Abstract
Objective
Intercellular conduction of electrical signals underlies spreading vasodilation of resistance arteries. Small and intermediate-conductance Ca2+ activated K+ channels (SKCa/IKCa) of endothelial cells serve a dual function by initiating hyperpolarization and modulating electrical conduction. We tested the hypothesis that the regulation of electrical signaling by SKCa/IKCa is altered with advancing age.
Approach and Results
Intact endothelial tubes (60 μm wide; 1-3 mm long) were freshly isolated from male C57BL/6 mouse (Young: 4-6 months; Intermediate: 12-14 months; Old: 24-26 months) superior epigastric arteries. Using dual intracellular microelectrodes, current was injected (±0.1-3 nA) at site 1 while recording membrane potential (Vm) at site 2 (separation distance: 50-2000 μm). Across age groups, greatest differences were observed between Young and Old. Resting Vm in Old (−38±1 mV) was more negative (P<0.05) than Young (−30±1 mV). Maximal hyperpolarization to both direct (NS309) and indirect (acetylcholine) activation of SKCa/IKCa was sustained (ΔVm ~ −40 mV) with age. The length constant (λ) for electrical conduction was reduced (P<0.05) from 1630±80 µm (Young) to 1320±80 μm (Old). Inhibiting SKCa/IKCa with apamin + charybdotoxin or scavenging H2O2 with catalase improved electrical conduction (P<0.05) in Old. Exogenous H2O2 (200 μM) in Young evoked hyperpolarization and impaired electrical conduction; these effects were blocked by apamin + charybdotoxin.
Conclusions
Enhanced current loss through KCa activation impairs electrical conduction along the endothelium of resistance arteries with aging. Attenuating the spatial domain of electrical signaling will restrict the spread of vasodilation and thereby contribute to blood flow limitations associated with advanced age.
Keywords: endothelial dysfunction, ion channels, oxidative stress
Introduction
Aging is associated with endothelial dysfunction 1,2, 3, a disorder characterized by impaired vasodilation in response to acetylcholine (ACh) 4-6, to muscular exercise 7, or to heating the skin 8. As a stimulus that is well-defined in its actions, ACh application triggers endothelium-dependent vasodilation by increasing the production of nitric oxide (NO) and/or activating small (KCa2.3, KCNN3)- and intermediate (KCa3.1, KCNN4)-conductance calcium-activated K+ channels (SKCa/IKCa). The bioavailability of NO decreases with advancing age 5, 11 and the function of endothelial SKCa/IKCa may be altered in pathological states 12, 13. However the effect of aging on endothelial SKCa/IKCa function has not been determined, particularly in light of impairments in blood flow that accompany advancing age 7, 14, 15. Cell-to-cell signaling through gap junctions is integral to endothelial function. Once initiated, hyperpolarization spreads rapidly along the endothelium and through myoendothelial junctions to relax smooth muscle cells (SMCs) 16, 17. By synchronizing vasomotor responses in resistance networks, the conduction of electrical signals along the endothelium serves to coordinate blood flow control along and among vessel branches 18, 19. Nevertheless, the spatial domain of endothelial signaling has received little attention in the context of aging.
In previous studies, conducted vasodilation in response to ACh 20 and ascending vasodilation in response to skeletal muscle contraction 14 were decreased in Old (20 month) vs. Young (3 month) male C57BL/6 mice. While the mechanism underlying this functional deficit has remained undefined, altered cell-to-cell coupling through gap junctions 21 could underlie impaired conduction. An alternative mechanism entails greater leakage of current through ion channels in plasma membranes, thereby precluding transmission of electrical signals along the endothelium 22. The activation of SKCa/IKCa initiates endothelial cell (EC) hyperpolarization and vasodilation 9, 10, 16, 23. Recent findings have revealed a role for SKCa/IKCa activation in modulating the spread of electrical signals along the endothelium of resistance arteries 22. Thus, changes in SKCa/IKCa function with advancing age may alter the ability of electrical signals to travel along the endothelium and thereby affect vasomotor control.
The present experiments were designed to define the ability of the endothelium to initiate and conduct electrical signals with advancing age. Using endothelial tubes freshly isolated from resistance arteries of skeletal muscle from Young (4-6 months), Intermediate (12-14 months) and Old (24-26 months) mice we tested the hypothesis that the dual function of SKCa/IKCa to initiate and modulate electrical signaling along the endothelium is altered with aging. Findings reported here are the first to show that, by enhancing ion channel activation (particularly IKCa) aging promotes hyperpolarization of the endothelium while impairing its ability to conduct electrical signals. This increase in ion channel activation is attributable to oxidative stress manifested through the actions of H2O2.
Methods and Materials
Animal care and use were approved by the University of Missouri Animal Care and Use Committee and comply with the Guide for the Care and Use of Laboratory Animals (National Research Council; 8th Ed., revised 2011). Male C57BL/6 mice were studied at 3-6 (n=60), 12-14 (n=9), and 24-26 (n=39) months of age. Mice received standard chow and tap water ad libitum. For experiments, endothelial tubes were freshly isolated from superior epigastric arteries of abdominal skeletal muscle. Details are provided in the online Supplemental Materials and Methods.
Results
Figures 1-7 illustrate Old vs. Young mice. Key values for the Intermediate age group are stated in the text with their summary data shown in Supplemental Figures II, III and V. Across experiments, resting Vm was more negative (P < 0.05) in Old (−38 ± 1; n=28) vs. Young (−30 ± 1; n=29) or Intermediate (−31 ± 1 mV; n=9).
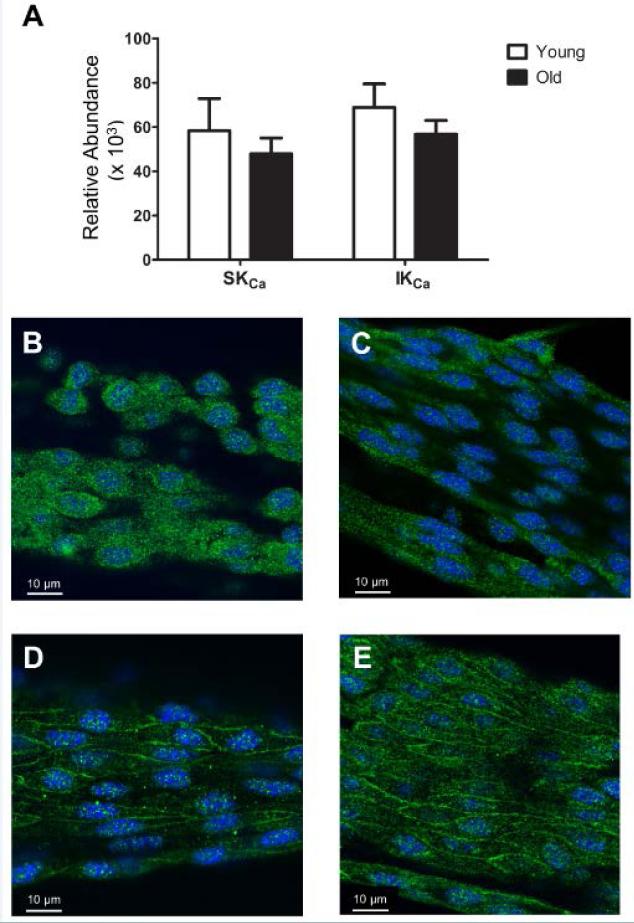
Figure 1. Expression of SKCa/IKCa in endothelial tubes of resistance arteries from Young and Old mice.
A, Abundance of mRNA for SKCa and IKCa relative to the expression of glucuronidase β (Gusb) in endothelium of Young and Old (n=5 per group). Summary data are means ± S.E. B, Single confocal slice image of an isolated endothelial tube from Young indicating SKCa in green and nuclei in blue. C, As in B, for Old. D, As in B for IKCa in Young. E, As in D for Old; each image represents at least three independent experiments.
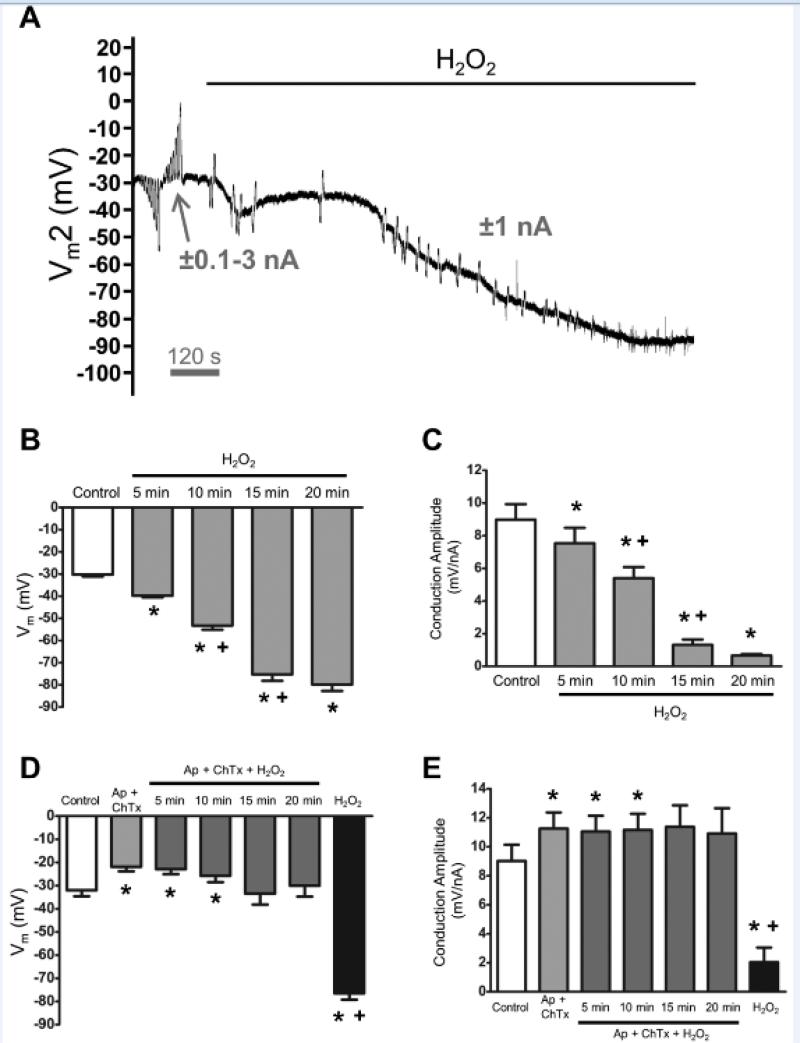
Figure 7. Endothelial hyperpolarization and loss of electrical conduction via SKCa/IKCa activation with H2O2.
A, Representative recording of membrane potential responses at 500 μm (Vm2) from current injected at site 1 before and during H2O2 (200 μmol/L) exposure. Note progressive hyperpolarization and loss Vm2 responses (with residual capacitance spikes). B, Summary data before (Control) and during effect of H2O2 on resting Vm over 20 minutes. C, Summary data before (Control) and during effect of H2O2 on Conduction Amplitude (distance = 500 μm) at times corresponding to those in B. D, Summary data for Vm before (Control) and during Ap (300 nmol/L) + ChTx (100 nmol/L), during H2O2 with Ap + ChTx for 20 minutes (note lack of hyperpolarization), and after washout of Ap + ChTx with H2O2 still present (note hyperpolarization to ~−80 mV). E, Conduction Amplitude (distance = 500 μm) at times corresponding to those in D. During H2O2 exposure, note maintenance of CA with Ap + ChTx present and loss of CA following their washout. *P < 0.05 vs. Control; +P < 0.05 vs. preceding time point. Summary data are means ± S.E.; n=6-8 per group. Data in B and C were obtained together in one set of experiments; Data in D and E were obtained together in a separate set of experiments. All data in this Figure are based upon continuous recordings from endothelial tubes of Young mice. See complementary data in Supplemental Figure VIII.
Expression of connexins and SKCa/IKCa in endothelial tubes
The mRNA transcript expression for SKCa and IKCa (Figure 1A) and connexins (Cx37, Cx40, and Cx43) (Supplemental Figure IA) was similar between Young and Old. Fluorescence immunolabeling confirmed the presence of respective KCa (Figures 1B-1E) and connexin proteins (Supplemental Figures IB-IG).
Endothelial hyperpolarization to acetylcholine is sustained with aging while intercellular transmission of electrical signals is reduced
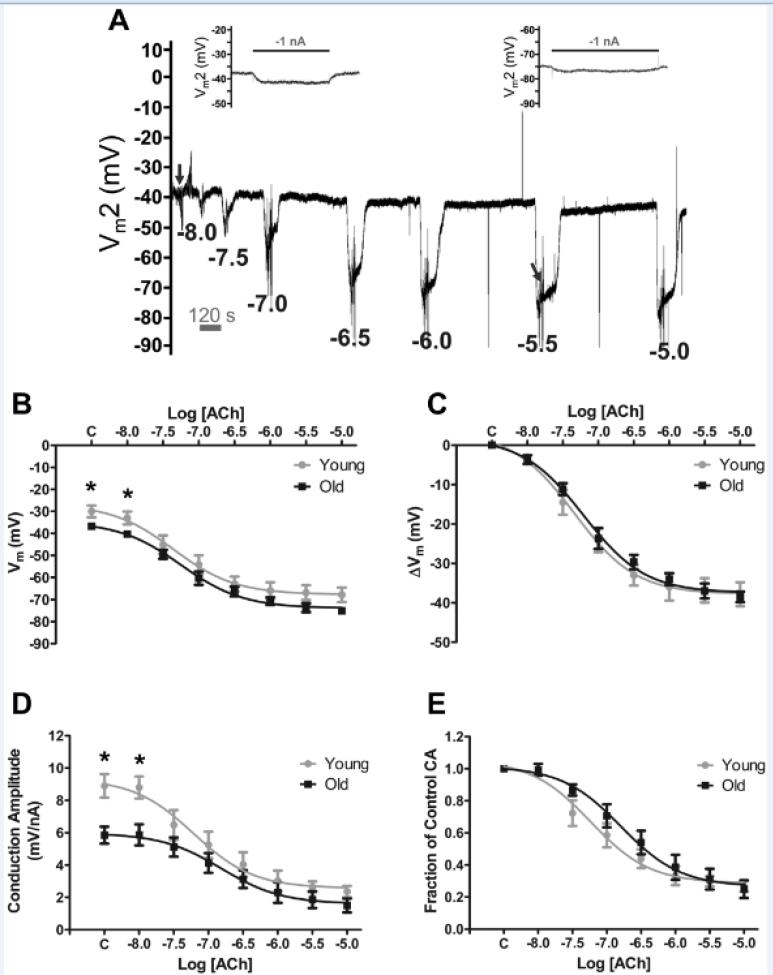
It is unknown whether the ability of ACh to initiate hyperpolarization in resistance artery endothelium is altered with advancing age. Thus, we determined whether hyperpolarization to ACh was affected by age. Figure 2A illustrates progressive hyperpolarization in response to cumulative [ACh] from 10−8 to 10−5 M with washout and recovery between exposures. Neither the pEC50 for hyperpolarization (Young: 7.29 ± 0.10, Intermediate: 7.48 ± 0.11, Old: 7.16 ± 0.11) nor the maximum response to ACh (ΔVm; Young: −38 ± 3 mV, Intermediate: −41 ± 3 mV, Old: −39 ± 1 mV; n=6 per group) differed between groups (Figures 2B, 2C; Supplemental Figures IIA, IIB).
Figure 2. Effects of ACh on membrane potential and electrical conduction in endothelial tubes of resistance arteries from Young and Old mice.
A, Representative membrane potential recording within an endothelial tube from an Old mouse illustrating hyperpolarization to increasing [ACh] with washout and recovery between each [ACh]. Electrical responses to current injected (±1-3 nA pulses, 2s each) at site 1 were recorded at site 2 (Vm2) with separation distance = 500 μm during control (left inset corresponds to trace under left arrow; see Figure 3A for expanded trace) and during peak hyperpolarization to each [ACh] (right inset corresponds to trace under right arrow). During hyperpolarization, note loss of Vm2 responses with residual capacitance spikes. For B-E, “C” on X-axes refers to Control values at rest; B, Effect of [ACh] on resting Vm in Young and Old. C, Effect of [ACh] on the change in Vm (ΔVm) from Control for Young and Old. D, Effect of [ACh] on Conduction Amplitude (CA) in Young and Old (for −1nA). E, Effect of [ACh] on Fraction of CA (= CA at each [ACh] / respective Control value in D. *P < 0.05, Young vs. Old. (n = 6 per group). Summary data are means ± S.E.
To test the efficacy of cell-to-cell electrical coupling along the endothelium, current (±1-3 nA) was injected into one EC (site 1) while Vm was recorded from site 2 at constant separation distance (500 μm). Under control conditions conduction amplitude (CA) for Old (5.9 ± 0.5 mV/nA) was ~66% of Young (8.9±0.7 mV/nA) and ~57% of Intermediate (10.4 ± 0.7 mV/nA) (Figure 2D; Supplemental Figure IIC). Acetylcholine reduced CA in all groups in a concentration-dependent manner (Figure 2D; Supplemental Figure IIC). Attributable to their lower initial values for CA (Figure 2D), endothelial tubes from Old tended to maintain a higher Fraction of Control CA compared to Young during exposure to submaximal concentrations (< 1 μmol/L) of ACh (Figure 2E). This difference between age groups was significant (P < 0.05) for Old vs. Intermediate (Supplemental Figure IID).
Aging decreases the length constant for electrical conduction
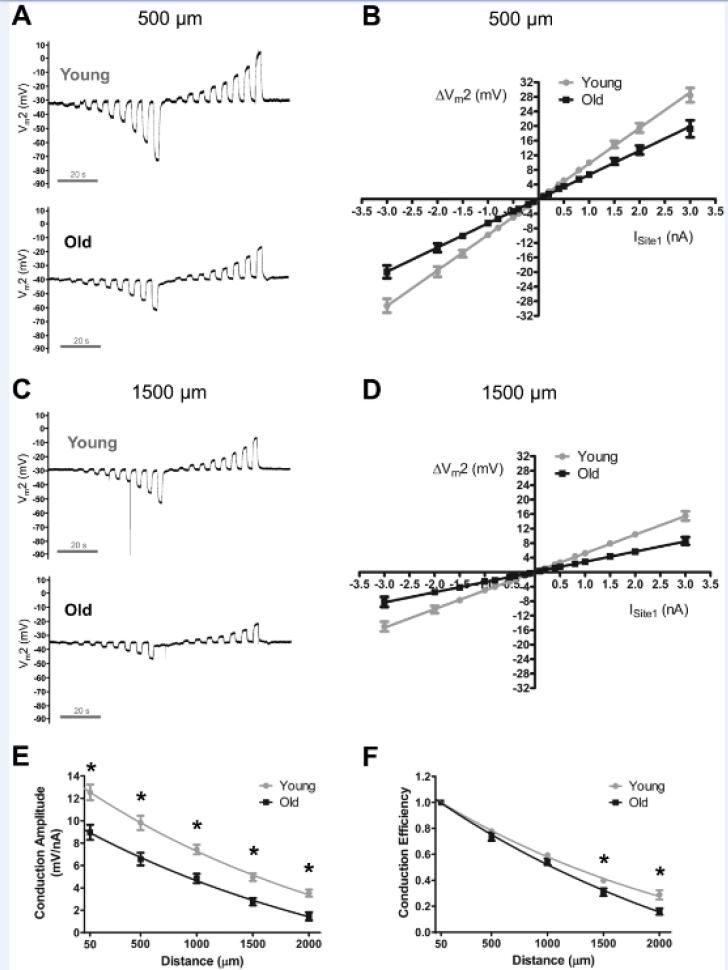
In light of electrical conduction along the endothelium being integral to conducted vasodilation 23, 24, and finding that CA at a constant separation distance was impaired in Old during control conditions, we investigated whether aging would alter the effective distance of electrical conduction. As shown in Figures 3B and 3D, ΔVm at site 2 (ΔVm2) was related linearly (R2 ≥ 0.99) to the amplitude and polarity of current injected at site 1 for Young and Old. The slope of the current-voltage (I-V) relationship decreased with age and with distance (Figure 3B vs. 3D; Supplemental Figure IIIA vs. IIIB). Nevertheless, CA at each distance was reduced (P < 0.05) for Old compared to Young (Figure 3E; Table 1) or Intermediate (Supplemental Figure IIIC). The calculated length constant for electrical conduction (λ) was greater (P < 0.05) in Young (1630 ± 80 μm, n=12) and Intermediate (1900 ± 90, n=8) compared to Old (1320 ± 80 μm, n=9). When CA at each distance was normalized to respective local values, Conduction Efficiency was reduced (P<0.05) at 1500-2000 μm for Old vs. Young (Figure 3F) or Intermediate (Supplemental Figure IIID). Increasing the amount of current injected for Old (by 40%) to achieve the same absolute CA at the local site as Young confirmed significantly greater spatial decay in Old (Table 1).
Figure 3. Electrical conduction along the endothelium of resistance arteries is impaired in Old vs. Young mice.
A, Membrane potential was recorded at site 2 (Vm2) located 500 μm from current injected at site 1 (±0.1 to 3 nA). Changes in Vm2 were related linearly to the amplitude and polarity of current injected at site 1. Note more negative Vm and diminished (~30%) ΔVm responses in Old vs. Young at each level of current injection. B, Summary data for experiments illustrated in A at 500 μm distance. Note lower (P < 0.05) slope of Old vs. Young (Young: 9.8 ± 0.7 mV/nA, n=12; Old: 6.6 ± 0.6 mV/nA, n=9). C, As in A with site 2 located 1500 μm from site 1. Note lower responses compared to A. D, As in B for site 2 at distance = 1500 μm [(Young: 5.0 ± 0.3 mV/nA (n=12), Old: 2.8 ± 0.3 mV/nA (n=9)]; the slope of respective I-V relationships decreased as distance increased. E, Conduction Amplitude vs. distance. At each distance, values for Old were depressed relative to values for Young; data are in response to −1 nA current injection. F, Conduction Efficiency = data from E normalized to respective CA at local (50 μm) site; note relatively greater decay with distance in Old. Calculated length constant for electrical conduction (λ) was greater (P < 0.05) in Young (1630 ± 80 μm, n=12) versus Old (1320 ± 80 μm, n=9). *P < 0.05, Young vs. Old. Resting Vm was greater (P < 0.05) in Old (−36 ± 2 mV) vs. Young (−28 ± 2 mV). Summary data are means ± S.E.
Table 1.
Spatial decay of electrical conduction is greater in Old vs. Young.
| Distance (μm) | Young (-1nA) ΔVm2 (mV) | Old (-1nA) ΔVm2 (mV) | Old (-1.4nA) ΔVm2 (mV) |
|---|---|---|---|
| 50 | -12.5 ± 0.7 | -9.0 ± 0.7* | -12.5 ± 0.9 |
| 500 | -9.8 ± 0.6 | -6.6 ± 0.6* | -9.1 ± 0.8 |
| 1000 | -7.4 ± 0.4 | -4.8 ± 0.4* | -6.7 ± 0.6 |
| 1500 | -5.0 ± 0.3 | -2.7 ± 0.3* | -3.8 ± 0.5* |
| 2000 | -3.5 ± 0.3 | -1.5 ± 0.4* | -2.0 ± 0.5* |
The standard current pulse microinjected at site 1 to evaluate a change in membrane potential at site 2 (ΔVm2) at distances of 50-2000 μm was -1 nA. The ΔVm2 response to -1 nA was reduced at all distances in Old (Column 3) vs. Young (Column 2). To achieve the same ΔVm2 at the nearest distance (50 μm) required ~ 40% more current (-1.4 nA; Column 4) in Old. Despite the same ΔVm2 at 50 μm; note progressively greater signal loss with distance in Old vs. Young (compare Column 2 with Column 4). These data are complementary to Figure 3E, F.
P < 0.05 vs. Young ΔVm2 responses to -1 nA at the same distances (n = 12 for Young, n = 9 for Old).
Aging decreases the effect of SKCa/IKCa activation on electrical conduction
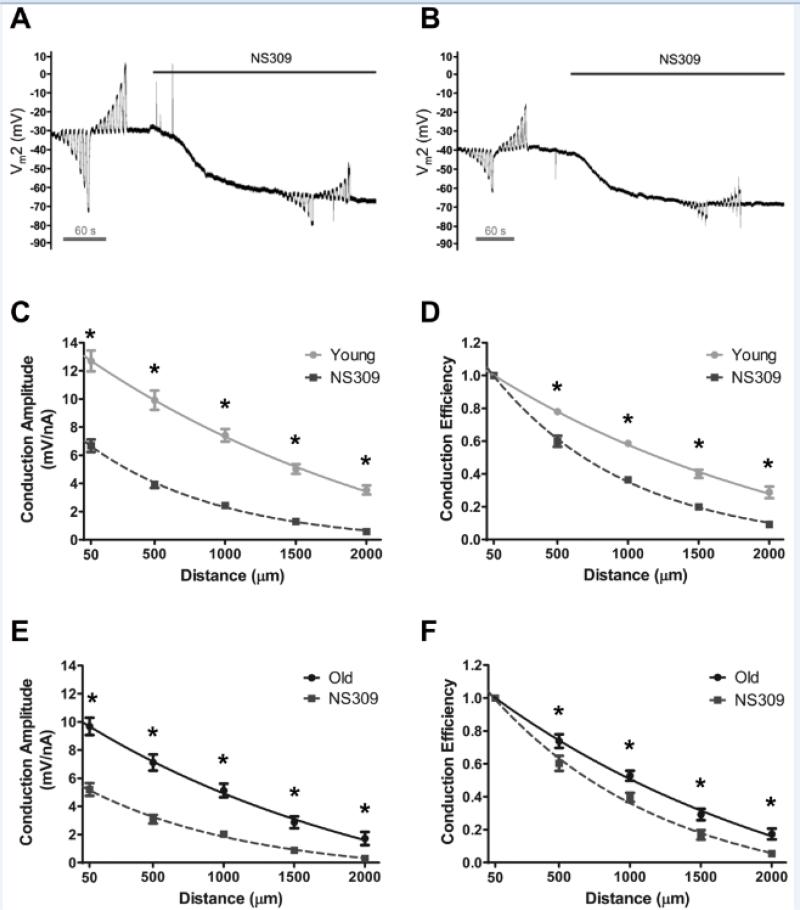
Given that aging reduced CA and increased spatial decay, we tested whether the effect of direct SKCa/IKCa activation on electrical conduction would vary with age. NS309 (1 μmol/L) reduced CA at all distances (P < 0.05) for endothelial tubes from Young and Old (Figures 4C, 4E. See Supplemental Figures IVC, IVD for concentration-dependent effects of NS309 on CA at 500 μm) as well as from the Intermediate group (Supplemental Figure V). For all age groups, the reduction in electrical conduction during NS309 treatment was also significant (P<0.05) with data expressed as Conduction Efficiency (Figures 4D, 4F; Supplemental Figure VB). The difference between Control and NS309 treatment was greater in Young than in Old (Figure 4). Thus the reduction in λ by NS309 was also greater in Young vs. Old (diminished by 690 ± 110 μm vs. 320 ± 90 μm, respectively; P < 0.05). Nevertheless, and consistent with ΔVm responses to ACh, the magnitude of hyperpolarization to NS309 did not differ between age groups (ΔVm = −33 ± 2 mV each; See Supplemental Figures IVA, IVB for concentration-dependent effects of NS309 on Vm).
Figure 4. Impairment of electrical conduction during SKCa/IKCa activation is greater along endothelium of Young vs. Old mice.
A, Representative recording of membrane potential responses at 500 μm (Vm2) from current injected at site 1 (±0.1 to 3 nA) before and during SKCa/IKCa activation with NS309 (1 μmol/L) in endothelial tube of Young. B, As in A for Old. The effect of SKCa/IKCa activation with NS309 on Conduction Amplitude (Panels C and E) and Conduction Efficiency (Panels D and F) vs. distance. Panels C and D, Young; Panels E and F, Old. NS309 reduced the amplitude and efficiency of electrical conduction across age groups with relatively greater effects in Young vs. Old (n=7 for Old, n=10 for Young). *P < 0.05 vs. NS309. Summary data are means ± S.E.
SKCa/IKCa blockade restores electrical conduction in Old
In light of evidence indicating greater SKCa/IKCa activity in Old, we hypothesized that blocking SKCa/IKCa would enhance electrical conduction to a greater extent in endothelial tubes of Old vs. Young. This was tested by injecting current and recording Vm2 continuously (at distance = 500 μm) before and during exposure to apamin (Ap; 300 nmol/L) and/or charybdotoxin (ChTx; 100 nmol/L) (Figures 5A and 5B). Blockade of SKCa/IKCa depolarized Old by 16 ± 2 mV (to −24 ± 2 mV; n=11) which was a greater (P < 0.05) effect than for Young (10±1 mV depolarization to −22 ± 1 mV; n=11). During Ap + ChTx, CA increased more (P < 0.05) in Old (57 ± 6%) vs. Young (24 ± 4%) thus CA in Old was restored to that of Young (Figure 5C). Expressing CA values as Fraction of Control (Figure 5D) illustrated the relatively greater effect of SKCa/IKCa blockade on electrical conduction in Old vs. Young. Experiments in which apamin or charybdotoxin were applied individually indicated a prominent role for IKCa in dissipating injected current (Figure 5C and 5D).
Figure 5. Enhanced electrical conduction during SKCa/IKCa blockade is greater along endothelium of Old vs. Young mice.
A, Representative recording of membrane potential responses at 500 μm (Vm2) from current injected at site 1 (±0.1 to 3 nA) in endothelial tube of Young. Note slight depolarization and enhanced Vm2 responses during apamin (Ap, 300 nmol/L) + charybdotoxin (ChTx, 100 nmol/L). B, As in A for Old. Note more negative resting Vm with reduced Vm2 responses vs. Young. During Ap + ChTx, note greater depolarization and enhancement of Vm2 responses vs. Young. C, Summary data (means ± S.E.) for conduction amplitude (CA) at rest (Control) and during Ap alone (n=5), ChTx alone (n=5) and in combination (n=11) within respective age groups. D, Data from C expressed as Fraction of Control CA within respective age groups. In C and D, note greater effect of ChTx vs, Ap on restoring CA in Old. *P<0.05 vs. Old for respective condition; +P<0.05 vs. respective Young Control; #P<0.05 vs. respective Old Control.
Scavenging H2O2 with catalase improves electrical conduction in Old
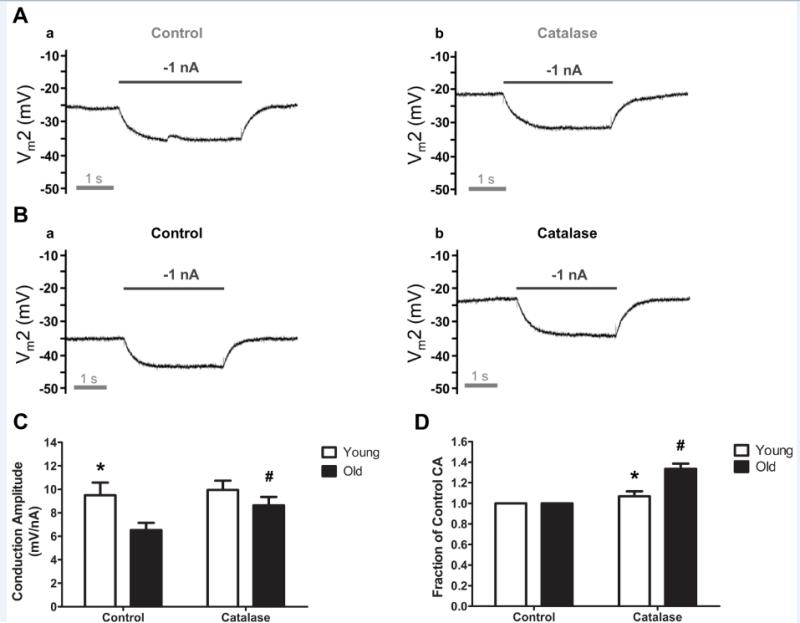
Aging and endothelial dysfunction have been attributed to heightened oxidative stress 11, 25, 26 including excess production of hydrogen peroxide (H2O2) 11, 27, 28. As H2O2 may alter the activity of Kca29, 30, we tested whether scavenging H2O2 with membrane-permeant catalase would improve electrical conduction. Catalase (500 U/ml) depolarized Vm by 3 ± 1 mV in Young (n=6; e.g., Figure 6A) and by 9 ± 1 mV in Old (n=7; e.g., Figure 6B) such that resting Vm (~ −28 mV) was no longer different between age groups. With negligible effect on CA of Young, catalase increased both absolute (Figure 6C) and relative CA (Figure 6D) of Old by ~30% (P<0.05). In complementary experiments (Supplemental Figure VI), the presence of catalase had little effect on hyperpolarization to ACh (3 µmol/L) for either age group (n=4 each). In response to NS309 (1 μmol/L), the presence of catalase reduced hyperpolarization of Young by 8 ± 1 mV and increased hyperpolarization of Old by 4 ± 1 mV (Supplemental Figure VI).
Figure 6. Catalase improved electrical conduction along endothelium of Old vs. Young mice.
A, Representative recording of membrane potential responses at 500 μm (Vm2) from current injected at site 1 in Young illustrating response to −1 nA before (a) and during catalase (500 U/ml, 20 minutes) to scavenge H2O2. (b). Note slight depolarization during catalase while V m2 response (−10 mV) to −1 nA current was maintained. B, As in A for Old. Note greater depolarization from Control vs. Young and enhanced Vm2 responses during catalase (Control: −7 mV, catalase: −10 mV). C, Summary data (means ± S.E.) for Conduction Amplitude at 500 μm distance under respective conditions for Young (n=6) and Old (n=7). D, Data from C normalized as Fraction of Control. * P<0.05 vs. Old for respective condition; #P<0.05 vs. Old Control.
Inhibiting endothelial nitric oxide synthase does not improve electrical conduction in Old
As the bioavailability of endothelium-derived NO decreases with advancing age 5, 11, we tested whether uncoupled endothelial nitric oxide synthase was a source of H2O2 31. Endothelial tubes from Old treated were treated with the inhibitor NG-nitro-L-arginine methyl ester (L-NAME; 100 μmol/L, 20 minutes). We found no significant effect on either resting Vm (Control: −41 ± 2 mV; L-NAME: −40 ± 4 mV, n = 3) or CA (at 500 μm: Control = 7.0 ± 1.0 mV/nA; L-NAME = 7.6 ± 1.2 mV/nA, n = 3).
SKCa/IKCa activation by H2O2 impairs electrical conduction
In light of catalase restoring Vm and conduction of Old to approximate values of Young, we tested whether exogenous H2O2 would hyperpolarize endothelial tubes and impair electrical conduction of Young. Addition of H2O2 (200 μmol/L) hyperpolarized Vm progressively over time, approximating the equilibrium potential for K+ (EK; ~−90 mV) after ~ 20 minutes (Figures 7A and 7B). Conduction Amplitude decreased as hyperpolarization increased (Figures 7A and 7C). In separate experiments, inclusion of Ap + ChTx prevented changes in Vm and CA during H2O2 exposure (Figures 7D and 7E; Supplemental Figures VII and VIII). Upon washout of Ap + ChTx, H2O2 evoked hyperpolarization and inhibited CA (Figures 7D and 7E; Supplemental Figures VII and VIII).
Discussion
Blood flow to skeletal muscle is attenuated with aging but the underlying mechanisms have remained poorly defined. Evidence has pointed to a role for enhanced sympathetic neuroeffector signaling 14, 15 however little is known of changes that may occur within the vascular wall. Intrinsic to blood flow control in resistance networks is the conduction of electrical signals along the endothelium to coordinate SMC relaxation 17, 32, 33. The present study has determined that the ability of the endothelium of skeletal muscle resistance arteries to conduct electrical signals is impaired with aging. The use of intact endothelial tubes freshly isolated from mouse superior epigastric arteries 22, 34, 35 enabled these changes to be resolved independent of blood flow or surrounding cells. Remarkably, conduction along the endothelium of Old was restored to that of Young upon selective blockade of SKCa/IKCa, particularly IKCa. Complementary experiments demonstrate that either direct (with NS309) or indirect (with ACh) activation of SKCa/IKCa in endothelial tubes of Young produced effects that mimicked the behavior of Old. In light of the association between aging and oxidative stress 11, 25-27 and reports that H2O2 may alter the activity of KCa 29, 30, treating endothelial tubes of Old with catalase restored electrical conduction in a manner consistent with the effects of blocking IKCa with charybdotoxin. In a reciprocal manner, treating endothelial tubes of Young with H2O2 impaired electrical conduction and this effect was also inhibited with SKCa/IKCa blockade. These data collectively support the hypothesis that, via the actions of H2O2, more KCa channels are open under resting conditions in Old vs. Young. In turn, the diminished resistance of cell membranes enables electrical signals to “leak” as they travel along the endothelium, reducing the spatial domain of electrical signaling 22.
Impact of age on electrical conduction: Effects of SKCa/IKCa activation
Endothelial dysfunction is characterized by impaired endothelium-dependent vasodilation 1. While such changes have been attributed to impaired NO bioavailability and signaling 2, 5, endothelium dependent hyperpolarization (EDH) initiated through SKCa/IKCa activation predominates as a signal (via myoendothelial coupling) for SMC relaxation in resistance vessels 10, 17, 32, 33. Alterations in SKCa/IKCa function have been associated with vascular disease 12, 13; nevertheless, it has not been determined how these ion channels may be affected by aging. Nor has it been determined what consequences such changes may have on endothelial function, particularly in regard to the initiation and conduction of electrical signals. We show here that the ability of either direct (NS309; Supplemental Figure IVB) or indirect (ACh; Figure 2C) SKCa/IKCa activation to produce hyperpolarization was preserved in endothelial tubes of Old. Despite a more negative resting Vm in Old vs. Young, the consistency of hyperpolarization to ACh indicates that the G-protein coupled signaling events underlying SKCa/IKCa activation 9, 10, 12 were maintained in endothelial tubes of Old. This finding is in contrast to reports that ACh-induced hyperpolarization of mesenteric arteries preconstricted with norepinephrine was greater in Young (1-8 month) vs. Old (20-26 month) rats 36, 37. Others have reported diminished sensitivity for relaxation to SKCa/IKCa activation (NS309) in saphenous arteries of 64-week vs. 12-week male mice 38. However a differential effect of NS309 was not apparent for hyperpolarization of endothelial tubes of Old vs. Young (Supplemental Figure IV). Such differences between preparations illustrates the use of the endothelial tube as a model to evaluate properties intrinsic to the endothelium to avoid the influence of smooth muscle activation, which can alter endothelial function via signaling through myoendothelial gap junctions 39.
In accordance with the biophysical determinants of the electrical length constant [λ = (rm/ra)1/2], the ability of electrical signals to spread along the endothelium reflects: (1) the axial resistance to current flow between cells (i.e., ra), which is determined primarily by the patency of gap junctions; and (2) the “leakiness” of plasma membranes (i.e., rm; the membrane resistance to current flow), which can be determined by the activation of ion channels (e.g., SKCa/IKCa 22). Respective signaling proteins are well-expressed in endothelial tubes of both Old and Young (Figure 1, Supplemental Figure I). Throughout our experiments, resting Vm was consistently 5-10 mV more negative in endothelial tubes of Old vs. Young. These findings are consistent with the ~6 mV more negative resting Vm of hippocampal pyramidal neurons from Old (>36 months) vs. Young (2-3 months) rabbits, also attributed to enhanced Ca2+-activated K+ current in neurons of Old 40.
The linearity and stability of electrical responses (Figures 3B and 3D; Supplemental Figures IIIA and IIIB) enabled electrical recordings throughout a full range of current injections (±0.1-3 nA) at multiple distances (50 to 2000 μm). Under resting conditions, the λ we determined for endothelial tubes of Old was depressed by ~20% compared to Young (Figures 3E and 3F; Supplemental Figure III). Our functional experiments illustrate that activating SKCa/IKCa reduced λ (and CA) to a greater extent in Young (~40%) when compared to Old (~25%) (Figure 4). Further, depolarization and augmentation of electrical conduction during selective blockade of SKCa/IKCa with Ap + ChTx was significantly greater in Old vs. Young and attributable primarily to actions on IKCa (Figure 5). We therefore suggest that, compared to Young (or Intermediate, which exhibited properties close to those of Young; Supplemental Figures II and III), the endothelium of Old has more IKCa open at rest, thereby allowing greater current leak and signal dissipation 22. In addition, the restoration of electrical conduction along endothelial tubes of Old to levels not different from Young upon selective channel blockade (Figure 5C) indicates that gap junction patency was sufficient across age groups to maintain intercellular electrical coupling along the endothelium.
The endothelium can conduct depolarization as effectively as hyperpolarization (Figures 3A-3D; Supplemental Figures IIIA and IIIB) 22, 34. Such linearity of the I-V relationship indicates a negligible functional expression of voltage-sensitive ion channels [e.g. large-conductance Ca2+-activated K+ channels (BKCa)] within EC plasma membranes. However, this behavior is not the case in intact feed arteries of hamster skeletal muscle, where depolarization conducted with less efficacy compared to hyperpolarization 32. This deviation from linearity can also be explained by myoendothelial coupling to SMCs 32, as the latter express voltage-gated K+ channels which increase conductance (i.e., current leakage) upon depolarization 41. Further, given the ability of α1-adrenoreceptor activation on SMCs to activate SKCa/IKCa of ECs via myoendothelial coupling 39, the impairment of conducted vasodilation along intact vessels 20 can be depressed even further than the effects of aging on SKCa/IKCa by an associated increase in sympathetic drive 42. In turn, the present findings suggest how impaired ability of the endothelium to conduct electrical signals may underlie earlier in vivo observations in mouse skeletal muscle of decreased conducted vasodilation along arterioles 20 and impaired ascending dilation of feed arteries 14 with aging. Collectively, such effects may contribute to the restriction of blood flow and compromised oxygen delivery during physical activity in older (e.g., > 60 years) humans 2, 7.
Impact of oxidative stress on an isolated endothelial syncytium
Aging and endothelial dysfunction are associated with increased oxidative stress 11, 25, 26, 43-45. Lower production of reactive oxygen species (ROS; e.g. superoxide) was reported in aortae from long-living mice (Peromyscus leucopus) vs. conventional house mice (Mus musculus) 25. The ROS signaling pathway begins with superoxide produced by mitochondria, NADPH/xanthine oxidases, and uncoupled eNOS 11, 27, 31, 46. Excessive and highly reactive superoxide levels are converted to the stable intermediate H2O2 either spontaneously or through actions of superoxide dismutases 11, 27, 28. Catalase and glutathione peroxidase convert H2O2 into water and these enyzmes are expressed at higher levels in P. leucopus vs. M. musculus 25, underscoring the ability to metabolize H2O2 as a determinant of maximum lifespan potential.
Intracellular H2O2 can alter protein thiol groups to form disulfide bridges in and between proteins 28. Thus, the actions of H2O2 may be promiscuous in modifying ion channel function. The physiological actions of H2O2 may thereby be manifested through its actions on the ion channels that are expressed in a given cell type (Figure 1). This reasoning is consistent with the actions of H2O2 on endothelial tubes from Young (Figure 7), where the consequences of activating SKCa/IKCa (i.e., hyperpolarization and impaired electrical conduction) were similar to the actions of either indirect (with ACh; Figure 2) or direct (with NS309; Figure 4) channel activation. In turn, these findings are consisted with the ability of catalase to restore resting Vm and electrical conduction of Old to values not different from Young (Figure 6). The concentration of H2O2 used in the present study (200 μmol/L) is consistent with that used by others (~100 to 300 μmol/L) to evoke dilation of coronary arterioles 30, 47. Further, the effects we observed for H2O2 (as well as aging) were sensitive to SKCa/IKCa blockade with Ap + ChTx (Figures 7D and 7E; Supplemental Figures VII and VIII). Altogether, our study indicates that H2O2 activate SKCa/IKCa of resistance artery endothelium. However, it should also be recognized that the effects of oxidative stress on the endothelium shown here may be “masked” in intact vessels by the presence of SMCs and their activation of BKCa channels in response to H2O2 30. As the inhibition of eNOS had no effect (see Results), key questions raised by the present findings point to resolving mitochondrial vs. non-mitochondrial sources of H2O2 and direct vs. indirect (i.e., via increases in [Ca2+]i48 or altered phosphorylation by protein kinase G I-α 49) activation of KCa by oxidative stress.
Summary
Our goal in this study was to determine the ability of the endothelium of resistance arteries from mouse skeletal muscle to initiate and conduct electrical signals with advancing age independent from the prevailing influence of blood flow, smooth muscle cells or other vasoactive stimuli. We focused on the roles of SKCa/IKCa to initiate hyperpolarization and to modulate the transmission of electrical signaling. Our findings demonstrate that the function of SKCa/IKCa to generate hyperpolarization was sustained with advancing age. However, more SKCa/IKCa (particularly IKCa) were open at rest in the endothelium of old animals which resulted in a more negative resting membrane potential and diminished electrical conduction, attributable to greater signal dissipation via charge loss through the plasma membrane. Scavenging H2O2 or blocking SKCa/IKCa channels (particularly IKCa) depolarized the endothelium of Old and restored electrical conduction to values not different from the endothelium of Young. Conversely, exposing endothelial tubes of Young to H2O2 produced hyperpolarization and reduced electrical conduction and these effects were also prevented by blocking IKCa alone or together with SKCa. The present findings are the first to highlight the effect of aging on the role of KCa channels in governing the initiation 9, 10 and transmission of electrical signals 22 within vascular endothelium. Endothelial SKCa/IKCa function thereby serves both to generate hyperpolarization underlying smooth muscle relaxation and to modulate the spread of vasodilatation along resistance networks. With aging, attenuating the spatial domain of electrical signaling will restrict spreading vasodilation and thereby contribute to blood flow limitations.
Supplementary Material
Significance.
Aging is associated with endothelial dysfunction, a disorder contributing to restricted muscle blood flow and compromised oxygen delivery during physical activity. The endothelium is instrumental in coordinating dilation within resistance networks by conducting electrical signals [e.g. hyperpolarization via activation of small- and intermediate- Ca2+ activated K+ channels (SKCa/IKCa)] that dilate resistance arteries to increase peak tissue blood flow. How advancing age impacts electrical signals underlying vasodilation is unknown. Using endothelial tubes freshly isolated from mouse superior epigastric arteries, we show that the initiation of hyperpolarization through SKCa/IKCa activation is sustained in old age while the spread of electrical signals is impaired. This functional decrement in electrical conduction along the endothelium is explained by loss of current through activated SKCa/IKCa (particularly IKCa) in response to oxidative stress. Attenuating the spatial domain of electrical signaling will impair spreading dilation of resistance arteries and can thereby restrict tissue blood flow.
Acknowledgments
None.
Sources of Funding
This work was supported by the National Heart, Lung and Blood Institute of the National Institutes of Health under award numbers R01-HL086483 (SSS), R37-HL041026 (SSS), F32-HL110701 (EJB) and F32-HL107050 (MJS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- ACh
acetylcholine
- Ap
apamin
- CA
conduction amplitude
- ChTx
charybdotoxin
- EC
endothelial cell
- eNOS
endothelial nitric oxide synthase
- H2O2
hydrogen peroxide
- SKCa/IKCa
small- and intermediate Ca2+-activated K+ channels
- NO
nitric oxide
- NS309
6,7-dichloro-1H-indole-2,3-dione 3-oxime
- ri
internal resistance to current flow between cells
- rm
membrane resistance to current flow
- Vm
membrane potential
Footnotes
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feletou M, Vanhoutte PM. Endothelial dysfunction: A multifaceted disorder (the Wiggers Award Lecture). Am J Physiol Heart Circ Physiol. 2006;291:H985–1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 2.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 2011;120:357–375. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gates PE, Strain WD, Shore AC. Human endothelial function and microvascular ageing. Exp Physiol. 2009;94:311–316. doi: 10.1113/expphysiol.2008.043349. [DOI] [PubMed] [Google Scholar]
- 4.James MA, Tullett J, Hemsley AG, Shore AC. Effects of aging and hypertension on the microcirculation. Hypertension. 2006;47:968–974. doi: 10.1161/10.1161/01.HYP.0000209939.05482.61. [DOI] [PubMed] [Google Scholar]
- 5.Muller-Delp JM. Aging-induced adaptations of microvascular reactivity. Microcirculation. 2006;13:301–314. doi: 10.1080/10739680600619023. [DOI] [PubMed] [Google Scholar]
- 6.Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- 7.Proctor DN, Parker BA. Vasodilation and vascular control in contracting muscle of the aging human. Microcirculation. 2006;13:315–327. doi: 10.1080/10739680600618967. [DOI] [PubMed] [Google Scholar]
- 8.Kenney WL, Morgan AL, Farquhar WB, Brooks EM, Pierzga JM, Derr JA. Decreased active vasodilator sensitivity in aged skin. Am J Physiol. 1997;272:H1609–1614. doi: 10.1152/ajpheart.1997.272.4.H1609. [DOI] [PubMed] [Google Scholar]
- 9.Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: Bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- 10.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 2006;21:69–78. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- 11.Muller-Delp JM, Gurovich AN, Christou DD, Leeuwenburgh C. Redox balance in the aging microcirculation: New friends, new foes, and new clinical directions. Microcirculation. 2012;19:19–28. doi: 10.1111/j.1549-8719.2011.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feletou M. Calcium-activated potassium channels and endothelial dysfunction: Therapeutic options? Br J Pharmacol. 2009;156:545–562. doi: 10.1111/j.1476-5381.2009.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grgic I, Kaistha BP, Hoyer J, Kohler R. Endothelial Ca2+-activated K+ channels in normal and impaired EDHF-dilator responses--relevance to cardiovascular pathologies and drug discovery. Br J Pharmacol. 2009;157:509–526. doi: 10.1111/j.1476-5381.2009.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson DN, Moore AW, Segal SS. Blunting of rapid onset vasodilatation and blood flow restriction in arterioles of exercising skeletal muscle with ageing in male mice. J Physiol. 2010;588:2269–2282. doi: 10.1113/jphysiol.2010.189811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinenno FA, Joyner MJ. Alpha-adrenergic control of skeletal muscle circulation at rest and during exercise in aging humans. Microcirculation. 2006;13:329–341. doi: 10.1080/10739680600618843. [DOI] [PubMed] [Google Scholar]
- 16.Domeier TL, Segal SS. Electromechanical and pharmacomechanical signalling pathways for conducted vasodilatation along endothelium of hamster feed arteries. J Physiol. 2007;579:175–186. doi: 10.1113/jphysiol.2006.124529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bagher P, Segal SS. Regulation of blood flow in the microcirculation: Role of conducted vasodilation. Acta Physiol (Oxf) 2011;202:271–284. doi: 10.1111/j.1748-1716.2010.02244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segal SS. Regulation of blood flow in the microcirculation. Microcirculation. 2005;12:33–45. doi: 10.1080/10739680590895028. [DOI] [PubMed] [Google Scholar]
- 19.Segal SS, Jacobs TL. Role for endothelial cell conduction in ascending vasodilatation and exercise hyperaemia in hamster skeletal muscle. J Physiol. 2001;536:937–946. doi: 10.1111/j.1469-7793.2001.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bearden SE, Payne GW, Chisty A, Segal SS. Arteriolar network architecture and vasomotor function with ageing in mouse gluteus maximus muscle. J Physiol. 2004;561:535–545. doi: 10.1113/jphysiol.2004.068262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Figueroa XF, Duling BR. Dissection of two Cx37-independent conducted vasodilator mechanisms by deletion of Cx40: Electrotonic versus regenerative conduction. Am J Physiol Heart Circ Physiol. 2008;295:H2001–2007. doi: 10.1152/ajpheart.00063.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Behringer EJ, Segal SS. Tuning electrical conduction along endothelial tubes of resistance arteries through Ca2+-activated K+ channels. Circ Res. 2012;110:1311–1321. doi: 10.1161/CIRCRESAHA.111.262592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emerson GG, Segal SS. Endothelial cell pathway for conduction of hyperpolarization and vasodilation along hamster feed artery. Circ Res. 2000;86:94–100. doi: 10.1161/01.res.86.1.94. [DOI] [PubMed] [Google Scholar]
- 24.Looft-Wilson RC, Payne GW, Segal SS. Connexin expression and conducted vasodilation along arteriolar endothelium in mouse skeletal muscle. J Appl Physiol. 2004;97:1152–1158. doi: 10.1152/japplphysiol.00133.2004. [DOI] [PubMed] [Google Scholar]
- 25.Csiszar A, Labinskyy N, Zhao X, Hu F, Serpillon S, Huang Z, Ballabh P, Levy RJ, Hintze TH, Wolin MS, Austad SN, Podlutsky A, Ungvari Z. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell. 2007;6:783–797. doi: 10.1111/j.1474-9726.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- 26.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 27.Bachschmid MM, Schildknecht S, Matsui R, Zee R, Haeussler D, R AC, Pimental D, Loo BV. Vascular aging: Chronic oxidative stress and impairment of redox signaling-consequences for vascular homeostasis and disease. Ann Med. 2013;45:17–36. doi: 10.3109/07853890.2011.645498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bychkov R, Pieper K, Ried C, Milosheva M, Bychkov E, Luft FC, Haller H. Hydrogen peroxide, potassium currents, and membrane potential in human endothelial cells. Circulation. 1999;99:1719–1725. doi: 10.1161/01.cir.99.13.1719. [DOI] [PubMed] [Google Scholar]
- 30.Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res. 2003;92:e31–40. doi: 10.1161/01.res.0000054200.44505.ab. [DOI] [PubMed] [Google Scholar]
- 31.Drouin A, Thorin-Trescases N, Hamel E, Falck JR, Thorin E. Endothelial nitric oxide synthase activation leads to dilatory H2O2 production in mouse cerebral arteries. Cardiovasc Res. 2007;73:73–81. doi: 10.1016/j.cardiores.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Emerson GG, Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: Role in vasomotor control. Circ Res. 2000;87:474–479. doi: 10.1161/01.res.87.6.474. [DOI] [PubMed] [Google Scholar]
- 33.Garland CJ, Hiley CR, Dora KA. EDHF: Spreading the influence of the endothelium. Br J of Pharmacol. 2011;164:839–852. doi: 10.1111/j.1476-5381.2010.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Behringer EJ, Socha MJ, Polo-Parada L, Segal SS. Electrical conduction along endothelial cell tubes from mouse feed arteries: Confounding actions of glycyrrhetinic acid derivatives. Br J Pharmacol. 2012;166:774–787. doi: 10.1111/j.1476-5381.2011.01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Socha MJ, Hakim CH, Jackson WF, Segal SS. Temperature effects on morphological integrity and Ca2+ signaling in freshly isolated murine feed artery endothelial cell tubes. Am J Physiol Heart Circ Physiol. 2011;301:H773–783. doi: 10.1152/ajpheart.00214.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujii K, Ohmori S, Tominaga M, Abe I, Takata Y, Ohya Y, Kobayashi K, Fujishima M. Age-related changes in endothelium-dependent hyperpolarization in the rat mesenteric artery. Am J Physiol. 1993;265:H509–516. doi: 10.1152/ajpheart.1993.265.2.H509. [DOI] [PubMed] [Google Scholar]
- 37.Goto K, Fujii K, Kansui Y, Iida M. Changes in endothelium-derived hyperpolarizing factor in hypertension and ageing: Response to chronic treatment with renin-angiotensin system inhibitors. Clin Exp Pharmacol Physiol. 2004;31:650–655. doi: 10.1111/j.1440-1681.2004.04054.x. [DOI] [PubMed] [Google Scholar]
- 38.Chennupati R, Lamers WH, Koehler SE, Mey JG. Endothelium-dependent hyperpolarization-related relaxations diminish with age in murine saphenous arteries of both sexes. Br J Pharmacol. 2013 doi: 10.1111/bph.12175. doi: 10.1111/bph.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tran CH, Taylor MS, Plane F, Nagaraja S, Tsoukias NM, Solodushko V, Vigmond EJ, Furstenhaupt T, Brigdan M, Welsh DG. Endothelial ca2+ wavelets and the induction of myoendothelial feedback. Am J Physiol Cell Physiol. 2012;302:C1226–1242. doi: 10.1152/ajpcell.00418.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Power JM, Wu WW, Sametsky E, Oh MM, Disterhoft JF. Age-related enhancement of the slow outward calcium-activated potassium current in hippocampal CA1 pyramidal neurons in vitro. J Neurosci. 2002;22:7234–7243. doi: 10.1523/JNEUROSCI.22-16-07234.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation. 2005;12:113–127. doi: 10.1080/10739680590896072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Behringer EJ, Segal SS. Spreading the signal for vasodilatation: Implications for skeletal muscle blood flow control and the effects of aging. J Physiol. 2012;590:6277–84. doi: 10.1113/jphysiol.2012.239673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fleenor BS, Seals DR, Zigler ML, Sindler AL. Superoxide-lowering therapy with tempol reverses arterial dysfunction with aging in mice. Aging Cell. 2012;11:269–276. doi: 10.1111/j.1474-9726.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: Impact of acute ascorbic acid administration. J Physiol. 2009;587:1989–2003. doi: 10.1113/jphysiol.2008.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dai DF, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circ Res. 2012;110:1109–1124. doi: 10.1161/CIRCRESAHA.111.246140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Widlansky ME, Gutterman DD. Regulation of endothelial function by mitochondrial reactive oxygen species. Antioxid Redox Signal. 2011;15:1517–1530. doi: 10.1089/ars.2010.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang LS, Reyes RA, Muller-Delp JM. Aging impairs flow-induced dilation in coronary arterioles: Role of NO and H2O2. Am J Physiol Heart Circ Physiol. 2009;297:H1087–1095. doi: 10.1152/ajpheart.00356.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bogeski I, Kappl R, Kummerow C, Gulaboski R, Hoth M, Niemeyer BA. Redox regulation of calcium ion channels: Chemical and physiological aspects. Cell Calcium. 2011;50:407–423. doi: 10.1016/j.ceca.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 49.Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, Schroder E, Browning DD, Eaton P. Cysteine redox sensor in PKG-I α enables oxidant-induced activation. Science. 2007;317:1393–1397. doi: 10.1126/science.1144318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.