Abstract
Objectives
To determine whether cardiac magnetic resonance (CMR) in vivo T1-mapping can measure myocardial area at risk (AAR) compared with microspheres or T2-mapping CMR.
Background
If T2-weighted CMR is abnormal in the AAR due to edema related to myocardial ischemia, then T1-weighted CMR should also be able to detect and accurately quantify AAR.
Methods
Dogs (n=9) underwent a 2 hour coronary occlusion followed by 4 hours of reperfusion. CMR of the left ventricle was performed for mapping of T1 and T2 prior to any contrast administration. AAR was defined as regions which had a T1 or T2 value (ms) greater than 2SD from remote, and regions with microsphere blood flow (ml/min/g) during occlusion less than 2SD from remote. Infarct size was determined by triphenyltetrazolium chloride staining.
Results
The relaxation parameters T1 and T2 were increased in the AAR compared to remote myocardium (T1: 1133±55 vs. 915±33ms, T2: 71±6 vs. 49±3 ms; mean±SD). On a slice-by-slice basis (n=78 slices), AAR by T1- and T2-mapping correlated (R2=0.95, p<0.001) with good agreement (0.4±16.6 % of slice, mean±2SD). On a whole-heart analysis, T1 measurements of left ventricular mass, AAR and myocardial salvage correlated to microsphere measures (R2=0.94) with good agreement (−1.4±11.2 g of myocardium; mean±2SD). Corresponding T2 measurements of left ventricular mass, AAR, and salvage correlated to microsphere analysis (R2=0.96, agreement 1.6±9.2 g of myocardium; mean±2SD). Median infarct size was 30% of the AAR (range 12–52).
Conclusions
For determining area at risk after acute myocardial infarction, non-contrast T1-mapping and T2-mapping sequences yield similar quantitative results, and both agree well with microspheres. The relaxation properties T1 and T2 both change in a way that is consistent with the presence of myocardial edema following myocardial ischemia/reperfusion.
Keywords: magnetic resonance imaging, ischemia, myocardium at risk, myocardial infarction, microspheres
Introduction
Myocardial area at risk (AAR) is defined as myocardium which becomes ischemic upon coronary occlusion.(1) Accurate quantification of AAR is important in studies aimed at determining the efficacy of infarct size reduction therapies. Measurement of AAR and infarct size allows determination of myocardial salvage -- a measure of therapeutic efficacy.(1)
Edema is a consequence of even short periods of myocardial ischemia.(2) Myocardial T2 relaxation properties determined by magnetic resonance imaging (MRI) are sensitive to changes in tissue water content.(3) In vivo MRI can accurately delineate the AAR using a number of imaging techniques which utilize T2-weighting.(4–9) Furthermore, ischemic injury also leads to an increase in myocardial T1, which is also related to increased tissue water content.(3,10,11) Similarly, non-contrast T1-weighted MRI has been shown to be capable of quantifying the AAR ex vivo(12) and detecting regions of acute myocardial infarction in vivo.(13) Notably, that in vivo study(13) did not try to differentiate between infarction and AAR. Recently, clinically relevant methods have been developed that can produce quantitative maps of both T1(14) and T2(15) with high signal to noise. The resulting images and parametric maps translate signal intensities into absolute T1 or T2 relaxation times, respectively. These methods have potential to improve objectivity of T1 and T2 imaging.
The aim of this study was to determine the accuracy for quantifying AAR with clinically available T1- and T2-mapping sequences when compared to microsphere blood flow analysis as an independent reference standard. We hypothesized that if T2-weighted MRI is abnormal in the AAR due to edema related to myocardial ischemia, then T1-weighted MRI should also be able to detect and accurately quantify AAR.
Methods
Animal Preparation
Nine dogs weighing 10–15 kg were studied after institutional approval. Anesthesia was induced by subcutaneous acepromazine (0.2 mg/kg), followed by intravenous thiopental sodium (15 mg/kg). Anesthesia was sustained by inhaled isoflurane (0.5%–2.0%). The animals were intubated and surgical preparation included venous catheters, arterial lines, a left atrial catheter, and a snare around the left anterior descending coronary artery, typically positioned distal to the first diagonal branch. Coronary occlusion was maintained for two hours. Fluorescent microspheres (IMT Stason, Irvine, California, USA) were injected into the left atrium during simultaneous withdrawal of a reference femoral artery blood sample. Reperfusion was maintained for four hours prior to commencing imaging. Animals were euthanized with potassium chloride following heparin administration.
MRI Imaging
The entire left ventricle was imaged in contiguous short-axis slices at 1.5T (Magnetom Avanto, Siemens Healthcare Sector, Erlangen, Germany) with an eight-channel coil prior to administration of any MRI contrast agents. Quantitative T1-mapping was performed with a Modified Look-Locker Inversion-recovery (MOLLI) sequence(14) using the following typical imaging parameters: TR/TE 220/1.14 ms, flip angle 35 degrees, field of view 270×185 mm2, matrix 192×132 pixels, slice thickness 6 mm, parallel imaging factor 2, acquisition in late diastole on every other heart beat, minimal inversion time 120 ms, increment 80 ms, voxel size 1.5×1.4×6.0 mm, temporal resolution 221 ms. The T1-mapping scheme included 3 acquisitions after the first inversion pulse, followed by a 3 heart beat pause, a second inversion followed by 3 acquisitions, a third 3 heartbeat pause, and a third inversion for the last 5 acquisitions.
Quantitative T2-mapping was performed using a T2-prepared steady-state free precession (SSFP) sequence(15) and the following imaging parameters: TR/TE 240/1.19 ms, flip angle 70 degrees, field of view 270×185 mm2, matrix 192×132 pixels, slice thickness 6 mm, parallel imaging factor 2, acquisition in late diastole on every fourth heart beat, T2 preparations; 0 ms, 24 ms, 55 ms, 90 ms, size 1.9×1.4×6.0 mm, temporal resolution 239 ms.
Image Analysis
T1 pixel maps were generated using MRmap (version 1.0, http://mrmap.sourceforege.net).(16) T2 pixel maps were automatically generated on the MR scanner. T1 map and T2 map images were analyzed using the software Segment (version 1.8 R1289, freely available for research use http://segment.heiberg.se).(17) The epicardial and endocardial borders of the left ventricle were manually delineated. AAR was semi-automatically identified as left ventricular myocardium with pixel values (T1 or T2, respectively) >2 SD from remote myocardium. Spurious non-contiguous pixels comprising less than 10% of the left ventricular myocardium were automatically excluded.(18) Tissue weight in grams for imaging results was calculated as the volume of myocardium multiplied by the density of myocardium (1.05g/cm3). One observer performed all image analysis twice for assessment of intraobserver variability, and an additional blinded observer performed image analysis for assessment of interobserver variability. Overall image quality was excellent or good in all animals.
Histopathology Preparation and Quantification
After explantation, hearts were set in 2% agarose gel and sliced in the short-axis plane using a commercially available meat slicer. Slices were stained with 1% triphenyltetrazolium chloride (TTC) and then photographed. In the TTC stained slices, the borders of the epicardium, endocardium and non-stained regions were delineated manually to determine infarct size.(19)
Microsphere Analysis
Each slice was photographed, sectioned into 16 transmural radial sectors, and sent for quantification of blood flow (IMT Stason, Irvine, California, USA). T1 and T2 maps, and microspheres were visually matched by comparing photographs of ex vivo slices with each in vivo image. This matching was performed blinded to the results of microsphere analysis. Microsphere blood flow analysis was performed blinded to the results of T1 and T2 data. Myocardial sectors with blood flow 2 SD below blood flow in remote myocardium was defined as AAR. Basal and apical slices were not used to determine the remote myocardial blood flow due to greater heterogeneity of flow measurements. The summed weight of sectors with blood flow below the 2SD threshold was divided by the weight of all sectors in the slice to quantify the percent of slice comprised of AAR. Salvaged myocardium was determined as AAR minus infarct size by TTC. The use of the 2SD threshold for both T1- and T2-maps and microspheres was postulated a priori, and was further determined to be reasonable upon visual inspection of the quality of the images and the microsphere data.
Statistical Analysis
Statistical analysis was performed using SPSS version 17 (IBM, Somers, New York, USA). Linear regression analysis was performed using Pearson’s coefficient and expressed as its square (R2). Data are presented as mean ± SD unless specified. Bland Altman analysis and agreement are average difference ± 2SD. Comparison of mean differences was performed with ANOVA or Wilcoxon’s test as appropriate. The F test was used to test the significance of differences in variability. Intra- and interobserver variability are presented as mean difference between observations ± SD. Statistical significance was defined as p<0.05.
Results
Determination of AAR and infarct size
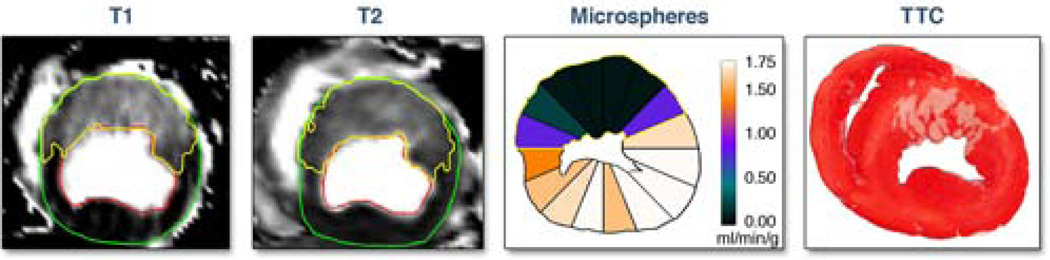
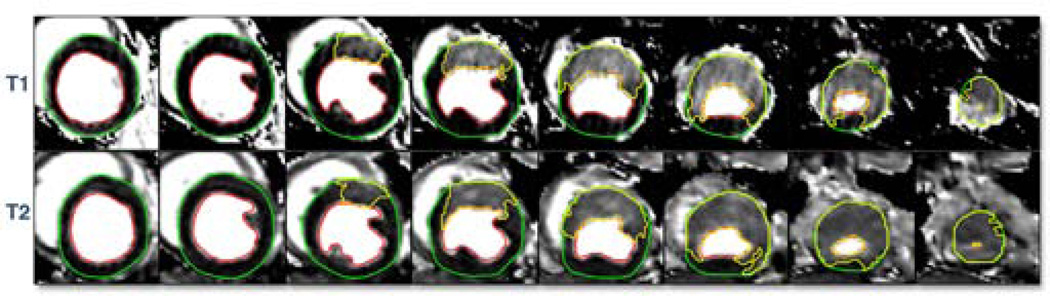
Figure 1 illustrates the image and tissue analysis methodology. AAR was quantified on T1-maps and T2-maps. Microspheres provided the independent measure of AAR. Infarct size was by TTC. Figure 2 illustrates the regions of interest used to quantify LV mass and AAR on a whole heart by T1- and T2-maps.
Figure 1. Myocardium at risk (AAR) by T1-mapping, T2-mapping and microspheres compared to infarct size.
Images are shown for one representative short-axis slice. In the images of the T1 map and T2 map, the epicardium and endocardium are delineated and the AAR is identified in yellow. Blood flow from microspheres injected during occlusion are displayed on the contours of the ex vivo slice, for 16 radial sectors which are color coded according to the color scale for blood flow quantification in ml/min/g. Sectors with microsphere blood flow 2SD below remote myocardium are defined as AAR appear in the blue to black range of the color scale for this experiment. The triphenyl tetrazolium chloride (TTC) image shows non-infarcted myocardium stained red and the region of myocardial infarction is white. Note that the area identified as AAR by T1-mapping and T2-mapping corresponds closely with the results of the microsphere blood flow analysis, suggesting that both imaging sequences agree with microsphere results. Also note how the area of infarction is much smaller than the AAR, indicating substantial salvage.
Figure 2. Slice-by-slice comparison of T1 and T2 maps.
Short-axis images are shown from the base (left) to the apex (right) for quantification of myocardial area at risk (yellow region of interest). Note the excellent spatial correspondence between the T1 and T2 methods for area at risk.
Assessment of the infarct model
The blood flow in the core of the occluded area was assessed by microspheres in order to ensure the validity of the infarct model. Blood flow in this area was 6.5±5.3 % of the blood flow in normally perfused remote myocardium. AAR by microspheres was larger than infarct size by TTC in all cases (AAR: 35±5 % of left ventricular myocardium (LVM), infarct size 12±6 % of LVM). This yielded a median infarct size of 30% of the AAR (range 12–52 % AAR), indicating substantial myocardial salvage in this canine model of 2 hours coronary occlusion under anesthesia.
Agreement for AAR between T1- and T2-mapping
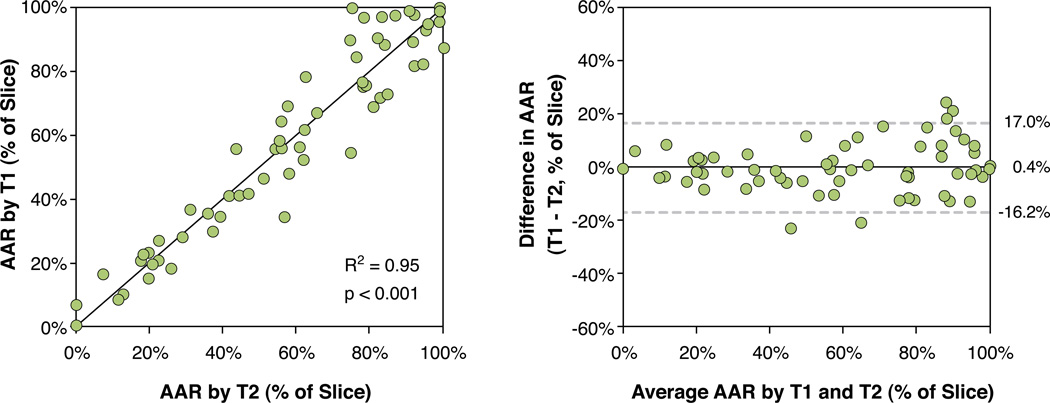
Figure 2 illustrates the slice-by-slice agreement for AAR by T1- and T2-mapping for the whole left ventricle. Figure 3 illustrates this quantitatively, AAR by T1- and T2–mapping correlated well (R2= 0.95, p<0.001). In Bland Altman analysis (Figure 3), the measurements of the size the AAR from T1- and T2-maps agreed well with each other (bias 0.4±16.6 % of slice, ±2SD).
Figure 3. Slice-by-slice comparison of myocardium at risk by T1-mapping and T2- mapping.
The figure shows a scatter plot (left) and Bland-Altman analysis (right) of myocardial area at risk (AAR) by T1-mapping versus T2-mapping. Data represent 78 slices from 9 dogs. The solid line denotes the line of identity in the scatter plot and the bias in te Bland Altman plot. Note the excellent agreement between the methods.
Accuracy of LV mass, AAR, and Myocardial Salvage by T1, T2, and microspheres
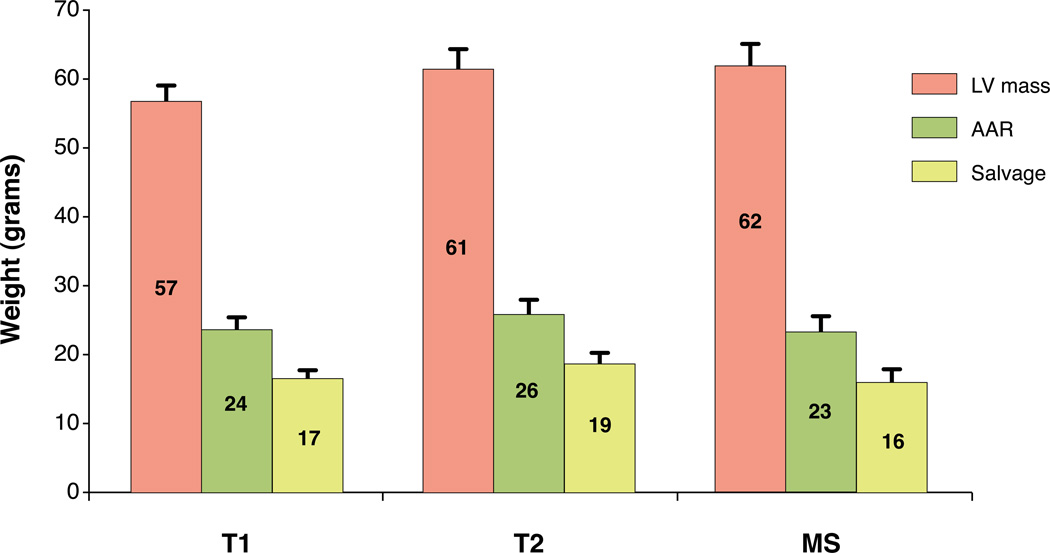
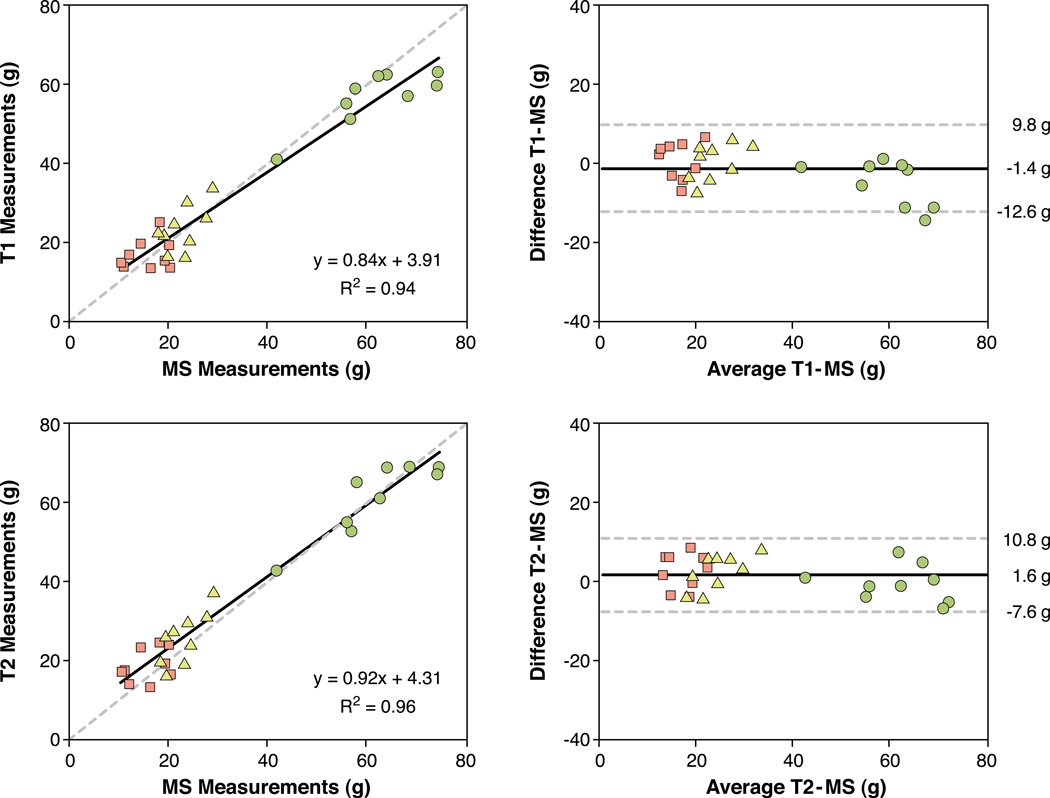
Whole heart volumetric determinations of LV mass, AAR, and myocardial salvage were determined using each of the three methods (Figure 4). The absolute weight in grams of the total left ventricular myocardium ± standard error of the mean (57±2g, 61±3g, 62±3g), AAR (24±2g, 26±2g, 23±1g) and salvaged myocardium (17±1g, 19±1g, 16±1g) was quantified by T1- mapping, T2-mapping and microspheres, respectively. In terms of the average mass of each zone, there were no statistically significant differences in the size of LV mass by ANOVA (p=0.13). Similarly, none of the measures of AAR (p=0.58) or the amount of myocardial salvage (p=0.50) differed significantly by ANOVA. The patterns of AAR and amount of myocardial salvage were similar for the three sets of measurements in absolute grams (Figure 4).
Figure 4. Comparison of absolute mass of left ventricular myocardium (LV mass), area at risk (AAR), and salvaged myocardium by T1- and T2-mapping and microspheres (MS).
Error bars denote SEM. There were no significant differences between the imaging and microsphere methodologies for the respective measures of LV mass, AAR, or salvage by analysis of variance.
Intraobserver variability for AAR by T1- and T2-mapping were 0.4±1.3g (2.1±1.8% of LV) and 0.6±2.6g (0.6±3.8% of LV), respectively. Interobserver variability for AAR by T1- and T2-mapping were 1.7±3.9g (1.4±4.6% of LV) and 1.4±4.4g (0.3±5.4% of LV), respectively.
Volumetric T1 measurements of LV mass, AAR and myocardial salvage correlated to microsphere measures (Figure 5, R2=0.94). The T1 measurements showed good agreement in Bland Altman analysis and tended to under estimate total LV mass marginally (bias −1.4±11.2 g of myocardium, ±2 SD).
Figure 5. Correlation and Bland Altman analysis of whole heart measures of left ventricular mass (circles), area at risk (AAR, triangles), and myocardial salvage (squares) by T1- and T2-mapping compared to AAR by microspheres.
AAR by T1-mapping and T2- mapping are compared to AAR by microspheres expressed in grams (g). The mean difference between imaging methods and microspheres was 1.4 g for the T1 measurements and 1.6 g for the T2 measurements. The dashed lines denote the line of identify in the scatter plots and the mean±2SD in the Bland-Altman plots.
Volumetric T2 measurements of LV mass, AAR, and myocardial salvage also correlated with the microsphere measurements (Figure 5, R2=0.96). The linear regression was close to the line of identity. Bland Altman analysis confirmed the good agreement between T2 and microspheres on a volumetric or mass basis.
T1 and T2 Changes Directionally Consistent with Edema
Data on T1 and T2 values in remote and AAR for all animals is presented in Table 1. Both T1 and T2 were greater in AAR compared to remote.
Table 1.
T1 and T2 values for AAR and remote myocardium in all animals.
| Animal | T1 AAR (ms) |
T1 remote (ms) |
T2 AAR (ms) |
T2 remote (ms) |
|---|---|---|---|---|
| 1 | 1129 | 914 | 62 | 46 |
| 2 | 1186 | 961 | 81 | 47 |
| 3 | 1132 | 899 | 69 | 48 |
| 4 | 1030 | 857 | 68 | 51 |
| 5 | 1162 | 924 | 72 | 48 |
| 6 | 1079 | 891 | 66 | 46 |
| 7 | 1132 | 910 | 68 | 51 |
| 8 | 1231 | 903 | 77 | 47 |
| 9 | 1116 | 971 | 76 | 56 |
| mean | 1133 | 915 | 71 | 49 |
| SD | 55 | 33 | 6 | 3 |
AAR denotes area at risk.
Discussion
This is the first study to show that in vivo non-contrast T1-mapping by MR imaging can accurately quantify AAR versus an independent microsphere reference standard. In terms of determining area at risk, the T1-mapping method was essentially equivalent to the T2-mapping method. The study confirms our hypothesis that T1 and T2 MRI relaxation properties both change sufficiently to measure area at risk. Furthermore, the changes in T1 and T2 are consistent with myocardial edema as a common pathophysiological mechanism leading to the CMR image appearances. This study takes an important step beyond prior work by including whole heart volumetric measurements of area at risk by imaging and microspheres. As is often the case with volumetric measurements, the variability of the measurements are smaller compared with prior limited slice-by-slice measurements.(6) Finally, the T1- and T2-mapping sequences were developed as works-in-progress for clinical scanning and thus should be available for quantitative imaging of patients soon.
T2 for quantifying AAR
The utility of T2-weighted cardiovascular MR imaging in acute cardiac disease has recently been extensively reviewed.(20) Several investigators have experimentally determined that T2 increases in proportion to myocardial edema measured as tissue water content.(3,21,22) However, the use of T2-weighted MRI for quantifying the AAR was experimentally determined using a T2-weighted double inversion recovery fast spin echo (also called turbo spin echo, TSE) sequence compared to microsphere blood flow in the dog for both reperfused(6) and non-reperfused infarcts.(5) Subsequently, the T2-weighted imaging methodology has been improved upon with the introduction of T2-prepared steady state free precession (SSFP)(8), and a T2-prepared hybrid TSE-SSFP sequence.(4) Validation of T2- weighted MRI for quantifying AAR in patients has been performed by comparison with myocardial perfusion SPECT using either T2-weighted short tau inversion recovery (STIR),(7) post-gadolinium contrast cine SSFP,(9) or T2-prepared SSFP compared to angiographic risk score.(23) For purposes of quantitative analysis, image quality in T2-weighted sequences can prove challenging. The current study used a T2-prepared SSFP-based T2-mapping sequence,(15) and provides the experimental validation that T2-mapping indeed can accurately quantify the AAR. Furthermore, the T2 values for acute ischemic injury and remote myocardium found in the current study were similar to those reported in humans and pigs using similar methodology.(15,24)
T1 for quantifying AAR
T1-weighted MRI should also be able to detect myocardial edema associated with acute myocardial occlusion and reperfusion. Notably, previous experimental studies have shown that T1 values also increase with increasing myocardial water content.(3,11) Furthermore, prolonged T1 values have been experimentally demonstrated in ischemic myocardium in the dog,(10) and in patients with acute myocardial infarction.(13) However, non-contrast T1-weighted imaging has not previously been used to quantify AAR in vivo, and our current study provides experimental validation that AAR by T1- and T2-mapping agree with microspheres as an independent reference standard.
Agreement between T1 and T2
The current study shows excellent quantitative agreement between the T1- and T2-mapping for determining AAR. Previous studies comparing the correlation between myocardial water content and T1 and T2, respectively, have shown results suggesting that T2 has a higher linear correlation,(3) indicating that T2 may be the preferred approach for quantifying AAR. However, those results were performed in ex vivo myocardium and using an early generation 0.35T MRI scanner which may have limited signal to noise compared to the current state-of-the-art methods. Importantly, our findings were achieved in vivo using clinical-grade sequences which yield high signal-to-noise image maps.
There are factors that might influence whether one selects T1 or T2 to image AAR. Microvascular obstruction or intramyocardial hemorrhage were absent in our mode, but are frequently detectable on T2 weighted images.(25) Although this has not yet been studied, these pathophysiological states would be expected to influence pre-contrast T1 to a lesser extent than T2. Quantitative image analysis of T2-maps in such cases would require including the lower T2 regions in the core into total AAR. However, in a setting where it may be of value to assess the myocardial extracellular volume fraction,(26) this would require acquiring T1-maps both before and after contrast. It appears that while both mapping techniques provide similar information with regards to AAR, and could be used interchangeably for this purpose, they both provide additional and unique complementary pathophysiologically relevant information.
The infarct model
The current study was performed in a model where infarct size was approximately 30% of AAR and predominantly subendocardial. Acute myocardial infarction changes both T1 and T2,(3,11,21) and the T2 changes represent an area of edema which is larger than the acute infarction and corresponds quantitatively to the AAR.(6) Thus, it is important that experimental model produces infarctions that do not fully encompass the AAR. Our model fulfills this criterion, adding confidence to results indicating that the T1- and T2-mapping methodologies detect changes in the AAR beyond those which are induced by infarction.
Limitations
Our study only represents changes present at one time point about four hours after reperfusion. Future studies are needed to determine the validity of the quantitative result beyond that time point. Despite this, the time course of myocardial edema between 1–7 days post-infarction has been shown to be suitable for detection by CMR.(7) The current results were obtained in mechanically ventilated dogs, and translation to human patients may be hampered by challenges related to optimal breath holding which may affect the image quality in the T1 or T2 maps. Furthermore, this study did not present segmented data on the mean and SD of T1 and T2 in healthy non-infarcted dogs, which might be of benefit to determine the robustness of T1 and T2 in truly normal myocardium, and this is a limitation.
Conclusions
Non-contrast T1-mapping and T2-mapping using clinical-grade sequences show excellent agreement with each other and microsphere blood flow for quantification of AAR following acute myocardial infarction. The relaxation properties T1 and T2 both change in a way which is consistent with the myocardial edema which occurs following myocardial ischemia/reperfusion
Acknowledgment
The authors thank Joni Taylor and Katherine Lucas for expert animal care. This work was funded by the Intramural Research Program of the National Heart, Lung, and Blood Institute, National Institutes of Health, USA (1 Z01 HL004607-08 CE).
Abbreviations
- AAR
area at risk
- CMR
cardiac magnetic resonance
- MRI
magnetic resonance imaging
- MS
microspheres
- SPECT
single photon emission computed tomography
- SSFP
steady-state free precession
- STIR
short tau inversion recovery
- T
Tesla
- T1
magnetic resonance time constant for longitudinal relaxation
- T2
magnetic resonance time constant for transverse relaxation
- TE
echo time
- TR
repetition time
- TSE
turbo spin echo (also known as fast spin echo)
- TTC
triphenyltetrazolium chloride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Arai is a principal investigator on a US government Cooperative Research and Development Agreement (CRADA) with Siemens Medical Solutions (HL-CR-05-004).
References
- 1.Lowe JE, Reimer KA, Jennings RB. Experimental infarct size as a function of the amount of myocardium at risk. Am J Pathol. 1978;90:363–379. [PMC free article] [PubMed] [Google Scholar]
- 2.Basuk WL, Reimer KA, Jennings RB. Effect of repetitive brief episodes of ischemia on cell volume, electrolytes and ultrastructure. Journal of the American College of Cardiology. 1986;8:33A–41A. doi: 10.1016/s0735-1097(86)80026-2. [DOI] [PubMed] [Google Scholar]
- 3.Higgins CB, Herfkens R, Lipton MJ, et al. Nuclear magnetic resonance imaging of acute myocardial infarction in dogs: alterations in magnetic relaxation times. Am J Cardiol. 1983;52:184–188. doi: 10.1016/0002-9149(83)90093-0. [DOI] [PubMed] [Google Scholar]
- 4.Aletras AH, Kellman P, Derbyshire JA, Arai AE. ACUT2E TSE-SSFP: a hybrid method for T2-weighted imaging of edema in the heart. Magn Reson Med. 2008;59:229–235. doi: 10.1002/mrm.21490. [DOI] [PubMed] [Google Scholar]
- 5.Tilak GS, Hsu LY, Hoyt RF, Jr, Arai AE, Aletras AH. In vivo T2-weighted magnetic resonance imaging can accurately determine the ischemic area at risk for 2-day-old nonreperfused myocardial infarction. Invest Radiol. 2008;43:7–15. doi: 10.1097/RLI.0b013e3181558822. [DOI] [PubMed] [Google Scholar]
- 6.Aletras AH, Tilak GS, Natanzon A, et al. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation. 2006;113:1865–1870. doi: 10.1161/CIRCULATIONAHA.105.576025. [DOI] [PubMed] [Google Scholar]
- 7.Carlsson M, Ubachs JF, Hedstrom E, Heiberg E, Jovinge S, Arheden H. Myocardium at risk after acute infarction in humans on cardiac magnetic resonance: quantitative assessment during follow-up and validation with single-photon emission computed tomography. JACC Cardiovasc Imaging. 2009;2:569–576. doi: 10.1016/j.jcmg.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 8.Kellman P, Aletras AH, Mancini C, McVeigh ER, Arai AE. T2-prepared SSFP improves diagnostic confidence in edema imaging in acute myocardial infarction compared to turbo spin echo. Magn Reson Med. 2007;57:891–897. doi: 10.1002/mrm.21215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorensson P, Heiberg E, Saleh N, et al. Assessment of myocardium at risk with contrast enhanced steady-state free precession cine cardiovascular magnetic resonance compared to single-photon emission computed tomography. J Cardiovasc Magn Reson. 2010;12:25. doi: 10.1186/1532-429X-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams ES, Kaplan JI, Thatcher F, Zimmerman G, Knoebel SB. Prolongation of proton spin lattice relaxation times in regionally ischemic tissue from dog hearts. J Nucl Med. 1980;21:449–453. [PubMed] [Google Scholar]
- 11.Brown JJ, Peck WW, Gerber KH, Higgins CB, Strich G, Slutsky RA. Nuclear magnetic resonance analysis of acute and chronic myocardial infarction in dogs: alterations in spinlattice relaxation times. Am Heart J. 1984;108:1292–1297. doi: 10.1016/0002-8703(84)90756-7. [DOI] [PubMed] [Google Scholar]
- 12.Buda AJ, Aisen AM, Juni JE, Gallagher KP, Zotz RJ. Detection and sizing of myocardial ischemia and infarction by nuclear magnetic resonance imaging in the canine heart. American heart journal. 1985;110:1284–1290. doi: 10.1016/0002-8703(85)90025-0. [DOI] [PubMed] [Google Scholar]
- 13.Goldfarb JW, Arnold S, Han J. Recent myocardial infarction: assessment with unenhanced T1-weighted MR imaging. Radiology. 2007;245:245–250. doi: 10.1148/radiol.2451061590. [DOI] [PubMed] [Google Scholar]
- 14.Messroghli DR, Greiser A, Frohlich M, Dietz R, Schulz-Menger J. Optimization and validation of a fully-integrated pulse sequence for modified look-locker inversion-recovery (MOLLI) T1 mapping of the heart. J Magn Reson Imaging. 2007;26:1081–1086. doi: 10.1002/jmri.21119. [DOI] [PubMed] [Google Scholar]
- 15.Giri S, Chung YC, Merchant A, et al. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson. 2009;11:56. doi: 10.1186/1532-429X-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Messroghli DR, Rudolph A, Abdel-Aty H, et al. An open-source software tool for the generation of relaxation time maps in magnetic resonance imaging. BMC Med Imaging. 2010;10:16. doi: 10.1186/1471-2342-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heiberg E, Sjogren J, Ugander M, Carlsson M, Engblom H, Arheden H. Design and validation of Segment - freely available software for cardiovascular image analysis. BMC Med Imaging. 2010;10:1. doi: 10.1186/1471-2342-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heiberg E, Engblom H, Engvall J, Hedstrom E, Ugander M, Arheden H. Semi-automatic quantification of myocardial infarction from delayed contrast enhanced magnetic resonance imaging. Scand Cardiovasc J. 2005;39:267–275. doi: 10.1080/14017430500340543. [DOI] [PubMed] [Google Scholar]
- 19.Hsu LY, Natanzon A, Kellman P, Hirsch GA, Aletras AH, Arai AE. Quantitative myocardial infarction on delayed enhancement MRI. Part I: Animal validation of an automated feature analysis and combined thresholding infarct sizing algorithm. J Magn Reson Imaging. 2006;23:298–308. doi: 10.1002/jmri.20496. [DOI] [PubMed] [Google Scholar]
- 20.Eitel I, Friedrich MG. T2-weighted cardiovascular magnetic resonance in acute cardiac disease. J Cardiovasc Magn Reson. 2011;13:13. doi: 10.1186/1532-429X-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boxt LM, Hsu D, Katz J, et al. Estimation of myocardial water content using transverse relaxation time from dual spin-echo magnetic resonance imaging. Magn Reson Imaging. 1993;11:375–383. doi: 10.1016/0730-725x(93)90070-t. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Dorado D, Oliveras J, Gili J, et al. Analysis of myocardial oedema by magnetic resonance imaging early after coronary artery occlusion with or without reperfusion. Cardiovasc Res. 1993;27:1462–1469. doi: 10.1093/cvr/27.8.1462. [DOI] [PubMed] [Google Scholar]
- 23.Berry C, Kellman P, Mancini C, et al. Magnetic resonance imaging delineates the ischemic area at risk and myocardial salvage in patients with acute myocardial infarction. Circ Cardiovasc Imaging. 3:527–535. doi: 10.1161/CIRCIMAGING.109.900761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verhaert D, Thavendiranathan P, Giri S, et al. Direct T2 quantification of myocardial edema in acute ischemic injury. JACC. Cardiovascular imaging. 2011;4:269–278. doi: 10.1016/j.jcmg.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Payne AR, Berry C, Kellman P, et al. Bright-blood t2-weighted MRI has high diagnostic accuracy for myocardial hemorrhage in myocardial infarction: a preclinical validation study in Swine. Circulation. Cardiovascular imaging. 2011;4:738–745. doi: 10.1161/CIRCIMAGING.111.965095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ugander M, Oki AJ, Hsu LY, et al. Eur Heart J. In press; Extracellular Volume Imaging by MRI Provides Insight into Overt and Subclinical Myocardial Pathology. [DOI] [PMC free article] [PubMed] [Google Scholar]