg B. Multiple points of interaction between retinoic acid and FGF signaling during embryonic axis formation. Development. 2004;131:2653–67. doi: 10.1242/dev.01129. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Early Development of Xenopus laevis: A Laboratory Manual. New York: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- Skromne I, Thorsen D, Hale M, Prince VE, Ho RK. Repression of the hindbrain developmental program by Cdx factors is required for the specification of the vertebrate spinal cord. Development. 2007;134:2147–58. doi: 10.1242/dev.002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–40. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Smith WC, Knecht AK, Wu M, Harland RM. Secreted noggin protein mimics the Spemann organizer in dorsalizing Xenopus mesoderm. Nature. 1993;361:547–9. doi: 10.1038/361547a0. [DOI] [PubMed] [Google Scholar]
- Sommerfeldt DW, Zhi J, Rubin CT, Hadjiargyrou M. Proline-rich transcript of the brain (prtb) is a serum-responsive gene in osteoblasts and upregulated during adhesion. J Cell Biochem. 2002;84:301–8. doi: 10.1002/jcb.10018. [DOI] [PubMed] [Google Scholar]
- Tsui S, Dai T, Roettger S, Schempp W, Salido EC, Yen PH. Identification of two novel proteins that interact with germ-cell-specific RNA-binding proteins DAZ and DAZL1. Genomics. 2000;65:266–73. doi: 10.1006/geno.2000.6169. [DOI] [PubMed] [Google Scholar]
- Wilson PA, Melton DA. Mesodermal patterning by an inducer gradient depends on secondary cell-cell communication. Curr Biol. 1994;4:676–86. doi: 10.1016/s0960-9822(00)00152-4. [DOI] [PubMed] [Google Scholar]
- Yang W, Mansour SL. Expression and genetic analysis of prtb, a gene that encodes a highly conserved proline-rich protein expressed in the brain. Dev Dyn. 1999;215:108–16. doi: 10.1002/(SICI)1097-0177(199906)215:2<108::AID-DVDY3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 – Dazap2 is conserved in the deuterostome clade.
(A)Dazap2 encodes a 168 amino acid protein that contains several conserved SH2 (YxxΦ, indicated by light grey boxes) and SH3 (PxxP, indicated by dark blue boxes) consensus target sequences, as well as a polyproline rich region (red box). (B) The alignment of fourteen Dazap2 proteins found throughout the deuterostome clade shows a high conservation (Metazome.net). The conserved domains are highlighted in red, with the annotation based on the Xenopus laevis protein sequence. (Hsa, Homo sapiens; Cfa, Canis familiaris; Rno, Rattus norvegicus; Mmu, Mus musculus; Xtr, Xenopus tropicalis; Xla, Xenopus laevis; Dre, Danio rerio; Gac, Gasterosteus aculeatus; Tru, Takifugu rubripes; Ola, Oryzias latipes; Csa, Ciona savignyi; Cin, Ciona intestinalis; Bfl, Branchiostoma floridae; Spu, Strongylocentrotus purpuratus.). (C) A phylogenic tree was generated to demonstrate the relative distances of proteins using a UPGMA best tree method (MacVector).
Supplemental Figure 2 – Dazap2 splice-blocking and translation blocking morpholinos: design and activity.
(A) Two antisense morpholino oligonucleotides were designed to reduce the level of endogenous Dazap2 protein. The scheme represents the Xenopus dazap2 gene, with light blue indicating UTRs and dark blue indicating coding sequence (arrow above the line is the translation start site). The translation blocking oligonucleotide (DZ2-MO) disrupts the binding of translation machinery to the spliced dazap2 mRNA. The splice blocking oligonucleotide (SP-MO) was used to block the second exon’s splice acceptor site, thus preventing proper splicing and ultimately leading to degradation of the pre-mRNA. (B) Semi-quantitative RT-PCR was used to monitor levels of endogenous dazap2 mRNA in vivo after SP-MO injections. A range of SP-MO was injected into both cells at the 2-cell stage, embryos were aged until stage 21 when RT-PCR was performed (total amount of SP-MO injected is indicated (ng) above each lane of the PCR radiograph, EF1a was used as a loading control). Quantification was acquired using the Typhoon 8600 system (Molecular Dynamics) and ratios spotted on the graph (C).


