Abstract
Exogenous proteolytic enzyme supplementation is required in certain disease conditions in humans and animals and due to compelling reasons on use of more plant protein ingredients and profitability in animal feed industry. However, limitations on their utility in diet are imposed by their pH specificity, thermolabile nature, inhibition due to a variety of factors and the possibility of intestinal damage. For enhancing the efficacy and safety of exogenous trypsin, an efficient chitosan (0.04%) nanoencapsulation-based controlled delivery system was developed. An experiment was conducted for 45 days to evaluate nanoencapsulated trypsin (0.01% and 0.02%) along with 0.02% bare trypsin and 0.4% chitosan nanoparticles against a control diet on productive efficiency (growth rate, feed conversion and protein efficiency ratio), organo-somatic indices, nutrient digestibility, tissue enzyme activities, hematic parameters and intestinal histology of the fish Labeo rohita. All the synthesized nanoparticles were of desired characteristics. Enhanced fish productive efficiency using nanoencapsulated trypsin over its bare form was noticed, which corresponded with enhanced (P<0.01) nutrient digestibility, activity of intestinal protease, liver and muscle tissue transaminases (alanine and aspartate) and dehydrogenases (lactate and malate), serum blood urea nitrogen and serum protein profile. Intestinal tissues of fish fed with 0.02% bare trypsin showed broadened, marked foamy cells with lipid vacuoles. However, villi were healthier in appearance with improved morphological features in fish fed with nanoencapsulated trypsin than with bare trypsin, and the villi were longer in fish fed with 0.01% nanoencapsulated trypsin than with 0.02% nanoencapsulated trypsin. The result of this premier experiment shows that nanoencapsulated trypsin mimics zymogen-like proteolytic activity via controlled release, and hence the use of 0.01% nanoencapsulated trypsin (in chitosan nanoparticles) over bare trypsin can be favored as a dietary supplement in animals and humans.
Introduction
Advances in the understanding of gastrointestinal tract physiology have enabled the manipulation of digestibility and absorption of food constituents, chemicals and biomolecules to meet individual needs and goals in farm animal production. Exogenous enzyme supplementation is an important area of research in humans and animals. People with exocrine pancreatic insufficiency and cystic fibrosis frequently require supplemental pancreatic enzymes (which include proteolytic enzymes, lipases, and amylases). In addition, some people suffering from celiac disease [1] or Crohn’s disease [2] and perhaps from indigestion [3] may be deficient in pancreatic enzymes. Exogenous enzymes in animal feed are used for compelling reasons: shortage of preferred animal protein sources (fish meal), coupled with greater use of plant protein ingredients as well as the profitability in the fish, poultry and pig sectors, which are compete for feed resources among themselves and also with humans. Because global fish production is in decline, these industries will increasingly rely on protein sources of plant origin. In addition, the aquaculture sector, the fastest growing food sector [4], requires rapid growth of aquafeed production using cheaper plant-based ingredients to satisfy its demands. Exogenous enzymes enable them to digest plant-based ingredients containing more fiber, low quality protein and various anti-nutritional factors, which have been shown to lower nutrient digestibility [5]–[7]. Moreover, the metabolic activity during the growth phase across species including fish and shellfish (juvenile stage) is quite high, and consequently requires a nutrient-dense (mainly high protein) diet in order to optimise survival and growth. However, the digestive system is not developed enough to digest a nutrient-dense diet. Improper digestion and malabsorption of nutrients often have far-reaching effects that include reduced growth, impaired immunity, allergic reactions and poor wound healing. These problems can be rectified by incorporating exogenous enzymes and nutraceuticals in aquafeed. Enzymes manufactured by synthetic means or derived from plants, animals or microbial sources are increasingly being used as additives in animal feeds [8]–[9]. Supplementation of diet with pancreatic enzymes stimulated growth rate and protein utilization in Salmo salar [10] and with bovine trypsin significantly increased the proteolytic activity in common carp [11].
Although in theory exogenous proteolytic enzymes would not cause health problems, a serious condition called fibrosing colonopathy involving damage to the large intestines has resulted from the use of pancreatic enzymes in children with cystic fibrosis. In some cases, the problem was linked to the use of high supplemental amounts of enzymes [12]–[14]. However, the amount of enzymes used has not been linked to the problem in all reports [15]. In some cases, lower amounts of enteric-coated enzymes have caused fibrosing colonopathy [16]. It is believed to be the result of unknown interactions between the enteric coating and the enzymes themselves causing damage to the intestines of children with cystic fibrosis [17]. In animals, damage to mucosa can increase the chance of infection. In animals, studies discussing safety of proteolytic enzymes are scarce.
Applications of exogenous enzymes in food/feed have certain limitations due to their thermolabile nature, pH specificity, heavy metal salt, various inhibitors etc. Additionally, use of unregulated proteolytic enzymes may cause damage to intestines and cause stress. To overcome these problems, an efficient delivery system has to be developed to maximize the efficiency of exogenous enzymes and enhance safety. A controlled release system for robust proteolytic enzyme (like monoproteases) is an option which has not been tried so far. A number of polymeric compounds and lipids are being used in medical fields to improve the delivery efficiency of enzymes, drugs, hormones, vaccines (DNA and RNA) or genes. Among those investigated, chitosan acts as an excellent carrier system for nutraceuticals and other biomolecules because of its versatile properties including biocompatibility, biodegradability, membrane forming ability, reactive surface functional groups for easy surface modification, low toxicity, immunomodulation, membrane adhesiveness, improved stability and enhanced permeability of epithelial membrane [18]–[19]. Chitosan is a base material for immobilization of several enzymes, as it exhibits increased thermostability compared to the free enzymes [19] and may help in protecting the biomolecules from the adverse effect of temperature, pH and endogenous enzyme activity [20].
Nanotechnology is ushering a new era in the field of medicine and the agri-food and feed sectors. Nanoscale drug systems have the advantage that they can circumvent rapid recognition by the immune system and deliver drugs to cells with high efficiency compared with microparticle based systems [21]. Nanoparticles have been studied as carriers for oral drug delivery to improve drug stability and bioavailability across the GI mucosa [22]. A number of polymeric nanoparticles have been synthesized and studied in the past few years as promising drug delivery systems which improve delivery efficiency and reduce side-effects of drug toxicity [23]–[24] including chitosan nanoparticles for controlled delivery of vitamin C in fish [25].
Fish are becoming popular as a research model of human pathologies because of their easy availability, high fecundity, rapid breeding and ease of maintaining and their high genetic and organ system homology to humans. One of the areas where fish models have contributed enormously to the understanding of human pathologies is the area of gastrointestinal diseases and microbe-host interactions [26]. The zebrafish model of inflammatory bowel disease [27]–[29] and glafenine-induced intestinal injury amelioration are effective as the gastrointestinal system of fishes, like humans, possess liver, pancreas, gall bladder and a linearly segmented intestinal track with absorptive and secretory functions [30]–[31] with proximal-distal functional specification and containing absorptive enterocytes having apical microvilli, an intestinal brush border [32] and other structures, goblet cells and enteroendocrine cells [30]–[32]. Information generated in fish research also has important applications for aquaculture and the fishery industry itself which contributes to food and nutritional security.
This premier report on fish model, with implications for animal species and humans, discusses the results of the hypothesis that nanoencapsulation of trypsin with chitosan releases enzyme in controlled manner and biomimics zymogen-like activity, thus improving safety of use in addition to production efficiency in fish.
Materials and Methods
Chemicals
Chitosan with a deacetylation degree of 90% was purchased from Sigma-Aldrich (St. Louis, MO). All other chemicals were of analytical and reagent grade.
Purification of chitosan
One gram of chitosan was dissolved in 15 mL of 1 M sodium hydroxide (NaOH) solution by heating at 50°C with vigorous stirring for 2 hours. The chitosan solution was filtered using a Nalgene filter (Nalgene, Rochester, NY) followed by washing with deionized water. The filtered chitosan was dried overnight at 40°C and then dissolved in 0.1 M acetic acid. Using a Buchner filter unit (Fisher Scientific, Houston, TX) insoluble residues were removed from the chitosan solution. The pH of the solution was adjusted to 7.0 using 1 M NaOH, which resulted in purified chitosan precipitate. The precipitated chitosan was collected by centrifugation and washed with deionized water. The chitosan was freeze-dried in lyophilizer (Labconco, Mo). This lyophilized chitosan was further used for nanoparticle preparation [33]–[34].
Synthesis of chitosan nanoencapsulated trypsin nanoparticles
Chitosan nanoparticles (NPs) were prepared according to the ionotropic gelation procedure [35] with slight modification. Chitosan (0.4 g) flakes were dissolved in 10 ml glacial acetic acid and the pH was adjusted to 7.0 by addition of sodium hydroxide. This solution was vigorously stirred with a magnetic stirrer at ambient temperature until the solution became clear. The total volume was made up to 100 ml. It was then sterile-filtered using a 0.22 µm syringe filter. Then tripolyphosphate (TPP) (1 mg/ml) solution was added drop-wise to the chitosan solution while stirring. Nanoparticles were formed immediately upon mixing of TPP and chitosan solutions as molecular linkages were formed between TPP phosphate and chitosan amino groups. The solution was stored at 4°C for further use. Enzyme solution was prepared by dissolving enzyme trypsin (10 mg/ml) in 67 mM phosphate buffer at pH 7.6. Trypsin was encapsulated in chitosan NPs by drop-wise addition of enzyme into the chitosan-TPP solution and homogenized for 5 min at 4°C. These nanoparticles were used for preparation of experimental diets. Enzyme encapsulation efficiency of nanoparticles [36] was determined by the separation of nanoparticles from the aqueous medium containing non-associated enzyme by centrifugation at 15000 rpm at 4°C for 30 min. The amount of free enzyme in the supernatant was measured by UV spectrophotometry at 280 nm using the supernatant of the non-encapsulated enzyme nanoparticles as basic correction. Trypsin encapsulation efficiency percentage (EE %) was calculated using the following formula:
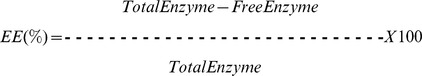 |
Characterization of chitosan and encapsulated trypsin nanoparticles
Mean particle size, poly-dispersity index (size distribution) and zeta-potential of chitosan-encapsulated trypsin nanoparticles were determined by Delsa™ Nano-C (Brea, CA) at room temperature in the triplicate. The morphological characteristics of the chitosan-encapsulated trypsin nanoparticles were examined by using a tapping-mode atomic force microscope (AFM) (JPK NanoWizard® AFM, Berlin, Germany). One drop of chitosan solution was placed onto a freshly cleaved piece of mica and air-dried.
Ethics Statement
The research undertaken complies with the current animal welfare laws in India. The study was approved by the Board of Studies and authorities of the Central Institute of Fisheries Education (Deemed University), Mumbai-61. The care and treatment of animals used in this study were in accordance with the guidelines of the CPCSEA [(Committee for the Purpose of Control and Supervision of Experiments on Animals), Ministry of Environment & Forests (Animal Welfare Division), Govt of India] on care and use of animals in scientific research. As the experimental fish i.e. L. rohita is a commercially important and not endangered fish, the provisions of the Govt of India’s Wildlife Protection Act of 1972 are not applicable for experiments on this fish.
Fish procurement, rearing and experimental conditions
Fingerlings of Labeo rohita were brought from Aarey fish farm (Goregaon, MS, India) and acclimated in a circular tank with a capacity of 2 tonnes for a period of 15 days and fed a diet of 30% crude protein. The experiment was conducted for a period 45 days. At the beginning of the study, L. rohita fingerlings were weighed individually and fifteen fishes (mean wt 2.8 g) were stocked in each of fifteen plastic tubs. Two hundred twenty five L. rohita fingerlings were randomly distributed into five experimental groups with three replicates each, following a completely randomised design. Round the clock aeration was provided to all the tubs and manual water exchange was done every other day. Water temperature, pH, dissolved oxygen, nitrite-N, nitrate-N and phosphate were analyzed every week using standard methods. Water quality parameters recorded during the study [37] were as follows: dissolved oxygen between 5.8–7.3 mg/L, and temperature range of 23.6–27.5°C (dissolved oxygen and temperature meter, Merck, Germany); pH 7.5–8.4 (digital pH meter, LABINDIA, Mumbai); negligible free carbon dioxide (titrimetric method); total hardness228–245 mg L− 1 (carbonate hardness test kit, Merck, Germany); ammonia 0.16–0.25 (at 635 nm by phenate method); nitrite 0.002–0.004 and nitrate 0.02–0.06 (543 nm wavelength).
Experimental diets and feeding
Five experimental groups were fed one of the following diets: a control (basal diet), or a basal diet supplemented with 0.4% chitosan NPs; 0.02% bare trypsin and 0.01% trypsin encapsulated with chitosan (0.4%) NPs; or 0.02% trypsin encapsulated with chitosan (0.4%) NPs. The composition of the basal diet by percentage was as follows: soy bean meal, 39.5; fish meal, 5; wheat flour, 12; mustard oilcake meal, 21; rice polish 14.5; oil mix, 6; carboxy methyl cellulose, 1; vitamin and mineral mixture 0.5; and chromic oxide, 0.5. The mean proximate (n = 3) composition (% DM basis): crude protein 28.49±0.29, ether extract 9.13±0.07, ash 8.55±0.11, total carbohydrate 53.81±0.40 and digestible energy 411±0.5 kcal. All the diets were isonitrogenous and isocaloric and were supplemented with butylated hydroxytoluene (0.02%).
All the ingredients were weighed properly as per requirements and mixed properly in a plastic container to form dough with the addition of the necessary quantity of water. When the dough was formed, the calculated quantity of the oils were added to it and mixed well. The dough was then transferred to an aluminum container, which was then placed in a pressure cooker for steaming/cooking. The steaming was done for 30 min. The pressure cooker was then removed from the flame and set aside to cool. The steamed dough was then taken out and was cooled further. To prevent their loss, vitamins and minerals along with the enzymes were added when the steamed dough was completely cooled. After incorporation of these elements, the dough was mixed properly and was pressed through a hand pelletizer to get uniform sized pellets, which were spread on a sheet of paper and were initially air dried. After that the feed was transferred to trays and was kept in a hot air oven overnight for complete drying at 35°C – 40°C. After drying, the pellets were packed in polythene bags and were sealed airtight and labeled according to the treatments. Feeding was carried out twice daily (9:00 a.m. and 6:00 p.m.) at 5% of body weight initially, and subsequently adjusted based on daily intake. Fish in each tub were weighed every 15 days to assess their growth.
Digestibility and sampling
Apparent digestibility coefficients (ADCs) were measured by the indicator method using 0.5% chromium oxide as a marker [38] during the last 20 days of study. Following the initial five-day acclimation to the diets; faecal materials were collected daily from the 30th to 45th day, for determining the digestibility of nutrients. Faecal material was collected manually by siphoning and straining through a fine-meshed net. Three hours after the first (9:00 a.m) feeding, uneaten feed, together with the faeces, was siphoned out and discarded. At 2:00 p.m., faecal material was collected and dried to constant weight at 60°C. Wet ashing of diets and faecal matters was carried out according to AOAC [39], and the chromium content of feed and faecal matters was determined with a flame ionization atomic absorption spectrophotometer (AAS 4129, Electronics Corporation of India, Hyderabad). The ADC of dry matter was calculated as:
While ADC of a nutrient was calculated as:
Chemical analysis of experimental diets and faecal samples
The proximate composition of all the diets was determined by the standard methods of the Association of Official Analytical Chemists [39]. In brief, moisture content was determined by drying at 105°C to a constant weight, nitrogen content was estimated by Kjeltec (2200 Kjeltec Auto distillation, Foss Tecator, Sweden), crude protein (CP) was estimated by multiplying nitrogen percentage by 6.25, ether extract (EE) was measured with a Soxtec system (1045 Soxtec extraction unit, Tecator, Sweden) using diethyl ether (boiling point 40–60°C) as a solvent, and ash content was determined by incinerating samples in a muffle furnace at 600°C for 6 h. Total carbohydrate (CHO) was calculated by difference:
Growth study
Fish in each tub were bulk-weighed every 15 days to assess their growth. Growth performance of fingerlings was evaluated in terms of specific growth rate (SGR), protein efficiency ratio (PER) and food conversion ratio (FCR) based on the following standard formulae.
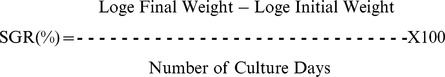 |
 |
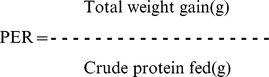 |
Blood collection
At the end of the feeding trial, a few fishes from each treatment group were anaesthetized with clove oil (50µl L−1) until they become motionless. Blood was collected from the caudal vein using a syringe, and then transferred immediately to the test tube containing a pinch of EDTA powder (as anticoagulant) and shaken gently in order to prevent haemolysis. For serum, blood was collected from the same treatment groups and transferred to the new test tube, allowed to clot for 2 hours, centrifuged (3000 x g for 5 min) and then kept at –80 °C until use.
Organo-Somatic Indices
After the clove oil anaesthetic treatment, the fish were removed from water when they were unresponsive to external stimuli. Liver and viscera were dissected out and immediately placed on ice. They were weighed separately with accuracy up to 1 mg for estimation of somatic indices. Hepato-somatic index and viscero-somatic index were determined as a proportion of weight of organ/tissue to the total body weight multiplied by 100.
Blood glucose and blood urea nitrogen (BUN)
Blood (500µl) was de-proteinized by mixing with 4.75 ml zinc sulphate followed by addition of 4.75 ml barium hydroxide. The solution was mixed vigorously and filtered and the supernatant was used for colorimetric estimation of glucose by the Nelson-Somogyi method at 540 nm against a blank. The BUN was determined colorimetrically by the diacetyl monoxime method at 525 nm.
Serum total protein, albumin, and globulin and A: G ratio
Total serum protein (by the biuret method) and albumin (by the bromocresol green binding method) were determined colorimetrically at 630 nm using the diagnostic kits (Qualigens Diagnostics, Mumbai, India). Globulin (G) was calculated by subtracting albumin (A) values from total serum protein and the A: G ratio was subsequently calculated. The detailed methodology followed for these estimations have been described elsewhere [5].
Digestive and metabolic enzyme analysis
The liver, muscle and intestine of the fish were removed carefully and were weighed as mentioned above. A 5% homogenate was prepared with chilled sucrose solution (0.25 M) in a glass tube using a Teflon-coated mechanical tissue homogenizer. The homogenate was centrifuged at 5000 rpm for 10 minutes at 4°C in a cooled centrifuge machine. The supernatant was stored at 4°C for further analysis.
Protease activity was determined as described by [40]. The reaction mixture consisted of 1% casein in 0.05 M Tris-PO4 buffer (pH 7.8) and was incubated for 5 min at 37°C. The tissue homogenate was then added. Ten minutes later the reaction was stopped by the addition of 10% TCA, followed by filtration of the samples. The reagent blank was made by the addition of tissue homogenate just before stopping the reaction without incubation. One unit of enzyme activity was defined as the amount of enzyme needed to release acid-soluble fragments equivalent to 0.001 A280 per minute at 37°C and pH 7.8.
Standard methods that were followed in our laboratory for biochemical analysis of lactate dehydrogenase (LDH) (E.C. 1.1.1.27), malate dehydrogenase (MDH) (E.C. 1.1.1.37), aspartate amino transferase (AST) (E.C.2.6.1.1) and alanine amino transferase (ALT) (E.C.2.6.1.2), and protein concentration have been recently published [41].
Preparation of tissue samples and histology of intestine
The histological study of intestinal tissue was conducted by sacrificing the fish from each experimental group at the end of the experiment. The fish were sacrificed and tissues were collected as mentioned above. Intestine of fish from different experimental groups were taken and fixed separately in 10% buffered formalin and were dehydrated in 90% alcohol for one hour, and three times in absolute alcohol for 45 minutes each. The samples were then cleared two more times in xylene for 30 minutes and embedded in paraffin for 45 minutes three times. The samples were then blocked and were allowed to cool. They were then cut on a rotator microtome at 7 μm and the mounted sections were dewaxed and dehydrated serially in alcohol. Then the slides were washed in tap water for 1 minute, stained in haematoxylene for 12 minutes, washed in tap water, dipped in 2% acid alcohol and washed in tap water, followed by Scott’s tap water substitute. The sections were dehydrated with 50%, 70% and 90% alcohol for 1 minute. Finally the stained sections were cleared in xylene for 5 minutes and mounted with DPX. Prepared slides were examined and photographed under a light microscope.
Statistical analysis
Mean values of all parameters were subjected to one-way ANOVA to study the treatment effect, and Duncan's multiple range tests were used to determine the significant differences between any two means, if they were significant. Comparisons were made at 5% probability level. All the data were analyzed using statistical package SPSS (Version 19).
Results
Synthesized nanoencapsulated trypsin has desired characteristics
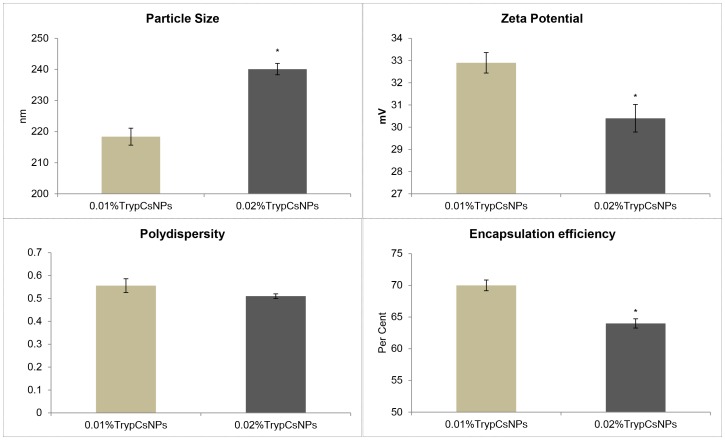
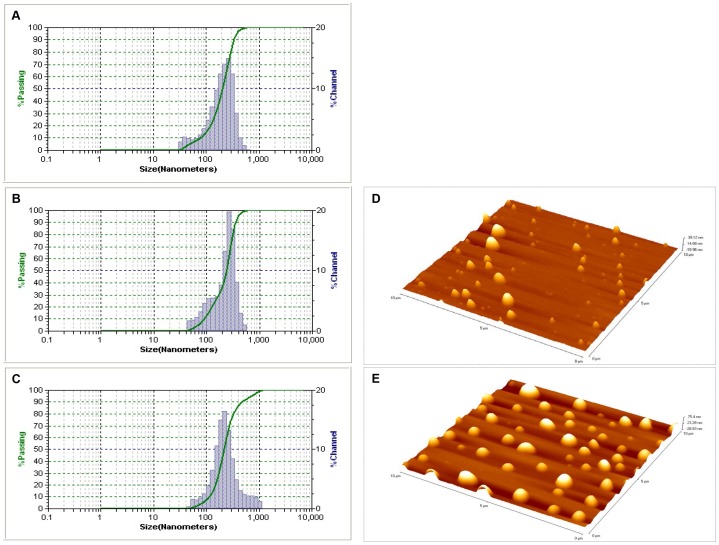
The 0.4% chitosan nanoparticles had a mean (n = 3) particle size of 147±2.25 nm, zeta potential of 37.1±0.54 mV and poly dispersity index of 0.667±0.01. Enzyme nanoparticles employing two different levels of trypsin (0.1% and 0.02% trypsin) using 0.4% chitosan as a vehicle were prepared. The physical and chemical characteristics of nanoencapsulated trypsin are given in Fig 1 and particle size distributions are given in Fig 2 (A to C). Comparison of 0.01% and 0.02% nanoencapsulated trypsin indicated that increasing the percentage of trypsin increased (P<0.05) the particle size (by 22 nm), and lowered the zeta potential (P<0.05) and encapsulation efficiency (P<0.05) (by 6%). Three-dimensional (3-D) imaging and analysis by atomic force microscope (AFM) of the two chitosan-nanoencapsulated trypsins revealed that the nanoparticles exhibited spherical shape with a homogenous distribution (Figure 2D and E).
Figure 1. Physico-chemical characteristics of 0.01% and 0.02% trypsin encapsulated in 0.4% chitosan nanoparticles (TrypCsNPs).
Means bearing different superscript letters differ significantly (*P<0.05 by student t test).Values represent means ± standard error of triplicate observations.
Figure 2. Particle size distribution.
A] 0.4% chitosan nanoparticles B] 0.01% trypsin encapsulated in 0.4% chitosan nanoparticles, C] 0.02% trypsin encapsulated in 0.4% chitosan nanoparticles and three-dimensional images D] 0.01% trypsin encapsulated in 0.4% chitosan nanoparticles, E] 0.02% trypsin encapsulated in 0.4% chitosan nanoparticles.
Nanoencapsulated trypsin enhanced fish performance over its bare form
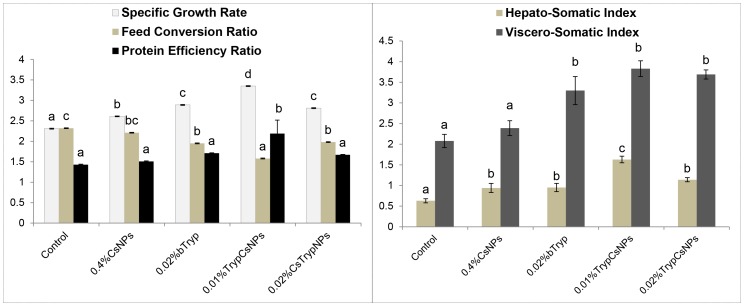
The data on growth and performance (Fig 3) indicated that feeding bare trypsin increased specific growth rate (SGR) and improved feed conversion ratio (FCR). Nanoencapsulation of trypsin at low doses further potentiated its nutritional benefits with regard to productive performance. Even at half the dose level of bare trypsin, in nanoencapsulated form, the productive efficiency in terms of SGR, FCR and PER was significantly (P<0.05) higher than with bare trypsin. Moreover, nanoencapsulation of trypsin at the same dose as bare trypsin (i.e. 0.02%) offered no additional production benefits as these attributes were similar in bare and nanoencapsulated 0.02% trypsin. Hepatosomatic index (HSI) and viscero-somatic index (VSI) data (Fig 3) indicated that chitosan NPs, bare and nanoencapsulated trypsin significantly increased HSI. The 0.01% nanoencapsulated trypsin had significantly highest HSI among all the groups. Trypsin, in either form, increased VSI significantly (P<0.05).
Figure 3. Productive performance and hepatosomatic- and viscero-somatic index of fish on diets containing 0.4% chitosan nanoparticles (CsNPs), 0.02% bare trypsin (bTryp), and 0.01% or 0.02% trypsin encapsulated in 0.4% chitosan nanoparticles (TrypCsNPs).
Means bearing different superscript letters differ significantly (P<0.05). Values represent means ± standard error of triplicate observations.
Nanoencapsulated trypsin enhanced nutrient digestibility in fish over its bare form
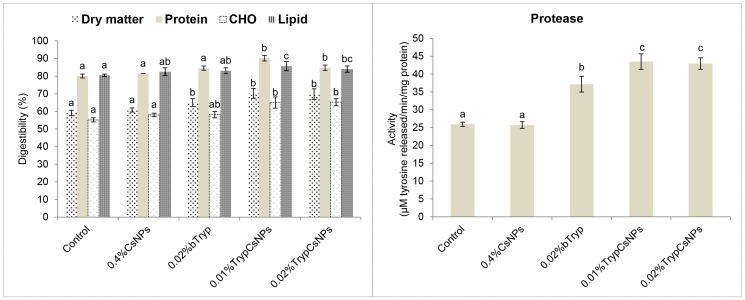
Feeding chitosan NPs had no effect on digestibility of dry matter and other nutrients. Feeding trypsin in either form increased the digestibility of dry matter (Figure 4). The apparent digestibility of protein was significantly increased with nanoencapsulated trypsin supplementation. While digestibility of carbohydrate in the nanoencapsulated trypsin group was significantly higher than the control and chitosan NPs-fed groups, it was not different from bare trypsin-fed group. The digestibility of lipid in the nanoencapsulated 0.01% trypsin group was significantly higher than in control, chitosan NPs and bare trypsin groups, and that of nanoencapsulated 0.02% trypsin was higher than control but similar to the other groups. Protein digestibility corresponded with intestinal protease activity (Fig 4). Feeding chitosan NPs had no effect on protease activity. However, feeding the nanoencapsulated form of trypsin at both half the dose rate and at the same dose rate of bare trypsin resulted in a significant increase (P<0.05) of intestinal protease activity.
Figure 4. Digestibility of diets and intestinal protease activity in fish fed these diets containing 0.4% chitosan nanoparticles (CsNPs), 0.02% bare trypsin (bTryp), and 0.01% or 0.02% trypsin encapsulated in 0.4% chitosan nanoparticles (TrypCsNPs).
Means bearing different superscript letters differ significantly (P<0.05). Values represent means ± standard error of triplicate observations.
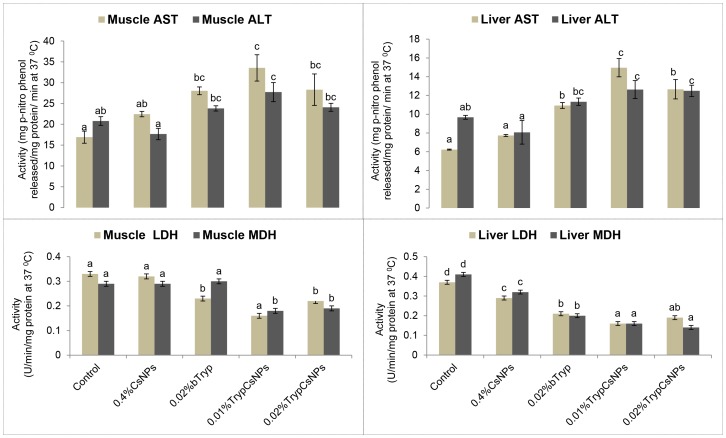
Enhanced transaminases and reduced dehydrogenases with nanoencapsulated trypsin over its bare form
Activity of the liver and muscle transaminases and the dehydrogenases of L. rohita fingerlings in the different experimental groups are presented in Fig 5. Fish fed nanoencapsulated trypsin at both 0.01 and 0.02% levels had higher liver ALT and the 0.01% dose showed higher AST activity than bare trypsin. Muscle ALT, but not muscle AST, in fish fed nanoencapsulated trypsin at both the 0.01 and 0.02% level was higher than in fish fed bare trypsin. Compared to control, LDH and MDH activity in the liver was reduced in groups fed chitosan or trypsin in either form. While trypsin in either form reduced muscle LDH, only trypsin in nanoencapsulated form reduced both muscle LDH and MDH.
Figure 5. Activities of aminotransferases and dehydrogenases in fish fed diets containing 0.4% chitosan nanoparticles (CsNPs), 0.02% bare trypsin (bTryp), and 0.01% or 0.02% trypsin encapsulated in 0.4% chitosan nanoparticles (TrypCsNPs).
Abbreviations: Aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH) and malate dehydrogenase (MDH). Means bearing different superscript letters differ significantly (P<0.05). Values represent means ± standard error of six observations.
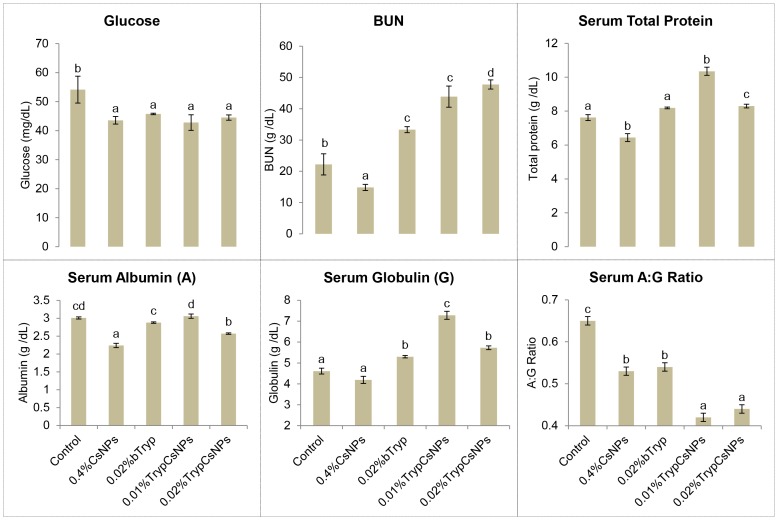
Enhanced plasma components and serum protein profile with nanoencapsulated trypsin over its bare form
The plasma components and serum protein levels of the different experimental groups are given in Fig 6. Compared to control, blood glucose was decreased in all of the treatment groups. While BUN was decreased in chitosan NPs-fed group, BUN was increased in both trypsin-fed groups. Significantly higher total serum protein level was found in chitosan nanoencapsulated trypsin groups. Trypsin in bare as well as nanoencapsulated form increased serum globulin. Chitosan NPs and trypsin in either form decreased serum A: G ratio.
Figure 6. Plasma components and serum protein profile in fish fed diets containing 0.4% chitosan nanoparticles (CsNPs), 0.02% bare trypsin (bTryp), and 0.01% or 0.02% trypsin encapsulated in 0.4% chitosan nanoparticles (TrypCsNPs).
Means bearing different superscript letters differ significantly (P<0.05). Values represent means ± standard error of six observations.
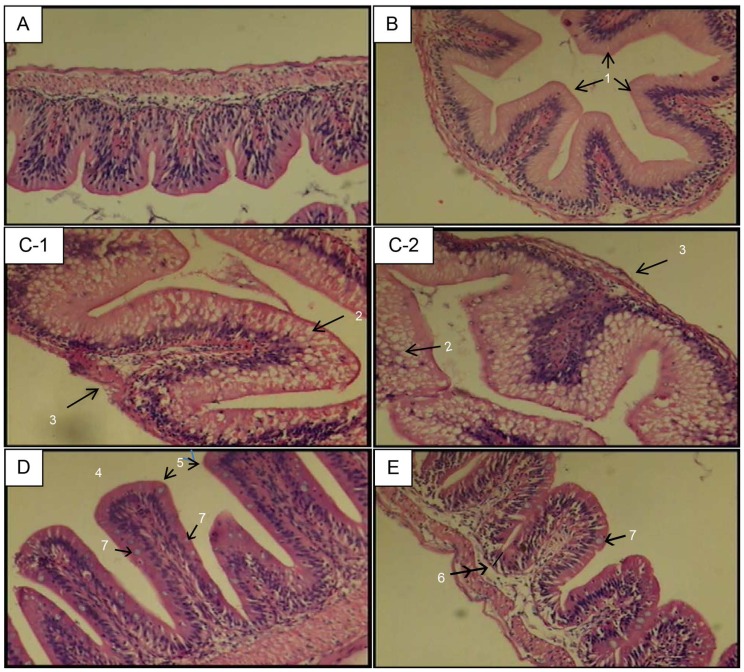
Histological study of intestine indicates safety of nanoencapsulated trypsin over its bare form
Intestine histology of Labeo rohita fingerlings fed the control diet showed intact architecture (Fig 7A), and histology of 0.4% chitosan NPs-fed fish did not show much difference from control (Fig 7B). Intestinal tissues of fish fed with the 0.02% bare trypsin supplemented diet showed broadened villi, marked foamy cells with lipid vacuoles, atrophied submucosal layer and muscularis (Fig 7C-1 and 7C-2). In general, villi were healthier in appearance and had improved morphological features after being fed chitosan nanoencapsulated trypsin compared to bare trypsin. The villi were longer in fish fed with 0.01% chitosan nanoencapsulated trypsin (Fig 7D) than 0.02% chitosan nanoencapsulated trypsin, which slightly resembled the control group (Fig 7E).
Figure 7. Intestine histology of fish fed control, 0.4% chitosan nanoparticles, 0.02% bare trypsin and and 0.01% or 0.02% nanoencapsulated trypsin containing diets (H&E, 40X).
In control group (A), intestinal mucosa is lined by regularly-packed villi with continuous basement membrane. In 0.4% chitosan nanoparticles fed fish (B), mildly swollen apical surface of villi (1) are noticed. In 0.02% bare trypsin (C-1 and C2), broadened villi, marked foamy cells with lipid vacuoles (2), atrophied submucosal layer and muscularis (3) were evident. In 0.01% trypsin encapsulated in 0.4% chitosan nanoparticles (D), longer villi (4) with healthy apical surface (5) and improved morphological features besides continuous basement membrane are evident. Crypt depth was less in treated groups (6). Distinct mucin producing goblet cells (7) distributed along the villi in nanoencapsulated trypsin groups indicates better gastro-intestinal health. Villi in 0.02% trypsin encapsulated in 0.4% chitosan nanoparticles (E) resembled those in control group (A). Effectiveness and safety of dietary nanoencapsulated trypsin at half the dose rate (0.01%) (D) of bare trypsin (0.02%) (C-1 and C-2) is evident from healthier villi with more height and absorptive surface.
Discussion
Nanoparticles have been studied as carriers for oral drug delivery to improve drug stability and bioavailability across the GI mucosa [22]. However, there are no reports on controlled release systems for proteolytic enzymes with potential use in the biomedical and animal feed industries. The present study clearly shows benefits for productive performance and the healthiness of intestinal villi from chitosan-encapsulated trypsin more so than from bare trypsin. Such a positive impact on enzyme stability and its bioavailability was expected from nanoencapsulation prepared by ionic gelation method. Ionotropic gelation between chitosan and tripolyphosphate leads to the formation of nano-sized colloidal systems. The colloidal particles have electrostatic interactions with charged molecules under aqueous physiological conditions [19] and incorporated protein, in this case trypsin, is protected against degradation in in vivo applications [34], [42].
Nanoencapsulated trypsin had satisfactory characteristics. Chun et al. [43] reported that chitosan nanoparticles sized between 35–190 nm and having zeta potential 35–42 mv are promising carriers for delivery of proteins. The zeta potential figures of nanoparticles were indicative of good particle stability. Increasing trypsin concentration from 0.01% to 0.02% decreased the encapsulation efficiency of chitosan nanoparticles, indicating that 0.4% chitosan NPs can hold more than 0.01% but less than 0.02% trypsin.
Increased specific growth rate, improved feed conversion and protein efficiency ratio in the experimental animals suggest that even after processing during feed manufacture the chitosan nanoencapsulated trypsin remained stable and active within the gut of the fish. The observations corroborate with Gole et al. [44], who reported that pepsin immobilized on a gold nanoparticle surface was more stable when compared with the free enzyme. Increased growth was shown in shrimp Penaeus japonicas [45] which were supplemented with amylase in a freeze-dried nylon-protein microcapsule in order to circumvent potential inactivation.
The apparent nutrient digestibility values corresponded with the growth performance of fish fingerlings in this study. While studies with enzyme mixtures are abundant, and though limited studies with mono-protease supplementation [46] also exist, there are no in vivo studies of mono-component proteolytic enzymes, including encapsulated enzymes, depicting the overall health of the intestine through histological investigation. Gut enzyme profile is the indicator of nutrient digestibility and utilization. The significantly higher activity of protease in fish fed nanoencapsulated trypsin (0.01% and 0.02%) clearly indicates a correlation between formulated diet intake and digestive enzyme activity, resulting in diet-related growth differences. Similar diet-related differences in growth and digestive enzyme activity have been reported with rainbow trout [47]. Common carp fed a diet supplemented with bovine trypsin showed increased proteolytic activity, and the increase in the activity was correlated with the bovine trypsin proportion [46]. In studies by Dalsgaard et al. [48], supplementing with glucanase or protease in a salmonid diet containing soybean meal significantly improved the apparent digestibility of all dietary nutrients. In another study [49], a commercial protease supplementation of a diet containing a mixture of rapeseed and pea was found to increase protein, lipid, energy and dry matter digestibility. Thus, the influence on digestibility of protein, fat and lipids in response to dietary proteolytic enzyme supplementation appears to depend on composition of the diet. In chickens, mono-component protease supplementation improved feed to gain as well as the digestibility of fat and CP, regardless of dietary protein or energy concentration [50]. Unaffected protease activity in the chitosan-fed group agrees with Han et al. [51]. However, the increased specific growth rate and feed conversion ratio in fish may be result of its antibacterial activity, and the findings corroborate with the reported results [52] in Tilapia.
The chitosan NPs and nanoencapsulated trypsin significantly increased hepatosomatic index (HSI) compared with control. The 0.01% nanoencapsulated trypsin had the significantly highest HSI. Trypsin, in either form, significantly (P<0.05) increased viscero-somatic index (VSI). The HSI and VSI was decreased in fish supplemented with a 0.1% and 0.15% commercial enzyme complex (neutral protease, b-glucanase and xylanase) during a 12-week study [53]. It is to be noted that the enzyme concentrations used in the feed in this study were 5 to 15 times lower than used by Lin et al. [53].
The major source of energy in teleosts is amino acids [54]. Provision of more gluconeogenic substrates through increased ALT and AST [55] indicates increased demand for energy, which occurs under conditions of increased growth or stress. In the present study, the activities of amino acid metabolism enzymes AST and ALT in muscle and especially liver were increased in chitosan NPs, trypsin- and nanoencapsulated trypsin-fed fish, which indicated increased utilization of dietary protein corresponding with an increase in growth (protein synthesis) in respective groups. In a 60-day feeding trial investigating the effects of dietary rapeseed meal levels on feed intake, growth, digestion and protein metabolism in juvenile cobia, Luo et al. [56] has shown significantly decreased activity of liver ALT and AST with decreased growth rate. Aminotransferases catabolize amino acids and transfer amino groups to alpha-keto acids. This can be attributed to high protein digestibility in these groups and controlled breakdown and release of amino acids in encapsulated trypsin-fed fish, creating a surplus pool of the available essential amino acids, and so the keto acids may be oxidised, thereby increasing the activities of ALT and AST [56]–[57]. There is a negative correlation between growth rates and LDH and MDH activity levels and a positive correlation between AST and growth [58]–[59]. This relationship was noticed in this study not only for transaminases but also for dehydrogenases (LDH and MDH), especially in nanoencapsulated trypsin-fed groups where growth was significantly more pronounced than in others.
Blood glucose was decreased in all the treatments. Change in the glucose levels is a secondary stress response providing required energy to cope with stressful conditions [60]. Hyperglycemia is due to stimulation of glycolysis and gluconeogenesis from protein and lipid sources [60]. Changes in serum blood urea nitrogen (BUN) concentration are indicative of the whole body status of amino acid metabolism and utilization in animals [61]. While BUN was decreased in the chitosan NPs-fed group, BUN was increased in either form of trypsin-fed groups. In early weaned piglets BUN was found to be reduced (P < 0.05) in response to dietary supplementation of chitosan and galactomannan-oligosaccharides during a two week study [62]. Similarly, Jo et al. [63] reported increased (P<0.05) BUN in growing pigs fed diets with protease-containing exogenous enzymes. Increased serum BUN suggests a potential breakdown of protein in animals due to trypsin supplementation after satisfying nutritional requirements.
An increase in the value of serum total protein, albumin and globulin levels is an indication of better immunity in animals [64]. Significantly higher total serum protein levels were found in chitosan nanoencapsulated trypsin groups. Trypsin feeding in its bare form as well as in the nanoencapsulated forms increased serum globulin. Serum A: G ratio indicated that chitosan NPs and trypsin in bare form and encapsulated forms improved serum A: G ratio and thus immunity. Dietary chitosan has been reported [65] to enhance the immunological properties of C. carpio. Similarly, serum total protein concentration was increased (P<0.05) in response to dietary supplementation of chitosan and galactomannan-oligosaccharids in early weaned piglets during a two week study [62]. In another study [66], 0.25 to 0.5% dietary supplementation of chitosan enhanced hematological profiles (lymphocyte count and total WBC) and 0.25% chitosan reduced (P<0.05) experimental mortality from environment-induced stresses (low oxygen, salinity and temperature) in rainbow trout. Abd El-Latif et al. [67] reported serum total protein and globulin in chickens fed a commercial multienzyme product.
Good intestinal mucosal morphology is the foundation of nutrient absorption and animal growth. Histologically, the intestine was not significantly altered by supplementation with 0.4% chitosan except for slightly swollen villi. In another study, statistically nonsignificant change from smooth in control to rough apical surface of intestinal villi and increased growth of broiler chickens fed 4 g/kg dietary chitosan was reported [68]. The 0.02% bare trypsin-fed group showed dilated swollen mucosal epithelium (villous broadening), marked foamy cells with lipid vacuoles, reduced crypt depth, and atrophied submucosal layer and muscularis. Compared to 0.02% bare trypsin fed group, in the nanoencapsulated trypsin group villi were longer in height with more surface area, showed more architectural symmetry and had a healthy appearance with distinct goblet cells distributed along the villi indicating better gastro-intestinal health. These cells secrete mucin, which destroys pathogens [69], into the mucus layer and also help maintain a suitable thickness of this layer in the intestine despite normal slough off. A greater number of goblet cells per villus have been reported to correlate with improved feed conversion ratio, growth performance and intestinal proteolytic enzyme activity in rainbow trout fed different nutritional supplements (yeast, S. cerevisiae and seaweeds) [70]–[71]. The villi were longer with more improved morphological features in the 0.01% than in the 0.02% nanoencapsulated trypsin group, which slightly resembled control. Crypt depth was less in treated groups. Increased villus length and reduced crypt depth were observed in the germfree animals [72]. The crypt epithelium primarily functions in epithelial cell renewal/regeneration [73]. This improvement of small intestine morphology corresponded with increased nutrient absorption in the gut and growth performance of fish. Chitosan nanoencapsulated trypsin was safe to feed, as indicated by protected normal mucosal epithelium. Increasing concentration from 0.01% to 0.02 had no effect on these parameters of intestinal morphology. These results are consistent with that of Iji et al. [74] who showed that addition of a high dose of enzyme to wheat-based diets had no effect on villus height, crypt depth or villus surface area of intestine of broilers. Reduction of villus height and surface area can reduce the absorption of nutrients. Therefore, the improved performance observed in fish fed with 0.01% nanoencapsulated trypsin may also be correlated with the morphology (increased absorptiveness) of small intestine [75] along with the release of simple sugars and protein.
The preceding discussion of significant positive effects on the performance of animals along with the safety of chitosan nanoencapsulated functional trypsin in the present experiment supports the reasons why chitosan is the most valued and preferred vehicle for preparing compounds intended for controlled release in the intestine through oral delivery in so many investigations. This delivery system has been successfully employed for the controlled oral delivery of proteins like insulin [76], drugs like the antitumor compound Gemcitabine [77] and other compounds of nutritional significance such as vitamin C [25] and yeast RNA/nucleotide [our unpublished data] with more pronounced desired effects. Recently, we reported pronounced in vivo biological effects due to the controlled release of a hormonal protein preparation encapsulated in chitosan nanoparticles compared to bare hormone [78]. Controlled delivery using chitosan nanoparticles also allows the preparation of organic solvent-free mucoadhesive particles [79].
In conclusion, enhanced performance and intestinal histological safety in the studied fish indicates that trypsin nanoencapsulated with chitosan mimics zymogen-like proteolytic activity, and the use of 0.01% nanoencapsulated trypsin over bare trypsin can be favored as a dietary supplement in animals and humans. Controlled release of enzyme in the study also indicates that chitosan nanocarriers are good vehicles for delivery of proteolytic enzymes or proteins where slow release is desired.
Acknowledgments
The authors are grateful to the Director, Central Institute of Fisheries Education, Mumbai, for providing necessary facilities for carrying out this experiment.
Funding Statement
The authors have no support or funding to report.
References
- 1. Patel RS, Johlin FC Jr, Murray JA (1999) Celiac disease and recurrent pancreatitis. Gastrointest Endosc 50: 823–7. [DOI] [PubMed] [Google Scholar]
- 2. Gullo L (1993) Indication for pancreatic enzyme treatment in non-pancreatic digestive diseases. Digestion 54 (suppl 2)43–47. [DOI] [PubMed] [Google Scholar]
- 3. Suarez F, Levitt MD, Adshead J, Barkin JS (1999) Pancreatic supplements reduce symptomatic response of healthy subjects to a high fat meal. Dig Dis Sci 44: 1317–21. [DOI] [PubMed] [Google Scholar]
- 4.FAO (2009) The State of World Fisheries and Aquaculture 2008. FAO, Rome.
- 5. Kumar S, Sahu NP, Pal AK, Choudhury D, Yengkokpam S, et al. (2005) Effect of dietary carbohydrate on hematology, respiratory burst activity, histological changes in L. rohita juveniles. Fish Shellfish Immunol 19: 331–344. [DOI] [PubMed] [Google Scholar]
- 6. Leeson S, Yersin A, Volker L (1993) Nutritive value of the corn crop. J Appl Poultry Res 2: 208–313. [Google Scholar]
- 7. Douglas MW, Parsons CM, Bedford MR (2000) Effect of various soybean meal sources and Avizyme on chick growth performance and ileal digestible energy. J Appl Poultry Res 9: 74–80. [Google Scholar]
- 8.Anon (1988) Enzymes in pig diets. Feed Int. March. 11 pp.
- 9. Adeola O, Cowieson AJ (2011) Board-Invited Review: opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J Anim Sci 89: 3189–3218. [DOI] [PubMed] [Google Scholar]
- 10. Carter CG, Houlihan DF, Buchanan B, McCarthy IA (1994) Growth and feed utilization efficiencies of seawater Atlantic salmon Salmo salar L. fed a diet containing supplementary enzymes. Aquacult Fish Manage 25: 37–49. [Google Scholar]
- 11. Dabrowska H, Grudniewski H, Dabrowski K (1979) Artificial diets for common carp: effect of the addition of enzyme extracts. Prog Fish Cult 41(4): 196–200. [Google Scholar]
- 12. Stevens JC, Maguiness KM, Hollingsworth J Heilman DK, Chong SK (1998) Pancreatic enzyme supplementation in cystic fibrosis patients before and after fibrosing colonopathy. J Pediatr Gastroenterol Nutr 26: 80–4. [DOI] [PubMed] [Google Scholar]
- 13. Oades PJ, Bush A, Ong PS, Brereton RJ (1994) High-strength pancreatic enzyme supplements and large-bowel stricture in cystic fibrosis. Lancet 343: 109. [PubMed] [Google Scholar]
- 14. Campbell CA, Forrest J, Muscgrove C (1994) High-strength pancreatic enzyme supplements and large-bowel stricture in cystic fibrosis. Lancet 343: 109–10. [PubMed] [Google Scholar]
- 15. Milla CE, Wielinski CL, Warwick WJ (1994) High-strength pancreatic enzymes. Lancet 343: 599. [PubMed] [Google Scholar]
- 16. Jones R, Franklin K, Spicer R, Berry J (1995) Colonic strictures in children with cystic fibrosis on low-strength pancreatic enzymes. Lancet 346: 499–500. [PubMed] [Google Scholar]
- 17. Powell CJ (1999) Pancreatic enzymes and fibrosing colonopathy. Lancet 354: 251. [DOI] [PubMed] [Google Scholar]
- 18. Dodane V, Vilivalam VD (1998) Pharmaceutical applications of chitosan. Pharm Sci Technol Today 1(6): 246–253. [Google Scholar]
- 19. Agnihotri SA, Mallikarjuna NN, Aminabhavi TM (2004) Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J Control Release 100: 5–28. [DOI] [PubMed] [Google Scholar]
- 20. Nakorn PN (2008) Chitin nanowhisker and chitosan nanoparticles in protein immobilization for biosensor applications. J Metals Mater Minerals 18(2): 73–77. [Google Scholar]
- 21. LaVan DA, McGuire T, Langer R (2003) Small scale systems for in vivo drug delivery. Nature Biotechnol 21: 1184–91. [DOI] [PubMed] [Google Scholar]
- 22. Jung T, Kamm W, Breitenbach A, Kaiserling E, Xiao JX, et al. (2000) Biodegradable nanoparticles for oral delivery of peptides: is there a role for polymers to affect mucosal uptake? Eur J Pharm Biopharm 50: 147–160. [DOI] [PubMed] [Google Scholar]
- 23. Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM (1999) Polymeric systems for controlled drug release. Chem Rev 99 3181–98. [DOI] [PubMed] [Google Scholar]
- 24. Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE (2001) Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release 70: 1–20. [DOI] [PubMed] [Google Scholar]
- 25.Alishahi A, Mirvaghefi A, Tehrani MR, Farahmand H, Koshio S, et al. (2011) Chitosan nanoparticle to carry vitamin C through the gastrointestinal tract and induce the non-specific immunity system of rainbow trout (Oncorhynchus mykiss). Carbohyd Polym 86: : 142– 146. [Google Scholar]
- 26.Goldsmith JR, Jobin C (2012) Think small: zebrafish as a model system of human pathology. J Biomed Biotechnol doi:10.1155/2012/817341. [DOI] [PMC free article] [PubMed]
- 27. Fleming A, Jankowski J, Goldsmith P (2010) In vivo analysis of gut function and disease changes in a zebrafish larvae model of inflammatory bowel disease: a feasibility study. Inflamm Bowel Dis 16: 1162–1172. [DOI] [PubMed] [Google Scholar]
- 28. Oehlers SH, Flores MV, Okuda KS, Hall CJ, Crosier KE, et al. (2011) A chemical enterocolitis model in zebrafish larvae that is dependent on microbiota and responsive to pharmacological agents. Dev Dyn 240: 288–298. [DOI] [PubMed] [Google Scholar]
- 29. Brugman S, Liu KY, Lindenbergh-Kortleve D, Samsom JN, Furuta GT, et al. (2009) Oxazolone-induced enterocolitis in zebrafish depends on the composition of the intestinal microbiota. Gastroenterology 137(5): 1757–67. [DOI] [PubMed] [Google Scholar]
- 30. Ng AN, de Jong-Curtain TA, Mawdsley DJ, White SJ, Shin J, et al. (2005) Formation of the digestive system in zebrafish: III. Intestinal epithelium morphogenesis. Dev Biol 286: 114–135. [DOI] [PubMed] [Google Scholar]
- 31. Wallace KN, Akhter S, Smith EM, Lorent K, Pack M (2005) Intestinal growth and differentiation in zebrafish. Mech Dev 122: 157–173. [DOI] [PubMed] [Google Scholar]
- 32.William H, Detrich I, Westerfield M, Zon L (2011) The zebrafish: Cellular and Developmental Biology, New York: Academic Press. [DOI] [PubMed]
- 33. Panyam J, Labhasetwar V (2003) Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev 55: 329–347. [DOI] [PubMed] [Google Scholar]
- 34. Gan Q, Wang T, Cochrane C, McCarron P (2005) Modulation of surface charge, particle size and morphological properties of chitosan–TPP nanoparticles intended for gene delivery. Colloids Surfs B: Biointerfaces 44: 65–73. [DOI] [PubMed] [Google Scholar]
- 35. Calvo P, Remunan-Lopez C, Vila-Jato JL, Alonso MJ (1997) Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J Appl Polym Sci 63: 125–132. [Google Scholar]
- 36. Xu Y, Du Y (2003) Effect of molecular structure of chitosan on protein delivery properties of chitosan nanoparticles. Int J Pharm 250: 215–226. [DOI] [PubMed] [Google Scholar]
- 37.APHA (1998) Standard methods for the estimation of water and waste water, 20th edn., Clesceri, LS, Greenberg AE, Eaton AD, Editors. American Public Health Association, American Water Works Association, Water Environment Federation, Washington DC.
- 38. Mohapatra S, Chakraborty T, Prusty AK (2012) Use of different microbial probiotics in the diet of rohu, Labeo rohita fingerlings: effects on growth, nutrient digestibility and retention, digestive enzyme activities and intestinal microflora. Aquacult Nutr 18: 1–11. [Google Scholar]
- 39.AOAC (1995) Official Methods of Analysis of AOAC International, Vol. 1, 16th edn, Cunniff PA, Editors. Arlington: AOAC International.
- 40.Drapean G (1974) Protease from Staphylococcus aureus. In: Methods in Enzymology Vol 45 B, Lorand L, Editor. NewYork: Academic Press, 469p.
- 41. Verma AK, Pal AK, Manush SM, Das T, Dalvi RS, et al. (2007) Persistent sub-lethal chlorine exposure elicits the temperature induced stress responses in Cyprinus carpio early fingerlings. Pestic Biochem Physiol 87: 229–237. [Google Scholar]
- 42. Atkas Y, Andrieux K, Alonso MJ, Calvo P, Gursoy RN, et al. (2005) Preparation and in vitro evaluation of chitosan nanoparticles containing a caspase inhibitor. Int J Pharm 298: 378–383. [DOI] [PubMed] [Google Scholar]
- 43.Chun W, Xiong F, Lian SY (2007) Water-soluble chitosan nanoparticles as a novel carrier system for protein delivery. Chin Sci Bull 52: : 883– 889. [Google Scholar]
- 44. Gole A, Dash C, Soman C, Sainkar SR, Rao M, et al. (2001) On the preparation, characterization, and enzymatic activity of fungal protease-gold colloid bioconjugates. Bioconj Chem 12 (5): 684–690. [DOI] [PubMed] [Google Scholar]
- 45. Maugle PD, Deshimaru O, Katayama T, Simpson K (1983) The use of amylase supplements in shrimp diets. J World Maric Soc 14: 25–37. [Google Scholar]
- 46. Dabrowski K, Glogowski J (1977) Studies on the role of exogenous proteolytic enzymes in digestion processes in fish. Hydrobiologia 54: 129–134. [Google Scholar]
- 47. Kawai S, Ikeda S (1973) Studies on digestive enzymes of fishes. III. Development of digestive enzymes of rainbow trout after hatching and effect of dietary change on the activities of digestive enzymes in the juvenile stage. Bull Jpn Soc Sci Fish 39: 817–823. [Google Scholar]
- 48. Dalsgaard J, Verlhac V, Hjermitslev NH, Ekmann KS, Fischer M, et al. (2012) Effects of exogenous enzymes on apparent nutrient digestibility in rainbow trout (Oncorhynchus mykiss) fed diets with high inclusion of plant-based protein. Animal Feed Sci Technol 171 181–191. [Google Scholar]
- 49.Drew MD, Racz VJ, Gauthier R, Thiessen DL (2005) Effect of adding protease to coextruded flax:pea or canola:pea products on nutrient digestibility and growth performance of rainbow trout (Oncorhynchus mykiss). Anim Feed Sci Technol 119: , 117–128. [Google Scholar]
- 50. Freitas DM, Vieira SL, Angel CR, Favero A, Maiorka A (2011) Performance and nutrient utilization of broilers fed diets supplemented with a novel mono-component protease. J Appl Poult Res 20: 322–334. [Google Scholar]
- 51. Han XY, Du WL, Huang QC, Xu ZR, Wang YZ (2012) Changes in small intestinal morphology and digestive enzyme activity with oral administration of copper-loaded chitosan nanoparticles in rats. Biol Trace Elem Res 145: 355–360. [DOI] [PubMed] [Google Scholar]
- 52. Hong KN, Park NY, Lee SH, Meyers SP (2002) Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int J Food Microbiol 4: 65–72. [DOI] [PubMed] [Google Scholar]
- 53.Lin S, Mai K, Tan B (2007) Effects of exogenous enzyme supplementation in diets on growth and feed utilization in tilapia, Oreochromis niloticus x O. aureus Aquacult Res 38: : 1645– 1653. [Google Scholar]
- 54.Bilinski E (1974) Biochemical aspects of fish swimming. In: Biochemical perspectives in marine biology, Vol 1, Malines BC, Sergent JR, Editors. New York: Academic Press, pp. 239–288.
- 55.Knox WE, Greengard O (1965) An introduction to enzyme physiology. Advan. Enzyme Regul. Vol. 3, Weber G, Editor. New York/London: Pergamon Press, pp. 247–248.
- 56. Luo Y, Ai QH, Mai KS, Zhang WB, Xu W, et al. (2012) Effects of dietary rapeseed meal on growth performance, digestion and protein metabolism in relation to gene expression of juvenile cobia (Rachycentron canadum). Aquaculture 368: 109–116. [Google Scholar]
- 57. Cheng ZY, Ai QH, Mai KS, Xu W, Ma HM, et al. (2010) Effects of dietary canola meal on growth performance, digestion and metabolism of Japanese seabass, Lateolabrax japonicus . Aquaculture 305: 102–108. [Google Scholar]
- 58. Pelletier D, Dutil JD, Blier P, Guderley H (1994) Relation between growth-rate and metabolic organization of white muscle, liver and digestive-tract in cod, Gadus morhua . J Comp Physiol B 164: 179–190. [Google Scholar]
- 59. Koedijk RM, Le François NR, Blier PU, Foss A, Folkvord A, et al. (2010) Ontogenetic effects of diet during early development on growth performance, myosin mRNA expression and metabolic enzyme activity in Atlantic cod juveniles reared at different salinities. . Comp Biochem Physiol A Mol Integr Physiol. 156(1): 102–9. [DOI] [PubMed] [Google Scholar]
- 60. Wendelaar Bonga SE (1997) The stress response in fish. Physiol Rev 77: 591–625. [DOI] [PubMed] [Google Scholar]
- 61. Eggum RO (1970) Blood urea measurement as a technique for assessing protein quality. Br J Nutr 24: 983–9. [DOI] [PubMed] [Google Scholar]
- 62. Tang ZR, Yin YL, Nyachoti CM, Huang RL, Li TJ, et al. (2005) Effect of dietary supplementation of chitosan and galacto-mannan-oligosaccharide on serum parameters and the insulin-like growth factor-I mRNA expression in early-weaned piglets. . Domest Anim Endocrinol. 28(4): 430–41. [DOI] [PubMed] [Google Scholar]
- 63. Jo JK, Ingale SL, Kim JS, Kim YW, Kim KH, et al. (2012) Effects of exogenous enzyme supplementation to corn- and soybean meal-based or complex diets on growth performance, nutrient digestibility, and blood metabolites in growing pigs. . J Anim Sci. 90(9): 3041–8. [DOI] [PubMed] [Google Scholar]
- 64. Wiegertjes GF, Stet RJM, Parmentier HK, Muiswinkel WB (1996) Immunogenetics of disease resistance in fish: a comparative approach. Dev Com Immunol 20: 365–381. [DOI] [PubMed] [Google Scholar]
- 65. Gopalakannan A, Arul V (2006) Immunomodulatory effects of dietary intake of chitin, chitosan and levamisole on the immune system of Cyprinus carpio and control of Aeromonas hydrophila infection in ponds. Aquaculture 255: 179–187. [Google Scholar]
- 66.Meshkini S, Tafy AA, Tukmechi A, Farhang-Pajuh F (2012) Effects of chitosan on hematological parameters and stress resistance in rainbow trout (Oncorhynchus mykiss). Veterinary Research Forum 3 (1): 49 – 54. [PMC free article] [PubMed] [Google Scholar]
- 67. Abd El-Latif SA, Mohammed KA, Ghali KA, Abd El-Latif MA (2008) Effect of using commercial enzymes on performance and some metabolic functions of rabbits fed grade levels of crude fiber. Egypt Poultry Sci J 28(4): 1003–1022. [Google Scholar]
- 68. Khambualai O, Yamauchi K, Tangtaweewipat S, Cheva-Isarakul B (2009) Growth performance and intestinal histology in broiler chickens fed with dietary chitosan. Brit Poultry Sci 50(5): 592–7. [DOI] [PubMed] [Google Scholar]
- 69.Vechklang K, Boonanuntanasarn S, Ponchunchoovong S, Pirarat N, Wanapu C (2011) The potential for rice wine residual as an alternative protein source in a practical diet for Nile tilapia (Oreochromis niloticus) at the juvenile stage. Aquacult. Nutr., 17, : 685–694. [Google Scholar]
- 70. Heidarieh M, Mirvaghefi AR, Akbari M, Sheikhzadeh N, Kamyabi-Moghaddam Z, et al. (2012) Evaluations of Hilyses™, fermented Saccharomyces cerevisiae, on rainbow trout (Oncorhynchus mykiss) growth performance, enzymatic activities and gastrointestinal structure. Aquaculture Nutr 19: 343–348 doi: 10.1111/j.1365-2095.2012.00973.x [Google Scholar]
- 71. Heidarieh M, Mirvaghefi AR, Akbari M, Farahmand H, Sheikhzadeh N, et al. (2012) Effect of dietary Ergosan on growth performance, digestive enzymes, intestinal histology, hematological parameters and body composition of rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 38(4): 1169–74 doi: 10.1007/s10695-012-9602-8 [DOI] [PubMed] [Google Scholar]
- 72. Furuse M, Okumura J (1994) Nutritional and physiological characteristics in germ-free chickens. Comp Biochem Physiol A Physiol 109 547–556. [PubMed] [Google Scholar]
- 73.Neutra MR, Padykula HK (1984) The gastrointestinal tract. In: Weiss L, editor. Modern Concepts of Gastrointestinal Histology. New York: Elsevier, pp 688–706.
- 74. Iji PA, Hughes RJ, Choct M, Tivey DR (2001) Intestinal structure and function of broiler chickens on wheat-based diets supplemented with a microbial enzyme. Asian-Aust J Anim Sci 14: 54–60. [Google Scholar]
- 75. Onderci M, Sahin N, Sahin K, Cikim G, Aydín A, et al. (2006) Efficacy of supplementation of α-amylase producing bacterial culture on the performance, nutrient use, and gut morphology of broiler chicken fed a corn-based diet. Poult Sci 85: 505–510. [DOI] [PubMed] [Google Scholar]
- 76. Bhumkar DR, Joshi HM, Sastry M, Pokharkar VB (2007) Chitosan reduced gold nanoparticles as novel carriers for transmucosal delivery of insulin. Pharm Res 24: 1415–26. [DOI] [PubMed] [Google Scholar]
- 77. Derakhshandeh K, Fathi S (2012) Role of chitosan nanoparticles in the oral absorption of Gemcitabine. Int J Pharm 437: 172–177. [DOI] [PubMed] [Google Scholar]
- 78. Rather MA, Sharma R, Gupta S, Ferosekhan S, Ramya VL, et al. (2013) Chitosan-nanoconjugated hormone nanoparticles for sustained surge of gonadotropins and enhanced reproductive output in female fish. PLoS ONE 8(2): e57094 doi:10.1371/journal.pone.0057094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. des Rieux A, Fievez V, Garinot M, Schneider YJ, Préat V (2006) Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Control Release 116: 1–27. [DOI] [PubMed] [Google Scholar]