Abstract
Objective
High-frequency oscillations (HFOs) in the intracerebral electroencephalogram (EEG) have been linked to the seizure onset zone (SOZ). We investigated whether HFOs can delineate epileptogenic areas even outside the SOZ by correlating the resection of HFO-generating areas with surgical outcome.
Methods
Twenty patients who underwent a surgical resection for medically intractable epilepsy were studied. All had presurgical intracerebral EEG (500Hz filter and 2,000Hz sampling rate), at least 12-month postsurgical follow-up, and a postsurgical magnetic resonance imaging (MRI). HFOs (ripples, 80 –250Hz; fast ripples, >250Hz) were identified visually during 5 to 10 minutes of slow-wave sleep. Rates and extent of HFOs and interictal spikes in resected versus nonresected areas, assessed on postsurgical MRIs, were compared with surgical outcome (Engel’s classification). We also evaluated the predictive value of removing the SOZ in terms of surgical outcome.
Results
The mean duration of follow-up was 22.7 months. Eight patients had good (Engel classes 1 and 2) and 12 poor (classes 3 and 4) surgical outcomes. Patients with a good outcome had a significantly larger proportion of HFO-generating areas removed than patients with a poor outcome. No such difference was seen for spike-generating regions or the SOZ.
Interpretation
The correlation between removal of HFO-generating areas and good surgical outcome indicates that HFOs could be used as a marker of epileptogenicity and may be more accurate than spike-generating areas or the SOZ. In patients in whom the majority of HFO-generating tissue remained, a poor surgical outcome occurred.
Thirty percent to 40% of patients with focal epilepsy are medically intractable,1 and for some, surgical removal of epileptogenic areas is the best option to gain seizure freedom. Intracranial electroencephalographic (iEEG) investigations are indicated for patients in whom noninvasive methods fail to identify a single focal seizure generator.2
iEEG is used to define the seizure onset zone (SOZ).3 Removal of the SOZ alone, however, does not always predict the surgical benefit.4,5 It is uncertain whether the outcome can be improved by removing areas of interictal spiking, often more widespread than the SOZ.6,7 Intracranial studies also have limitations, as their results depend on electrode location and type of implantation (intracortical vs subdural). For instance, iEEG electrodes only record neuronal activity in their direct vicinity and are blind for other areas,8 making it hard to judge whether the activity at seizure onset really represents the seizure generator or is the result of propagation from else-where. Thus the actual focus and its extent may be missed, leading to unsuccessful surgery.
Microelectrode-recorded high-frequency oscillations (HFOs), ripples (80 –250Hz), and fast ripples (FRs, 250 –500Hz), were found predominantly in epileptogenic tissue.9 –11 They can also be recorded with macroelectrodes during clinical iEEG investigation.12,13 HFOs were more specific in indicating the SOZ than spikes.14 Additionally, they were linked to the SOZ independently of the underlying lesion and were infrequent in lesional areas outside the SOZ.15 Evidence therefore suggests that HFOs are good markers of epileptic tissue and may help to identify epileptogenic areas. We hypothesize that removing areas generating HFOs results in good surgical outcome. The correlation between removal of HFO-generating areas and seizure outcome was compared to that coming from spikes and to the current gold standard, removing the SOZ.
Materials and Methods
Patient Selection
Between 2004 and 2008, 47 patients underwent iEEG with 2,000Hz sampling (see below). The decision to perform iEEG was taken exclusively for clinical reasons. The study was performed retrospectively and patient inclusion criteria were: 1) surgery following the intracranial investigation; 2) 12 months postsurgical follow-up; and 3) postsurgical magnetic resonance imaging (MRI).
This study was approved by the Montreal Neurological Institute and Hospital Research Ethics Committee, and all patients signed an informed consent.
Recording Methods and Contact Selection
Depth electrodes were implanted stereotactically using an image-guidance system (SNN Neuronavigation System, Mississauga, Canada).16 Electrodes were manufactured onsite (9 contacts per electrode; contact surface 0.8mm2) as described earlier.14,17 iEEGs were low-pass filtered at 500Hz, sampled at 2,000Hz, and recorded using Harmonie (Stellate, Montreal, Canada). We recorded electro-oculogram (EOG) and electromyogram (EMG) to facilitate sleep staging. The recording was performed referentially with an epidural reference electrode placed in the parietal lobe of the hemisphere contralateral to the main epileptic focus. Analyses were performed on bipolar montages.
All electrode contacts without any prolonged artifacts and clearly located within the brain were retained. We analyzed interictal samples of slow-wave sleep lasting 5 to 10 minutes.18 Periods of slow-wave sleep were selected using EEG, EOG, and EMG as described before.14
Marking Spikes and HFOs
We selected and marked 5 minutes of slow-wave sleep in all patients. Interictal EEG segments were separated at least 4 hours from any seizure activity. The first minute of EEG of each patient was marked by 2 reviewers (J.J. and M.Z.) separately, and the concordance between marked events (spikes and HFOs) was assessed using Cohen’s kappa coefficient for each contact. Both observers jointly reviewed the events in contacts with kappa <0.519 and established a consensus. Based on this consensus, the remaining 4 minutes of EEG were marked by 1 of the reviewers. In patients for whom a stable measurement of HFOs was not reached during 5 minutes according to the method of Zelmann et al,18 10 minutes were marked.
For identifying HFOs, contacts were displayed with the maximum time resolution of the computer monitor (0.6 seconds, 1,200 samples). The display was split vertically with an 80Hz high-pass filter on the left side and a 250Hz high-pass filter on the right side, using finite impulse response filters to eliminate ringing. A ripple was marked if an event was clearly visible on the left (80Hz) and not on the right (250Hz). An event was regarded as an FR if it was visible on the right (250Hz). Only events containing at least 4 consecutive oscillations were regarded as HFOs, and 2 events were considered distinct when separated by at least 2 non-HFO oscillations (Supplementary Fig 1).
Classification of Contacts
The SOZ was defined by a clinical neurophysiologist as the area showing the first ictal activity (seizure onset) during iEEG recording. This was done independently from this research project. The traditional display of the iEEG does not allow visualization of HFOs (see Supplementary Fig 1), and HFOs that may have been present at seizure onset were therefore not taken into account for the determination of the SOZ. If seizures were originating from >1 area independently, all contacts within the different SOZs were regarded as SOZ contacts.
The electrode and contact localizations derived from the preimplantation Neuronavigation calculations and the postsurgical MRIs were used to determine whether electrode contacts were included in the brain tissue eventually removed at surgery. Postsurgical MRIs consisted of the following sequences: global T1 with gadolinium, T1 sagittal, T2 axial and coronal, fluid-attenuated inversion recovery coronal. Electrode tracts were best seen on the T1 sagittal views. Electrode contacts were classified as removed if they were within the surgical cavity and could not be found on the postsurgical MRI. Additionally, contacts were regarded as removed contacts if they were in disconnected brain tissue, which was for example the case for the innermost amygdala contacts after selective amygdalohippocampectomies.
Surgical Outcome
The decision for surgery resulted from the intracranial investigation as well as noninvasive investigations. HFO occurrence and rates were not used for surgical planning. In some cases, surgery was palliative, as the SOZ was within functional areas, widespread or multifocal. Documents of the last visit of the patient to the clinic were used for classifying the patient’s outcome according to Engel’s classification20: class 1 if patients were seizure free, class 2 in the case of rare disabling seizures, class 3 if a worthwhile improvement was reached, and class 4 for patients without improvement. Outcomes 1 and 2 were considered good, and 3 and 4 poor.
Statistical Analysis
HFOS AND SURGICAL OUTCOME
Rates of ripples, FRs, and spikes were calculated for each contact. The purpose was to investigate the correlation between HFOs and surgical outcome (Fig 1A). It was unclear whether only contacts with high HFO rates are important or if contacts with sparsely occurring HFOs should also be taken into account to identify epileptogenic areas. Often we see a small number of contacts with high rates of HFOs and more contacts with low HFO rates (Fig 1B). We thus quantified first the HFO rates in removed and nonremoved contacts, and then the number of removed contacts with HFOs regardless of HFO rates (ie, the extent of tissue showing HFOs).
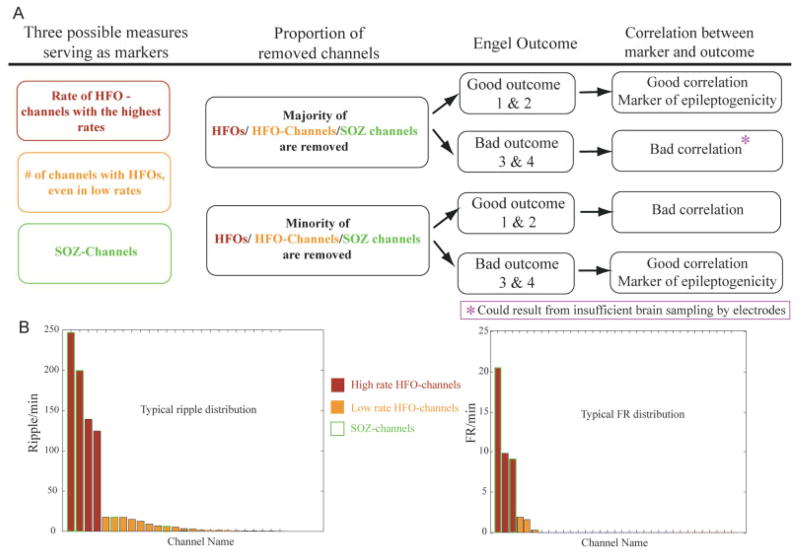
FIGURE 1.
This figure demonstrates our hypothesis. (A) The diagram illustrates the assumed relationship between the removal of the majority of measured events and the surgical outcome. Our hypothesis does not assume that all patients with a poor outcome must have remaining high-frequency oscillation (HFO)-generating regions. It might be that the majority of recorded HFOs are removed, but HFOs are generated in brain areas in which there are no electrodes. (B) Histograms in which channels are ranked according to event rates. The 2 graphs illustrate a typical distribution of ripples (left), some contacts having very high rates and many contacts having low rates, and fast ripples (FRs) (right), with similar numbers of contacts with high and low rates. In all patients, the distribution of FRs was restricted to much fewer channels than that of ripples. SOZ = seizure onset zone.
First, we investigated whether high rates of HFOs are indicative of epileptogenic areas. For this purpose, we calculated a ratio between rates of HFO in removed contacts and those in nonremoved contacts:
where ev is the type of event (ripple, FR or spike), RemCh are the contacts that were removed, and NonRemCh are the contacts that were not.
This ratio identifies the relationship between event rates in removed contacts compared with event rates in nonremoved contacts. For instance, a value close to +1 identifies patients in whom the majority of HFOs have been removed, whereas a value close to −1 indicates that the majority HFOs remained untouched. In patients with a value around zero, a similar total amount of HFOs were removed and remained untouched. According to our hypothesis, we expected patients with a value around 1 for ripples or FR to have a good outcome, and patients with a value around −1 to have a poor outcome.
Second, we evaluated the importance of the extent of removed HFO contacts regardless of the rate, reflecting the spatial extent of the HFO-generating area that was removed. We calculated the ratio between the number of removed and nonremoved contacts showing HFOs as follows:
where #ChannRem is the number of removed contacts with events (ripple, FR, or spike), and #ChannNonRem is the number of nonremoved contacts with events.
SOZ and Surgical Outcome
The determination of whether a channel belongs to the SOZ was described above. Knowledge about the SOZ is normally used to predict surgical outcome. We calculated whether removing the SOZ contacts would result in a better outcome than their nonremoval. SOZ channels were classified as removed or nonremoved regardless of other factors, such as reasons for nonremoval or frequency of seizures generated from each SOZ in the case of multiple SOZs in the same patient.
The following ratio was calculated:
where #ChannSOZRem is the number of removed contacts within the SOZ, and #ChannSOZNonRem is the number of nonremoved contacts within the SOZ. This ratio increases as the proportion of removed to nonremoved channels increases.
A Wilcoxon rank sum test was used to compare both ratios in patients with good (classes 1 and 2) versus those with poor surgical outcome (classes 3 and 4) for all 3 analyses above.
Results
Patient Inclusion and Surgical Outcome
Twenty of 47 patients were included. Twenty-seven were excluded for various reasons: 3 did not undergo surgery after the iEEG investigation because no clear SOZ was identified or it could not be removed for functional reasons; 11 had postsurgical follow-up shorter than 12 months; and 13 did not have a postsurgical MRI. This exclusion criterion did not bias the study in regard to surgical outcome, as the mean surgical outcome of the patients investigated (class 2.7 ± 0.7) was not different from those excluded (class 2.6 ± 1.1). Three patients (patients #10, #17, and #19) underwent 2 surgeries after iEEG, and the data presented here relate to the second surgery.
Clinical details are given in the Table. Mean time follow-up was 22.7 ± 6.8 months. Eight patients had a good (classes 1 and 2) and 12 a poor (classes 3 and 4) surgical outcome. Only 2 patients had a class 4 outcome, but only 1 patient became seizure free (class 1).
TABLE 1.
Clinical Information for All Patients and Position of the Intracranial Electroencephalographic Electrodes
| Patient | Age, y | MRI | Electrode Positions | Ictal Onset | Type of Surgery | Removed Contacts | Follow-up, mo | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 50 | Bilateral mesial T atrophy | L-A, HC, PHC; R-A, HC, PHC | L-A, HC, PHC; R- A,a HC,a PHCa | R SAH | R-HC 1-4 | 25 | 3 |
| 2 | 30 | Remote ant. T resection; gliotic lesion behind surgical cavity | LPH, OF, C | Middle cont LPH | add. resection 2nd and 3rd T gyri + PHC gyrus | L-PHC complete | 18 | 3 |
| 3 | 44 | FCD L-F F2 | L-OF, C, Le1, Le2; ROF, C | Inner cont Le1 and Le2 | Lesionectomy | Le2 complete | 18 | 2 |
| 4 | 42 | Normal (PS) | LPH, OT, PC, AC, SMA, PSMA | Inner cont L-SMA and middle cont LCP | F resection | L-AC and SMA complete | 13 | 3 |
| 5 | 40 | Normal (PS); resection R-T | R-PHC, OT | External cont R- PHC, OT | add. resection 2nd, 3rd, and 4th T gyri + PHC gyrus | R-PHC complete; R-OT 1-3 | 29 | 1 |
| 6 | 48 | Normal | L-A, HC, PHC, TP; R-A, HC, PHC, TP | R-A, HC, PHC; L- A,a HC,a PHCa | L-SAH | L-HC 1-3 | 27 | 3 |
| 7 | 39 | L-FCD, F2 | L-SMA (2×), F2 and Le; R-SMA (2×), F2 | Middle cont L-Le | Lesionectomy | Le complete | 25 | 3 |
| 8 | 45 | Oligodendroglioma L-T | L-Le and T in lesion | Inner cont L-T | Lesionectomy | L-T 3-7 | 37 | 3 |
| 9 | 21 | Normal | L-A, HC, PHC; R-A, HC, PHC | L-A, HC, PHC; R- A,a HC,a PHCa | R-SAH | R-A 3-7, R-HC 1-2 | 18 | 4 |
| 10 | 43 | Sturge-Weber syndrome, L-O pial angiomatosis and O-P atrophy | R-A, HC, IC, SC | R-IC and external cont SC | Incomplete lesionectomy, O | R-IC complete, R-SC 3-9 | 20 | 2 |
| 11 | 50 | R hippocampal malrotation | L-A, HC; R-A, HC | R-A, HC | R-SAH | R-A 1-2, R-HC 1-3 | 26 | 2 |
| 12 | 27 | Gliotic changes (Ps); resection L-T | L-PHC, OT | Middle cont L- PHC and OT | add. resection 2nd and 3rd T gyri | L-PHC complete | 36 | 4 |
| 13 | 36 | Tuberous sclerosis; tubers in L-OF, ant. T and TP, and R sup. F | L-A, HC, OF, C; R-A, HC, OF, C | Inner cont L-OF | ant. L-T resection | L-HC 1-3 | 22 | 3 |
| 14 | 23 | Normal | R-A, HC, AC, PC, OF, IC, SC, | Inner cont R-SCa and external cont R-HC | R-T-O resection | R-IC 4-9, R-SC 4-9 | 18 | 2 |
| 15 | 47 | Neurocutaneous syndrome | L-A, HC, PHC; R-A, HC, PHC | L-A, HC, PHC; R- A,a HC,a PHCa | R-SAH | R-A 1-2, R-HC 1-3 | 24 | 2 |
| 16 | 55 | FCD L-C | L-SM, IM, SPC, IPC | Inner cont L-IM and SM | Partial lesionectomy | L-IM and IPC complete | 28 | 3 |
| 17 | 28 | R hemi. atrophy | R-A, HC, PHC, and Heschl gyrus | R-HC, A, PHC | R-SAH | R-A and HC complete | 18 | 2 |
| 18 | 34 | Bilateral OT PNH | L-A, HC, OT, O; R-A, HC, PHC, OT | L-A, HC; R-A,a HC,a PHCa | R-SAH | R-A 1-3, R-HC 1-3 | 14 | 3 |
| 19 | 57 | R mesial T atrophy; gliotic lesion OT | R-A, HC, PHC, OT1, OT2 | R-A, HC, PHC | R-SAH | R-A complete, R-HC 1-4 | 25 | 3 |
| 20 | 28 | PNH with 2 nodules in R atrium | R-A, HC, PHC, R ant. nodule, R post. nodule | R-HC, A, PHC | R-SAH | R-A and HC complete | 13 | 2 |
The removed contacts are determined according to the postsurgical MRI. Time of follow-up relates to the last visit of the patient to our hospital. Surgical outcomes are given according to Engel’ classification.20 Patient #13 underwent a palliative approach, because his seizure onset was within his speech area.
More common seizure onset zone.
MRI = magnetic resonance imaging; T = temporal; L = left; A = amygdala; HC = hippocampus; PHC = posterior hippocampus; R = right; SAH = selective amygdalohippocampectomy; ant. = anterior; OF = orbitofrontal; C = cortex; cont = contacts; add. = additional; FCD = focal cortical dysplasia; F = frontal; Le = lesion; PS = postsurgical, in these cases patients with surgical cavity due to prior surgery; OT= occipitotemporal junction; PC = posterior cingulate; AC = anterior cingulate; SMA = supplementary motor area; PSMA = pre-SMA; L-PC = left posterior cingulated; TP = temporal pole; FCD = focal cortical dysplasia; O = occipital; P = parietal; IC = infracalcarine; SC = supracalcarine; L-T = left temporal; OF = orbitofrontal; sup. = superior; SM = superior motor; IM = inferior motor; SPC = superior postcentral; IPC = inferior postcentral; hemi. = hemisphere; PNH = periventricular nodular heterotopias; post. = posterior.
Events Rates and Surgical Outcome
The ratio between HFO rates in removed and nonremoved contacts was significantly higher in patients with a good outcome than in those with a poor outcome (Fig 2A). This was especially the case for ripples (good outcome ratio, 0.31 ± 0.; poor outcome ratio, −0.3 ± 0.4; p = 0.006), whereas the difference was larger but less significant for FRs (good outcome ratio, 0.7 ± 0.4; poor outcome ratio, −0.2 ± 0.7; p = 0.04). Figure 3 gives the ratios in all patients. In all but 1 patient with a good outcome, ratios >0 were seen for ripples and FRs, in agreement with our hypothesis (Fig 4, Supplementary Fig 1). Eight of 12 patients with a poor outcome had ratios <0 for ripples and FRs (Fig 5). Only 1 patient (#19) with a poor outcome showed positive values for both ripples and FRs. Two patients (#12, #16) showed clearly positive values for FRs, and a good outcome might have been expected. This disagreement between a large removal of HFO-generating tissue and bad outcome in these 3 patients may have resulted from insufficient electrode coverage. For spikes, there was no significant difference between patients with good and poor outcome.
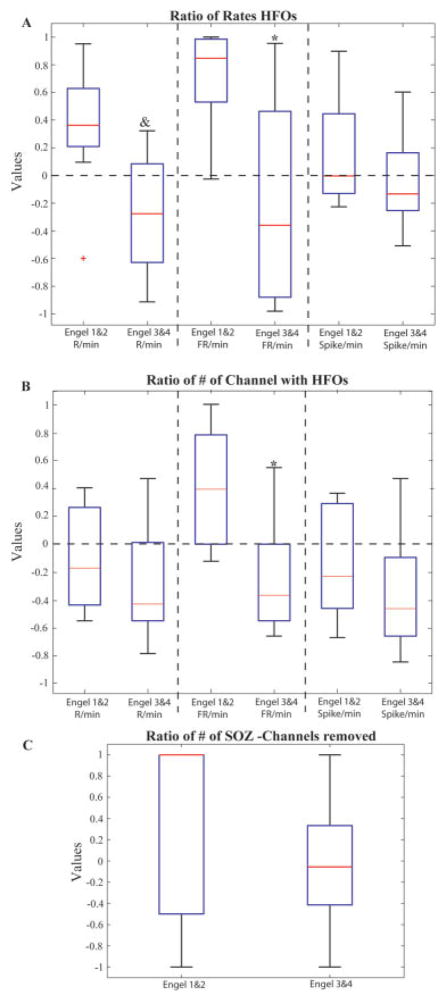
FIGURE 2.
Overview of the results of the study. (A) Ratio of event rates in the removed areas to rates in the non-removed areas for patients with good versus bad outcome (see formula in text). Regions generating high rates of ripples (R) and fast ripples (FRs) where more likely to have been removed in patients with good outcome. (B) Ratio of the number of contacts with events to the number of contacts without any event in patients with good versus poor surgical outcomes (see formula in text). Only the number of contacts carrying FRs showed a significant difference. (C) The ratio of removed versus nonremoved seizure onset zone (SOZ) contacts is compared for the 2 outcome groups. No significant difference was seen. *Significantly different from Engel classes 1 and 2 (p = 0.04). &Significantly different from Engel classes 1 and 2 (p = 0.008). Red lines signify median. HFO = high-frequency oscillation. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
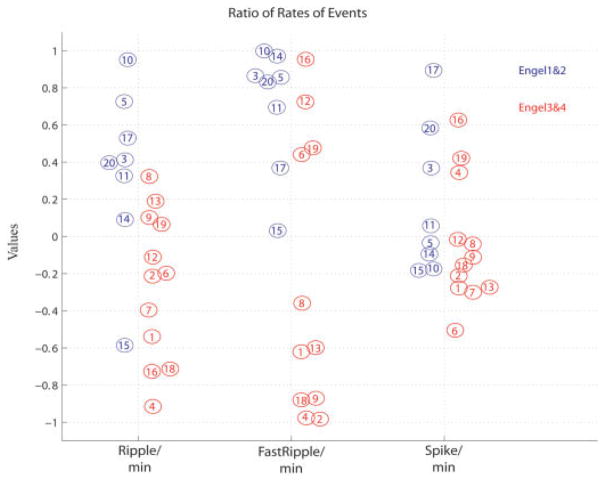
FIGURE 3.
Same data as in Figure 2A, but showing all patients. All patients with good outcome are in blue and patients with poor outcome in red circles. A number identifies each patient, so that it is possible to follow which marker (ripples on the left, fast ripples [FRs] in the middle, spikes on the right) is indicative of the outcome in each patient. Values >0 indicate that the majority of high-frequency oscillations (HFOs) are removed and patients should therefore have a good outcome (blue circles). One patient did not have any FRs (#7). The chart shows that, except for patient #15, all patients with good outcome had the majority of HFOs removed (values >0). This result can be stated differently: all patients with most HFOs remaining (values <0) had a poor outcome (except patient #15). We also notice that for patients with poor outcome, all but 1 (#19) had the majority of ripples or FRs remaining.
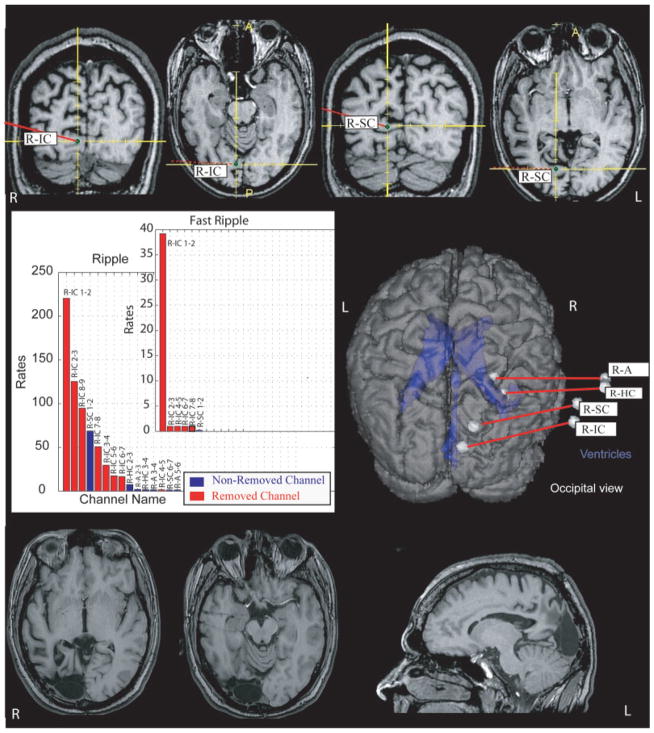
FIGURE 4.
Pre- and postsurgical magnetic resonance imaging (MRI) of patient #10 as an example of a patient with removal of most of high-frequency oscillation-generating tissue and a good outcome. The postsurgical MRI clearly shows that the areas around electrode R-IC were removed, whereas the tissue around the inner contacts of R-SC remained untouched. The axial view of the postsurgical MRI also demonstrates the electrode traces of R-A and R-HC. The graph illustrates the rates of ripples/fast ripples in contacts that were removed (red) and those that remained (blue). Only channels that had events are shown; ripples are found in more channels than fast ripples. A = amygdala; HC = hippocampus; IC = infracalcarine; L = left; R = right; SC = supracalcarine.
FIGURE 5.
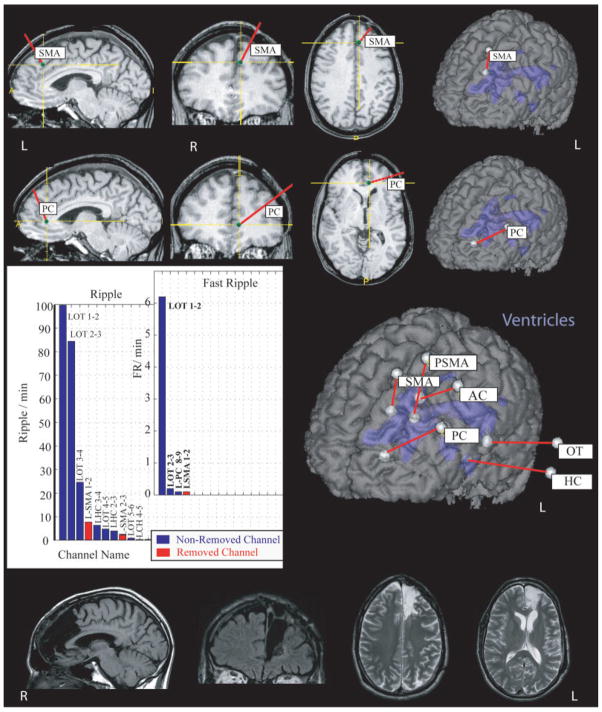
Patient #4 underwent a resection in the left frontal lobe with removal of the brain areas around the electrodes in the left anterior cingulate (L-AC) and left supplementary motor area (L-SMA). High-frequency oscillations were most frequently seen in the contacts placed in the occipitotemporal junction. The patient had a poor outcome, with reduced but persisting complex partial seizures. HC = hippocampus; OT= occipitotemporal junction; PC = posterior cingulate; PSMA = posterior SMA; R = right.
Number of Contacts Removed With Events and Surgical Outcome
The difference between patients with good and poor outcomes was borderline significant for the ratio between the number of removed and nonremoved contacts with FRs (good outcome, 0.4 ± 0.4; poor outcome, −0.2 ± 0.4; p = 0.06) (Fig 2B). This finding is interesting, as contacts with FRs were significantly less frequent and less distributed than those with ripples (Fig 1B). The average number of contacts with FRs was 5.9 ± 3.2 per patient, compared with 21 ± 7.8 contacts with ripples per patient ( p < 0.001). Thus, FRs in most patients were seen in a few contacts but with relatively high rates, and there was only a negligible difference between the set of all contacts with FR and the set of contacts with high rates of FR. No significant difference for ripples and spikes was found. Contacts showing low rates of ripples therefore seem to be less important indicators of epileptogenicity.
SOZ and Postsurgical Outcome
The removal of the SOZ was not different for patients with good or poor outcomes (Fig 2C), indicating that SOZ contacts that are not removed do not necessarily result in poor outcome. The ratio of removed versus non-removed SOZ channels varied for good as well as for bad outcomes between +1 (complete removal) and −1 (no removal). In a larger group a patients and with more statistical power, grading of different SOZs and reasons for nonremoval could be analyzed and may shed light on these results.
The difference in correlation with postsurgical outcome between removal of HFO-generating tissue and SOZ-generating tissue underlines that the extents of SOZ- and HFO-generating tissues do not completely overlap. Nevertheless, rates of HFOs are significantly higher in SOZ than non-SOZ areas ( p < 0.001) (Supplementary Fig 2).
Conclusion
A significant correlation was found between the removal of contacts with high rates of HFOs and good surgical outcome. For FRs, the removal of the majority of contacts with FRs also resulted in good outcomes. Patients with remaining HFO-generating tissue had bad outcomes. Surprisingly, the SOZ in this specific group of patients appeared to be a weak indicator of epileptogenicity.
Discussion
Interictal HFOs, especially at high rates, seem to be reliable markers of epileptogenic tissue. Patients in whom most HFO-generating tissue was removed experienced a good outcome.
Our surgical results were worse than other series, with only 40% of patients in Engel class 1 or 2.21–23 Surgical outcome, however, is less favorable in patients with neocortical,24 nonlesional,25 and multifocal or bitemporal epilepsy.4 All our patients had incongruent or contradictory preimplantation findings. Five had nonlesional epilepsy, and 10 had multiple or diffuse lesions. Three had previous unsuccessful resections. Only 10% of our patients with refractory focal epilepsy undergo intracranial investigations. Our selection criteria for iEEG may therefore be more stringent compared with other centers, where up to 30% of patients have iEEG.26,27 This group consists almost entirely of patients with unclear or poorly localized SOZs, which probably explains the surgical outcome.
The definition of the SOZ by iEEG is an important measure in presurgical planning, and interictal spikes are also used to guide the surgical extent.6,7,28 But how reliable are these markers for evaluating epileptogenic areas?
In our study, removing the SOZ, or part of it, was not necessarily correlated with a good outcome, and some patients continued to have seizures even when the SOZ was removed (eg, patients #7 and #19). This may be explained by the spatially limited sampling of intracranial investigations.5 On the other hand, good outcome was observed in some patients in whom part of the SOZ was not included in the resection. This was seen in patients with mesial bitemporal (patients #11 and #15) and with extended neocortical seizure onset (patients #3 and #14). In patients with bilateral mesial-temporal epilepsy, good outcome can occur with unilateral resection,4 but it remains unclear why remaining epileptogenic areas become inactive over time. The definition of the SOZ used in this study (all regions with a seizure onset, independently of iEEG morphology and number of seizures) does not necessarily correspond to the definition used in practice for surgical planning. This may also explain the unexpected poor relationship between removal of the SOZ and outcome.
Interictal spikes are also potential markers of epileptogenic tissue, but their involvement in seizure generation is unclear. Some studies indicate that removal of all spike-generating tissue is necessary to achieve good outcome,6 but others indicate that seizure freedom is independent of interictal activity.7 This study did not find a difference in the amount of spike-generating tissue removed between patients with good and bad outcomes. Thus, spikes were not a good marker for epileptogenicity. Spikes may be generated in areas secondarily involved in the epileptogenic network, which are not responsible for seizure generation.29,30 It has even been hypothesized that spikes may have a protective effect and prevent seizure occurrence.31,32 They decrease with antiepileptic medication withdrawal.24
The weakness of spikes as epileptogenic markers raises the question of whether interictal HFOs are independent enough of spikes to be better markers. HFOs often occur with spikes,33 and some are visible in the unfiltered EEG as “riding on spikes.”13 Around 40% of HFOs occur outside spikes, however, and contacts without spikes can show high HFO rates.14 Additionally, HFOs behave differently from spikes in response to anti-epileptic medication reduction and after seizures.34 Thus, HFOs are possibly generated independently of spikes even if they often co-occur. They may result from gamma-aminobutyric acidergic interneurons or an imbalance between excitatory and inhibitory interneurons within epileptic tissue.10,35
Although HFOs do not increase prior to seizures,15 animal studies have shown a relationship between their presence and number of seizures.36 Additionally, more frequent ictal HFOs were observed in seizure onset areas compared with remote areas.12,37 These results indicate that HFOs may be linked to seizure generation. The strongest indicator that HFOs are a pathological correlate of epileptogenic tissue, however, remains a correlation with surgical outcome.
We hypothesized that the majority of HFO-generating areas were removed in patients with good surgical outcome. We did not expect to predict the outcome, because brain coverage with depth electrodes is limited, and patients with removal of HFO-generating areas may continue to have HFOs outside the recording zone. All 3 patients (#12, #16, #19), who had a bad outcome despite a good removal of HFO-generating areas had extended SOZ areas and limited lesional coverage by the electrodes due to the extent (#12, #19) or localization (#16) of the lesion. This disagreement may therefore be dependent on the extent and type of electrode placement chosen for each patient. Although the implantation of subdural grids often results in coverage of large surface areas, activity from deeper cortical structures can be better recorded by depth electrodes. Additionally, the extent and number of electrodes varies strongly from center to center. Future studies will have to determine which type of iEEG implantation and recording technique best allows interpreting HFO findings and predicting postsurgical outcome.
We considered HFOs a reliable marker of epileptogenicity if most HFO-generating areas were removed in patients with good outcome and if these areas were not removed in patients with poor outcome. Patients in whom HFO-generating tissue remained postsurgically had unfavorable outcomes. On the contrary, in all but 1 patient (#15) with a good outcome, most HFO-generating tissue was removed. In this patient, with bitemporal seizure onsets and right selective amygdalohippocampectomy, the majority of ripples were not removed. In patients with bitemporal implantations (#1, #6, #9, #15, and #18), HFOs were generally bitemporal and had more remaining ripples than in patients with neocortical implantations. Some remaining ripples may therefore have been correlates of healthy ripples,38 and in mesial temporal structures may not be as indicative for epileptogenic areas as in neocortex.
Do we have to distinguish between HFO frequencies, and are ripples or FRs the better marker of epileptogenicity? Although FRs have been more strongly linked to epileptogenicity than ripples,11,33 we found that both were increased in the SOZ.14 Functionally related FRs have been described after sensory stimulation,39 but not spontaneously, and therefore most likely do not interfere with our study, which was conducted during sleep. In mesial temporal structures, there is the issue of differentiating between memory-related38,40 and pathologic ripples.41 Normal ripples synchronize neuronal activity over long distances and thus facilitate memory consolidation. Pathological HFOs, which most likely result from abnormal synchronously bursting neurons, cannot be distinguished by simple frequency analysis from healthy ripples.41 Rates of HFOs in the present study therefore may in some contacts be increased by healthy HFOs, which we cannot distinguish from pathological ones at the moment.
Our study, however, suggests that removal of ripple-generating tissue is important to gain seizure relief; the difference in HFO rates in removed areas between patients with good and poor outcomes was higher for ripples than for FRs. In patients with a poor outcome, only in 1 (#19) was the majority of tissue generating both event types removed. In 3 others (#8, #9, and #13), the majority of FRs but not ripples were removed. In these cases, either FRs were generated outside the recording area and thus epileptogenic tissue was not removed, or ripples were also indicators of epileptogenicity and should have been removed.
Ripples and FRs also showed very different distribution patterns. Whereas FRs were more localized, ripples tended to occur in larger regions. Furthermore, the distribution of ripples seems to be less indicative of the epileptogenic zone than areas with high rates of ripples. Hence, a differentiation between ripples and FRs seems necessary for interpreting rates as a marker of epileptogenicity.
We conclude that good surgical outcome is better correlated with the removal of HFO-generating tissue than with the removal of spike-generating tissue or the SOZ. This indicates that HFOs should be good markers of epileptic tissue. Prospective studies are needed to evaluate the value of HFOs for postsurgical outcome prediction.
Supplementary Material
Acknowledgments
This project was supported by grant MOP-10189 from the Canadian Institutes of Health Research. J.J. received the Preston Robb Fellowship of the Montreal Neurological Institute. M.Z. was supported by the Netherlands Organization of Scientific Research, AGIKO-grant No. 92003481, the University Medical Center Utrecht (internationalization grant), and the “Stichting de drie lichten.” C.-E.C. received a postdoctoral fellowship from “La Fondation Savoy pour l’Épilepsie.”
Footnotes
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Schuele SU, Lüders HO. Intractable epilepsy: management and therapeutic alternatives. Lancet Neurol. 2008;7:514–524. doi: 10.1016/S1474-4422(08)70108-X. [DOI] [PubMed] [Google Scholar]
- 2.Diehl B, Lüders HO. Temporal lobe epilepsy: when are invasive recordings needed? Epilepsia. 2000;41(suppl 3):61–74. doi: 10.1111/j.1528-1157.2000.tb01536.x. [DOI] [PubMed] [Google Scholar]
- 3.Rosenow F, Lüders H. Presurgical evaluation of epilepsy. Brain. 2001;124(pt 9):1683–1700. doi: 10.1093/brain/124.9.1683. [DOI] [PubMed] [Google Scholar]
- 4.Boling W, Aghakhani Y, Andermann F, Sziklas V, Olivier A. Surgical treatment of independent bitemporal lobe epilepsy defined by invasive recordings. J Neurol Neurosurg Psychiatry. 2009;80:533–538. doi: 10.1136/jnnp.2008.155291. [DOI] [PubMed] [Google Scholar]
- 5.Prasad A, Pacia SV, Vazquez B, et al. Extent of ictal origin in mesial temporal sclerosis patients monitored with subdural intracranial electrodes predicts outcome. Clin Neurophysiol. 2003;20:243–248. doi: 10.1097/00004691-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Bautista RE, Cobbs MA, Spencer DD, Spencer SS. Prediction of surgical outcome by interictal epileptiform abnormalities during intracranial EEG monitoring in patients with extrahippocampal seizures. Epilepsia. 1999;40:880–890. doi: 10.1111/j.1528-1157.1999.tb00794.x. [DOI] [PubMed] [Google Scholar]
- 7.Hufnagel A, Dümpelmann M, Zentner J, et al. Clinical relevance of quantified intracranial interictal spike activity in presurgical evaluation of epilepsy. Epilepsia. 2000;41:467–478. doi: 10.1111/j.1528-1157.2000.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 8.Quesney LF, Gloor P. Localization of epileptic foci. Electroencephalogr Clin Neurophysiol Suppl. 1985;37:165–200. [PubMed] [Google Scholar]
- 9.Bragin A, Engel J, Jr, Wilson CL, et al. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid-treated rats with chronic seizures. Epilepsia. 1999;40:127–137. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- 10.Bragin A, Wilson CL, Staba RJ, et al. Interictal high-frequency oscillations (80–500 Hz) in the human epileptic brain: entorhinal cortex. Ann Neurol. 2002;52:407–415. doi: 10.1002/ana.10291. [DOI] [PubMed] [Google Scholar]
- 11.Staba RJ, Frighetto L, Behnke EJ, et al. Increased fast ripple to ripple ratios correlate with reduced hippocampal volumes and neuron loss in temporal lobe epilepsy patients. Epilepsia. 2007;48:2130–2138. doi: 10.1111/j.1528-1167.2007.01225.x. [DOI] [PubMed] [Google Scholar]
- 12.Jirsch JD, Urrestarazu E, LeVan P, et al. High-frequency oscillations during human focal seizures. Brain. 2006;129:1593–1608. doi: 10.1093/brain/awl085. [DOI] [PubMed] [Google Scholar]
- 13.Urrestarazu E, Chander R, Dubeau F, Gotman J. Interictal high-frequency oscillations (100–500 Hz) in the intracerebral EEG of epileptic patients. Brain. 2007;130:2354–2366. doi: 10.1093/brain/awm149. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs J, LeVan P, Chander R, et al. Interictal high-frequency oscillations (80–500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia. 2008;49:1893–1907. doi: 10.1111/j.1528-1167.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs J, Levan P, Châtillon CE, et al. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain. 2009;132(pt 4):1022–1037. doi: 10.1093/brain/awn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olivier A, Germano IM, Cukiert A, Peters T. Frameless stereotaxy for surgery of the epilepsies: preliminary experience. J Neurosurg. 1994;81:629–633. doi: 10.3171/jns.1994.81.4.0629. [DOI] [PubMed] [Google Scholar]
- 17.Urrestarazu E, Jirsch JD, LeVan P, et al. High-frequency intracerebral EEG activity (100–500 Hz) following interictal spikes. Epilepsia. 2006;47:1465–1476. doi: 10.1111/j.1528-1167.2006.00618.x. [DOI] [PubMed] [Google Scholar]
- 18.Zelmann R, Zijlmans M, Jacobs J, Châtillon CE, Gotman J. Improving the identification of high frequency oscillations. Clin Neurophysiol. 2009;120:1457–1464. doi: 10.1016/j.clinph.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landis JR, Koch GG. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics. 1977;33:363–374. [PubMed] [Google Scholar]
- 20.Engel J, Van Ness PC, Rasmussen TB, et al. Outcome with respect to epileptic seizures. In: Engel J Jr, editor. Surgical Treatment of Epilepsies. New York, NY: Raven Press; 1993. pp. 609–621. [Google Scholar]
- 21.Cossu M, Lo Russo G, Francione S, et al. Epilepsy surgery in children: results and predictors of outcome on seizures. Epilepsia. 2008;49:65–72. doi: 10.1111/j.1528-1167.2007.01207.x. [DOI] [PubMed] [Google Scholar]
- 22.Cohen-Gadol AA, Wilhelmi BG, Collignon F, et al. Long-term outcome of epilepsy surgery among 399 patients with nonlesional seizure foci including mesial temporal lobe sclerosis. J Neurosurg. 2006;104:513–524. doi: 10.3171/jns.2006.104.4.513. [DOI] [PubMed] [Google Scholar]
- 23.Uijl SG, Leijten FS, Arends JB, et al. Prognosis after temporal lobe epilepsy surgery: the value of combining predictors. Epilepsia. 2008;49:1317–1323. doi: 10.1111/j.1528-1167.2008.01695.x. [DOI] [PubMed] [Google Scholar]
- 24.Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7:525–537. doi: 10.1016/S1474-4422(08)70109-1. [DOI] [PubMed] [Google Scholar]
- 25.Wetjen NM, Marsh WR, Meyer FB, et al. Intracranial electroencephalography seizure onset patterns and surgical outcomes in nonlesional extratemporal epilepsy. J Neurosurg. 2009;110:1147–1152. doi: 10.3171/2008.8.JNS17643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zumsteg D, Wieser HG. Presurgical evaluation: current role of invasive EEG. Epilepsia. 2000;41(suppl 3):S55–S60. doi: 10.1111/j.1528-1157.2000.tb01535.x. [DOI] [PubMed] [Google Scholar]
- 27.Holmes MD, Miles AN, Dodrill CB, et al. Identifying potential surgical candidates in patients with evidence of bitemporal epilepsy. Epilepsia. 2003;44:1075–1079. doi: 10.1046/j.1528-1157.2003.58302.x. [DOI] [PubMed] [Google Scholar]
- 28.Holmes MD, Kutsy RL, Ojemann GA, et al. Interictal, unifocal spikes in refractory extratemporal epilepsy predict ictal origin and postsurgical outcome. Clin Neurophysiol. 2000;111:1802–1808. doi: 10.1016/s1388-2457(00)00389-8. [DOI] [PubMed] [Google Scholar]
- 29.Bagshaw AP, Jacobs J, LeVan P, Dubeau F, Gotman J. Effect of sleep stage on interictal high-frequency oscillations recorded from depth macroelectrodes in patients with focal epilepsy. Epilepsia. 2009;50:617–628. doi: 10.1111/j.1528-1167.2008.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Usui N, Mihara T, Baba K, et al. Intracranial EEG findings in patients with lesional lateral temporal lobe epilepsy. Epilepsy Res. 2008;78:82–91. doi: 10.1016/j.eplepsyres.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Avoli M. Do interictal discharges promote or control seizures? Experimental evidence from an in vitro model of epileptiform discharge. Epilepsia. 2001;42(suppl 3):2–4. doi: 10.1046/j.1528-1157.2001.042suppl.3002.x. [DOI] [PubMed] [Google Scholar]
- 32.Avoli M, Biagini G, de Curtis M. Do interictal spikes sustain seizures and epileptogenesis? Epilepsy Curr. 2006;6:203–207. doi: 10.1111/j.1535-7511.2006.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Staba RJ, Wilson CL, Bragin A, et al. High-frequency oscillations recorded in human medial temporal lobe during sleep. Ann Neurol. 2004;56:108–115. doi: 10.1002/ana.20164. [DOI] [PubMed] [Google Scholar]
- 34.Zijlmans M, Jacobs J, Zelmann R, et al. High frequency oscillations mirror disease activity in patients with epilepsy. Neurology. 2009;72:979–986. doi: 10.1212/01.wnl.0000344402.20334.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones MS, Barth DS. Effects of bicuculline methiodide on fast (>200Hz) electrical oscillations in rat somatosensory cortex. J Neurophysiol. 2002;88:1016–1025. doi: 10.1152/jn.2002.88.2.1016. [DOI] [PubMed] [Google Scholar]
- 36.Bragin A, Wilson CL, Almajano J, et al. High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia. 2004;45:1017–1023. doi: 10.1111/j.0013-9580.2004.17004.x. [DOI] [PubMed] [Google Scholar]
- 37.Ochi A, Otsubo H, Donner EJ, et al. Dynamic changes of ictal high-frequency oscillations in neocortical epilepsy: using multiple band frequency analysis. Epilepsia. 2007;48:286–296. doi: 10.1111/j.1528-1167.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- 38.Buzsaki G. Rhythms of the Brain. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 39.Curio G, Mackert BM, Burghoff M, et al. Localization of evoked neuromagnetic 600 Hz activity in the cerebral somatosensory system. Electroencephalogr Clin Neurophysiol. 1994;91:483–487. doi: 10.1016/0013-4694(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 40.Axmacher N, Elger CE, Fell J, et al. Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain. 2008;7:1806–1817. doi: 10.1093/brain/awn103. [DOI] [PubMed] [Google Scholar]
- 41.Engel J, Bragin A, Staba R, Mody I. High-frequency oscillations: what is normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.