Abstract
Recent reports of functional impairment in the ‘unaffected’ limb of stroke patients have suggested that these deficits vary with the side of lesion. This not only supports the idea that the ipsilateral hemisphere contributes to arm movements, but also implies that such contributions are lateralized. We have previously suggested that the left and right hemispheres are specialized for controlling different features of movement. In reaching movements, the non-dominant arm appears better adapted for achieving accurate final positions and the dominant arm for specifying initial trajectory features, such as movement direction and peak acceleration. The purpose of this study was to determine whether different features of control could characterize ipsilesional motor deficits following stroke. Healthy control subjects and patients with either left- or right-hemisphere damage performed targeted single-joint elbow movements of different amplitudes in their ipsilateral hemi-space. We predicted that left-hemisphere damage would produce deficits in specification of initial trajectory features, while right-hemisphere damage would produce deficits in final position accuracy. Consistent with our predictions, patients with left, but not right, hemisphere damage showed reduced modulation of acceleration amplitude. However, patients with right, but not left, hemisphere damage showed significantly larger errors in final position, which corresponded to reduced modulation of acceleration duration. Neither patient group differed from controls in terms of movement speed. Instead, the mechanisms by which speed was specified, through modulation of acceleration amplitude and modulation of acceleration duration, appeared to be differentially affected by left- and right-hemisphere damage. These findings support the idea that each hemisphere contributes differentially to the control of initial trajectory and final position, and that ipsilesional deficits following stroke reflect this lateralization in control.
Keywords: lateralization, stroke, control, arm movements
Introduction
While contralateral motor deficits are common following stroke, a number of studies have also revealed more subtle ipsilateral motor deficits that emerge acutely (Jones et al., 1989; Yelnik et al., 1996; Sunderland et al., 1999) and persist chronically (Winstein and Pohl, 1995; Carey et al., 1998; Sunderland, 2000; Haaland et al., 2004; Yarosh et al., 2004; Wetter et al., 2005). These deficits likely reflect the fact that both hemispheres contribute to unilateral limb movements, an interpretation supported by neural activation studies in humans (Kawashima et al., 1994; Dassonville et al., 1997; Kawashima et al., 1998) and by electrophysiology in other animals (Tanji et al., 1988; Donchin et al., 2002; Cisek et al., 2003). It should be noted that the contribution of ipsilateral cortex to unilateral movement does not appear to be symmetric (Kawashima et al., 1993; Kim et al., 1993; Verstynen et al., 2005), which suggests that ipsilesional deficits following unilateral brain damage might also vary with lesion side.
Some previous studies have shown that damage to the left hemisphere selectively impairs the acceleration phase of motion, while right hemisphere damage might selectively impair the deceleration phase (Fisk and Goodale, 1988; Haaland and Harrington, 1989a; Winstein and Pohl, 1995). This has led to the idea that the left and right hemispheres may be differentially specialized for ‘open- and closed-loop processing,’ respectively. However, Haaland et al. (2004) recently challenged this notion by failing to show deficits in visual-based movement corrections in patients with right-hemisphere damage. In addition, studies using clinical measures of motor performance, such as the Jebsen–Taylor Hand Function Test, have reported that functional ipsilesional deficits do not vary with the side of lesion (Desrosiers et al., 1996; Wetter et al., 2005). It may be that describing hemispheric specialization as lateralization of open- and closed-loop sensorimotor processes may be too general of an approach to effectively characterize ipsilesional motor deficits following stroke.
We have recently reported systematic interlimb differences in specific trajectory features, as well as in the final position accuracies of reaching movements made by healthy young right-handers. These studies showed differences between the dominant and non-dominant arms in the coordination of intersegmental dynamics, adaptation to novel inertial configurations, and achieving stable final positions, even in the presence of unexpected perturbations (Sainburg and Kalakanis, 2000; Bagesteiro and Sainburg, 2002; Sainburg and Wang, 2002; Bagesteiro and Sainburg, 2003). Our findings have led to the proposal that each hemisphere might be specialized for controlling different features of movement (Sainburg, 2002, 2005). More specifically, we hypothesize that the left (dominant) hemisphere has become better adapted for anticipating aspects of limb and task dynamics that are required for efficient coordination. We also propose that the right (non-dominant) hemisphere may be better adapted for specifying and achieving steady-state positions through impedance control mechanisms (Gribble and Ostry, 2000).
We have previously reported advantages in dominant arm performance for some tasks and advantages in non-dominant arm performance for different tasks. However, more recently we have reported single tasks for which each limb displays advantages for different aspects of performance. For example, during adaptation to novel inertial conditions, the dominant arm shows faster improvements in initial trajectory direction, while the non-dominant arm shows faster improvements in final position accuracy (Duff and Sainburg, 2006). In addition, we recently reported distinct differences in the mechanisms by which each limb achieves different amplitude movements during a targeted single-joint task (Sainburg and Schaefer, 2004). While both arms moved with similar speeds and accuracies, the acceleration profiles, which vary directly with joint torque profiles, were different between the arms. The dominant arm varied initial torque amplitude with movement distance, while the non-dominant arm primarily varied torque duration. Peak torque, which occurred within the first 50 ms of movement, has been shown to reflect the amplitude of initial agonist electromyography (EMG), and is specified prior to movement (Lestienne, 1979; Cooke and Brown, 1990). In contrast, torque duration has been shown to reflect the shift from agonist to antagonist muscle activities (Lestienne, 1979; Cooke and Brown, 1990) and can be substantially modified by sensory events occurring early in movement (Brown and Cooke, 1981b). Our interpretation of these results was that each arm relied differently on each of these processes in order to control movement distance.
These separate features of control have previously been attributed to distinct mechanisms. In an isometric elbow joint task, Gordon and Ghez reported that peak rate of force production and the amplitude of initial agonist EMG varied systematically with the amplitude of force targets (Gordon and Ghez, 1987a). However, force rise-time, which corresponded to the onset of antagonist EMG, varied inversely with peak rate of force production within a given target amplitude (Gordon and Ghez, 1987b). This suggested that force rise-time (duration) compensated for incorrect scaling of peak force rate, in order to achieve accurate steady-state force levels. The authors speculated that initial rate of force was preprogrammed, while compensatory adjustments in force rise-time depended on information about the initial force profile. Thus, during single-joint movements, modulation of torque duration appears to be essential for achieving accuracy relative to a target. Likewise, Brown and Cooke (1981a) used targeted elbow joint movements to demonstrate that the amplitude of initial agonist EMG scales with movement distance, but is resistant to peripheral sensory input, such as unexpected forces prior to movement. In contrast, the duration of agonist EMG is extensively modified by peripheral sensory influences, such as mechanical perturbations (Brown and Cooke, 1981b). This suggests that the amplitude and timing of muscle activities reflect independent control mechanisms (Cooke and Brown, 1990).
Our previous findings in healthy young subjects suggest that the mechanisms that underlie control of movement distance during targeted elbow joint movements are lateralized (Sainburg and Schaefer, 2004). Whereas the dominant arm specifies movement distance primarily by varying the amplitude of initial torque, the non-dominant arm does so primarily by varying torque duration. In both cases, movement velocity and distance were similar between the limbs, indicating that both control strategies were equally effective in achieving the performance criterion of the experimental task. We now employ this task to examine whether sensorimotor stroke will differentially affect specification of torque amplitude or of torque duration in the ipsilesional arm, depending on the hemisphere that is damaged. We will test two hypotheses: (i) Anticipatory modulation of torque amplitude reflects a dominant hemisphere specialization for controlling limb dynamics, while modulation of torque duration reflects non-dominant hemisphere advantages in controlling final limb position; (ii) Each hemisphere contributes to the control of the ipsilateral limb, and therefore sensorimotor stroke results in predictable deficits in the ipsilesional arms. Because tangential hand acceleration is linearly related to joint torque during single-joint movements, this task allows us to quantify tangential acceleration in order to examine control strategy. We predict that left-hemisphere damage will produce reductions in modulation of initial acceleration amplitude, relative to age-matched controls, while right-hemisphere damage will produce reductions in modulation of acceleration duration. Because acceleration duration has been shown to reflect compensations for errors in acceleration amplitude, we expect that right-hemisphere damage will also result in greater errors in final position.
Materials and methods
Participants
Ten male right-handed hemiparetic stroke patients and 16 male right-handed healthy control subjects were examined after obtaining approval from the Human Research and Review Committee of the University of New Mexico School of Medicine and informed consent from each participant, according to the Declaration of Helsinki. All subjects were screened and excluded based on history of (i) substance abuse and/or psychiatric diagnosis; (ii) non-stroke neurological diseases for the stroke patients and all neurological diagnoses for the control subjects; and (iii) peripheral movement restrictions, such as neuropathy or orthopaedic disorders. Five stroke patients had left-hemisphere damage, and five patients had right-hemisphere damage. All stroke patients completed the experiment with their ipsilesional arm. All stroke patients were hemiparetic in the contralesional arm, as defined by a contralesional grip strength 1.5 standard deviations below normal and at least 1.5 SDs less than ipsilesional grip strength using a hand dynamometer. Additional measures of hemiparesis (Fugl-Meyer et al., 1975), language comprehension (Kertesz, 1982) and limb apraxia (Haaland and Flaherty, 1984) were also used. Sixteen age- and education-matched healthy control subjects completed the experiment with their left arm (n =8) or right arm (n =8).
MRIs (Phillips Edge 1.5 tesla scanner) were obtained in 9 of the 10 stroke patients with slice thickness of 5 mm and a slice gap of 1 mm. One patient had a CT scan (Phillips PQ 6000 scanner) with slice thickness of 8 mm and no gaps between slices. A board-certified neurologist, who was blinded to the behavioural characteristics of the patients, outlined the area of damage for each patient on 11 standardized horizontal sections derived from the DeArmond atlas (DeArmond et al., 1989) using T1-weighted MRI images for anatomical detail and T2-weighted images to specify borders of the damaged tissue (Fig. 1). These tracings were retraced on a digitizing tablet for input into a computer program that used an algorithm to calculate lesion volume and location within each hemisphere (Frey et al., 1987). This information was used to ensure comparable lesion size and intrahemispheric location between the two stroke groups.
Fig. 1.
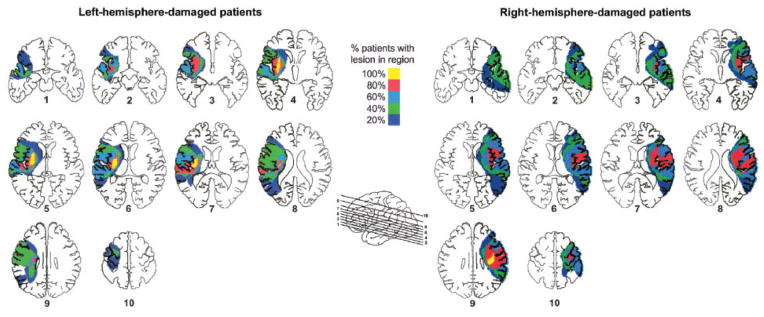
Lesion locations based on tracing lesions from MRI or CT scans were superimposed on axial slices, separately for left-hemisphere-(displayed on left) and right-hemisphere-damaged (displayed on right) patients. Colors of shaded regions denote percentage (20, 40, 60, 80 or 100%) of left- and right-hemisphere-damaged patients with lesion in the corresponding area.
Table 1 summarizes the characteristics of each subject group. Age and education were similar across groups (age: P =0.11; education: P =0.26). Patients with left or right-hemisphere damage did not significantly differ in number of years post stroke (P =0.61), lesion volume (P =0.41), limb apraxia (P =0.53), auditory comprehension (P =0.17) or degree of hemiparesis based on contralesional grip strength (P =0.52) or Fugl–Meyer score (P =0.33). All patients had strokes in the middle cerebral artery distribution. Figure 1 shows that there was cortical and/or subcortical damage to the motor system. All patients with left hemisphere damage had damage in the internal capsule, basal ganglia, and some part of the insula (yellow), while 3 of these 5 patients had damage in Brodmann areas 6, 4, 3, 1, and 2 (red). The two patients who did not have damage in areas 4 and 6 had damage in the internal capsule. Four of the five right-hemisphere-damaged patients had damage in the insula and the same Brodmann areas as the left-hemisphere-damaged group. The one right-hemisphere-damaged patient who did not have damage in these regions had damage in the internal capsule. Four of the five patients in both left- and right-hemisphere-damaged groups also had damage in the supramarginal gyrus (area 40) inferior to the intraparietal sulcus.
Table 1.
Summary of participant information
| Variable (mean ±SD) | Healthy control
|
Hemisphere damaged
|
||
|---|---|---|---|---|
| Left | Right | Left | Right | |
| n | 8 | 8 | 5 | 5 |
| Age (years) | 59.6 ±9.0 | 67.6 ±9.2 | 53.6 ±9.6 | 59.8 ±13.2 |
| Education (years) | 15.0 ±1.2 | 15.8 ± 2.4 | 15.4 ±1.9 | 13.6 ±1.7 |
| Years post-strokea | 9.8 ±5.6 | 7.8 ± 6.3 | ||
| Lesion volume (cm3)b | 99.3 ± 61.4 | 148.0 ±108.2 | ||
| Total upper-extremity Fugl^Meyer scorec | 86.2 ± 24.6 | 61.0 ±32.4 | ||
| Language comprehensiond | 79 ± 2.8 | 80 ± 0 | 68.6 ±14.0 | 80 ± 0 |
| Apraxiae | 13.0 ±1.5 | 13.0 ±1.5 | 11.0 ±2.7 | 11.4 ±2.6 |
| Grip strength rightf | 47.9 ±5.0 | 48.8 ±14.8 | 10.4 ±12.6 | 43.4 ±12.2 |
| Grip strength leftf | 47.8 ± 6.2 | 50.3 ±7.0 | 48.8 ± 4.7 | 5.8 ± 8.6 |
Note: Values are means ±SD.
Years post-stroke are calculated as time elapsed between incidence of stroke and day of data collection.
Lesion volume is computed from MRI or CT scans using a computer algorithm.
Maximum score on the total upper-extremity Fugl^Meyer score is 126.
Language comprehension was assess using the Western Aphasia Battery.
Apraxia is designated as mean number correct out of 15 items using a validated apraxia battery.
Grip strength from dynamometer are expressed as standardized t scores.
Experimental setup
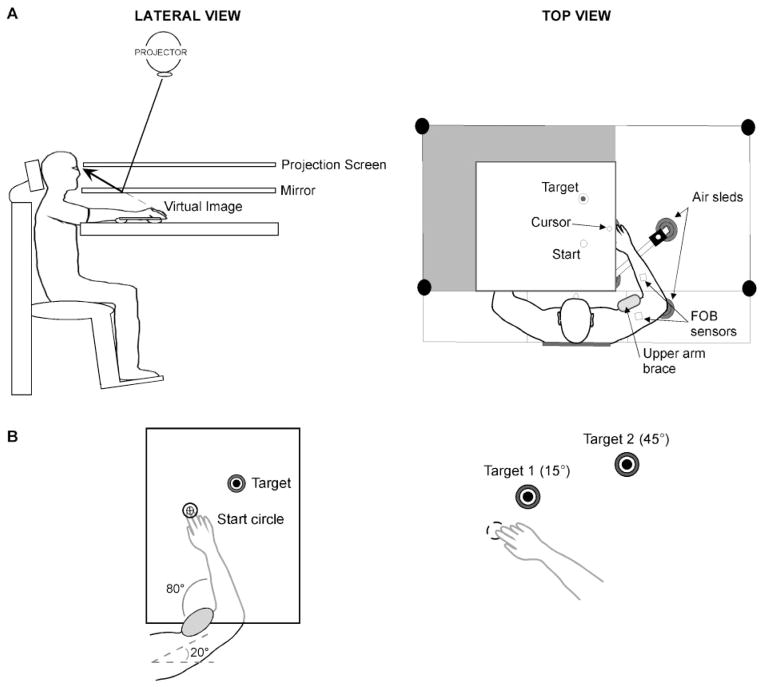
Figure 2A illustrates the experimental setup. Participants sat facing a projection screen with either their left or right arm supported over a horizontal surface by an air-jet system to reduce the effects of friction and gravity. The arm was positioned just below shoulder height. The start circle, a target, and a cursor that represented finger position were projected on a horizontal back-projection screen positioned above the arm, with a horizontal mirror positioned below this screen. The mirror reflected the visual display to give the illusion that the display was in the same horizontal plane as the fingertip. Calibration of the display assured that this projection was veridical.
Fig. 2.
(A) Lateral and top view of experimental apparatus are shown. (B) Experimental task required movement of cursor from start circle to 1 of 2 target circles, with upper arm restrained.
All joints distal to the elbow were immobilized using an adjustable brace. Position and orientation of the segments proximal and distal to the elbow joint were sampled using a Flock of Birds (FoB)® (Ascension-Technology) magnetic six-degree-of-freedom (6-DOF) movement recording system. A single sensor was attached to the upper-arm segment via an adjustable plastic cuff, while another sensor was fixed to the air sled where the forearm was fitted. The sensors were positioned approximately at the centre of each arm segment. The 3-D positions of the following three bony landmarks were digitized using a stylus that was rigidly attached to a FoB sensor: (i) index finger tip; (ii) the lateral epicondyle of the humerus; (iii) the acromion, directly posterior to the acromioclavicular joint. The 3-D position of the finger tip was projected into the plane of the display in order to drive the cursor position. Screen redrawing occurred fast enough to maintain the cursor on the fingertip throughout the sampled arm movements. Digital data were collected at 103 Hz using a Macintosh computer, which controlled the sensors through separated serial ports, and stored on disk for further analysis. The data were digitally resampled at 100 Hz for analysis. Custom computer algorithms for experiment control and data analysis were written in REAL BASIC™ (REAL Software, Inc.), C and IgorPro™ (Wavemetric, Inc.).
Experimental task
All targets were 2.5 cm in diameter, and the target locations were determined according to subjects’ shoulder and elbow angles, and were unique for each subject. For all subjects, the upper arm was positioned at 20°, and stabilized by a brace attached to the table (Fig. 2B). The starting elbow angle was 80°, while the targets were placed at the fingertip locations that corresponded to elbow angles of 95 and 125°, respectively. Therefore, the targets required 15 and 45° of elbow extension, respectively. Although target positions were individually set for each subject according to elbow angles, the average Euclidean distances were 7 and 30 cm, respectively. These target distances were similar to those in our previous study in young healthy subjects (Sainburg and Schaefer, 2004) in order to systematically vary velocity and distance of the hand in the mid-range of elbow joint motion. We used an intermediate target (25°) in order to randomize target amplitude during the session.
The cursor, which corresponded to the real-time position of the index finger tip, the start circle and the target were displayed on the screen prior to each trial. Subjects were to hold the cursor within the starting circle for 200 ms to trigger the audiovisual ‘go’ signal, which initiated each trial. They were instructed to move their finger (cursor) to ‘the centre of the target and stop, using a single, uncorrected motion.’ Feedback regarding the fingertip position (cursor display) was given to allow subjects to position the hand in the start circle, and was then removed at the ‘go’ signal. No visual feedback was given during the movement, nor was explicit knowledge of results provided at the end of the movement. However, for motivational purposes, subjects received a numerical score at the end of each trial, which was based on final position accuracy. Final position errors of <1.25 cm from the centre of the target (i.e. within the target circle) were awarded 10 points, while errors between 1.25 and 2.5 cm were awarded 3 points, and errors between 2.5 cm and 3.75 cm were awarded 1 point. The purpose of awarding points to each trial was merely to motivate our subjects; these points were not analysed as dependent variables, and all trials were recorded and saved. Following the display of the numerical score after each trial, the cursor was redisplayed for accurate positioning of the fingertip back at the start circle for the next trial. Targets were presented in a pseudorandom order, such that no single target was presented consecutively. The first 45 trials of each session allowed for task familiarity, while kinematic and statistical analyses were conducted on the following 50 trials to each target.
Kinematic data
The 3D position of the index finger, elbow point and shoulder point were calculated from sensor position and orientation data. Then, joint angles were calculated from these data. All kinematic data were low-pass filtered at 8 Hz (3rd order, dual-pass Butterworth), and differentiated to yield tangential velocity and acceleration values. Movement start was determined by identifying the time of peak velocity and searching backward in time for the first minimum below 6% of peak tangential velocity, or for zero velocity, whichever was identified first. Movement end was similarly determined by searching forward in time from peak velocity to find the first minimum below 6% of peak tangential velocity, thereby excluding any small, corrective submovements.
Dependent measures
The following measures were calculated for each trial: Absolute and variable final position error, movement time, velocity, acceleration, acceleration duration, deceleration and deceleration duration. Absolute final position error, a measure of accuracy, was calculated as the absolute value of the distance from the finger tip at movement end to the centre of the target. Variable error, a measure of consistency, was calculated as the distance from the finger tip at movement end to the mean final position for each target. Movement time was defined as the elapsed time from movement start to movement end. Peak velocity was defined as the maximum tangential velocity. Peak acceleration was defined as the maximum tangential acceleration, and then normalized for each subject. Similarly, peak deceleration was defined as the minimum tangential acceleration, and then normalized for each subject. Acceleration duration was defined as the elapsed time from movement start to time of peak velocity. Deceleration duration was defined as the elapsed time from time of peak velocity to movement end. In order to focus our analysis on within-subject variations in acceleration measures, we normalized peak acceleration to the maximum acceleration recorded within the experimental session (see Hu et al., 2006). A similar algorithm was employed for quantification of peak deceleration, normalizing to minimum acceleration.
where accmax = largest peak acceleration produced by that subject during session acci = peak acceleration of trial i
Statistical analysis
Our task was designed to vary the required amplitude of movements between the shortest (15°) and longest (45°) target distance, which served as our independent variable for this study. We interposed an intermediate length target (25°) in order to randomize target amplitudes. However, the actual movement displacements for the intermediate target extensively overlapped the distributions of the 15 and 45° targets (Ranges: 15°: 3.6–13.8 cm; 25°: 7.2–25.9 cm; 45°: 14.6–39.7 cm). Thus, we limited our statistical analysis to the short and long target distances, which corresponded to substantial changes in our independent variables.
The individual dependent measures were analysed using 3-way repeated-measure ANOVA, with arm (left or right) and group (healthy control or hemisphere-damaged) as between-subject factors and target (15 or 45°) as the within-subject factor. Based upon our hypothesis, we predicted significant 3-way interactions for acceleration and final position parameters, which should reflect changes in acceleration amplitude, acceleration duration and movement error as a function of target and hemisphere of damage. Mean data were subjected to 3-way, repeated measures ANOVA in JMP® statistical software (SAS®). When warranted by significant interaction, post hoc analyses were performed using student’s t-tests.
Results
Task performance
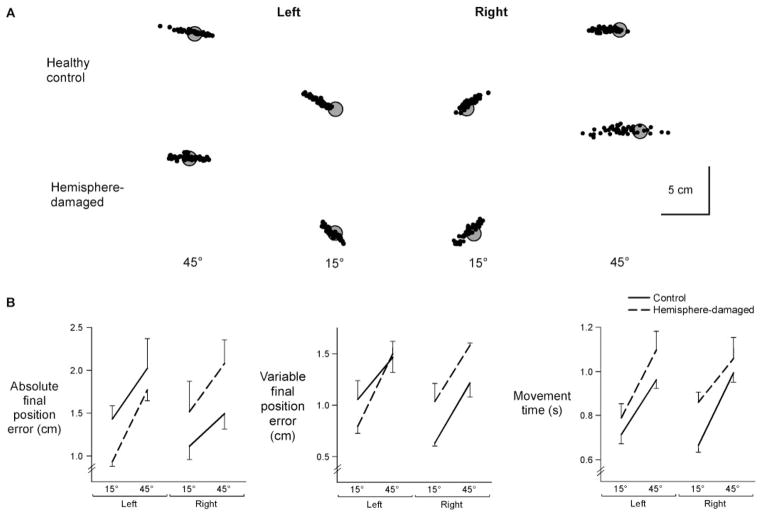
Figure 3A shows the distributions of final position from representative subjects, relative to each target. Final positions of the left-hemisphere-damaged patient’s left (ipsilesional) arm were more accurate than the left arm of the control subject for both targets. However, the right arm of the right-hemisphere-damaged patient was substantially less accurate, and more variable, in final position as compared to the control subject’s right arm. Thus, there were significant interactions between arm (left or right) and group (healthy control or hemisphere-damaged) for both constant final position error [F(1,22) =4.36, P<0.05] and variable error [F(1,22) =4.33, P<0.05] (Fig. 3B). Further analyses revealed that the ipsilesional arm of right-hemisphere-damaged patients was significantly less accurate than the right arm of control subjects (constant error: P<0.05; variable error: P<0.05). In contrast, left-hemisphere-damaged patients were just as accurate as control subjects (constant error: P =0.20; variable error: P =0.50). In other words, damage to the right, but not left, hemisphere produced specific deficits in final position accuracy of the ipsilesional arm, relative to performance in age- and arm-matched controls.
Fig. 3.
(A) Final positions at movement end for each trial (dot) are displayed relative to gray targets for a representative subject from each experimental group. (B) Mean absolute final position error, mean variable final position error and movement time for each target is displayed for the left and right arms of control subjects and the ipsilesional arms of left- and right-hemisphere-damaged patients. Bars indicate standard error of mean.
Movement time was systematically longer for patients than for control subjects, irrespective of arm [F(1,22) =5.63, P<0.05] (Fig. 3B). Thus, regardless of the side of the lesion, the stroke patients showed prolonged movement durations as compared to the control subjects.
Velocity and acceleration
Previous studies have consistently reported a strong tendency for subjects to scale movement speed with movement distance when reaching to different target distances (Bouisset and Lestienne, 1974; Ghez, 1979; Brown and Cooke, 1981a, 1990; Cooke and Brown, 1990). This relationship appears to be maintained in our hemisphere-damaged patients, as well as in our age-matched control subjects (Fig. 4A). For all subjects, velocity profiles tended to be unimodal, skewed to the left and scaled in magnitude with target [F(1,22) =419.9, P<0.0001] (Fig. 4B). However, this scaling did not depend on arm or on side of lesion, indicating that the differences in final error between left- and right-hemisphere-damaged patients could not be accounted for by speed-accuracy tradeoffs.
Fig. 4.
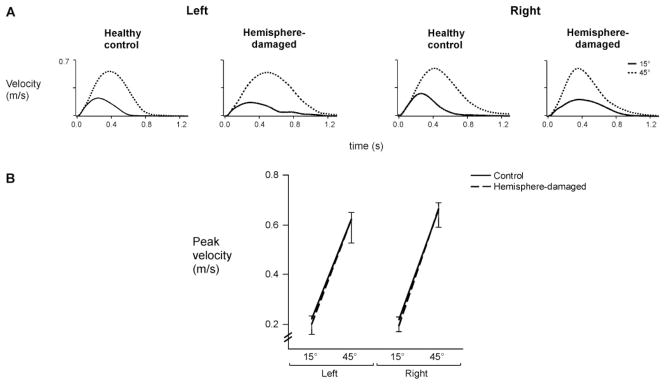
(A) Average tangential velocity profiles for each target for representative control subjects and hemisphere-damaged patients. (B) Mean peak velocity for each target is displayed for the left and right arms of control subjects and the ipsilesional arms of left- and right-hemisphere-damaged patients. Bars indicate standard error of mean.
There were, however, mechanistic differences in how each group effectively scaled velocity with target distance. Peak velocity is determined by both the amplitude and duration of acceleration, which have been shown to reflect different aspects of movement control (Ghez, 1979; Brown and Cooke, 1981b, 1984b, 1990; Cooke and Brown, 1990). As shown in Fig. 5A, the amplitude of acceleration increased substantially with target distance for all subjects, except for the left-hemisphere-damaged patient. Instead, this patient increased the duration of acceleration (acceleration cross-zero) with target distance, resulting in effective scaling of peak tangential velocity with intended movement distance. In contrast, the right-hemisphere-damaged patient showed extensive change in acceleration amplitude between targets with minimal adjustment of acceleration duration.
Fig. 5.
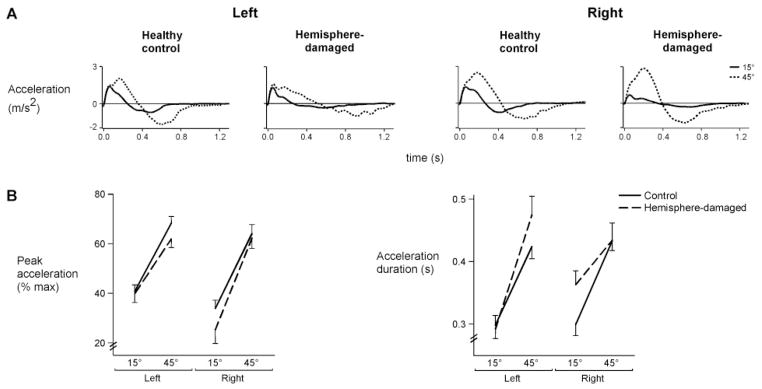
(A) Average tangential acceleration profiles for each target for representative control subjects and hemisphere-damaged patients. (B) Mean peak acceleration (normalized to% max) and acceleration duration for each target is displayed for the left and right arms of control subjects and the ipsilesional arms of left- and right-hemisphere-damaged patients. Bars indicate standard error of mean.
These differences in acceleration were consistent across subjects, as shown in Fig. 5B (left). The left (ipsilesional) arm of left-hemisphere-damaged patients showed less change in acceleration amplitude across targets as compared to that of control subjects, whereas the right arm of right-hemisphere-damaged patients showed greater change in acceleration amplitude relative to control subjects, as reflected by a significant 3-way interaction of arm, group and target [F(1,22) =4.45, P<0.05]. Figure 5B (right) shows a reverse pattern of results for acceleration duration: Greater target-dependent change in acceleration duration was present in the left-hemisphere-damaged patients as compared to their control group, while less change was present in the right-hemisphere-damaged patients, as reflected by a significant 3-way interaction of arm, group and target [F(1,22) =14.56, P<0.001]. Thus, left-hemisphere-damaged patients showed restrictions in scaling of acceleration amplitude across targets, while right-hemisphere-damaged patients showed restricted scaling of acceleration duration. Interestingly, these two features of acceleration modulation have previously been hypothesized to reflect independent feedforward and feedback control processes (Brown and Cooke, 1981b; Gordon and Ghez, 1987a, b; Bermejo and Zeigler, 1989).
Previous studies have characterized the relationship between acceleration duration and onset of antagonist EMG during single joint movement. The function of antagonist muscle activity is to decelerate the limb and to achieve a stable final position (Brown and Cooke, 1986; Cooke and Brown, 1990; Berardelli et al., 1996). Therefore, we examined the amplitude and duration of the deceleration phase to determine whether variations in deceleration could have contributed to the observed differences in error at final position. Changes in peak deceleration across targets did not depend on arm or on side of lesion [F(1,22) =2.58, P =0.12] (Fig. 6, left). Thus, the amplitude of deceleration did not appear to be differentially affected by left or right-hemisphere damage, nor did the duration of deceleration. As shown in Fig. 6 (right), the stroke patients had longer deceleration durations than did the control subjects, regardless of side of lesion [F(1,22) =5.47, P<0.05]. Thus, neither the amplitude nor duration of deceleration could account for the arm- and lesion-dependent differences in final position errors.
Fig. 6.
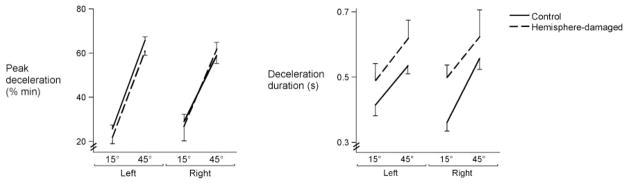
Mean peak deceleration (normalized to% min) and deceleration duration for each target is displayed for the left and right arms of control subjects and the ipsilesional arms of left- and right-hemisphere-damaged patients. Bars indicate standard error of mean.
Error as a function of timing modulation
Previous work has described changes in acceleration amplitude and duration relative to changes in velocity, torque and distance (Ghez and Vicario, 1978; Ghez, 1979; Brown and Cooke, 1984, 1986; Gordon and Ghez, 1987a, b; Gottlieb et al., 1989, 1990; Brown and Cooke, 1990; Cooke and Brown, 1990). Acceleration amplitude corresponds to initial agonist EMG amplitude, whereas acceleration duration corresponds to the duration of agonist activity and onset of antagonist activity. It has been suggested that such temporal adjustments of muscle activity, in part, compensate for errors in initial specification of agonist amplitude (Brown and Cooke, 1981b; Gordon and Ghez, 1987b); thus, we expected that larger errors in final position might be more dependent on a reduced ability to modulate acceleration duration throughout the task, rather than acceleration amplitude. As reported earlier, patients with left-hemisphere damage demonstrated the largest change in acceleration duration with movement distance (Fig. 5B) and also the smallest errors (Fig. 3B). In contrast, patients with right-hemisphere damage had the smallest change in acceleration duration (Fig. 5B) and the largest errors (Fig. 3B). Figure 7 depicts the relationship between constant final position error and acceleration duration within individual trials for representative subjects from each group. In this figure, final position error is signed, with positive values indicating target overshoot, and negative values indicating target undershoot. The left and right arms of the control subjects produced similar ranges of acceleration duration and similar distributions of final position error, as reflected by the similar size and orientation of their density ellipses (CI =0.99). However, the distribution for the left-hemisphere-damage patient was such that large modulation of acceleration duration was associated with greater accuracy, as reflected by the horizontal orientation of the final position density ellipse. The vertically elongated ellipse for the right-hemisphere-damaged patient indicates that the larger error range was associated with a restricted modulation of acceleration duration. This striking spatial-temporal relationship supports our hypothesis of right hemisphere specialization for the control of final steady-state position, given that the intact right hemisphere appears to effectively maintain movement accuracy, as in the case of our left-hemisphere-damaged patients. This is especially compelling, given that (i) the large extent of damage within the dominant (left) hemisphere of these patients does not appear to selectively impair final accuracy; and (ii) when the dominant (left) hemisphere is intact, as in our right-hemisphere-damaged patients, it does not appear to be advantageous for achieving accurate final positions.
Fig. 7.
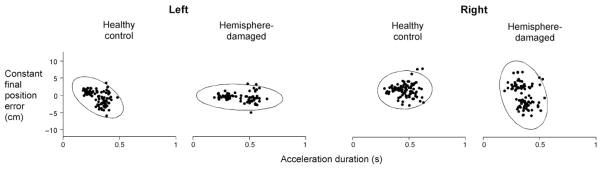
Constant final position error (y axis) and acceleration duration (x axis) of each trial (dot) are displayed for representative control subjects and hemisphere-damaged patients. Final position error is signed, such that positive values indicate overshoot of target, and negative values indicate undershoot of target. Ellipses represent the 99% confidence interval of the data from each subject.
Discussion
This study confirmed that ipsilesional motor deficits in patients with left or right-hemisphere damage vary as a function of lesion side. Based on our previous findings of interlimb differences in healthy young right-handers (Sainburg, 2002, 2004), we hypothesized that ipsilesional motor deficits following stroke should reflect hemispheric specializations for controlling different aspects of movement. We confirmed that during targeted single-joint reaching, left-hemisphere-damaged patients showed reductions only in the modulation of acceleration amplitude with target distance, while right-hemisphere-damaged patients showed reductions only in the modulation of acceleration duration. We also found that deficits in final position accuracy were specific to right-hemisphere-damaged patients, and we speculated that these larger errors in final position could be related to the restricted ability of these patients to modulate acceleration duration. Together, these findings support the idea that asymmetric ipsilesional deficits following stroke could be attributed to the differential contribution of the left and right hemispheres to the control of a single limb. This interpretation is consistent with recent functional imaging reports of asymmetric hemispheric activation during unimanual tasks (Kawashima et al., 1993; Kim et al., 1993; Dassonville et al., 1997; Verstynen et al., 2005). Our current results also support previous findings from our laboratory, which demonstrated dominant arm advantages for anticipating aspects of limb and task dynamics, and non-dominant arm advantages for achieving steady-state position in healthy subjects (Sainburg and Kalakanis, 2000; Bagesteiro and Sainburg, 2002; Sainburg, 2002; Bagesteiro and Sainburg, 2003). These results substantially extend our hypothesis of hemispheric specialization by demonstrating differential effects of left and right hemisphere stroke on modulation of acceleration amplitude and duration, respectively.
Right-hemisphere-damaged patients showed errors in final position that were associated with restricted modulation of acceleration duration. The idea that modulation of acceleration duration might be causally related to movement accuracy is consistent with a number of previous studies. For a single joint movement, the termination of joint acceleration corresponds to the onset of antagonist muscle activity, which serves to decelerate the limb as it approaches the target (Brown and Cooke, 1986; Cooke and Brown, 1990; Berardelli et al., 1996). More importantly, a number of studies have indicated that variations in the duration of acceleration, torque or force are inversely related to the initial magnitude of these variables, and thus appear to compensate for inaccuracies in planning (Brown and Cooke, 1981b; Gordon and Ghez, 1987a, b; Bermejo and Zeigler, 1989). Our current study extends these findings to show that right hemisphere damage resulted in restricted modulation of acceleration duration, and was associated with substantial final position errors. In contrast, left-hemisphere-damaged patients had preserved modulation of acceleration duration, and smaller final position errors.
It is plausible that the final position errors of right-hemisphere-damaged patients resulted from inaccurate targeting of the movements due to perceptual deficits. In fact, previous studies have attributed spatial errors in right-hemisphere-damaged patients to perceptual deficits (Heilman et al., 1986; Benton and Tranel, 1993). However, these patients preserved the scaling of acceleration amplitude with target distance, which clearly reflects the ability to modulate torque amplitude with target distance. We thus conclude that the current deficits in movement accuracy, associated with both reduced modulation of acceleration duration and intact modulation of acceleration amplitude, result from deficits in regulating the timing of muscle actions in order to compensate variations in initial torque, rather than from deficits in perceived target location.
Our findings indicate reduced modulation of peak acceleration across movement directions in left-hemisphere-damaged patients. Because apraxias are commonly associated with left-hemisphere damage (Koski et al., 2002), the question of whether this finding might be related to apraxia should be addressed. Ideomotor limb apraxia has been characterized as kinematic impairment during gesture imitation, reflecting spatial and temporal deficits in multijoint coordination (Poizner et al., 1995), and has been previously attributed to deficits in motor planning (Harrington and Haaland, 1992). In the current study, our finding of reduced modulation of acceleration amplitude in our left-hemisphere-damaged patients does associate deficits in motor planning with damage in the left hemisphere. However, limb apraxia is characterized by disorganization of complex movements, while the movements in the current study should not elicit the same organizational requirements as gestures that are typically associated with apraxic symptoms. It is, however, plausible that fundamental deficits in specifying joint torques could result in deficits in intersegmental coordination (Sainburg et al., 1993, 1995; Bastian et al., 1996, 2000). Thus, it remains possible that the deficits in torque specification revealed by the current study could contribute to, or interact with, the more complex coordination deficits associated with limb apraxia. Further research would be necessary to distinguish the interaction between such deficits. It should be noted that only 3 of our 5 left-hemisphere-damaged patients were clinically diagnosed with ideomotor limb apraxia.
Our current findings may, however, provide a context for understanding the functional motor deficits that result from unilateral stroke. Many previous studies have reported ipsilateral motor impairment in patients with unilateral brain damage (Wyke, 1967; Haaland et al., 1977; Haaland and Delaney, 1981; Haaland and Harrington, 1989a, b; Winstein and Pohl, 1995; Haaland and Harrington, 1996; Carey et al., 1998; Haaland et al., 2004), as well as in animals with ipsilateral lesions (Grabowski et al., 1993; Gonzalez et al., 2004). While these deficits are substantially less severe than contralateral deficits, they have been shown to produce significant functional impairments, including problems performing activities of daily living (Desrosiers et al., 1996; Sunderland, 2000; Wetter et al., 2005; Sainburg and Duff, 2006). The association of ipsilesional deficits with functional impairment may seem counter-intuitive, but longitudinal studies have shown that only a minority of hemiplegic stroke patients demonstrates full functional recovery in the contralesional limbs (Wade et al., 1983; Parry et al., 1999; Kwakkel et al., 2003; Gillen and Burkhardt, 2004). Thus, when stroke patients have moderate to severe hemiplegia, the ipsilesional limb usually serves as the lead controller for bilateral activities such as unscrewing a jar lid, fastening buttons, slicing food or as the only controller in unilateral activities, such as transporting a cup of coffee to the mouth. In fact, Bonifer et al. (2005) reported that the ipsilesional arm of moderately impaired patients continued to be used as the lead controller in unimanual and bimanual tasks, even after an effective constraint-induced therapy trial. Moreover, Vega-Gonzalez and Granat (2005) continuously monitored spontaneous use of both arms of chronic stroke patients and found that hemiplegic patients used the ipsilesional limb 3–6 times more frequently than the impaired contralesional limb. Thus, for many hemiplegic patients, functional recovery relies heavily on ipsilesional limb function. Our current findings reveal systematic differences in the ipsilesional deficits that result from left and right-hemisphere damage that may reflect specialization for controlling different features of movement. Further research is necessary to determine how these different deficits might affect functional performance, and what intervention strategies could ameliorate such dysfunction.
Acknowledgments
This research was supported by the National Institutes of Health, National Institute for Child Health and Human Development (#RO1HD39311), National Institute on Aging training grant, Interdisciplinary Training in Gerontology (#T32AG00048) and the Department of Veterans Affairs, Merit Review Grant. This project was also funded, in part, under a grant with the Pennsylvania Department of Health using Tobacco Settlement Funds. The Department specifically disclaims responsibility for any analyses, interpretations or conclusions. Further acknowledgments are to (i) Jennifer Hogan, Rena Singleton and Monica Stump for data collection; (ii) Drs Robert Knight and Blaine Hart for MRI tracings and neuroanatomical consultation; (iii) Dr Joseph Sadek for statistical consultation and (iv) Drs John Adair and Sally Harris, as well as HealthSouth Rehabilitation Hospital and Lovelace Medical Center, for patient referral.
Abbreviations
- EMG
electromyography
References
- Bagesteiro LB, Sainburg RL. Handedness: dominant arm advantages in control of limb dynamics. J Neurophysiol. 2002;88:2408–21. doi: 10.1152/jn.00901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagesteiro LB, Sainburg RL. Nondominant arm advantages in load compensation during rapid elbow joint movements. J Neurophysiol. 2003;90:1503–13. doi: 10.1152/jn.00189.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian AJ, Martin TA, Keating JG, Thach WT. Cerebellar ataxia: abnormal control of interaction torques across multiple joints. J Neurophysiol. 1996;76:492–509. doi: 10.1152/jn.1996.76.1.492. [DOI] [PubMed] [Google Scholar]
- Bastian AJ, Zackowski KM, Thach WT. Cerebellar ataxia: torque deficiency or torque mismatch between joints? J Neurophysiol. 2000;83:3019–30. doi: 10.1152/jn.2000.83.5.3019. [DOI] [PubMed] [Google Scholar]
- Benton AL, Tranel D. Visuoperceptual, visuospatial, and visuoconstructive disorder. In: Heilman KM, Valenstein E, editors. Clinical neuropsychology. New York: Oxford University Press; 1993. [Google Scholar]
- Berardelli A, Hallett M, Rothwell JC, Agostino R, Manfredi M, Thompson PD, et al. Single-joint rapid arm movements in normal subjects and in patients with motor disorders. Brain. 1996;119 (Pt 2):661–74. doi: 10.1093/brain/119.2.661. [DOI] [PubMed] [Google Scholar]
- Bermejo R, Zeigler HP. Prehension in the pigeon. II Kinematic analysis. Exp Brain Res. 1989;75:577–85. doi: 10.1007/BF00249909. [DOI] [PubMed] [Google Scholar]
- Bonifer NM, Anderson KM, Arciniegas DB. Constraint-induced therapy for moderate chronic upper extremity impairment after stroke. Brain Inj. 2005;19:323–30. doi: 10.1080/02699050400004302. [DOI] [PubMed] [Google Scholar]
- Bouisset S, Lestienne F. The organisation of a simple voluntary movement as analysed from its kinematic properties. Brain Res. 1974;71:451–7. doi: 10.1016/0006-8993(74)90988-3. [DOI] [PubMed] [Google Scholar]
- Brown SH, Cooke JD. Amplitude- and instruction-dependent modulation of movement-related electromyogram activity in humans. J Physiol. 1981a;316:97–107. doi: 10.1113/jphysiol.1981.sp013775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SH, Cooke JD. Responses to force perturbations preceding voluntary human arm movements. Brain Res. 1981b;220:350–5. doi: 10.1016/0006-8993(81)91224-5. [DOI] [PubMed] [Google Scholar]
- Brown SH, Cooke JD. Initial agonist burst duration depends on movement amplitude. Exp Brain Res. 1984;55:523–7. doi: 10.1007/BF00235283. [DOI] [PubMed] [Google Scholar]
- Brown SH, Cooke JD. Initial agonist burst is modified by perturbations preceding movement. Brain Res. 1986;377:311–22. doi: 10.1016/0006-8993(86)90874-7. [DOI] [PubMed] [Google Scholar]
- Brown SH, Cooke JD. Movement-related phasic muscle activation. I Relations with temporal profile of movement. J Neurophysiol. 1990;63:455–64. doi: 10.1152/jn.1990.63.3.455. [DOI] [PubMed] [Google Scholar]
- Carey JR, Baxter TL, Di Fabio RP. Tracking control in the nonparetic hand of subjects with stroke. Arch Phys Med Rehabil. 1998;79:435–41. doi: 10.1016/s0003-9993(98)90146-0. [DOI] [PubMed] [Google Scholar]
- Cisek P, Crammond DJ, Kalaska JF. Neural activity in primary motor and dorsal premotor cortex in reaching tasks with the contralateral versus ipsilateral arm. J Neurophysiol. 2003;89:922–42. doi: 10.1152/jn.00607.2002. [DOI] [PubMed] [Google Scholar]
- Cooke JD, Brown SH. Movement-related phasic muscle activation. II Generation and functional role of the triphasic pattern. J Neurophysiol. 1990;63:465–72. doi: 10.1152/jn.1990.63.3.465. [DOI] [PubMed] [Google Scholar]
- Dassonville P, Zhu XH, Uurbil K, Kim SG, Ashe J. Functional activation in motor cortex reflects the direction and the degree of handedness. Proc Natl Acad Sci USA. 1997;94:14015–8. doi: 10.1073/pnas.94.25.14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeArmond S, Fusco M, Dewey M. Structure of the human brain: a photographic atlas. New York: Oxford University Press; 1989. [Google Scholar]
- Desrosiers J, Bourbonnais D, Bravo G, Roy PM, Guay M. Performance of the ‘unaffected’ upper extremity of elderly stroke patients. Stroke. 1996;27:1564–70. doi: 10.1161/01.str.27.9.1564. [DOI] [PubMed] [Google Scholar]
- Donchin O, Gribova A, Steinberg O, Mitz AR, Bergman H, Vaadia E. Single-unit activity related to bimanual arm movements in the primary and supplementary motor cortices. J Neurophysiol. 2002;88:3498–517. doi: 10.1152/jn.00335.2001. [DOI] [PubMed] [Google Scholar]
- Duff SV, Sainburg RL. Lateralization of motor adaptation reveals independence in control of trajectory and steady-state position. Exp Brain Res. 2006 doi: 10.1007/s00221-006-0811-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisk JD, Goodale MA. The effects of unilateral brain damage on visually guided reaching: hemispheric differences in the nature of the deficit. Exp Brain Res. 1988;72:425–35. doi: 10.1007/BF00250264. [DOI] [PubMed] [Google Scholar]
- Frey R, Woods D, Knight R, Scabini D, Clayworth C. Defining functional areas with averaged CT scans. Social Neurosci. 1987;13:1266. [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1 a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- Ghez C. Contributions of Central Programs to Rapid Limb Movement in the Cat. In: Asanuma H, Wilson VJ, editors. Integration in the nervous system. Tokyo, New York: Igaku-Shoin; 1979. [Google Scholar]
- Ghez C, Vicario D. The control of rapid limb movement in the cat. II Scaling of isometric force adjustments. Exp Brain Res. 1978;33:191–202. doi: 10.1007/BF00238059. [DOI] [PubMed] [Google Scholar]
- Gillen G, Burkhardt A. Stroke rehabilitation: a function-based approach. St. Louis: Elsevier Science/Mosby; 2004. [Google Scholar]
- Gonzalez CL, Gharbawie OA, Williams PT, Kleim JA, Kolb B, Whishaw IQ. Eur J Neurosci. 2004;20:3442–52. doi: 10.1111/j.1460-9568.2004.03751.x. [DOI] [PubMed] [Google Scholar]
- Gordon J, Ghez C. Trajectory control in targeted force impulses. II Pulse height control. Exp Brain Res. 1987a;67:241–52. doi: 10.1007/BF00248546. [DOI] [PubMed] [Google Scholar]
- Gordon J, Ghez C. Trajectory control in targeted force impulses. III Compensatory adjustments for initial errors. Exp Brain Res. 1987b;67:253–69. doi: 10.1007/BF00248547. [DOI] [PubMed] [Google Scholar]
- Gottlieb GL, Corcos DM, Agarwal GC. Organizing principles for single-joint movements. I A speed-insensitive strategy. J Neurophysiol. 1989;62:342–57. doi: 10.1152/jn.1989.62.2.342. [DOI] [PubMed] [Google Scholar]
- Gottlieb GL, Corcos DM, Agarwal GC, Latash ML. Organizing principles for single joint movements. III Speed-insensitive strategy as a default. J Neurophysiol. 1990;63:625–36. doi: 10.1152/jn.1990.63.3.625. [DOI] [PubMed] [Google Scholar]
- Grabowski M, Brundin P, Johansson BB. Paw-reaching, sensorimotor, and rotational behavior after brain infarction in rats. Stroke. 1993;24:889–95. doi: 10.1161/01.str.24.6.889. [DOI] [PubMed] [Google Scholar]
- Gribble PL, Ostry DJ. Compensation for loads during arm movements using equilibrium-point control. Exp Brain Res. 2000;135:474–82. doi: 10.1007/s002210000547. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Cleeland CS, Carr D. Motor performance after unilateral hemisphere damage in patients with tumor. Arch Neurol. 1977;34:556–9. doi: 10.1001/archneur.1977.00500210058010. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Delaney HD. Motor deficits after left or right hemisphere damage due to stroke or tumor. Neuropsychologia. 1981;19:17–27. doi: 10.1016/0028-3932(81)90040-3. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Flaherty D. The different types of limb apraxia errors made by patients with left vs. right hemisphere damage. Brain Cogn. 1984;3:370–84. doi: 10.1016/0278-2626(84)90029-0. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL. Hemispheric control of the initial and corrective components of aiming movements. Neuropsychologia. 1989a;27:961–9. doi: 10.1016/0028-3932(89)90071-7. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL. The role of the hemispheres in closed loop movements. Brain Cogn. 1989b;9:158–80. doi: 10.1016/0278-2626(89)90027-4. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL. Hemispheric asymmetry of movement. Curr Opin Neurobiol. 1996;6:796–800. doi: 10.1016/s0959-4388(96)80030-4. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Prestopnik JL, Knight RT, Lee RR. Hemispheric asymmetries for kinematic and positional aspects of reaching. Brain. 2004;127:1145–58. doi: 10.1093/brain/awh133. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Haaland KY. Motor sequencing with left hemisphere damage. Are some cognitive deficits specific to limb apraxia? Brain. 1992;115 (Pt 3):857–74. doi: 10.1093/brain/115.3.857. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Bowers D, Valenstein E, Watson RT. The right hemisphere: neuropsychological functions. J Neurosurg. 1986;64:693–704. doi: 10.3171/jns.1986.64.5.0693. [DOI] [PubMed] [Google Scholar]
- Hu X, Tong K, Tsang VS, Song R. Joint-angle-dependent neuromuscular dysfunctions at the wrist in persons after stroke. Arch Phys Med Rehabil. 2006;87:671–9. doi: 10.1016/j.apmr.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Jones RD, Donaldson IM, Parkin PJ. Impairment and recovery of ipsilateral sensory-motor function following unilateral cerebral infarction. Brain. 1989;112 (Pt 1):113–32. doi: 10.1093/brain/112.1.113. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Matsumura M, Sadato N, Naito E, Waki A, Nakamura S, et al. Regional cerebral blood flow changes in human brain related to ipsilateral and contralateral complex hand movements–a PET study. Eur J Neurosci. 1998;10:2254–60. doi: 10.1046/j.1460-9568.1998.00237.x. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Roland PE, O’Sullivan BT. Fields in human motor areas involved in preparation for reaching, actual reaching, and visuomotor learning: a positron emission tomography study. J Neurosci. 1994;14:3462–74. doi: 10.1523/JNEUROSCI.14-06-03462.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima R, Yamada K, Kinomura S, Yamaguchi T, Matsui H, Yoshioka S, et al. Regional cerebral blood flow changes of cortical motor areas and prefrontal areas in humans related to ipsilateral and contralateral hand movement. Brain Res. 1993;623:33–40. doi: 10.1016/0006-8993(93)90006-9. [DOI] [PubMed] [Google Scholar]
- Kertesz A. Western Aphasia battery. New York: The Psychological Corporation; 1982. [Google Scholar]
- Kim SG, Ashe J, Hendrich K, Ellermann JM, Merkle H, Ugurbil K, et al. Functional magnetic resonance imaging of motor cortex: hemispheric asymmetry and handedness. Science. 1993;261:615–7. doi: 10.1126/science.8342027. [DOI] [PubMed] [Google Scholar]
- Koski L, Iacoboni M, Mazziotta JC. Deconstructing apraxia: understanding disorders of intentional movement after stroke. Curr Opin Neurol. 2002;15:71–7. doi: 10.1097/00019052-200202000-00011. [DOI] [PubMed] [Google Scholar]
- Kwakkel G, Kollen BJ, van der Grond J, Prevo AJ. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke. 2003;34:2181–6. doi: 10.1161/01.STR.0000087172.16305.CD. [DOI] [PubMed] [Google Scholar]
- Lestienne F. Effects of inertial loading and velocity on the braking process of voluntary limb movements. Exp Brain Res. 1979;35:407–18. doi: 10.1007/BF00236760. [DOI] [PubMed] [Google Scholar]
- Parry RH, Lincoln NB, Vass CD. Effect of severity of arm impairment on response to additional physiotherapy early after stroke. Clin Rehabil. 1999;13:187–98. doi: 10.1177/026921559901300302. [DOI] [PubMed] [Google Scholar]
- Poizner H, Clark M, Merians AS, Macauley B, Rothi LJ, Heilman KM. Joint coordination deficits in limb apraxia. Brain. 1995;118:227–42. doi: 10.1093/brain/118.1.227. [DOI] [PubMed] [Google Scholar]
- Sainburg RL. Evidence for a dynamic-dominance hypothesis of handedness. Exp Brain Res. 2002;142:241–58. doi: 10.1007/s00221-001-0913-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL. Handedness: differential specializations for control of trajectory and position. Exercise Sport Sci Rev. 2005;33:206–13. doi: 10.1097/00003677-200510000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Duff SV. Does motor lateralization have implications for stroke rehabilitation? J Rehabil Res Dev. 2006;43:311–22. doi: 10.1682/jrrd.2005.01.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Ghilardi MF, Poizner H, Ghez C. Control of limb dynamics in normal subjects and patients without proprioception. J Neurophysiol. 1995;73:820–35. doi: 10.1152/jn.1995.73.2.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Kalakanis D. Differences in control of limb dynamics during dominant and nondominant arm reaching. J Neurophysiol. 2000;83:2661–75. doi: 10.1152/jn.2000.83.5.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Poizner H, Ghez C. Loss of proprioception produces deficits in interjoint coordination. J Neurophysiol. 1993;70:2136–47. doi: 10.1152/jn.1993.70.5.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Schaefer SY. Interlimb differences in control of movement extent. J Neurophysiol. 2004;92:1374–83. doi: 10.1152/jn.00181.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Wang J. Interlimb transfer of visuomotor rotations: independence of direction and final position information. Exp Brain Res. 2002;145:437–47. doi: 10.1007/s00221-002-1140-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland A. Recovery of ipsilateral dexterity after stroke. Stroke. 2000;31:430–3. doi: 10.1161/01.str.31.2.430. [DOI] [PubMed] [Google Scholar]
- Sunderland A, Bowers MP, Sluman SM, Wilcock DJ, Ardron ME. Impaired dexterity of the ipsilateral hand after stroke and the relationship to cognitive deficit. Stroke. 1999;30:949–55. doi: 10.1161/01.str.30.5.949. [DOI] [PubMed] [Google Scholar]
- Tanji J, Okano K, Sato KC. Neuronal activity in cortical motor areas related to ipsilateral, contralateral, and bilateral digit movements of the monkey. J Neurophysiol. 1988;60:325–43. doi: 10.1152/jn.1988.60.1.325. [DOI] [PubMed] [Google Scholar]
- Vega-Gonzalez A, Granat MH. Continuous monitoring of upper-limb activity in a free-living environment. Arch Phys Med Rehabil. 2005;86:541–8. doi: 10.1016/j.apmr.2004.04.049. [DOI] [PubMed] [Google Scholar]
- Verstynen T, Diedrichsen J, Albert N, Aparicio P, Ivry RB. Ipsilateral motor cortex activity during unimanual hand movements relates to task complexity. J Neurophysiol. 2005;93:1209–22. doi: 10.1152/jn.00720.2004. [DOI] [PubMed] [Google Scholar]
- Wade DT, Langton-Hewer R, Wood VA, Skilbeck CE, Ismail HM. The hemiplegic arm after stroke: measurement and recovery. J Neurol Neurosurg Psychiatry. 1983;46:521–4. doi: 10.1136/jnnp.46.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetter S, Poole JL, Haaland KY. Functional implications of ipsilesional motor deficits after unilateral stroke. Arch Phys Med Rehabil. 2005;86:776–81. doi: 10.1016/j.apmr.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Winstein CJ, Pohl PS. Effects of unilateral brain damage on the control of goal-directed hand movements. Exp Brain Res. 1995;105:163–74. doi: 10.1007/BF00242191. [DOI] [PubMed] [Google Scholar]
- Wyke M. Effect of brain lesions on the rapidity of arm movement. Neurology. 1967;17:1113–20. doi: 10.1212/wnl.17.11.1113. [DOI] [PubMed] [Google Scholar]
- Yarosh CA, Hoffman DS, Strick PL. Deficits in movements of the wrist ipsilateral to a stroke in hemiparetic subjects. J Neurophysiol. 2004;92:3276–85. doi: 10.1152/jn.00549.2004. [DOI] [PubMed] [Google Scholar]
- Yelnik A, Bonan I, Debray M, Lo E, Gelbert F, Bussel B. Changes in the execution of a complex manual task after ipsilateral ischemic cerebral hemispheric stroke. Arch Phys Med Rehabil. 1996;77:806–10. doi: 10.1016/s0003-9993(96)90261-0. [DOI] [PubMed] [Google Scholar]