Summary
Increased amino acid supplementation (0.5×, 1.0×, and 5.0× recommended concentrations or additional proline) was hypothesized to increase the collagen content in engineered cartilage. No significant differences were found between groups in matrix content or dynamic modulus. Control constructs possessed the highest compressive Young’s modulus on day 42. On day 42, compared to controls, decreased type II collagen was found with 0.5×, 1.0×, and 5.0× supplementation and significantly increased DNA content found in 1.0× and 5.0×. No effects were observed on these measures with added proline. These results lead us to reject our hypothesis and indicate that the low collagen synthesis in engineered cartilage is not due to a limited supply of amino acids in media but may require a further stimulatory signal. The results of this study also highlight the impact that culture environment can play on the development of engineered cartilage.
Keywords: Agarose, Cartilage tissue engineering, Chondrocyte, Amino acids, Cell proliferation, Dedifferentiation, Cell culture media
Introduction
Cartilage tissue engineering using a temporal application of transforming growth factor β3 (TGF-β3) can produce an engineered tissue that approaches a physiologic magnitude in both compressive Young’s modulus and glycosaminoglycan content (Byers et al., 2006). However, the collagen content and dynamic modulus of these engineered tissues are only approximately ¼ that of the native tissue (Mankin et al., 2000; Byers et al., 2006). Given the relationship between collagen and functional mechanical properties in cartilage (Wong et al., 2000; Williamson et al., 2003), improving the quality and quantity of the collagen network in engineering cartilage may be necessary to develop a functional, clinical replacement for damaged cartilage.
It is unknown whether nutrient limitations during the development of engineered cartilage are responsible for the low collagen content. Protein synthesis of various cell types has been reported to be modulated by increasing medium amino acid concentration (Park et al., 1976; Ledford and Jacobs, 1985; Lopes and Larbier, 1994), up to as much as 15-fold for selected amino acids of the basal medium (Park and Chandler, 1976; Roh et al., 1994). It has also been shown that increased concentrations of amino acids in culture media can increase their transport and incorporation into protein by chondrocytes (Handley et al., 1980; Barker et al., 1999). In a preliminary study, we showed that supplementation of a serum-containing growth media with essential amino acids increases collagen content of engineered cartilage after 14 days in culture, though this trend was not sustained over prolonged culture duration (Ng et al., 2006a) and may be partially attributed to the variability and undefined nature of serum as a culture supplement (Honn et al., 1975; Price and Gregory, 1982).
Therefore, in the present study, it is hypothesized that supplementation with amino acids combined with the temporal application of TGF-β3 in a well-defined, serum-free culture media will increase the collagen production in engineered cartilage. To test this hypothesis, chondrocytes were seeded in agarose, a well-characterized and robust scaffold system (Benya and Shaffer, 1982; Mouw et al., 2005) and cultured in the presence of additional L-proline, an amino acid directly involved in collagen formation (Hausmann and Neuman, 1961), or varying concentrations of essential and non-essential amino acids.
Materials and methods
Creation and culture of engineered constructs
Articular bovine chondrocytes were isolated via enzymatic digestion as described previously (Ng et al., 2005). Briefly, chondrocytes were isolated from calf carpometacarpal joints via serial digestion of full thickness cartilage slices in 0.25% pronase (Calbiochem, San Diego, CA) and 0.05% collagenase (Sigma Aldrich, St. Louis, MO). Cells were resuspended and mixed with molten type VII agarose (Sigma) in phosphate buffered saline (PBS, Sigma) at 40 °C to yield a 2% agarose suspension with 30 × 106 chondrocytes=ml. This suspension was cast between two glass plates and allowed to cool for 20 min. Disks were cored out (Ø 4.0× 2.3mm) and cultured at 37 °C and 5% CO2 in 35 ml of chondrogenic media (high glucose DMEM, 1% ITS+, 0.1µM dexamethasone, 110 µg/ml sodium pyruvate, 50 µg/ml L-proline, 50 µg/ml ascorbate-2-phosphate, sodium bicarbonate, and antibiotics (Byers et al., 2006)) with or without supplementation of additional essential and non-essential amino acids (Mediatech, Herndon, VA) at 0.5×, 1×, or 5× recommended concentration or an additional 50 µg/ml L-proline (“proline” group). All media pH was adjusted to 7.2 and the osmolality of control media and each amino acid media formulation (n = 3 independent media batches per group) was determined via an Advanced 3D3 Osmometer (Advanced Instruments Inc., Norwood, MA). Media was changed every other day. For the first 14 days in culture, 10 ng/ml of TGF-β3 (R&D Systems, Minneapolis, MN) was added with each media change (Byers et al., 2006). Day 0 mechanical testing was performed prior to amino acid and TGF-β3 treatment. Constructs (n = 4–5 per group) were then removed from culture on day 14, 28, and 42 for evaluation of mechanical properties and bio-chemical composition.
Mechanical testing
Mechanical testing was performed in unconfined compression as previously described (Mauck et al., 2003c). Constructs were placed in a custom testing device and were first equilibrated under a creep tare load of ~0.02N followed by a stress relaxation test with a ramp displacement of 1 µm/sec to 10% strain (based on the measured post-creep thickness). After equilibrium was reached (2000 sec), a sinusoidal displacement of 40 µm amplitude was applied at 1Hz. The compressive Young’s modulus (EY), a property that indicates a material’s resistance to deformation, was determined from the equilibrium response of the stress relaxation test by dividing the equilibrium stress (minus the tare stress) by the applied strain. The dynamic modulus (G*) at 1Hz, which represents a material’s resistance to vibratory deformation at the given frequency, was calculated from the ratio of the measured stress amplitude and the applied strain amplitude of the dynamic loading. Following mechanical testing, samples were halved, with each half either fixed for histology or frozen at −30 °C for biochemistry.
Biochemical analysis
The samples were thawed, weighed wet, and digested for 16 h at 56 °C with 1mg/ml proteinase K (EMD Biosciences, San Diego CA) in 50 mM Tris buffered saline containing 1mM EDTA, 1mM iodoacetamide and 10 µg/ml pepstatin A (Sigma) (Riesle et al., 1998). These digests were used to determine sample GAG content via the 1,9-dimethylmethylene blue (Sigma) dye-binding assay (Farndale et al., 1982) and for DNA content via the PicoGreen assay (Invitrogen, Carlsbad, CA). The overall collagen content was measured using orthohydroxyproline (OHP) colorimetric assay (Stegemann and Stalder, 1967). Collagen content was calculated by assuming a 1:10 OHP-to-collagen mass ratio (Hollander et al., 1994). Assays were adapted for use in 96-well, micro-titer plates. Biochemical content was normalized to the construct wet weight (% ww) and DNA content.
The type II collagen content of samples was determined from the proteinase K digests via a custom ELISA (Riesle et al., 1998; Kelly et al., 2006). Briefly, bovine type II collagen standards (0–3 µg/ml; Rockland Immunochemical, Gilbertsville, PA) and samples were coated onto the surface of separate wells in a 96-well multi-titer plate. Immobilized type II collagen was labeled with biotinylated type II collagen antibody (Rockland Immunochemical), incubated with streptavidin-conjugated horseradish peroxidase (Pierce Biotechnology, Rockford, IL) and reacted with 3,3′,5,5′-tetramethylbenzidine reagent (Vector Laboratories, Burlingame, CA). The reaction was stopped with 1N sulfuric acid and read at 450 nm in a microtiter plate reader. The type II collagen content was normalized to the total collagen content of the disks as determined by the OHP assay.
Histology
Samples were fixed in acid-ethanol-formalin (Lin et al., 1997) for 48 h at 4 °C, dehydrated in a graded series of ethanol, cleared, embedded in Tissue Prep embedding media (Fisher Scientific, Pittsburgh, PA), and sectioned at 6 µm. Sections were then stained in hematoxylin and eosin to study cell proliferation and spatial distribution.
Statistical analysis
Statistics were performed using the Statistica (Statsoft, Inc., Tulsa, OK) software package. For media osmolality, groups were examined using multivariate analysis of variance with osmolality as the dependent variable and media formulation as the independent variable. For mechanical and biochemical data, groups were examined using multivariate analysis of variance with EY, GAG, collagen, DNA, and type II collagen as the dependent variables and culture time and amino acids as the independent variables. Fisher’s least-significant different (LSD) post hoc tests were carried out with statistical significance set at α = 0.05, n = 4–5 per group. All data is presented as an average with standard deviation.
Results
Osmolality testing of media formulations found no statistical differences between control media (336 ± 3mOsm) and media supplemented with either 0.5× (333 ± 6 mOsm), 1.0× (339 ± 4mOsm), 5.0× (325 ± 9mOsm) amino acids or additional proline (338 ± 2mOsm).
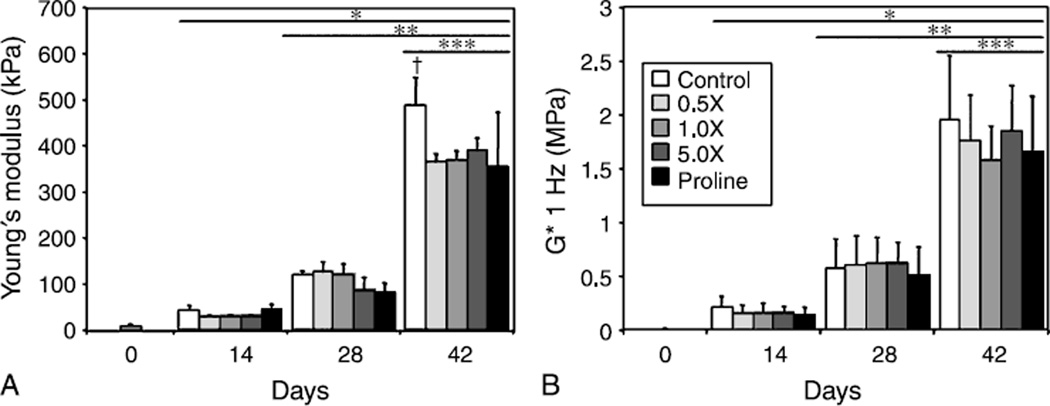
Mechanical properties of constructs (Fig. 1A, B) increased significantly over time in culture, with no differences among the groups through 28 days in culture (EY~100 kPa and G* ~0.6MPa at day 28). By day 42, however, the EY of untreated controls (487 ± 61 kPa) was ~30% greater than that of amino acid and proline supplemented constructs (Fig. 1A, p < 0.05). The G* of constructs also increased over time, with the highest overall value reached on day 42 by controls (Fig. 1B, 1.96 ± 0.60 MPa), though there were no significant differences observed between groups.
Fig. 1.
The Young’s modulus (A, EY) and dynamic modulus (B, G*) of engineered cartilage tissue increased with time in culture. On day 42, control tissue possessed a significantly greater EY than tissue cultured with increased amino acids, reaching a value of 487 ± 61 kPa. The dynamic modulus increased significantly with each time point to ~2MPa on day 42, however no significant differences were found between experimental groups. *p<0.05 vs. d0, **p<0.05 vs. d14, ***p<0.05 vs. d28, †p<0.05 vs. all other groups
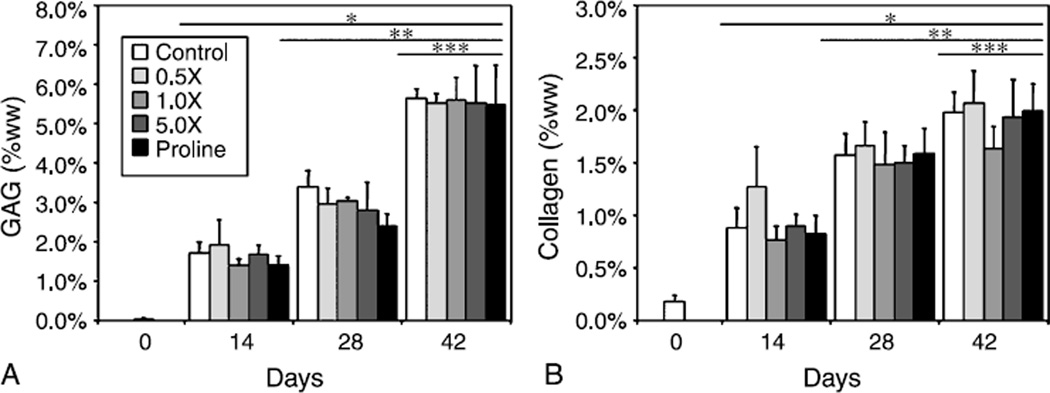
GAG and collagen content were observed to increase over time in culture, reaching values of ~5.5% ww and ~2.0% ww on day 42, respectively, with no significant differences found between culture conditions (Fig. 2A, B). Given the extremely low collagen content of day 0 constructs, no meaningful measurements for type II collagen could be made. However, on days 14 and 28, type II collagen made up ~70% of the total synthesized collagen in control constructs. On day 42, increasing amino acid supplementation was found to significantly decrease the synthesis of type II collagen when compared to controls, with type II collagen in 5.0× constructs representing only 50% of the total collagen (Fig. 3A, p < 0.05). No differences were observed due to proline concentration. DNA was found to increase over time in culture (Fig. 3B), with no significant differences noted between control and proline groups. However, both 1.0× and 5.0× amino acids supplementation resulted in significantly greater DNA content over controls by day 42 (p < 0.05). At this time point, DNA-normalized biochemical content (GAG/DNA, DNA, collagen/DNA) was significantly lower for 1.0× (122.6 ± 16.0 g/g, 35.8 ± 6.0 g/g) and 5.0× (103.3 ± 20.6 g/g, 35.8 ± 5.4 g/g) compared to controls (156.1 ± 5.0 g/g, 54.6 ± 2.5 g/g, p < 0.05), with no differences noted at other time points or between other groups on day 42.
Fig. 2.
GAG content (A) and collagen content (B) of engineered cartilage tissue increased with time in culture, with no differences found between experimental groups. GAG content appeared to roughly double approximately every 2 weeks attaining a maximum value of ~5.5% ww, whereas collagen content increased at a slower rate to a final value of ~2.0% ww. *p<0.05 vs. d0, **p<0.05 vs. d14, ***p<0.05 vs. d28
Fig. 3.
Amino acids at 1.0× and 5.0× were found to decrease type II collagen content (A) compared to control on day 28 and day 42. Amino acids at 0.5× diminished type II collagen only on day 42, though this value was the lowest value between all groups and time points. Proline was found to have no effect. DNA (B) was found to increase with 1.0× and 5.0× amino acid supplementation over controls. *p<0.05 vs. day 0, **p<0.05 vs. day 14, †p<0.05 vs. control of respective time point, ‡p<0.05 vs. all other groups and previous time points
Histological analysis revealed that the chondrocytes proliferated in dense clusters throughout the construct (Fig. 4A, B). The periphery of constructs did not possess a fibrous capsule that has been reported to form on agarose constructs cultured in fetal bovine serum (Fig. 4C) (Kisiday et al., 2005; Ng et al., 2006c).
Fig. 4.
Hematoxylin and eosin staining (day 56, 5.0× shown) indicated that chondrocytes in the center (A) and peripheral (B) regions of constructs cultured with transient TGF-β3 proliferated in dense clusters. The periphery of these constructs did not show the highly cellular, fibrous outgrowth previously found to form in agarose constructs cultured in fetal bovine serum with amino acid supplementation (C, arrows, from Ng et al. (2006a)). Scale bar 200 µm (for a color reproduction of this figure, the reader is referred to the online version of this paper under www.springerlink.com)
Discussion
The current study examining amino acid supplementation was motivated by research showing that increased concentrations of amino acids in culture media can increase their transport and usage in chondrocytes and other cell types (e.g., Handley et al., 1980; Lopes and Larbier, 1994; Barker et al., 1999; Washizu et al., 2000). The results from the current study lead us to reject our hypothesis and indicate that the concentration of amino acids in DMEM is not a limiting factor in the matrix synthesis of chondrocytes seeded in agarose hydrogels. It is known that collagen remodeling in healthy cartilage occurs over extremely long periods of time, with a half life estimated to be>100 years (Maroudas et al., 1992; Verzijl et al., 2000), yet injury can trigger collagen remodeling that can form repair tissue on the order of weeks (Eyre et al., 1980). These previous data and the results of the presented study imply that the collagen synthesis in tissue engineered cartilage may not be limited by nutritional limitations but rather the lack of the appropriate stimuli to induce rapid tissue remodeling. The results of temporal application of TGF-β3 would support this as the tissues increase rapidly in GAG and compressive modulus with the addition and removal of the growth factor. The temporal application may be an important aspect as growth factor and cytokine expression in vivo during healing is transient (Bos et al., 2001) and in vitro studies of continuous growth factor expression do not show this trend (Mauck et al., 2003a; Byers et al., 2006).
The control tissue grown in the current study with a chemically-defined media and TGF-β3 appeared to possess a physiologic Young’s modulus, GAG content, and proportion of type II collagen (~70% of total collagen) (Mankin et al., 2000). This last result is very positive given that previously characterized constructs cultured in free-swelling with serum-containing media possessed a lower type II collagen content (~50–60% total collagen) and a physiologic value could only be obtained with the application of dynamic loading (Kelly et al., 2006). The control constructs in the current study also lacked the highly cellular, fibrous capsule found to form in serum-containing culture media (Kisiday et al., 2005; Ng et al., 2006c). In addition, this outgrowth layer became thicker (>100 µm) and more pronounced with amino acids supplementation in serum-containing media (Ng et al., 2006a). This fibrous tissue, composed of flattened, dedifferentiated chondrocytes, may play a role in impeding nutrient transport and adds variability in the composition and properties of the engineered cartilage. These results show that serum-free culture conditions better maintain the differentiation state of the control chondrocytes and is important in minimizing variability for future clinical treatments. Though no fibrous layer was present, increased DNA was measured in the serum-free conditions of the current study with high concentrations of amino acids, implying that the proliferation is an effect of the amino acid treatment and can be synergistically upregulated with serum.
Though the research is limited, the published literature imply a signaling role for amino acids for chondrocytes and other cell types. Amino acids have been shown be capable of activating signaling pathways (e.g., mTOR pathway via branched-chain amino acids (Naomoto et al., 2005)) and affecting a variety of downstream cellular responses such as protein synthesis (e.g., (Park et al., 1976; Ledford and Jacobs, 1985; Lopes and Larbier, 1994)), mRNA expression (Thissen et al., 1994), and apoptogen sensitivity (Tonomura et al., 2006). This signaling role may explain the apparent progressive dedifferentiation in chondrocyte phenotype with prolonged culture in increasing concentrations of amino acids, as indicated by the changes in type II collagen production and DNA. The increased proliferation and dedifferentiation has also been previously observed in chondrocyte monolayer cultures supplemented with non-essential amino acids (Hendriks et al., 2006). Interestingly, in this study by Hendriks et al. (2006), the dedifferentiation of these chondrocytes in monolayer culture, as measured by the absence of GAG, could not be reversed when these chondrocytes were returned to a 3D environment via pellet culture, an outcome contrary to the established redifferentiating effects of a 3D culture environment (Benya and Shaffer, 1982). This result implies a change in chondrocyte memory, where the chondrocytes appear to be irrevocably changed due to the amino acids in the culture media. These earlier results are insightful; however, since no diminished GAG content was measured in the current study, it appears that the effect of amino-acid supplementation observed in monolayer culture is not necessarily the same as in 3D culture. Though the current study focused on the impact of amino acids on the functional properties of engineered cartilage, it is clear that the effects of amino acids on the chondrocyte itself also deserves specific study such as changes in chondrocyte apoptogen sensitivity, autophagy, and hypertrophy.
Though the magnitude of the equilibrium compressive modulus of cartilage is generally associated with the proteoglycan content, it is interesting that the 30% reduction in EY observed at day 42 in all treatment groups (Fig. 1A) is not predicated by changes in GAG content (Fig. 2A). This is not necessarily surprising, in light of earlier findings that the correlation between GAG content and compressive equilibrium modulus is generally moderate. Indeed, it is possible that other matrix molecules, such as cartilage oligomeric matrix proteins, also contribute to the compressive stiffness of cartilage (Mauck et al., 2003) and that the synthesis of such molecules is affected by the amino-acid concentration in the culture medium.
The results of this study highlight the impact that culture environment can play on the development of engineered cartilage. The concentration of amino acids does not appear to be a limiting factor in the collagen production in engineered cartilage in vitro and indicates that alternative strategies must be sought to increase collagen synthesis. The reported findings lend more support towards the adoption of a well-defined culture media for use in tissue engineering applications. They also hint to the possibility that the agarose hydrogel may impede deposition of collagen beyond the values observed in this study, possibly due to pore size; this alternative hypothesis is currently being investigated (Ng et al., 2006b). Long term studies to elucidate the exact mechanisms behind the observed results in this study are worthy of future discovery and may help to better our understanding of chondrocyte behavior in response to changes in environment that may occur with joint disease.
Acknowledgements
This study was supported with funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR 46568).
References
- 1.Barker GA, Wilkins RJ, Golding S, Ellory JC. Neutral amino acid transport in bovine articular chondrocytes. J Physiol. 1999;514(Pt 3):795–808. doi: 10.1111/j.1469-7793.1999.795ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 3.Bos PK, van Osch GJ, Frenz DA, Verhaar JA, Verwoerd-Verhoef HL. Growth factor expression in cartilage wound healing: temporal and spatial immunolocalization in a rabbit auricular cartilage wound model. Osteoarthr Cartilage. 2001;9:382–389. doi: 10.1053/joca.2000.0399. [DOI] [PubMed] [Google Scholar]
- 4.Byers BA, Mauck RL, Chiang I, Tuan RS. Temporal exposure of TGF-beta3 under serum-free conditions enhances biomechanical and biochemical maturation of tissue-engineered cartilage. Trans Orthop Res. 2006;31:43. doi: 10.1089/ten.tea.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eyre DR, McDevitt CA, Billingham ME, Muir H. Biosynthesis of collagen and other matrix proteins by articular cartilage in experimental osteoarthrosis. Biochem J. 1980;188:823–837. doi: 10.1042/bj1880823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 7.Handley CJ, Speight G, Leyden KM, Lowther DA. Extracellular matrix metabolism by chondrocytes. 7. Evidence that L-glutamine is an essential amino acid for chondrocytes and other connective tissue cells. Biochim Biophys Acta. 1980;627:324–331. doi: 10.1016/0304-4165(80)90463-8. [DOI] [PubMed] [Google Scholar]
- 8.Hausmann E, Neuman WF. Conversion of proline to hydroxyproline and its incorporation into collagen. J Biol Chem. 1961;236:149–152. [PubMed] [Google Scholar]
- 9.Hendriks J, Riesle J, Vanblitterswijk CA. Effect of stratified culture compared to confluent culture in monolayer on proliferation and differentiation of human articular chondrocytes. Tissue Eng. 2006;12:2397–2405. doi: 10.1089/ten.2006.12.2397. [DOI] [PubMed] [Google Scholar]
- 10.Hollander AP, Heathfield TF, Webber C, Iwata Y, Bourne R, Rorabeck C, Poole AR. Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J Clin Invest. 1994;93:1722–1732. doi: 10.1172/JCI117156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honn KV, Singley JA, Chavin W. Fetal bovine serum: a multivariate standard. Proc Soc Exp Biol Med. 1975;149:344–347. doi: 10.3181/00379727-149-38804. [DOI] [PubMed] [Google Scholar]
- 12.Kelly TA, Ng KW, Wang CC, Ateshian GA, Hung CT. Spatial and temporal development of chondrocyte-seeded agarose constructs in free-swelling and dynamically loaded cultures. J Biomech. 2006;39:1489–1497. doi: 10.1016/j.jbiomech.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 13.Kisiday JD, Kurz B, DiMicco MA, Grodzinsky AJ. Evaluation of medium supplemented with insulin-transferrin-selenium for culture of primary bovine calf chondrocytes in three-dimensional hydrogel scaf-folds. Tissue Eng. 2005;11:141–151. doi: 10.1089/ten.2005.11.141. [DOI] [PubMed] [Google Scholar]
- 14.Ledford BE, Jacobs DF. Translation kinetics in cultured mouse hepatoma cells. Regulation of albumin synthesis by amino acids. Eur J Biochem. 1985;152:611–618. doi: 10.1111/j.1432-1033.1985.tb09239.x. [DOI] [PubMed] [Google Scholar]
- 15.Lin W, Shuster S, Maibach HI, Stern R. Patterns of hyaluronan staining are modified by fixation techniques. J Histochem Cytochem. 1997;45:1157–1163. doi: 10.1177/002215549704500813. [DOI] [PubMed] [Google Scholar]
- 16.Lopes JM, Larbier M. Influence of amino-acid concentration in the culture medium on the rate of amino-acid incorporation into protein and amino-acid oxidation of cultured chicken hepatocytes. Reprod Nutr Dev. 1994;34:157–164. doi: 10.1051/rnd:19940207. [DOI] [PubMed] [Google Scholar]
- 17.Mankin HJ, Mow VC, Buckwalter JA, Iannotti JP, Ratcliffe A. Articular cartilage structure, composition, and function. In: Buckwalter JA, Einhorn TA, Simon SR, editors. Orthopaedic basic science. Biology and biomechanics of the musculoskeletal system. Rosemont: American Academy of Orthopaedic Surgeons; 2000. pp. 443–470. [Google Scholar]
- 18.Maroudas A, Palla G, Gilav E. Racemization of aspartic acid in human articular cartilage. Connect Tissue Res. 1992;28:161–169. doi: 10.3109/03008209209015033. [DOI] [PubMed] [Google Scholar]
- 19.Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003a;9:597–611. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 20.Mauck RL, Wang CC, Chen FH, Lu HH, Ateshian GA, Hung CT. Dynamic deformational loading of chondrocyte-seeded agarose hydro-gels modulates deposition and structural organization of matrix constituents. Proc ASME BIO. 2003b:531–532. [Google Scholar]
- 21.Mauck RL, Wang CC, Oswald ES, Ateshian GA, Hung CT. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthr Cartilage. 2003c;11:879–890. doi: 10.1016/j.joca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Mouw JK, Case ND, Guldberg RE, Plaas AH, Levenston ME. Variations in matrix composition and GAG fine structure among scaf-folds for cartilage tissue engineering. Osteoarthr Cartilage. 2005;13:828–836. doi: 10.1016/j.joca.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 23.Naomoto Y, Yamatsuji T, Shigemitsu K, Ban H, Nakajo T, Shirakawa Y, Motok T, Kobayashi M, Gunduz M, Tanaka N. Rational role of amino acids in intestinal epithelial cells (Review) Int J Mol Med. 2005;16:201–204. [PubMed] [Google Scholar]
- 24.Ng KW, Bian LM, Lin EY, Kelly TA, Ateshian GA, Hung CT. Essential amino acids supplementation modulates chondrocyte behavior in 3D agarose culture. Trans Orthop Res. 2006a;31:791. [Google Scholar]
- 25.Ng KW, Kugler LE, Kelly TA, DeFrancis JG, Ateshian GA, Hung CT. Enzymatic removal of agarose scaffold for tissue engineered cartilage. Proc ASME BIO. 2006b:157473. [Google Scholar]
- 26.Ng KW, Mauck RL, Statman LY, Lin EY, Ateshian GA, Hung CT. Dynamic deformational loading results in selective application of mechanical stimulation in a layered, tissue-engineered cartilage construct. Biorheology. 2006c;43:497–507. [PubMed] [Google Scholar]
- 27.Ng KW, Wang CC, Mauck RL, Kelly TA, Chahine NO, Costa KD, Ateshian GA, Hung CT. A layered agarose approach to fabricate depth-dependent inhomogeneity in chondrocyte-seeded constructs. J Orthop Res. 2005;23:134–141. doi: 10.1016/j.orthres.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Park CS, Chandler PT. Response to labeled precursor amino acids, varying cell density, and graded amino acid complement for protein synthesis in mammary cell culture. J Dairy Sci. 1976;59:216–223. doi: 10.3168/jds.S0022-0302(76)84187-2. [DOI] [PubMed] [Google Scholar]
- 29.Park CS, Chandler PT, Clark RM. Optimum amino acid complement for protein synthesis by rat mammary cells in tissue culture. J Dairy Sci. 1976;59:1758–1763. doi: 10.3168/jds.S0022-0302(76)84434-7. [DOI] [PubMed] [Google Scholar]
- 30.Price PJ, Gregory EA. Relationship between in vitro growth promotion and biophysical and biochemical properties of the serum supplement. In Vitro. 1982;18:576–584. doi: 10.1007/BF02810081. [DOI] [PubMed] [Google Scholar]
- Riesle J, Hollander AP, Langer R, Freed LE, Vunjak-Novakovic G. Collagen in tissue-engineered cartilage: types, structure, and crosslinks. J Cell Biochem. 1998;71:313–327. doi: 10.1002/(sici)1097-4644(19981201)71:3<313::aid-jcb1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 32.Roh SG, Baik MG, Choi YJ. The effect of lactogenic hormones on protein synthesis and amino acid uptake in rat mammary acinar cell culture at various physiological stages. Int J Biochem. 1994;26:479–485. doi: 10.1016/0020-711x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 33.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 34.Thissen JP, Pucilowska JB, Underwood LE. Differential regulation of insulin-like growth factor I (IGF-I) and IGF binding protein-1 .messenger ribonucleic acids by amino acid availability and growth hormone in rat hepatocyte primary culture. Endocrinology. 1994;134:1570–1576. doi: 10.1210/endo.134.3.7509741. [DOI] [PubMed] [Google Scholar]
- 35.Tonomura H, Takahashi KA, Mazda O, Arai Y, Inoue A, Terauchi R, ShinYa M, Kishida T, Imanishi J, Kubo T. Glutamine protects articular chondrocytes from heat stress and NO-induced apoptosis with HSP70 expression. Osteoarthr Cartilage. 2006;14:545–553. doi: 10.1016/j.joca.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Verzijl N, DeGroot J, Thorpe SR, Bank RA, Shaw JN, Lyons TJ, Bijlsma JW, Lafeber FP, Baynes JW, TeKoppele JM. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275:39027–39031. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- 37.Washizu J, Chan C, Berthiaume F, Tompkins RG, Toner M, Yarmush ML. Amino acid supplementation improves cell-specific functions of the rat hepatocytes exposed to human plasma. Tissue Eng. 2000;6:497–504. doi: 10.1089/107632700750022143. [DOI] [PubMed] [Google Scholar]
- 38.Williamson AK, Masuda K, Thonar EJ, Sah RL. Growth of immature articular cartilage in vitro: correlated variation in tensile biomechanical and collagen network properties. Tissue Eng. 2003;9:625–634. doi: 10.1089/107632703768247322. [DOI] [PubMed] [Google Scholar]
- 39.Wong M, Ponticiello M, Kovanen V, Jurvelin JS. Volumetric changes of articular cartilage during stress relaxation in unconfined compression. J Biomech. 2000;33:1049–1054. doi: 10.1016/s0021-9290(00)00084-1. [DOI] [PubMed] [Google Scholar]