Abstract
Background
Myocardial fibrosis is a hallmark of hypertrophic cardiomyopathy (HCM) and a potential substrate for arrhythmias and heart failure. Sarcomere mutations appear to induce profibrotic changes before left ventricular hypertrophy (LVH) develops. To further evaluate these processes, we used cardiac magnetic resonance (CMR) with T1 measurements on a genotyped HCM population to quantify myocardial extracellular volume (ECV).
Methods and Results
Sarcomere mutation carriers with LVH (G+/LVH+, n = 37) and without LVH (G+/LVH−, n = 29); HCM patients without mutations (sarcomere-negative HCM, n = 11); and healthy controls (n = 11) underwent contrast CMR, measuring T1 times pre- and post-gadolinium infusion. Concurrent echocardiography and serum biomarkers of collagen synthesis, hemodynamic stress, and myocardial injury were also available in a subset. Compared to controls, ECV was increased in patients with overt HCM, as well as G+/LVH− mutation carriers (ECV= 0.36±0.01, 0.33±0.01, 0.27±0.01 in G+/LVH+, G+/LVH−, controls, respectively, P≤0.001 for all comparisons). ECV correlated with NT-proBNP levels (r = 0.58, P<0.001) and global E’ velocity (r = −0.48, P<0.001). Late gadolinium enhancement (LGE) was present in >60% of overt HCM patients but absent from G+/LVH− subjects. Both ECV and LGE were more extensive in sarcomeric HCM than sarcomere-negative HCM.
Conclusions
Myocardial ECV is increased in HCM sarcomere mutation carriers even in the absence of LVH. These data provide additional support that fibrotic remodeling is triggered early in disease pathogenesis. Quantifying ECV may help characterize the development myocardial fibrosis in HCM and ultimately assist in developing novel disease-modifying therapy, targeting interstitial fibrosis.
Keywords: hypertrophic cardiomyopathy, genetics, magnetic resonance imaging, fibrosis, gadolinium
Hypertrophic cardiomyopathy (HCM) is caused by mutations in genes encoding sarcomere proteins.1, 2 The clinical diagnosis of HCM hinges on the presence of unexplained left ventricular hypertrophy (LVH). In contrast, genetic testing uniquely identifies family members with pathogenic sarcomere gene mutations (G+) who are at risk for developing HCM even when LV wall thickness is normal (LVH−). By studying this subclinical genotype-positive, LVH− negative (G+/LVH−) population, early phenotypes of sarcomere mutations can be characterized, without the confounding influence of pathologic changes that accompany overt disease. Impaired LV relaxation and altered myocardial energetics have been demonstrated in G+/LVH− subjects, indicating that sarcomere mutations cause primary cardiac abnormalities independently of LVH.3–5
Myocardial fibrosis is a histopathologic hallmark of HCM, present as both dense replacement fibrosis (scar) and interstitial fibrosis. Fibrosis is presumed to play a role in important outcomes such as sudden cardiac death, ventricular tachyarrhythmias, LV dysfunction, and heart failure.6–10 However, the precise triggers that lead to the development of fibrosis are unknown. Transcriptional profiling performed in mouse models of HCM, genetically engineered to carry sarcomere mutations that cause human disease, have demonstrated that pathways involved in fibrosis and collagen deposition are activated early, before gross or histologic LVH was detectable.11 More recent human studies have shown that serum levels of carboxy-terminal propeptide of procollagen type I (PICP), a biomarker of collagen type I synthesis, were increased in G+/LVH− subjects.12 These studies suggest that a profibrotic milieu is present in the early stages of disease pathogenesis, when cardiac morphology appears normal.
Gadolinium enhanced cardiac magnetic resonance (CMR) allows noninvasive visualization of extracellular volume expansion and myocardial fibrosis.13 Gadolinium diffuses into the interstitial space between cells, but does not cross intact cell membranes. In scar and when the extracellular space is expanded (fibrosis, edema, infiltration), the volume of distribution of gadolinium is increased, resulting in accumulation, delayed clearance, and persistently higher concentrations of gadolinium relative to normal myocardium.14, 15 Late gadolinium enhancement (LGE) is visualized in over 60% of patients with clinically overt HCM and likely represents dense replacement fibrosis.8,16–23 However, there are important limitations of current LGE-based techniques, including the requirement to null the signal to a normal myocardial reference region.13 In conditions such as HCM and aortic stenosis, fibrosis is diffuse and thus the reference myocardium is likely abnormal, leading to underestimation of the presence, severity, and extent of myocardial involvement.24, 25
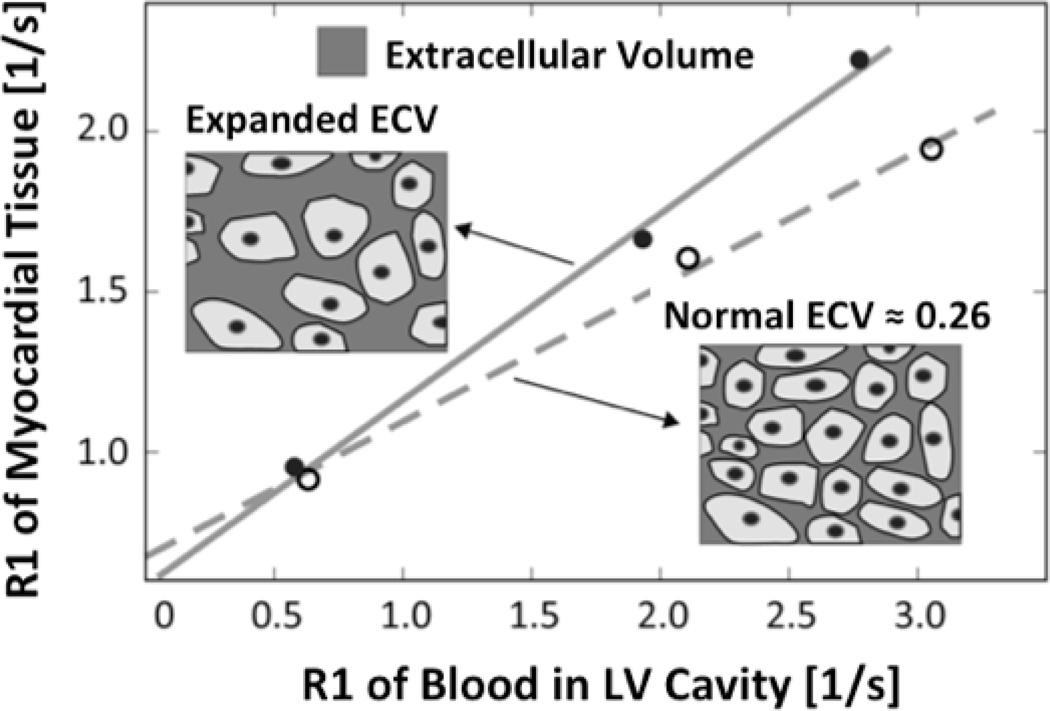
Techniques for measuring myocardial and blood T1 times or rates (R1=1/T1) before and after gadolinium administration have been applied to overcome limitations related to the binary detection of LGE. With these techniques, diffuse myocardial abnormalities, including interstitial fibrosis, can be quantified and are reflected by an increased volume of distribution of gadolinium. As illustrated in Figure 1, myocardial extracellular volume (ECV) can be robustly quantified by comparing changes in relaxation rates between pre- and post-contrast states in the myocardium relative to blood.25–29 As such, these techniques provide a non-invasive means of assessing extracellular volume expansion and diffuse myocardial fibrosis. T1 measurements have been validated against histological measures of myocardial collagen content in patients with aortic stenosis and HCM.25 Shorter contrast-enhanced T1 times may also identify subclinical myocardial dysfunction in diabetic patients.30 In this study, we aimed to test whether myocardial ECV is increased in sarcomere mutation carriers with overt HCM, as well as at-risk individuals without LVH. We also examined the pathophysiologic impact of ECV expansion by investigating their correlation with tissue Doppler echocardiography and serum biomarkers of collagen type I synthesis, myocardial injury, and hemodynamic stress.
Figure 1. Estimating the myocardial extracellular volume from T1 measurements.
CMR T1 measurements before and after gadolinium contrast administration are used to determine the change of the relaxation rate (R1=1/T1) in myocardial tissue relative to blood. Data are derived from the slope of a least-squares linear regression that is fit to the measured R1 data. The slope corresponds to the partition-coefficient for the extracellular gadolinium contrast in myocardial tissue. By multiplying the partition coefficient with (1-hematocrit), to adjust for the blood contrast volume of distribution, the extracellular volume (ECV) fraction of myocardial tissue can be estimated. An expansion of the extracellular space corresponds to an increase of the slope of the regression line. The figure insets illustrate the extracellular space and intact cardiac myocytes (from which gadolinium is excluded) in normal myocardium, and with expanded ECV.
Methods
Study Population
Written informed consent was obtained from all participants in protocols approved by the Institutional Review Board of Brigham and Women’s Hospital/ Partners Healthcare, Boston, MA. Genotyped HCM patients and relatives were identified via research protocols or clinical evaluation. Sarcomere mutations carriers (G+) had a pathogenic or presumed pathogenic HCM mutation, as defined by standard criteria.4 Subjects were designated as having overt HCM (G+/LVH+) or subclinical HCM (G+/LVH−) based on echocardiographically-determined LV wall thickness, as previously described.12 The overt HCM cohort included sarcomere mutation carriers (G+) with a maximal LV wall thickness by ≥12 mm. The G+/LVH− HCM cohort consisted of sarcomere mutation carriers without LVH (maximal LV wall thickness <12 mm). More rigorous criteria were used to define study groups than routinely used to diagnose clinical HCM31 in order to avoid including individuals with borderline LVH and emerging or mild cardiomyopathy in the G+/LVH− group. All G+/LVH− subjects were confirmed to have normal LV wall thickness by CMR.
Control subjects were healthy volunteers and relatives who did not carry a sarcomere mutation (G-/LVH−). Sarcomere-negative HCM patients had a clinical diagnosis of HCM, based on echocardiographic evidence of unexplained LVH, but had negative genetic testing. In these subjects, direct DNA sequence analysis of 8 sarcomere genes (MYH7, MYBPC3, TNNT2, TNNI3, TPM1, ACTC, MYL2, MYL3), PRKAG2, or LAMP2 failed to identify a pathogenic or likely pathogenic mutation. Exclusion criteria included systemic hypertension (SBP >140 or DBP>90 mmHg or on medical therapy), infiltrative or storage disease, coronary artery disease, valvular heart disease other than that associated with systolic anterior motion of the mitral valve, prior septal myectomy or alcohol septal ablation, presence of a permanent pacemaker or implantable cardioverter-defibrillator, contraindications to gadolinium administration, and atrial fibrillation.
Standard CMR Protocol
All image acquisitions were done with the subject in the supine position and utilizing both EKG-gating and breath-holding. All subjects were imaged on a 3.0 Tesla system (Tim Trio, Siemens, Erlangen, Germany). The standard protocol consisted of cine steady-state free precession (SSFP) imaging (repetition time, 3.4 ms; echo time, 1.2 ms; in-plane spatial resolution, 1.6 × 2 mm, temporal resolution 40–50 msec) for LV function and LV mass.32 Cine imaging was obtained in 8 to 14 matching short-axis planes (8 mm thick with no gap) and 3 radial long-axis planes. For the calculation of LV mass and function, the endocardial and epicardial borders of the LV myocardium were manually traced on successive short-axis cine images at end-diastole and systole. The papillary muscles were excluded in the LV mass calculation. LV mass was derived by the summation of discs method and multiplying the myocardial muscle volume by 1.05 g/cm and indexed to BSA.33 LV wall thickness was measured in at least four sections – anterior and posterior septal, lateral and inferior – in all subjects. The location and measurement of maximal LV wall thickness were also noted.
Late Gadolinium Enhancement
All patients underwent an LGE imaging protocol (TR, 4.8 ms; TE, 1.3 ms; inversion time, 200 to 300 ms) to detect focal myocardial fibrosis. A segmented inversion-recovery pulse sequence for LGE was used starting 10–15 minutes after cumulative 0.15-mmol/kg dose of gadolinium-DTPA (Bayer HealthCare Pharmaceuticals Inc, Wayne, New Jersey). LGE images were obtained in 8 to 14 matching short-axis (8 mm thick with no gap) and 3 radial long-axis planes. LGE was quantified using a semi-automated gray-scale threshold technique using a cutoff of 6 SDs above the mean signal intensity.24 The quantity of LGE was expressed in grams and as a percentage of the total LV myocardial mass. All LGE analyses were performed using commercially available software (QMassMR, version 7.4; Medis, Leiden, the Netherlands).
T1 Protocol and Analysis
T1 measurements were performed with a Look-Locker sequence (5,6) with a non-slice-selective adiabatic inversion pulse, followed by segmented gradient-echo acquisition for 17 cardiac phases/times after inversion (TI’s), spread over 2 cardiac cycles (temporal resolution 80–100 ms pre-contrast, and 45–55 ms post-contrast, slice thickness 8 mm, TR > 3 RR intervals pre-contrast and 2 RR intervals post-contrast). The Look-Locker sequence was repeated in three parallel mid LV short-axis slices (basal, mid, and distal), once before, and 3–5 additional times after the injection of gadolinium spanning a 30-minute period. For each Look-Locker sequence the endo- and epicardial borders of the three short axis slices were traced and divided into the standard 16-segment model (QMassMR, version 7.4; Medis, Leiden, the Netherlands). The signal intensity versus time curves for each segment and the blood pool were used to determine segmental T1 through fitting to an analytical expression for the inversion recovery, and correction for the radiofrequency pulse alteration of the inversion recovery.34, 35
The accuracy of T1 measurements with the Look-Locker method was tested in Gd-doped phantoms, against the standard inversion-recovery spin-echo technique. The mean difference of R1 in 10 phantoms, using the Look-Locker and IR-spin-echo techniques was 0.069 s−1 (Bland-Altman 5% and 95% limits of agreement: −0.152 to 0.013), with the mean R1 covering a range from 0.36 to 6.7 s−1. The intraclass correlation coefficient was 0.997 with a 95% confidence interval of 0.992 to 0.999.
T1 relaxation rate, calculated as the reciprocal of T1 (R1=1/T1), was used to plot the myocardial R1 against the R1 in the blood pool, and calculate the slope by linear regression, using all measurement points with an R1 of less than 3 s−1. The slope of the linear relationship defines the partition coefficient for gadolinium, λGd (Figure 1). From the slope of this relationship, the myocardial extracellular volume (ECV), was obtained by multiplying each of the segmental λGd by (1-hematocrit in percent/100) as reported previously.28 An ECV fraction for each subject was then calculated by averaging the 16 myocardial segmental values from the 3 short-axis slices. Due to technical limitations in 18 subjects, a single representative mid-LV slice was used for analysis. In subjects with regional fibrosis detected by LGE, we also calculated a second ECV after excluding regions that contained LGE.
Echocardiographic Protocol
The Vivid-7 ultrasound system (GE Medical Systems, Milwaukee, WI) was used for standard echocardiography and tissue Doppler interrogation (TDI). Standard measures of cardiac dimensions and function from 2D and spectral Doppler analysis were determined using the mean of 3 cardiac cycles, in accordance with guidelines of the American Society of Echocardiography.36 Tissue Doppler myocardial velocities in early diastole (E’) at the lateral, septal, anterior, and inferior aspects of the mitral annulus in the apical 4- and 2-chamber views. Global values of E’ were calculated as the average of these 4 measurements and reflect the velocity of early myocardial relaxation. Images were analyzed by two observers blinded to clinical and genetic status. Echocardiographic studies performed within 1 year of CMR imaging were included in this study.
Measurement of Serum Biomarkers
Blood samples (serum and K3-EDTA plasma) were available on an unselected subset of subjects who also participated in research protocols that included collection of blood for biomarker analysis. Samples were obtained at the time of cardiac imaging, processed within 60 minutes of phlebotomy, and stored at −80° C prior to analysis. All assays were performed using commercially available reagents by personnel who were blinded to clinical and genetic status. The following markers were analyzed: PICP (Quidel Corporation, San Diego, CA), amino terminal propeptide of B-type natriuretic peptide (NT-pro BNP; Roche, Indianapolis, IN), and supersensitive cardiac troponin I (Singulex, Atlanta, GA).
Statistical Analysis
To test for differences between the 3 status groups or between overt HCM patients with and without sarcomere mutations, analysis of variance (ANOVA) and logistic regression were performed with clustering to adjust for family relationships. An exchangeable correlation structure was assumed within families, except in one case where convergence issues required initial estimates based on independence, with correlation accounted for in the standard errors in the final estimates. Age-dependent parameters of tissue Doppler, ECV, PICP, and NT-proBNP levels were adjusted for age as well as family relationships; analyses were also adjusted for gender. Except where noted, values are expressed as adjusted mean ± standard error. For analyses across the 3 status groups (G+/LVH+, G+/LVH−, control), a P-value <0.017 was considered statistically significant to apply post-hoc Bonferroni correction for multiple comparisons. Pearson’s correlation was used to evaluate for associations between continuous measures. All analyses were performed with SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Clinical Characteristics
Eighty-eight individuals from 68 unrelated families were included in this study. These included 37 G+/LVH+ overt HCM subjects, 29 G+/LVH− subjects, 11 normal controls, and 11 sarcomere-negative HCM subjects. General clinical characteristics of sarcomere mutation carriers and normal controls are summarized in Table 1. Forty-eight different mutations in 6 sarcomere genes were represented, listed in the Supplemental Table: β-myosin heavy chain (MYH7, n=20), cardiac myosin binding protein C (MYBPC3, n=39), cardiac troponin T (TNNT2, n=5), cardiac troponin I (TNN13, n=1), myosin essential light chain (MYL2, n=1), and myosin regulatory light chain (MYL3, n=1).
Table 1.
Clinical Characteristics
| Control n=11 |
P-value† Control vs G+/LVH− |
G+/LVH n=29 |
P-value† G+/LVH− vs G+/LVH+ |
G+/LVH+ n=37 |
P-value† Control vs G+/LVH+ |
|
|---|---|---|---|---|---|---|
| Age, years ± Standard Deviation (range) | 27.2 ± 7.1 (19–39) | 0.50 | 28.1 ± 9.5 (16–48) | <0.001 | 41.3 ± 14.1 (19–73) | <0.001 |
| Female, % (#female/ #male) | 60% (6/4) | 0.67 | 59% (17/12) | 0.03 | 32% (12/25) | 0.19 |
| Causal Gene, n | ||||||
| MYH7 | 12 | 8 | ||||
| MYBPC3 | 14 | 25 | ||||
| TNNT2 | 3 | 2 | ||||
| TNNI3 | 0 | 1 | ||||
| MYL2 | 0 | 1 | ||||
| MYL3 | 0 | 1 | ||||
| BSA, m2 | 1.89 ± 0.07 | 0.13 | 1.79 ± 0.04 | 0.03 | 1.95 ± 0.04 | 0.52 |
| CMR Maximal LV wall thickness, mm | 7.8 ± 0.4 | 0.54 | 8.1 ± 0.4 | <0.001 | 18.1 ± 0.8 | <0.001 |
| CMR LV Mass, g | 106 ± 8 | 0.20 | 96 ± 7 | <0.001 | 156 ± 9 | <0.001 |
| CMR LV Mass index, g/m2 | 52 ± 5 | 0.63 | 49 ± 3 | <0.001 | 84 ± 5 | <0.001 |
| CMR LVEDV, ml | 175 ± 11 | 0.06 | 154 ± 7 | 0.30 | 164 ± 6 | 0.49 |
| CMR LVESV, ml | 70 ± 4 | 0.09 | 61 ± 3 | 0.78 | 60 ± 4 | 0.13 |
| CMR LV EF, % | 59 ± 1 | 0.08 | 62 ± 1 | 0.35 | 63 ± 1 | 0.03 |
Data other than age are presented as means ± standard error, adjusted for family relations, gender, and age
p values <0.017 are statistically significant and adjusted for age and family relations
CMR= Cardiac magnetic resonance; BSA = body surface area; LV = left ventricle; LVEDV= LV end-diastolic volume; LVESV= LV end-systolic volume; EF = ejection fraction
Echocardiographic studies were available in 76 subjects; 67% of studies were performed within 24 hours of CMR (median time difference 0 ± 73 days; range 0–349 days). Serum biomarker analysis was additionally available in 53 of these subjects (G+/LVH+, n=20; G+/LVH−, n=27; controls, n=6) and results are presented in Table 2. Serum PICP levels were significantly higher in G+/LVH+ and G+/LVH− subjects compared to healthy controls. Serum NT-proBNP levels were significantly higher in G+/LVH+ subjects compared to G+/LVH− and healthy controls. There were no significant differences in supersensitive cardiac troponin I levels across the cohorts.
Table 2.
CMR Metrics of Fibrosis and Serum Biomarker Levels
|
Control n=11 |
P-value* Control vs G+/LVH− |
G+/LVH n=29 |
P-value* G+/LVH− vs G+/LVH+ |
G+/LVH+ n=37 |
P-value* Control vs G+/LVH+ |
|
|---|---|---|---|---|---|---|
| ECV (range) | 0.27 ± 0.01 (0.24–0.31) | <0.001 | 0.33 ± 0.01 (0.23–0.38) | 0.001 | 0.36 ± 0.01 (0.31–0.51) | <0.001 |
| LGE present, % (#yes / #no) | 0% (0/11) | 0% (0/29) | 78% (29/8) | |||
| LGE, g ± Standard Deviation (range) | 0 | 0 | 0.004 | 15.6 ± 25.8 (0.0–105.6) | 0.003 | |
| LGE, % LV mass ± Standard Deviation (range) | 0 | 0 | <0.001 | 7.4 ± 10.4 (0.0–44.6%) | 0.001 | |
| PICP, µg/L | 69.3 ± 4.1 | <0.001 | 89.1 ± 4.7 | 0.48 | 97.37 ± 9.5 | 0.015 |
| Singulex cTnI, pg/ml | 3.1 ± 1.5 | 0.38 | 6.0 ± 3.7 | 0.24 | 14.2 ± 5.5 | 0.09 |
| NT-proBNP, pg/ml | 52.9 ± 52.0 | 0.24 | 81.3 ± 44.5 | 0.002 | 325.8 ± 79.2 | 0.002 |
LGE data are presented as unadjusted mean ± standard deviation
All other data are presented as means ± standard error, adjusted for family relations, gender, and age
p values <0.017 are statistically significant and adjusted for age and family relations
ECV= extracellular volume; LGE = late gadolinium enhancement; PICP= carboxy-terminal propeptide of procollagen type I; cTnI= cardiac troponin I; NTproBNP= amino terminal propeptide of B-type natriuretic peptide
T1 Measurement Results
Overall Cohort
CMR variables are presented in Table 2. Compared to normal controls, the ECV was increased in sarcomere mutation carriers, both with overt HCM and with normal LV wall thickness (Figure 2). There were significant correlations between ECV and serum NT-proBNP levels (Pearson r = 0.54, P<0.001 for NT-proBNP; r = 0.58, P<0.001 for log NT-proBNP) and global E’ velocity (Pearson r = −0.48, P<0.001), illustrated in Figure 3. There was a weak but significant correlation between ECV and myocardial mass (Pearson r = 0.36, p<0.001). There was a weak correlation between LGE, but not ECV, and LVEF (Pearson r= −0.37, p<0.001). No significant correlation was seen between ECV or LGE and serum PICP or supersensitive troponin levels. No obvious genotypic influence was detected. No significant differences in ECV were seen comparing MYH7 and MYBPC3 mutation carriers, with or without LVH (data not shown). For the overall cohort, the average pre-contrast T1 was ∼1087±81 ms (mean ± SD) in myocardial segments, and ∼1556±152 ms in the LV blood pool.
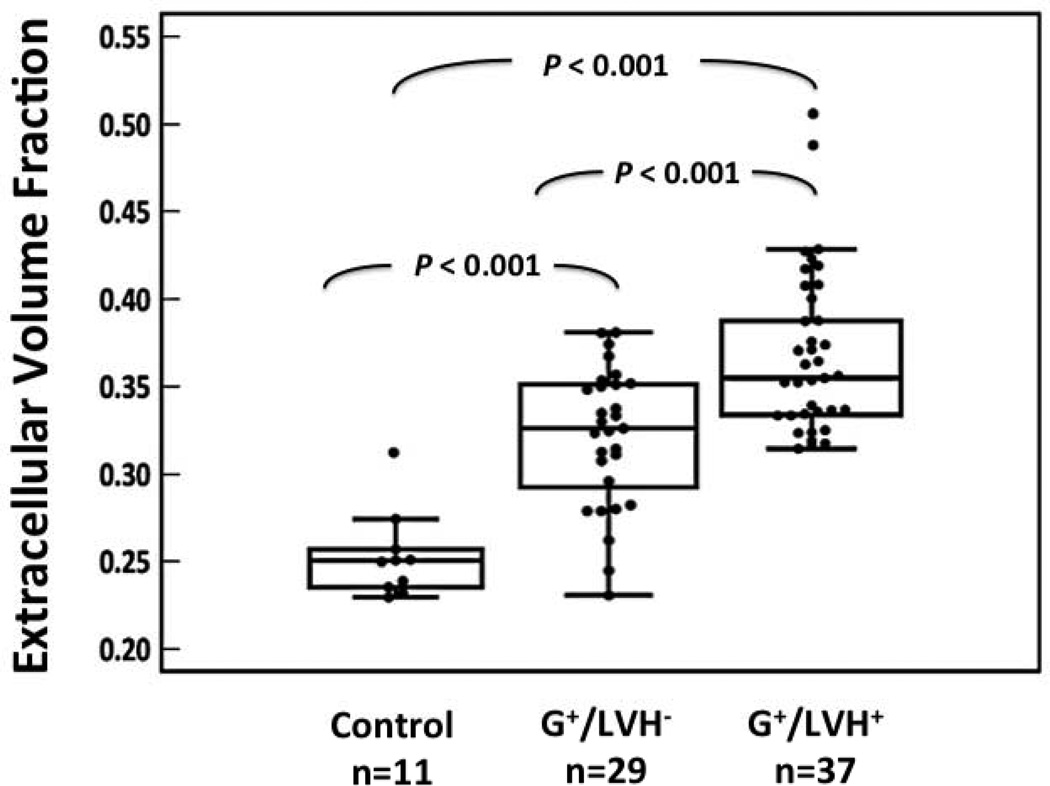
Figure 2. The myocardial extracellular volume is significantly increased in HCM sarcomere mutation carriers with and without LVH.
Compared to normal controls, ECV was 27% higher in G+/LVH− subjects and 42% higher in G+/LVH+ HCM patients. ECV in overt HCM subjects was 12% higher than G+/LVH− subjects (P ≤0.001 for all comparisons).
Figure 3. The myocardial extracellular volume is correlated to serum NT-proBNP levels and E’ velocity.
a) ECV was significantly positively correlated to serum NT-proBNP levels in the overall cohort excluding sarcomere-negative HCM patients (serum biomarkers not available for this cohort).
b) ECV was significantly inversely correlated echocardiographic global E’ velocities in the overall cohort
G+/LVH− Cohort
The mean age of G+/LVH− subjects was 28.1 ± 9.5 years (range 16–48 years) and 59% were female. The myocardial ECV was 0.06 (22%) higher in G+/LVH− subjects (ECV = 0.33 ± 0.01) compared to controls (ECV = 0.27 ± 0.01; P<0.001; Table 2 and Figure 2). Despite increased ECV and serum PICP levels, LGE was not detected in any G+/LVH− subjects (Figure 4). Compared to normal controls, there were no significant differences in age, LV wall thickness or LV mass. Subjects with MYH7 mutations were younger than those with MYBPC3 mutations (24.6 vs 31.9 years, p=0.04). MYH7 mutation carriers also had significantly lower global E’ velocity (13.2 ± 0.7 vs 15.4 ± 0.4 cm/sec, p=0.003).
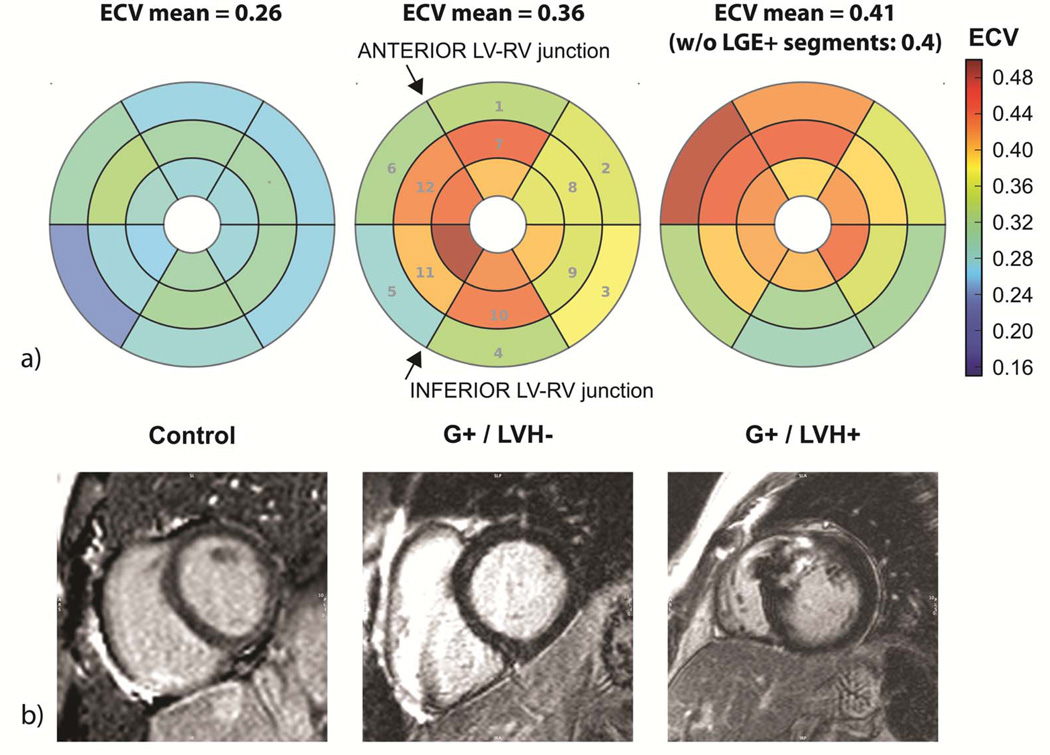
Figure 4. Graphic comparison of T1 measurements and late gadolinium enhancement.
a) Bull’s eye representation of results from segmental analysis (numbers correspond to AHA segment numbering for basal and mid-level slices) of T1 measurements before and after gadolinium contrast administration. Compared to healthy controls (images on left), the myocardial extracellular volume was elevated in sarcomere mutation carriers (G+), both in the absence (middle) or presence (right) of LVH. In the G+/LVH+ subject, the ECV remained highly abnormal if LGE+ segments were excluded.
b) Representative LGE images from the same subjects, in mid-level locations corresponding to one of the levels for the T1 measurements of ECV. LGE is not seen in the normal control or the G+/LVH− subject, despite significantly increased ECV in the latter. The G+/LVH+ subject with overt HCM demonstrates prominent LGE near the anterior LV-RV junction.
Overt HCM Cohort
The mean age of G+/LVH+ patients was 41.3 ± 14.1 years (range 19–73 years) and 32% were female. The myocardial ECV was highest in this subgroup, even after excluding segments with LGE in subjects with overt HCM. In G+/LVH+ subjects, ECV measured 0.36 ± 0.01; 0.09 (33%) higher than controls, and 0.03 (9%) higher than G+/LVH− subjects (P≤ 0.001 for all comparisons; Table 2 and Figure 2). LGE was present in 78% of sarcomere mutation carriers with overt HCM (Table 2 and Figure 4). Age, LV wall thickness, and LV mass were significantly higher in G+/LVH+ subjects compared to G+/LVH− subjects and normal controls.
G+/LVH+ patients with sarcomeric HCM were compared to patients with sarcomere-negative HCM. Results are summarized in Table 3. Patients with sarcomeric HCM had more pronounced abnormalities in CMR metrics of myocardial fibrosis than HCM patients without sarcomere mutations. Although there was no significant difference in the prevalence of LGE, the extent was greater in patients with sarcomere mutations. ECV was also higher in patients with sarcomeric HCM, even after excluding segments with LGE. There was a strong correlation between ECV and the extent of LGE (Pearson r = 0.76, P<0.001), and a weak correlation between both ECV and LGE and LVEF (Pearson r= −0.42– −0.5, p≤0.003).
Table 3.
Comparison of patients with clinically overt HCM with and without sarcomere mutations
|
Sarcomere (+) HCM n=37 |
Sarcomere (−) HCM n=11 |
P-value* | |
|---|---|---|---|
| Age, years ± Standard Deviation (range) | 41.3 ± 14.1 (19–73) | 44.6 ± 11.1 (25–63) | 0.29 |
| Female, % (#female/ #male) | 32% (12/25) | 9% (1/10) | 0.17 |
| BSA, m2 | 1.93 ± 0.04 | 2.21 ± 0.06 | <0.0001 |
| Maximal LV wall thickness, mm | 18.6 ± 0.8 | 17.7 ± 1.2 | 0.54 |
| LV Mass, g | 169 ± 12 | 200 ± 15 | 0.11 |
| LV Mass index, g/m2 | 87 ± 5 | 91 ± 7 | 0.65 |
| LV EF, % | 62 ± 1 | 69 ± 2 | 0.01 |
| ECV | 0.37 ± 0.01 | 0.35 ± 0.01 | 0.03 |
| ECV, excluding LGE | 0.37 ± 0.00 | 0.34 ± 0.01 | 0.04 |
| LGE present, % (#yes/#no) | 78% (29/8) | 64% (7/4) | 0.35 |
| LGE, g | 15.9 ± 4.2 | 4.3 ± 2.2 | 0.02 |
| LGE, % LV mass | 18.2 ± 0.04 | 5.2 ± 3.7 | <0.001 |
Data are presented as means ± standard error, adjusted for family relations and age
p values <0.05 are statistically significant and adjusted for age and family relations
BSA = body surface area; ECV = extracellular volume; LGE = late gadolinium enhancement
Discussion
We report evidence of abnormal extracellular volume expansion in hypertrophic cardiomyopathy. Cardiac magnetic resonance imaging with pre- and post-contrast T1 measurements demonstrated a significant increase in myocardial ECV not only in patients with clinically overt disease, but notably also in sarcomere mutation carriers with normal LV wall thickness. These results suggest that HCM sarcomere mutations lead to myocardial abnormalities, potentially reflecting increased interstitial fibrosis, independently of LVH. These findings are an important advance from LGE methodology that identifies dense, focal scar but cannot capture more diffuse expansion of the extracellular space, such as caused by interstitial fibrosis, edema, inflammation, or infiltrative processes. Indeed, myocardial ECV appears to be more discriminating for the presence of a sarcomere mutation than LGE, as LGE is very rarely seen in mutation carriers prior to the development of LVH, and was not present in our cohort of G+/LVH− subjects (Figure 4).
We have postulated that myocardial fibrosis is a fundamental and early consequence of sarcomere mutations rather than simply a downstream response to the LVH, outflow obstruction, and ischemia associated with clinically overt HCM.12 This hypothesis was initially based on unbiased transcriptional profiling in a mouse model of HCM. These studies demonstrated that young animals with sarcomere mutations had significant and early upregulation of genes involved in extracellular matrix formation.11 Genetic pathways were activated well before histologic or gross LVH or fibrosis developed. More recent human studies similarly indicated that myocardial type I collagen synthesis is increased in sarcomere mutation carriers, in the absence of LVH or LGE.12 We now describe expansion of myocardial extracellular volume in the at-risk G+/LVH− population. Although the pathways have not yet been characterized, collectively these findings suggest that sarcomere gene mutations drive a profibrotic state early in disease pathogenesis.
We also explored the potential functional and pathophysiological impact of increased myocardial ECV. There was a significant positive correlation between ECV and serum NT-proBNP levels, and a significant negative correlation between ECV and global E’ velocities. These observations are consistent with the theory that extracellular volume expansion results in diastolic dysfunction. These changes may contribute to the reduced E’ velocities seen in G+/LVH− mutation carriers.3, 4
Our findings additionally indicate that the fibrotic burden is higher in HCM patients with sarcomere mutations compared to those without a sarcomere mutation. Both LGE and ECV were significantly more extensive in sarcomeric HCM. These results may partly underlie the worse outcomes reported in sarcomere-positive versus sarcomere-negative HCM, where an increased risk of cardiovascular death, nonfatal stroke, or worsening NYHA functional class was observed in patients with sarcomere mutations.37
Important limitations of this study are the relatively small population and the cross-sectional design, measuring T1 values in two distinct stages of HCM: G+/LVH− and G+/LVH+. Moreover, T1 quantification of ECV is not specific for myocardial fibrosis. Myocardial edema, inflammation, infiltrative processes, and increased sarcolemmal permeability will also lead to increased ECV. In this study, there was no significant positive correlation between ECV and serum PICP levels, a biomarker of collagen synthesis. Comprehensive and longitudinal studies in larger populations, anchored by genotype, are needed to better define the processes that truly underlie abnormalities of serum biomarkers, altered myocardial gadolinium distribution, and myocardial fibrosis in the pathogenesis of HCM. Such critical studies will also allow assessment of their impact on key disease manifestations, such as the development of arrhythmias and heart failure.
CMR T1 measurement is another tool to study disease biology, identifying and quantifying myocardial extracellular volume expansion. Previous reports have demonstrated abnormal expansion of the extracellular space using pre-contrast T1 mapping methods,38 as well as pre- and post-contrast T1 mapping methods 39–41 in patients with phenotypically-established HCM and left ventricular hypertrophy. In this report, we extend this experience to at-risk individuals without overt LVH to test the hypothesis that expansion of the extracellular space precedes the development of gross structural changes. Along with diastolic dysfunction,3, 4 impaired energetics,5 and increased collagen synthesis,12 these findings of myocardial ECV expansion provide further evidence that sarcomere gene mutations detrimentally impact the heart prior to the development of left ventricular hypertrophy. A profibrotic state appears to a fundamental feature of HCM and an early phenotype of sarcomere mutations. This profibrotic milieu may contribute importantly to diastolic abnormalities and adverse clinical outcomes in HCM. However, in contrast to dense scar, formation of interstitial fibrosis may be a more dynamic and reversible process.42–44 As such, monitoring ECV could provide a means of following disease progression as well as response to future, disease-modifying therapy. Developing treatments targeted at interstitial fibrosis may have the potential to change the pathophysiologic substrate, thereby diminishing disease progression and reducing the risk of arrhythmias and heart failure in HCM.
Supplementary Material
Clinical Summary.
Hypertrophic cardiomyopathy (HCM) is caused by mutations in sarcomere genes. Increased myocardial fibrosis is a histopathologic hallmark of HCM and thought to contribute to important outcomes such as sudden cardiac death, ventricular tachyarrhythmias, and heart failure. However, the triggers that lead to its development are unknown. Animal and human investigations suggest that a profibrotic milieu is present early in disease pathogenesis, even when cardiac morphology appears normal. In this study, we measured T1 times by cardiac magnetic resonance imaging to characterize extracellular volume (ECV) expansion in HCM and to test the hypothesis that expansion of the extracellular space precedes the development of cardiac hypertrophy. We found that myocardial ECV was significantly increased not only in patients with clinically overt disease, but also in at-risk mutation carriers with normal LV wall thickness. We also explored the potential functional impact of increased ECV. There was a significant positive correlation between ECV and serum NT-proBNP levels, and a negative correlation between ECV and global E’ velocities, suggesting that increased ECV impairs LV filling. Along with diastolic dysfunction, altered energetics, and increased collagen synthesis, these findings of myocardial ECV expansion provide further evidence that sarcomere gene mutations detrimentally impact the heart before gross structural changes develop. A profibrotic state appears to be a fundamental, early phenotype of sarcomere mutations and may contribute to diastolic abnormalities and adverse clinical outcomes in HCM. Developing treatments to reduce interstitial fibrosis may diminish disease progression and decrease the risk of arrhythmias and heart failure in HCM.
Acknowledgments
Sources of Funding
This work was supported by grants from the National Institutes of Health (CYH and RYK); the agreement between FIMA and Unión Temporal de Empresas project CIMA (AG, BL, JD); Ministry of Economy and Competitiveness, Spain (RECAVA and Ramon y Cajal Program) (AG, BL, JD) and the European Union (MEDIA and EU-MASCARA projects) (AG, BL, JD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Dr. Jerosch-Herold is listed as co-inventor on a pending patent application related to detection of diffuse fibrosis by MRI. Otherwise, no other authors have any relevant financial disclosures.
References
- 1.Seidman JG, Seidman C. The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell. 2001;104:557–567. doi: 10.1016/s0092-8674(01)00242-2. [DOI] [PubMed] [Google Scholar]
- 2.Richard P, Villard E, Charron P, Isnard R. The genetic bases of cardiomyopathies. J Am Coll Cardiol. 2006;48:A79–A89. [Google Scholar]
- 3.Ho CY, Sweitzer NK, McDonough B, Maron BJ, Casey SA, Seidman JG, Seidman CE, Solomon SD. Assessment of diastolic function with Doppler tissue imaging to predict genotype in preclinical hypertrophic cardiomyopathy. Circulation. 2002;105:2992–2997. doi: 10.1161/01.cir.0000019070.70491.6d. [DOI] [PubMed] [Google Scholar]
- 4.Ho CY, Carlsen C, Thune JJ, Havndrup O, Bundgaard H, Farrohi F, Rivero J, Cirino AL, Andersen PS, Christiansen M, Maron BJ, Orav EJ, Kober L. Echocardiographic Strain Imaging to Assess Early and Late Consequences of Sarcomere Mutations in Hypertrophic Cardiomyopathy. Circulation: Cardiovascular Genetics. 2009;2:314–321. doi: 10.1161/CIRCGENETICS.109.862128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crilley JG, Boehm EA, Blair E, Rajagopalan B, Blamire AM, Styles P, McKenna WJ, Ostman-Smith I, Clarke K, Watkins H. Hypertrophic cardiomyopathy due to sarcomeric gene mutations is characterized by impaired energy metabolism irrespective of the degree of hypertrophy. J Am Coll Cardiol. 2003;41:1776–1782. doi: 10.1016/s0735-1097(02)03009-7. [DOI] [PubMed] [Google Scholar]
- 6.Basso C, Thiene G, Corrado D, Buja G, Melacini P, Nava A. Hypertrophic cardiomyopathy and sudden death in the young: pathologic evidence of myocardial ischemia. Human pathology. 2000;31:988–998. doi: 10.1053/hupa.2000.16659. [DOI] [PubMed] [Google Scholar]
- 7.Shirani J, Pick R, Roberts WC, Maron BJ. Morphology and significance of the left ventricular collagen network in young patients with hypertrophic cardiomyopathy and sudden cardiac death. J Am Coll Cardiol. 2000;35:36–44. doi: 10.1016/s0735-1097(99)00492-1. [DOI] [PubMed] [Google Scholar]
- 8.Choudhury L, Mahrholdt H, Wagner A, Choi KM, Elliott MD, Klocke FJ, Bonow RO, Judd RM, Kim RJ. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;40:2156–2164. doi: 10.1016/s0735-1097(02)02602-5. [DOI] [PubMed] [Google Scholar]
- 9.Varnava AM, Elliott PM, Mahon N, Davies MJ, McKenna WJ. Relation between myocyte disarray and outcome in hypertrophic cardiomyopathy. The American journal of cardiology. 2001;88:275–279. doi: 10.1016/s0002-9149(01)01640-x. [DOI] [PubMed] [Google Scholar]
- 10.Varnava AM, Elliott PM, Sharma S, McKenna WJ, Davies MJ. Hypertrophic cardiomyopathy: the interrelation of disarray, fibrosis, and small vessel disease. Heart. 2000;84:476–482. doi: 10.1136/heart.84.5.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JB, Porreca GJ, Song L, Greenway SC, Gorham JM, Church GM, Seidman CE, Seidman JG. Polony multiplex analysis of gene expression (PMAGE) in mouse hypertrophic cardiomyopathy. Science. 2007;316:1481–1484. doi: 10.1126/science.1137325. [DOI] [PubMed] [Google Scholar]
- 12.Ho CY, Lopez B, Coelho-Filho OR, Lakdawala NK, Cirino AL, Jarolim P, Kwong R, Gonzalez A, Colan SD, Seidman JG, Diez J, Seidman CE. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N Engl J Med. 2010;363:552–563. doi: 10.1056/NEJMoa1002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 14.Flacke SJ, Fischer SE, Lorenz CH. Measurement of the gadopentetate dimeglumine partition coefficient in human myocardium in vivo: normal distribution and elevation in acute and chronic infarction. Radiology. 2001;218:703–710. doi: 10.1148/radiology.218.3.r01fe18703. [DOI] [PubMed] [Google Scholar]
- 15.Kim RJ, Chen EL, Lima JA, Judd RM. Myocardial Gd-DTPA kinetics determine MRI contrast enhancement and reflect the extent and severity of myocardial injury after acute reperfused infarction. Circulation. 1996;94:3318–3326. doi: 10.1161/01.cir.94.12.3318. [DOI] [PubMed] [Google Scholar]
- 16.Moon JC, McKenna WJ, McCrohon JA, Elliott PM, Smith GC, Pennell DJ. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol. 2003;41:1561–1567. doi: 10.1016/s0735-1097(03)00189-x. [DOI] [PubMed] [Google Scholar]
- 17.Moon JC, Mogensen J, Elliott PM, Smith GC, Elkington AG, Prasad SK, Pennell DJ, McKenna WJ. Myocardial late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy caused by mutations in troponin I. Heart. 2005;91:1036–1040. doi: 10.1136/hrt.2004.041384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adabag AS, Maron BJ, Appelbaum E, Harrigan CJ, Buros JL, Gibson CM, Lesser JR, Hanna CA, Udelson JE, Manning WJ, Maron MS. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51:1369–1374. doi: 10.1016/j.jacc.2007.11.071. [DOI] [PubMed] [Google Scholar]
- 19.Maron MS, Appelbaum E, Harrigan CJ, Buros JL, Gibson CM, Hanna CA, Lesser JR, Udelson JE, Manning WJ, Maron BJ. Clinical Profile and Significance of Delayed Enhancement in Hypertrophic Cardiomyopathy. Circ Heart Fail. 2008;1:184–191. doi: 10.1161/CIRCHEARTFAILURE.108.768119. [DOI] [PubMed] [Google Scholar]
- 20.Rudolph A, Abdel-Aty H, Bohl S, Boye P, Zagrosek A, Dietz R, Schulz-Menger J. Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy relation to remodeling. J Am Coll Cardiol. 2009;53:284–291. doi: 10.1016/j.jacc.2008.08.064. [DOI] [PubMed] [Google Scholar]
- 21.Bruder O, Wagner A, Jensen CJ, Schneider S, Ong P, Kispert EM, Nassenstein K, Schlosser T, Sabin GV, Sechtem U, Mahrholdt H. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:875–887. doi: 10.1016/j.jacc.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 22.O'Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, Webb J, Kulkarni M, Dawson D, Sulaibeekh L, Chandrasekaran B, Bucciarelli-Ducci C, Pasquale F, Cowie MR, McKenna WJ, Sheppard MN, Elliott PM, Pennell DJ, Prasad SK. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:867–874. doi: 10.1016/j.jacc.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Rubinshtein R, Glockner JF, Ommen SR, Araoz PA, Ackerman MJ, Sorajja P, Bos JM, Tajik AJ, Valeti US, Nishimura RA, Gersh BJ. Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circ Heart Fail. 2010;3:51–58. doi: 10.1161/CIRCHEARTFAILURE.109.854026. [DOI] [PubMed] [Google Scholar]
- 24.Flett AS, Hasleton J, Cook C, Hausenloy D, Quarta G, Ariti C, Muthurangu V, Moon JC. Evaluation of techniques for the quantification of myocardial scar of differing etiology using cardiac magnetic resonance. Jacc. 2011;4:150–156. doi: 10.1016/j.jcmg.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 25.Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, McGregor C, Moon JC. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122:138–144. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 26.Jerosch-Herold M, Sheridan DC, Kushner JD, Nauman D, Burgess D, Dutton D, Alharethi R, Li D, Hershberger RE. Cardiac magnetic resonance imaging of myocardial contrast uptake and blood flow in patients affected with idiopathic or familial dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2008;295:H1234–H1242. doi: 10.1152/ajpheart.00429.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iles L, Pfluger H, Phrommintikul A, Cherayath J, Aksit P, Gupta SN, Kaye DM, Taylor AJ. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol. 2008;52:1574–1580. doi: 10.1016/j.jacc.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 28.Broberg CS, Chugh SS, Conklin C, Sahn DJ, Jerosch-Herold M. Quantification of diffuse myocardial fibrosis and its association with myocardial dysfunction in congenital heart disease. Circ Cardiovasc Imaging. 2010;3:727–734. doi: 10.1161/CIRCIMAGING.108.842096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ugander M, Oki AJ, Hsu LY, Kellman P, Greiser A, Aletras AH, Sibley CT, Chen MY, Bandettini WP, Arai AE. Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub-clinical myocardial pathology. Eur Heart J. 33:1268–1278. doi: 10.1093/eurheartj/ehr481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng AC, Auger D, Delgado V, van Elderen SG, Bertini M, Siebelink HM, van der Geest RJ, Bonetti C, van der Velde ET, de Roos A, Smit JW, Leung DY, Bax JJ, Lamb HJ. Association between diffuse myocardial fibrosis by cardiac magnetic resonance contrast-enhanced T(1) mapping and subclinical myocardial dysfunction in diabetic patients: a pilot study. Circ Cardiovasc Imaging. 2012;5:51–59. doi: 10.1161/CIRCIMAGING.111.965608. [DOI] [PubMed] [Google Scholar]
- 31.McKenna WJ, Spirito P, Desnos M, Dubourg O, Komajda M. Experience from clinical genetics in hypertrophic cardiomyopathy: proposal for new diagnostic criteria in adult members of affected families. Heart. 1997;77:130–132. doi: 10.1136/hrt.77.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steel K, Broderick R, Gandla V, Larose E, Resnic F, Jerosch-Herold M, Brown KA, Kwong RY. Complementary prognostic values of stress myocardial perfusion and late gadolinium enhancement imaging by cardiac magnetic resonance in patients with known or suspected coronary artery disease. Circulation. 2009;120:1390–1400. doi: 10.1161/CIRCULATIONAHA.108.812503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, Pennell DJ. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. The American journal of cardiology. 2002;90:29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 34.Deichmann R, Hahn D, Haase A. Fast T1 mapping on a whole-body scanner. Magn Reson Med. 1999;42:206–209. doi: 10.1002/(sici)1522-2594(199907)42:1<206::aid-mrm28>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 35.Messroghli DR, Rudolph A, Abdel-Aty H, Wassmuth R, Kuhne T, Dietz R, Schulz-Menger J. An open-source software tool for the generation of relaxation time maps in magnetic resonance imaging. BMC medical imaging. 2010;10:16. doi: 10.1186/1471-2342-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Olivotto I, Girolami F, Ackerman MJ, Nistri S, Bos JM, Zachara E, Ommen SR, Theis JL, Vaubel RA, Re F, Armentano C, Poggesi C, Torricelli F, Cecchi F. Myofilament protein gene mutation screening and outcome of patients with hypertrophic cardiomyopathy. Mayo Clin Proc. 2008;83:630–638. doi: 10.4065/83.6.630. [DOI] [PubMed] [Google Scholar]
- 38.Dass S, Suttie JJ, Piechnik SK, Ferreira VM, Holloway CJ, Banerjee R, Mahmod M, Cochlin L, Karamitsos TD, Robson MD, Watkins H, Neubauer S. Myocardial tissue characterization using magnetic resonance noncontrast t1 mapping in hypertrophic and dilated cardiomyopathy. Circ Cardiovasc Imaging. 2012;5:726–733. doi: 10.1161/CIRCIMAGING.112.976738. [DOI] [PubMed] [Google Scholar]
- 39.Amano Y, Takayama M, Kumita S. Contrast-enhanced myocardial T1-weighted scout (Look-Locker) imaging for the detection of myocardial damages in hypertrophic cardiomyopathy. J Magn Reson Imaging. 2009;30:778–784. doi: 10.1002/jmri.21921. [DOI] [PubMed] [Google Scholar]
- 40.Ellims AH, Iles LM, Ling LH, Hare JL, Kaye DM, Taylor AJ. Diffuse myocardial fibrosis in hypertrophic cardiomyopathy can be identified by cardiovascular magnetic resonance, and is associated with left ventricular diastolic dysfunction. J Cardiovasc Magn Reson. 2012;14:76. doi: 10.1186/1532-429X-14-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kellman P, Wilson JR, Xue H, Bandettini WP, Shanbhag SM, Druey KM, Ugander M, Arai AE. Extracellular volume fraction mapping in the myocardium, part 2: initial clinical experience. J Cardiovasc Magn Reson. 2012;14:64. doi: 10.1186/1532-429X-14-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zannad F, Alla F, Dousset B, Perez A, Pitt B. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES) Rales Investigators. Circulation. 2000;102:2700–2706. doi: 10.1161/01.cir.102.22.2700. [DOI] [PubMed] [Google Scholar]
- 43.Diez J, Querejeta R, Lopez B, Gonzalez A, Larman M, Martinez Ubago JL. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation. 2002;105:2512–2517. doi: 10.1161/01.cir.0000017264.66561.3d. [DOI] [PubMed] [Google Scholar]
- 44.Lopez B, Querejeta R, Gonzalez A, Sanchez E, Larman M, Diez J. Effects of loop diuretics on myocardial fibrosis and collagen type I turnover in chronic heart failure. J Am Coll Cardiol. 2004;43:2028–2035. doi: 10.1016/j.jacc.2003.12.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.