Abstract
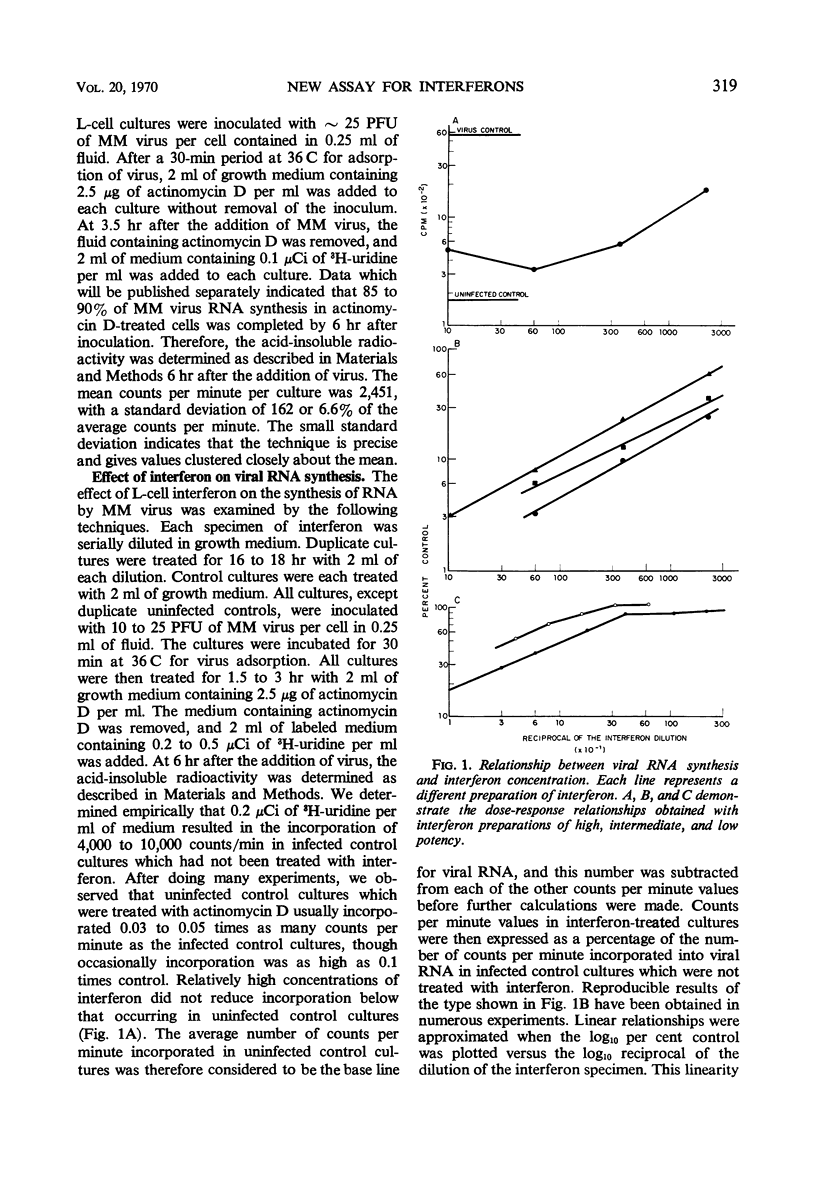
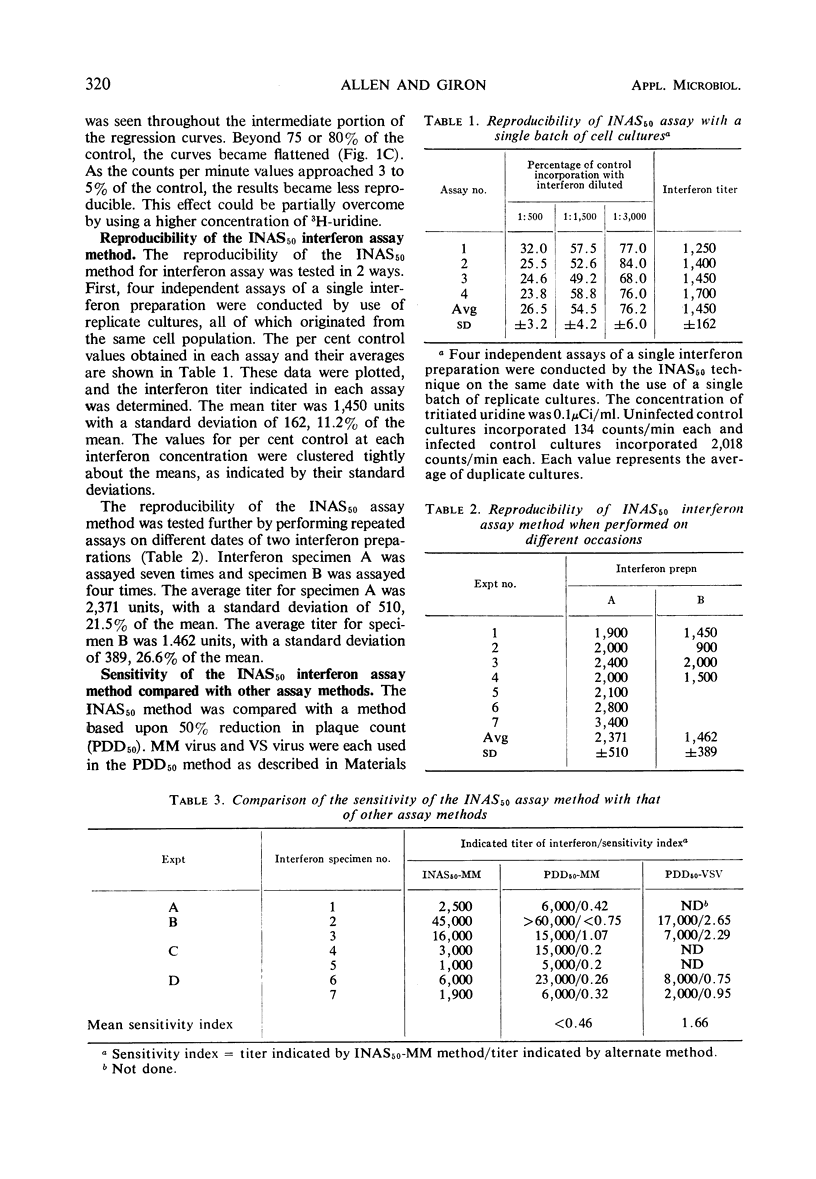
A method for assaying mouse interferon based on the inhibition of viral ribonucleic acid (RNA) synthesis was devised. The amount of MM virus and RNA synthesized in interferon-treated L-cell cultures was determined by measuring the amount of 3H-uridine converted into a trichloroacetic acid-insoluble form after treatment of the infected cultures with 2.5 μg of actinomycin D per ml. The amount of RNA synthesized was inversely related to the concentration of interferon used for treatment. A linear dose-response regression curve was obtained by plotting the log of the amount of RNA made, expressed as a percentage of the control, versus the log of the reciprocal of the interferon dilution. A unit of interferon was defined as that concentration which inhibited nucleic acid synthesis by 50% (INAS50). The concentration of mouse interferon could be determined within 24 hr. This assay method, on the average, was approximately half as sensitive as the method which measured the 50% reduction of MM virus plaque number (PDD50-MM method), but was, on the average, almost 1.7 times as sensitive as the PDD50-VSV method. It averaged approximately 20 times the sensitivity of the methods which used as end points the 70% reduction in yield of MM virus or the complete inhibition of cytopathic effect by MM virus. The reproducibility of the INAS50 technique was tested in two ways. (i) Four independent assays of an interferon specimen were performed with replicate cultures. The standard deviation was 11.2% of the mean titer. (ii) On different dates, one interferon specimen was assayed seven times and another was assayed four times. The standard deviations were 21.5 and 26.6% of the respective mean titers.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell J. B., Colter J. S. Sensitivity to, and production of interferon by three variants of Mengo virus. Can J Microbiol. 1967 Aug;13(8):931–937. doi: 10.1139/m67-125. [DOI] [PubMed] [Google Scholar]
- DE SOMER P., PRINZIE A., DENYS P., Jr, SCHONNE E. Mechanism of action of interferon. I. Relationship with viral ribonucleic acid. Virology. 1962 Jan;16:63–70. doi: 10.1016/0042-6822(62)90202-7. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Nutrition needs of mammalian cells in tissue culture. Science. 1955 Sep 16;122(3168):501–514. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- FINTER N. B. QUANTITATIVE HAEMADSORPTION, A NEW ASSAY TECHNIQUE. I. ASSAY OF INTERFERON. Virology. 1964 Dec;24:589–597. doi: 10.1016/0042-6822(64)90212-0. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Sonnabend J. A. Inhibition by interferon of production of double-stranded Semliki forest virus ribonucleic acid. Nature. 1965 May 1;206(983):532–532. doi: 10.1038/206532a0. [DOI] [PubMed] [Google Scholar]
- GROSSBERG S. E., HOLLAND J. J. Interferon and viral ribonucleic acid. Effect on virus-susceptible and insusceptible cells. J Immunol. 1962 Jun;88:708–714. [PubMed] [Google Scholar]
- Giron D. J., Pindak F. F. Propagation of MM virus in L cells. Appl Microbiol. 1969 Jun;17(6):811–814. doi: 10.1128/am.17.6.811-814.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giron D. J. Role of interferon in the propagation of MM virus in L cells. Appl Microbiol. 1969 Oct;18(4):584–588. doi: 10.1128/am.18.4.584-588.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I., Chenault S. S., Stevenson D., Acton J. D. Effect of interferon on polymerization of single-stranded and double-stranded mengovirus ribonucleic acid. J Bacteriol. 1966 Mar;91(3):1230–1238. doi: 10.1128/jb.91.3.1230-1238.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERMODSSON S., PHILIPSON L. A SENSITIVE METHOD FOR INTERFERON ASSAY. Proc Soc Exp Biol Med. 1963 Dec;114:574–579. doi: 10.3181/00379727-114-28735. [DOI] [PubMed] [Google Scholar]
- LINDENMANN J., GIFFORD G. E. Studies on vaccinia virus plaque formation and its inhibition by interferon. III. A simplified plaque inhibition assay of interferon. Virology. 1963 Mar;19:302–309. doi: 10.1016/0042-6822(63)90068-0. [DOI] [PubMed] [Google Scholar]
- LOCKART R. Z., Jr, SREEVALSAN T., HORN B. Inhibition of viral RNA synthesis by interferon. Virology. 1962 Nov;18:493–494. doi: 10.1016/0042-6822(62)90042-9. [DOI] [PubMed] [Google Scholar]
- TAYLOR J. STUDIES ON THE MECHANISM OF ACTION OF INTERFERON. I. INTERFERON ACTION AND RNA SYNTHESIS IN CHICK EMBRYO FIBROBLASTS INFECTED WITH SEMLIKI FOREST VIRUS. Virology. 1965 Mar;25:340–349. doi: 10.1016/0042-6822(65)90053-x. [DOI] [PubMed] [Google Scholar]


