Abstract
Background
Although task-specific training is emerging as a viable approach for recovering motor function after stroke, there is little evidence for whether the effects of such training transfer to other functional motor tasks not directly practiced in therapy.
Objective
The purpose of the current study was to test whether training on one motor task would transfer to untrained tasks that were either spatiotemporally similar or different in individuals with chronic hemiparesis post-stroke.
Methods
Eleven participants with chronic mild-to-moderate hemiparesis following stroke completed five days of supervised massed practice of a feeding task with their affected side. Performance on the feeding task, along with two other untrained functional upper extremity motor tasks (sorting, dressing) was assessed before and after training.
Results
Performance of all three tasks improved significantly after training exclusively on one motor task. The amount of improvement in the untrained tasks was comparable, and was not dependent on the degree of similarity to the trained task.
Conclusions
Because the number and type of tasks that can be practiced are often limited within standard stroke rehabilitation, results from this study will be useful for designing task-specific training plans to maximize therapy benefits.
Keywords: task-specific training, transfer, stroke, upper extremity
INTRODUCTION
Task-specific training is emerging as a viable neurorehabilitative approach for improving motor function after stroke1,2. Task-specific training is based on the fundamental principle that repeated practice is the best way to learn a particular task3. Unfortunately, an individual often loses the ability to perform a number of tasks after stroke, many more so than can be practiced feasibly within the current rehabilitation setting4,5. It is assumed that the effects of training on one task in therapy will transfer to other tasks that have not undergone training, yet very little is known about whether this is true. Transfer, in this sense, is the gain in the proficiency of one motor task as a result of practice on some other motor task6. To what degree does motor training transfer after task-specific training? Given that “there is currently a dearth of evidence on which to construct rehabilitation interventions that are accurately targeted, properly framed, and credibly measured”7 p. 2033), it is critical to understand 1) if task-specific training transfers and 2) how much transfer occurs.
One challenge in addressing these questions experimentally is the substantial differences between functional upper extremity motor tasks that may be practiced within task-specific training. For example, an individual may repeatedly practice sorting coins with the affected limb8. This task would be markedly different from other tasks such as feeding or dressing oneself, not only in terms of movement goals but also in terms of spatiotemporal characteristics of the movements9. Previous studies in neurologically-intact individuals have used point-to-point reaching paradigms to demonstrate how the effects of motor training can transfer, but the amount of transfer is incomplete even when the context is similar to the training condition10,11. Such results from motor tasks bearing little resemblance to real-world actions suggest that little to no transfer of training would occur between more naturalistic upper extremity actions involving the entire upper extremity. Naturalistic actions are purposeful and multi-step12–15, often recruiting many degrees of freedom and differing substantially in how “successful” performance is defined. We recently found, however, that performance on one novel yet naturalistic motor task (simulated dressing) improved significantly following a single session of training on another task that was spatiotemporally quite different (simulated feeding)16. Performance on a novel, nonmotor cognitive task (associative recognition) did not, however, improve following training on the feeding task, indicating that the transfer effects were specific to the motor domain and not due to changes in overall arousal. Moreover, no improvements on the dressing task were observed in individuals who did not train on the feeding task, indicating that the transfer effects were experience-dependent. By establishing this proof-of-principle in neurologically-intact individuals, we now address whether motor training can transfer in individuals with chronic post-stroke hemiparesis, and whether the amount of transfer depends on the degree of similarity between the trained and untrained tasks.
The purpose of this study was to test whether five consecutive days of training on one motor task would transfer to two other untrained tasks in individuals with chronic hemiparesis post-stroke. All tasks were performed with the affected upper extremity. We hypothesized that training on the feeding task would improve motor performance not only on that task, but also on two tasks that were not trained (sorting, dressing). We also hypothesized that because the sorting task was spatiotemporally similar to the feeding task, the amount of transfer would be greater in the sorting task than in the dressing task. In this study, the amount of transfer was measured as the degree of improvement from before to after training on the feeding task. Results from this study have direct and immediate implications for maximizing the benefits of task-specific training after stroke by providing evidence that can be used to guide clinicians' selection of which tasks to practice.
METHODS
Participants
Eleven adults with chronic upper extremity hemiparesis following stroke participated in this study. Nine participants were right-handed, based on self-report. Five participants had right-side hemiparesis; 6 had left-side hemiparesis. Participants were recruited from the Brain Recovery Core Stroke Registry at Washington University in St. Louis based on the presence of unilateral hemiparesis. Potential stroke participants were included if they (1) had a diagnosis of ischemic or hemorrhagic stroke by a stroke neurologist, (2) had persistent hemiparesis with a score of 1–3 on the Motor Arm item of the National Institutes of Health Stroke Scale (NIHSS), indicating mild-to-moderate impairment, and (3) had the ability to follow 2-step commands. Potential participants were excluded from the study if they (1) had severe hemispatial neglect as evidenced by a score of 2 on the Extinction and Inattention items of the NIHSS, or (2) were unable to give informed consent. This study was approved by the Washington University Human Research Protection Office, and was conducted in compliance with the Helsinki Declaration. All participants provided informed consent prior to beginning the study.
Several clinical tests were used to characterize participants. Maximum grip strength (kg) of the affected and unaffected sides was measured via dynamometer (Jamar, Sammons-Preston-Rolyan)17,18 before and after training. The Action Research Arm Test (ARAT) was used to quantify upper extremity function19–24. An ARAT score of 57 indicates normal function. Spasticity of the elbow flexors was assessed on the affected side using the Modified Ashworth Scale25. Cutaneous sensation was measured with Semmes Weinstein monofilaments (Touch-TestTM, North Coast Medical, Inc) applied to the palmar surface on the distal phalange of the index finger. The Trail-Making Test B (TMT-B) was used to assess cognitive function, specifically switching attention26,27. Results from these tests are shown in Table 1, along with other descriptive characteristics. Time post-stroke ranged from 12 to 156 months, and severity of sensorimotor impairment and functional limitation ranged from mild-to-moderate, as shown by measures of strength and sensation and scores on the Action Research Arm test (ARAT).
Table 1.
Participant characteristics.
| Number of participants (n) | 11 (4 female, 7 male) |
| Age (years) | 58.9 ± 7.5 |
| Gender | 4 female, 7 male |
| Affected (Tested) side | 5 dominant, 6 nondominant (5 right, 6 left) |
| Stroke type | 8 ischemic, 2 hemorrhagic, 1 unknown |
| Months post-stroke | 52.7 ± 53.4 (range: 12 to 156) |
| Grip strength, affected hand (% unaffected hand) a | Pre-test: 53.0 ± 15.8 |
| Post-test: 59.0 ± 21.7b | |
| Action Research Arm Test, affected side c | 35.6 ± 7.5 (range: 23 to 44) |
| Spasticityd(affected side) | 0: n=4 (normal) |
| 1: n=2 | |
| 2 :n=3 | |
| 3: n=2 | |
| 4: n=0 (rigid) | |
| Sensatione(affected side) | 2.83: n=6 (normal) |
| 3.61: n=0 | |
| 4.31: n=1 | |
| 4.56: n=1 | |
| 6.65: n=3 (deep pressure sensation only) | |
| Trails B testf(time to completion, sec) | 140.7 ± 55.3 |
| (#,% participants with normal scores) | 10 out of 11 (91%) |
Unless otherwise indicated, values are mean ± SD for the affected upper extremity
average of 3 consecutive measurements
post-test grip strength not significantly different from pre-test grip strength (F1, 10=2.60; p=. 14)
normal (maximum) score =57
Modified Ashworth scale, elbow flexors
Semmes Weinstein monofilaments
Scores normalized for gender, age, and education level
Experimental design
General procedure
This study used a mixed model design to evaluate the transfer of training from one motor task to two other untrained motor tasks16. For all tasks, participants sat in a chair behind a table (76 × 51cm) that was adjusted to be as low as possible without contacting the thighs. All motor tasks were completed with the affected upper extremity, and were graded according to motor ability on an individual basis.
Each participant completed the study over the course of 5 days (Fig. 1). Prior to training on Day 1, participants completed one 30-second trial of all three tasks to establish their pre-test motor performance with their affected upper extremity. Participants then completed 50 more trials of the trained task with their affected upper extremity in a massed practice training session. On Days 2–5, participants received additional training only on that task (50 trials/day), resulting in 250 total trials over the course of five days. Immediately following the training session on Day 5, participants completed one 30-second trial of all three tasks to determine their post-test motor performance. In both the pre- and post-test sessions the order of the motor tasks was randomized using the random permutation function in MATLAB (MathWorks, Inc., Natick, MA) for each participant.
Figure 1.

Diagram of training schedule across five days. Training sessions were comprised of fifty 30-sec trials of only one motor task. Gray shading indicates sessions (Pre-test and Post-test) in which all motor tasks were completed, under both single and dual task conditions. Order of trials and conditions within each session was randomized.
Trained motor task: Feeding
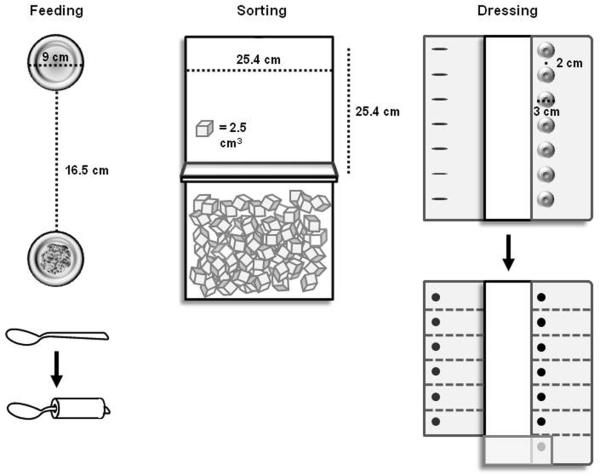
The trained motor task was a simulated feeding task that required spooning beans from one cup to another (Fig. 2, left panel). At the start of each feeding trial, participants picked up a metal spoon with their most affected hand and spooned one bean at a time from one cup away from their body to another cup. The start cup contained 70 beans that were distributed evenly across the bottom. The cups were secured to a wooden board, which was centered in line with the participant's shoulder. Participants spooned as many beans as possible per trial in the target direction, and the total number of beans in the target cup was recorded after each trial. Only one bean was counted per repetition if >1 bean was transferred at a time. That is, a successful repetition in the feeding task was one in which at least one bean was transferred. Participants were given no information about their performance strategy during training, and were only given verbal feedback about the number of successful repetitions after each trial. Thus, a `discovery learning approach' was taken in this study28–30 in which participants adapt their movement strategies based on trial and error over time. The measure of performance for each feeding trial was the number of successful repetitions. Prior to the experiment, participants were tested on whether they could spoon at least two beans (two successful repetitions) within 30 seconds. Only four of the 11 participants did so. For the remaining 7 participants, a piece of cylindrical foam was used to cover the spoon's handle (Fig. 2, left panel). This graded the feeding task according to the individual's ability.
Figure 2.
Motor tasks. Top view of the trained task (`Feeding') and untrained tasks (`Sorting', 'Dressing'). Adapted versions of the feeding task and dressing task are shown beneath each task, respectively, as indicated by arrows.
Untrained motor tasks: Sorting and dressing
Participants were also tested on two other motor tasks before and after training: sorting and dressing. Participants did not train on either task. The sorting task required participants to transport blocks one-by-one from one box to another box away from their body (Fig. 2, middle panel). Each box was 8.5 × 25.4 × 24.4cm. The boxes were separated by a partition that was 18cm high, and were centered in line with the participant's shoulder. Participants transported as many blocks as possible per trial with their most affected hand. The measure of performance for each sorting trial was the number of blocks transported.
The apparatus used in this task was the Box and Blocks Test31. In its standardized clinical use, the boxes are oriented side-by-side, requiring individuals to transport blocks in the mediolateral direction across midline. In this study, however, the boxes were oriented such that participants were required to transport blocks in the anteroposterior direction in the hemispace ipsilateral to the affected side. This configuration required participants to move in a similar movement direction to the feeding task.
The second untrained motor task was a dressing task in which buttons were fastened sequentially. At the start of each dressing trial, participants began buttoning the bottom of seven buttons (3cm diameter) that were sewn 2cm apart to plain-weave cotton fabric (Fig. 2, right panel). Both fabric pieces were secured to a wooden board, with the placket centered in line with the participant's shoulder. The button-side of the fabric was folded onto the board, while the buttonhole-side of the fabric was unfolded onto the table prior to each trial, lateral to the affected upper extremity. Participants fastened as many consecutive buttons as possible per trial with their affected hand. If all seven buttons were fastened in less than 30 seconds, participants were instructed to completely unfasten each button in the reverse order until the trial ended. The measure of performance for each dressing trial was the number of buttons fastened/unfastened.
Prior to the experiment, participants were tested on whether they could fasten a single button within 30 seconds. Only two of the 11 participants did so. The remaining 9 participants were tested on an adapted dressing task in which medium-stress dot-on-dot Velcro® was fastened rather than buttons. This enabled the dressing task to be graded down according to the individual's ability.
Task similarity
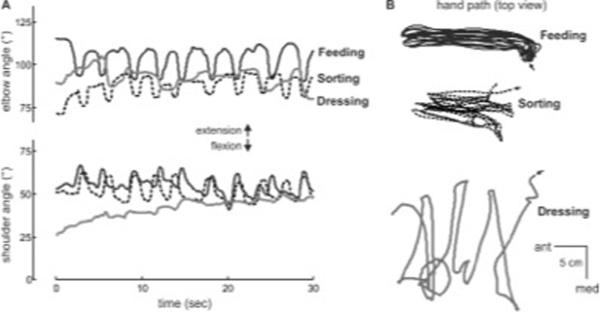
Movement kinematics were similar between the feeding task and the sorting task, whereas kinematics in the dressing task were different. To illustrate the similarities/differences between tasks, shoulder and elbow flexion angles during individual trials of each motor task in a single participant are provided (Fig. 3A); more positive values indicate extension direction. Relative to the feeding task (black line), the sorting task (dashed line) is spatiotemporally similar given the repetitive and consistent shoulder and elbow flexion/extension patterns as the hand moves back and forth between the cups or boxes in the anterior-posterior direction, respectively (Fig. 3B). The dressing task (gray line) is different, seen both in its joint angle patterns (Fig. 3A) and hand paths (Fig. 3B) as this participant fastened buttons over the course of the 30-second trial. Thus, the sorting task was considered more similar to the feeding task than the dressing task was. All three motor tasks, however, were selected because they simulate activities of daily living32–37 that are relevant and meaningful to one's ability for self-care38,39.
Figure 3.
Spatiotemporal characteristics and training schedule of motor tasks: Feeding (black), sorting (dashed), and dressing (gray). (A) Shoulder and elbow flexion angle and (B) hand path in the horizontal plane (top view; arrows indicate start of trial) during individual 30-second trials for a single participant. Note similarity between feeding and sorting tasks in terms of movement kinematics. 3D position data of the upper extremity segments were collected with an electromagnetic tracking system with four sensors (The Motion Monitor, Innovative Sports Training, Chicago, IL). Sensor locations were: midsternum, upper arm, forearm, and back of hand. Kinematic data were collected at 50 Hz and low-pass filtered at 6 Hz using a second-order Butterworth filter.
Task automaticity: An additional probe of transfer
It is possible that over the course of training, participants' motor performance on the feeding task would become more “automatic” with practice. This is based on the theory that the more learned a task is, the more automatic it is40. Thus, if the effects of training did transfer to the two untrained tasks, they too should become more automatic than prior to training. Automaticity is easily tested using a dual task condition41–43 and may be operationalized by the amount of dual task interference (i.e. degradation in performance of at least one task under dual task condition compared to performance by itself44,45). We recently tested whether dual task conditions were feasible for detecting transfer in neurologically-intact individuals16, and found that both the trained and untrained tasks showed improved automaticity following a single-session of training. To test whether similar effects occurred in this study following 5 sessions of training in individuals with chronic post-stroke hemiparesis, we evaluated individuals' motor performance under dual task conditions at pre- and post-test in the same manner shown in Figure 1. This was to further probe the transfer of training.
In the dual task condition, participants performed each motor task (feeding, sorting, dressing) and a non-motor auditory vigilance task concurrently. Prior to performing any motor task, all participants performed two trials of the auditory task in which they listened to recordings of 35-letter sequences. Each 30-letter sequence consisted of a random series of the same four letters (A, G, M, and O) at 1 Hz. Prior to each sequence, participants were instructed to pay attention to the number of times a target letter was heard. The target letter was A, G, M, or O, and was changed for each trial. Immediately after each sequence, participants were asked to verbally report the number of times a target letter was heard. The primary measure of performance for the auditory task was the number of listening errors per trial, which was calculated as the difference between the reported and correct number of times the target letter was heard. This difference was expressed as an absolute value, such that a score of one error was the over- or underestimating the number of target letters by one. Our previous study16 provides additional methodology of this task.
Data analysis
JMP 8.0 (SAS Institute Inc., Carey, NC) was used for all statistical analyses. The Shapiro-Wilk test was used to verify normal distribution of each variable. To test whether training on the feeding task 1) improved feeding performance and 2) transferred to the sorting and dressing tasks, we used one-way repeated-measures ANOVAs with time (pre-test vs. post-test) as the within-subject factor. Because the units of performance (i.e. beans, blocks, buttons) differed between tasks, separate ANOVAs were performed for the feeding, sorting, and dressing tasks. Significant differences between pre- and post-test performance of the untrained motor tasks would indicate that training had transferred.
To test whether the dual task condition interfered with each task's motor performance, we used 2×2 mixed model ANOVAs with time (pre-test vs. post-test) and condition (motor task only vs. dual task) as within-subject factors. Separate ANOVAs were performed for the feeding, sorting, and dressing tasks.
All participants' pre- and post-test motor performance was normalized to their unaffected side's performance (normal=100%). This normalization allowed for direct comparison of how much each task improved relative to other tasks. Improvement was calculated as normalized post-test performance minus normalized pre-test performance. To determine whether more transfer occurred to a spatiotemporally similar task (sorting) compared to a different task (dressing), we used a one-way repeated-measures ANOVA with task (feeding vs. sorting vs. dressing) as the within-subject factor. For all ANOVAs in this study, Tukey-Kramer Honestly Significant Different (HSD) tests46,47 were used for post hoc analysis when warranted based on the criterion for statistical significance (α=0.05).
RESULTS
Effects of motor training
As expected, feeding performance improved with training (Fig. 4A). There was an effect of time on the number of successful repetitions per trial (F1,10=68.5; p<.0001), indicating that participants spooned more beans per trial after training (post-test) compared to before training (pre-test) (Fig. 4B). Over the five days of training on the feeding task, the median number of successful repetitions per trial was 9. Thus, the number of repetitions achieved by each participant with the affected upper extremity per day was approximately 450 (9 reps/trial × 50 trials/day).
Figure 4.
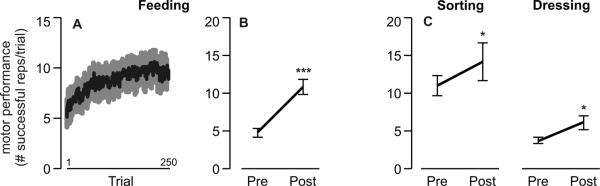
Effects of motor training. Mean ± SE motor performance on the (A) feeding task per trial over the course of 250 training trials; (B) feeding task before (pre) and after (post) training; and (C) untrained sorting and dressing tasks before (pre) and after (post) training (***p<.0001; *p<.05).
Although participants did not train on the sorting or dressing tasks with their affected arm, performance on these tasks also improved from pre-test to post-test (Fig. 4C). There was an effect of time on the number of successful repetitions per trial in the sorting (F1,10=5.4; p<.05) and dressing (F1,10=8.2; p<.05) tasks.
Preserved effects of training under dual task conditions
Pre- and post-test performance on all three motor tasks was also evaluated under dual task conditions. Results presented here focus on the statistical comparisons that determined whether automaticity of the trained and untrained tasks improved. There was a significant effect of time on listening performance (F2,10=3.8; p<.05), such that listening errors were greater during pre-test compared to listening only (post hoc p<.05; Fig. 5A). This occurred even though participants were instructed to prioritize the auditory task. Instead, participants appeared to maintain their motor performance during pre-test at the cost of the listening performance, as evidenced by no significant main effect of condition (single vs. dual task) for the sorting (F1,10=1.0; p=.32) and dressing (F1,10=1.1; p=.31) tasks. Thus, prior to any motor training, the motor tasks appeared to disrupt the auditory task. By post-test, however, listening performance under dual task conditions (Fig. 5A) was comparable to listening only (p=.57). Motor performance for all tasks under dual task conditions also improved significantly after training on only one task (Fig. 5B), as shown by the main effect of time (pre-test vs. post-test) for feeding (F1,10=187.07; p<.0001), sorting (F1,10=7.48; p<.01) and dressing (F1,10=16.27; p<.001) performance. Collectively, these results show that after training on one motor task, the automaticity of the untrained tasks improved as well, allowing for enough attention to be re-allocated back the auditory task under dual task conditions with minimal interference.
Figure 5.
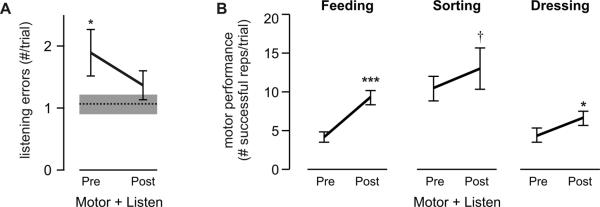
Preserved training effects under dual task conditions. (A) Mean listening error under dual task conditions ('Listen + Motor'; collapsed across all motor tasks) before (pre) and after (post) motor training. Dashed line indicates mean listening error for `Listening only' condition; gray box indicates SE. (B) Mean motor performance per trial before (pre) and after (post) training for the trained ('Feeding') and the untrained ('Sorting', `Dressing') tasks under dual task conditions ('Listen + Motor'). Error bars indicate SE. Note similarity in trend to Figure 3B (***p<.0001; *p<.05;† p<.1).
Magnitude of improvement across motor tasks
The magnitude of improvement across tasks was examined with normalized scores (see Methods). Figure 6A shows a participant in all three motor tasks at pre- and post-test. Not surprisingly, the trained feeding task improved the most. Although the untrained tasks (sorting, dressing) also improved, the amount of improvement was 1) comparable and 2) less than that of the feeding task. This effect was consistent across participants (Fig. 6B). There was main effect of motor task (feeding vs. sorting vs. dressing) on the amount of improvement in motor performance (F2,10=9.38; p<.01). Post hoc analyses revealed that the trained feeding task showed more improvement compared to the untrained sorting (p<.01) and dressing (p<.05) tasks. The amounts of improvement on the two untrained tasks were not different from each other (p=.50).
Figure 6.
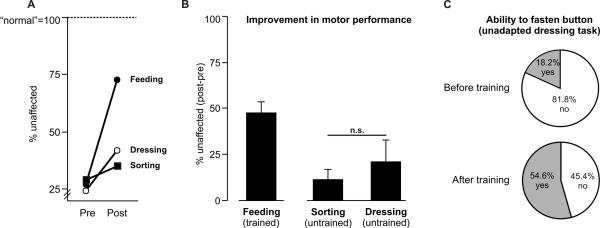
Magnitude of improvement due to transfer. (A) Motor performance on all three tasks for a single participant with moderate hemiparesis (ARAT score=26) before (pre) and after (post) training, normalized to unaffected arm performance (100%=normal). (B) Mean improvement in motor performance from pre- to post-test on all three motor tasks as a result of training only on one (Feeding). Amount expressed as percent change relative to the unaffected side of the unaffected side. Error bars indicate SE. (C) Change in ability to fasten buttons from before to after training on the dressing task. Percent of participants based on n=11.
Prior to the experiment, participants were tested on whether they could fasten a single button within 30 seconds. Only two of 11 participants did so. After training only on the feeding task, more participants (six of 11) were able to fasten >1 buttons within 30 seconds compared to before training (χ2 (2, n=22)=3.28; p<.10), indicating progression to a more challenging, unadapted version of the dressing task. These data are represented in Figure 6C, further illustrating improvement on a motor task that was substantially different than that which was practiced during training.
DISCUSSION
The purpose of this study was to test whether five consecutive days of training on one motor task would transfer to two other untrained tasks in individuals with chronic mild-to-moderate hemiparesis post-stroke. Results supported the first hypothesis that training on the feeding task would improve motor performance not only on that task, but also on two other tasks that were not trained (sorting, dressing). Results did not, however, support the second hypothesis that the amount of transfer would be greater in the sorting task than in the dressing task due to its spatiotemporal similarity to the feeding task. Instead, the amount of transfer (i.e. improvement from pre- to post-test) was comparable. Collectively, these results provide evidence for the effects of task-specific training after stroke to potentially generalize to a wider range of motor tasks beyond that which is practiced.
Transfer of training after stroke: Clinical importance yet little evidence
Given the increased prevalence of task-specific training in neurorehabilitation after stroke1, the question of whether the effects of training on one motor task will transfer to other unpracticed tasks is important. Numerous factors may limit exactly how many tasks can be practiced during standard therapy (e.g. time, available space, patient/clinician characteristics), but undoubtedly the number and range of tasks needing practice exceeds that which can be practiced feasibly within the allotted amount of rehabilitation4,5. Thus, any task-specific training treatment plan must consider the amount of expected transfer in order to maximize its benefits.
While the clinical importance of transfer is indisputable, little research has been done directly in 1) the clinical populations currently being treated with task-specific training and 2) the tasks currently being used within task-specific training. Previous studies in neurologically-intact adults have demonstrated in experimental tasks with few degrees of freedom (i.e. planar point-to-point reaching or sequential finger flexion with limb support), the amount of transfer to novel conditions of the same task is relatively small48–53. Nevertheless, this work has provided considerable evidence that the nervous system can and does generalize movement-related information. This work is, however, unable to predict the following: Does training on a more complex, functional task that recruits more degrees of freedom facilitate more transfer? Or does the diversity among movement patterns and goals minimize or even prevent any transfer? Prior to the current study, it was unclear whether transfer would be expected between more functional tasks involving many steps and degrees of freedom, i.e. tasks that are practiced during task-specific training after stroke, such as sorting silverware or drinking from a glass2,8,54. We recently tested this in a group of neurologically intact individuals, and found that the effects of repetitive training on one functional task are transferrable to another functional task16. The tasks in this previous study were similar to the tasks performed in the current study. By demonstrating the effects of transfer between functional upper extremity motor tasks as a result of repetitive training in individuals with chronic mild-to-moderate hemiparesis after stroke, we now provide novel quantitative findings that address both a specific population and a task set that are relevant clinically.
Transfer of training after stroke: More task similarity, more transfer?
In the current study, participants trained on one motor task (feeding) but were tested on two other untrained motor tasks (sorting, dressing) before and after training. The sorting task was relatively similar to the feeding task in terms of movement kinematics, whereas the dressing task was not (Fig. 3A). Although we expected that the degree of similarity would predict the amount of transfer between tasks55–57, we found that the sorting and dressing tasks showed comparable improvement. Although the sample size for this study was small (n=11), these novel findings may suggest that matching tasks' spatiotemporal characteristics may not necessarily promote additional transfer. Moreover, the motor tasks had different movement goals. The goals of the feeding, sorting, and dressing tasks were to spoon beans, pick up blocks, and fasten buttons, respectively. Previous work has suggested that movements with similar motor goals share common adaptive structures11,58, and while more transfer might be expected experimentally when the goals of tasks overlap, this study demonstrates that transfer can occur even when the goals are quite disparate. More research across a broader range of both upper and lower extremity motor tasks is needed to test the limits of transfer. Nevertheless, these results are encouraging, as clinicians will often take an individualized approach when determining activities of interest and specific tasks to address during treatment sessions8,59.
Transfer of training after stroke: More task practice, more transfer?
From this study we now provide evidence that the benefits of a high, but relatively brief, dose of functional upper extremity training (approx. 2,250 repetitions over 5 days) can transfer to other untrained upper extremity tasks that are markedly different than the task that was practiced. This is consistent with recent findings demonstrating that following stroke, high doses of task-specific locomotor training over a 6-week intervention also improved other lower extremity tasks that were not practiced, and that were functionally very different from the trained task60. Collectively these findings support that, even after 1 or more years post-stroke, transfer can and does occur as a result of training. It is likely that the generalized benefits in the 6-week intervention are in part due to increased limb strength and/or coordination60–62. The training in the current study was only 5 days and no significant changes in strength (i.e. grip strength, Table 1) were observed. This suggests that the mechanism underlying transfer in the current study may be related more to neural reorganization, rather than muscular conditioning, following high doses of functional activity over only a few days.
It is still unclear whether even higher doses of training will yield even more transfer. In this study, the median number of successful repetitions achieved per day of training on the feeding task was 450 repetitions, which is comparable with other work in which high doses of task-specific upper extremity training (average 322 repetitions/day8) have yielded sustained improvements in clinically-evaluated motor function. Future research in a larger sample is needed to determine whether and how the amount of transfer can be enhanced with more practice to maximize the functional benefits of a given intervention.
CONCLUSIONS
Results from this study suggest that the effects of upper extremity task-specific training can transfer to other untrained tasks in individuals with chronic mild-to-moderate hemiparesis after stroke. Because the number and type of tasks that can be practiced are often limited within standard stroke rehabilitation, our findings will be useful for optimizing the design of task-specific training plans to maximize the generalization of training to other tasks that may also need improvement.
ACKNOWLEDGEMENTS
This work was supported in part by National Institutes of Health R01HD055964, T32HD007434 to the Program in Physical Therapy at Washington University School of Medicine, and American Heart Association 10POST4140091.
Footnotes
CONFLICTS OF INTEREST: None
REFERENCES
- 1.French B, Thomas L, Leathley M, et al. Does repetitive task training improve functional activity after stroke? A Cochrane systematic review and meta-analysis. J Rehabil Med. 2010 Jan;42(1):9–14. doi: 10.2340/16501977-0473. [DOI] [PubMed] [Google Scholar]
- 2.Hubbard IJ, Parsons MW, Neilson C, Carey LM. Task-specific training: evidence for and translation to clinical practice. Occup Ther Int. 2009;16(3–4):175–189. doi: 10.1002/oti.275. [DOI] [PubMed] [Google Scholar]
- 3.Bayona NA, Bitensky J, Salter K, Teasell R. The role of task-specific training in rehabilitation therapies. Top Stroke Rehabil. 2005 Summer;12(3):58–65. doi: 10.1310/BQM5-6YGB-MVJ5-WVCR. [DOI] [PubMed] [Google Scholar]
- 4.Kimberley TJ, Samargia S, Moore LG, Shakya JK, Lang CE. Comparison of amounts and types of practice during rehabilitation for traumatic brain injury and stroke. J Rehabil Res Dev. 2010;47(9):851–862. doi: 10.1682/jrrd.2010.02.0019. [DOI] [PubMed] [Google Scholar]
- 5.Lang CE, Macdonald JR, Reisman DS, et al. Observation of amounts of movement practice provided during stroke rehabilitation. Arch Phys Med Rehabil. 2009 Oct;90(10):1692–1698. doi: 10.1016/j.apmr.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt RA, Lee TD. Motor Control and Learning: a behavioral emphasis. 3rd ed Human Kinetics; Champaign, IL: 1999. [Google Scholar]
- 7.Daly JJ, Ruff RL. Construction of efficacious gait and upper limb functional interventions based on brain plasticity evidence and model-based measures for stroke patients. ScientificWorldJournal. 2007;7:2031–2045. doi: 10.1100/tsw.2007.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birkenmeier RL, Prager EM, Lang CE. Translating animal doses of task-specific training to people with chronic stroke in 1-hour therapy sessions: a proof-of-concept study. Neurorehabil Neural Repair. 2010 Sep;24(7):620–635. doi: 10.1177/1545968310361957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Timmermans AA, Seelen HA, Willmann RD, et al. Arm and hand skills: training preferences after stroke. Disabil Rehabil. 2009;31(16):1344–1352. doi: 10.1080/09638280902823664. [DOI] [PubMed] [Google Scholar]
- 10.Thoroughman KA, Taylor JA. Rapid reshaping of human motor generalization. J Neurosci. 2005 Sep 28;25(39):8948–8953. doi: 10.1523/JNEUROSCI.1771-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wada Y, Kawabata Y, Kotosaka S, Yamamoto K, Kitazawa S, Kawato M. Acquisition and contextual switching of multiple internal models for different viscous force fields. Neurosci Res. 2003 Jul;46(3):319–331. doi: 10.1016/s0168-0102(03)00094-4. [DOI] [PubMed] [Google Scholar]
- 12.Giovannetti T, Libon DJ, Buxbaum LJ, Schwartz MF. Naturalistic action impairments in dementia. Neuropsychologia. 2002;40(8):1220–1232. doi: 10.1016/s0028-3932(01)00229-9. [DOI] [PubMed] [Google Scholar]
- 13.Hartmann K, Goldenberg G, Daumuller M, Hermsdorfer J. It takes the whole brain to make a cup of coffee: the neuropsychology of naturalistic actions involving technical devices. Neuropsychologia. 2005;43(4):625–637. doi: 10.1016/j.neuropsychologia.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Leipmann H. Das Krankheitsbild der Apraxie (`motorischen Asymbolie') auf Grund eines Falles von einseitiger Apraxie. Monatsschrift für Psychiatrie und Neurologie. 1900;8:15–44. [Google Scholar]
- 15.Schwartz MF, Montgomery MW, Buxbaum LJ, et al. Naturalistic action impairment in closed head injury. Neuropsychology. 1998 Jan;12(1):13–28. doi: 10.1037//0894-4105.12.1.13. [DOI] [PubMed] [Google Scholar]
- 16.Schaefer SY, Lang CE. Using dual tasks to test immediate transfer of training between naturalistic movements: A proof-of-principle study. J Mot Behav. 2012 Sep;44(5):313–327. doi: 10.1080/00222895.2012.708367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrews AW, Thomas MW, Bohannon RW. Normative values for isometric muscle force measurements obtained with hand-held dynamometers. Phys Ther. 1996 Mar;76(3):248–259. doi: 10.1093/ptj/76.3.248. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt RT, Toews JV. Grip strength as measured by the Jamar dynamometer. Arch Phys Med Rehabil. 1970 Jun;51(6):321–327. [PubMed] [Google Scholar]
- 19.Hsieh CL, Hsueh IP, Chiang FM, Lin PH. Inter-rater reliability and validity of the action research arm test in stroke patients. Age Ageing. 1998 Mar;27(2):107–113. doi: 10.1093/ageing/27.2.107. [DOI] [PubMed] [Google Scholar]
- 20.Lang CE, Wagner JM, Dromerick AW, Edwards DF. Measurement of upper-extremity function early after stroke: properties of the action research arm test. Arch Phys Med Rehabil. 2006 Dec;87(12):1605–1610. doi: 10.1016/j.apmr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res. 1981;4(4):483–492. doi: 10.1097/00004356-198112000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Van der Lee JH, Beckerman H, Lankhorst GJ, Bouter LM. The responsiveness of the Action Research Arm test and the Fugl-Meyer Assessment scale in chronic stroke patients. J Rehabil Med. 2001 Mar;33(3):110–113. doi: 10.1080/165019701750165916. [DOI] [PubMed] [Google Scholar]
- 23.Van der Lee JH, De Groot V, Beckerman H, Wagenaar RC, Lankhorst GJ, Bouter LM. The intra- and interrater reliability of the action research arm test: a practical test of upper extremity function in patients with stroke. Arch Phys Med Rehabil. 2001 Jan;82(1):14–19. doi: 10.1053/apmr.2001.18668. [DOI] [PubMed] [Google Scholar]
- 24.Yozbatiran N, Der-Yeghiaian L, Cramer SC. A standardized approach to performing the action research arm test. Neurorehabil Neural Repair. 2008 Jan-Feb;22(1):78–90. doi: 10.1177/1545968307305353. [DOI] [PubMed] [Google Scholar]
- 25.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987 Feb;67(2):206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 26.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Neuropsychology Press; Tucson, AZ: 1993. [Google Scholar]
- 27.Heaton RK, Grant I, Matthew CG. Comprehensive norms for an expanded Halstead-Reitan Battery. Psychological Assessment Resources; Odessa, FL: 1991. [Google Scholar]
- 28.Orrell AJ, Eves FF, Masters RS. Implicit motor learning of a balancing task. Gait Posture. 2006 Jan;23(1):9–16. doi: 10.1016/j.gaitpost.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Taubert M, Draganski B, Anwander A, et al. Dynamic properties of human brain structure: learning-related changes in cortical areas and associated fiber connections. J Neurosci. 2010 Sep 1;30(35):11670–11677. doi: 10.1523/JNEUROSCI.2567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wulf G, Weigelt M, Poulter D, McNevin N. Attentional focus on suprapostural tasks affects balance learning. Q J Exp Psychol A. 2003 Oct;56(7):1191–1211. doi: 10.1080/02724980343000062. [DOI] [PubMed] [Google Scholar]
- 31.Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the Box and Block Test of manual dexterity. Am J Occup Ther. 1985 Jun;39(6):386–391. doi: 10.5014/ajot.39.6.386. [DOI] [PubMed] [Google Scholar]
- 32.Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud. 1988;10(2):61–63. doi: 10.3109/09638288809164103. [DOI] [PubMed] [Google Scholar]
- 33.Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The stroke impact scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke. 1999 Oct;30(10):2131–2140. doi: 10.1161/01.str.30.10.2131. [DOI] [PubMed] [Google Scholar]
- 34.Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Arch Phys Med Rehabil. 1969 Jun;50(6):311–319. [PubMed] [Google Scholar]
- 35.Keith RA, Granger CV, Hamilton BB, Sherwin FS. The functional independence measure: a new tool for rehabilitation. Adv Clin Rehabil. 1987;1:6–18. [PubMed] [Google Scholar]
- 36.Taub E, Miller NE, Novack TA, et al. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993 Apr;74(4):347–354. [PubMed] [Google Scholar]
- 37.Walker MF, Lincoln NB. Reacquisition of dressing skills after stroke. Int Disabil Stud. 1990 Jan-Mar;12(1):41–43. doi: 10.3109/03790799009166603. [DOI] [PubMed] [Google Scholar]
- 38.Blennerhassett JM, Carey LM, Matyas TA. Clinical measures of handgrip limitation relate to impaired pinch grip force control after stroke. J Hand Ther. 2008 Jul-Sep;21(3):245–252. doi: 10.1197/j.jht.2007.10.021. quiz 253. [DOI] [PubMed] [Google Scholar]
- 39.Duncan PW, Wallace D, Studenski S, Lai SM, Johnson D. Conceptualization of a new stroke-specific outcome measure: the stroke impact scale. Top Stroke Rehabil. 2001 Summer;8(2):19–33. doi: 10.1310/BRHX-PKTA-0TUJ-UYWT. [DOI] [PubMed] [Google Scholar]
- 40.Fitts PM, Posner MI. Human Performance. Brooks/Cole; Belmont, CA: 1967. [Google Scholar]
- 41.Gopher D, Sanders AF. S-Oh-R: Oh Stages! Oh Resources! In: Prinz W, Sanders AF, editors. Cognition and Motor Processes. Springer-Verlag; Berlin Heidelberg: 1984. [Google Scholar]
- 42.Neumann O. Automatic processing: a review of recent findings and a plea for an old theory. In: Prinz W, Sanders AF, editors. Cognition and Motor Processes. Springer-Verlag; Berlin Heidelberg: 1984. [Google Scholar]
- 43.Passingham RE. Attention to action. Philos Trans R Soc Lond B Biol Sci. 1996 Oct 29;351(1346):1473–1479. doi: 10.1098/rstb.1996.0132. [DOI] [PubMed] [Google Scholar]
- 44.Kinsbourne M. Single-channel Theory. In: Holding D, editor. Human Skills. John Wiley & Sons Ltd.; New York: 1981. [Google Scholar]
- 45.Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002 Aug;16(1):1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- 46.Kramer CY. Extension of Multiple Range Tests to Group Means with Unequal Numbers of Replications. Biometrics. 1956;12(3):307–310. [Google Scholar]
- 47.Stoline MR. The Status of Multiple Comparisons: Simultaneous Estimation of All Pairwise Comparisons in One-Way ANOVA Designs. The American Statistician. 1981;35(3):134–141. [Google Scholar]
- 48.Abeele S, Bock O. Transfer of sensorimotor adaptation between different movement categories. Exp Brain Res. 2003 Jan;148(1):128–132. doi: 10.1007/s00221-002-1317-0. [DOI] [PubMed] [Google Scholar]
- 49.Cotti J, Guillaume A, Alahyane N, Pelisson D, Vercher JL. Adaptation of voluntary saccades, but not of reactive saccades, transfers to hand pointing movements. J Neurophysiol. 2007 Aug;98(2):602–612. doi: 10.1152/jn.00293.2007. [DOI] [PubMed] [Google Scholar]
- 50.Howard IS, Ingram JN, Wolpert DM. Context-dependent partitioning of motor learning in bimanual movements. J Neurophysiol. 2010 Oct;104(4):2082–2091. doi: 10.1152/jn.00299.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ikegami T, Hirashima M, Taga G, Nozaki D. Asymmetric transfer of visuomotor learning between discrete and rhythmic movements. J Neurosci. 2010 Mar 24;30(12):4515–4521. doi: 10.1523/JNEUROSCI.3066-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krakauer JW, Pine ZM, Ghilardi MF, Ghez C. Learning of visuomotor transformations for vectorial planning of reaching trajectories. J Neurosci. 2000 Dec 1;20(23):8916–8924. doi: 10.1523/JNEUROSCI.20-23-08916.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tomi N, Gouko M, Ito K. Impedance control complements incomplete internal models under complex external dynamics. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:5354–5357. doi: 10.1109/IEMBS.2008.4650424. [DOI] [PubMed] [Google Scholar]
- 54.Arya KN, Verma R, Garg RK, Sharma VP, Agarwal M, Aggarwal GG. Meaningful task-specific training (MTST) for stroke rehabilitation: a randomized controlled trial. Top Stroke Rehabil. 2012 May-Jun;19(3):193–211. doi: 10.1310/tsr1903-193. [DOI] [PubMed] [Google Scholar]
- 55.Bills AG, McTeer W. Transfer of fatigue and identical elements. J Exp Psychol. 1932;15(1):23–36. [Google Scholar]
- 56.Deese JE. Principles of Psychology. Allyn and Bacon, Inc.; Boston: 1964. [Google Scholar]
- 57.Thorndike EL. Educational Psychology: Briefer Course. Columbia University Press; New York: 1914. [Google Scholar]
- 58.Weigelt C, Bock O. Adaptation of the precision grip orientation to a visual-haptic mismatch. Exp Brain Res. 2010 Apr;201(4):621–630. doi: 10.1007/s00221-009-2076-y. [DOI] [PubMed] [Google Scholar]
- 59.Higgins J, Salbach NM, Wood-Dauphinee S, Richards CL, Cote R, Mayo NE. The effect of a task-oriented intervention on arm function in people with stroke: a randomized controlled trial. Clin Rehabil. 2006 Apr;20(4):296–310. doi: 10.1191/0269215505cr943oa. [DOI] [PubMed] [Google Scholar]
- 60.Hornby TG, Straube DS, Kinnaird CR, et al. Importance of specificity, amount, and intensity of locomotor training to improve ambulatory function in patients poststroke. Top Stroke Rehabil. 2011 Jul-Aug;18(4):293–307. doi: 10.1310/tsr1804-293. [DOI] [PubMed] [Google Scholar]
- 61.Lewek MD, Cruz TH, Moore JL, Roth HR, Dhaher YY, Hornby TG. Allowing intralimb kinematic variability during locomotor training poststroke improves kinematic consistency: a subgroup analysis from a randomized clinical trial. Phys Ther. 2009 Aug;89(8):829–839. doi: 10.2522/ptj.20080180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Michael K, Goldberg AP, Treuth MS, Beans J, Normandt P, Macko RF. Progressive adaptive physical activity in stroke improves balance, gait, and fitness: preliminary results. Top Stroke Rehabil. 2009 Mar-Apr;16(2):133–139. doi: 10.1310/tsr1602-133. [DOI] [PMC free article] [PubMed] [Google Scholar]