Abstract
Cheliensisin A (Chel A), a novel styryl-lactone isolated from Goniothalamus cheliensis Hu, has been shown to induce of apoptosis in human promyelocytic leukemia HL-60 cells with Bcl-2 downregulation. Yet the potential chemopreventive effect of Chel A has not been explored. Here we demonstrated that Chel A treatment with various concentrations (0.5, 1.0, 2.0, and 4.0 μM) for 3 weeks could dramatically inhibit EGF-induced cell transformation in Cl41 cells (IC50 approximately 2.0 μM). Also, co-incubation of Cl41 cells with Chel A (2.0 and 4.0 μM) for 48 hours could induce cell apoptosis in a caspase-3-dependent manner. Mechanically, Chel A treatment could result in increased p53 phosphorylation at Ser15 and elevated p53 total protein expression. Moreover, we found that p53 induction by Chel A was regulated at the protein degradation level, but not at either the transcription or the mRNA level. Further studies showed that p53 stabilization by Chel A was mediated via induction of phosphorylation and activation of Chk1 protein at Ser345. This notion was substantiated by the results that transfection of dominant negative mutant of Chk1 (GFP-Chk1 D130A) significantly attenuated the p53 protein expression, cell apoptosis, and inhibition of cell transformation by Chel A. Finally, increased hydrogen peroxide was found to mediate Chk1 phosphorylation at Ser345, p53 protein induction, cell apoptotic induction, and transformation inhibition following Chel A treatment. Taken together, our studies identify Chel A as a chemopreventive agent with the understanding of the molecular mechanisms involved.
Keywords: Cheliensisin A, apoptosis, anti-cancer, p53, chemoprevetion, cell transformation
Introduction
The continuing high magnitude of the cancer incidence and the failure of treatments on cancers at advanced stage highlight the dire need for new approaches to control such diseases. Chemoprevention, which is a pharmacological approach to intervention in an effort to arrest or reverse carcinogenesis at tumor promotion stage, has become increasingly appreciated as a new strategy in the fight against cancer (1-3). Since diverse compounds isolated from plants were reported to interfere with a specific stage of the carcinogenic process or possess immense oncological value, numerous efforts have been devoted to identifying such phytochemicals and phytochemical-derived agents with cancer preventive properties (4, 5).
Chiliensisine A (Chel A), a novel styryl-lactone isolated from Goniothalamus cheliensis Hu, has been shown to possess potent cytotoxicity against human promyelocytic leukemia HL-60 cells (6). Mechanically, Chel A was capable of inducing apoptosis of leukemia cell by downregulation of Bcl-2 expression (7). Additionally, a recent study in our laboratory has demonstrated that Chel A also displayed profound cytotoxic effects against HCT116 colon cancer cell line (data not shown). These results together suggest the potential of Chel A as an agent for cancer chemotherapy. Yet there has been no exploration of Chel A for chemopreventive intervention thus far, to the best of our knowledge. Hence, the study here sought to evaluate the potential inhibitory effect of Chel A on EGF-induced cell transformation in JB6 Cl41 cell culture model.
p53 is a tumor suppressor that is inactivated or severely damaged during carcinogenesis of most human cancers (8). A multitude of studies have confirmed its pivotal role in anticancer functions, as well as there having been many studies of the multiple mechanisms that are involved, e.g. activating DNA repair proteins, inducing cell cycle arrest, and initiating apoptosis (9-11). Despite being abrogated in some cancer types, p53 can become activated in response to a myriad of stress types, which suggests that activating p53 by targeting p53-related pathways is a potential strategy for cancer prevention and therapy (12-14). Herein, we demonstrated that Chel A exerted a chemopreventive effect on EGF-induced cell transformation with induction of apoptosis in Cl41 cells. Moreover, our results indicated that Chel A-induced apoptosis is mediated through stabilization and activation of p53 by hydrogen peroxide/Chk1-dependent axis.
Material and Methods
Chemicals
Chel A [6(7,8-epoxy-styryl)-5-acetoxy-5,6-dihydro-2-pyrone] (Fig 1A) was isolated from Goniothalamus cheliensis by the Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, China. Chel A is a white crystalline with a purity of greater than 99.0%, as previously described (7). The chemicals MG132 and cycloheximide (CHX) were purchased from Calbiochem (San Diego, CA, USA). Dichlorofluorescein diacetate (DCFH-DA) and hydroethidine (HE) were from purchased from Invitrogen (Carlsbad, CA, USA).
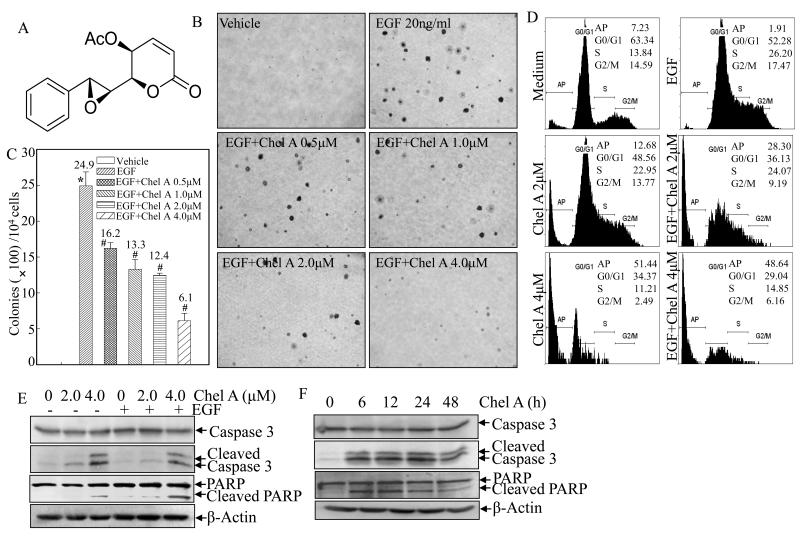
Fig. 1. The inhibition on EGF-induced cell transformation and cell apoptotic induction by Chel A in Cl41 cells.
(A) Chemical structure of Chel A. (B and C) Cl41 cells were exposed to indicate concentrations of Chel A in combination with EGF for cell transformation assay in soft agar as described in Material and Methods. After being cultured in 37 °C with 5% CO2 for 3 weeks, the colony formation was observed under inverted microscope and photographed (B). The number of colonies was scored, and presented as colonies per 104 seeded cells (C). The symbol (*) indicates a significant increase as compared with that of vehicle control (p<0.05). The symbol (#) indicates a significant decrease as compared with that of EGF treatment alone (p<0.05). Each bar indicates the mean and standard deviation of three independent experiments. (D) Cl41 cells (2×105) were seeded into each well of a six-well plate and cultured with MEM containing 5% FBS at 37 °C overnight. After being cultured in MEM containing 0.1% FBS for 48 hours, the cells were treated with various concentrations of EGF and Chel A as indicated, for 48 hours, and then were fixed and stained with propidium iodide (PI) as described in Materials and Methods. Cell apoptosis was determined by flow cytometry. The result showing was representative one from three independent experiments. (E and F) Cl41 cells were seeded into each well of six-well plates and cultured the same as those for flow cytometry assay. Then cells were treated with EGF and Chel A at different concentrations (E) or for different time periods (F). The cell extracts were subjected to Western blotting as described in Materials and Methods. β-Actin was used as a control for protein loading. The result showing was a representative data from three independent experiments.
Cell culture
Normal mouse epidermal Cl41 cells has been described before (15). Cl41 cells and their stable transfectants were cultured in 5% Fetal Bovine Serum (FBS) MEM containing with 1% penicillin/streptomycin, and 2 mM L-glutamine (Life Technologies), and were maintained at 37°C in 5% CO2 incubator. Normal mouse embryo fibroblasts with wild-type p53 expression (p53+/+) and p53 deficient mouse embryo fibroblasts (p53−/−) have been described before (16). Both (p53+/+) and (p53−/−) mouse embryonic fibroblasts were cultured in DMEM with 10% FBS. The cultures were dissociated with trypsin and transferred to new 25 cm2 culture flasks twice a week. No authentication was done with the cell lines by the authors. FBS was purchased from Life Technologies, Inc. (Gaithersburg, MD, USA); MEM and DMEM were from Calbiochem (San Diego, CA, USA).
Plasmids and transfection
The pEGFP-Chk1 kinase-inactive mutant (GFP-Chk1 D130A) plasmids were kindly provided by Dr. Helen Piwnica-Worms (Department of Cell Biology and Physiology & Howard Hughes Medical Institute, Washington University School of Medicine) (17). Mitochondria Catalase expression plasmid (pZeo/mCat) and its parental control vector were kindly provided by Dr. J. Andres Melendez (Center for Immunology and Microbial Disease, Albany Medical College) as described in our previous publications (18, 19). The p53-promoter luciferase plasmid was kindly provided by Dr. Xianglin Shi (Graduate Center for Toxicology, University of Kentucky, Lexington) (20).
JB6 Cl41 cells were transfected with mCat and its corresponding control vector by using LipofectAMINE transfection kit (Gibco BRL, Gaithersburg, MD, USA), and stable transfectants were selected by Zeocin-resistant selection. Cl41 cells stably transfected with p53 promoter-driven luciferase reporter plasmid were established using FuGENE HD (Roche Applied Science, Indianapolis, IN, USA), following the manufacturer’s instructions. The basal level of luciferase activity in Cl41 p53 transfectant was verified to be significantly higher than that in Cl41 WT cells. DN-Chk1 plasmid and its corresponding control vector were transfected into Cl41 cells by using PolyJet™ DNA In Vitro Transfection Reagent (SignaGen Laboratories, Rockville, MD, USA) following the manufacturer’s instructions and stable transfectants were established by G418-resistant selection.
Anchorage-independent growth
Soft agar colony formation assay was performed as previously described (15). Briefly, 2.5 ml of 0.5% agar in basal modified Eagle’s medium(BMEM) supplemented with 10% FBS, and 20 ng/ml EGF, as well as Chel A, at indicated concentrations, was layered onto each well of 6-well tissue culture plates. 3×104 Cl41 cells or their stable transfectants were mixed with 1 ml of 0.5% agar BMEM supplemented with 10% FBS with or without 20 ng/ml EGF, as well as with or without Chel A, and layered on top of the 0.5% agar layer. The plates were incubated at 37 °C in 5% CO2 for three weeks. The colonies were then counted under microscopy. Those with more than 32 cells were scored. The results were presented as colonies/104 seeded cells.
Western blottings
Cl41 cells, MEFs, and their transfectants were cultured in each well of 6-well plates with normal medium until they reached 70%-80% confluence. Cell culture medium was replaced to medium with 0.1% FBS for 24 hours. Then the culture medium was changed to medium with 1% FBS and cells were treated with Chel A for 0.5 h followed by treatment with Chel A and/or EGF with indicated concentrations and time periods. After exposure to EGF and Chel A, cells were washed with ice-cold PBS, and then extracted with cell lysis buffer (10 mM Tris-HCl, pH 7.4, 1%SDS, 1mM Na3VO4, and proteasome inhibitor). The cell extracts were subjected to Western blotting with each of the antibodies for determination of PARP, Cleaved PARP, Caspase 3, Cleaved Caspase 3, p53, p-p53 ser15, p-MDM2 Ser166, p-Chk1 Ser345, Chk1, p-Chk2 Thr68 (Cell Signaling, Beverly, MA, USA), Catalase (Calbiochem, EMD Biosciences, Inc., La Jolla, CA, USA), MDM2, β-Actin (Sigma, St. Louis, MO, USA), as indicated. The protein bands specifically bound to the primary antibodies were detected using an alkaline phosphatase-linked secondary antibody and an ECF (enhanced chemifluorescence) Western blotting analysis system (Amersham Pharmacia Biotech, Piscataway, NJ, USA), as previously described (15).
Reverse transcription polymerase chain reaction (RT-PCR)
Cl41 cells were cultured in 6-well plates until reaching 70%-80% confluence. Cell culture medium was changed to 0.1% FBS medium for 24 h. Then cells were exposed to Chel A using the same method as was used for the cells that were treated for Western blotting assay. After treatment for the indicated time periods, total RNAs were extracted from cells using Trizol reagent (Invitrogen, Carlsbad, California, USA). Total cDNAs were synthesized by using oligdT(20) primer by SuperScript™ First-Strand Synthesis system (Invitrogen, Carlsbad, California, USA). p53 and β-actin mRNA presented in the cells were determined by semiquantative RT-PCR assay. Mouse p53 (Forward: 5′-CACGTACTCTCCTCCCCTCA-3′, Reverse 5′-CTTCTGTACGGCGGTCTCTC-3′) and β-actin (Forward: 5′-CCTGTGGCATCCATGAAACT-3′, Reverse: 5′-GTGCTAGGAGCCAGAGCAGT-3′) primers (Invitrogen) were used to determine the mRNA amount of p53 and β-actin, respectively. The PCR products were separated onto 3% agarose gels, stained with EB, and scanned the images from a UV light as described previously (21).
Luciferase assay
Cl41 and its stable transfectant transfected with p53-promoter luciferase reporter plasmid were seeded into 96-well plates. The cell culture medium was replaced with 0.1% FBS MEM for 24 hrs, and then cells were exposed to Chel A at the indicated time periods and concentrations. The cells were then lysed for luciferase assay using luciferase substrate as previously described (21).
Flow cytometry assay
Cl41 cells were cultured in 6-well plates until 70%–180% confluent. Cell culture medium was replaced with 0.1% FBS medium for 36 h. The cells were then treated with EGF (20 ng/ml) with or without Chel A at indicated concentrations in the medium containing 0.1% FBS. Cells were fixed in ice-cold 70% ethanol and stained with PI buffer (0.1% Triton X-100, 0.2mg/ml RNase A, and 0.05mg/ml PI) for 15 min. The samples were subjected to flow cytometry (Beckman) for apoptosis analysis.
ROS detection by fluorescence spectrophotometer analysis
Cl41 cells were seeded into 96-well plates until 75-80% confluent. The cell culture medium was replaced with 0.1% FBS MEM for 36 hrs. The cells were incubated with DCFH-DA or HE for 0.5 h. Cells were then exposed to Chel A at indicated time periods followed by washing with PBS to remove the extracellular compounds and the fluorescence Intensities were detected by using SpectroMax M2 Microplate Reader (Molecular Devices Corp.). The cells incubated with DCFH-DA were employed to monitor the production of hydrogen peroxide, with the excitation of 488 nm and emission of 530 nm, while cells treated with HE were used to the measure of superoxide, with excitation of 510 nm and emission of 600 nm (19, 22, 23).
Statistical analysis
The student’s t-test was used to determine the significance between Chel A-treated vs. untreated group, or between specific gene transfectant vs. vector transfectant. The results are expressed as mean ± SD from at least three independent experiments. p<0.05 was considered as a significant difference between comparison groups.
Results
Chel A inhibited EGF-induced cell transformation with induction of apoptosis in Cl41 cells
Although Chel A has been reported to possess the apoptosis-inducing effect on human promyelocytic leukemia HL-60 cells (6), its chemopreventive effect has not been explored yet. Thus, we employed an EGF-induced Cl41 cell transformation model to evaluate the inhibitory effect of Chel A. As shown in Figs. 1B and 1C, co-incubation of Cl41 cells with Chel A at various concentrations (0.5, 1.0, 2.0, and 4.0 μM) for 3 weeks significantly inhibited EGF-induced anchorage-independent colony formation in mouse epidermal Cl41 cells, suggesting that Chel A might have a chemopreventive effect. To investigate whether the inhibitory effect of Chel A on EGF-induced cell transformation is due to its induction of apoptosis or cell cycle arrest, the cell cycle of Cl41 cells were first examined upon Chel A and/or EGF co-treatment. Cells were treated with Chel A in concentrations of 0, 2, and 4μM for 48h with and without exposure to EGF. High-resolution flow cytometry analysis of PI-stained nuclei displayed a substantial increase in apoptotic peak in a dose-dependent manner (Fig. 1D). Given that poly (ADP-ribose) polymerase (PARP) cleavage and caspase-3 activation are well-characterized markers often associated with apoptosis (24), western blot analysis was further carried out to check the induction of cleavage of caspase-3 and PARP. Consistent with flow cytometry analysis, Chel A treatment resulted in an increased cleavage of caspase-3 and PARP in both dose-dependent and time-dependent manners (Figs. 1E and 1F). Our results strongly demonstrated that the Chel A treatment inhibited cell transformation with induction of apoptosis.
p53 played a critical role in Chel A-induced cell apoptosis
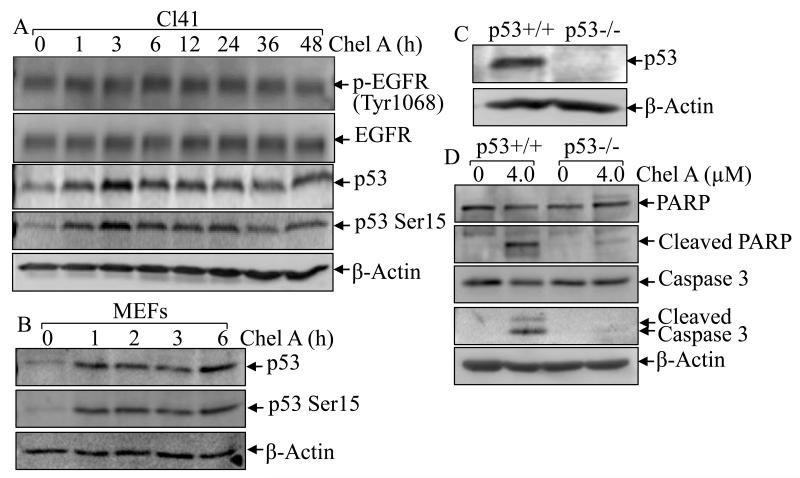
p53, a tumor suppressor, has been reported to participate directly in the mediation of caspase-dependent apoptosis (25). To explore the mechanism underlying Chel A-induced apoptosis, phosphorylation of p53 at ser15, which reflects its functional response to cellular stress and leads to apoptosis (26), and total p53 protein expression were examined in Chel A-treated Cl41 cells, which express wild-type p53. Western blot analysis revealed that Chel A treatment induced both p53 phosphorylation at ser15 and total p53 expression within as early as 1 hour and reaching maximum 3 hours (Fig. 2A), indicating that p53 participates in Chel A response. To further buttress this point, p53 phosphorylation and total expression were verified in MEF cells, which express wild-type p53. Chel A treatment upregulated levels of both Ser15 phosphorylated p53 and total p53 protein in MEF cells (Fig. 2B), which is consistent with the results obtained from the Cl41cells. To determinate whether p53 activation is necessary for Chel A-induced apoptosis, we used p53 knockout cells. Western blot analysis showed that p53 protein expression was depleted in MEF p53−/− cells in compared with that in p53+/+ cells (Fig. 2C). Moreover, p53 knockout significantly abrogated Chel A-induced cleavage of PARP and caspase-3, suggesting that p53 was critical in Chel A-induced apoptosis (Fig. 2D). In addition, we also examined the Chel A’s effect on EGFR activation. As shown in Fig. 2A, Chel A treatment elicited neither increased EGFR phosphorylation at Tyr1068, which is crucial to the EGF-induced Ras/MAPK activation (27), nor elevated EGFR total protein expression, indicating that EGFR was not involved in Chel A-induced cell transformation inhibition and apoptotic induction.
Fig. 2. p53 induction was required for apoptotic induction by Chel A.
(A and B) Cl41 cells and MEF/WT cells were treated with Chel A for indicated time periods and cell extracts were subjected to Western blotting for the detection of p53 protein expression, EGFR protein phosphorylation and expression. β-Actin was used as a control for protein loading. (C and D) p53+/+ and p53−/− cells were seeded into six-well plates. The cells were treated with Chel A for 48 hrs and the cell extracts were applied to Western blotting to determine the expression and cleavages of PARP and Caspase 3. β-Actin was used as a control for protein loading. The result showing was a representative one from three independent experiments.
Chel A specifically inhibited p53 protein degradation
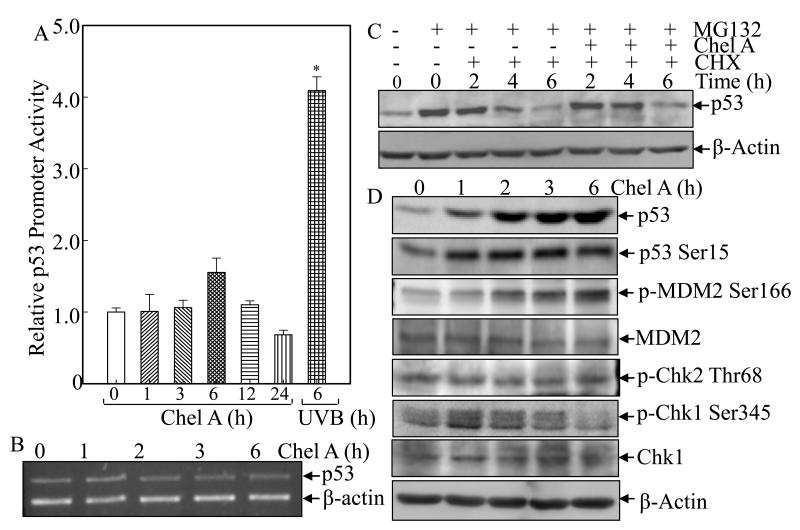
To elucidate the molecular mechanisms for Chel A upregulation of p53 protein expression, we first examined the effect of Chel A on p53 transcription by using Cl41 p53 promoter-driven luciferase reporter stable transfectant. As shown in Fig. 3A, Chel A treatment did not significantly promote p53 promoter-driven luciferase reporter activity, while UVB radiation significantly induced p53 promoter-driven luciferase activity, revealing that Chel A did not upregulate p53 at transcription level. Further, Chel A exerted no impact on p53 mRNA level within 6 hours along the time course by RT-PCR assay (Fig. 3B). Thus, we anticipated that p53 induction by Chel A might be regulated at the protein degradation level or translational level.
Fig. 3. Chel A induces p53 protein expression by inhibiting p53 protein degradation.
(A) Cl41 cells stably transfected with p53 promoter-driven luciferase reporter were exposed to Chel A for indicated time periods or to UVB as positive control. The luciferase activity was determined and the results were presented as relative p53 promoter activity. The symbol (*) indicates a significant increase as compared with that of medium control (p<0.05). Each bar indicates the mean and standard deviation of three independent experiments. (B) Cl41 cells were exposed to Chel A for indicated time periods, and p53 mRNA was determined by RT-PCR. (C) Cl41 cells were treated with MG132 for 4 h, followed by exposure with CHX combined with Chel A or CHX alone as indicated. Then cell extracts were subjected to Western Blotting and β-Actin protein expression was used as a protein loading controls. (D) Cl41 cells were exposed to Chel A for indicated time periods, and cell extracts were subjected to Western Blotting and β-Actin protein expression was used as a protein loading controls.
In an attempt to clarify whether Chel A treatment is capable of preventing p53 protein from degradation, Cl41 cells were first pretreated with proteasome inhibitor MG132 to accumulate p53 protein. Then MG132 was removed and the protein synthesis inhibitor CHX was added to the cells, alone or in combination with Chel A. The effect of Chel A on the dynamic of p53 protein degradation was determined during the indicated time periods. As shown in Fig. 3C, the p53 protein level at 4 hrs in cells co-treated with Chel A and CHX is much higher than that observed in the cells treated with CHX alone, demonstrating that Chel A treatment significantly inhibited p53 protein degradation rate, further suggesting that Chel A treatment promoted p53 expression via inhibiting p53 protein degradation.
Chk1 mediated p53 stabilization and activation upon Chel A treatment
Several proteins, such as MDM2, Chk1, and Chk2, have been reported to be capable of regulating p53 protein degradation (26, 28). MDM2 serves as a p53-specific E3 ubiquitin ligase functioning to mediating p53 protein degradation, by which MDM2 limits p53-induced apoptosis (28). Posttranslational phosphorylation of the MDM2 protein at Ser166 promotes its mediation of p53 protein degradation (29). Thus, we examined whether Chel A had an effect on MDM2 phosphorylation at Ser166. The results showed that MDM2 total protein expression was not affected upon Chel A treatment, whereas its phosphorylation at Ser166 was increased in a time-dependent manner following Chel A treatment (Fig. 3D). These data suggest that Chel A did not show any inhibition of MDM2 phosphorylation at Ser166, therefore did not account for accumulation of p53 protein upon Chel A treatment (Fig. 3D). Given the negative results received with MDM2, we then examined the kinases that can mediate p53 protein accumulation, such as Chk1 and Chk2 (30). Chk1 and Chk2 are kinases capable of activating and stabilizing p53 protein by mediating phosphorylation of p53 protein, whereas the phosphorylation of Chk1 at Ser345 or of Chk2 at Thr68, is required for their activation (31). Our results demonstrated that Chk1 phosphorylation at Ser345 was notably induced by Chel A treatment, whereas total Chk1 protein was slightly upregulated (Fig. 3D). In contrast, the phosphorylation of Chk2 at Thr68 did not show marked alteration following Chel A treatment (Fig. 3D). These results suggested that Chel A treatment might result in Chk1 phosphorylation and activation, subsequently mediating p53 stabilization.
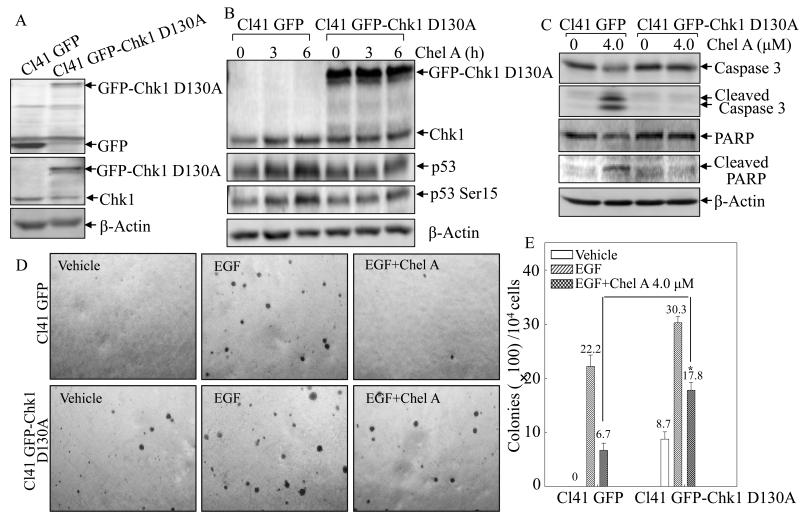
To further substantiate this point, we analyzed protein levels of p53 protein phosphorylation and expression in the Chel A-treated Cl41 cells transfected with the plasmid encoding kinase inactive Chk1 mutant, GFP-Chk1 D130A, which can out-compete the endogenous Chk1 protein (17). Verification of GFP-Chk1 D130A expression in Cl41 cells stably transfectant was shown in Fig 4A and ectopic expression of kinase inactive GFP-Chk1 D130A attenuated induction of p53 protein phosphorylation at Ser15 and expression by Chel A (Fig. 4B), strongly indicating that Chk1 mediated p53 stabilization. Furthermore, GFP-Chk1 D130A expression abrogated cheliensisin A-induced cleavages of caspase-3 and PARP (Fig. 4C), as well as inhibiting of EGF-induced Cl41 cell transformation (Figs. 4D and 4E).
Fig. 4. Ectopic expression of GFK-Chk1 D130A reversed the biological effect of Chel A in Cl41 cells.
(A) Identification of stable expression of GFP-Chk1 D130A in comparison to its scramble vector GFP transfectant in Cl41 cells. (B-C) The stable transfectant of Cl41 cells transfected with kinase inactive Chk1 (Cl41 GFP-Chk1 D130A) and its scramble vector (Cl41 GFP) were treated with Chel A as indicated, and the cell extracts were applied to Western blotting for determination of the protein expressions using specific antibosies. β-Actin was used as protein loading controls. (D and E) Effect of Chel A on EGF-induced cell transformation in Cl41 DN-Chk1 and Cl41 vector cells were determined in Soft Agar assays. The colony formation was observed under inverted microscope and photographed (D). The numbers of colonies were scored, and presented as colonies per 10,000 seeded cells (E). The symbol (*) indicates a significant increase in Cl41 GFP-Chk1 D130A cells compared with Cl41 GFP cells (p<0.05). Each bar indicates the mean and standard deviation from three independent experiments.
Hydrogen peroxide generation was required for Chel A induction of Chk1 phosphorylation at Ser315 and its mediation of p53 protein phosphorylation at Ser15 and biological effects
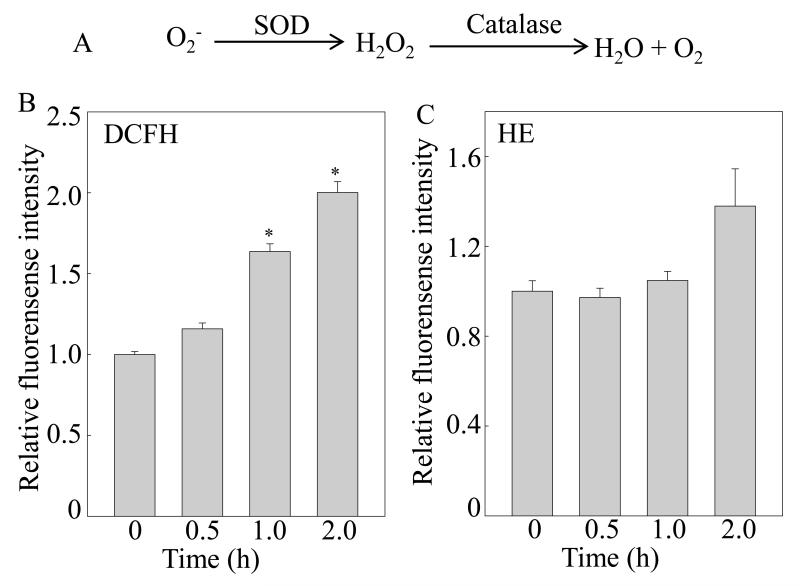
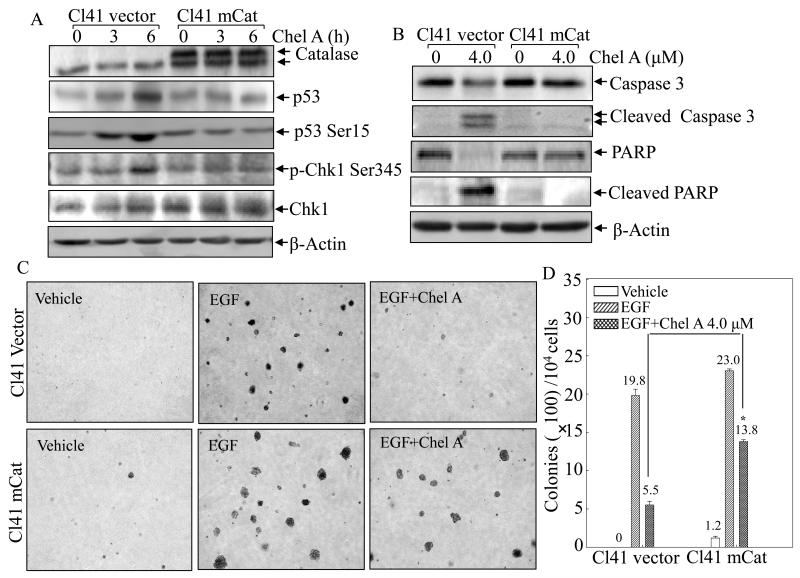
Since reactive oxygen species (ROS) has been well-characterized as a positive regulator in activating Chk1 and p53 (32), it is tempting to speculate whether ROS is also implicated in Chel A-induced Chk1 and p53 activation. Thus, two dyes, 2′,7′-dichlorofluorescein diacetate (DCFH-DA) and hydroethidine (HE), were utilized to determine the intracellular hydrogen peroxide and superoxide anion following Cl41 treated with Chel A, as illustrated in Fig. 5A. As a result, the amount of hydrogen peroxide significantly increased upon Chel A exposure in a time-dependent manner (Fig 5B), whereas the generation of superoxide was not observed under the same experimental conditions (Fig. 5C). To further evaluate whether generation of hydrogen peroxide contributed to the induction of Chk1 and p53 by Chel A, Cl41 cells stably transfected with mitochondria catalase (Cl41 mCat) were employed. The results showed that mCat overexpression attenuated the induction of Chk1 phosphorylation at Ser345 with consistent reduction of p53 protein phosphorylation at Ser15 and p53 protein accumulation following Chel A treatment (Fig. 6A), revealing that the increase in the intracellular level of hydrogen peroxide was required for Chk1 phosphorylation at Ser345, and subsequently leading to p53 protein accumulation by Chel A. Most importantly, Chel A-induced cleavages of caspase-3 and PARP were almost blocked in the Cl41 cells with mCat overexpressing (Fig. 6B). Consequently, the inhibition of cell transformation by Chel A was partially reversed by ectopic expression of mCat (Figs.6C and 6D). Together, our results indicated that generation of hydrogen peroxide was required for Chel A inhibition of EGF-induced cell transformation in Cl41 cells.
Fig. 5. Induction of hydrogen peroxide by Chel A in Cl41 cells.
(A) diagram of ROS generation and decomposition. (B and C) Cl41 cells were pretreated with 2′,7′-dichlorfluorescein-diacetate (DCFH) (B) or dihydroethidium (HE) (C), respectively, for 0.5 h, followed by being treated with Chel A for indicated time periods. Then cells were washed with PBS, and the fluorescence intensity was detected. The results were shown as the induction of each time point relative to 0 min. The symbol (*) indicates a significant increase as compared with that of 0 min (p<0.05). Each bar indicates the mean and standard deviation from three independent experiments.
Fig. 6. mCat overexpression blocked the biological effect of Chel A in Cl41 cells.
(A and B) Cl41 mCat and Cl41 vector were treated with Chel A for 0-6 h (A) or 48h (B). The cell extracts were subjected to Western blotting. β-Actin was used as protein loading controls. (C and D) Effect of Chel A on EGF-induced cell transformation in Cl41 mCat cells and Cl41 vector cells were determined by Soft Agar assay. The colony formation was observed under inverted microscope and photographed (C). The numbers of colonies were scored, and presented as colonies per 10,000 seeded cells (D). The symbol (*) indicates a significant increase in Cl41 mCat cells as compared with Cl41 vector(p<0.05). Each bar indicates the mean and standard deviation from three independent experiments.
Discussion
Chemoprevention has become increasingly appreciated as a new strategy to fight against cancers. Discovering chemopreventive agents to reverse carcinogenesis is therefore of high significance (1-3). Chel A, a novel styryl-lactone isolated from Goniothalamus cheliensis Hu, has been shown to induce apoptosis in human promyelocytic leukemia HL-60 cells, and this process is mediated through the downregulation of Bcl-2 expression (6, 7). Nevertheless, the chemopreventive effect and mechanisms of Chel A on skin tumors promotion has not been explored. In this study, we have demonstrated that Chel A is capable of inhibiting EGF-induced cell transformation in mouse epidermal JB6 Cl41 cells, suggesting the chemopreventive effect of Chel A on skin carcinogenesis. Our results indicated that Chel A exerted its inhibition of cell transformation via stabilization and activation of p53 with induction of apoptosis. Further studies showed that Chel A effect on p53 stabilization, apoptosis, and inhibition of cell transformation was resulted from generation of hydrogen peroxide and subsequent mediation of Chk1 phosphorylation at Ser345. Therefore, we identify a novel function of Chel A as a chemopreventive agent that is mediated by ROS/Chk1/p53 axis.
p53, a tumor suppressor, is inactivated or severely damaged during carcinogenesis of most human cancers (8). A multitude of studies have confirmed its pivotal role in anticancer functions, and multiple mechanisms have been involved, including activation of DNA repair proteins, induction of cell cycle arrest, and initiation apoptosis (9-11). Inactivation of p53 by point mutations has been found to occur in more than 50% human cancers (33). Despite being abrogated in some cancer types, p53 can become activated in response to a myriad of stress types, suggesting that activating p53 by targeting p53-related pathways is a potential strategy for cancer prevention and therapy (12-14). In cancer harboring a WT p53, p53 often loses its function due to the degradation by overexpressed MDM2. Hence, targeting the p53-relavant pathway by reactivating its mutant or activating WT p53 could serve as an attractive strategy in both cancer prevention and therapy (34-36). Previous study showed that Chel A could trigger apoptosis in human promyelocytic leukemia HL-60 cells, which are deficient of p53 expression (37) and are unlikely to examine Chel A’s effect on p53-dependent pathway. In the current study, Cl41 cells and MEF cells with expression of wild-type p53 were utilized to explore whether Chel A could exert an impact on p53-associated anti-cancer effects. Our results strongly suggested that Chel A treatment could lead to both the accumulation and activation of p53 proteins. Additional studies indicated that Chel A regulated p53 by preventing p53 proteins from degradation.
Several factors may result in stress-induced p53 stabilization and activation (26, 28). Our results implied that activation of Chk1 by phosphorylation at Ser345 mediates Chel A-induced p53 stabilization and activation in Cl41 cells. However, the mechanism underlying p53 stabilization and activation regulated by Chk1 remains elusive. Building upon the previous study, that Chk1 is capable of phosphorylating p53 at Ser15 (38), it is likely that p53 phosphorylation at Ser15 is directly phosphorylated by Chk1 in our experimental system. If this is the case, Chel A-induced p53 stabilization may result from p53 phosphorylation at Ser15, because p53 phosphorylation at Ser15 can lead to a reduced interaction of p53 with its negative regulator MDM2 (28). On the other hand, our data likewise suggested that MDM2 was activated due to its increased phosphorylation at Ser166. Although MDM2 activation would mediate p53 protein degradation, the overall p53 protein level is determined by multiple factors (26, 28). The results in the current study presented the elevated total p53 protein levels in Chel A-treated cells, thus indicating that the force augmenting p53 protein stability, such as p53 phosphorylation at Ser15 by Chk1, outweighed that leading to p53 reduction.
ROS are products of cellular metabolism and have the potential to elicit DNA damage, as well as playing an important role in apoptosis induction under both physiologic and pathologic conditions (39-41). Thus far, a multitude of stimulations has been identified that can induce an elevated level of intracellular ROS, thereby leading to cell apoptosis (42-44). Similarly, our studies showed that Chel A treatment resulted in hydrogen peroxide generation, which played a critical role in Chel A-induced Chk1 phosphorylation at Ser345, p53 phosphorylation at Ser15 and apoptosis, since induction of these biological effects by Chel A were almost completely impaired by ectopic expression of mCat in Cl41 cells. Consistently, overexpression of mCat could also reverse Chel A inhibition of EGF-induced cell transformation, suggesting that Chel A-induced apoptosis via H2O2/ChK1/p53 was crucial for the chemopreventive effect of Chel A. However, the mechanism underlying hydrogen peroxide generation by Chel A is still unclear, and might be due to alteration of either oxidation-reduction metabolic pathways or interference with mitochondrial electron transport system (42). These possibilities are currently under investigation in our laboratory.
In summary, our studies demonstrated that Chel A exerted a chemopreventive effect on EGF-induced cell transformation by inducing apoptosis in JB6 Cl41 cells. Mechanical insight into induction of apoptosis suggested that the signaling pathway is through hydrogen peroxide generation, which subsequently mediates Chk1 phosphorylation at Ser345 and p53 phosphorylation at Ser15. Taken together, these findings implicate the potential utilization of Chel A as a cancer chemopreventive agent.
Acknowledgements
We thank Dr. Helen Piwnica-Worms from Department of Cell Biology and Physiology & Howard Hughes Medical Institute, Washington University School of Medicine for the gift of GFP-Chk1 D130A construct, Dr. Xianglin Shi from Graduate Center for Toxicology, University of Kentucky for the gift of the p53-promoter luciferase plasmid, and Dr. J. Andres Melendez from The Center for Immunology and Microbial Disease, Albany Medical College for his generosity in providing us with the construct of mitochondria Catalase (pZeo/mCat). This work was partially supported by grants from NIH/NCI RO1 CA112557, R01 CA177665 and NIH/NIEHS ES000260; NSFC81229002 and NSFC 90813004.
References
- 1.Kelloff GJ, Crowell JA, Steele VE, Lubet RA, Malone WA, Boone CW, et al. Progress in cancer chemoprevention: development of diet-derived chemopreventive agents. J Nutr. 2000;130:467S–471S. doi: 10.1093/jn/130.2.467S. [DOI] [PubMed] [Google Scholar]
- 2.Patterson SL, Colbert Maresso K, Hawk E. Cancer chemoprevention: successes and failures. Clin Chem. 2013;59:94–101. doi: 10.1373/clinchem.2012.185389. [DOI] [PubMed] [Google Scholar]
- 3.Sporn MB, Suh N. Chemoprevention of cancer. Carcinogenesis. 2000;21:525–30. doi: 10.1093/carcin/21.3.525. [DOI] [PubMed] [Google Scholar]
- 4.Wiart C. Lead Compounds from Medicinal Plants for the Treatment of Cancer. Elsevier Inc.; 2013. [Google Scholar]
- 5.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–80. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 6.Li CM, Mu Q, Sun HD, Xu B, Tang WD, Zheng HL, et al. A new anti-cancer constituent of Goniothalamus cheliensis. Acta Bot Yunnan. 1998;20:102–4. [Google Scholar]
- 7.Zhong L, Li CM, Hao XJ, Lou LG. Induction of leukemia cell apoptosis by cheliensisin A involves down-regulation of Bcl-2 expression. Acta Pharmacol Sin. 2005;26:623–8. doi: 10.1111/j.1745-7254.2005.00077.x. [DOI] [PubMed] [Google Scholar]
- 8.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 9.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–31. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 10.Schmitt CA, Fridman JS, Yang M, Baranov E, Hoffman RM, Lowe SW. Dissecting p53 tumor suppressor functions in vivo. Cancer Cell. 2002;1:289–98. doi: 10.1016/s1535-6108(02)00047-8. [DOI] [PubMed] [Google Scholar]
- 11.Sengupta S, Harris CC. p53: traffic cop at the crossroads of DNA repair and recombination. Nat Rev Mol Cell Biol. 2005;6:44–55. doi: 10.1038/nrm1546. [DOI] [PubMed] [Google Scholar]
- 12.Fuster JJ, Sanz-González SM, Moll UM, Andrés V. Classic and novel roles of p53: prospects for anticancer therapy. Trends Mol Med. 2007;13:192–9. doi: 10.1016/j.molmed.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Shangary S, Wang S. Small-molecule inhibitors of the MDM2-p53 protein-protein interaction to reactivate p53 function: a novel approach for cancer therapy. Annu Rev Pharmacol Toxicol. 2009;49:223–41. doi: 10.1146/annurev.pharmtox.48.113006.094723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.She QB, Bode AM, Ma WY, Chen NY, Dong Z. Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular-signal-regulated protein kinases and p38 kinase. Cancer Res. 2001;61:1604–10. [PubMed] [Google Scholar]
- 15.Zhang J, Ouyang W, Li J, Zhang D, Yu Y, Wang Y, et al. Suberoylanilide hydroxamic acid (SAHA) inhibits EGF-induced cell transformation via reduction of cyclin D1 mRNA stability. Toxicol Appl Pharmacol. 2012;263:218–24. doi: 10.1016/j.taap.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Z, Chen F, Huang C, Shi X. Vanadate induces G2/M phase arrest in p53-deficient mouse embryo fibroblasts. J Environ Pathol Toxicol Oncol. 2002;21:223–31. [PubMed] [Google Scholar]
- 17.Leung-Pineda V, Ryan CE, Piwnica-Worms H. Phosphorylation of Chk1 by ATR is antagonized by a Chk1-regulated protein phosphatase 2A circuit. Mol Cell Biol. 2006;26:7529–38. doi: 10.1128/MCB.00447-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodríguez AM, Carrico PM, Mazurkiewicz JE, Meléndez JA. Mitochondrial or cytosolic catalase reverses the MnSOD-dependent inhibition of proliferation by enhancing respiratory chain activity, net ATP production, and decreasing the steady state levels of H(2)O(2) Free Radic Biol Med. 2000;29:801–13. doi: 10.1016/s0891-5849(00)00362-2. [DOI] [PubMed] [Google Scholar]
- 19.Ding J, Zhang X, Li J, Song L, Ouyang W, Zhang D, et al. Nickel compounds render anti-apoptotic effect to human bronchial epithelial Beas-2B cells by induction of cyclooxygenase-2 through an IKKbeta/p65-dependent and IKKalpha- and p50-independent pathway. J Biol Chem. 2006;281:39022–32. doi: 10.1074/jbc.M604798200. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, Shi X, Mechanisms of. Cr(VI)-induced p53 activation: the role of phosphorylation, mdm2 and ERK. Carcinogenesis. 2001;22:757–62. doi: 10.1093/carcin/22.5.757. [DOI] [PubMed] [Google Scholar]
- 21.Zhang D, Li J, Gao J, Huang C. c-Jun/AP-1 pathway-mediated cyclin D1 expression participates in low dose arsenite-induced transformation in mouse epidermal JB6 Cl41 cells. Toxicol Appl Pharmacol. 2009;235:18–24. doi: 10.1016/j.taap.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li F, Wang H, Li L, Huang C, Lin J, Zhu G, et al. Superoxide plays critical roles in electrotaxis of fibrosarcoma cells via activation of ERK and reorganization of the cytoskeleton. Free Radic Biol Med. 2012;52:1888–96. doi: 10.1016/j.freeradbiomed.2012.02.047. [DOI] [PubMed] [Google Scholar]
- 23.Huang C, Li J, Ke Q, Leonard SS, Jiang BH, Zhong XS, et al. Ultraviolet-induced phosphorylation of p70(S6K) at Thr(389) and Thr(421)/Ser(424) involves hydrogen peroxide and mammalian target of rapamycin but not Akt and atypical protein kinase C. Cancer Res. 2002;62:5689–97. [PubMed] [Google Scholar]
- 24.Boulares AH, Yakovlev AG, Ivanova V, Stoica BA, Wang G, Iyer S, et al. Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J Biol Chem. 1999;274:22932–40. doi: 10.1074/jbc.274.33.22932. [DOI] [PubMed] [Google Scholar]
- 25.Schuler M, Bossy-Wetzel E, Goldstein JC, Fitzgerald P, Green DR. p53 induces apoptosis by caspase activation through mitochondrial cytochrome c release. J Biol Chem. 2000;275:7337–42. doi: 10.1074/jbc.275.10.7337. [DOI] [PubMed] [Google Scholar]
- 26.Appella E, Anderson CW. Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem. 2001 May;268(10):2764–72. doi: 10.1046/j.1432-1327.2001.02225.x. [DOI] [PubMed] [Google Scholar]
- 27.Rojas M, Yao S, Lin YZ. Controlling epidermal growth factor (EGF)-stimulated Ras activation in intact cells by a cell-permeable peptide mimicking phosphorylated EGF receptor. J Biol Chem. 1996;271:27456–61. doi: 10.1074/jbc.271.44.27456. [DOI] [PubMed] [Google Scholar]
- 28.Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91:325–34. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 29.Zhou BP, Liao Y, Xia W, Zou Y, Spohn B, Hung MC. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol. 2001;3:973–82. doi: 10.1038/ncb1101-973. [DOI] [PubMed] [Google Scholar]
- 30.Ou YH, Chung PH, Sun TP, Shieh SY. p53 C-terminal phosphorylation by CHK1 and CHK2 participates in the regulation of DNA-damage-induced C-terminal acetylation. Mol Biol Cell. 2005;16:1684–95. doi: 10.1091/mbc.E04-08-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–96. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- 32.Kulkarni A, Das KC. Differential roles of ATR and ATM in p53, Chk1, and histone H2AX phosphorylation in response to hyperoxia: ATR-dependent ATM activation. Am J Physiol Lung Cell Mol Physiol. 2008;294:L998–L1006. doi: 10.1152/ajplung.00004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–10. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 34.Chène P. Inhibiting the p53-MDM2 interaction: an important target for cancer therapy. Nat Rev Cancer. 2003;3:102–9. doi: 10.1038/nrc991. [DOI] [PubMed] [Google Scholar]
- 35.Hursting SD, Perkins SN, Phang JM, Barrett JC. Diet and cancer prevention studies in p53-deficient mice. J Nutr. 2001;131:3092S–4S. doi: 10.1093/jn/131.11.3092S. [DOI] [PubMed] [Google Scholar]
- 36.Bai L, Zhu W. p53: Structure, Function and Therapeutic Applications. journal of cancer molecules. 2006;2:141–153. [Google Scholar]
- 37.Wolf D, Rotter V. Major deletions in the gene encoding the p53 tumor antigen cause lack of p53 expression in HL-60 cells. Proc Natl Acad Sci U S A. 1985;82:790–4. doi: 10.1073/pnas.82.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shieh SY, Ahn J, Tamai K, Taya Y, Prives C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 2000;14:289–300. [PMC free article] [PubMed] [Google Scholar]
- 39.Barker GF, Manzo ND, Cotich KL, Shone RK, Waxman AB. DNA damage induced by hyperoxia: quantitation and correlation with lung injury. Am J Respir Cell Mol Biol. 2006;35:277–88. doi: 10.1165/rcmb.2005-0340OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cacciuttolo MA, Trinh L, Lumpkin JA, Rao G. Hyperoxia induces DNA damage in mammalian cells. Free Radic Biol Med. 1993;14:267–76. doi: 10.1016/0891-5849(93)90023-n. [DOI] [PubMed] [Google Scholar]
- 41.Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–8. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]
- 42.Pérez-Galán P, Roué G, Villamor N, Montserrat E, Campo E, Colomer D. The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood. 2006;107:257–64. doi: 10.1182/blood-2005-05-2091. [DOI] [PubMed] [Google Scholar]
- 43.Shen SC, Lee WR, Yang LY, Tsai HH, Yang LL, Chen YC. Quercetin enhancement of arsenic-induced apoptosis via stimulating ROS-dependent p53 protein ubiquitination in human HaCaT keratinocytes. Exp Dermatol. 2012;21:370–5. doi: 10.1111/j.1600-0625.2012.01479.x. [DOI] [PubMed] [Google Scholar]
- 44.Shimizu T, Numata T, Okada Y. A role of reactive oxygen species in apoptotic activation of volume-sensitive Cl(-) channel. Proc Natl Acad Sci U S A. 2004;101:6770–3. doi: 10.1073/pnas.0401604101. [DOI] [PMC free article] [PubMed] [Google Scholar]