Abstract
Continued androgen receptor (AR) expression and signaling is a key driver in castration resistant prostate cancer (CRPC) after classical androgen ablation therapies have failed, and therefore remains a target for the treatment of progressive disease. Here we describe the biological characterization of AZD3514, an orally bioavailable drug that inhibits androgen-dependent and–independent AR signaling. AZD3514 modulates AR signaling through two distinct mechanisms, an inhibition of ligand driven nuclear translocation of AR and a down-regulation of receptor levels, both of which were observed in vitro and in vivo. AZD3514 inhibited testosterone-driven seminal vesicle development in juvenile male rats and the growth of androgen-dependent Dunning R3327H prostate tumors in adult rats. Furthermore, this class of compound demonstrated anti-tumor activity in the HID28 mouse model of CRPC in vivo. AZD3514 is currently in Phase I clinical evaluation.
Introduction
Prostate cancer is the most common form of malignancy in men, and remains the second leading cause of male cancer related death (1). This disease is dependent upon the hormone testosterone (2) which activates androgen receptor (AR) signaling, with surgical (orchiectomy) and chemical anti-androgen approaches being established as first-line therapy. Although treatments are initially highly effective, resistance ultimately develops in the majority of patients. Despite castrate levels of androgen, in many cases, this resistance is still dependent upon AR signaling (3, 4).
The AR is a member of the steroid hormone receptor family and functions as a ligand dependent transcription factor. In the absence of androgens AR is predominantly inactive and present in the cytoplasm bound to heat shock proteins. Binding of androgens to AR induces conformational changes, dimerization and translocation into the nucleus. In the nucleus, AR binds to androgen response elements (AREs) within regulatory elements of target genes (such as prostate specific antigen (PSA) and TMPRSS2) and regulates gene expression through the recruitment of co-factors (5-7).
There are a number of potential mechanisms by which AR signaling can occur in the presence of anti-androgen therapies. These include; an increase in the expression of AR protein which can sensitize cells to low levels of androgen (8-11), AR mutations that can alter transactivation or sensitize AR to alternative ligands (3,5) and those that cause classical AR antagonists such as flutamide and bicalutamide to behave as agonists (12,13). Additionally prostate tumors may also synthesize their own androgens thereby increasing the local intra-tumoral testosterone levels available to activate the AR (4,5,14-16).
Prostate cancer acquiring resistance to conventional treatment was initially termed androgen-independent or hormone-refractory, but is now widely described as castration-resistant prostate cancer (CRPC). The change in terminology reflects emerging data that shows prostate cancer in this resistant state to have a continued dependency upon AR signaling. This is exemplified by the recent overall survival benefit demonstrated in CRPC patients following treatment with either abiraterone acetate or enzalutamide (17-22). Abiraterone acetate inhibits 17-α-hydroxylase/17,20-lyase (CYP17) activity resulting in a reduction in residual androgens synthesized by the adrenals and potentially the prostate tumor itself (23). Enzalutamide inhibits the key events that must occur to enable AR signaling in that it prevents AR; binding testosterone, translocating to the nucleus, and binding to DNA (24). However, although the activity of these agents in CRPC is very encouraging, neither works in all patients and both are associated with the development of additional resistance. Whilst the mechanisms by which tumors become refractory to abiraterone acetate and enzalutamide treatment have not yet been clearly elucidated, current clinical (4,25) and preclinical (26-28) data suggests that re-activation of the AR will remain a primary mechanism. Thus, there is a continued need to identify alternative therapies for the treatment of CRPC, and in particular those that have a mechanistically distinct inhibitory effect on AR signaling.
Here we describe the characterization of AZD3514, an orally-bioavailable drug that; binds to AR, inhibits its nuclear translocation, and ligand-dependent and independent transcriptional activity. Additionally, AZD3514 is differentiated from existing pharmacological approaches in that it is also able to induce AR down-regulation in vitro and in vivo.
Materials and Methods
AZD3514
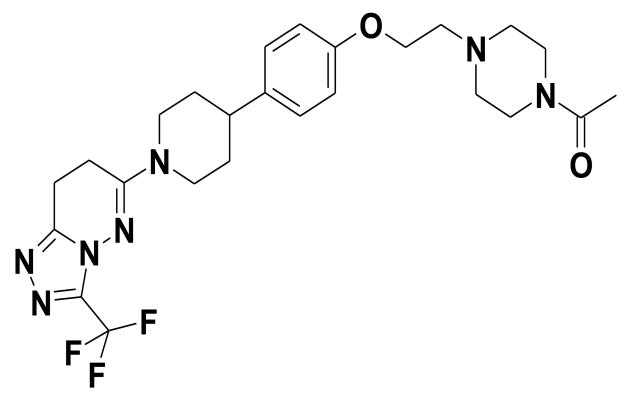
6-(4-{4-[2-(4-Acetylpiperazin-1-yl)ethoxy]phenyl}piperidin-1-yl)-3-(trifluoromethyl)-7,8-dihydro[1,2,4]triazolo[4,3-b]pyridazine (AZD3514, AstraZeneca; Fig. 1) was synthesized according to published procedures (29). For in vivo studies AZD3514 was formulated in a solution of 20% Captisol (β- cyclodextrin sulfobutyl ethers sodium salts) at pH4.
Figure 1.
Chemical structure of AZD3514.
ARD1
6-[4-(4-Cyanobenzyl)piperazin-1-yl]-3-(trifluoromethyl)[1,2,4]triazolo[4,3-b]pyridazine (ARD1, AstraZeneca; Fig. 6C) was obtained by the published procedure (30). For in vivo studies ARD1 was prepared in a suspension of 1% polysorbate 80.
Figure 6.
(A-B) ARD1 was administered at 100 mg/kg by oral gavage twice daily (bid) to either (A) intact or (B) castrated male nude mice bearing established HID28 tumors. Tumor volumes are plotted against time. Error bars are SEM.
(C) Chemical structure of ARD1.
(D) LNCaP cells grown in stripped serum conditions were treated for 24 hours with a dose response to ARD1. Effects on total AR levels were assessed by Western blot and normalized to levels of GAPDH.
(E) LNCaP cells in steroid depleted media were treated with a dose response to AZD3514 and the indicated levels of DHT for 24 hours. Expression of PSA was determined by quantitative RT-PCR and normalized to levels of 18s. Data shown is expression relative to the vehicle control in the absence of ligand and is representative data.
(F) Inhibition of DHT induced AR translocation in AR U2OS cells, recombinant U2OS cells stably expressing human androgen receptor (AR) fused to the C-terminus of enhanced green fluorescent protein. Cells were dosed with ARD1 30 minutes before treatment with 0.3 nM DHT for 2 hours prior to fixation. Error bars are SD.
Abiraterone acetate
Abiraterone acetate was obtained according to published procedures (31) and was prepared in a suspension of 1% polysorbate 80. Animals were given between 4-40mg/kg, administered once daily by oral gavage.
Enzalutamide
Enzalutamide was obtained according to published procedures (32).
Testosterone proprionate
Testosterone proprionate (TP) was prepared in stripped corn oil at 0.2 mg/ml, and administered subcutaneously (s.c.) at 0.4 mg/kg once a day.
Cell Culture
LNCaP, DU145, HCT116 cells were purchased from ATCC, LAPC4 were obtained from Dr Beth Pflug, Indiana, USA, and AR U2OS were puchased from ThermoScientific. All cell lines were maintained under standard cell culture conditions and were tested and authenticated at AstraZeneca cell banking using DNA fingerprinting short tandem repeat (STR) assays. All revived cells were used within 15 passages, and less than six months.
Assessment of Protein and mRNA levels
Cell lysates were prepared with Lysate Buffer (25 mM Tris pH 6.8, 3 mM EDTA, 3 mM EGTA, 50 mM NaF, 2 mM Na3VO4, 0.27 M Sucrose, 10 mM β-glycerolphosphate, 5 mM pyrophosphate and 0.5% Triton X-100) containing EDTA-free protease inhibitor cocktail (Cat# 11873580001 Roche, Burgess Hill, UK) for asessement of total protein by western bloting following 24hrs treatment appropriate test compound. Western blotting was performed using standard SDS-PAGE procedures, with antibodies to AR (AR441, cat#M3562, Dako, Cambridgeshire, UK), GAPDH (Clone6C5, Bio Scientific, Austin, USA), PSA (Cat#AF1344, R&D Systems, Abingdon, UK) or PARP (cat#51-6639GR, BD Pharmingen, Oxford, UK). Protein levels were determined using the ChemiGenius imaging system and GeneTools analysis software (Syngene, Cambridge, UK). For mRNA analysis cell lysates were prepared using FastLane Cell RT-PCR kit (Qiagen, Crawley, UK) and Taqman RT-PCR performed to measure mRNA levels of PSA and TMPRSS2. Expression was normalized to 18s ribosomal protein and quantified using the 2−ΔΔCT method. Data was analyzed relative to a DMSO treated control in the absence of DHT. Primer probe assays used were: 18S Hs99999901, PSA Hs00426859 and TMPRSS2 Hs00237175 from Applied Biosystems (Life Technologies, Paisley UK).
Western Translocation assays
LNCaP cells were plated in media containing steroid free serum and treated with AZD3514, MDV3100 or DMSO for 2 hours prior to treating with 1nM DHT or DMSO for 30 minutes. Nuclear and cytoplasmic cell lysates were prepared using the NE-PER extraction kit (Pierce, Fisher Scientific, Loughborough, UK) according to the manufacturer’s instruction. Western blotting was used to determine levels of nuclear and cytoplasmic proteins
Proliferation assays
For ligand driven LNCaP and LAPC4 proliferation assays, cells grown in RPMI + 5% charcoal stripped serum were incubated with a dose response of AZD3514, MDV3100 or DMSO ± 1 nM of DHT and incubated for 6-7 days. LNCaP cells were fixed in 3.7% formaldehyde, stained with Hoechst 33342 (Invitrogen, Paisley, UK) and cell number determined on the Cellomics ArrayScan using a cell count algorithm. LAPC4 cell growth was measured on an Incucyte (Essen Instruments, Hertfordshire, UK) to measure cell confluence. For DU145 and HCT116 proliferation assays cells were plated in RPMI + 5% (DU145) or 10% (HCT116) FBS and treated with a dose response of AZD3514 or DMSO. Cell growth was monitored on an Incucyte (Essen Instruments, Hertfordshire, UK) to measures cell confluence over time.
AR Translocation Imaging
AR U20S cells were in steriod free media were fixed using 4% formaldehyde and imaged using an ArrayScan VTI (Cellomics, USA), using compartmental analysis Bioapplication. Cytonuclear differences were calculated by the software by subtracting the average cytosolic fluorescence pixel intensity from the average nuclear fluorescence pixel intensity. Nuclear masks were generated from the Hoechst 33342 stained nuclei and a user defined threshold was applied to detected AR foci within the nuclear masks.
Measurement of AR degradation and synthesis rates by Mass Spectrometry
LNCaPs were grown for at least 3 passages in SILAC media (Pierce, Thermo Scientific, Cramlington, UK) containing 13C615N4 arginine and 13C6 lysine to ensure maximal labeling of proteins. For the assay, cells were plated at 5 × 105 cells/well into 6-well TC treated plates in steroid free media containing 5% dialyzed FBS 13C615N4 arginine and 13C6 lysine for 24 hours before treating for 24 hours with AZD3514 or DMSO control. Media was then switched to SILAC media containing unlabeled arginine and lysine containing the appropriate treatment and samples collected over a 24h period. Protein samples were prepared in Lysate Buffer. Equal concentrations of sample proteins spiked with equal concentrations of internal standards (lysate from LNCaPs labeled with 13C6 lysine only, ‘medium’ labeled) were then immunoprecipitated overnight at 4°C with a PG21 polyclonal AR antibody (cat#06-680 Millipore, Watford, UK) coated on a Nunc MaxiSorp plate (Fisher Scientific, Loughborough, UK). Wells were washed 4 x with a HEPES:RIPA buffer 4 x with PBS and then 2 x with water. Samples were digested by addition of 30 μl well sequencing grade trypsin (Promega, Southampton, UK) diluted to 16.7 μg/ml in 10 mM TEAB buffer (Sigma, Gillingham, UK) at 37°C for a minimum of 3 hours before analyzing by mass spectrometry using relative peptide quantification by selected reaction monitoring (SRM).
Relative peptide quantification by selected reaction monitoring (SRM)
Relative quantification of two AR peptides was performed by SRM using an Ultimate 3000 nano liquid chromatography (LC) system (Dionex) coupled to either a TSQ Vantage (Thermo, experiment 3) or a 4000 QTRAP (Applied Biosystems, experiments 1 and 2) triple quadrupole mass spectrometer. Further details of instrument set up are included in supplementary methods for reference. Both the TSQ Vantage and 4000 QTRAP were operated in SRM mode to selectively measure the following AR peptides: GAFQNLFQSVR (21-31) and LLDSVQPIAR (862-871). The unlabeled, ‘heavy’ labeled (13C6, 15N4 R, +10 Da), and ‘medium’ labeled (13C6, +6 Da) forms of both peptides were measured in parallel. Instrument settings are shown in supplementary Table 1 and Table 2.
AR degradation and synthesis by radio-labeled pulse chase
LNCaPs were seeded in RPMI containing 5% dialyzed charcoal stripped FBS. Radio-labeling of AR protein used Labeling Media (RPMI without amino acids methionine and cysteine supplemented with 100 μCi [35S] methionine/cysteine labeling mix (Perkin Elmer, Beaconsfield, UK), with cells incubated 1 hour. Lysates were prepared from the cells by the addition of Lysate Buffer supplemented with 0.1% SDS and AR protein immunoprecipitated by incubating 500 μg protein with 50 μl Protein G coated paramagnetic beads (Invitrogen, Paisley, UK) bound with 4 μg PG21 anti-AR antibody. The beads were washed three times in Lysate Buffer without SDS supplemented with 500 mM NaCl, and AR eluted in LDS Loading Buffer (Invitrogen, Paisley, UK) at 95°C, 5 min. Samples were run on polyacrylamide gels, transferred to nitrocellulose and levels of radio-labeled AR present determined by autoradiography.
In vivo physiological studies
Male 33-day-old Hans Wistar/RCC Hans Wistar rats were obtained from Harlan, UK and randomized into cages of five at AstraZeneca. All experiments were carried out in full accordance with the UK Home Office Animal (Scientific Procedures) Act 1986.
For intact studies 49-day-old rats (Hans Wistar) were assigned to vehicle or treatment groups and dosed for 6 days, animal body weight and condition were recorded daily. On day 7, 24h after the last dose, animals were terminated and seminal vesicle weight recorded. For castrated rat studies 42-day-old rats (RCC Hans Wistar) were surgically castrated under isoflurane anesthesia and left for a period of 7 days. Animals were assigned to vehicle or treatment groups and dosed for 6 days, animal body weight and condition were recorded daily. On day 7, 24h after the last dose, animals were terminated and seminal vesicle weight recorded.
In vivo tumor studies
All animals were housed in specific-pathogen-free facilities and all experiments were performed in accordance with approved ethical standards appropriate to the establishment. R3327H tumor studies were performed by Oncodesign, France. R3327H tumors were established by implanting Male Copenhagen rats (8- to 12-week-old; obtained from Harlan, France) with fresh tumor fragments obtained from donor rats. When tumors reached the determined size animals were randomized into control and treated groups. Tumor volume (measured by caliper), animal body weight and tumor condition were monitored twice weekly for the duration of the study.
HID28 tumor studies were performed by Xentech, France. HID28 tumors were established by implanting male athymic nude mice (Hsd:Athymic Nude-Fox1nu, Harlan; 6- to 9-week-old) with HID28 tumor fragments established previously in either intact (for intact study) or castrated (for castrated study) mice. When tumors reached the determined size animals were randomized into control and treated groups.
Tumor volume (measured by caliper), animal body weight and tumor condition were monitored twice weekly. Tumor volumes were assessed by bilateral Vernier caliper measurement at least twice weekly and calculated using the formula (length × width) × √ (length × width) × (π/6), where length was taken to be the longest diameter across the tumor and width the corresponding perpendicular.
IHC Methods
Antigen retrieval was performed on formalin-fixed, paraffin-embedded 4 micron sections using an RHS microwave vacuum processor (Milestone) at 110°C on the 2 minute cycle in EDTA (pH8) retrieval buffer. Slides were blocked, prior to incubation for 1h with anti-AR antibody (RB-9030-P1; 1:500; Thermo Fisher Scientific, Cheshire, UK). AR was detected using Dako Envision + System-HRP labeled Polymer (30min, Dako, Cambridge, UK), followed by incubation with DAB chromagen (Dako) and counterstained with Carazzi’s haematoxylin. Nuclear AR expression in tumor cells was assessed in whole sections by a pathologist for IHC brown staining intensity (0+ - none, 1+ - weak, 2+ - moderate, 3+ - strong) and for percentage (%) distribution, to calculate an H-score (sum of 1 × % 1+, 2 × % 2+ and 3 × % 3+) Nuclear AR expression in tumor cells was also quantified using a nuclear image analysis algorithm (Aperio ePathology Solutions, Oxford, UK) combined with Genie™ pattern recognition software. Thresholds were set for different intensities of AR expression and the % distribution of each intensity was used to calculate an H score as described for the pathologist’s assessment.
Results
AZD3514 inhibits AR signaling in vitro and in vivo
AZD3514 was identified using a cellular assay designed to detect compounds with the ability to cause AR down-regulation (described previously for a related series of compounds, (30)). Details relating to the identification of AZD3514 compound have been recently described (29)
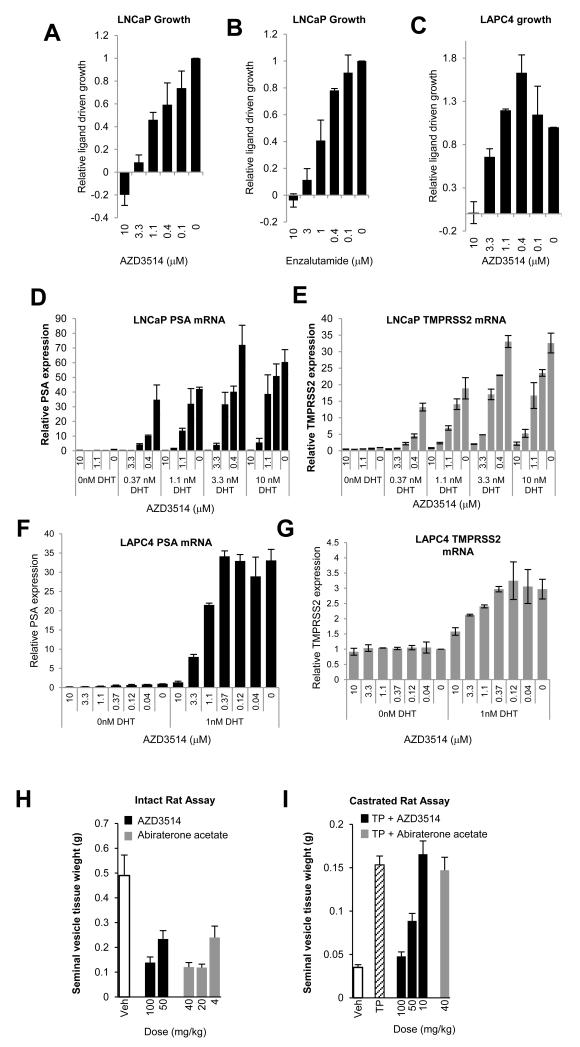
The ability of AZD3514 to inhibit AR function was assessed in LNCaP and LAPC4 prostate cancer cells that express the T877A ligand binding domain mutation of AR and wild type AR respectively (33,34). AZD3514 and AR antagonist Enzalutamide inhibited DHT driven proliferation of LNCaP cells in a dose-dependent manner (Fig.2A-B). The DHT driven proliferation of LAPC4 cells was also inhibited by 10 μM of AZD3514 (Fig. 2C). AZD3514 inhibited the ligand-driven expression of known AR regulated genes PSA and TMPRSS2 in both LNCaP and LAPC4 cells (Fig. 2D-G). In LNCaP cells inhibition of PSA and TMPRSS2 expression by AZD3514 was shown under a range of DHT conditions (0-10 nM) (Fig. 2D and E). AZD3514 was less potent with increasing DHT concentrations, although near complete inhibition of PSA and TMPRSS2 was observed with 10 μM AZD3514 at all concentrations. Importantly, the basal expression of PSA, and TMPRSS2 in LNCaP cells, and PSA in LAPC4s cells, observed in the absence of DHT was also reduced by treatment with AZD3514 (Fig. 2D-G; data re-plotted in Sup. Fig. 1A-D to provide greater clarity). In LNCaP cells AZD3514 inhibited ligand driven PSA and TMPRSS2 expression within 2-4 hours of treatment (Sup. Fig. 1E-G). AZD3514 had minimal effects on the growth of DU145 (Sup. Fig. 2A), an AR negative prostate cancer cell line, and in HCT116 (Sup. Fig. 2B), a colon cell line negative for AR and other nuclear hormone receptors, ER, PR and GR.
Figure 2.
(A-C) LNCaP and LAPC4 growth in steriod free media supplemented with 1 nM DHT and AZD3514 or enzalutamide. Cell number determined after 7 days. Data shown is growth relative to vehicle control and is the mean of 3 (AZD3514) or 2 (enzalutamide) independent experiments. Error bars are SEM.
(D-G) PSA and TMPRSS2 gene expression normalized to levels of 18s after AZD3514 treatment of cells +/− DHT. Data shown is gene expression relative to the vehicle control in the absence of ligand and is a mean of 2 independent experiments. Error bars are SEM.
(H) AZD3514 or abiraterone acetate was administered by oral gavage once daily for 6 days to intact 42 day old male rats at the doses indicated. On day 7 animals were terminated and seminal vesicles weighed. Data shown are the mean values of 5 animals. Error bars are SEM.
(I) Testosterone proprionate (TP) (0.4 mg/kg) was administered by subcutaneous injection along with either vehicle; AZD3514 or abiraterone acetate were administered by oral gavage once daily for 6 days to castrated 42d old male rats at the doses indicated. On day 7 animals were terminated and seminal vesicles weighed. Data shown are the mean values of 5 animals. Error bars are SEM.
We explored the ability of AZD3514 to inhibit AR functional activity in vivo using physiological assays that measure the activity against endogenous (intact rat) or exogenous (castrated rat) testosterone-induced growth of seminal vesicles (35). In the intact rat assay AZD3514 reduced seminal vesicle weight, similar to the CYP17 inhibitor of testosterone production abiraterone acetate (Fig. 2H). In the castrated rat assay AZD3514 inhibited the ability of exogenous testosterone proprionate (TP) to cause an increase in seminal vesicle weight, consistent with a direct inhibition of AR signaling. Abiraterone acetate was inactive in this assay consistent with its mechanism of action (Fig. 2I).
Mechanism of action of AZD3514
The mechanism by which AZD3514 inhibits AR function was explored in vitro. With 24h treatment, AZD3514 reduced AR protein expression in LNCaPs in a dose dependent manner (Fig. 3A and B) although complete reduction of AR protein was not observed at any dose. A time-course of treatment with 10 μM AZD3514 in LNCaPs showed that no down-regulation of AR protein was observed within the first 6h (Fig. 3C). However, down-regulation was observed either with or without DHT at 24h. In contrast enzalutamide had no significant effect on AR expression at any time point (Fig. 3C). Down-regulation of AR protein was also observed in LAPC4 cells treated with 10 μM AZD3514 for 24h (Sup. Fig 3).
Figure 3.
(A-B) LNCaP cells treated with AZD3514 for 24 hours. Graph shows AR levels normalized to GAPDH and relative to the vehicle control. Data is represented as Mean of 3 independent experiments. Error bars are SEM.
(C) LNCaP cells grown in steroid free conditions were incubated with 10 μM AZD3514, enazalutamide or vehicle control for the indicated times. Cells were then treated −/+ 1 nM DHT for 30 minutes. Effects on total AR were assessed by Western blot and normalized to levels of GAPDH. * indicates reduced AR expression.
(D) LNCaP cells grown in steroid free conditions were treated with 1 or 10 μM of AZD3514 or enzalutamide for 2 hours and then −/+ 1 nM of DHT for 30 minutes. Levels of cytoplasmic and nuclear AR were assessed by Western blotting. Levels of PARP and GAPDH were used to assess nuclear and cytoplasmic fractionation.
(E-G) AR U2OS cells, Recombinant U2OS cells stably expressing human androgen receptor (AR) fused to the C-terminus of enhanced green fluorescent protein, were dosed with AZD3514 or enzalutamide for 30 minutes before treatment of cells with 0.3 nM DHT for 2 hours prior to fixation. Representive images at 20x magnification include (i) Vehicle (ii) DHT (iii) 10 μM AZD3514 + DHT (iv) 10 μM enzalutamide + DHT. (F-G) Inhibition of DHT induced AR foci formation and AR translocation as measured on Arrayscan VTI using a compartmental analysis bioapplication. Data are representative from one experiment. Error bars are SD.
Since the effects of AZD3514 on AR protein expression did not appear to fully account for its ability to inhibit AR signaling, we explored whether AZD3514 impacted on AR nuclear translocation, a key process in AR transactivation in response to androgens. By western blot analysis in the absence of DHT AR is predominantly cytoplasmic in LNCaPs, with a small fraction detected in the nucleus (Fig 3D). In response to 30 minute treatment with 1 nM DHT an increase in the levels of nuclear AR was detected (Fig. 3D). AZD3514 caused a dose dependent reduction in this ligand driven AR nuclear localization, with 10 μM of AZD3514 almost completely inhibiting the increase in nuclear AR (Fig. 3D). As has been reported previously, enzalutamide was also able to inhibit ligand driven translocation of nuclear AR in this assay (Fig. 3D) (24). No effects of AZD3514 on total levels of AR were observed following 2 hours treatment (Fig. 3C). AZD3514 also inhibited ligand induced AR nuclear translocation in engineered U2OS cells expressing GFP tagged AR (Fig 3E and F and Sup Fig. 7). In addition, AZD3514 inhibited ligand driven AR foci formation in the AR U2OS cell line (Fig. 3G and Sup. Fig 7), these AR foci have previously been reported and correlate with AR-mediated activity (36-38).
Mechanism of AR down-regulation by AZD3514
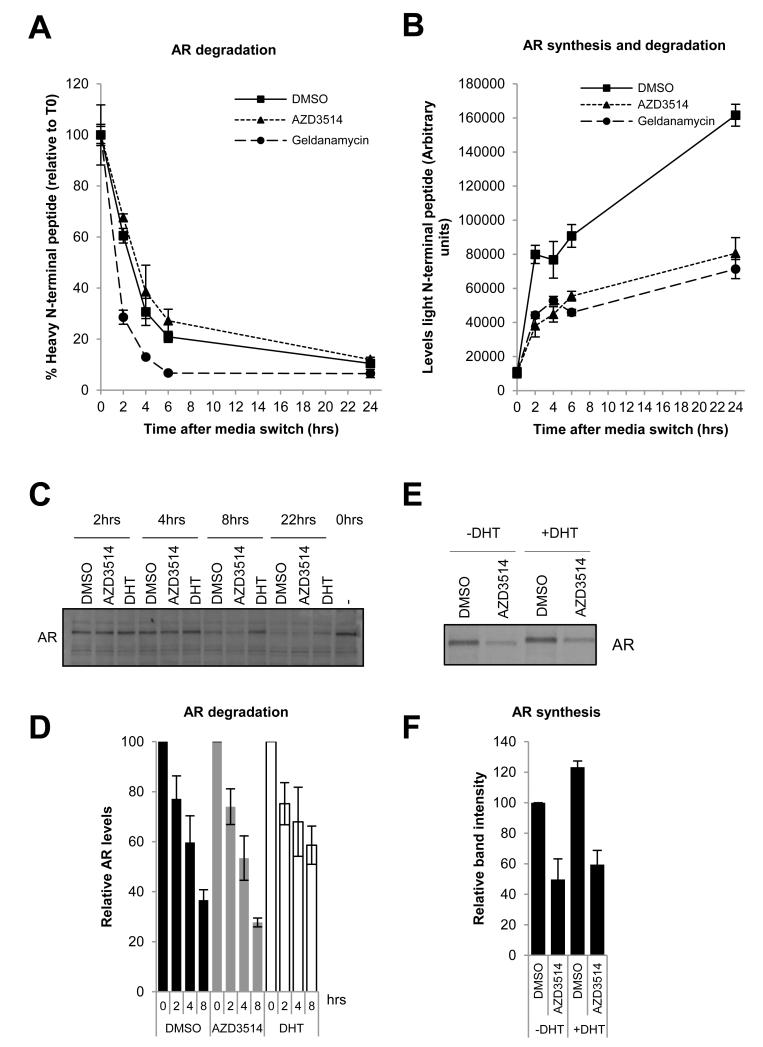
We next investigated whether the mechanism of AR down-regulation by AZD3514 was via an increase in the rate of AR degradation or a decrease in AR synthesis. A mass spectrometry approach was used which measured AR degradation by tracking the decrease of two 13C615N4 arginine labeled (heavy) AR peptides. A composite measure of AR degradation and synthesis was obtained by tracking the increase of the unlabeled (light) AR peptides. Treatment of LNCaPs with 10 μM of AZD3514 was unable to increase the rate of AR degradation compared to vehicle control (Fig. 4A and Sup Fig. 4A). However, geldanamycin, an Hsp90 inhibitor, was able to increase the rate of AR degradation as previously reported (Fig. 4A and Sup Fig.4A) (39). The half-life of AR in LNCaPs quantified using this approach for both vehicle and geldanamycin treated cells is consistent with that previously reported (Sup. Table 3) (11,39). Levels of light AR peptide were significantly reduced following AZD3514 treatment (Fig. 4B and Sup Fig. 4B). No increase in the rate of AR degradation was observed (Fig. 4A and Sup Fig. 4A) therefore this data suggests that AZD3514 is reducing the rate of AR synthesis.
Figure 4.
(A-B) LNCaP cells grown in steroid free conditions in SILAC media containing 5% dialyzed fetal calf serum, 13C615N4 arginine and 13C6 lysine (to label proteins as “heavy”) were treated with 10 μM AZD3514 or vehicle for 24 hours, then switched to grow in SILAC media containing unlabeled arginine (to label newly synthesized protein as “light”). Cells previously dosed with AZD3514 were treated for a further 24 hours with 10 μM AZD3514. Cells previously dosed with vehicle were treated for a further 24 hours with either 3 μM geldanamycin or vehicle. Samples were collected at indicated timepoints following the media switch and protein lysates prepared. AR protein levels were measured in the samples by immunoprecipitation with an AR specific antibody followed by liquid chromatography– mass spectrometry quantifying levels of N-terminal peptide of AR. (A) AR shown is % of heavy labeled AR peptide at each time point relative to the level of AR present at the time of the media switch. (B) AR shown is the increase in light labeled AR peptide at each time point following the media switch. Both graphs are representative data of 3 separate experiments −/+ SD. Half-life values obtained in 3 independent experiments are shown in supplementary table 3.
(C-D) Radio-labeled pulse chase analysis of AR degradation in LNCaP cells. Cells were incubated with radiolabel, washed and label-free media added containing 10 μM AZD3514 or 1 nM DHT. Lysates were prepared, levels of radio-labeled AR measured and expressed relative to AR levels at time 0. Data are represented as Mean levels from 2 independent experiments. Error bars are SD.
(E-F) Radio-labeled pulse chase analysis of AR synthesis in LNCaP cells. Cells were treated with 10 μM AZD3514 or vehicle control −/+ 1 nM DHT for 24 hr. Labeling Media containing treatments was added, and the cells incubated 1 hr. Lysates were prepared and levels of radio-labeled AR measured and expressed relative to the DMSO control -DHT. The graph shows the mean AR levels from 2 independent experiments. Error bars are SD.
These observations were confirmed measuring AR turnover by pulse chase labeling of LNCaPs with 35S-methionine and cysteine. AZD3514 had no effect on AR degradation rate although DHT stabilized AR as expected (Fig 4C and D) (40,41). In contrast a 24h treatment with 10μM AZD3514 was shown to decrease AR synthesis in the absence or presence of ligand (Fig. 4E and F).
The effects of AZD3514 on AR mRNA levels were variable between experiments with 0-50% reduction in steady state AR mRNA observed as measured by RT-PCR (data not shown), suggesting that an effect on AR transcription is unlikely to be the only mechanism by which AZD3514 regulates AR protein levels.
AZD3514 modulates nuclear AR protein and inhibits growth of the androgen dependent Dunning R3327H rat prostatic adenocarcinoma model in vivo
To assess AZD3514 anti-tumor activity in vivo against an androgen dependent model, male Copenhagen rats bearing Dunning R3327H prostate tumors (42) were randomized and treated with AZD3514. Once daily oral administration of AZD3514 at 50 mg/kg caused a statistically significant inhibition of tumor growth (64 %, P<0.001) compared with vehicle treated controls (Fig. 5A). AZD3514 also reduced nuclear AR protein expression in these tumors (data not shown). However, since tumor size and morphology was modulated by chronic treatment, we investigated the impact of AZD3514 on AR protein expression in R3327H tumors in vivo following acute dosing. Rats were treated with 50 mg/kg or 100 mg/kg AZD3514 for 3 days and tumors were harvested 24h after the last dose for assessment of AR protein expression by IHC (Fig. 5B). No conclusive results were obtained from the analysis of cytoplasmic AR expression, which was highly variable and challenging to assess (data not shown). However, three days of dosing AZD3514 caused a significant reduction in nuclear AR protein at both the 50 mg/kg and 100 mg/kg dose, with 100 mg/kg having the greatest effect (Fig. 5B). Nuclear AR expression was quantified by both conventional pathology scoring and the use of Aperio Genie™ software to determine the proportion of tumor cells expressing different intensities of AR, and both analyses confirmed a reduction in nuclear AR by AZD3514 (Fig. 5C and D).
Figure 5.
(A-D) AZD3514 was administered by oral gavage once daily to Copenhagen rats bearing established R3327H tumors at the doses indicated. (A) tumor volumes are plotted against time. (B) i, representative images showing AR IHC from animals administered with vehicle or AZD3514 at 50 or 100 mg/kg for 3 days and ii, the associated Genie™ annotation grading intensity of nuclear AR. Graphical representation of the percentage of tumor nuclei with 0+, 1+, 2+ or 3+ intensity of AR protein as quantified by Genie™ (C) and scored by a pathologist (D).
ARD1, a compound with similar biological properties to AZD3514 inhibits androgen independent HID28 prostate tumor growth in vivo
We subsequently wanted to assess whether the AZD3514 mechanism of action could deliver in vivo efficacy in a castrate resistant setting using the murine HID28 model, an androgen-independent variant of PAC120 prostate tumors (43). Oral dosing with AZD3514 at 100mg/kg in the mouse had poor pharmacokinetic profile (Sup Fig. 5C, Sup Table. 4). This was such that we would not have been able to increase the dose sufficiently to a dose that would have been required for adequate exposure. Thus the hypothesis was tested with ARD1, a compound from the same chemical series which had improved mouse PK versus AZD3514 (Sup Fig. 5C).
Intact male nude mice bearing the HID28 tumors were randomized and treated with ARD1 or vehicle. Twice daily oral administration of ARD1 at 100mg/kg caused a statistically significant inhibition of tumor growth (54 %, P<0.01) compared with vehicle treated controls (Fig. 6A). AR expression in these tumors detected by IHC was highly variable and not quantified, however some modulation of nuclear AR levels was observed with ARD1 (Sup Fig. 6A). In this HID28 study the AR antagonist bicalutamide was inactive, however the gonadotropin releasing hormone antagonist degarelix (44) also caused a significant inhibition of tumor growth (40 %, P<0.05, Sup Fig. 6B) suggesting androgens were contributing to HID28 tumor growth in intact animals. However ARD1 was also able to inhibit HID28 growth (95%, P<0.05) in castrated mice (Fig. 6B), suggesting that ARD1 is able to inhibit tumor growth under castrate resistant levels of androgen.
Discussion
A body of data now indicates that AR continues to play a key role in driving progression of CRPC after classical anti-androgen therapies have failed. Multiple mechanisms have been implicated in the re-activation of AR signaling within castrate levels of androgen, including over-expression, mutation or alternative splicing of the receptor, or the intra-tumoral synthesis of testosterone. Given the diversity of available mechanisms, re-activated AR signaling may also have the potential to mediate intrinsic and acquired resistance to alternative inhibitors of AR signaling such as those described recently to have activity in CRPC (19,20). Thus a strategy involving the removal of AR protein should have utility within different stages of current prostate cancer treatment practice, such an approach having the ability to inhibit both androgen-dependent and –independent AR signaling. To obtain such an inhibitor, we established a cellular assay that was designed to identify compounds that modulated the level of AR in the nucleus of LNCaP prostate cancer cells in androgen-free conditions (30). This work yielded AZD3514, a small molecule inhibitor of AR signaling that is also able to down-regulate AR.
Consistent with inhibitors of AR, AZD3514 was shown to inhibit ligand-driven proliferation and expression of AR-dependent genes (PSA and TMPRSS2) in LNCaPs and LAPC4s. Whilst the potency of AZD3514 was influenced by the concentration of DHT, consistent with an antagonist mechanism of action, inhibition of AR signaling was still observed at the highest level of DHT tested (10 nM). AZD3514 was also able to inhibit the basal expression of PSA and TMPRSS2 in LNCaPs consistent with a mode of action that is dependent upon AR but independent of androgens. In vivo AZD3514 treatment inhibited the growth of Dunning R3327H tumors, an androgen-dependent prostate cancer model. The compound also inhibited the increase in seminal vesicle weight in juvenile male rats that was driven by either physiological testosterone, or exogenous testosterone in castrated rats. This is in contrast to the androgen synthesis inhibitor abiraterone acetate, which was active against physiological testosterone only and is consistent with AZD3514 having a direct effect on the AR.
A number of approaches were used to gain insight into the mechanisms through which AZD3514 drives modulation of AR function. AZD3514 inhibited ligand-driven translocation of AR to the nucleus in LNCaPs and in engineered U2OS cells expressing AR-GFP. This occurred within 2 hours of AZD3514 treatment and is consistent with the early effects observed on AR-driven gene expression. The mechanism by which AR nuclear translocation is inhibited has not been explored fully, but is also reported to occur following treatment with the AR antagonist enzalutamide (24). Ligand displacement assays using either rat AR ligand binding domain or full length AR derived from LNCaP lysates has shown that AZD3514 binds to AR (data not shown). In addition, since AZD3514 also competes with DHT-induced responses, these acute effects are anticipated to be a consequence of a direct interaction with AR. Longer term treatment with AZD3514 in vitro (24hours in LNCaPs) caused a reduction in total AR protein levels in both the absence and presence of androgen, providing an additional mechanism by which AZD3514 could reduce AR-signaling independent of androgens. These effects on AR were also evident in vivo. In the Dunning R3327H tumor model, AR staining in the nucleus of tumor cells was significantly reduced following 3 days of AZD3514 treatment.
AR down-regulation could be achieved by enhancing the rate of AR degradation and/or reducing the rate of synthesis. Although the effect of AZD3514 in the cellular assay was attenuated by proteosome inhibition (data not shown), no effects on the rate of AR degradation were observed in our assays. Indeed our data suggests that AZD3514 treatment reduces the rate of AR synthesis, which is perhaps consistent with the timeframe that the AR down-regulation is observed. These observations were made using two approaches, a mass spectrometry pulse chase method and a radio-labeled pulse chase method, and consistent results were obtained. Importantly in these studies the AR half-life measured, and the impact of DHT and geldanamycin on AR stability was consistent with published data. Whether the reduced rate of protein synthesis by AZD3514 is due to inhibition of mRNA transcription or translation is as yet unclear and inconsistent effects on steady state levels of AR mRNA were observed. MicroRNAs have been suggested to regulate AR levels in prostate cancer cells (45) and EBP1 has been reported to inhibit the translation of AR mRNA (46,47). These and other potential mechanisms cannot be ruled out and will be the focus of future investigation.
Using ARD1, which is structurally-related to AZD3514 and is similarly able to inhibit AR nuclear translocation and reduce AR levels, we were able to examine the effectiveness of this class of compound in the HID28 tumor model; an androgen-independent variant of the PAC120 patient derived prostate tumor model grown in nude mice (40). ARD1 was active in this model when grown in both intact and castrated mice, suggesting that this class of compound is active under low/castrate levels of androgen. Although the circulating testosterone levels in rat are not affected by the administration of pharmacologically active doses of ARD1 (data not shown), we cannot conclusively exclude the possibility of a direct effect on androgen synthesis in the tumor. In addition, although AR protein expression is variable in the HID28 tumors, we were able to detect a reduction in nuclear AR protein following ARD1 treatment, consistent with a direct modulation of AR signaling. This effect on AR protein, independent of effects on androgen synthesis differentiates the mechanism of AZD3514 from other known agents that target AR signaling such as abiraterone.
Collectively, these pre-clinical data indicate that AZD3514 has a novel mechanism and is able to inhibit AR signaling in androgen-dependent and androgen-independent conditions. The compound prevents nuclear translocation of AR and inhibits AR synthesis, with sustained exposure leading to a detectable down-regulation of the receptor. Since intrinsic or acquired resistance to current anti-androgen therapies is frequently mediated by AR, an inhibitor that also reduces AR levels may conceivably be beneficial in circumventing, or helping to delay the emergence of resistance. Such an approach may deliver benefit in castration resistant prostate cancer either alone or in combination with other therapies.
Supplementary Material
Acknowledgments
MRC Industrial Collaboration Award, G0800889/1 awarded to C.N. Robson, L. Gaughan, S. A. Loddick, N.A. Brooks
Footnotes
All Authors have declared no conflict of interest pertaining to the publication of this article.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Huggins C, Hodges CV. Studies on prostatic cancer I the effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22:232–40. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 3.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: Directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–61. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 4.Attard G, Cooper CS, de Bono JS. Steroid hormone receptors in prostate cancer: A hard habit to break? Cancer Cell. 2009;16:458–62. doi: 10.1016/j.ccr.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Knudsen KE, Penning TM. Partners in crime: Deregulation of AR activity and androgen synthesis in prostate cancer. Trends Endocrinol Metab. 2010;21:315–24. doi: 10.1016/j.tem.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett NC, Gardiner RA, Hooper JD, Johnson DW, Gobe GC. Molecular cell biology of androgen receptor signalling. Int J Biochem Cell Biol. 2010;42:813–27. doi: 10.1016/j.biocel.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Taplin ME. Drug insight: Role of the androgen receptor in the development and progression of prostate cancer. Nat Clin Pract Oncol. 2007;4:236–44. doi: 10.1038/ncponc0765. [DOI] [PubMed] [Google Scholar]
- 8.Koivisto P, Visakorpi T, Kallioniemi OP. Androgen receptor gene amplification: A novel molecular mechanism for endocrine therapy resistance in human prostate cancer. Scand J Clin Lab Invest Suppl. 1996;226:57–63. [PubMed] [Google Scholar]
- 9.Linja MJ, Savinainen KJ, Saramaki OR, Tammela TL, Vessella RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001;61:3550–5. [PubMed] [Google Scholar]
- 10.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 11.Gregory CW, Johnson RT, Jr, Mohler JL, French FS, Wilson EM. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res. 2001;61:2892–8. [PubMed] [Google Scholar]
- 12.Taplin ME, Bubley GJ, Ko YJ, Small EJ, Upton M, Rajeshkumar B, et al. Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer Res. 1999;59:2511–5. [PubMed] [Google Scholar]
- 13.Hara T, Miyazaki J, Araki H, Yamaoka M, Kanzaki N, Kusaka M, et al. Novel mutations of androgen receptor: A possible mechanism of bicalutamide withdrawal syndrome. Cancer Res. 2003;63:149–53. [PubMed] [Google Scholar]
- 14.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: A mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–54. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–15. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 16.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–25. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 17.Danila DC, Morris MJ, de Bono JS, Ryan CJ, Denmeade SR, Smith MR, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28:1496–501. doi: 10.1200/JCO.2009.25.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reid AH, Attard G, Danila DC, Oommen NB, Olmos D, Fong PC, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010;28:1489–95. doi: 10.1200/JCO.2009.24.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: A phase 1-2 study. Lancet. 2010;375:1437–46. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Effect of MDV3100, an androgen receptor signaling inhibitor (ARSI), on overall survival in patients with prostate cancer postdocetaxel: Results from the phase III AFFIRM study. J Clin Oncol. 2012;30(suppl 5) abstr LBA1. [Google Scholar]
- 22.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 23.Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–71. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 24.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–90. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, Clegg NJ, Scher HI. Anti-androgens and androgen-depleting therapies in prostate cancer: New agents for an established target. Lancet Oncol. 2009;10:981–91. doi: 10.1016/S1470-2045(09)70229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richards J, Lim AC, Hay CW, Taylor AE, Wingate A, Nowakowska K, et al. Interactions of abiraterone, eplerenone, and prednisolone with wild-type and mutant androgen receptor: A rationale for increasing abiraterone exposure or combining with MDV3100. Cancer Res. 2012;72:2176–82. doi: 10.1158/0008-5472.CAN-11-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai C, Chen S, Ng P, Bubley GJ, Nelson PS, Mostaghel EA, et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011;71:6503–13. doi: 10.1158/0008-5472.CAN-11-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mostaghel EA, Marck BT, Plymate SR, Vessella RL, Balk S, Matsumoto AM, et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: Induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res. 2011;17:5913–25. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradbury RH, Acton DG, Broadbent NL, Brooks AN, Carr GR, Hatter G, et al. Discovery of AZD3514, a small-molecule androgen receptor downregulator for treatment of advanced prostate cancer. Bioorg Med Chem Lett. 2013;23:1945–8. doi: 10.1016/j.bmcl.2013.02.056. [DOI] [PubMed] [Google Scholar]
- 30.Bradbury RH, Hales NJ, Rabow AA, Walker GE, Acton DG, Andrews DM, et al. Small-molecule androgen receptor downregulators as an approach to treatment of advanced prostate cancer. Bioorg Med Chem Lett. 2011;21:5442–5. doi: 10.1016/j.bmcl.2011.06.122. [DOI] [PubMed] [Google Scholar]
- 31.Potter GA, Hardcastle IR, Jarman M. A Convenient, Large-Scale Synthesis of Abiraterone Acetate [h-Acetoxy-17-(3- pyridyl)androsta-5,16-dien], A Potential New Drug for the Treatment of Prostate Cancer. Organic Preperations and Procedures Int. 1997;29:123–128. [Google Scholar]
- 32.Jung ME, Ouk S, Yoo D, Sawyers CL, Chen C, Tran C, et al. Structure Activity relationship for thiohydantoin androgen receptor antagonists for castration-resistant prostate cancer (CRPC) J Med Chem. 2010;53:2779–96. doi: 10.1021/jm901488g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veldscholte J, Berrevoets CA, Ris-Stalpers C, Kuiper GG, Jenster G, Trapman J, et al. The androgen receptor in LNCaP cells contains a mutation in the ligand binding domain which affects steroid binding characteristics and response to antiandrogens. J Steroid Biochem Mol Biol. 1992;41:665–9. doi: 10.1016/0960-0760(92)90401-4. [DOI] [PubMed] [Google Scholar]
- 34.Klein KA, Reiter RE, Redula J, Moradi H, Zhu XL, Brothman AR, et al. Progression of metastatic human prostate cancer to androgen independence in immunodeficient SCID mice. Nat Med. 1997;3:402–8. doi: 10.1038/nm0497-402. [DOI] [PubMed] [Google Scholar]
- 35.Hershberger LG, Shipley EG, Meyer RK. Myotrophic activity of 19-nortestosterone and other steroids determined by modified levator ani muscle method. Proc Soc Exp Biol Med. 1953;83:175–80. doi: 10.3181/00379727-83-20301. [DOI] [PubMed] [Google Scholar]
- 36.Roy AK, Tyagi RK, Song CS, Lavrovsky Y, Ahn SC, Oh TS, et al. Androgen receptor: Structural domains and functional dynamics after ligand-receptor interaction. Ann N Y Acad Sci. 2001;949:44–57. doi: 10.1111/j.1749-6632.2001.tb04001.x. [DOI] [PubMed] [Google Scholar]
- 37.Saitoh M, Takayanagi R, Goto K, Fukamizu A, Tomura A, Yanase T, et al. The presence of both the amino- and carboxyl-terminal domains in the AR is essential for the completion of a transcriptionally active form with coactivators and intranuclear compartmentalization common to the steroid hormone receptors: A three-dimensional imaging study. Mol Endocrinol. 2002;16:694–706. doi: 10.1210/mend.16.4.0812. [DOI] [PubMed] [Google Scholar]
- 38.Tomura A, Goto K, Morinaga H, Nomura M, Okabe T, Yanase T, et al. The subnuclear three-dimensional image analysis of androgen receptor fused to green fluorescence protein. J Biol Chem. 2001;276:28395–401. doi: 10.1074/jbc.M101755200. [DOI] [PubMed] [Google Scholar]
- 39.Vanaja DK, Mitchell SH, Toft DO, Young CY. Effect of geldanamycin on androgen receptor function and stability. Cell Stress Chaperones. 2002;7:55–64. doi: 10.1379/1466-1268(2002)007<0055:eogoar>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Syms AJ, Norris JS, Panko WB, Smith RG. Mechanism of androgen-receptor augmentation. analysis of receptor synthesis and degradation by the density-shift technique. J Biol Chem. 1985;260:455–61. [PubMed] [Google Scholar]
- 41.Yeap BB, Krueger RG, Leedman PJ. Differential posttranscriptional regulation of androgen receptor gene expression by androgen in prostate and breast cancer cells. Endocrinology. 1999;140:3282–91. doi: 10.1210/endo.140.7.6769. [DOI] [PubMed] [Google Scholar]
- 42.Smolev JK, Heston WD, Scott WW, Coffey DS. Characterization of the dunning R3327H prostatic adenocarcinoma: An appropriate animal model for prostatic cancer. Cancer Treat Rep. 1977;61:273–87. [PubMed] [Google Scholar]
- 43.de Pinieux G, Legrier ME, Poirson-Bichat F, Courty Y, Bras-Goncalves R, Dutrillaux AM, et al. Clinical and experimental progression of a new model of human prostate cancer and therapeutic approach. Am J Pathol. 2001;159:753–64. doi: 10.1016/S0002-9440(10)61746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinberg M. Degarelix: A gonadotropin-releasing hormone antagonist for the management of prostate cancer. Clin Ther. 2009;31:2312–31. doi: 10.1016/j.clinthera.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 45.Östling P, Leivonen SK, Aakula A, Kohonen P, Mäkelä R, Hagman Z, et al. Systemic analysis of microRNAs targeting the AR in prostate cancer cells. Cancer Research. 2011;71:1956–67. doi: 10.1158/0008-5472.CAN-10-2421. [DOI] [PubMed] [Google Scholar]
- 46.Zhou H, Zhang Y, Hamburger AW. EBP1 inhibits translation of AR mRNA in castration resistant prostate cancer. Anticancer Res. 2011;31:3129–35. [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou H, Mazan-Mamczarz K, Martindale JL, Barker A, Liu Z, Gorospe M, et al. Postranscription regulation of AR mRNA by an ErbB3-binding protein 1 in prostate cancer. Nucleic Acids Res. 2010;38:3619–31. doi: 10.1093/nar/gkq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.