Abstract
The genus Scaptomyza is emerging as a model lineage in which to study biogeography and ecological adaptation. To place future research on these species into an evolutionary framework we present the most comprehensive phylogeny of Scaptomyza to date, based on 5042 bp of DNA sequence data and representatives from 13 of 21 subgenera. We find evidence that the lineage originated in the Hawaiian Islands and subsequently dispersed to the mainland and other remote oceanic islands. We also identify that many of the unique ecological niches exploited by this lineage (e.g., herbivory, spider predation) arose singly and independently. We find strong support for the monophyly of almost all subgenera with exceptions corroborating hypotheses of conflict inferred from previous taxonomic studies.
Keywords: Scaptomyza, Hawaiian Drosophilidae, dispersal, Hawaii, leaf-mining
1. Introduction
1.1 Dispersal in island-adapted species
Colonists that become established on oceanic islands can adapt to a wide variety of ecological niches, develop a wide range of phenotypes, and comprise radiations of hundreds of species (Baldwin and Sanderson, 1998; Grant, 1999; Shaw, 2002; Jordan, et al, 2003; Lerner, et al, 2011; O'Grady, et al, 2011). However, oceanic islands have historically been considered a “dead end” for diversity (Wilson, 1961; Mayr and Diamond, 2001). Island-adapted taxa are poor dispersers (Darwin, 1859) and as an island erodes and is submerged many resident lineages go extinct. Furthermore, island endemics are considered too naïve to compete with continental species (Cox, 1999), and in many lineages energetically expensive defensive structures (e.g. Hawaiian thornless raspberries) and compounds (e.g. Hawaiian “mintless” mints) are abandoned in the absence of predators (Carlquist, 1974). However, new evidence suggests that island endemics are capable of escaping islands, colonizing continents and other remote islands, and diversifying (Heaney, 2007; Bellemain and Ricklefs, 2008).
The Hawaiian Archipelago is one of the most remote oceanic island chains in the world. Its isolation, located 3200 km from the nearest landmass or archipelago, has led to its characteristically disharmonic and diverse set of biota (Simberloff and Wilson, 1969; Gillespie and Roderick, 2002; Price and Clague, 2002; Cowie and Holland, 2008). Examples of emigrations of lineages that arose in the Hawaiian Islands are emerging from species groups as diverse as sandalwoods, birds and snails (Filardi and Moyle, 2005; Harbaugh and Baldwin, 2007; Rundell and Price, 2009). Another example of this dispersal out of Hawaii is found within the hyper-diverse Hawaiian Drosophilidae (Diptera), a clade that includes two monophyletic groups, the endemic Hawaiian Drosophila and its cosmopolitan sister genus Scaptomyza. The common ancestor of both the Hawaiian Drosophila and Scaptomyza lineages colonized the archipelago ca. 25 million years ago and has subsequently diversified into a lineage of an estimated 1,000 species (Throckmorton, 1966; Grimaldi, 1990; Russo, et al, 1995; Remsen and DeSalle, 1998; Da Lage, et al, 2007; O'Grady and DeSalle, 2008; Van der Linde, et al, 2010). The present cosmopolitan distribution of Scaptomyza is hypothesized to be the result of an ancient dispersal event out of Hawaii (O'Grady and DeSalle, 2008). The majority of the 272 (80%) described Scaptomyza species occur on remote oceanic islands including the Hawaiian Islands, the Marquesas, Tristan da Cunha, Ogasarawa Islands, St. Helena Islands, and Juan Fernandez Islands. The remaining 55 species are found on all continents except Antarctica (Evenhuis and Samuelson, 2007; O'Grady, et al, 2010). Most drosophilid lineages of similar age are more restricted in their distributions (Russo, et al, 1995; Tamura, et al, 2004, Morales-Hojas and Vieira, 2012) and lineages like the immigrans species group that have dispersed to a similar degree are less speciose (Markow and O'Grady, 2006). The relatively recent origin of the Hawaiian Drosophilidae clade (Hawiian Drosophila + Scaptomyza), ca. 23–35 million years ago (mya) (Russo, et al, 1995; Tamura, et al, 2004; Morales-Hojas and Vieira, 2012) provides a temporal interval during which Scaptomyza arose and dispersed around the world. The ability of Scaptomyza species to disperse large distances may be explained by their derived physiological characteristics. O'Grady and DeSalle (2008) analyzed potential characters that could have predisposed Scaptomyza species to be dispersers, allowing them to exist in marginal habitats and to traverse long geographic distances. They found that Scaptomyza species are generally smaller and develop more rapidly than other species of Hawaiian Drosophilidae, facilitating the use of ephemeral breeding and feeding substrates.
Dispersal between the Hawaiian Islands may also be a factor in diversification in the Hawaiian Scaptomyza, as is seen in other Hawaiian Drosophilidae lineages (Bonacum, et al, 2005; Carson, 1997). The Hawaiian Emperor Seamount chain is a volcanic archipelago that has existed for over 80 million years and extends approximately 5500 km across the Pacific Ocean. Islands form in the southeast over a volcanic hotspot near the current position of the island of Hawaii, then migrate northwest on the Pacific plate, erosive forces causing the islands to eventually subside. Many Hawaiian lineages follow the progression rule (Hennig, 1966) where basally branching lineages are found on older islands and more recently derived species are present on younger islands. As new islands form, taxa from older neighboring islands can colonize, leading to clades that have diversified “down” the chain (e.g. Jordan, 2003; Pons and Gillespie, 2004; Rubinoff, 2008). Here, we test the hypothesis that the progression rule can explain the biogeographic history of the Hawaiian endemic Scaptomyza.
1.2 Scaptomyza larval ecology
The majority of drosophilid species, including many members of the genus Scaptomyza, have saprophagous larvae that feed on yeasts, other fungi, and bacteria living on decomposing plant material (Markow and O'Grady, 2008). In addition to this general saprophagous habit, larvae of some Scaptomyza species utilize a variety of substrates not found in other Drosophila clades (Markow and O'Grady, 2008). For example larvae of some Scaptomyza species are animal predators. Larvae of species in the subgenus Titanochaeta were reared from thomisid spider egg sacs (Hardy, 1965), and some species in the subgenus Elmomyza feed on insects (Magnacca, et al, 2008). The subgenus Scaptomyza is an herbivorous lineage whose larvae are leaf-miners of mustards (Brassicaceae) and other plant families (Hackman, 1959; Wheeler and Takada, 1966; Maca, 1972; Brncic, 1983; Whiteman, et al, 2011), including the genetic reference plant, Arabidopsis thaliana (Chittenden, 1902). Species in the subgenera Hemiscaptomyza and Dentiscaptomyza are also known to associate with mustards, but whether they actively mine the leaf is unclear (Wheeler and Takada, 1966; Brncic, 1983). Larvae of species in the subgenus Exalloscaptomyza specialize on the microflora living on the corollas of Hawaiian Ipomoea spp. flowers (Montague, 1984). These larval ecologies have never been examined in a phylogenetic context.
1.3 Scaptomyza taxonomy and systematics
Hardy erected the genus Scaptomyza in 1849 for the type species S. graminum Fallen. Scaptomyza has a complex taxonomic history, characterized by various shifts in status from genus to subgenus and species transfers from one subgenus to another. Species delimitation in Scaptomyza has also been difficult, because of the wide distributions and similar morphology of many taxa. For example, the type series of S. graminum contains both S. pallida and S. graminum specimens (Hackman, 1959). Dissections of male genitalia are required to reliably identify many closely related species in this genus. Scaptomyza is currently divided into 21 subgenera, several of which (e.g., Bunostoma, Celidosoma, Grimshawomyia, Titanochaeta) were originally described as distinct genera (Malloch, 1932; Throckmorton, 1966), but have since been synonymized with Scaptomyza due to other morphological characters and molecular evidence (Hackman, 1959; O'Grady, et al, 2003).
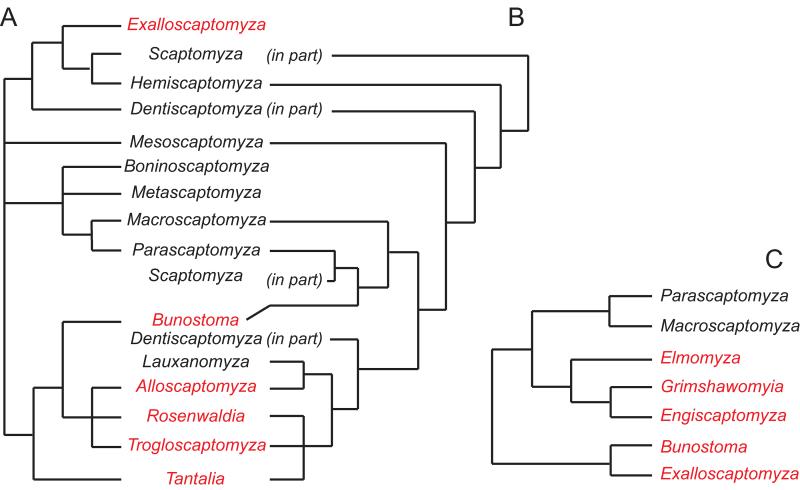
Researchers have previously examined the phylogenetic relationships within Scaptomyza to place these species into a taxonomic framework. Okada (Okada, 1973) used a phenetic algorithm to propose relationships within the genus Scaptomyza. His analysis suggested that all Hawaiian subgenera, with the exception of Exalloscaptomyza, were monophyletic and divided into 3 major lineages (Fig 1A). This analysis agreed with previously posited taxonomic hypotheses (Throckmorton, 1966). However, neither analyses employed an outgroup and therefore the monophyly of the genus Scaptomyza was not tested. Grimaldi's (Grimaldi, 1990) morphological study was an improvement over the previous phenetic work because it tested the monophyly of the genus Scaptomyza and it's subgenera through more extensive sampling of outgroups and multiple members of subgenera (Fig 1B). There are two areas of concordance between these morphological studies: both suggest (a) close relationships among the Hawaiian subgenera Alloscaptomyza, Tantalia, Rosenwaldia, and Elmomyza and (b) that Macroscaptomyza and Parascaptomyza are members of the same larger clade. The most recent treatment of this group, based on phylogenetic analyses of molecular characters (O'Grady and DeSalle, 2008), provides evidence that this lineage arose in the Hawaiian Islands, but the paucity of mainland taxa sampled in this study indicates that a more complete sampling of Scaptomyza diversity would better test monophyly and relationships among the subgenera (Fig 1C).
Figure 1.
Previous phylogenetic hypotheses. Subgenera in red include species endemic to the Hawaiian Islands. Relationships within the genus Scaptomyza based on A. Okada's phenetic analysis (Okada, 1973), B. Grimaldi's cladistic analysis (Grimaldi, 1990) C. O'Grady and DeSalle's (2008) molecular phylogenetic analysis.
1.4 Objectives
The genus Scaptomyza is a diverse lineage that is becoming a useful model system for studies of biogeography and ecological diversification. Here, we present the most comprehensive phylogeny of the genus Scaptomyza to date, employing more than twice as many subgenera and molecular characters than any previous molecular analysis to test the relationships within and between the subgenera of Scaptomyza. We use this phylogeny to test hypotheses of how and when Scaptomyza dispersed globally, and explore when and how often various larval ecologies evolved.
2. Materials and methods
2.1 Specimen collection and vouchers
We included 63 Scaptomyza taxa in this analysis, including representatives of 13 of the 21 subgenera. This is the most comprehensive molecular phylogeny of the genus to date, and these species encompass the ecological variation and geographic range of the genus. Twelve Hawaiian Drosophila species, including multiple taxa from the four major lineages (O'Grady, et al, 2011), were used as outgroups. Species were collected via sweeping or reared from plants and immediately placed into 100% EtOH. Voucher specimens are stored at −80° C in 100% EtOH at the University of Arizona and the University of California, Berkeley in the collections of NKW and PMO, respectively (Table 1).
Table 1.
Taxon and Character Sampling. “Spnr” refers to taxa that are not identified to a described species, but morphologically similar to the described species following spnr. Accession numbers in italics are for sequences new to this paper.
Detailed information on specimens can be found by contacting the authors with the reference barcodes. Hd: Hawaiian Drosophila project. 0: P.M. O'Grady. L: R.T. Lapoint.
2.2 DNA amplification and sequencing
DNA was extracted following standard Qiagen DNeasy blood and tissue kits and protocols. We created alignments of the Scaptomyza flava transcriptome (Whiteman, et al, 2012) with orthologous sequences from the completely sequenced genomes of the closely related D. mojavensis, D. virilis and D. grimshawi from Flybase (Tweedie, et al, 2009). We used the program primer3 (Rozen and Skaletsky, 2000), as implemented in Geneious 5.5.5, to design or modify PCR primers that would amplify partial fragments of four nuclear genes: gstd1, cad-r, marf and n(l)tid based on these alignments. These loci were included based on their use in other studies (O'Grady and Zilversmit, 2004; Gloss et al, in review). Four additional mitochondrial genes (16s, co1, co2 and nd2) were also PCR-amplified using universal mitochondrial primers (Simon, et al, 1994). See Table 2 for relevant details for each gene.
Table 2.
Details of loci used in this study. Tm is the annealing temperature. More than one temperature is listed for primers that were used in touchdown PCRs.
| Gene | Primer | Tm | # Ind | # Sbgn | Chars(PIC)d |
|---|---|---|---|---|---|
| nuclear | |||||
|
| |||||
| n(l)tid a | ntidL-GGGYCGCATCTTTGAGCACAAATGG | 60C | 26 | 9 | 618(71) |
| ntidR-TGCTGGGATAGGTGTTCCARCARTA | |||||
| cad-r a | Cad787F-GGSAATACGACNGCCTGYTTTGARCC | 62C,58C,54C | 36 | 12 | 849(208) |
| Cad1098R-TTNGGCAGCTGRCCNCCCAT | |||||
| Marf a | MarfF1-ATGGCGGCCTAYTTGAAYCGCA | 62C,58C,54C | 51 | 12 | 371(84) |
| MarfR1-AAGAAGGCGACCTTCATGTGRTC | |||||
| gstd-1 b | gstd1FB-TGTGCTYTTCTAATTATAG | 38C | 12 | 7 | 630(76) |
| gstd1RA-GAATACWCTTTTATTWTAAG | |||||
|
| |||||
| mitochondrial | |||||
|
| |||||
| 16s c | 16sF-CCGGTTTGAACTCAGATCACGT | 56C | 43 | 12 | 550(25) |
| 16sR-CGCCTGTTTAACAAAAACAT | |||||
| co1 c | 2183-CAACATTTATTTTGATTTTTTGG | 56C | 71 | 12 | 765(246) |
| 3041-TYCATTGCACTAATCTGCCATATTAG | |||||
| co2 c | 3037-ATGGCAGATTAGTGCAATGG | 56C | 72 | 13 | 700(214) |
| 3791-GTTTAAGAGACCAGTACTTG | |||||
| nd2 c | 192-AGCTATTGGGTTCAGACCCC | 56C | 35 | 12 | 559 (102) |
| 732-GAAGTTTGGTTTAAACCTCC | |||||
A. Gloss, in prep.
Maximum sequence length. Number of parsimony informative characters for ingroup taxa are in parentheses.
#Ind refers to the number of individuals sequenced for that locus for this study. #Sbgn refers to the number of subgenera that had representatives sequenced for that locus in this study.
PCR conditions included an initial denaturation step at 95°C for five minutes, followed by 30 cycles of the following amplification sequence: 1) denature at 95°C for 30 seconds, 2) anneal (between 52–64°C depending on gene) for 30 seconds, and 3) extension at 72°C for 1 minute. A final round of extension at 72°C was performed for 5 minutes. A touch down PCR was performed to obtain enough specific PCR product for cad-r and marf. In these cases, three rounds of amplification sequences were performed: 1) An initial high specificity sequence with a high annealing temperature round for 5 cycles, 2) a second sequence at the calculated annealing temperature for 10 cycles, and 3) a final low specificity sequence at a low annealing temperature at 20 cycles. PCR products were visualized on 1% agarose TAE gels and cleaned using Fermentas ExoSAP-it following manufacturers instructions. Cleaned PCR products were Sanger sequenced in both directions at the University of Arizona Genetics Core Sequencing Facility or the University of California, Berkeley Sequencing Facility using the same primers for PCR amplification and ABI dye terminator chemistry. Raw sequences were assembled into contigs and trimmed in Geneious 5.5.5 (Biomatters). All sequences have been deposited on GenBank (Table 1).
2.3 Phylogenetic Inference
Additional drosophilid DNA sequences were downloaded from GenBank and Flybase (Table 1), added to the newly sequenced genes, and aligned in MUSCLE (Edgar, 2004) using the default parameters. The large concatenated DNA sequence matrix was comprised of 5042 bp. Alignment of each gene was trivial, though the intronic region in marf was found to be highly variable between species and was removed due to a high degree of ambiguity in that portion of the alignment. The coding regions of marf were retained. Most loci contained a high proportion of parsimony informative characters (Table 2). The data were analyzed both using individual genes and in a concatenated, partitioned phylogenetic analysis under Bayesian (MrBayes v3.1.2) (Ronquist and Huelsenbeck, 2003) and Maximum Likelihood (RAxML) (Stamatakis, 2006) frameworks. To explore the effects of missing data we analyzed a reduced dataset without gstd1 (which had the lowest level of coverage) of 32 taxa that were sequenced for at least five of seven genes. We will refer to this smaller, higher coverage matrix (~85% complete data matrix) as the “small dataset” and the larger matrix with more gaps (~60% complete data matrix) as the “large dataset.” Phylogenies were rooted with Hawaiian Drosophila species. Phylogenetic analyses were performed on the CIPRES Science Gateway workbench (Miller, et al, 2010).
Partitioned genealogies were estimated for each gene. The genes were partitioned by codon position, RNA sequences were given their own partition, and models of sequence evolution were selected for each partition using MrModeltest (Nylander, 2004). The substitution matrix, base frequencies, and gamma shape parameter were unlinked for each data partition, and the rate prior was set to variable. MrBayes analyses were run twice for 5×106 generations, and sampled every 500 generations. Convergence was assessed via a low (<0.01) average standard deviation in split frequencies and by assessing the cumulative split frequency in AWTY (Nylander, et al, 2008). Appropriate levels of burn-in were discarded – generally the first 10% to 20% of the sampled data. RAxML was used to simultaneously infer the optimal topology and to assess bootstrap support. The individual gene datasets were partitioned in the same manner for the maximum likelihood analysis as described for the Bayesian analyses, but the GTRGAMMA model was applied to all partitions. A total of 1000 bootstrap replicates were generated to assess node confidence. Each dataset was analyzed five times with different random starting seeds in RAxML to identify if significant changes in topology and support occurred between runs.
All loci were combined into a partitioned, concatenated dataset. Both the large and small datasets were partitioned by gene, codon position, and RNA – 16s and part of tRNA-Lys which is sequenced at the end of co2 – resulting in a total of 23 partitions for the large dataset and 20 partitions for the small dataset. The concatenated phylogenetic analyses were run as described above except that the MrBayes analysis was run for 5×107 generations and sampled every 5000 generations and the chain temperature was reduced to 0.15 to improve chain mixing. Convergence was assessed in the same way and the first 30% was discarded as burn-in for the large dataset and 15% was discarded as burn-in for the small dataset.
2.4 Dating
Divergence date estimates were inferred using BEAST v1.6.2 (Drummond and Rambaut, 2007). The concatenated, partitioned dataset was used and the models of substitution identified by MrModeltest were implemented as above. A relaxed clock with an uncorrelated lognormal model of rate variation was implemented. Both a birth-death and Yule speciation process for branching rates were tested, and after comparing Bayes Factors (non significant for rejecting either model) a birth death model was implemented in all subsequent analyses. The analysis was run for 5×107 generations and sampled every 1000 generations. The analysis was run twice independently, to refine the tuning operators and weights for maximum efficiency. After all weights and operators were optimized the analysis was run twice more and outputs were combined using LogCombiner v1.6.1 (Drummond and Rambaut, 2007). Tracer v1.5 (Rambaut and Drummond, 2004) was used to visually assess convergence and stationarity, and to observe if the effective sample size (ESS) for all parameters was sufficiently high.
To estimate the timing of divergence events we analyzed the large dataset employing 2 calibration points – a fossil and biogeographic information. Biogeographic calibrations points have been previously used for dating most drosophilid lineages (Russo, et al, 1995; Tamura, et al, 2004; Bonacum, 2005; Morales-Hojas and Vieira, 2012), but there is evidence that this may introduce a large amount of error (Obbard, et al, 2012). We used only the fossil calibration to date the small dataset due to a lack of appropriate taxa on which to place the biogeographic calibration. Given the limitations of our dataset we caution against too strict an interpretation of our inferred dates (Parham, et al, 2012).
Unlike in many drosophilid lineages, a well-described Scaptomyza fossil (Scaptomyza dominicana) from Dominican amber is available (Grimaldi, 1987). Placement of fossil calibration points can strongly influence the divergence time estimates, and while this specimen shares many synapomorphies with modern Scaptomyza species, including four rows of acrostichal setae, long legs and longitudinal thoracic color patterning, the placement of S. dominicana within any Scaptomyza subgenus is uncertain. To identify whether the fossil belongs to either the crown or stem group of Scaptomyza we performed a partitioned analysis using a mixed data matrix, including our molecular dataset and Grimaldi's (1990) morphological character matrix. We used the morphological matrix of 218 morphological characters compiled by Grimaldi (1990) for Scaptomyza subgenera and Hawaiian Drosophila (Table S1) including species for which we were not able to get DNA for. Scaptomyza dominicana was coded based on characters and states described for the fossil (Table S2) (Grimaldi, 1987). This same partitioning scheme was used for this analysis and the concatenated, partitioned phylogenetic analysis, with the addition of another partition for morphology and applying the standard discrete model to the morphological partition. The analyses were run in MrBayes using the same settings as for the concatenated dataset, and convergence was similarly assessed. The first 20% of the dataset was excluded as burn-in. The phylogeny inferred via this method was poorly resolved, especially at deeper nodes, and the fossil's placement as either a stem or crown group species could not be ascertained due to a lack of resolution (Fig S1).
To explore how the placement of this fossil calibration as either a stem or crown group species effects the dates of divergence we ran the BEAST analysis twice for both the large and small datasets. We placed the fossil calibration as belonging to the crown group of Scaptomyza in one analysis and the stem in another analysis. Given the uncertainty in the dating of Dominican amber (Iturralde-Vinent and MacPhee, 1996) we calibrated these nodes with a lognormal distribution prior that allows for a range of divergence dates from 15 to 45 mya with a the median at 24.5 mya, minimum at 15 mya and a 95% HPD greater than 45 mya. To obtain this distribution the log (mean) was set to 2.251, Log (std dev) was set to 0.75, and the offset was 15.
We applied two calibration points to the large dataset dating analyses: 1) the fossil (described above) and 2) the biogeography of the Hawaiian Islands. Clades endemic to the island of Hawaii are not expected to be older than that island (Fleischer, et al, 1998), making the most probable time of divergence between Hawaiian endemic lineages and their sister species on the next nearest island, Maui, about 0.5 mya (Price and Clague, 2002). Since the Hawaiian lineage could have diverged before the formation of Hawaii and sister species on Maui Nui went extinct, or the island of Hawaii could have been colonized later than the island's initial formation, we calibrated the time to most recent common ancestor of these groups with a normal distribution prior with a mean of 0.5 and a standard deviation of 0.15 mya. This creates a distribution where the most probable time of divergence is 0.5 mya, but allows for divergence from almost the present and up to 0.9 mya. The ancestral node of Scaptomyza scoloplichas and S. exigua was calibrated using this prior since both species are found on the island of Hawaii and are sister to a Maui Nui species. We were not able to use this calibration in the small dataset due to the exclusion of S. scoloplichas.
2.5 Ancestral State Reconstructions
We used two methods to infer the biogeographic history of Scaptomyza. For Hawaiian endemic taxa we used the program Lagrange v2.0.1 (Ree and Smith, 2008). Lagrange models dispersal, extinction and cladogenesis (DEC) in a likelihood framework and allows for modeling multiple biogeographic scenarios. This method has proven to be powerful in reconstructing ancestral ranges in simulation and empirical studies (Buerki, et al, 2011) and is appropriate for Hawaiian taxa, where dispersal to adjacent islands is associated with diversification (Bonacum, et al, 2005; Ree and Smith, 2008; Holland and Cowie, 2009), and vicariant diversification is possible within island groups like Maui Nui (which includes the islands of Maui, Molokai and Lanai) that are historically connected or isolated during periods of glacial maxima and minima respectively (Price and Clague, 2002).
The chronogram for the large dataset calibrated with Hawaiian biogeography and the fossil placed as part of the crown Scaptomyza was entered into the Lagrange configurator and pruned to include only Hawaiian Scaptomyza species (reelab.net/lagrange/configurator). Species' present day ranges were coded based on known ranges (Hardy, 1965; Wheeler and Takada, 1966) and collection localities (Table S1). Each species was coded as being from Hawaii, Maui Nui, Oahu, or Kauai. Species from either Maui, Molokai, Lanai or any combination thereof were treated as being from one island since these islands were connected together into the larger island known as Maui Nui in the past, facilitating dispersal between islands (Price and Elliot-Fisk, 2004). A stepping stone model of evolution was applied in Lagrange: possible node ranges were restricted to single islands or two adjacent islands.
Since the above assumptions are not realistic for lineages separated by large distances (e.g. Hawaiian Island species and North American species), Lagrange was not used to reconstruct biogeographic history of the more distant dispersal events. We instead used SIMMAP v1.5 (Bollback, 2006) to stochastically map the ancestral ranges in a Bayesian framework. Ancestral state reconstructions used 1000 post burn-in BEAST trees and their branch lengths. Species areas were coded as Hawaiian Islands, North America, New Zealand and Tristan da Cunha. The overall substitution rate of each morphological character was modeled using a gamma distribution whose priors α and β were estimated using the two-step procedure suggested in SIMMAP 1.5. An MCMC analysis was used to sample overall rate parameter values. Next, the results of this analysis were analyzed with the R Statistical Package and the sumprmcmc.r script provided with SIMMAP 1.5 to find the best fitting gamma and beta distributions. Based on these analyses, we obtained an α=0.668 and a β=0.005 which were used to parameterize further analyses.
To identify how often unique larval ecologies have evolved in Scaptomyza we reconstructed the ancestral states for lineages using SIMMAP v1.5. Ecological status was coded based on published sources (Stalker, 1945; Maca, 1972; Collinge and Louda, 1989; Martin, 2004; Magnacca, et al, 2008) (Table S1). Species were coded as being saprophagous, spider predators, flower-specialists or leaf-miners. Species with no information were coded as unknown. We used the same method as described above to identify substitution model parameters. We obtained an α=3.361 and a β=0.482 which were used to parameterize further analyses.
3. Results
3.1 Phylogenetics
The concatenated phylogeny for the large dataset is supported throughout most of the topology and the maximum likelihood estimate (−lnL= −36674.8637) is similar to the Bayesian topology (Fig 2 and S2). The small dataset corroborates the results of the larger dataset, though with fewer taxa it is not as rigorous a test of monophyly for most groups (Fig S3). Wiens and colleagues (Wiens 2003, 2006; Wiens and Moen, 2008) have shown that modest amounts of missing data have little impact on phylogenetic inference but a lack of overlapping characters can influence branch lengths. In the larger analyses comparisons between the small and large phylogenies demonstrate little impact of missing data, either in terms of topology or support. Individual gene genealogies broadly agree with one another (Fig S4a–e) and the concatenated dataset. The conflict observed between individual loci is poorly supported and may be due to stochasticity, incomplete lineage sorting or hybridization.
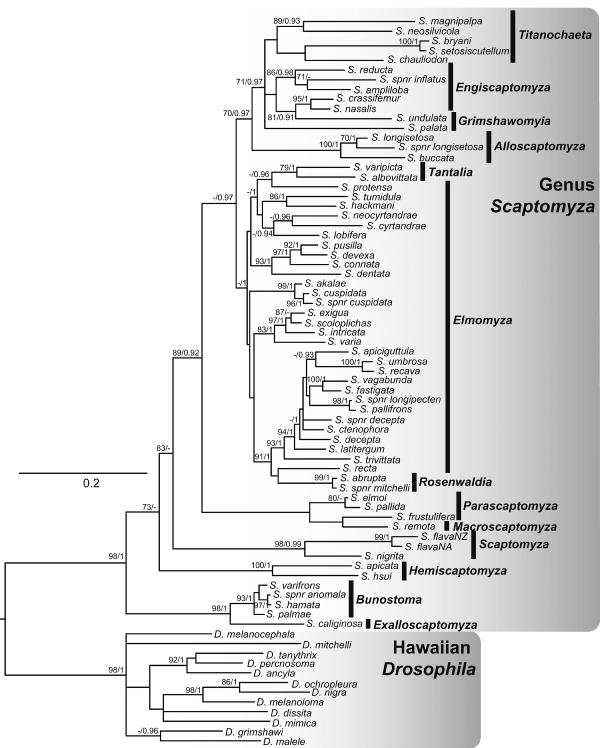
Figure 2.
Bayesian analysis of phylogenetic relationships in the genus Scaptomyza based on combined mitochondrial and nuclear loci. Support is indicated at nodes. Bayesian posterior probabilities are to the right, maximum likelihood bootstrap support is to the left. A lack of either posterior probability or bootstrap support is indicated by a -. Nodes with no numbers are unsupported.
Almost all subgenera are monophyletic, except: (1) Elmomyza is rendered paraphyletic by Rosenwaldia and Tantalia, (2) Grimshawomyia and Engiscaptomyza are paraphyletic with respect to one another; and (3) Macroscaptomyza is nested within Parascaptomyza. This analysis recovered two well-supported clades comprising Hawaiian Scaptomyza (Fig 2), one including the majority of Hawaiian subgenera, and another including only Bunostoma and Exalloscaptomyza. The non-Hawaiian subgenera are separated by long branches, and relationships between these subgenera are poorly supported in this phylogeny (posterior probability <0.90).
3.2 Divergence dating
Ages of divergence varied depending on the placement of the fossil calibration, and the completeness of the dataset, though there was overlap between estimated age ranges of all analyses (Table 3, Fig 3, S5, and S6). The dates inferred with the small dataset had similar 95% highest posterior density (HPD) ranges as the full dataset, even with the exclusion of the biogeographic calibration from the small dataset. The placement of the fossil has the strongest effect on the dating inference. Placing the S. dominicana fossil as part of the stem lineage of Scaptomyza reduces the timing of divergences of extant Scaptomyza species, and if placed as part of the crown group, increases the age of the Scaptomyza species. Given the uncertainty of the taxonomic placement of S. dominicana, dates should be treated with caution.
Table 3.
Dates of nodes of interest based on alternate fossil placements. Dates are in millions of years. The numbers in parentheses are 95% HPD values.
| Node | Large Dataset fossil calibrate stem of Scaptomyza | Large Dataset fossil calibrate crown of Scaptomyza | Small Dataset fossil calibrate stem of Scaptomyza | Small Dataset fossil calibrate crown of Scaptomyza |
|---|---|---|---|---|
|
| ||||
| Hawaiian Drosophilidae | 23.9815 (18.4171,30.8063) | 27.5188 (19.7108,36.4494) | 22.094 (16.9855,30.4300) | 26.7188 (18.5686,38.9109) |
| Hawaiian Drosophila | 13.5124 (8.5068,18.991) | 16.2589 (10.5708,22.9984) | 7.3783 (3.7371,12.3473) | 8.8566 (4.2898,15.3252) |
| Genus Scaptomyza | 17.7695 (12.0609,23.8934) | 23.3117 (18.2039,29.3288) | 18.0608 (12.0566,26.6593) | 22.0651 (17.0523,30.5186) |
| Subgenus Bunostoma/Exalloscaptomyza | 5.0245 (2.6018,8.0539) | 6.2836 (3.2876,9.9992) | ||
| Subgenus Hemiscaptomyza | 6.3179 (3.1055,9.8527) | 7.9487 (3.9896,12.3632) | 7.3128 (3.2918,12.6882) | 8.9594 (4.3753,15.3327) |
| Subgenus Scaptomyza | 6.2578 (3.5528,9.3058) | 7.8196 (4.2868,11.6125) | 6.0421 (2.8436,10.2698) | 7.3262 (3.8031,12.5439) |
| Subgenus Parascaptomyza/Macroscaptomyza | 5.0038 (2.3353,8.2453) | 6.2199 (2.8278,10.1529) | ||
| Major Hawaiian Scaptomyza clade and Parascaptomyza | 12.4499 (8.6459,16.8388) | 15.6811 (11.3817,20.5619) | 13.2164 (8.5173,19.3361) | 16.1853 (11.5786,23.1262) |
| Major Hawaiian Scaptomyza clade | 10.1232 (7.0774,13.5957) | 12.5643 (9.0474,16.5584) | 10.7234 (6.992,15.8132) | 13.1557 (9.1929,18.7188) |
| Subgenus Titanochaeta | 7.3799 (5.0047,10.2295) | 9.1050 (6.2861,12.2000) | 7.2832 (4.9255,10.9465) | 8.9665 (6.0078,13.3242) |
| Subgenus Alloscaptomyza | 3.6752 (1.9896,5.6053) | 4.5275 (2.4184,6.8349) | ||
| Subgenus Engiscaptomyza/Grimshawomyia | 7.3432 (4.8311,10.0851) | 9.0934 (6.1614,12.2354) | 7.0578 (4.359,10.8391) | 8.6677 (5.7311,12.9715) |
| Subgenus Tantalia | 3.9689 (2.1777,5.8193) | 4.8385 (2.6327,7.1144) | ||
| Subgenus Rosenwaldia | 0.5273 (0.1952,0.9471) | 0.6553 (0.2310,1.1546) | 0.5342 (0.1785,0.9632) | 0.6526 (0.2304,1.2757) |
| Subgenus Elmomyza sensu lato | 8.4941 (5.9130,11.4224) | 10.5345 (7.4580,13.9344) | 8.2522 (5.1581,12.2088) | 10.0645 (6.9321,14.7174) |
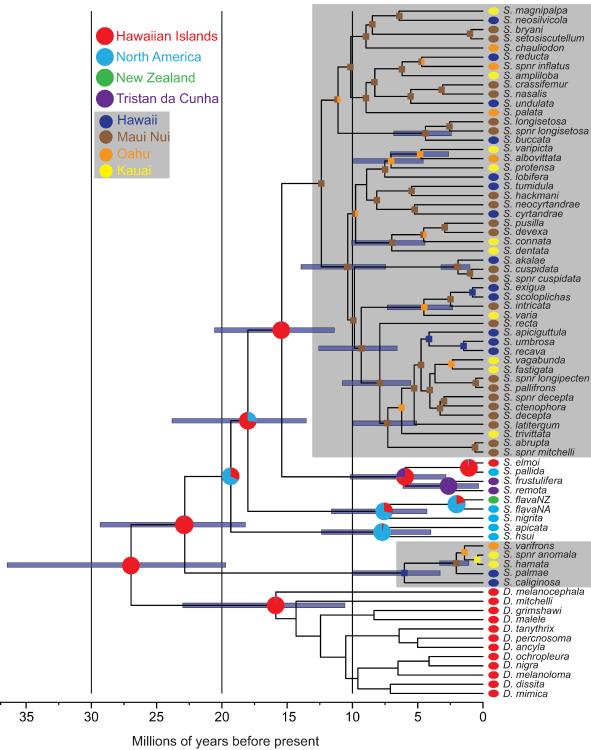
Figure 3.
Chronogram with biogeographic reconstructions. Calibrations include the age of Hawaii for range restricted species of Elmomyza, and a Dominican amber calibration placed at the crown of the genus Scaptomyza. 95% HPD age estimate distributions are drawn on nodes of interest with a BEAST BI PP >0.90. Pie charts on nodes indicate biogeographic reconstructions. Ancestral ranges inferred using Lagrange (nodes in gray boxes) or SIMMAP (nodes not in gray boxes). States inferred via SIMMAP indicate posterior probability of each range. Ranges inferred by Lagrange are the most likely state given likelihood reconstructions – divided circles indicate a range of multiple islands.
Dates of divergence between Scaptomyza and Hawaiian Drosophila were estimated in all analyses within the range of those estimated from previous analyses using biogeographic or external calibrations (Russo, et al, 1995; Tamura, et al, 2004; Obbard, et al, 2012; Morales-Hojas and Vieira, 2012). The genus Scaptomyza is estimated to have arisen between 20–30 mya with the majority of diversification in the Hawaiian lineages occurring 7–16 mya, around the time that the islands of Gardner and Necker were high enough to support rainforests (Price and Clague, 2002). Divergence times within the non-Hawaiian subgenera were variable, and ranged from ca 3 to 12 mya, although sparse sampling in some of these lineages reduces confidence in these estimates.
3.3 Biogeography
Stochastically mapped reconstructions of range suggest a Hawaiian origin for the genus Scaptomyza (Fig 3). Due to the lack of support at nodes leading to the mainland subgenera Hemiscaptomyza and Scaptomyza it is difficult to quantify the number of dispersal events from Hawaii. Stochastic mapping reconstructions indicate that at least one dispersal from the island chain occurred, followed by subsequent worldwide diversification of mainland subgenera. The split between Hemiscaptomyza and the remaining Scaptomyza is reconstructed as most likely to have occurred on North America. The node at the base of all non-Hawaiian subgenera is weakly supported in all analyses, and improved sampling of mainland subgenera may change the relationships inferred for this node.
The greatest diversity of Hawaiian Scaptomyza species belongs to the clade including Elmomyza, Tantalia, Rosenwaldia, Titanochaeta, Engiscaptomyza, Alloscaptomyza, and Grimshawomyia. Most species in these subgenera are inferred as originating on Maui Nui (Fig 2). However, other analyses do not support a Maui Nui ancestral range for these subgenera, because the Maui Nui islands are younger than these subgenera.
3.4 Evolution of larval ecologies
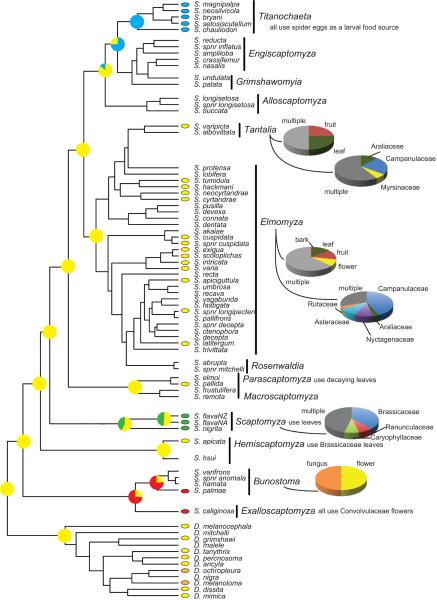
Figure 4 shows the reconstruction of ecological preference (leaf-mining, spider predation, flower-specialization, etc) using stochastic character mapping. We defined character states for ~40% of the Scaptomyza species included in this study using the available rearing records (Stalker, 1945; Maca, 1972; Collinge and Louda, 1989; Martin, 2004; Magnacca, et al, 2008; Table S1). The majority (~60%) of these species are saprophagous and use only one or few rotting host substrates. We only differentiated between flower and non-flower based saprophagy in this analysis. The ancestral state of the Scaptomyza larval substrate is saprophagy, the few clades with unique larval ecologies are monophyletic, and the ecologies are reconstructed to their most basal node with high support. Switches between larval ecologies occurred only rarely.
Figure 4.
Ancestral state reconstruction of larval host ecologies. Pie charts at nodes indicate posterior probability of stochastically mapped ancestral states. Yellow = saprophagy, green = leaf-mining, blue = spider predation, red = flower specialist, orange = fungus. Charts to the right of subgenera indicate subgenus wide rearing records parsed by either part of plant that Scaptomyza were reared from, or family of plant that Scaptomyza were reared from.
4. Discussion
4.1 Subgeneric relationships
Our results suggest that the majority of subgenera included in our analysis are monophyletic, and the relationships within each group are congruent with morphological and ecological affinities (See figure 2). However, there are subgenera that were not recovered as monophyletic. Parascaptomyza is rendered paraphyletic by Macroscaptomyza. This is not entirely surprising: the close relationship between these two subgenera has been proposed by multiple prior morphological phylogenetic analyses (Okada, 1973; Grimaldi, 1990). Also, Rosenwaldia and Tantalia are reconstructed as nested within Elmomyza. Again, this is not unexpected, given that at least one previous analysis allied these subgenera (Grimaldi, 1990). Grimshawomyia is also not monophyletic, but suffers from poor support at the base of the clade including Grimshawomyia and part of Engiscaptomyza. Increased taxa and character sampling could improve the resolution of these relationships. While previous analyses had conflicted on whether Parascaptomyza and Scaptomyza rendered each other paraphyletic or were distinct clades, we found support for both subgenera belonging to distinct clades.
We identified three clades of Hawaiian Scaptomyza separated by well supported nodes: 1) Bunostoma and Exalloscaptomyza, 2) Elmomyza, Rosenwaldia and Tantalia, and 3) Grimshawomyia, Alloscaptomyza, Titanochaeta and Engiscaptomyza. The first clade includes species of flower specialists, though they have been reared from divergent plant families (Magnacca, et al, 2008). The second clade of Elmomyza, Rosenwaldia and Tantalia has been combined in the past based on morphological cladistics and genitalic similarity (Hackman, 1959; Grimaldi, 1990). The final clade has been identified as monophyletic in a previous molecular analysis, and while gross morphology had originally placed them as distantly related, scanning electron microscopy of genitalia and polytene chromosome morphology revealed synapomorphies between subgenera (O'Grady, et al, 2003).
The non-Hawaiian endemic subgenera included in this study - Parascaptomyza, Macroscaptomyza, Scaptomyza, and Hemiscaptomyza - belong to distinct clades, despite a wide overlap in range and morphology. Their monophyletic relationships in regard to each other (excluding Macroscaptomyza and Parascaptomyza) and the Hawaiian subgenera agree well with previous phylogenetic hypotheses (Okada, 1973; Grimaldi, 1990). The exact branching relationships between these subgenera are suspect, however, given the low support at a node intermediate between them and relatively sparse taxon sampling. Future studies that include more non-Hawaiian taxa are required to more thoroughly explore these taxonomic relationships.
4.2 Biogeography of Scaptomyza
Throckmorton (1975) suggested that the ancestors of the Hawaiian Drosophilidae arrived in either one or two colonization events. The single colonist theory is supported by the sister relationship between the Hawaiian Drosophila and Scaptomyza lineages (Russo, et al, 1995; Remsen and DeSalle, 1998; Da Lage, et al, 2007; O'Grady and DeSalle, 2008; Van der Linde, et al, 2010). The two-event colonization hypothesis is supported if Hawaiian Drosophila and Scaptomyza were not sister taxa, a result that is only seen in a single study (Grimaldi, 1990). O'Grady and DeSalle (2008) further expanded on the biogeographic history in Scaptomyza by proposing a single escape from Hawaii by the ancestor of a subset of mainland subgenera. After emigrating from the Hawaiian Islands this lineage dispersed to remote Atlantic and Pacific Islands, and all continents except Antarctica. Our phylogenetic analyses are an improvement over previous studies in both taxonomic and character sampling. We inferred a more complex pattern of dispersal, but one that cannot reject the one-event colonization hypothesis.
4.2.1 Dispersal from and to Hawaii
The Hawaiian Drosophilidae is recognized as the oldest terrestrial Hawaiian lineage to have persisted to the present day (Price and Clague, 2002), though little dating has been done to identify when subgenera within Scaptomyza diverged. Our analyses confirm that the divergence of Scaptomyza and the Hawaiian Drosophila predates the emergence of the present day high islands and occurred when the atolls of Midway and Laysan were high islands (peak heights 28.7 and 20.7 myo, respectively (Price and Clague, 2002)). The ancestral range of the genus Scaptomyza was inferred to be the Hawaiian Islands, and the divergence between the Hawaiian Drosophila and Scaptomyza fits within the previously estimated range of dates of 23–35 mya (Russo, et al, 1995; Tamura, et al, 2004).
Our phylogeny recovered a Hawaiian origin of Scaptomyza (pp = 0.99), and we inferred an emigration of Scaptomyza from the Hawaiian Islands around 20 mya (pp = 0.68). Given the locality of the fossil species in Dominican amber, the dispersal of Scaptomyza was rapid. The pattern of movement to and from the Hawaiian Islands is more complicated than expected. One possible conclusion from these analyses is that there is one dispersal event from Hawaii to the mainland by the ancestor of the subgenera Scaptomyza, Hemiscaptomyza, Parascaptomyza and Macroscaptomyza. The split between the ancestor of Bunostoma and Exalloscaptomyza and the ancestor of the remaining genus Scaptomyza represents the oldest divergence event in our topology. Following this split a lineage emigrated from Hawaii and another lineage remained in Hawaii. Given the low support at important nodes of our topology we cannot entirely reject this hypothesis (Fig 2). Alternatively, a second colonization of Hawaii by the ancestor of the large Hawaiian Scaptomyza clade may have occurred ca. 12 mya. This Hawaiian clade is found to be sister to the globally distributed subgenera Macroscaptomyza and Parascaptomyza in our analyses. However, this interpretation requires another long distance colonization event to Hawaii – one colonization event is already an unlikely event (but see Rundell, et al, 2004; Arnedo, et al, 2007) – and a subsequent radiation.
Other instances of long distance dispersal have occurred. The subgenus Bunostoma includes non-Hawaiian endemic species, and future collections will identify if dispersal from or to Hawaii explains this range (Hackman, 1982). Similarly the Hawaiian subgenus Rosenwaldia includes species found throughout the Pacific, and represents another independent dispersal event. Further sampling is required to test these hypotheses.
4.2.2 Hawaiian biogeography
Movement of Scaptomyza within the Hawaiian Islands is also reconstructed as more complex than hypothesized. Most Hawaiian Scaptomyza species are single island endemics, although they display a higher incidence of multi-island endemism than species in their sister lineage, the Hawaiian Drosophila. Approximately 25% of described Hawaiian Scaptomyza species are multi-island endemics, as compared to 10% of Hawaiian Drosophila species (Hardy, 1965). The progression rule is a pattern found in the Hawaiian Drosophila (Bonacum, et al, 2005) and is observed in our study in one clade of Elmomyza including S. exigua, S. intricate, S. scoloplichas, and S. varia which is corroborated by by our dating and biogeographic inferences. The remainders of the Hawaiian Scaptomyza species do not conform to this pattern. The high vagility of Scaptomyza species is one potential explanation for why there is almost no signal of a progression rule for many Hawaiian Scaptomyza clades. In contrast, the subgenus Bunostoma appears to follow a reverse progression rule, with earlier divergences occurring on younger islands. It is possible that this lineage originated on the island of Hawaii and back-colonized up the island chain. This movement is surprising since this back colonization pattern is not normally observed among Hawaiian taxa (but see Magnacca and Danforth, 2006), and given the young age of the island of Hawaii. This pattern may be a symptom of the high vagility displayed by Scaptomyza.
Our analyses indicate that the larger clade of Hawaiian Scaptomyza – which includes Elmomyza, Rosenwaldia, Tantalia, Grimshawomyia, Alloscaptomyza, Titanochaeta and Engiscaptomyza – started to diversify across the Hawaiian Islands approximately 10–12 mya. This was the last time multiple islands in the Hawaiian Island chain were as high in elevation as they are today - the islands of Gardner, LaPerouse and Necker were above sea-level and large (Price and Clague, 2002). Given the mobility of this lineage we propose that this increased topographic diversity created more habitats and allowed for increased ecological diversification, rather than an increased incidence of vicariance. This lineage presently comprises the majority of described Hawaiian Scaptomyza species (90%).
Maui Nui is reconstructed as the area of origin for many of the Hawaiian Scaptomyza subgenera, which is probably an artifact of the present high species diversity within subgenera on Maui Nui. Maui Nui has experienced a high degree of geological heterogeneity as the sea level falls during ice ages and rises during interglacial periods. The islands of Maui, Molokai, Lanai and Kahoolawe were intermittently connected over the past 1.2 million years, and the opportunity for communities to intermingle and then diverge in allopatry via vicariant events is credited with facilitating speciation in many different taxa (Jordan, et al, 2003; Gillespie, 2005; Holland and Cowie, 2007). However, this scenario is unlikely given the age of the Hawaiian Scaptomyza subgenera and the dispersal capabilities of these species. The subgenera of Hawaiian Scaptomyza are not derived from Maui Nui ancestors – we reconstruct these lineages as older than the formation of the current main islands. We hypothesize instead that the Hawaiian subgenera had diversified during periods when older Hawaiian Islands were high enough in elevation to support cloud forests, and these subgenera persisted onto the current high islands. The present diversity seen on Maui Nui may be due to a combination of its size (second largest island in the archipelago in total area today) and age (1.2 myo to present).
4.3 Evolution of larval ecologies
Saprophagy is hypothesized to be the ancestral larval ecology in the family Drosophilidae (Throckmorton, 1975) and our analysis supports the hypothesis that saprophagy was the ancestral state for the genus Scaptomyza with high confidence (pp = 0.99). Our analyses conceal a high level of specialization within Scaptomyza: many of the saprophagous species utilize decaying plant matter of specific plant families and are restricted to specific parts of the plant (e.g. rotting leaf or stem), similar to their sister lineage, the Hawaiian Drosophila (O'Grady et al. 2011). This specialization may be due to Hawaiian microflora specializing on rotting tissues of specific parts of specific plant families (Ort et al, 2012), which may attract specific species of Scaptomyza.
Several specialized larval ecologies evolved in Scaptomyza that are found only rarely, if at all, in other drosophilid lineages. Our analysis provides basic information about the evolution of these Scaptomyza ecologies that have previously been untested. We provide the first phylogenetic evidence for the conservation of ecologies within subgenera of Scaptomyza. In almost all cases novel ecologies arise once and relatively recently. This pattern is not surprising given the large number of morphological and physiological changes required of these new lifestyles. These adaptations do not appear to be solely associated with dispersal events, and may be due to other factors such as exploiting newly available resources, or as a potential escape from predation or parasitism (Connor and Taverner, 1997).
Larvae of species in the subgenus Titanochaeta are spider egg predators and the group reconstructs strongly as such (pp = 0.98). The subgenus Titanochaeta and its spider predation ranges from ca. 7–9 mya. This is interesting given that the Hawaiian Thomisidae radiation, the group of spiders that Titanochaeta larvae feed on, are inferred to have colonized the Hawaiian Islands during this same time period, between 7–9 mya and subsequently radiated across the Hawaiian Archipelago (Garb and Gillespie, 2009). Given the coincident date of diversification of Thomisidae and the origination of spider predation it is possible that Titanochaeta radiated alongside the Thomisidae. Titanochaeta is phylogenetically nested within a lineage of Hawaiian Scaptomyza with completely unknown ecologies - including Engiscaptomyza, Grimshawomyia and Alloscaptomyza. While none of the species from these other subgenera have been reared from spider egg sacs, the ancestor of these subgenera are reconstructed as possibly being predatory (pp = 0.76). Understanding the ecologies of their relatives may illuminate the evolution of this larval ecology unique to Drosophila.
The subgenus Scaptomyza includes leaf-mining species, and is also monophyletic, although sampling within this group is currently very limited, and the sister lineage is poorly resolved. Leaf-mining originated at least 6 mya in Scaptomyza (pp = 0.47). Adapting to an herbivorous lifestyle requires overcoming multiple hurdles, especially for leaf-mining species in the subgenus Scaptomyza, some of which specialize on mustard oil-rich plant leaves in the Brassicaceae. Extant herbivorous insects are among the most evolutionarily successful radiations, and comprise nearly 25% of all eukaryote species (Bernays and Graham, 1988; Bernays, 1998). The transition to herbivory occurred at least 50 times in the Insecta (Labandeira and Sepkoski, 1993; Labandeira, 2005) and was accompanied by major changes that allowed nascent herbivores to feed on nutritionally different hosts with potent phytotoxins. Moreover, the evolution of herbivory in insects is associated with increases in the rate of lineage diversification (Mitter, et al, 1988; Farrell, 1998; Farrell and Sequeira, 2004). However, in many cases, these evolutionary transitions occurred so long ago that understanding the biogeographic, morphological and physiological underpinnings of these transitions is difficult. The relatively recent origin of herbivory in Scaptomyza indicates that it might be a good system in which to study these transitions (Whiteman, et al, 2012).
Identifying where and when obligate flower specialization arose is not yet possible given that we have only sampled one species of Exalloscaptomyza, but we did find that this lineage is sister to Bunostoma, a subgenus with species that have also been reared from flowers but are not specialists and reconstruct the most probable life history for this group as flower breeding (pp = 0.76). Placing the mainland lineage Dentiscaptomyza, another Scaptomyza subgenus that includes flower specialist (Brncic, 1983), in a phylogenetic framework would allow further insight into how this life history evolved. Specialization on morning glory flowers (Ipomoea spp.) has occurred at least twice in the Drosophilidae (Remsen and O'Grady, 2002; O'Grady and DeSalle, 2008); once in Scaptomyza (subgenus Exalloscaptomyza) and independently in the genus Drosophila (subgenus Phloridosa). These morning glory-adapted taxa converged on similar phenotypes, including dark body color with lightening at the tip of the abdomen and a general shortening and reduction in chaetotaxy. Both species have low fecundity, larvae that feed and adults that breed on yeast on the surface of corollas, and rapid metamorphosis. These species compete for the same resources in the Hawaiian Islands, where D. floricola is introduced and breed in Ipomoea spp. (Montague, 1984). Whether there are convergent molecular evolutionary underpinnings of these convergent phenotypes is unknown.
4.4 Conclusions
The genus Scaptomyza is a model for future studies of biogeography, the evolution of ecology and the associated morphological and physiological changes accompanying these adaptations. To begin exploring these questions and placing the associated research into an evolutionary context we have produced the most complete phylogenetic analysis of this group to date. This study also allows us to identify where future taxonomic sampling will improve our inference of the phylogeny of this genus. Our analyses are an important step in the evaluation of this group, but will be improved by further research. We find support for the Hawaiian origin of Scaptomyza, and its subsequent emigration back to the mainland, in accordance with previous studies, but we also find a more complex biogeography than previously predicted. The vagility of this group has been attributed to desiccation tolerance and ecology of Scaptomyza (O'Grady and DeSalle, 2008), however a better understanding of the biology of this clade is required to fully explain how these species are able to disperse across such vast distances and if Scaptomyza is pre-adapted to long range dispersal. By including specimens of other ecologically important lineages - such as the flower specializing and possibly leaf-mining Dentiscaptomyza and the predacious Elmomyza species – we will be able to explore how these ecologies have evolved in relation to those included in this study. Species from more remote areas of the broad range of this genus – such as other subgenera from Tristan da Cunha in the South Atlantic and species of the subgenus Bunostoma from around the Pacific Basin – will further refine our understanding of how Scaptomyza have dispersed throughout the world.
Supplementary Material
Supplementary Table 1. Additional information for taxa. Locality = Locality information used in range reconstruction. Ecology = Larval ecology used in ancestral state reconstructions. Morphology = Information used for the combined morphological and molecular phylogeny. Symbols in morphology matrix: + refers to having both morphological and molecular coverage, - refers to only having morphological characters in matrix, / refers to only having molecular characters in matrix. 1. Magnacca, et al, 2008. 2. Personal observation. 3. Martin, 2004. 4. Stalker, 1945. 5. Maca, 1972. 6. Collinge and Louda, 1989.
Supplementary Table 2. Morphological matrix for Scaptomyza dominicana. Characters are in the exact order as in the matrix from Grimaldi, 1990. ? indicate where no state was able to be determined from either the description or drawing from Grimaldi, 1987.
Supplementary Figure 1. Combined morphological and molecular phylogeny. States coded for S. dominicana from Grimaldi, 1987. Character states from other species drawn from Grimaldi, 1990. Bayesian posterior probabilities indicated at nodes.
Supplementary Figure 2. Maximum Likelihood topology of full dataset. Partitions same as for topology inferred using Bayesian inference. Bootstrap support indicated on nodes.
Supplementary Figure 3. Maximum Likelihood and Bayesian Inference phylogeny of the small dataset. Numbers at nodes are bootstrap support values and posterior probabilities for the topology inferred using RAxML and MrBayes, respectively.
Supplementary Figure 4a–e. Gene genealogies. Nodes with greater than 70% Maximum Likelihood bootstraps and greater than 90% posterior probabilities indicated with an *.
Supplementary Figure 5. Chronogram of the genus Scaptomyza. Calibrations include the age of Hawaii Island for range restricted species of Elmomyza, and a Dominican amber calibration placed at the stem of the genus Scaptomyza. 95% HPD age estimate distributions are drawn on nodes. Numbers on nodes indicate posterior probabilities.
Supplementary Figure 6. Chronogram of the genus Scaptomyza using the small dataset. Calibrations include the age of Hawaii for range restricted species of Elmomyza, and a Dominican amber calibration placed at a) the stem of the genus Scaptomyza and b) the crown of the genus Scaptomyza. 95% HPD age estimate distributions are drawn on nodes. Numbers on nodes indicate posterior probabilities.
Phylogeny of genus Scaptomyza, based on 5 kbp of sequence and taxa from 13 subgenera.
Scaptomyza originated in the Hawaiian Islands and dispersed from there around the world
The ecological niches this lineage exploits arose independently
support for the monophyly of all subgenera with some exceptions
Acknowledgements
The authors would like to thank the insightful and helpful advice of two anonymous reviewers. We thank the Hawaii Natural Area Reserves and Department of Fish and Wildlife for their help with permitting. Thanks to A. Gloss, K. Magnacca, B. Ort, and A. Gidaya for indispensible help with specimen collecting and sequencing. Thanks to both the Whiteman and O'Grady labs for advice and editing on a previous version of the manuscript. This work was supported by PERT funds from the University of Arizona to RTL, Walker funds from the University of California, Berkeley, to RTL, NSF DEB0842348 awarded to PMO, University of Arizona Faculty Seed Grant to NKW, University of Arizona Center for Insect Science Seed Grant to NKW, and a Faculty Seed Grant to NKW, University of Arizona laboratory setup funds to NKW and NSF DEB 1256758 awarded to NKW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnedo MA, Agnarsson I, Gillespie RG. Molecular insights into the phylogenetic structure of the spider genus Theridion (Araneae, Theridiidae) and the origin of the Hawaiian Theridion-like fauna. Zool. Scr. 2007;36:337–352. [Google Scholar]
- Baldwin BG, Sanderson MJ. Age and rate of diversification of the Hawaiian silversword alliance (Compositae) Proc Natl Acad Sci U S A. USA. 1998;95:9402–9406. doi: 10.1073/pnas.95.16.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellemain E, Ricklefs RE. Are islands the end of the colonization road. Trends Ecol. Evol. 2008;23:461–468. doi: 10.1016/j.tree.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Bernays E. Evolution of feeding behavior in insect herbivores. BioSci. 1998;48:35–44. [Google Scholar]
- Bernays E, Graham M. On the evolution of host specificity in phytophagous arthropods. Ecol. 1988;69:886–892. [Google Scholar]
- Bollback JP. SIMMAP: stochastic character mapping of discrete traits on phylogenies. BMC Bioinforma. 2006;7:88. doi: 10.1186/1471-2105-7-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonacum J, DeSalle R, O'Grady PM, Olivera D, Wintermute J, Zilversmit M. New nuclear and mitochondrial primers for systematics and comparative genomics in Drosophilidae. Drosophila Inform. Serv. 2001;84:201–204. [Google Scholar]
- Bonacum J, O'Grady PM, Kambysellis M, DeSalle R. Phylogeny and age of diversification of the planitibia species group of the Hawaiian Drosophila. Mol. Phylogenet. Evol. 2005;37:73–82. doi: 10.1016/j.ympev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Brncic D. A review of the genus Scaptomyza Hardy (Diptera, Drosophilidae) in Chile with description of a new species. Revista Chilena de Historia Natural. 1983;56:71–76. [Google Scholar]
- Buerki S, Forest F, Alvarez N, Nylander JAA, Arrigo N, Sanmartín I. An evaluation of new parsimony-based versus parametric inference methods in biogeography: a case study using the globally distributed plant family Sapindaceae. J. Biogeogr. 2011;38:531–550. [Google Scholar]
- Carlquist S. Island Biology. Columbia University Press; New York and London: 1974. [Google Scholar]
- Carson HL. Sexual Selection: A driver of genetic change in Hawaiian Drosophila. J. Hered. 1997;88:343–352. doi: 10.1093/oxfordjournals.jhered.a023115. [DOI] [PubMed] [Google Scholar]
- Chittenden FH. Some insects injurious to vegetable crops. Bulletin of the U.S. Department of Agriculture, Division of Entomology; 1902. [Google Scholar]
- Collinge SK, Louda SM. Scaptomyza nigrita Wheeler (Diptera: Drosophilidae), a Leaf Miner of the Native Crucifer, Cardamine cordifolia A. Gray (Bittercress) J Kans. Entomol. Soc. 1989;62:1–10. [Google Scholar]
- Conner EF, Taverner MP. The evolution and adaptive significance of the leaf-mining habit. Oikos. 1997;79:6–25. [Google Scholar]
- Cowie RH, Holland BS. Molecular biogeography and diversification of the endemic terrestrial fauna of the Hawaiian Islands. Philos Trans R Soc Lond B Biol Sci. 2008;363:3363–3376. doi: 10.1098/rstb.2008.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox GW. Alien Species in North America and Hawaii. Island Press; Washington, DC: 1999. [Google Scholar]
- Da Lage JL, Kergoat GJ, Maczkowiak F, Silvain JF, Cariou ML, Lachaise D. A phylogeny of Drosophilidae using the Amyrel gene: questioning the Drosophila melanogaster species group boundaries. J. Zool. Syst. Evol. Res. 2007;45:47–63. [Google Scholar]
- Darwin C. On the Origin of Species by Means of Natural Selection, Or the Preservation of Favoured Races in the Struggle for Life. John Murray; London: 1859. [PMC free article] [PubMed] [Google Scholar]
- Drummond A, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nuc. Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenhuis NL, Samuelson A. The insect and spider collections of the world website of the Bishop Museum. Honolulu, Hawaii: 2007. [Google Scholar]
- Farrell BD. “Inordinate Fondness” explained: why are there So many beetles? Science. 1998;281:555–559. doi: 10.1126/science.281.5376.555. [DOI] [PubMed] [Google Scholar]
- Farrell BD, Sequeira AS. Evolutionary rates in the adaptive radiation of beetles on plants. Evol. 2004;58:1984–2001. doi: 10.1111/j.0014-3820.2004.tb00484.x. [DOI] [PubMed] [Google Scholar]
- Filardi CE, Moyle RG. Single origin of a pan-Pacific bird group and upstream colonization of Australasia. Nature. 2005;438:216–219. doi: 10.1038/nature04057. [DOI] [PubMed] [Google Scholar]
- Fleischer RC, McIntosh CE, Tarr CL. Evolution on a volcanic conveyor belt: using phylogeographic reconstructions and K-Ar-based ages of the Hawaiian Islands to estimate molecular evolutionary rates. Mol. Ecol. 1998:533–545. doi: 10.1046/j.1365-294x.1998.00364.x. [DOI] [PubMed] [Google Scholar]
- Garb JE, Gillespie RG. Diversity despite dispersal: colonization history and phylogeography of Hawaiian crab spiders inferred from multilocus genetic data. Mol. Ecol. 2009;18:1746–1764. doi: 10.1111/j.1365-294X.2009.04125.x. [DOI] [PubMed] [Google Scholar]
- Gillespie RG. Geographical context of speciation in a radiation of Hawaiian Tetragnatha spiders (Araneae, Tetragnathidae) J. Arachnol. 2005;33:313–322. [Google Scholar]
- Gillespie RG, Roderick GK. Arthropods on islands: colonization, speciation, and conservation. Ann. Rev. Entomol. 2002;47:595–632. doi: 10.1146/annurev.ento.47.091201.145244. [DOI] [PubMed] [Google Scholar]
- Grant PR. Ecology and Evolution of Darwin's Finches. Princeton University Press; Princeton, New Jersey: 1999. [Google Scholar]
- Grimaldi DA. A phylogenetic, revised classification of genera in the Drosophilidae (Diptera) Bulletin of the American Museum of Natural History. 1990;197:123–128. [Google Scholar]
- Grimaldi D. Amber fossil Drosophilidae (Diptera), with particular reference to the Hispaniolan taxa. American Museum Novitates. 1987;1 [Google Scholar]
- Hackman W. The relation between the genera Scaptomyza and Drosophila (Diptera, Drosophilidae) Annales Entomologicae Fennicae. 1982;48:97–104. [Google Scholar]
- Hackman W. On the genus Scaptomyza Hardy (Diptera, Drosophildae) Acta. Zool. Fenn. 1959;97:3–73. [Google Scholar]
- Harbaugh DT, Baldwin BG. Phylogeny and biogeography of the sandalwoods (Santalum, Santalaceae): repeated dispersals throughout the Pacific. Am. J Bot. 2007;94:1028–1040. doi: 10.3732/ajb.94.6.1028. [DOI] [PubMed] [Google Scholar]
- Hardy DE. Diptera: Cyclorrhapha II, Series Schizophora, Section Acalypterae I, Family Drosophilidae. Insects of Hawaii. 1965;12:1–814. [Google Scholar]
- Hardy J. Notes on the remedies of the turnip-fly amongst the ancients, a new genus (Scaptomyza) and species (S. graminum and S. apicalis) of Diptera. Proc. Berwicksh. Nat. Club. 1849;2:359–362. [Google Scholar]
- Heaney LR. Is a new paradigm emerging for oceanic island biogeography? J. Biogeogr. 2007;34:753–757. [Google Scholar]
- Hennig W. Phylogenetic Systematics. Univ. Illinois Press; Urbana: 1966. [Google Scholar]
- Holland BS, Cowie RH. A geographic mosaic of passive dispersal: population structure in the endemic Hawaiian amber snail Succinea caduca (Mighels, 1845) Mol. Ecol. 2007;16:2422–2435. doi: 10.1111/j.1365-294X.2007.03246.x. [DOI] [PubMed] [Google Scholar]
- Holland BS, Cowie RH. Land snail models in island biogeography: A tale of two snails. Am. Malac. Bull. 2009;27(1–2):59–68. [Google Scholar]
- Iturralde-Vinent MA, MacPhee RDE. Age and paleographical origin of Dominican amber. Science. 1996:1850–1852. [Google Scholar]
- Jordan S, Simon C, Polhemus D. Molecular Systematics and Adaptive Radiation of Hawaii's Endemic Damselfly Genus Megalagrion (Odonata: Coenagrionidae) Syst. Biol. 2003;52:89–109. doi: 10.1080/10635150390132803. [DOI] [PubMed] [Google Scholar]
- Labandeira CC. Invasion of the continents: cyanobacterial crusts to tree-inhabiting arthropods. Trends Ecol Evol. 2005;20:253–262. doi: 10.1016/j.tree.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Labandeira CC, Sepkoski JJ. Insect diversity in the fossil record. Science. 1993;261:310–315. doi: 10.1126/science.11536548. [DOI] [PubMed] [Google Scholar]
- Lerner HL, Meyer M, James H, Hofreiter M, Fleischer R. Multilocus resolution of phylogeny and timescale in the extant adaptive radiation of Hawaiian honeycreepers. Curr. Biol. 2011;21:1838–1844. doi: 10.1016/j.cub.2011.09.039. [DOI] [PubMed] [Google Scholar]
- Maca J. Czechoslovak species of the genus Scaptomyza Hardy (Diptera, Drosophilidae) and their bionomics. Acta entomologica Bohemoslovaca. 1972;69:119–132. [Google Scholar]
- Magnacca KN, Danforth BN. Evolution and biogeography of the native Hawaiian Hylaeus bees (Hymenoptera: Colletidae) Clad. 2006;22(5):393–411. [Google Scholar]
- Magnacca KN, Foote D, O'Grady PM. A review of the endemic Hawaiian drosophilidae and their host plants. Zootaxa. 2008;1728:1–58. [Google Scholar]
- Malloch JR. New species and other records of Otitidae (Ortalidae), Piophilidae, clusiidae, chloropidae, and drosophilidae from the Marquesas. Bulletin of the Bernice P. Bishop Museum. 1932;98:205–223. [Google Scholar]
- Markow TA, O'Grady PM. Drosophila: A Guide to Species Identification and Use. Academic Press; London: 2006. [Google Scholar]
- Markow TA, O'Grady P. Reproductive ecology of Drosophila. Funct. Ecol. 2008;22:747–759. [Google Scholar]
- Martin NA. History of an invader, Scaptomyza flava (Fallen, 1823) (Diptera: Drosophilidae) N.Z. J Zool. 2004;31:27–32. [Google Scholar]
- Mayr E, Diamond J. The Birds of Northern Melanesia. Oxford University Press; New York: 2001. [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Gateway Computing Environments Workshop (GCE) 2010;2010:1–8. [Google Scholar]
- Mitter C, Farrell BD, Wiegmann B. The phylogenetic study of adaptive zones: has phytophagy promoted insect diversification? Am. Nat. 1988;132:107–128. [Google Scholar]
- Montague JR. The ecology of Hawaiian flower-breeding drosophilids. I. Selection in the larval habitat. Am Nat. 1984;124:712–722. [Google Scholar]
- Morales-Hojas R, Vieira J. Phylogenetic patterns of geographical and ecological diversification in the subgenus Drosophila. PLoS One. 2012;7(11):e49552. doi: 10.1371/journal.pone.0049552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander JAA. MrModeltest v2. 2004. [Google Scholar]
- Nylander JAA, Wilgenbusch JC, Warren DL, Swofford DL. AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bio informa. 2008;24:581–583. doi: 10.1093/bioinformatics/btm388. [DOI] [PubMed] [Google Scholar]
- Obbard DJ, Maclennan J, Kim KW, Rambaut A, O'Grady PM, Jiggins FM. Estimating the divergence dates and substitution rates in the Drosophila phylogeny. Mol. Biol. Evol. 2012;29:3459–3473. doi: 10.1093/molbev/mss150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Grady PM, Bonacum J, Val FC. The placement of the Engiscaptomyza, Grimshawomyia, and Titanochaeta, three clades of endemic Hawaiian Drosophilidae. Zootaxa. 2003;159:1–16. [Google Scholar]
- O'Grady PM, Magnacca KN, Lapoint RT. Taxonomic relationships within the endemic Hawaiian Drosophilidae. Records of the Hawaii Biological Survey. 2010;108:3–35. [Google Scholar]
- O'Grady P, DeSalle R. Out of Hawaii: The biogeographic history of the genus Scaptomyza (Diptera: Drosophilidae) Biol. Lett. 2008;4:195–199. doi: 10.1098/rsbl.2007.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Grady PM, Lapoint RT, Bonacum J, Lasola J, Owen E, Wu Y, DeSalle R. Phylogenetic and ecological relationships of the Hawaiian Drosophila inferred by mitochondrial DNA analysis. Mol. Phylogenet. Evol. 2011;58:244–256. doi: 10.1016/j.ympev.2010.11.022. [DOI] [PubMed] [Google Scholar]
- O'Grady PM, Zilversmit M. phylogenetic relationships within the haleakalae species group inferred by molecular and morphological characters (Diptera: Drosophilidae) Bishop Museum Bulletins in Entomology. 2004;10:117–134. [Google Scholar]
- Okada T. Descriptions of four new species of drosophilidae of the Bonins, with taxometrical analyses of the Scaptomyza species (Diptera) Kontyu. 1973;41:83–90. [Google Scholar]
- Ort BS, Bantay RM, Pantoja NA, O'Grady PM. Fungal diversity associated with Hawaiian Drosophila host plants. PLoS One. 2012;7:e40550. doi: 10.1371/journal.pone.0040550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham JF, Donoghue PCJ, Bell CJ, Calway TD, Head JJ, Holroyd PA, Inoue JG, Irmis RB, Joyce WG, Ksepka DT, et al. Best practices for justifying fossil calibrations. Syst. Biol. 2012;61:346–359. doi: 10.1093/sysbio/syr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons J, Gillespie RG. Evolution of satellite DNAs in a radiation of endemic Hawaiian spiders: does concerted evolution of highly repetitive sequences reflect evolutionary history? J. Mol. Evol. 2004;59:632–641. doi: 10.1007/s00239-004-2655-2. [DOI] [PubMed] [Google Scholar]
- Price JP, Elliot-Fisk D. Topographic history of the Maui Nui complex, Hawaii, and it's implications for biogeography. Pac. Sci. 2004;58:27–45. [Google Scholar]
- Price JP, Clague DA. How old is the Hawaiian biota? Geology and phylogeny suggest recent divergence. Philos Trans R Soc Lond B Biol Sci. 2002;269:2429–2435. doi: 10.1098/rspb.2002.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut A, Drummond AJ. Tracer v1.4. 2004. [Google Scholar]
- Ree RH, Smith SA. Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Syst Biol. 2008;57:4–14. doi: 10.1080/10635150701883881. [DOI] [PubMed] [Google Scholar]
- Remsen J, O'Grady PM. Phylogeny of Drosophilidae (Diptera), with comments on combined analysis and character support. Mol. Phylogenet. Evol. 2002;24:248–263. doi: 10.1016/s1055-7903(02)00226-9. [DOI] [PubMed] [Google Scholar]
- Remsen J, DeSalle R. Character congruence of multiple data partitions and the origin of the Hawaiian Drosophilidae. Mol. Phylogenet. Evol. 1998;9:225–235. doi: 10.1006/mpev.1997.0484. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinforma. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000;132:365. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Rubinoff D. Phylogeography and ecology of an endemic radiation of Hawaiian aquatic case-bearing moths (Hyposmocoma: Cosmopterigidae) Phil. Trans. R. Soc. B. 2008;363:3459–3465. doi: 10.1098/rstb.2008.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundell RJ, Price TD. Adaptive radiation, nonadaptive radiation, ecological speciation and nonecological speciation. Trends Ecol Evol. 2009;24:394–399. doi: 10.1016/j.tree.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Russo CAM, Takezaki N, Nei M. Molecular phylogeny and divergence times of drosophilid species. Mol. Biol. Evol. 1995;12:391–404. doi: 10.1093/oxfordjournals.molbev.a040214. [DOI] [PubMed] [Google Scholar]
- Shaw KL. Conflict between nuclear and mitochondrial DNA phylogenies of a recent species radiation: what mtDNA reveals and conceals about modes of speciation in Hawaiian crickets. Proc. Natl. Acad. Sci. U S A. 2002:16122–16127. doi: 10.1073/pnas.242585899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simberloff DS, Wilson EO. Experimental zoogeography of islands: The colonization of empty islands. Ecol. 1969;50:278–296. [Google Scholar]
- Simon C, Frati F, Beckenbach A, Crespi B, Liu H, Flook P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 1994;87:651. [Google Scholar]
- Stalker HD. On the biology and genetics of Scaptomyza graminum Fallen (Diptera, Drosophilidae) Genet. 1945;30:266–279. doi: 10.1093/genetics/30.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinforma. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Tamura K, Subramanian S, Kumar S. Temporal patterns of fruit fly (Drosophila) evolution revealed by mutation clocks. Mol. Biol. Evol. 2004;21:36–44. doi: 10.1093/molbev/msg236. [DOI] [PubMed] [Google Scholar]
- Throckmorton LH. The phylogeny, ecology, and geography of Drosophila. In: King RC, editor. Handbook of genetics. Volume 3. Plenum; New York: 1975. pp. 421–469. [Google Scholar]
- Throckmorton LH. The relationships of the endemic Hawaiian Drosophilidae. Univ. Tex. Publ. 1966;6615:335–396. [Google Scholar]
- Tweedie S, Ashburner M, Falls K, Leyland P, McQuilton P, Marygold S, Millburn G, Osumi-Sutherland D, Schroeder A, Seal R, et al. FlyBase: enhancing Drosophila Gene Ontology annotations. Nucleic Acids Res. 2009;37:D555–D559. doi: 10.1093/nar/gkn788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Linde K, Houle D, Spicer GS, Steppan J. A supermatrix-based molecular phylogeny of the family Drosophilidae. Genet. Res. 2010;92:25–38. doi: 10.1017/S001667231000008X. [DOI] [PubMed] [Google Scholar]
- Wheeler MR, Takada H. The Nearctic and Neotropical species of Scaptomyza Hardy (Diptera: Drosophilidae) Univ. Tex. Publ. 1966;6615:35–78. [Google Scholar]
- Whiteman NK, Groen SC, Chevasco D, Bear A, Beckwith N, Gregpry TR, Denoux C, Mammarella N, Ausubel FM, Pierce NE. Mining the plant-herbivore interface with a leafmining Drosophila of Arabidopsis. Mol. Ecol. 2011;20:995–1014. doi: 10.1111/j.1365-294X.2010.04901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman NK, Gloss AD, Sackton TB, Groen SC, Humphrey PT, Lapoint RT, Sønderby IE, Halkier BA, Kocks C, Ausubel FM, et al. Genes involved in the evolution of herbivory by a leaf-mining, drosophilid fly. Genome Biol. Evol. 2012;4:788–804. doi: 10.1093/gbe/evs063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens JJ. Missing data, incomplete taxa, and phylogenetic accuracy. Syst. Biol. 2003;52:528–538. doi: 10.1080/10635150390218330. [DOI] [PubMed] [Google Scholar]
- Wiens JJ. Missing data and the design of phylogenetic analyses. J. Biomed. Inf. 2006;39:34–42. doi: 10.1016/j.jbi.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Wiens JJ, Moen DS. Missing data and the accuracy of Bayesian phylogenetics. J. Syst. Evol. 2008;46:307–314. [Google Scholar]
- Wilson EO. The nature of the taxon cycle in the Melanesian ant fauna. Am. Nat. 1961;95:169–193. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Additional information for taxa. Locality = Locality information used in range reconstruction. Ecology = Larval ecology used in ancestral state reconstructions. Morphology = Information used for the combined morphological and molecular phylogeny. Symbols in morphology matrix: + refers to having both morphological and molecular coverage, - refers to only having morphological characters in matrix, / refers to only having molecular characters in matrix. 1. Magnacca, et al, 2008. 2. Personal observation. 3. Martin, 2004. 4. Stalker, 1945. 5. Maca, 1972. 6. Collinge and Louda, 1989.
Supplementary Table 2. Morphological matrix for Scaptomyza dominicana. Characters are in the exact order as in the matrix from Grimaldi, 1990. ? indicate where no state was able to be determined from either the description or drawing from Grimaldi, 1987.
Supplementary Figure 1. Combined morphological and molecular phylogeny. States coded for S. dominicana from Grimaldi, 1987. Character states from other species drawn from Grimaldi, 1990. Bayesian posterior probabilities indicated at nodes.
Supplementary Figure 2. Maximum Likelihood topology of full dataset. Partitions same as for topology inferred using Bayesian inference. Bootstrap support indicated on nodes.
Supplementary Figure 3. Maximum Likelihood and Bayesian Inference phylogeny of the small dataset. Numbers at nodes are bootstrap support values and posterior probabilities for the topology inferred using RAxML and MrBayes, respectively.
Supplementary Figure 4a–e. Gene genealogies. Nodes with greater than 70% Maximum Likelihood bootstraps and greater than 90% posterior probabilities indicated with an *.
Supplementary Figure 5. Chronogram of the genus Scaptomyza. Calibrations include the age of Hawaii Island for range restricted species of Elmomyza, and a Dominican amber calibration placed at the stem of the genus Scaptomyza. 95% HPD age estimate distributions are drawn on nodes. Numbers on nodes indicate posterior probabilities.
Supplementary Figure 6. Chronogram of the genus Scaptomyza using the small dataset. Calibrations include the age of Hawaii for range restricted species of Elmomyza, and a Dominican amber calibration placed at a) the stem of the genus Scaptomyza and b) the crown of the genus Scaptomyza. 95% HPD age estimate distributions are drawn on nodes. Numbers on nodes indicate posterior probabilities.