Abstract
Since the existence of the occipitofrontal fascicle (OFF) in humans has remained controversial, we utilized diffusion tensor imaging (DT-MRI)-based segmentation and tractography to investigate its trajectory in vivo in the human. We found that the OFF is distinct from the subcallosal fasciculus or Muratoff’s bundle (MB) and extends from the dorsal and medial parts of the occipital lobe as well as the dorsal, medial and inferior parietal lobules to the dorsal and medial part of the prefrontal and premotor regions. In most of its course, it remains parallel to the corpus callosum, the caudate nucleus and the lateral ventricle. In the coronal plane, the OFF is discerned in the core of the white matter medial to the corona radiata and the superior longitudinal fascicle II (SLF II) and lateral to MB and the corpus callosum. The volumetric measurements of the stem portion of the OFF indicate that the OFF is smaller than the SLF II and the cingulum bundle. Since DT-MRI allows the visualization of OFF fibers leading to the projection areas but not to the origin or termination of these fibers, this has been extrapolated from the experimental data in non-human primates. The OFF may have a role in visual spatial processing along with SLF II.
Keywords: DT-MRI, Segmentation, Tractography, Occipitofrontal fascicle, Fronto-occipital fascicle
Introduction
Several cortical association fiber pathways have been described in the human brain using gross dissection and myelin staining techniques. One of these fiber tracts, the occipitofrontal fascicle (OFF), has elicited a great deal of debate regarding its trajectory, origin and termination. Forel and his student Onufrowicz, while studying a case of callosal agenesis attributed the name of occipitofrontal fascicle to an intrahemispheric association fiber pathway, which they observed running in the sagittal orientation lateral to the fornix and internal to the corona radiata (Onufrowicz, 1887). This conclusion was corroborated by Kaufmann (1888) and Hochhaus (1893). Forel and Onufrowitz also stated that the tapetum was part of the OFF and further suggested that the OFF is part of the superior longitudinal (SLF) or arcuate fascicle of Burdach. However, other authors such as Sachs (1893) suggested that the pathway that Forel and Onufrowicz described was just aberrant fibers of the corpus callosum that failed to cross to the opposite hemisphere. Subsequently, this view was confirmed by Probst (1901a,b). Dejerine (1895), while describing different association fiber pathways, gave a detailed description of this fiber bundle as well. Dejerine identified the OFF in the normal human brain as a distinct fiber bundle, located medial to the corona radiata and SLF. However, he attributed the original discovery of the OFF to Forel and Onufrowicz and, like those investigators, considered the tapetum to be part of the OFF. Although in his monograph Dejerine illustrated the subcallosal fasciculus or Muratoff’s bundle (MB) and the OFF as two separate entities, in his textual description he considered them as equivalent. Since then several authors have considered these two fiber bundles as one and the same. Rosett (1933), using the gross dissection method, interpreted the OFF as contributing thalamo-cortical and cortico-pontine fibers to the cerebral peduncle. Yakovlev and Locke (1961) and Mufson and Pandya (1984) described Muratoff’s bundle in the macaque monkey as a separate entity that carries corticostriatal fibers. Recently, the fiber trajectory of the OFF has been outlined in the macaque monkey using the anterograde tracing technique (Schmahmann and Pandya, 2006; Yeterian and Pandya, unpublished data, 2007). According to these authors the OFF fibers originate from the dorsal and medial preoccipital areas and from the inferior parietal lobule and medial parietal region. Petrides and Pandya (2006) have shown that fibers from the caudal dorsolateral prefrontal areas and from the premotor regions project to the inferior and medial parietal as well as the medial occipital cortices. They have designated these fibers as the fronto-occipital fascicle-FOF (Petrides and Pandya, 2006).
With the availability of more refined neuroimaging techniques, several studies in recent years have described the location and course of the OFF in humans in vivo using diffusion tensor MRI (DT-MRI) (Makris et al., 1997; Catani et al., 2002; Mori, 2002). Although the available descriptions of this fiber bundle provide some basic anatomical information, two issues need to be addressed for a better understanding of this fiber system. The first issue is the relationship of the occipitofrontal fascicle with the nearby subcallosal fascicle or MB. So far, no attempt has been done to differentiate the OFF from the MB in the human. These two fiber bundles have been co-equated using DT-MRI (Catani et al., 2002; Mori, 2002). As noted earlier, the OFF and the MB have been identified as two separate entities in non-human primates (Schmahmann and Pandya, 2006; Yeterian and Pandya, unpublished data, 2007). The second issue regards the delineation of the precise origin and terminations of the OFF in humans. In non-human primates, the OFF has been described in terms of its trajectory, origin and terminations (Petrides and Pandya, 2006; Schmahmann and Pandya, 2006; Yeterian and Pandya, unpublished data, 2007) and its relation with MB has been clarified. Using this information derived from non-human primates, one can extrapolate and suggest the possible origin and termination of this fiber pathway on the basis of data obtained with DT-MRI in humans in vivo. The use of DT-MRI tractography along with cortical parcellation of the human brain allows a better delineation of the trajectory of fiber pathways (Makris et al., 2005).
We found that the OFF is present in the human brain as a distinct entity and is separate from the subcallosal fascicle or MB as has been shown in non-human primates. The trajectory and volume of the OFF in normal human subjects were determined in vivo using the tractographic and segmentation DT-MRI methods. We also have extrapolated the experimental findings for the OFF in non-human primates to the human OFF to provide the trajectory of this fiber pathway in terms of its origin and terminations. Detailed information regarding the trajectory and quantification of this fiber system would facilitate more precise anatomical–functional correlational studies as well as better evaluation of white matter structures in clinical conditions.
Methods
We used magnetic resonance imaging (MRI) to quantify the stem portions of the OFF and delineate their trajectories in six normal adult (age range 23 to 33 years) right-handed human subjects (5 males and 1 female). We combined two different DT-MRI-based techniques, specifically fiber tract segmentation and tractography and a T1-based technique for cortical parcellation of the human brain (Caviness et al., 1996). Additionally, in a second ‘illustrative’ experiment, we performed a tractographic analysis using a different DT-MRI protocol in one subject (22-year-old female).
MRI protocol
Magnetic resonance imaging was performed using a Siemens Trio 3 Tesla imaging system. Scans included a T1-weighted acquisition with the following parameters: TE=3.3 ms, TR=2530 ms, TI=1100 ms, flip angle=7°, slice thickness =1.33 mm, 128 contiguous sagittal slices, acquisition matrix=256×256, in-plane resolution=1×1 mm2 (i.e., FOV=256 mm×256 mm), 2 averages and pixel bandwidth=200 Hz/pixel. A DT-MRI echo-planar-based protocol was also acquired, and it included automatic magnetic field shimming and axial diffusion tensor imaging covering the entire brain (60 axial sections). We sampled the diffusion tensor, D, using a seven-shot echo-planar imaging (EPI) technique that samples the magnitude and orientation of the diffusion tensor. The following parameters were used: TR=8.9 s, TE=79 ms, averages=10, number of slices= 60 in the axial orientation, slice thickness=2 mm, no slice spacing, FOV=256 mm×256 mm (i.e., data matrix=128× 128, in-plane resolution=2 mm2), diffusion sensitivity b=600 s/mm . The total imaging time for DT-MRI was 10 min. For the second experiment, we used a Siemens Allegra 3 Tesla scanner, and the following DT-MRI acquisition protocol: TR=6.5 s, TE=68 ms, averages=10, number of slices=60 in the axial orientation, slice thickness=2 mm, no slice spacing, FOV=256 mm×256 mm (i.e., data matrix=128× 128, in-plane resolution=2 mm2 ), diffusion sensitivity b=700 s/mm 2. The total imaging time for DT-MRI was 10 min.
Segmentation
The stem portion of the OFF was segmented in six subjects based upon a priori anatomical information from human (Dejerine, 1895) and experimental animal literature (Schmahmann and Pandya, 2006; Yeterian and Pandya, unpublished data, 2007). This was done by progressing through the coronal sections in the rostrocaudal dimension and labeling voxels pertaining to the stem portion of the OFF. To determine whether the selected voxels were part of the stem portion of the OFF two criteria were used: (1) the relative topography of the voxels and (2) the orientation of diffusion properties of the tissue. In the coronal plane, the OFF consisted of voxels with tensor information of anterior–posterior orientation along the pericallosal region, which occupied a position in the core of the white matter medial to the corona radiata, the superior longitudinal fascicle II (SLF II) and the internal capsule, and lateral to the MB, the lateral ventricle and the corpus callosum (Figs. 1 and 6).
Fig. 1.
Six diagrams showing the topographic relationships of the occipitofrontal fascicle (OFF) with the subcallosal fascicle or Muratoff’s bundle (MB), the lateral ventricle (LV), the caudate nucleus and the corpus callosum (CC) from a normal human brain. (A) A frontal view showing the relationship of OFF with the caudate nucleus and the LV in the coronal plane; (B) a magnified view of (A) showing the relationship of OFF and MB with the LV; (C) an enlarged oblique view depicting the relationship of OFF and MB with the CC, LV and the caudate nucleus; (D) a lateral view demonstrating the course of the OFF in comparison with the LV and the caudate nucleus; (E) an enlarged oblique lateral view showing the course of the OFF and MB in relation to the LV and the caudate nucleus; (F) an oblique lateral view showing the relationship of the OFF and MB with the LV and the caudate nucleus.
Fig. 6.
Panel A shows in a superior oblique view, the composite topographic relationships of the OFF (occipitofrontal fascicle) with the lateral ventricle (LV) and the caudate nucleus as well as with neighboring fiber pathways such as the subcallosal fasciculus or Muratoff bundle (MB), the cingulum bundle (CB), the corpus callosum (CC), the corona radiata (CR), the superior longitudinal fascicle II (SLF II) and the arcuate fascicle (AF). Panel B shows the topographic relationship of OFF with adjacent fiber bundles and subcortical structures in various configurations (B, a–d). Each view emphasizes the relative location of OFF with one or more of the particular surrounding structures.
To differentiate the OFF from the MB more precisely, we attempted to delineate the MB as well following the same criteria used for the OFF. Given that the orientation of the MB and OFF is similar, their differential segmentation was based entirely upon their topographic location. MB is located immediately above the lateral ventricle and beneath the corpus callosum, whereas the OFF is located adjacent and lateral to the superior–lateral corner of the lateral ventricle as shown in Figs. 1 and 6.
Characterization of region of interest (ROI)
We calculated the size (as number of voxels) and the mean and standard deviation (SD) of anisotropy as fractional anisotropy index (FA) for each ROI (Basser and Pierpaoli, 1996; Pierpaoli and Basser, 1996). We also calculated left–right volumetric and FA symmetry based upon a symmetry coefficient (L–R)/[0.5(L + R)] (Galaburda et al., 1987). The trajectory of OFF was defined in each individual subject using the “color map approach” (Makris et al., 1997, 2002a; Mori, 2002). Three-dimensional reconstructions were done based on the voxels manually selected on each coronal section (Fig. 3). Moreover, the trajectory of each individual OFF (in 12 hemispheres) was determined in the Talairach coordinate system (Talairach and Tournoux, 1988). This was performed for the group as well by computing the Talairach coordinate of the center of mass for the OFF in each coronal slice in which it was observed (Fig. 4). Tkmedit was used for manual segmentation of the fiber tracts (Dale et al., 1999; Fischl et al., 1999).
Fig. 3.
Three-dimensional renderings showing the trajectory of the stem portion of the OFF (occipitofrontal fascicle) as resulted from DT-MRI-based segmentation in the twelve hemispheres (six subjects right and left) of all the subjects analyzed in the study in a lateral view. The OFF is shown in green projected on the background of a midsagittal T2-EPI section. Abbreviations: L: left; R: right.
Fig. 4.
Composite representations (A through D) of coronal (A), axial (B) and sagittal (C, D) planes of the OFF (occipitofrontal fascicle) in six subjects in the Talairach space (Talairach and Tournoux, 1988). The anterior commissure (AC) corresponds to 0 mm and the posterior commissure to −24 mm. For each individual subject, each OFF has been color coded as shown in the inset on the right. Note that in panels A, B, C and D the orientation of the tensors are co-aligned with the slice-to-slice progression of the centers of mass.
Tractography
To delineate more completely the trajectory of the OFF, we performed tractographic analysis using the Syngo DTI Task Card software (Massachusetts General Hospital; Makris et al., 2005). The algorithm used by the software is a streamline method that develops fiber tracts by following the direction of the principle eigenvector at each step starting from a seed point (Mori et al., 1999; Lori et al., 2002). Once segmentation was accomplished for the OFF and the MB, seed points were placed for the tractographic delineation of these fiber bundles. The seed points were placed at locations within the stem portions of the OFF and MB as indicated by their segmentation. Thus, tractography was based upon and guided by the segmentation results. Given that segmentation does not allow the delineation of fiber pathways beyond their stem portions (Makris et al., 1997, 2002b, 2005), tractography is necessary for a more complete delineation of their trajectories. Furthermore, tractography offers a better visualization of the relative topographic relations between fiber tracts as well as the relations of the fiber bundles with subcortical gray structures and, especially, the cerebral cortex.
Results
The term occipitofrontal fascicle requires clarification. The designation of a fascicle as occipito-frontal implies that fibers course from the occipital lobe towards the frontal lobe. In experimental animals, however, the OFF region has been shown to contain fibers directed from the frontal to the occipital lobe as well (Petrides and Pandya, 2006). These fibers have been designated as the fronto-occipital fascicle. The term occipitofrontal fascicle has been used interchangeably with fronto-occipital fascicle. Since the DT-MRI tractographic method allows the identification of fiber orientation, but not their directionality, our description of the OFF could involve both fronto-occipital and occipitofrontal fibers.
In this study we accomplished five principal goals. (1) We segmented the stem portion of the occipitofrontal fascicle in six normal adult subjects and made volumetric and fractional anisotropy (FA) measurements (Table 1, Figs. 2 and 3); (2) we mapped the OFF stems in the Talairach coordinate space (Fig. 4); (3) we traced the trajectory of the occipitofrontal fascicle using the tractographic technique (Fig. 5); (4) we differentiated the OFF from adjacent fiber tracts such as superior longitudinal fascicle II (SLF II), cingulum bundle (CB), corpus callosum, corona radiata and subcallosal fascicle (MB) by tracing them tractographically (Fig. 6); and (5) we followed the tractographic renderings into the cortical origin and termination of OFF fibers using cortical parcellation results (Figs. 7 and 8).
Table 1.
Statistical results of fractional anisotropy index (FA), volumetry, symmetry index and ratio of OFF to cerebral and white matter volume
| Region | Measure | Side | Subject 1 | Subject 2 | Subject 3 | Subject 4 | Subject 5 | Subject 6 | Group mean |
Group SD |
Symmetry index |
|---|---|---|---|---|---|---|---|---|---|---|---|
| OFF | FA | R | 0.39 | 0.39 | 0.44 | 0.37 | 0.33 | 0.35 | 0.38 | 0.04 | −0.04 |
| L | 0.42 | 0.34 | 0.37 | 0.35 | 0.34 | 0.38 | 0.36 | 0.03 | |||
| Average | 0.40 | 0.36 | 0.41 | 0.36 | 0.33 | 0.36 | 0.37 | 0.03 | |||
| Number of voxels | R | 126 | 91 | 143 | 163 | 84 | 98 | 117.50 | 31.61 | −0.07 | |
| L | 88 | 84 | 134 | 180 | 69 | 101 | 109.33 | 40.98 | |||
| L+R | 214 | 175 | 277 | 343 | 153 | 199 | 226.83 | 70.84 | |||
| Volume (cm3) | R | 1.01 | 0.73 | 1.14 | 1.30 | 0.67 | 0.78 | 0.94 | 0.25 | −0.07 | |
| L | 0.70 | 0.67 | 1.07 | 1.44 | 0.55 | 0.81 | 0.87 | 0.33 | |||
| L+R | 1.71 | 1.40 | 2.22 | 2.74 | 1.22 | 1.59 | 1.81 | 0.57 | |||
| Ratio of OFF to cerebral volume (%) | R | 0.15 | 0.12 | 0.18 | 0.21 | 0.12 | 0.13 | 0.15 | 0.04 | −0.14 | |
| L | 0.10 | 0.11 | 0.17 | 0.23 | 0.10 | 0.14 | 0.14 | 0.05 | |||
| L+R | 0.13 | 0.11 | 0.17 | 0.22 | 0.11 | 0.14 | 0.15 | 0.04 | |||
| Ratio of OFF to white matter volume (%) | R | 0.33 | 0.30 | 0.40 | 0.50 | 0.32 | 0.33 | 0.36 | 0.08 | −0.10 | |
| L | 0.23 | 0.29 | 0.38 | 0.57 | 0.26 | 0.35 | 0.35 | 0.12 | |||
| L+R | 0.28 | 0.29 | 0.39 | 0.53 | 0.29 | 0.34 | 0.35 | 0.10 |
Abbreviations: L=left; R=right; FA=fractional anisotropy index; SD=standard deviation. Symmetry index: (L–R)/[0.5(L+R)].
Fig. 2.
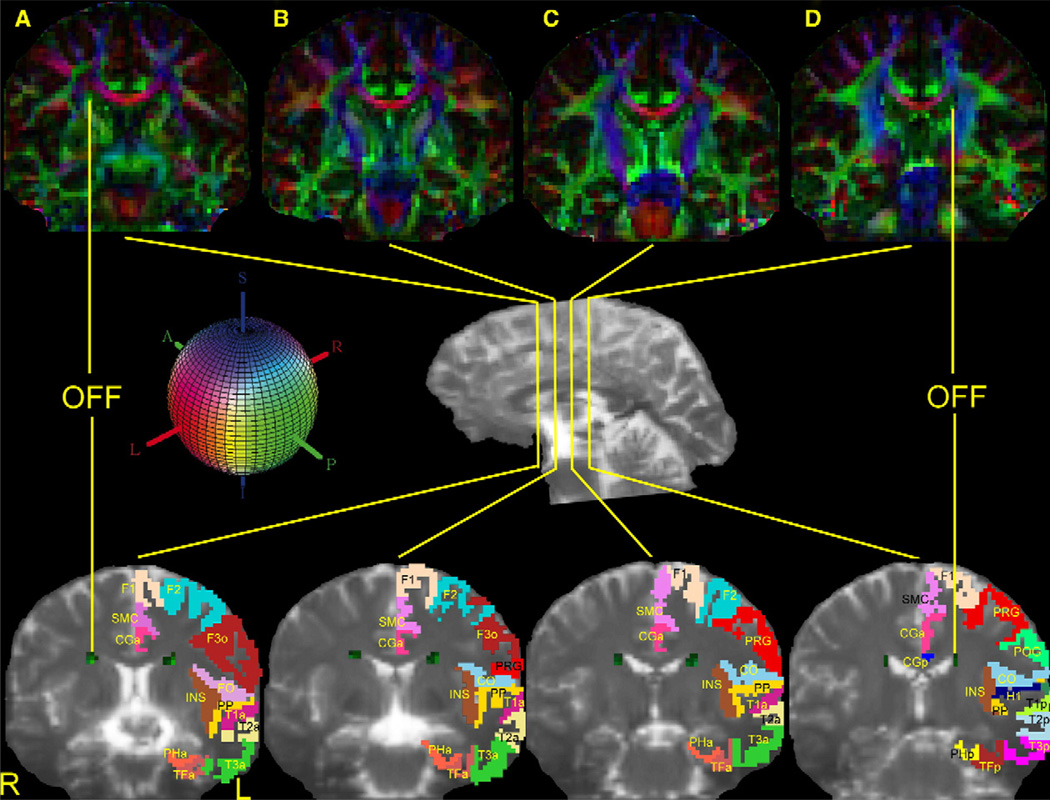
The course and topography of the OFF (occipitofrontal fascicle) are shown in four representative coronal sections (A through D) in the top and bottom panels. In the center of the figure is a lateral view of the hemisphere showing the rostrocaudal level of the selected sections (A–D). Coronal section (A) is a rostral section, located approximately at the level of the anterior commissure, whereas section D is more caudal, situated at the level of the pineal. The upper row of images is composed of the principal eigenvector maps (PEMS). The lower row shows T2-EPI sections corresponding to the DT-MRI of the upper panel to highlight the location and relative topography of the OFF. In both rows OFF is shown in green. The colored sphere in the middle row shows the color-coding scheme adopted in the DT-MRI data analysis. The left hemispheres of these four sections are utilized to show the topographic relationships of the OFF (occipitofrontal fascicle) with specific cortical regions. For this, the individual brain was segmented and parcellated using the Center for Morphometric Analysis neuroanatomic framework (Filipek et al., 1994; Caviness et al., 1996). Red is right–left, green is anterior–posterior and blue is superior–inferior orientations. Abbreviations: CGa: cingulate gyrus, anterior; CGp: cingulate gyrus, posterior; CO: central operculum; F1: superior frontal gyrus; F2: middle frontal gyrus; F3o: inferior frontal gyrus, pars opercularis; FO: frontal operculum; H1: Heschl’s gyrus; INS: insula; PHa: parahippocampal gyrus, anterior; PHp: parahippocampal gyrus, posterior; POG: postcentral gyrus; PP: planum polare; PRG: precentral gyrus; SMC: supplementary motor cortex; T1a: superior temporal gyrus, anterior; T1p: superior temporal gyrus, posterior; T2a: middle temporal gyrus, anterior; T2p: middle temporal gyrus, posterior; T3a: inferior temporal gyrus, anterior; T3p: inferior temporal gyrus, posterior; TFa: temporal fusiform, anterior; TFp: temporal fusiform, posterior; L: left; R: right.
Fig. 5.
The trajectories of the OFF (occipitofrontal fascicle) as resulted from DT-MRI-based tractography in six subjects (twelve hemispheres, 1 through 12) analyzed in the present study are shown in 3-D representations in a lateral view. The OFF is shown in green contrasting on the background of a midsagittal T2-EPI section. Abbreviations: L: left; R: right.
Fig. 7.
The trajectory of the occipitofrontal fascicle (OFF) including its frontal and medial parietal connecting cortical areas (which were derived using the cortical parcellation technique; Caviness et al., 1996) are shown in a lateral view of a representative hemisphere (i.e., hemisphere 6L of Fig. 3) of the six subjects analyzed in the study. The OFF is shown contrasting on the background of a midsagittal T2-EPI section. Abbreviations: F1: superior frontal gyrus; PCN: precuneus; BA: Brodmann’s area.
Fig. 8.
The trajectory of the occipitofrontal fascicle (OFF) and the medial and lateral connecting cortical areas are shown in a representative hemisphere of the single subject scanned in the second experiment of the present study (A). These cortical areas were derived using the cortical parcellation methodology (Caviness et al., 1996). (C and D) Oblique views captured from an angle as indicated in panel (B) where a superior view of the cerebral hemispheres is shown. Arrows in c* and d* demonstrate the angle from which the sections C and D were taken. Diagram C, shows the OFF and MB on a right hemisphere. In this diagram, OFF connects with lateral frontal areas such as caudal frontal pole (BA 8) and superior frontal gyrus (BA 8 and BA 6), whereas the subcallosal fascicle or Muratoff’s bundle (MB) connects with the supplementary motor area (BA 6). Diagram D, shows the OFF connecting with the precuneus (PCN, BA 7) in the medial as well as the angular gyrus (AG, BA 39) and superior parietal lobule (SPL, BA 5 and BA 7) on the lateral surface of the hemisphere. Abbreviations: BA: Brodmann’s area.
Segmentation observations
In the present study, we have been able to outline the location and course of the stem portion of the OFF using the DT-MRI segmentation method in six subjects. As shown in Figs. 2A–D (upper panel), in four representative rostrocaudal coronal sections taken at the levels depicted in the medial view of the hemisphere, we were able to outline the location of the OFF lateral to the lateral ventricle and medial to the corona radiata. In this part of the study, we were able to differentiate the OFF from the adjoining MB as shown in Fig. 1. We were not able, however, to completely define MB because of its relatively small size compared to the voxel resolution used in this study. The lower panel in Figs. 2A–D shows the T2-weighted EPI sections corresponding to the DT-MRI of the upper panel. Figs. 1B and D–F are 3-D reconstructions of the rostrocaudal extent of the OFF shown in lateral, upper and oblique views of the hemisphere. Fig. 3 shows the result of the OFF segmentation in 3-D projected on a lateral view in the twelve hemispheres of the six subjects in the present study.
The composite data set of each individual was placed in the Talairach coordinate space as shown in Figs. 4A–D, in which the stem portion of the OFF is represented in the three cardinal planes applying affine transformation. The extent of the OFF in each case was depicted in a color-coded fashion. It is striking to see the resemblance of the extent and topography of the OFF stem in all six subjects. Thus, the utilization of the segmentation DT-MRI method provided the general extent and location of OFF (Figs. 2–4).
Tractographic observations
In order to further elucidate the trajectory of the OFF, the DT-MRI tractographic technique was used. Fig. 5 shows the trajectory of the OFF in the sagittal plane in the 12 hemispheres (right and left in six subjects). Figs. 6A, B shows the topographic relationships of OFF with neighboring fiber tracts and subcortical structures. Fig. 6A is a superior–oblique view showing the relation of OFF with the several adjacent fiber pathways. Specifically, the OFF is lateral and ventral to the corpus callosum, the cingulum bundle and the subcallosal fasciculus (MB). It is also medial to the corona radiata and ventromedial to the superior longitudinal fascicle II (SLF II). Fig. 6B shows the relationship of the OFF with adjacent fiber bundles and subcortical structures in different planes (Fig. 6Ba–d). In this figure, each view emphasizes the relationship of the OFF with particular surrounding structures. Fig. 7 shows the trajectory of the OFF in the sagittal plane of a representative case demonstrating the connecting cortical areas, which were derived using the cortical parcellation technique by Caviness et al. (1996). Rostrally, the OFF fibers extended to the superior frontal gyrus (Brodmann’s areas BA 8 and BA 6) whereas caudally the OFF fibers reached the precuneal area (BA 7).
In the second experiment, we were able to demonstrate the existence of more detailed cortical connections for the OFF in one subject. In this case, the trajectories of the OFF and, to some extent, that of MB are outlined in Fig. 8. OFF fibers appear to be directed to the superior frontal region (BA 8 and BA 6) as noted above (Figs. 8A, C). Caudally the fibers extended into the inferior parietal lobule and the dorsal parietal and occipital areas (BA 7, BA 39 and BA 19) (Figs. 8A, d). The MB fibers appeared to travel above and medial to the OFF fibers leading to the supplementary motor area (Figs. 8A, C).
Quantitative analyses of the OFF
We also derived measurements of the volumes and average fractional anisotropy (FA) values as well as the symmetry coefficient for the stem of OFF in six normal subjects. FA, volume and symmetry coefficient measurements for OFF are shown in detail in Table 1 for each individual subject as well as for the group. The group mean left/right value for the FA was 0.36/0.38, volume was 0.87 cm3/0.94 cm3 and the symmetry coefficient was −0.04 (rightward) for the FA and −0.07 (rightward) for volume. Furthermore, the OFF was 0.15% of the total cerebral volume and 0.35% of the total cerebral white matter volume.
Discussion
As mentioned above the occipitofrontal fascicle (OFF) is a controversial cortico-cortical association fiber pathway. The term occipitofrontal fascicle was originally coined by Forel and Onufrowicz; however, they considered the tapetum to be part of the OFF (Onufrowicz, 1887). To designate the aberrant fibers as the occipitofrontal fascicle was inaccurate, as was pointed out by Sachs (1892), Wernicke (1897), Schröder (1901) and Probst (1901a,b) (for detailed historical discussion, see also Schmahmann and Pandya, 2006). Dejerine clearly identified this fascicle in myelin stained material as well as in a brain with a lesion of the occipital lobe. However, he did not differentiate this fiber tract from the adjoining subcallosal fascicle of Muratoff. Dejerine, like Onufrowicz, considered the tapetum to be part of the OFF. Subsequent investigators have maintained Dejerine’s description of the occipitofrontal fascicle in the literature, although they named it the superior occipitofrontal fasciculus (see, e.g., Crosby and Schnizlein, 1982). Recent experimental studies in macaque monkeys using anterograde tracer techniques have confirmed, in part, Dejerine’s observations regarding the location and trajectory of the OFF (Schmahmann and Pandya, 2006; Yeterian and Pandya, unpublished data, 2007). These experimental studies have also suggested that the OFF and Muratoff’s bundle (MB) are two separate pathways. Whereas the OFF is a cortical association fiber tract, the MB is a corticostriatal fiber pathway. Moreover, it has been shown that the tapetum is not associated with the OFF, but instead contains temporal and occipital callosal fibers (Schmahmann and Pandya, 2006; Yeterian and Pandya, unpublished data, 2007). The results of our study in humans using neuroimaging techniques are consistent with experimental observations.
In the present study, we have identified the OFF as a fiber bundle situated lateral and dorsal to the head and body of the caudate nucleus. It extends from the dorsomedial occipital and inferior parietal lobule to caudal, dorsal and medial frontal lobe areas. Its main body, i.e., the stem portion, courses parallel to the corpus callosum. Moreover, we have identified a more medially situated fiber bundle that occupies a position medial and dorsal to the body and head of the caudate nucleus, which corresponds to the Muratoff bundle. The present observations indicate that these two fiber bundles are distinct entities. Moreover, we did not observe the OFF being continued with the tapetum, which consists of temporal and occipital callosal fibers. Whereas the OFF is located adjacently and laterally to the superior corner of the lateral ventricle, the tapetum courses for most of its trajectory in a location adjacent and lateral to the temporal horn, the inferior part of the trigone and the occipital horn of the lateral ventricle (Dejerine, 1895; Crosby and Schnizlein, 1982; Talairach and Tournoux, 1988).
In a recent DT-MRI study (Catani et al., 2002), OFF has been also identified in a location similar to that of the present study. However, these authors have not differentiated OFF from the subcallosal fasciculus (MB) and have designated in their heading of OFF as “superior fronto-occipital (subcallosal) fasciculus”. This perpetuates the confusion regarding whether the OFF and MB are distinct entities. Mori (2002), also using the DT-MRI tractographic technique stated that they were unable to identify the superior fronto-occipital fasciculus. Their results seemed to suggest that the superior fronto-occipital fasciculus may not exist as a long association fiber bundle. It should be pointed out that the term “superior fronto-occipital fasciculus” is equivalent to what we have designated as “occipitofrontal fascicle” or OFF. We have avoided the term “superior fronto-occipital fasciculus” because our recent studies in the monkey have failed to identify the inferior occipitofrontal fasciculus, although its existence has been suggested in the human (Burgel et al., 2006; Nieuwenhuys et al., 1988).
DT-MRI tractography along with cortical parcellation allows better delineation of an association pathway in terms of the cortical areas it connects (Makris et al., 2005). With this approach, we have observed that the OFF fibers extend caudally up to the medial occipital and parietal areas as well as the inferior parietal lobule. Rostrally, the fibers of the OFF reach the medial and caudal lateral prefrontal and premotor cortices. Although DT-MRI tractography can provide information regarding most of the trajectory of the OFF, it does not allow the identification of precise origins and terminations of this fiber bundle. Therefore, we have extrapolated the information regarding the origin and terminations of OFF from the experimental data in monkeys. Experimental studies in macaque monkeys (Yeterian and Pandya, unpublished data, 2007) have shown that OFF fibers terminate in medial area 8B (preSMA), area 9, caudodorsal area 46 and dorsal area 8 (8Ad). It should be pointed out that although OFF does carry fibers from the occipital and parietal lobes to the frontal lobe, this fiber bundle is likely bidirectional. Petrides and Pandya (2006) have shown that frontal lobe areas 8Ad and 9/46d give rise to a fiber bundle that courses in the same location as the occipito-frontal fiber bundle. Interestingly, these fronto-occipital fibers course caudally to terminate in medial parietal and occipital areas. Some fibers of the OFF intermingle with SLF II fibers and presumably terminate in the caudal part of the inferior parietal lobule. Thus, the occipitofrontal fascicle contains bidirectional fibers originating from the frontal lobe and coursing to the parietal and occipital lobes, and vice versa.
It is clear from the present study that OFF is distinct from the laterally situated superior longitudinal fascicle II/arcuate fascicle (SLF II/AF) pathways and distinct from the medially adjoining cingulum bundle (CB) (Fig. 6). As shown in Fig. 6, the SLF II/AF is a major fiber system occupying a position lateral to the corona radiata (CR), whereas the CB is located medial to the OFF.
The limitations of the current acquisition analysis protocol used in the six subjects did not allow us to demonstrate the full range of cortical connections as predicted from the experimental animal observations. However, as shown in Fig. 8, we were able to demonstrate more detailed cortical connections for the OFF. Rostrally, OFF tractographic lines (which represent fibers) reached the superior frontal region (BA 8 and BA 6) as noted above (Figs. 8A, C). Caudally, these fibers reached the inferior parietal lobule and the dorsal parietal and occipital areas (BA 7, BA 39 and BA 19) (Figs. 8A, D). The MB appeared to course above and medial to the OFF connecting the supplementary motor area (Figs. 8A, C). From an anatomical perspective, this protocol allowed us to trace trajectories of fibers coursing through the corona radiata (CR), which made possible the visualization of connections between cortical regions located in the dorsal and medial aspect of the hemisphere such as the superior frontal region (BA 8 and BA 6) with laterally located cortical regions such as the angular gyrus (BA 39). An explanation for the improved results obtained using this protocol could be the following. Due to the high fiber density in the CR, we expect a high signal return. The orientation of fibers in the CR, however, is equally distributed due to the crossing of different classes of fiber tracts. It is probable that the critical factor to differentiate individual orientations within a voxel in the CR is contrast more than signal. Therefore, the higher b value of 700 (which provides higher contrast but lower signal) used in the single subject protocol over the b value of 600 (which provides higher signal but lower contrast) used in the six subject protocol was able to better resolve the problem of fiber orientations in such a high signal region as the corona radiata. Unfortunately, due to the experimental design we did not have more data sets at b=700. Future DT-MRI studies using higher b values would allow a better definition of the individual orientations of fibers within the CR.
Functional considerations
There are several long cortical association pathways connecting reciprocally the post-Rolandic cortices with the frontal lobe, especially the prefrontal and premotor areas. Each pathway is suggested to be involved in specific functions (e.g., Petrides and Pandya, 2002; Schmahmann and Pandya, 2006). With regard to the OFF, no definite functional role has thus far been ascribed, partly because of uncertainty regarding the existence of this pathway. In the past, several investigators have associated the occipitofrontal fascicle with the Muratoff’s bundle. The latter has been shown to be corticostriatal in nature (Schmahmann and Pandya, 2006). We would like to suggest the putative functional role of the OFF in view of its connections with specific cortical areas and some physiological observations.
The OFF connects the dorsal and medial occipital and parietal regions with the caudodorsal prefrontal cortex. All these areas have been shown to play a role in visuospatial function. Similarly, SLF II originates predominantly from the inferior parietal lobule (IPL) and connects with the caudodorsal prefrontal region. Both the medial preoccipital area and the IPL (BA 39) are considered part of the dorsal visual stream and are thought to play a role in visuospatial processing (Ungerleider and Mishkin, 1982). Rizzolatti and Matelli (2003) have proposed a functional dichotomy for these areas on the basis of local connections. These authors have suggested two different functional streams of the dorsal visual system. Accordingly, the dorso-dorsal visual stream is formed by medial extrastriate areas V6 and V6A (area PO) and area MIP of the superior parietal lobule (SPL) and suggested that these areas have a role in control of action “on line” or action tracking and reaching. In contrast, the ventro-dorsal stream is formed by extra-striate area MT (BA 37) and the IPL (BA 39). This cortical stream is suggested to play a role in action organization, space perception and action understanding. We would like to suggest that these two subcomponents of the dorsal visual stream, dorso-dorsal and ventro-dorsal, are connected to the frontal lobe by two distinct fiber systems, respectively, the occipitofrontal fascicle (OFF) and the SLF II. The OFF, by virtue of its connections, may be involved in action tracking and reaching, whereas the SLF II may have a role in the organization of action and space perception and in action understanding. Since both of these systems are reciprocally connected, they may have a role in focusing attention in visual space.
Apart from the dorsal stream visual pathways (i.e., OFF and SLF II) that serve mainly peripheral visual fields, there is evidence for ventral stream visual pathways stemming from the dorso-lateral and ventral preoccipital areas (Ungerleider and Mishkin, 1982). Unlike the dorsal visual pathway, which terminates in the caudo-dorsal prefrontal cortex (area 8Ad), the fibers of the ventral stream are shown to terminate in the ventral part of the frontal eye field area 8Av (Petrides and Pandya, 2006). The precise pathway carrying information of dorso-lateral and ventral preoccipital areas to the frontal lobe has not been established. It is suggested that these fibers would travel via the inferior longitudinal fasciculus and the SLF II pathways (Yeterian and Pandya, unpublished data, 2007) or via the extreme capsule fibers (Ungerleider et al., 1989). Since the occipital lobe areas that give origin to this connection serve primarily central visual fields and the area involved in visual motion, the OFF may have roles in object discrimination and object emotional reactivity (Pandya and Yeterian, 1985; Wilson et al., 1993). This fiber pathway could be lesioned in an array of clinical conditions such as stroke, neurodegenerative disorders, multiple sclerosis and tumors. Furthermore, given its relationship with occipital and multimodal association parietal cortical regions, it could well be involved when damaged in disorders affecting higher brain functions such as finger agnosia and Gerstmann’s syndrome (Gerstmann, 1924, 1940, 1942, 1957; Lezak, 1983; Benton, 1985).
Quantitative analysis
We performed measurements of biophysical parameters for fractional anisotropy (FA), and of volume and of symmetry for the stem portion of the OFF. FA ranged from 0.34 to 0.42 on the left and from 0.33 to 0.45 on the right. The FA values for the stem of the OFF were similar to the FA reported in other studies of different fiber tracts in the normal human brain. Pierpaoli and Basser (1996) reported FA values of 0.46 in subcortical white matter. Klingberg et al. (2000) showed mean unscaled fractional anisotropy values in temporoparietal white matter that ranged from 0.38 to 0.59. In previous studies of the cingulum bundle and the superior longitudinal fascicle, we have shown that FA values of the CB ranged from 0.45 to 0.54 (Makris et al., 2002a) and that the overall FA for the SLF (all four subcomponents combined) was 0.44 (Makris et al., 2005). Thus, it seems that our results of FA for the stem of the OFF are in agreement with data on other fiber pathways.
The overall volume of the OFF stem portion was 1.81 cm3. To render a better idea of its size in the context of the cerebral white matter, a comparison with other fiber tracts is needed. The OFF stem is considerably smaller than the total SLF stem (all four subcomponents combined), which is 32.21 cm3 (Makris et al., 2005) as well as the cingulum bundle stem, which is 12.06 cm3 (Makris et al., 2002a). When compared to the individual subcomponents of the SLF, the OFF is approximately half the size of both SLF III, which measures 4.29 cm3, and the vertical component of the arcuate fascicle, which is 4.34 cm3 (Makris et al., 2005). The SLF II is 18.78 cm3 in size, approximately ten times larger than the OFF. These values should be contrasted with measurements derived from purely topographic, constrained methods such as “white matter parcellation” (Makris et al., 1999). Specifically, the white matter of the superior sagittal stratum sector or “Ss total” as measured in 20 healthy adult subjects using T1-weighted MRI was estimated to be 28.42 cm3. This corresponds to approximately 7% of total cerebral white matter. The superior sagittal stratum sector corresponds approximately to the SLF (all four subcomponents combined) and these results are comparable in magnitude, although they reflect different underlying methodologies and anatomic considerations. Given that the OFF is approximately 18 times smaller than the SLF, the OFF may represent 0.4% of the total cerebral white matter. This is in agreement with the results of this study, which showed that the OFF is 0.35% of the total cerebral white matter (Table 1). Due to the small number of subjects in this report, however, care should be taken in the interpretation of the population variance of these volumetric observations. The stem of the OFF showed rightward asymmetry, which was not statistically significant.
The stem portion of the OFF is similar for all hemispheres across the six subjects in the study and between the two approaches, i.e., segmentation and tractography. In contrast, in the tractographic delineation of OFF there seems to be variability regarding the peripheral trajectory in all hemispheres. This may be due, in part, to the anatomic variability in cortical morphology among subjects as well as to the tractographic methodological approach. The latter depends on the details of the tracts on specific parameters such as anisotropy, angular thresholds and ROI selection as well as the fact that the streamline algorithms tend to follow the way of the major bundle. This can interrupt fiber tractographic identification in regions where large bundles such as the corticospinal tract or the thalamic cortical projections predominate over small tracts such as the peripheral fibers of OFF.
Conclusion
We have been able to consistently identify the occipitofrontal fascicle in every subject in our study. It seems that the OFF extends from a distinct frontal region (i.e., superior frontal gyrus (BA 8 and BA 6)) to the parietal (i.e., angular gyrus (BA 39), precuneus (BA 7)) and occipital (BA 19) regions. The OFF is shown to be distinct from Muratoff’s bundle (MB), which leads from the medial BA 6 or supplementary motor area (SMA) to the caudate nucleus. Its fractional anisotropy (FA) is consistent with the FA values of other white matter fiber pathways, whereas its volume is relatively small compared to other cortical association fiber bundles. It is suggested that OFF may have a role, along with SLF II, in processing visuospatial information.
Acknowledgments
Preparation of this article was supported in part by grants from: the National Association for Research in Schizophrenia and Depression (NARSAD) and the National Institutes of Health National Center for Complementary and Alternative Medicine (NCCAM) to Dr. Nikos Makris; NS34189 and the Fairway Trust to Dr. David Kennedy. The authors gratefully acknowledge Dr. Edward H. Yeterian, Dr. Larry Seidman, Dr. Andre van der Kouwe, Rudolph Pienaar, Steven Hodge and Ruopeng Wang for their valuable contributions to the preparation of the manuscript. Offprints: Nikos Makris, M.D., Ph.D., Center for Morphometric Analysis, Massachusetts General Hospital, 149 13th St., Room 6017, Charlestown, MA.
References
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. Ser. B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Benton A. Body schema disturbances: finger agnosia and right–left disorientation. In: Heilman KM, Valenstein E, editors. Clinical Neuropsychology. 2nd Edition. New York: Oxford Univ. Press; 1985. [Google Scholar]
- Burgel U, Amunts K, Hoemke L, Mohlberg H, Gilsbach JM, Zilles K. White matter fiber tracts of the human brain: three-dimensional mapping at microscopic resolution, topography and intersubject variability. NeuroImage. 2006;29:1092–1105. doi: 10.1016/j.neuroimage.2005.08.040. [DOI] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. NeuroImage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Caviness VSJ, Makris N, Meyer J, Kennedy D. MRI-based parcellation of human neocortex: an anatomically specified method with estimate of reliability. J. Cogn. Neurosci. 1996;8:566–588. doi: 10.1162/jocn.1996.8.6.566. [DOI] [PubMed] [Google Scholar]
- Crosby E, Schnizlein H. Comparative Correlative Neuroanatomy of the Vertebrate Telencephalon. New York: Macmillan; 1982. [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dejerine J. Anatomie des Centres Nerveux, 1980, Masson Edition. Paris, France: Rueff et Cie; 1895. [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, Caviness VS., Jr The young adult human brain: an MRI-based morphometric analysis. Cereb. Cortex. 1994;4:344–360. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis: II. Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Corsiglia J, Rosen GD, Sherman GF. Planum temporale asymmetry: reappraisal since Geschwind and Levitsky. Neuropsychologia. 1987;25:853–868. [Google Scholar]
- Gerstmann J. Fingeragnosie: cine umschriebene Storung der Orientierung am eigenen Korper. Wien. Klin. Wochenschr. 1924;37:1010–1012. [Google Scholar]
- Gerstmann J. Syndrome of finger agnosia, disorientation for the right and left, agraphia, and acalculia. Arch. Neurol. Psych. 1940;44:398–408. [Google Scholar]
- Gerstmann J. Problems of interception of disease and of impaired body territories with organic lesions. Arch. Neurol. Psych. 1942;48:890–913. [Google Scholar]
- Gerstmann J. Some notes on the Gerstmann syndrome. Neurology. 1957;7:866–869. doi: 10.1212/wnl.7.12.866. [DOI] [PubMed] [Google Scholar]
- Hochhaus H. Ueber Balkenmangel im menschlichen Gehirn. Dtsch. Z. Nervenheilkd. (Leipzig) 1893;4:79–93. [Google Scholar]
- Kaufmann E. Ueber Mangel des Balkens im menschlichen Gehirn. Arch. Psychiatr. 1888;18:769–781. [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JD, Moseley ME, Poldrack RA. Microstructure of temporo-parietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25:493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- Lezak M. Neuropsychological Assessment. New York: Oxford Univ. Press; 1983. [Google Scholar]
- Lori NF, Akbudak E, Shimony JS, Cull TS, Snyder AZ, Guillory RK, Conturo TE. Diffusion tensor fiber tracking of human brain connectivity: acquisition methods, reliability analysis and biological results. NMR Biomed. 2002;15:494–515. doi: 10.1002/nbm.779. [DOI] [PubMed] [Google Scholar]
- Makris N, Worth AJ, Sorensen AG, Papadimitriou GM, Wu O, Reese TG, Wedeen VJ, Davis TL, Stakes JW, Caviness VS, Kaplan E, Rosen BR, Pandya DN, Kennedy DN. Morphometry of in vivo human white matter association pathways with diffusion-weighted magnetic resonance imaging. Ann. Neurol. 1997;42:951–962. doi: 10.1002/ana.410420617. [DOI] [PubMed] [Google Scholar]
- Makris N, Meyer JW, Bates JF, Yeterian EH, Kennedy DN, Caviness VS. MRI-based topographic parcellation of human cerebral white matter and nuclei II. Rationale and applications with systematics of cerebral connectivity. NeuroImage. 1999;9:18–45. doi: 10.1006/nimg.1998.0384. [DOI] [PubMed] [Google Scholar]
- Makris N, Pandya DN, Normandin JJ. Quantitative DT-MRI investigations of the human cingulum bundle. Cent. Nerv. Syst. Spectr. 2002a;7:522–528. [Google Scholar]
- Makris N, Papadimitriou GM, Worth AJ, Jenkins BG, Garrido L, Sorensen AG, Wedeen V, Tuch DS, Wu O, Cudkowicz ME, Caviness VS, Jr, Rosen B, Kennedy DN. Diffusion tensor imaging. (Chapter 27) In: Nemeroff C, editor. Neuropsychopharmacology: The Fifth Generation of Progress. New York: Lippincott Williams, and Wilkins; 2002b. [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Jr, Pandya DN. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb. Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Massachusetts General Hospital. DTI Task Card v1.70. Boston, MA: A.A. Martinos Center for Biomedical Imaging. [Google Scholar]
- Mori S. Two and three-dimensional analyses of brain white matter architecture using diffusion imaging. Cent. Nerv. Syst. Spectr. 2002;7:529–534. [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann. Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Mufson E, Pandya DN. Some observations on the course and composition of the cingulum bundle in the rhesus monkey. J. Comp. Neurol. 1984;225:31–43. doi: 10.1002/cne.902250105. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Voogd J, van Huijzen C. he Human Central Nervous System. 3rd Edition. Berlin: Springer-Verlag; 1988. [Google Scholar]
- Onufrowicz W. Das balkenlose Mikrocephalengehirn Hoffman. Ein Beitrag zur pathologischen und normalen Anatomie des menschlichen Gehirnes. Arch. Psychiatr. 1887;18:305–328. [Google Scholar]
- Pandya DN, Yeterian EH. Architecture and connections of cortical association areas. In: Peters A, Jones EG, editors. Cerebral Cortex. New York: Plenum Press; 1985. pp. 3–61. [Google Scholar]
- Petrides M, Pandya DN. Association pathways of the prefrontal cortex and functional observations. In: Knight RT, editor. Principles of Frontal Lobe function. Oxford: Oxford Univ. Press; 2002. pp. 31–50. [Google Scholar]
- Petrides M, Pandya DN. Efferent association pathways originating in the caudal prefrontal cortex in the macaque monkey. J. Comp. Neurol. 2006;498:227–251. doi: 10.1002/cne.21048. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn. Reson. Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Probst M. Ueber den Bau des vollständig balkenlosen Grosshirnes sowie über Mikrogyrie und Heterotopie der grauen Substanz. Arch. Psychiatr. Nervenkr. 1901a;34:709–777. [Google Scholar]
- Probst M. Ueber den Verlauf der centralen Sehfasern (Rinden-Sehhugelfasern) und deren Endigung im Zwischenund Mittlehirne und über die Associations-und Commissuren-fasern der Sehsphäre. Arch. Psychiatr. Nervenkr. 1901b;35:22–43. [Google Scholar]
- Rizzolatti G, Matelli M. Two different streams form the dorsal visual system: anatomy and functions. Exp. Brain Res. 2003;153:146–157. doi: 10.1007/s00221-003-1588-0. [DOI] [PubMed] [Google Scholar]
- Rosett J. The myth of the occipitofrontal association tract. Arch. Neurol. Psych. 1933:1248–1258. [Google Scholar]
- Sachs H. I. Der Hinterhauptlappen [dissertation] Leipzig: Breslau. Universität. G. Thieme; 1892. Das Hemisphärenmark des menschlichen Grosshirns. [Google Scholar]
- Sachs H. Vorträge über Bau and Thätigkeit des Grosshirns und die Lehre von der Aphasie and Seelenblindheit, für Aerzte und Studirende. Breslau: Verlag von Preuss and Junger; 1893. [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. New York: Oxford Univ. Press; 2006. [Google Scholar]
- Schröder P. Das fronto-occipitale Associationsbündel. Ein kritischer Beitrag. Monatsschr. Psychiatr. Neurol. 1901;9:81–99. [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers, Inc; 1988. [Google Scholar]
- Ungerleider L, Mishkin M. Two cortical visual systems. In: Ingle D, Goodale M, Mansfield R, editors. Analysis of Visual Behavior. Cambridge, MA: MIT Press; 1982. pp. 549–586. [Google Scholar]
- Ungerleider LG, Gaffan D, Pelak VS. Projections from inferior temporal cortex to prefrontal cortex via the uncinate fascicle in rhesus monkeys. Exp. Brain Res. 1989;76:473–484. doi: 10.1007/BF00248903. [DOI] [PubMed] [Google Scholar]
- Wernicke C. Schletter’schen Buchhandlung (Franck and Weigert). Breslau. Abteilung II-20 Horizontalschnitte durch eine Grosshirnhemisphäre. Verlag der psychiatrischen Klinik. Breslau 1900. Abteilung III-21 Sagittalschnitte durch eine Grosshirnhemisphäre. Verlag der psychiatrischen Klinik. Breslau 1903. 1897. Photographischer Atlas de Gehirns. Schniktte durch das meschliche Gehirn in photographischen Originalen. Abteilung I-32 Frontalschnitte durch eine Grosshirnhemisphäre. [Google Scholar]
- Wilson FA, Scalaidhe SP, Goldman-Rakic PS. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science. 1993;260:1955–1958. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Locke S. Limbic nuclei of thalamus and connections of limbic cortex. III. Corticocortical connections of the anterior cingulate gyrus, the cingulum, and the subcallosal bundle in monkey. Arch. Neurol. 1961;5:364–400. doi: 10.1001/archneur.1961.00450160014002. [DOI] [PubMed] [Google Scholar]