Abstract
Secular trends towards earlier puberty, possibly caused by new environmental triggers, provide a basis for periodic evaluation of the influence and interaction of genetic and environmental effects on pubertal timing. In such studies, a practical marker that reflects timing of puberty in both genders needs to be used. We investigated genetic and environmental influences on pubertal timing by using change in the relative height between early and late adolescence (HD:SDS, height difference in standard deviations) as a new marker of pubertal timing. HD:SDS correlated well with age at peak height velocity in a population of men and women with longitudinal growth data. In 2,309 twin girls and 1,828 twin boys, HD:SDS was calculated between height SDs at age 11.5 and 17.5, and 14.0 and 17.5 years, respectively. Quantitative genetic models for twin data were fitted to estimate the genetic contribution to HD:SDS. We also investigated whether the same genetic factors influenced individual differences between HD:SDS and development of secondary sex characteristics prospectively collected by pubertal development scale (PDS). Genetic effects contributed to 86 and 82% of the variance in HD:SDS in girls and boys, respectively, when using the same model including additive genetic and specific environmental factors. In girls, 30% and in boys, 49% of the genetic factors affecting PDS and HD:SDS were the same. Future comparison of the results of periodic evaluations allows estimation of possible changes in the effects of environment on timing of puberty. In such studies, HD:SDS can be used as a practical marker of pubertal timing.
Timing of puberty has a 4–5-year variation in the general population (Tanner, 1962). This is influenced by genetic and environmental factors, evidenced by several family (Sedlmeyer et al., 2002) and twin studies (Beunen et al., 2000; Fischbein, 1977; Kaprio et al., 1995; Mustanski et al., 2004; Sharma, 1983; Sklad, 1977; Treloar and Martin, 1990). Previous twin studies have estimated that genetic variation accounts from 57 to 100% of all variation on pubertal timing. The choice of the marker used for recording timing of puberty and sample size affect the results significantly. Longitudinal investigations and recording the first signs of puberty [i.e., breast development in girls and testicular enlargement in boys (Grumbach and Styne, 1998; Tanner, 1962)] is possible in small studies (Sharma, 1983; Sklad, 1977), which are, however, less precise in their ability to estimate the role of genetic factors due to sample size and selection effects. In large studies, only less precise estimation of timing of puberty is possible for practical reasons. For example, commonly used age at menarche (Kaprio et al., 1995; Treloar and Martin, 1990) only moderately correlates with breast and pubic hair development (Biro et al., 2006). On the other hand, the appearance of pubic hair in girls is dependent primarily on adrenal androgens (Palmert et al., 2001; Sklar et al., 1980), and also breast growth may be stimulated independently of pituitary-gonadal activation. In addition, breast tissue might not be easily distinguished from fat in obese girls (de Ridder et al., 1992). Therefore, even the use of these markers of pubertal timing may be somewhat unreliable in estimating onset of central puberty. In boys, testicular enlargement can be reliably recorded by palpation only. Practical markers of pubertal timing in boys are few, and probably for that reason, fewer large sample studies investigating both genders exist. In one study by Mustanski et al. (2004), pubertal timing in both genders was assessed by pubertal development scale (PDS) questionnaires (Petersen et al., 1983), in which the stages of pubertal maturation were self-estimated by appearance of secondary sex characteristics. PDS scores showed high heritability, but self-estimation may cause some inaccuracy (Hergenroeder et al., 1999). In addition, the PDS requires prospective data collection impractical in periodic evaluations.
Periodic estimates of genetic and environmental correlates on pubertal timing may be needed because of secular trends towards earlier ages of puberty. A secular trend has been reported in several countries during the last 50 years (Anderson et al., 2003; Sanchez-Andres, 1997; Wyshak and Frisch, 1982), but, even more strikingly, there is evidence that in the United States, African-American girls in particular begin puberty at a much younger age than previously reported (Anderson and Must, 2005; Herman-Giddens et al., 1997; Wu et al., 2002). Existing data are, however, insufficient to establish such a trend in boys (de Muinck Keizer-Schrama and Mul, 2001; Herman-Giddens et al., 2001). Earlier breast development in African-American girls suggests that new environmental triggers, which can influence timing of pubertal maturation may exist (Blanck et al., 2000; Freni-Titulaer et al., 1986; Krstevska-Konstantinova et al., 2001; Teilmann et al., 2002). Because of possible environmental triggers, which may have sex-specific effects on the human endocrine axis, periodic population-based investigations on the interaction and effects of genes and environment on pubertal timing, and estimation of changes in these effects in both genders are required.
In this study, we examined the relative contribution of genetic and environmental influences on pubertal timing in a large sample of twin boys and girls born in the 1980s by using timing of pubertal growth, which reflects the onset of central puberty, as a marker of pubertal timing. The timing of growth spurt was estimated retrospectively by change in the relative height (standard deviations, SDs, in height) between early and late adolescence (HD:SDS, height difference in standard deviations), which correlates with age at pubertal peak height velocity (phv). In addition to data from a large population of both genders, we offer a new practical marker of pubertal timing, difference in height SDs between only two height measurements, which may be later used in similar studies allowing estimation of possible changes in the effects of genes or environment on timing of puberty. We also investigated the degree to which the same genetic or environmental factors influence both HD:SDS and development of secondary sex characteristics prospectively collected by PDS (Petersen et al., 1983).
SUBJECTS AND METHODS
Participants
The data were derived from the FinnTwin12–17 study, which includes five subsequent birth cohorts of Finnish twins born in 1983–1987 (Kaprio et al., 2002).We obtained information on 2,309 girls and 1,828 boys, including 457 monozygotic (MZ), 465 same-sex dizygotic (SSDZ), and 399 opposite sex dizygotic (OSDZ) complete twin pairs. Zygosity was determined by a deterministic algorithm using questions on physical similarity during school age, which has shown high validity in another Finnish twin cohort; agreement between the blood test and questionnaire was 100% in a sub sample of 104 twin pairs, and the probability of misclassification was estimated at 1.7% (Sarna et al., 1978). Persons with unknown zygosity (3% of the respondents in the data collection at age 17) were removed from all analyses.
Methods
The ethical committee of the Helsinki and Uusimaa Hospital-District and the IRB of Indiana University, Bloomington, Indiana both approved the study protocol and data collection. The parents or guardians of the twins provided their written informed consent.
Questionnaires were used to collect data on height and pubertal development (PDS) at mean ages of 11.4 years (SD 0.29) and 17.6 years (SD 0.27) in girls and 14.1 years (SD 0.08) and 17.6 years (SD 0.24) in boys. The reason for using responses at these ages is explained below. The heights in centimeters reported by the twins were changed into standard deviations by using growth data from British children as a reference (Child Growth Foundation, 1996) and standard deviations were adjusted by exact age at the time of response by calculating regression residuals to account for small age differences at the time of response (Cole and Green, 1992; Cole et al., 1998).
Assessment of pubertal timing
HD:SDS
Relative height (height SD) changes during puberty depending on the timing of pubertal growth spurt; it increases if maturation is early and decreases if pubertal development occurs late compared to those with average pubertal timing. At age when height growth peaks in the general population, height SD is influenced by timing of pubertal maturation, as well as genetic height potential (i.e., target height) (Karlberg et al., 2003; Tanner et al., 1976). By calculating the difference between height SD at phv age and at adulthood, the influence of genetic height potential can be excluded. Therefore, the change in height SDs between these ages reflects timing of puberty. In the present study, height SD at age 11.5 years in girls and 14.0 in boys was subtracted from height SD at age 17.5 creating the difference, HD:SDS. The age of 12 in girls and 14 in boys represent ages of phv in the general population (Tanner, 1976). Height at age 17.5 in twins was considered near-final height, since by that age 99.8% of girls and 99.1% of boys have reached their adult height (Greulich and Pyle, 1959). Therefore, HD:SDS calculated between 11.5 and 17.5 years in girls, and 14.0 and 17.5 years in boys, reflects timing of puberty. HD:SDS is negative for early maturers and positive for late maturers. HD:SDS as a marker is practical compared with assessment of pubertal timing by longitudinal investigations, may be used retrospectively, and reflects true activation of the hypothalamus-pituitary-gonadal axis in both genders.
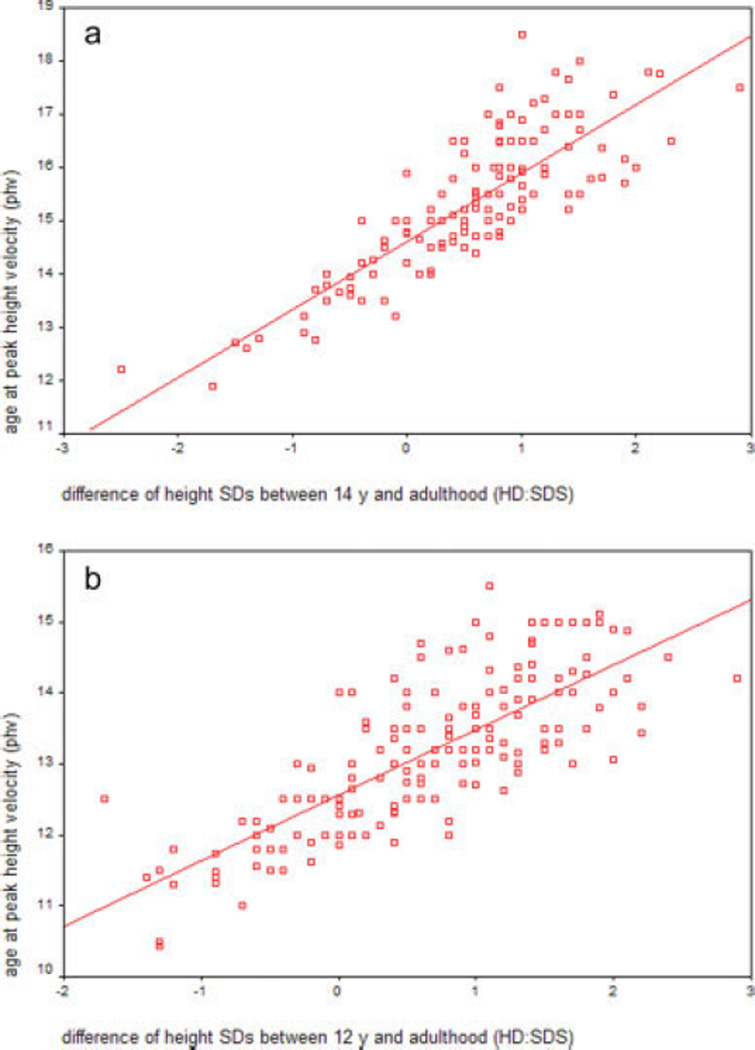
Because HD:SDS has not been previously used as a marker of pubertal timing, to validate it, we investigated its correlation with age at phv and age at acceleration of pubertal growth in 215 healthy men and 211 women from whom longitudinal growth data were available. In this cohort, age at phv was estimated from the point where height velocity was the fastest during puberty, and age at acceleration of growth from the point at which height velocity increased after the slowest growth velocity, resulting in an increase of more than 0.3 in height SDs (Karlberg et al., 2003). HD:SDS was calculated as the difference between height SDs at 14 years and adulthood in men, and at 12 years and adulthood in women. A significant correlation of HD:SDS with age at phv was observed both for men (r = 0.84, P < 0.001; Fig. 1a) and for women (r = 0.78, P < 0.001; Fig. 1b). The correlation of HD:SDS with age at acceleration of growth was 0.75 (P < 0.001) in men and 0.71 (P < 0.001) in women.
Fig. 1.
(a) Correlation of the chance in height SDs between 14 years and adulthood (HD:SDS) with age at peak height velocity (phv) in 215 healthy men (r = 0.84, P < 0.001). (b) Correlation of the change in height SDs between 12 years and adulthood (HD:SDS) with age at phv in 211 healthy women (r = 0.78, P < 0.001). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
PDS
Histories of pubertal timing were obtained using the PDS, explained in detail elsewhere (Dick et al., 2001; Petersen et al., 1983). The five-item PDS questionnaire assessed secondary body changes including body hair, skin changes and growth spurt for both sexes. For girls, additional items measured breast growth and menarche, whereas for boys, two additional items assessed facial hair and voice change. Development of each characteristic was rated on a four-point scale ranging from one (no development) to four (complete development) with the exception of menarche, which was dichotomous (1 = has not occurred or 4 = has occurred). At age 11.5, the fourth response option (development completed) for all items but menarche was omitted because few, if any, preteens were expected to have completed these indices of pubertal development by that age. The mean value of the ratings at age 11.5 in girls and 14.0 in boys was used as PDS score, describing the stage of pubertal maturation at that age (roughly representing the same stage of maturation between sexes). PDS score is large for early maturers and small for late maturers.
Twin genetic models
The classic twin study design is based on comparing the similarity of MZ and DZ twins (Boomsma et al., 2002). MZ twin pairs are genetically identical, whereas DZ twins share, on average, 50% of their segregating genes. The genetic component can be divided into additive genetic effects (A) and effects due to dominance (D), i.e., interaction between alleles in the same locus. Expected correlation within MZ pairs is 1 for both, and within DZ pairs, 0.5 and 0.25, for additive and dominance genetic effects, respectively. Environmental variation can further be divided into shared environment (C) and unique environment (E), including any measurement error; correlations for shared environmental effect are assumed to be 1 and for unique environmental effect 0 both within MZ and DZ twins. On the basis of these assumptions, it is possible to estimate values for each of these variance components treating them as latent standardized variables in linear structural equation model. However, since we have only twins reared together, we can not simultaneously estimate common environmental and dominance genetic effects.
Data analysis
Genetic modeling was started by selecting the best model using univariate models. The assumptions of the twin model, i.e., equal means and variances for MZ and DZ twins, as well as for the first and second born cotwins, were tested by comparing chi-square change between the twin model (ADE, AE, or ACE model) and the saturated model, which did not make any of these assumptions. After that we conducted bivariate modeling using Cholesky decomposition to examine whether the trait correlation between HD:SDS and PDS score was partly or completely due to the same or linked genetic factors (genetic correlation) or to the same or correlated environmental factors (environmental correlation) (Neale and Cardon, 1992). The significance of these correlations was studied by fitting nested models and examining the chi-square distributed change in the −22 log-likelihood values (Δχ2) between the models with and without the correlation parameter set to zero. All quantitative genetic models were carried out using the Mx statistical package (version 1.4.06) designed for studies of twin and family data (Neale, 2003).
RESULTS
Number of twins and means of the traits appear in Table 1. DZ boys were taller than MZ boys at ages 14.0 (0.20 SD vs. 0.10 SD, P = 0.036) and 17.5 (0.19 SD vs. 0.07 SD, P = 0.021), while no differences were seen for girls. Birth data (height, weight, and gestational age, data not shown) or PDS score and HD:SDS did not differ by zygosity within genders.
TABLE 1.
Number of twin individuals (N), means and standard deviations (SD) of the relative height (standard deviations, SDs in height) at age 11.5 years in girls or 14.0 years in boys (Height 12/14), and at age 17.5 years for both girls and boys (Height 17), pubertal development scale score at age 11.5 in girls or 14.0 in boys (PDS), and difference in height SDs between ages 11.5 and 17.5 in girls, and 14.0 and 17.5 in boys (HD:SDS, height difference in SDs, reflecting timing of pubertal growth) by sex and zygosity. P-values indicate the significance of the difference in means between monozygous (MZ) and dizygous (DZ) twins
| Height 2/14 | Height 17 | PDS | HD:SDS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | N | |
| Girls | ||||||||||||
| MZ | 0.08 | 1.01 | 778 | 0.25 | 1.00 | 706 | 1.66 | 0.43 | 798 | 0.18 | 0.78 | 673 |
| DZ | 0.15 | 1.04 | 1515 | 0.35 | 0.99 | 1292 | 1.67 | 0.43 | 1478 | 0.18 | 0.80 | 1244 |
| P | 0.21 | 0.11 | 0.80 | 0.94 | ||||||||
| Boys | ||||||||||||
| MZ | 0.10 | 1.11 | 666 | 0.07 | 0.91 | 779 | 2.01 | 0.48 | 668 | −0.01 | 0.79 | 570 |
| DZ | 0.20 | 1.00 | 1450 | 0.19 | 0.95 | 1679 | 2.03 | 0.48 | 1396 | −0.03 | 0.79 | 1247 |
| P | 0.036 | 0.021 | 0.62 | 0.85 | ||||||||
Age-adjusted intra-class correlation coefficients of height SDs at different ages were higher within MZ pairs (r = 0.89–0.92 in girls and 0.92 in boys) than within DZ pairs (0.51 and 0.49–0.52, respectively) (Table 2) indicating the effect of genetic factors. Similarly, correlations of HD:SDS were higher within MZ (0.82 in girls and 0.86 in boys) than DZ pairs (0.51 and 0.27, respectively). Within SSDZ twin pairs, the correlations of HD:SDS were higher in girls (0.51) than in boys (0.27). Correlations of PDS scores are also shown in Table 2. Common and item specific correlations of PDS have been reported in more detail elsewhere (Mustanski et al., 2004).
TABLE 2.
Intra-class trait correlations (r) of relative height (standard deviations, SDs, in height) at age 11.5 years in girls or 14.0 years in boys (Height 12/14), and at age 17.5 years for both girls and boys (Height 17), and difference in height SDs between ages 11.5 and 17.5 in girls, and 14.0 and 17.5 in boys (HD:SDS, height difference in SDs, reflecting timing of pubertal growth), pubertal development scale score at age 11.5 in girls and 14.0 in boys (PDS), and number of complete twin pairs (N) by sex and zygosity
| Girls | Boys | Girls | Boys | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MZ | MZ | SSDZ | SSDZ | OSDZ | ||||||
| r | N | r | N | r | N | r | N | r | N | |
| Height 12/14 | 0.92 | 381 | 0.92 | 319 | 0.51 | 357 | 0.52 | 362 | 0.46 | 668 |
| Height 17 | 0.89 | 344 | 0.92 | 284 | 0.51 | 313 | 0.49 | 321 | 0.46 | 604 |
| HD:SDS | 0.86 | 321 | 0.82 | 267 | 0.51 | 291 | 0.27 | 300 | 0.31 | 563 |
| PDS | 0.82 | 350 | 0.72 | 265 | 0.51 | 317 | 0.20 | 292 | 0.26 | 530 |
In univariate genetic models, the additive genetic/common environment/specific environment (ACE) model best fit in girls (Δχ26 = 6, P = 0.44) and the additive genetic/dominance genetic/specific environment (ADE) model in boys (Δχ26 = 4, P = 0.68) when compared with saturated models (Table 3). Statistically nonsignificant difference in the χ2-values between ACE/ADE models and saturated models suggest that the assumptions of twin modeling were not violated. Using the ACE model in girls, 71% (95% CI 56–87%) of the variance of HD:SDS was attributed to additive genetic effects, 14% (95% CI 12–17%) to specific environmental effects and 15% (95% CI 0–30%) to common environmental effects (Table 4). Using the ADE model in boys, 24% (95% CI 0–62%) of the variance in HD:SDS was attributed to additive genetic effects, 58% (95% CI 20–85%) to dominance genetic effect, and the remaining 18% (95% CI 15–22%) to specific environmental effects. As the use of same model allows better comparison between sexes, we repeated the analyses using also the AE model in both genders. This was permissible because the AE model yielded an adequate fit to the data in both girls and boys (P-values for the χ2 differences compared with the saturated models 0.28 and 0.06, respectively). In this case, 86% (95% CI 83–88%) of the variance in HD:SDS was attributed to additive genetic effects in girls and 82% (95% CI 78–85%) in boys. Specific environmental contributions were the same as in the ACE/ADE models. In table 4, variance component estimates for PDS scores reported previously are also shown (in more detail; Mustanski et al., 2004).
TABLE 3.
Fit of the saturated model and ACE, ADE, and AE univariate models (relative to saturated model, the most parsimonious model are indicated in bold)
| Saturated model | ACE modela | ADE-modela | AE-modela | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| −2LL | d.f. | Δχ2 | Δd.f. | P-value | Δχ2 | Δd.f. | P-value | Δχ2 | Δd.f. | P-value | |
| Girls | |||||||||||
| HD:SDS | 2518 | 1279 | 6 | 6 | 0.44 | 9 | 6 | 0.17 | 9 | 7 | 0.28 |
| PDS | 8262 | 1377 | 4 | 6 | 0.64 | 12 | 6 | 0.06 | 12 | 7 | 0.11 |
| Boys | |||||||||||
| HD:SDS | 2584 | 1213 | 13 | 6 | 0.04 | 4 | 6 | 0.68 | 13 | 7 | 0.06 |
| PDS | 8257 | 1138 | 13 | 6 | 0.04 | 3 | 6 | 0.81 | 13 | 7 | 0.06 |
Fit of the model compared to the saturated model.
TABLE 4.
Best fitting model (ACE model for girls and ADE model for boys) variance component estimates with 95% confidence intervals for difference in relative height (standard deviations, SDs in height) between 11.5 years and 17.5 years in girls and 14.0 years and 17.5 years in boys (HD:SDS, height difference in SDs, reflecting timing of pubertal growth) and pubertal development scale score at age 11.5 in girls and 14.0 in boys (PDS)
| Estimates of variance components | ||||
|---|---|---|---|---|
| a2 | d2 | c2 | e2 | |
| Girls | ||||
| HD:SDS | 0.71 (0.56–0.87) | – | 0.15 (0.00–0.30) | 0.14 (0.12–0.17) |
| PDS | 0.59 (0.45–0.75) | – | 0.22 (0.07–0.36) | 0.19 (0.16–0.22) |
| Boys | ||||
| HD:SDS | 0.24 (0.00–0.62) | 0.58 (0.20–0.85) | – | 0.18 (0.15–0.22) |
| PDS | 0.09 (0.00–0.47) | 0.62 (0.24–0.76) | – | 0.28 (0.24–0.34) |
a2=additive genetic variance, c2=common environmental variance, and e2=specific environmental variance.
In bivariate modeling, we first used the ACE model for girls and the ADE model for boys. In girls, the correlation between additive genetic factors affecting PDS score and HD:SDS was −0.55 (95% CI −0.68, −0.44), which suggests that 30% of the portions of genetic correlations accounting to PDS and HD:SDS, i.e., the square of the additive genetic correlation −0.55, were the same or closely linked. In boys, the dominance genetic correlation was very large [−0.96 (95% CI −1.00, −0.62)] under the ADE model, whereas additive genetic correlation was low (0.09, NS). Because we found this result not to be biologically plausible, we also conducted the AE model in boys; in this model, additive genetic correlation was −0.70 (95% CI −0.76, −0.65), suggesting that 49% of the portions of genetic correlations accounting to PDS and HD:SDS were the same or closely linked. The correlation between specific environmental factors affecting the two traits was −0.27 (95% CI −0.37, −0.16) in girls and −0.32 (95% CI −0.43, −0.21) in boys. This indicates that 7% of the specific environmental factors in girls and 10% in boys, affecting PDS score and HD:SDS were identical or correlated. In girls, the correlation between common environmental factors affecting PDS score and HD:SDS was −0.92 (95% CI −1.00, −0.32), which means that 83% of the common environmental factors affecting PDS score and HD:SDS were identical or correlated.
DISCUSSION
In this large sample of 922 twin pairs, pubertal timing was assessed by timing of pubertal growth spurt, estimated as change in the relative height between age when height growth peaks in the general population and adulthood (HD:SDS). Using this marker, 86 and 82% of the variance of pubertal timing was explained by genetic factors in girls and boys, respectively, when using the same additive genetic/specific environment model. A significant part of the same genetic effects, 30% in girls and 49% in boys, influenced both HD:SDS and PDS score.
Results of earlier studies estimating the relative influence of genetic and environmental factors affecting pubertal timing vary greatly. A few studies assessing timing of puberty by the appearance of secondary sex characteristics showed 88–100% concordance of sexual maturity in MZ twins compared with 30–39% concordance in DZ twins (Sharma, 1983; Sklad, 1977). Because of small numbers of twins in these studies, however, overestimation of concordance due to selection effects or sampling variation is possible. In contrast, large sample studies using the age of menarche as a marker of pubertal timing have shown lower genetic influence, from 61 to 75% (Kaprio et al., 1995; Treloar and Martin, 1990). These studies provide information only from females, and age at menarche has been recently shown to only moderately correlate with onset of breast or pubic hair development (Biro et al., 2006), which can be regarded as more reliable markers for timing of puberty than age at menarche. Large sample studies investigating both sexes are few. In our population-based twin cohort, Mustanski et al. (2004) investigated genetic and environmental influences on ages at achieving certain pubertal milestones, self-reported by prospectively collected PDS. MZ correlations showed large variation, from 0.57 to 0.82 depending on the PDS item in question. Self-estimation (Hergenroeder et al., 1999), development of pubic hair or breast tissue independently of the pituitary-gonadal maturation (Palmert et al., 2001; Sklar et al., 1980), or obesity interfering with breast growth assessment (de Ridder et al., 1992) may produce inaccuracy. PDS also, like most other pubertal markers, requires prospective data collection.
Timing of pubertal growth spurt, reflecting the onset of central puberty (Largo and Prader, 1983a,b; Marshall and Tanner, 1969, 1970), can be used as a marker of pubertal timing in both genders. A few longitudinal twin studies have estimated the magnitude of genetic effect on timing of pubertal growth (Beunen et al., 2000; Fischbein, 1977; Sklad, 1977). Timing of growth spurt occurred in the same year for 81% of MZ and for about 43% of DZ pairs (Sklad, 1977), and a greater similarity in height at every age was found within MZ pairs (correlation 0.90) than within DZ pairs (correlation 0.60–0.70) (Fischbein, 1977). Beunen et al. (2000) performed height measurements on 99 twin pairs semi-annually throughout puberty. They used the same novel twin genetic models as we did and the AE univariate model for both men and women. Genetic contribution to timing of acceleration of growth spurt was 89–93% in both genders. Measuring heights frequently to assess acceleration of growth or phv, however, is impractical in large study populations and in periodic evaluations. In contrast, HD:SDS can be calculated retrospectively between only two height measurements. Change in height SDs between age when growth peaks in the general population and adulthood correlated well with phv age in a cohort of healthy men (correlation 0.84) and women (correlation 0.78) provided with longitudinal growth data. This indicates that HD:SDS is a valid marker of timing of pubertal growth and therefore, timing of puberty. Comparing the results of previous and current twin studies on growth, the estimates of genetic contribution to timing of pubertal growth are close, irrespective of whether longitudinal height measurements or just two observations like in HD:SDS were used. In addition, the multivariate analysis of our study suggested that as much as 30% of the genetic effects in girls, and 49% in boys, influence both HD:SDS and PDS. Negative correlation (−0.55 in girls and −0.70 in boys) between factors affecting PDS and HD:SDS derive from late maturers having small PDS score and positive HD:SDS, and early maturers large PDS score and negative HD:SDS. The observed gender difference can be explained by small differences in stages of sexual maturation at ages of response and PDS ratings between sexes (see below). The twins self-reported their heights adding some inaccuracy to our data. Among 40 MZ Finnish twins, however, the correlation between self-reported and measured values of height from school records at age 17 years was as high as 0.97 (Pietiläinen et al., 2004).
In our study, using the ACE model in girls and ADE model in boys, the estimates of genetic contribution to HD:SDS (71% in girls and 82% in boys) differed between sexes. In contrast, AE model gave similar results in both genders (86% in girls and 82% in boys). The sex difference in model fits was probably caused by small differences in stages of sexual maturation at ages of response between boys and girls, which may result in increased estimates of dominance genetic or common environmental effects (Eaves and Silberg, 2003). This is also supported by the result that, in bivariate modeling under the ADE model, additive genetic component was not statistically significant in boys; for polygenetic inherited traits this is not a biologically plausible model and thus we also used the AE model. At age 17.5, some of the boys may not have ceased growth yet, while even the latest maturing girls have. Also, girls at age 11.5 may have been less mature than boys at age 14.0. Age of average phv in the general population is 12 in girls and 14 in boys (Tanner, 1976), which is, compared with the age of obtaining height in our twins, later in girls and the same in boys. PDS scores were also lower in girls at age 11.5 than in boys at age 14.0 (1.66 and 2.01 in MZ twin girls and boys, and 1.67 and 2.03 in DZ twin girls and boys, respectively). Because of differences in PDS ratings (dichotomous rating for menarche, three-point scale in girls and four-point scale in boys), however, exact comparison of PDS scores between genders is not possible. For these reasons, we excluded the opposite-sex twin pairs from the analysis. Recent advancement in timing of sexual maturation especially in girls (Anderson and Must, 2005; Herman-Giddens et al., 1997; Wu et al., 2002), but not convincingly in boys (de Muinck Keizer-Schrama and Mul, 2001; Herman-Giddens et al., 2001), however, suggests that there may be sex differences in genetic or environmental factors affecting pubertal timing. In addition, other traits such as height and adiposity have displayed such gender differences (Pietilänen et al., 1999; Silventoinen et al., 2003). Further investigations may, therefore, be required to determine whether gender differences in genetic or environmental factors affecting pubertal timing exist or not. Secular trends may also suggest that the contribution of environmental compared with genetic factors on timing of puberty is not constant. Estimation of possible changes in these effects by periodic evaluations is, therefore, needed. In the present study, we were not able to estimate possible changes in the effects of genetic or environmental factors on timing of puberty, because of differences in methods used between previous studies and ours. In future research, however, such an evaluation is possible if our analyses are repeated using the same marker of pubertal timing.
Our estimate of the genetic component in variation of pubertal timing, using an assessment of change in relative height, is 86% in girls and 82% in boys. In girls, 30% and in boys, 49% of the genetic factors affecting PDS and HD:SDS were the same. The secular trend towards earlier onset of puberty suggests changes in the human endocrine system, probably caused by environmental influences. Therefore, periodic evaluation of the influence and interaction of genetic and environmental effects on pubertal timing and estimation of possible changes in these effects in populations are necessary. We suggest that in such investigations, change in the standard deviation of height between age of average phv in the general population and age when growth has ceased may serve as a practical marker of pubertal timing in both genders.
Acknowledgments
Contract grant sponsor: National Institute on Alcohol Abuse and Alcoholism; Contract grant numbers: AA-12502, AA-00145, AA-09203, AA-015416; Contract grant sponsor: Academy of Finland; Contract grant numbers: 100499, 205585; Contract grant sponsor: European Union; Contract grant numbers: QLG2-CT-2002-01254, 513991; Contract grant sponsor: Academy of Finland; Contract grant number: 108297.
LITERATURE CITED
- Anderson SE, Dallal GE, Must A. Relative weight and race influence average age at menarche: results from two nationally representative surveys of US girls studied 25 years apart. Pediatrics. 2003;111:844–850. doi: 10.1542/peds.111.4.844. [DOI] [PubMed] [Google Scholar]
- Anderson SE, Must A. Interpreting the continued decline in the average age at menarche: results from two nationally representative surveys of US girls studied 10 years apart. J Pediatr. 2005;147:753–760. doi: 10.1016/j.jpeds.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Beunen G, Thomis M, Maes HH, Loos R, Malina M, Claessens AL, Vlietinck R. Genetic variance of adolescent growth in stature. Ann Hum Biol. 2000;27:173–186. doi: 10.1080/030144600282280. [DOI] [PubMed] [Google Scholar]
- Biro FM, Huang B, Crawford PB, Lucky AW, Striegel-Moore R, Barton BA, Daniels S. Pubertal correlates in black and white girls. J Pediatr. 2006;148:234–240. doi: 10.1016/j.jpeds.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Blanck HM, Marcus M, Tolbert PE, Rubin C, Henderson AK, Hertzberg VS, Zhang RH, Cameron L. Age at menarche and tanner stage in girls exposed in utero and postnatally to polybrominated biphenyl. Epidemiology. 2000;11:641–647. doi: 10.1097/00001648-200011000-00005. [DOI] [PubMed] [Google Scholar]
- Boomsma D, Busjahn A, Peltonen L. Classical twin studies and beyond. Nature Rev Genet. 2002;3:872–882. doi: 10.1038/nrg932. [DOI] [PubMed] [Google Scholar]
- Child Growth Foundation. UK-Cross-Sectional Reference Data: 1990/1. London: Child Growth Foundation; 1996. [Google Scholar]
- Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med. 1998;17:407–429. [PubMed] [Google Scholar]
- Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992;11:1305–1319. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- de Muinck Keizer-Schrama SM, Mul D. Trends in pubertal development in Europe. Hum Reprod Update. 2001;7:287–291. doi: 10.1093/humupd/7.3.287. [DOI] [PubMed] [Google Scholar]
- de Ridder CM, Thijssen JH, Bruning PF, Van de Brande JL, Zonderland ML, Erich WB. Body fat mass, body fat distribution, and pubertal development: a longitudinal study of physical and hormonal sexual maturation of girls. J Clin Endocrinol Metab. 1992;75:442–488. doi: 10.1210/jcem.75.2.1639945. [DOI] [PubMed] [Google Scholar]
- Dick DM, Rose RJ, Pulkkinen L, Kaprio J. Measuring puberty and understanding its impact: a longitudinal study of adolescent twins. J Youth Adolesc. 2001;30:385–399. [Google Scholar]
- Eaves LJ, Silberg JL. Modulation of gene expression by genetic and environmental heterogeneity in timing of a developmental milestone. Behav Genet. 2003;33:1–6. doi: 10.1023/a:1021060430942. [DOI] [PubMed] [Google Scholar]
- Fischbein S. Intra-pair similarity in physical growth of monozygotic and of dizygotic twins during puberty. Ann Hum Biol. 1977;4:417–430. doi: 10.1080/03014467700002401. [DOI] [PubMed] [Google Scholar]
- Freni-Titulaer LW, Cordero JF, Haddock L, Lebron G, Martinez R, Mills JL. Premature thelarche in Puerto Rico. A search for environmental factors. Am J Dis Child. 1986;140:1263–1267. doi: 10.1001/archpedi.1986.02140260065028. [DOI] [PubMed] [Google Scholar]
- Greulich WW, Pyle SI. Radiological atlas of skeletal development of the hand and wrist. 2nd ed. Stanford, California: Stanford University Press/London: Oxford University Press; 1959. [Google Scholar]
- Grumbach MM, Styne DM. Puberty: Ontogeny, neuroendocrinology, physiology, and disorders. In: Wilson JD, Foster DW, Kronenberg HM, Larsen PR, editors. William’s textbook of endocrinology. 9th ed. Philadelphia: W.B. Saunders; 1998. pp. 1509–1625. [Google Scholar]
- Hergenroeder AC, Hill RB, Wong WW, Sangi-Haghpeykar H, Taylor W. Validity of self-assessment of pubertal maturation in African-American and European-American adolescents. J Adolesc Health. 1999;24:201–205. doi: 10.1016/s1054-139x(98)00110-4. [DOI] [PubMed] [Google Scholar]
- Herman-Giddens ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, Koch GG, Hasemeier CM. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the pediatric research in office settings network. Pediatrics. 1997;99:505–512. doi: 10.1542/peds.99.4.505. [DOI] [PubMed] [Google Scholar]
- Herman-Giddens ME, Wang L, Koch G. Secondary sexual characteristics in boys: Estimates from the national health and nutrition examination survey III, 1988–1994. Arch Pediatr Adolesc Med. 2001;155:1022–1028. doi: 10.1001/archpedi.155.9.1022. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Pulkkinen L, Rose RJ. Genetic and environmental factors in health-related behaviors: studies on Finnish twins and twin families. Twin Res. 2002;5:366–371. doi: 10.1375/136905202320906101. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Rimpelä A, Winter T, Viken RJ, Rimpelä M, Rose RJ. Common genetic influences on BMI and age at menarche. Hum Biol. 1995;67:739–753. [PubMed] [Google Scholar]
- Karlberg J, Kwan CW, Gelander L, Albertsson-Wikland K. Pubertal growth assessment. Horm Res. 2003;60:27–35. doi: 10.1159/000071223. [DOI] [PubMed] [Google Scholar]
- Krstevska-Konstantinova M, Charlier C, Craen M, du Caju M, Heinrichs C, de Beaufort C, Blomteux G, Bourguignon JP. Sexual precocity after immigration from developing countries to Belgium: evidence of previous exposure to organochlorine pesticides. Hum Reprod. 2001;16:1020–1026. doi: 10.1093/humrep/16.5.1020. [DOI] [PubMed] [Google Scholar]
- Largo RH, Prader A. Pubertal development in Swiss girls. Helv Paediatr Acta. 1983a;38:229–243. [PubMed] [Google Scholar]
- Largo RH, Prader A. Pubertal development in Swiss boys. Helv Paediatr Acta. 1983b;38:211–228. [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustanski BS, Viken RJ, Kaprio J, Pulkkinen L, Rose RJ. Genetic and environmental influences on pubertal development: longitudinal data from Finnish twins at ages 11 and 14. Dev Psychol. 2004;40:1188–1198. doi: 10.1037/0012-1649.40.6.1188. [DOI] [PubMed] [Google Scholar]
- Neale MC. Mx: Statistical modeling. Richmond, VA: Department of Psychiatry; 2003. [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Dordrecht: Kluwer Academic; 1992. [Google Scholar]
- Palmert MR, Hayden DL, Mansfield MJ, Crigler JF, Jr, Crowley WF, Jr, Chandler DW, Boepple PA. The longitudinal study of adrenal maturation during gonadal suppression: evidence that adrenarche is a gradual process. J Clin Endocrinol Metab. 2001;86:4536–4542. doi: 10.1210/jcem.86.9.7863. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Tobin-Richards M, Boxer AM. Puberty: its measurement and its meaning. J Early Adolesc. 1983;3:47–62. [Google Scholar]
- Pietiläinen KH, Kaprio J, Rissanen A, Winter T, Rimpelä A, Viken RJ, Rose RJ. Distribution and heritability of BMI in Finnish adolescents aged 16y and 17y: a study of 4884 twins and 2509 singletons. Int J Obes Relat Metab Disord. 1999;23:107–115. doi: 10.1038/sj.ijo.0800767. [DOI] [PubMed] [Google Scholar]
- Pietiläinen KH, Rissanen A, Laamanen M, Lindholm AK, Markkula H, Yki-Järvinen H, Kaprio J. Growth patterns in young adult monozygotic twin pairs discordant and concordant for obesity. Twin Res. 2004;7:421–429. doi: 10.1375/1369052042335368. [DOI] [PubMed] [Google Scholar]
- Sanchez-Andres A. Genetic and environmental factors affecting menarcheal age in Spanish women. Anthropol Anz. 1997;55:69–78. [PubMed] [Google Scholar]
- Sarna S, Kaprio J, Sistonen P, Koskenvuo M. Diagnosis of twin zygosity by mailed questionnaire. Hum Hered. 1978;28:241–254. doi: 10.1159/000152964. [DOI] [PubMed] [Google Scholar]
- Sedlmeyer IL, Hirschhorn JN, Palmert MR. Pedigree analysis of constitutional delay of growth and maturation: determination of familial aggregation and inheritance patterns. J Clin Endocrinol Metab. 2002;87:5581–5586. doi: 10.1210/jc.2002-020862. [DOI] [PubMed] [Google Scholar]
- Sharma JC. The genetic contribution to pubertal growth and development studied by longitudinal growth data on twins. Ann Hum Biol. 1983;10:163–171. doi: 10.1080/03014468300006301. [DOI] [PubMed] [Google Scholar]
- Silventoinen K, Sammalisto S, Perola M, Boomsma DI, Cornes BK, Davis C, Dunkel L, de Lange M, Harris JR, Hjelmborg JV, Luciano M, Martin NG, Mortensen J, Nistico L, Pedersen NL, Skytthe A, Spector TD, Stazi A, Willemsen G, Kaprio J. Heritability of adult body height: a comparative study of twin cohorts in eight countries. Twin Res. 2003;6:399–408. doi: 10.1375/136905203770326402. [DOI] [PubMed] [Google Scholar]
- Sklad M. The rate of growth and maturing of twins. Acta Genet Med Gemellol. 1977;26:221–237. doi: 10.1017/s0001566000009703. [DOI] [PubMed] [Google Scholar]
- Sklar CA, Kaplan SL, Grumbach MM. Evidence for dissociation between adrenarche and gonadarche: studies in patients with idiopathic precious puberty, gonadal dysgenesis, isolated gonadotrophin deficiency, and constitutionally delayed growth and adolescence. J Clin Endocrinol Metab. 1980;51:170–179. doi: 10.1210/jcem-51-3-548. [DOI] [PubMed] [Google Scholar]
- Tanner JM. Growth at Adolescence. 2nd ed. Oxford, UK: Blackwell; 1962. [Google Scholar]
- Tanner JM, Whitehouse RH, Marubini E, Resele LF. The adolescent growth spurt of boys and girls of the Harpenden Growth Study. Ann Hum Biol. 1976;3:109–126. doi: 10.1080/03014467600001231. [DOI] [PubMed] [Google Scholar]
- Teilmann G, Juul A, Skakkebaek NE, Toppari J. Putative effects of endocrine disrupters on pubertal development in the human. Best Pract Res Clin Endocrinol Metab. 2002;16:105–121. doi: 10.1053/beem.2002.0184. [DOI] [PubMed] [Google Scholar]
- Treloar SA, Martin NG. Age at menarche as a fitness trait: nonadditive genetic variance detected in a large twin sample. Am J Hum Genet. 1990;47:137–148. [PMC free article] [PubMed] [Google Scholar]
- Wu T, Mendola P, Buck GM. Ethnic differences in the presence of secondary sex characteristics and menarche among US girls: the Third National Health and Nutrition Examination Survey. Pediatrics. 2002;110:752–757. doi: 10.1542/peds.110.4.752. [DOI] [PubMed] [Google Scholar]
- Wyshak G, Frisch RE. Evidence for a secular trend in age of menarche. N Engl J Med. 1982;306:1033–1035. doi: 10.1056/NEJM198204293061707. [DOI] [PubMed] [Google Scholar]