Abstract
We herein describe the development and validation of a high performance liquid chromatography (HPLC) method for the quantitation of 7-(benzylamino)-1, 3, 4, 8-tetrahydropyrrolo [4, 3, 2-de]quinolin-8(1H)-one (BA-TPQ), a newly synthesized iminoquinone anticancer agent. BA-TPQ was extracted from plasma and tissue samples by first precipitating proteins with acetonitrile followed by a liquid-liquid extraction with ethyl acetate. Chromatographic separation was carried out using a gradient flow rate on a Zorbax SB C-18 column, and the effluent was monitored by UV detection at 346 nm. The method was found to be precise, accurate, and specific, with a linear range from 3.91 to 1955.0 ng/mL in plasma, 19.55 to 1955.0 ng/mL in spleen, brain, and liver homogenates, and 19.55 to 3910.0 ng/mL in heart, lung and kidney homogenates. The method was stable under all relevant conditions. Using this method, we also carried out an initial study determining plasma pharmacokinetics and tissue distribution of BA-TPQ in mice following intravenous administration. In summary, this simple and sensitive HPLC method can be used in future preclinical and clinical studies of BA-TPQ.
Keywords: BA-TPQ, HPLC, iminoquinone, pharmacokinetics
Introduction
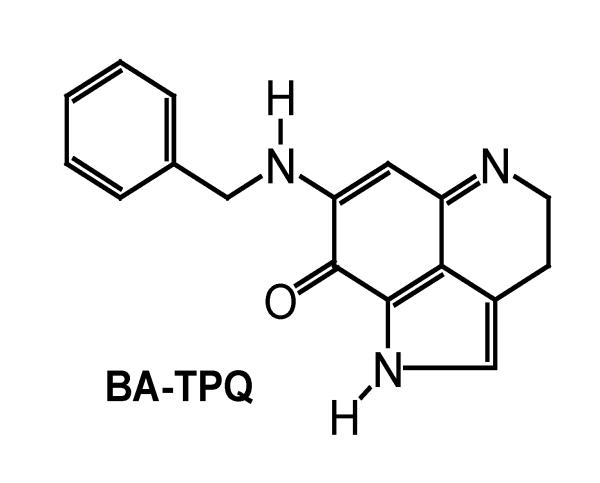
Cancer poses a major health problem worldwide, and chemotherapy remains one of the major approaches to the treatment of various human cancers. Although impressive progress has been made in improving both the safety and efficacy of cancer chemotherapeutic agents, there is an urgent need to develop novel agents for patients whose cancers are refractory to the currently available therapies. We have been interested in developing natural product-based anticancer agents, and have recently synthesized a series of novel iminoquinone compounds based on the chemical structures of marine sponge-derived natural products (Shinkre et al., 2007; Shinkre et al., 2008). Among these synthetic compounds, several analogs demonstrated in vitro and in vivo anticancer activity (Nadkarni et al., 2009; Wang et al., 2009). One of the most active compounds, 7-(benzylamino)-1,3,4,8-tetrahydropyrrolo [4,3,2-de]quinolin-8(1H)-one (BA-TPQ, Fig. 1), exhibited strong activity against various cancer cell lines in vitro, modulated the expression of several genes important for cancer cell proliferation, cell cycle progression and apoptosis in vitro and in vivo, and inhibited the growth of breast cancer xenograft tumors in vivo (Wang et al., 2009).
Figure 1.
(A) Structure of BA-TPQ [7-(benzylamino)-1,3,4,8-tetrahydropyrrolo [4,3,2-de]quinolin-8(1H)-one].
These compounds have now entered into preclinical development as novel anticancer agents. However, as these compounds represent a new class of drugs, there are no existing methods for their separation and quantitation from biological matrices. The development and validation of an analytical method is essential for the further pre-clinical and clinical development of the candidate therapeutic agent (Srinivas, 2006). Without a sensitive and reliable analytical protocol, it is not possible to determine key characteristics of the candidate compound, including its in vivo stability, pharmacokinetics, and sites of accumulation and potential toxicity in vivo. Therefore, there is a need for a validated analytical method for the detection and quantitation of BA-TPQ in biological matrices in order to facilitate its preclinical and clinical evaluation. Herein, we report the development and validation of a high performance liquid chromatographic (HPLC) method with UV detection for the quantitation of BA-TPQ in mouse plasma and tissues. To test the robustness and sensitivity of the method, we also carried out a pharmacokinetic study of BA-TPQ in CD-1 mice following intravenous administration. We believe that the results presented here provide a suitable method for future studies of this compound in preclinical and clinical settings.
Experimental
HPLC Analysis
Chemicals and reagents
BA-TPQ was synthesized and purified as previously reported, and the structure was confirmed by UV, IR, MS, and NMR spectroscopy (Shinkre 2007, Shinkre 2008). The purity of the compound was determined to be greater than 99%. HPLC-grade acetonitrile, ethyl acetate, and methanol were obtained from Fisher Scientific (Fair Lawn, NJ, USA). Formic acid was purchased from Sigma (St. Louis, MO, USA). Blank mouse plasma (heparinized, non-Swiss albino) was obtained from Lampire Biological Laboratories (Pipersville, PA, USA).
Instrumentation
Liquid chromatography experiments were conducted on an Agilent 1120 Compact HPLC system, including a gradient pump, mobile phase degasser, column oven, autosampler and UV detector (all from Agilent, CA, USA). Chromatographic separation was performed on a Zorbax SB C-18 column (4.6×150 mm, 5 μm, Agilent) with a guard-column cartridge (Agilent, SB C-18). All data were acquired and analyzed using the Agilent EZ-Chrom Elite Compact software (Agilent Technologies).
Chromatographic conditions
The mobile phase was composed of 28.0% acetonitrile in water and contained 0.03% formic acid (v/v). The solution was filtered through a Millipore glass filter system with a nylon membrane (0.2 μm) before use and was freshly prepared for each run. The column temperature was set at 35°C. The flow rate of the mobile phase was maintained at 0.5 mL/min for 7 min, then increased to 1.2 mL/min at 8 minutes, then decreased again to 0.5 mL/min at 11 minutes for the remainder of the 16 min run. Detection was accomplished at 346 nm.
Preparation of standard and quality control samples
A 391 μg/mL stock solution of BA-TPQ was prepared in methanol and stored at −80°C. The working solutions for calibration standards and quality control samples were obtained from the stock solution. Calibration curves were prepared at concentrations of 3.91-1955.0 ng/mL in mouse plasma, 19.55-1955.0 ng/mL in mouse spleen, brain and liver homogenates, and 19.55-3910.0 ng/mL for mouse heart, lung and kidney homogenates were prepared by adding the working solution to the plasma or homogenate, followed by immediate extraction. Quality control samples of low (19.55 ng/mL), medium (195.5 ng/mL) and high (1955.0 ng/mL) concentrations were prepared in the same way to assess intra-day and inter-day precision and accuracy, as well as recovery and stability
Sample preparation
BA-TPQ was extracted from plasma and homogenized tissues by precipitating proteins with acetonitrile, followed by liquid-liquid extraction with ethyl acetate. Birefly, ice-cold acetonitrile (600 μL) was added to 200 μL plasma or tissue homogenate, and samples were vortexed for at least 30 s. Samples were then centrifuged at 14,000 rpm for 10 min at 4°C. The supernatant was transferred to a glass tube and evaporated to dryness at 35°C under nitrogen flow using a TurboVap® LV Concentration Workstation (Caliper Lifesciences, Hopkinton, MA, USA). The residue was then reconstituted in 500 μL ethyl acetate and vortexed for 30 s. Samples were centrifuged again, and the resulting supernatant was transferred to a new glass tube and evaporated to dryness at 35°C under nitrogen flow. Finally, samples were reconstituted in 100 μL mobile phase, and the sample was vortex mixed for 1 min. After vortex mixing, the sample was centrifuged at 14,000 rpm for 10 min at 4°C. Finally, 50 μL was injected onto the HPLC.
Blank tissue homogenates were prepared by pooling six portions of tissue from different mice in a glass tube and adding two volumes (v/w) of PBS. The closed tubes were vigorously shaken, manually and by vortex mixing for 1 min, and then further mixed in an ultrasonic bath for 15 min. After centrifugation at 14000×g for 5 min, the remaining solid fraction was discarded.
Method Selectivity
The specificity of the method was assessed by analyzing blank plasma and tissue samples spiked with BA-TPQ to observe the possible endogenous interference from plasma and tissue samples with the analyte.
Linearity
The linearity of the relationship between the detector response and BA-TPQ concentrations was confirmed within the concentration range of 3.91-1955.0 ng/mL for mouse plasma, 19.55-1955.0 ng/mL for mouse spleen, brain and liver homogenates, and 19.55-3910.0 ng/mL for mouse heart, lung and kidney homogenates. Calibration curves of the slope, intercept, and the determination coefficients were calculated by plotting the peak area ratios (y) for BA-TPQ vs the nominal concentrations (x) in standard plasma or tissue by the 1/x2 weighted least-square linear regression.
Precision and accuracy
To evaluate the precision and accuracy of the method, QC samples at three concentration levels (19.55 ng/mL, 195.5 ng/mL and high 1955.0 ng/mL) were analyzed in five replicates on four separate days. Means, standard deviations (SD), and the ratio of the standard deviation to the mean (RSD) were calculated and used to evaluate the precision. The accuracy of the assay was assessed by comparing the calculated mean concentrations to the actual concentrations of serial dilutions. The accuracy was required to be within ±15%, and the intra- and inter-day precisions were not to exceed 15%.
Lower limits of detection and quantification
The lower limit of detection (LLOD) was defined as the peak signal of the compound equal to three times the average noise level. The lower limit of quantification (LLOQ) was defined as the lowest concentration of compound giving a signal-noise ratio of 5:1 with a precision and accuracy of 100% ± 20%.
Extraction recovery and stability
The percent recoveries of BA-TPQ at three QC levels (n = 5) from mouse plasma and six tissues were determined by comparing the mean peak area of the quality controls extracted from mouse plasma and various tissues with those of pure compound prepared in the mobile phase. The stability of BA-TPQ in mouse plasma and the six tissues were evaluated by analyzing replicates (n = 3) of the samples that were exposed to different conditions (time and temperature) at concentrations of 195.5 and 1955 ng/mL. These results were compared with those obtained for freshly prepared samples. Short-term stability was assessed by analyzing samples kept at 37°C for 8 h, which far exceeded the routine preparation time of samples. Long-term stability was determined by assaying samples after storage at −20°C for 24 h. Freeze-thaw stability was investigated after three freeze (−80°C)/thaw (room temperature) cycles for 1, 2, and 4 weeks before analysis. The analytes were considered stable in the biological matrix when 85%-115% of the initial concentration was retained.
Application of the Analytical Method
To demonstrate the utility of the HPLC method, the pharmacokinetics of the compound in mouse plasma, and the compound’s tissue distribution, were determined in male CD-1 mice (4-6 weeks) following intravenous administration of 5 mg/kg of BA-TPQ. The animal study protocol was approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. Plasma and tissue samples were collected at 0 (pre-dose), 5, 15, 30 and 60 min, and 2, 4, 8, and 24 hr after drug administration. Plasma was obtained from the retroorbital plexus of anesthetized mice. Various tissues (liver, heart, lungs, kidneys, spleen, and brain) were collected at necropsy, immediately trimmed of extraneous fat and connective tissue, blotted on filter paper, and weighed. Tissues were stored at −80°C until processing. For BA-TPQ extraction, tissue samples were homogenized in PBS. The samples were then processed as described above, and concentration-time curves were obtained for plasma and each tissue.
Results
Method Validation
Chromatographic separation and UV detection of BA-TPQ
The specificity of the assay was examined using the described chromatographic conditions. Representative chromatograms of blank mouse plasma, control (drug-free) mouse plasma spiked to contain 195.5 ng/mL BA-TPQ (Fig. 2A), a plasma sample obtained from CD-1 mice pre-dose (time 0) and five minutes after intravenous administration of 5 mg/kg (Fig. 2B), a blank heart sample and a heart tissue sample obtained from a CD-1 mouse 5 min after intravenous administration of 5mg/kg (Fig. 2C), and a blank spleen sample and a spleen tissue sample obtained from a CD-1 mouse 5 min after intravenous administration of 5mg/kg appear (Fig. 2D). The chromatograms were free from any endogenous interference at the retention time of the peak of interest (~5.3min) in plasma and tissue homogenates (Fig. 2). The theoretical plate number of the column was 11975 and the asymmetry factor was 1.04.
Figure 2.
Representative chromatograms of BA-TPQ in (A) control (drug-free) mouse plasma (solid line) and mouse plasma spiked to contain 195.5 ng/mL BA-TPQ (dotted line), (B) control (time 0) mouse plasma (solid line) and mouse plasma obtained 5 minutes after intravenous administration of 5 mg/kg BA-TPQ (dotted line), (C) control (drug-free) mouse heart homogenate (solid line) and mouse heart homogenate obtained from a mouse 5 minutes after intravenous administration of 5 mg/kg BA-TPQ (dotted line), (D) control (drug-free) mouse spleen tissue homogenate (solid line) and the homogenate of a mouse spleen tissue sample obtained 5 min after intravenous administration of 5 mg/kg BA-TPQ (dotted line).
Linearity of the calibration curve, lower limit of detection, and lower limit of quantitation
The linear least squares regression equation (1/c2) correlation coefficients of the standard curves for BA-TPQ in plasma and various tissue homogenates were all above 0.9945. All the linearity and range parameters and their related validation data are shown in Table 1.
Table 1.
Calibration data resulting from linear least squares regression analysis for the determination of BA-TPQ in plasma and various tissue samples
| Linear equation |
Slope (±SD) |
Intercept (±SD) |
Determination coefficient (R) |
Linear range (ng/mL) |
LOD (ng/mL) |
LOQ (ng/mL) |
|
|---|---|---|---|---|---|---|---|
| Plasma | Y=1379035.62 × + 10948.50 |
1379035.62 ± 14525.26 |
10948.50 ± 6450.02 |
0.9999 | 3.910–1955 | 2.19 | 3.91 |
| Liver | Y= 414597.29 × −3054.70 |
414597.29 ± 14602.89 |
−3054.70 ± 6528.10 |
0.9977 | 19.55–1955 | 7.82 | 19.55 |
| Brain | Y= 579717.02 × − 12593.05 |
579717.02± 11423.93 |
−12593.05 ± 1074.38 |
0.9945 | 19.55–1955 | 7.82 | 19.55 |
| Spleen | Y= 440809.45 × + 4942.39 |
440809.45 ± 29347.48 |
4942.39 ± 1823.16 |
0.9948 | 19.55–1955 | 7.82 | 19.55 |
| Heart | Y= 489626.21 × − 9316.55 |
489626.21± 11727.36 |
−9316.55 ± 3075.53 |
0.9961 | 19.55–3910 | 7.82 | 19.55 |
| Lung | Y=516549.20 × − 13337.27 |
516549.20 ± 4750.98 |
−13337.27 ± 3433.46 |
0.9960 | 19.55–3910 | 7.82 | 19.55 |
| Kidney | Y= 440809.45 ×− 13151.46 |
440809.45± 29347.4768 |
−13151.46 ± 520.35 |
0.9984 | 19.55–3910 | 7.82 | 19.55 |
The lower limit of detection (LLOD) and lower limit of quantitation (LLOQ) for BA-TPQ in plasma using the optimized conditions were 2.19 ng/mL (LLOD) and 3.91 ng/mL (LLOQ). The coefficient of variation (RSD) was 1.99 %, and the accuracy was 110.78 at the LLOQ (3.19 ng/mL).
The present method afforded a LLOD of 7.82 ng/mL and a LLOQ of 19.55 ng/mL for BA-TPQ in the mouse six tissues, with intra- and inter-day precision with less than 8.65% RSD, and accuracies were between 98.56 and 105.33 at the LLOQ.
Precision and accuracy
The intra- and inter-day precision and accuracy for detection of BA-TPQ in mouse plasma and tissue samples are presented in Table 2. Accuracy was defined as the percentage of the concentration calculated from peak areas compared to the known concentration of the prepared samples. Precision was defined as the variation between replicate samples. The RSD of the intra- and inter-day precision for BA-TPQ was 8.65, 9.27 and 4.80 for the low, moderate and high concentrations, respectively. The accuracy of the quantitative analysis of the compound ranged from 92.40 to 110.78% for intra-day and 93.77 to 99.61% for inter-day analyses. The precision and accuracy values were well within the acceptable range as described by the U.S. FDA (CDER/US FDA, 2001).
Table 2.
Accuracy and precision of the method for detection of BA-TPQ in plasma and tissue samples
| Intra-day assay (n = 5) | Inter-day assay (n = 4) | ||||||
|---|---|---|---|---|---|---|---|
| Nominal concentration (ng/mL) |
Mean measured concentration ± S.D. (ng/mL) |
(RSD) (%) |
Accuracy (%) |
Mean measured concentration ± S.D. (ng/mL) |
(RSD) (%) |
Accuracy (%) |
|
| 19.55 | 21.51 ± 1.955 | 1.99 | 110.78 | 19.16 ± 1.955 | 1.94 | 98.68 | |
| Plasma | 195.5 | 180.6 ± 8.993 | 4.19 | 92.40 | 183.38 ± 8.993 | 3.98 | 93.77 |
| 1955 | 2099 ± 40.27 | 1.84 | 107.37 | 1947 ± 26.59 | 1.28 | 99.61 | |
| Liver | 19.55 | 20.01 ±1.319 | 6.59 | 102.37 | 19.16 ± 0.5642 | 2.93 | 98.56 |
| 195.5 | 195.3 ± 9.475 | 4.87 | 99.81 | 203.3 ± 5.280 | 2.69 | 100.47 | |
| 1955 | 1952 ± 34.85 | 1.79 | 99.83 | 1985 ± 27.15 | 1.37 | 101.55 | |
| Brain | 19.55 | 19.75 ± 1.708 | 8.65 | 101.04 | 19.94 ± 0.7828 | 3.93 | 101.90 |
| 195.5 | 204.1 ± 14.77 | 7.23 | 104.40 | 202.4 ± 10.26 | 5.05 | 103.81 | |
| 1955 | 1877 ± 78.11 | 4.16 | 96.01 | 1968 ± 59.68 | 3.03 | 100.68 | |
| Spleen | 19.55 | 19.47 ± 1.536 | 7.88 | 99.61 | 19.94 ± 0.782 | 3.92 | 102.00 |
| 195.5 | 194.9 ± 15.19 | 7.80 | 99.67 | 194.7 ± 10.11 | 5.19 | 99.60 | |
| 1955 | 1850 ± 88.84 | 4.80 | 94.63 | 1906 ± 43.72 | 2.29 | 97.51a | |
| Heart | 19.55 | 19.92 ± 0.7827 | 3.93 | 101.89 | 20.33 ± 0.9840 | 4.87 | 103.33 |
| 977.5 | 948.8 ± 19.08 | 2.01 | 97.07 | 983.0 ± 51.19 | 5.20 | 100.56 | |
| 3910 | 3713 ± 75.30 | 2.03 | 94.97 | 3807 ± 59.42 | 1.56 | 97.37 | |
| Lung | 19.55 | 20.83 ± 1.012 | 3.01 | 102.56 | 20.73 ± 0.002 | 3.95 | 105.33 |
| 977.5 | 1017 ± 11.57 | 4.81 | 99.83 | 1007 ± 47.47 | 4.71 | 103.01 | |
| 3910 | 3828 ± 60.46 | 1.52 | 95.21 | 3859 ± 45.16 | 1.17 | 98.70a | |
| Kidney | 19.55 | 20.05 ± 0.6049 | 4.86 | 106.56 | 19.16 ± 220.6 | 2.93 | 98.56 |
| 977.5 | 975.8 ± 46.96 | 1.14 | 104.08 | 871.9 ± 80.83 | 9.27 | 89.18 | |
| 3910 | 3722 ± 56.59 | 1.58 | 97.92 | 3775 ± 41.33 | 1.09 | 96.56 | |
Extraction recovery
Extraction recoveries of the compound were determined at low (19.55 ng/mL), moderate (195.5 ng/mL), and high (1955 ng/mL) concentrations (in triplicate) in plasma, spleen, liver and brain, and were above 71.4 ± 3.99%, 75.1 ± 4.48%, and 86.3 ± 2.58%. The recovery determined at low (19.55 ng/mL), moderate (975.0 ng/mL), and high (3910 ng/mL) concentrations (in triplicate) in heart, lung and kidney yielded recoveries greater than 73.4 ± 2.75%, 78.1 ± 1.72%, and 81.5 ± 1.17%, respectively. The recovery percentages for the compound extracted from mouse plasma and various tissues at the 3 concentrations are shown in Table 3.
Table 3.
Recovery of BA-TPQ from plasma and tissue homogenates
| Concentration (ng/mL) |
Recovery (%) ± S.D. (%) (n = 5) |
R.S.D. (%) | |
|---|---|---|---|
| 19.55 | 72.1 ± 0.71 | 0.99 | |
| Plasma | 195.5 | 87.7 ± 1.36 | 1.55 |
| 1955 | 89.8 ± 2.84 | 2.91 | |
| Liver | 19.55 | 71.4 ± 3.99 | 5.58 |
| 195.5 | 75.1 ± 4.48 | 5.96 | |
| 1955 | 86.3 ± 2.58 | 2.99 | |
| Brain | 19.55 | 73.1 ± 3.21 | 4.39 |
| 195.5 | 81.9 ± 0.96 | 1.17 | |
| 1955 | 87.7 ± 0.58 | 0.66 | |
| Spleen | 19.55 | 74.0 ± 2.34 | 3.16 |
| 195.5 | 85.6 ± 2.06 | 2.41 | |
| 1955 | 87.6 ± 2.00 | 2.28 | |
| Heart | 19.55 | 75.1 ± 1.32 | 1.75 |
| 977.5 | 78.1 ± 1.72 | 2.20 | |
| 3910 | 81.5 ± 1.17 | 0.21 | |
| Lung | 19.55 | 74.3 ± 4.18 | 5.62 |
| 977.5 | 83.6 ± 0.74 | 0.32 | |
| 3910 | 88.1 ± 0.27 | 0.84 | |
| Kidney | 19.55 | 73.4 ± 2.75 | 3.74 |
| 977.5 | 78.7 ± 1.24 | 1.58 | |
| 3910 | 83.1 ± 1.54 | 1.86 |
The results of stability experiments showed that BA-TPQ was stable for 8 h after preparation at 37°C, for 24 h at −20°C and following three freeze/thaw cycles (−80 to 22°C), as the RE values were within ±15% for both the low and high concentrations. Taken together, the stability data indicate that BA-TPQ in plasma and tissue samples can be stored and prepared under routine laboratory conditions without special precautions.
Application of the Method to an Initial Pharmacokinetic Study
The concentrations of BA-TPQ in mouse plasma and tissues were determined after administration of a single intravenous (5 mg/kg) dose to CD-1 mice. Following administration, the concentration of the compound in plasma decreased rapidly, from 230 ng/mL at 5 min to 20 ng/mL at 1 hr, to undetectable levels at 4 hr after administration. The highest overall concentrations in the tissues examined were in the following order: Lungs> kidneys> heart> spleen> plasma≈liver> brain. BA-TPQ was still present in the lungs, kidneys, heart, and liver at 24 hr after administration, albeit at low concentrations. The distribution of the compound in plasma and various tissues is presented in Figure 3. We have also observed that the parent compound was minimally excreted in urine and feces (data not shown), suggesting that it is highly metabolized.
Figure 3.
Distribution of BA-TPQ in (A) plasma and (B) various tissues following intravenous administration of 5 mg/kg of the compound to CD-1 mice. For the plasma, concentrations at 4 hours and later were below the limit of detection, so they have not been plotted.
Discussion
During preclinical development of a new candidate drug, a diverse number of studies related to its chemistry, formulation, pharmacology, and toxicity are required (Gad, 2008). The establishment and validation of an analytical method is paramount for these studies. Since BA-TPQ is a novel compound, there are no existing methods for its detection and quantification in biological specimens. Thus, a sensitive and reliable method for determining the presence and concentration of the compound in biological matrices is essential for further studies of this agent. High performance liquid chromatography (HPLC) is a widely-used technique for the analysis of compounds, and has evolved into a primary technique for the evaluation of nonvolatile small molecules (Bansal et al., 2007; Lee, 2003). As BA-TPQ is a nonvolatile compound, and since HPLC methods are often used for the detection and quantitation of quinolone-based compounds (Kudo et al., 2001; Wolfender, 2009), we decided to develop a HPLC method, with an emphasis on its practical applications for future pre-clinical and clinical studies.
Since we used UV detection, the selection of an optimal wavelength was critical. Preliminary spectrophotometric scans of the compound revealed a significant absorbance band at 234 nm. However, since both DNA and proteins absorb within this range (220-300 nm), using this wavelength to analyze samples extracted from biological matrices was not feasible. Therefore, the second most prominent absorbance band (348 nm) was selected to monitor the compound. For any effective HPLC method, a sample preparation strategy that is rapid and does not compromise its efficiency and selectivity is desirable. In the current study, a two-step protein precipitation/extraction using acetonitrile followed by liquid-liquid extraction with ethyl acetate was selected as the pretreatment strategy. While this protocol increases manipulation of the samples, and results in significant loss of analyte, applying this sample preparation procedure led to better purification of the compound, improving the selectivity of the chromatographic technique, and extending the life of the analytical column.
To demonstrate the utility of the method, we wanted to analyze plasma and tissue samples obtained from animals. Mice are the most commonly used species for preclinical studies of compound safety, efficacy and distribution (Sinha et al., 2008; Zhou et al., 2002). In this study, we demonstrated that BA-TPQ has a relatively short half life in the plasma, and extensive tissue distribution following intravenous administration. Our previous in vitro studies of the compound demonstrated that it is effective against cancer cells at the high nanomolar level (Wang et al., 2009). The current pharmacokinetic study demonstrates that administration of the compound at an intravenous dose of 5 mg/kg yielded concentrations at or above the IC50 (for breast cancer cells) for a duration of only 0.5-1 hr in the plasma, but for a longer duration of time in the major tissues examined. We have previously demonstrated that 5 and 10 mg/kg intraperitoneal doses of BA-TPQ can inhibit tumor growth in mice (Wang et al., 2009). Since these doses may have resulted in short-term or low-dose exposure of the tumor to the agent, it is possible that optimization of the dose, dosing schedule, or a change in the formulation could lead to improved anti-tumor activity. Optimization of the administration of the compound may also decrease its toxicity to other host tissues. Future studies are needed to make these improvements; however, we now have a useful analytical method that will facilitate our future studies geared toward optimizing the administration, and investigating the stability, protein binding, metabolism, and detailed pharmacokinetics of the compound.
Conclusions
In summary, a sensitive and reliable HPLC method for the determination of BA-TPQ in mouse plasma was developed and validated according to U.S. Food and Drug Administration guidelines (CDER/US FDA 2001). The extraction and quantitation provide accurate and reproducible results, with acceptable intra- and inter-day precision. To evaluate the applicability of the analytical method in in vivo studies, we measured the concentrations of BA-TPQ in the plasma and tissues of CD-1 mice following intravenous administration. We believe that the method can be employed in additional investigative studies of BA-TPQ as it undergoes further preclinical development.
Acknowledgements
RZ was supported by NIH grants R01 CA112029 and R01 CA121211, and a grant (BCTR070731) from Susan G. Komen for the Cure.
References
- Shinkre BA, Raisch KP, Fan L, Velu SE. Analogs of the marine alkaloid makaluvamines: synthesis, topoisomerase II inhibition, and anticancer activity. Bioorganic & Medicinal Chemistry Letters. 2007;17:2890–2893. doi: 10.1016/j.bmcl.2007.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkre BA, Raisch KP, Fan L, Velu SE. Synthesis and antiproliferative activity of benzyl and phenethyl analogs of makaluvamines. Bioorganic & Medicinal Chemistry. 2008;16:2541–2549. doi: 10.1016/j.bmc.2007.11.051. [DOI] [PubMed] [Google Scholar]
- Nadkarni DH, Wang F, Wang W, Rayburn ER, Ezell SJ, Murugesan S, Velu SE, Zhang R. Synthesis and in vitro anti-lung cancer activity of novel 1, 3, 4, 8-tetrahydropyrrolo [4, 3, 2-de]quinolin-8(1H)-one alkaloid analogs. Medicinal Chemistry. 2009;5:227–236. doi: 10.2174/157340609788185873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Rayburn ER, Velu SE, Chen D, Nadkarni DH, Murugesan S, Chen D, Zhang R. A novel synthetic iminoquinone, BA-TPQ, as an anti-breast cancer agent: in vitro and in vivo activity and mechanisms of action. Breast Cancer Research & Treatment. 2009 Nov 21; doi: 10.1007/s10549-009-0638-0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas NR. Applicability of bioanalysis of multiple analytes in drug discovery and development: review of select case studies including assay development considerations. Biomedical Chromatography. 2006;20:383–414. doi: 10.1002/bmc.594. [DOI] [PubMed] [Google Scholar]
- CDER/US FDA [accessed December 15, 2009];Guidance for Industry: Bioanalytical Methods Validation. 2001 available at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guida nces/UCM070107.pdf.
- Gad SC. Preclinical Development Handbook: ADME and Biopharmaceutical Properties. John Wiley & Sons; New Jersey: 2008. [Google Scholar]
- Bansal S, DeStefano A. Key elements of bioanalytical method validation for small molecules. AAPS Journal. 2007;9:E109–114. doi: 10.1208/aapsj0901011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS. LC/MS Applications in Drug Development. John Wiley & Sons; New Jersey: 2003. [Google Scholar]
- Kudo M, Ohkubo T, Sugawara K. Development of measurement of new quinolones in body fluids by HPLC using column switching and their application to drug interaction. Yakugaku Zasshi. 2001;121:319–326. doi: 10.1248/yakushi.121.319. [DOI] [PubMed] [Google Scholar]
- Wolfender JL. HPLC in natural product analysis: the detection issue. Planta Medica. 2009;75:719–734. doi: 10.1055/s-0028-1088393. [DOI] [PubMed] [Google Scholar]
- Sinha VK, De Buck SS, Fenu LA, Smit JW, Nijsen M, Gilissen RA, Van Peer A, Lavrijsen K, Mackie CE. Predicting oral clearance in humans: how close can we get with allometry? Clinical Pharmacokinetics. 2008;47:35–45. doi: 10.2165/00003088-200847010-00004. [DOI] [PubMed] [Google Scholar]
- Zhou S, Kestell P, Paxton JW. Predicting pharmacokinetics and drug interactions in patients from in vitro and in vivo models: the experience with 5,6-dimethylxanthenone-4-acetic acid (DMXAA), an anti-cancer drug eliminated mainly by conjugation. Drug Metabolism Reviews. 2002;34:751–790. doi: 10.1081/dmr-120015693. [DOI] [PubMed] [Google Scholar]