Abstract
Objectives
To determine whether mean arterial blood pressure (MAP) excursions below the lower limit of cerebral blood flow (CBF) autoregulation during cardiopulmonary bypass (CPB) are associated with acute kidney injury (AKI) after surgery.
Setting
Tertiary care medical center.
Patients
Four hundred ten patients undergoing cardiac surgery with CPB.
Design
Prospective observational study.
Interventions
None.
Measurements and Main Results
Autoregulation was monitored during CPB by calculating a continuous, moving Pearson’s correlation coefficient between MAP and processed near-infrared spectroscopy signals to generate the variable cerebral oximetry index (COx). When MAP is below the lower limit of autoregulation, COx approaches 1, because CBF is pressure passive. An identifiable lower limit of autoregulation was ascertained in 348 patients. Based on the RIFLE criteria, AKI developed within 7 days of surgery in 121 (34.8%) of these patients. Although the average MAP during CPB did not differ, the MAP at the limit of autoregulation and the duration and degree to which MAP was below the autoregulation threshold (mmHg × min/hr of CPB) were both higher in patients with AKI than in those without AKI. Excursions of MAP below the lower limit of autoregulation (relative risk, 1.02, 95% confidence interval, 1.01 to 1.03, p<0.0001) and diabetes (relative risk, 1.78, 95% confidence interval, 1.27 to 2.50, p=0.001) were independently associated with for AKI.
Conclusions
Excursions of MAP below the limit of autoregulation and not absolute MAP are independently associated with for AKI. Monitoring COx may provide a novel method for precisely guiding MAP targets during CPB.
Keywords: Cerebral autoregulation, blood pressure, cardiac surgery, acute kidney injury
Introduction
Acute kidney injury (AKI) develops in 5% to 30% of patients after cardiac surgery, predisposing such patients to in-hospital and long-term mortality (1–3). Renal hypoperfusion resulting from hypotension during and after surgery is a potentially modifiable risk factor for AKI (4–6). Our group has evaluated whether real-time monitoring of cerebral blood flow (CBF) autoregulation may provide a more precise approach for determining mean arterial blood pressure (MAP) targets during cardiopulmonary bypass (CPB) than the current standard of care in which MAP targets are empirically chosen, often to 50 mmHg and transiently even lower (7–10). Importantly, we have found that slow waves (20 sec to 3 mins) of near-infrared spectroscopy (NIRS) signals provide a clinically suitable surrogate for CBF during autoregulation monitoring (11, 12). Since NIRS is noninvasive, continuous, and widely used during cardiac surgery for monitoring cerebral oxygen saturations, our methods could be widely applied. Like blood flow in the brain, blood flow to the kidney is autoregulated to ensure flow over a range of blood pressures (13). Optimizing MAP to ensure cerebral perfusion has been found to benefit the function of other organs, suggesting that the brain may serve as an “index organ” for determining appropriate MAP during CPB (14, 15). An additional aim of our ongoing investigations is to evaluate the relationship between blood pressure relative to the CBF autoregulation threshold and renal function. In this study we hypothesize that the duration and degree to which MAP remains below the lower limit of CBF autoregulation as determined with NIRS is associated with for AKI after cardiac surgery.
Methods
All procedures received the approval of the Institutional Review Board of The Johns Hopkins Medical Institutions and were performed after patients granted written informed consent. Patients undergoing elective coronary artery bypass graft (CABG) surgery and/or valvular surgery requiring CPB were enrolled between June 6, 2008, and June 29, 2011, as part of a parent study to evaluate the accuracy of NIRS for monitoring CBF autoregulation (7, 9). Patients with end-stage renal disease were excluded.
Perioperative Care
During surgery, the patients received routine institutional care that included direct radial artery blood pressure monitoring and a balanced anesthetic with midazolam, fentanyl, and isoflurane; pancuronium was administered for skeletal muscle relaxation (7, 8). CPB was achieved during surgery with nonpulsatile flow between 2.0 and 2.4 L/min/m2 by using a nonocclusive roller pump and a membrane oxygenator. The patients were managed with α-stat pH management. During CPB, isoflurane concentrations were held between 0.5% and 1.0% by using a vaporizer in line with oxygenator gas inflow. Normocarbia was maintained during CPB by varying gas flow based on arterial blood gas measurements from an oxygenator-based, continuous in-line arterial blood gas monitor that was calibrated hourly. Blood pressure levels during CPB and the rate of patient rewarming were determined by standard institutional practice. Postoperative care included continuous echocardiograph monitoring in the intensive care unit and on the postoperative ward.
NIRS-Based Autoregulation Monitoring
Before having anesthesia induced, the patients were connected to a NIRS monitor (INVOS, Somenetics, Inc., Boulder, CO) via sensors placed on the right and left sides of the forehead. The acquisition and analysis methods for processing NIRS signals and MAP have been described (7, 8). Briefly, analog arterial blood pressure signals were digitized and then processed along with digital NIRS signals by a personal computer that used ICM+ software (University of Cambridge, Cambridge, UK). Signals were filtered as non-overlapping 10-sec mean values that were time-integrated, a method that is equivalent to applying a moving average filter with a 10-sec time window and re-sampling at 0.1 Hz. The purpose of this process is to eliminate high-frequency components (e.g., the respiration and pulse waveforms). Additional high-pass filtering was performed with a DC cutoff set at 0.003 Hz to remove drifts such as those resulting from hem dilution. A continuous, moving Pearson’s correlation coefficient between MAP and NIRS signals was then calculated to generate the variable cerebral oximetry index (COx). Each calculation was carried out with consecutive, paired, 10-second averaged values from 300 sec duration, incorporating 30 data points for each index (7, 8). When MAP is within the limits of CBF autoregulation, COx approaches zero, but when MAP is outside the limits of autoregulation, COx approaches 1, indicating that CBF is blood pressure passive.
Acute Kidney Injury Definition
We defined AKI by comparing the maximal change in serum creatinine or estimated glomerular filtration rate (eGFR) in the first 7 postoperative days with baseline values measured before surgery. The primary outcome was AKI based on the RIFLE criteria: 1) Risk defined as an increase in plasma creatinine × 1.5 or decrease in eGFR by >25% from baseline; 2) Injury defined as an increase in plasma creatinine × 2 or a decrease in eGFR by >50% from baseline; and 3) Failure defined as an increase in plasma creatinine ×3, plasma creatinine ≥350 μmol/L, an acute rise in plasma creatinine of ≥44 μmol/L from baseline, or new renal replacement therapy (3, 16). Plasma creatinine was measured at the Clinical Chemistry Laboratory of The Johns Hopkins Hospital with commercial kits (Roche Diagnostics, Indianapolis, IN) that have a sensitivity of 0.1 mg/dL. Baseline serum creatinine was defined as the last value collected before surgery. Estimated glomerular filtration was calculated with the simplified Modification of Diet in Renal Disease (MDRD) formula (17).
Data Analysis
COx values for each patient during CPB were categorized into 5 mmHg MAP bins. The exact COx denoting the lower autoregulation threshold is not clear. The highest MAP associated with COx ≥0.3 was chosen as the lower limit of autoregulation (7, 11). Patients whose COx was ≥0.3 at all MAP and those without a clear autoregulation threshold were excluded from this analysis. Excursions below the lower limit of autoregulation were quantified considering both magnitude (mmHg) and duration (mins) as the area under the curve of the product of MAP versus time normalized for hour of CPB (mmHg × min/h) (18).
The patients were categorized according to whether they developed AKI. Continuous data between these groups were compared with analysis of variance (ANOVA) and with Bonferroni’s correction when multiple comparisons were performed. Dichotomous data were compared with Fisher’s exact test. Non-normally distributed data were log transformed before analysis. Generalized linear model with Poisson distribution and robust standard errors were used to identify variables independently associated with AKI and estimate the relative risk for AKI and the associated 95% confidence intervals. Variables with a value of p<0.2 based on univariate analysis were included in the modeling. Statistical analysis was performed with Stata software version 11 (StataCorp LP, College Station, TX).
Results
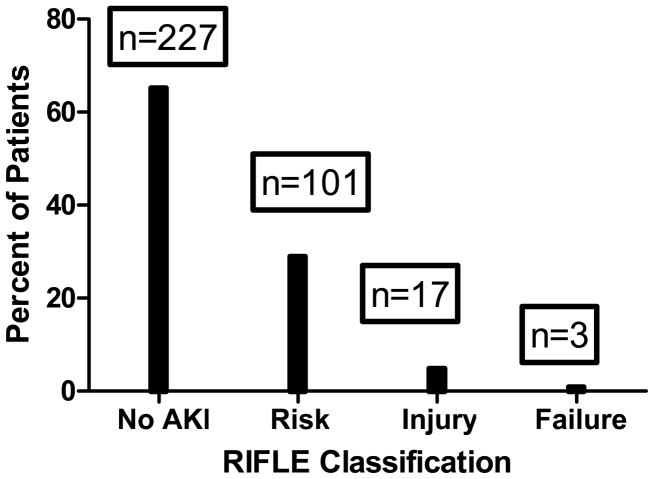
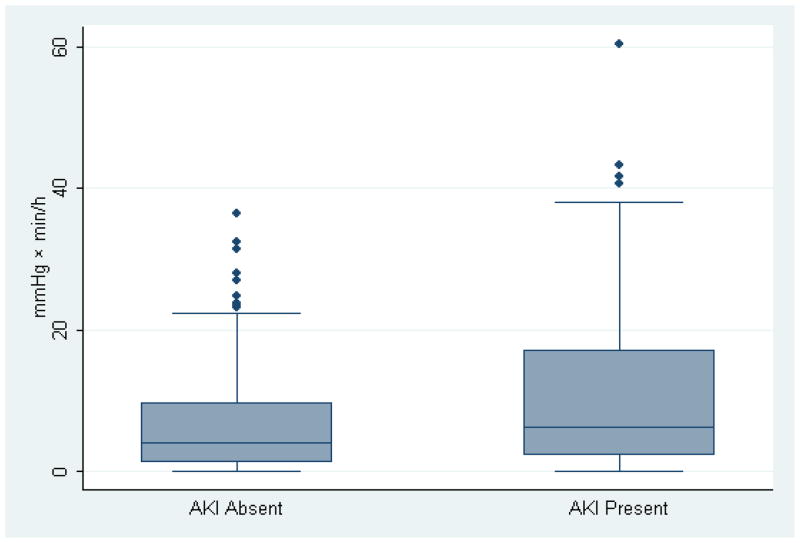
Autoregulation data from 410 patients were available for analysis. In 48 patients, COx was ≥0.3 at all MAPs, and in 14 patients, a clear autoregulation threshold with declining MAP did not occur. Of the remaining 348 patients, AKI occurred in 121 (34.8%) patients. Demographic and other patients characteristics of the patients included in this anlaysis are shown in Table 1. The percentage of patients with a history of diabetes, congestive heart failure, and aspirin use was greater in those with AKI than in those without AKI (Table 1). Compared to patients without AKI, those with AKI had a higher average pulse pressure, were more likely to have a pulse pressure > 60 mmHg, and had higher 30-day mortality. With the exceptions of more males and a lower frequency of preoperative angiotensin converting enzyme inhibitor therapy, there were no difference in between the 62 patients without a clear autoregulation threshold and the 348 patients included in the analysis (Table 2). A clinical COx recording from a patient during CPB demonstrating the methods for determining autoregulation thresholds is shown in Figure 1. Most episodes of AKI were in the risk category (Figure 2). Patients who developed AKI had similar MAPs during surgery but higher estimated lower limits of autoregulation (Table 3). Consequently, patients with AKI had a higher magnitude-duration of MAP below the lower limit of autoregulation. The distribution of the blood pressure data below the autoregulation limit for patients with and without AKI is shown in Figure 3. The data were skewed towards higher values with the median value lower in the patients without AKI compared with those with AKI. The normalized product of magnitude-duration of MAP below the lower limit of autoregulation during CPB (p<0.0001) and diabetes (p=0.001) were independently associated with AKI (Table 4).
Table 1.
Characteristics of patients who did and did not develop acute kidney injury (AKI) within 7 days after surgery.
| AKI (n=121) | No AKI (n=227) | P-value AKI vs No |
|
|---|---|---|---|
| Age (years) | 66±11 | 66±11 | 0.675 |
| Male gender (%) | 91 (75.2) | 173 (76.2) | 0.895 |
| Baseline creatinine (mg/dL) | 1.0±0.4 | 1.1±0.3 | 0.476 |
| Baseline eGFR (ml×min−1×1.73m−2) | 59±21 (53 to 66) | 56±23 (49 to 62) | 0.428 |
| Prior CVA (%) | 10 (8.3) | 20 (8.8) | 0.518 |
| COPD (%) | 11 (9.1) | 25 (11.0) | 0.359 |
| Current smoker (%) | 13 (10.7) | 43 (18.9) | 0.031 |
| PVD (%) | 14 (11.6) | 30 (13.2) | 0.398 |
| Hypertension (%) | 97 (80.2) | 161 (70.9) | 0.101 |
| Diabetes (%) | 52 (43.0) | 69 (30.4) | 0.013 |
| Congestive heart failure (%) | 25 (20.6) | 28 (12.3) | 0.030 |
| Prior myocardial infarction (%) | 37 (30.6) | 54 (23.8) | 0.107 |
| Prior cardiac surgery | 8 (6.6) | 17 (7.5) | 0.474 |
| Aspirin (%) | 91 (75.2) | 151 (66.5) | 0.057 |
| Beta-blockers (%) | 66 (54.5) | 135 (59.5) | 0.214 |
| ACE-I (%) | 119 (98.3) | 223 (98.2) | 0.279 |
| Statins (%) | 80 (66.1) | 141 (62.1) | 0.269 |
| Baseline pulse pressure (mmHg) | 68±20 (64 to 71) | 63±18 (61 to 65) | 0.029 |
| Pulse pressure ≥ 60 mmHg (%) | 84 (69.4) | 128 (56.4) | 0.01 |
| Type of Surgery | |||
| CABG (%) | 79 (65.3) | 138 (60.8) | 0.961 |
| CABG/AVR (%) | 13 (10.7) | 27 (11.9) | |
| CABG/MVR (%) | 3 (2.5) | 6 (2.6) | |
| AVR (%) | 19 (15.7) | 42 (18.5) | |
| MVR (%) | 7 (5.8) | 14 (6.2) | |
| CPB duration (min) | 106±38 (100 to 113) | 108±38 (103 to 113) | 0.787 |
| Cross clamp (min) | 68±25 (66 to 73) | 69±26 (65 to 72) | 0.978 |
| Postoperative atrial fibrillation (%) | 33 (27.2) | 65 (28.6) | 0.520 |
| Stroke (%) | 2 (1.7) | 8 (3.5) | 0.262 |
| Thirty-day mortality (%) | 6 (5.0) | 2 (0.9) | 0.023 |
eGFR, estimated glomerular filtration rate; CVA, cerebral vascular accident; COPD, chronic obstructive pulmonary disease; PVD, peripheral vascular disease; ACE-I, angiotensin converting enzyme inhibitors; statins, HMG-CoA reductase inhibitors; CABG, coronary artery bypass graft; AVR, aortic valve replacement; MVR, mitral valve replacement or repair.
Table 2.
Specific complication rates for patients with major morbidity and mortality after surgery and relationship with duration and magnitude of MAP below lower limit of cerebral blood flow autoregulation measured with cerebral oximetry index.
| Autoregulation Threshold (n=348) | No Threshold Determined (n=62) | P-value | |
|---|---|---|---|
| Age (years) | 66±12 | 65±9 | 0.4445 |
| Male gender (%) | 72.5 | 88.9 | 0.006 |
| Baseline creatinine (mg/dL) | 1.0± 0.3 | 1.1±0.3 | 0.7423 |
| Baseline eGFR (ml×min−1×1.73m−2) | 56±21 | 62±26 | 0.2853 |
| Prior CVA (%) | 8.3 | 9.7 | 0.802 |
| COPD (%) | 11.1 | 6.4 | 0.359 |
| Current smoker (%) | 16.0 | 16.1 | 1.0 |
| PVD (%) | 13.9 | 6.4 | 0.139 |
| Hypertension (%) | 74.2 | 75.8 | 0.896 |
| Diabetes (%) | 33.4 | 40.3 | 0.307 |
| Congestive heart failure (%) | 15.7 | 12.9 | 0.698 |
| Prior myocardial infarction (%) | 25.4 | 29.0 | 0.632 |
| Prior cardiac surgery | 7.0 | 8.1 | 0.786 |
| Aspirin (%) | 70.0 | 66.1 | 0.442 |
| Beta-blockers (%) | 58.2 | 54.8 | 0.569 |
| ACE-I (%) | 36.6 | 22.6 | 0.028 |
| Statins (%) | 62.0 | 71.0 | 0.305 |
| Baseline pulse pressure (mmHg) | 65±19 | 64±17 | 0.6989 |
| Pulse pressure ≥ 60 mmHg (%) | 62.0 | 55.5 | 0.393 |
| Type of Surgery | |||
| CABG (%) | 62.0 | 62.9 | 0.741 |
| CABG/AVR (%) | 11.8 | 9.7 | |
| CABG/MVR (%) | 2.1 | 4.8 | |
| AVR (%) | 18.1 | 16.1 | |
| MVR (%) | 5.9 | 6.4 | |
| CPB duration (min) | 108±39 | 105±32 | 0.6311 |
| Cross clamp (min) | 69±27 | 68±22 | 0.7204 |
| Postoperative Acute Kidney Injury (%) | 34.8 | 35.5 | 0.208 |
| Postoperative atrial fibrillation (%) | 40.8 | 36.2 | 0.622 |
| Stroke (%) | 3.6 | 0 | 0.219 |
| Thirty-day mortality (%) | 2.8 | 0 | 0.359 |
eGFR, estimated glomerular filtration rate; CVA, cerebral vascular accident; COPD, chronic obstructive pulmonary disease; PVD, peripheral vascular disease; ACE-I, angiotensin converting enzyme inhibitors; statins, HMG-CoA reductase inhibitors; CABG, coronary artery bypass graft; AVR, aortic valve replacement; MVR, mitral valve replacement or repair.
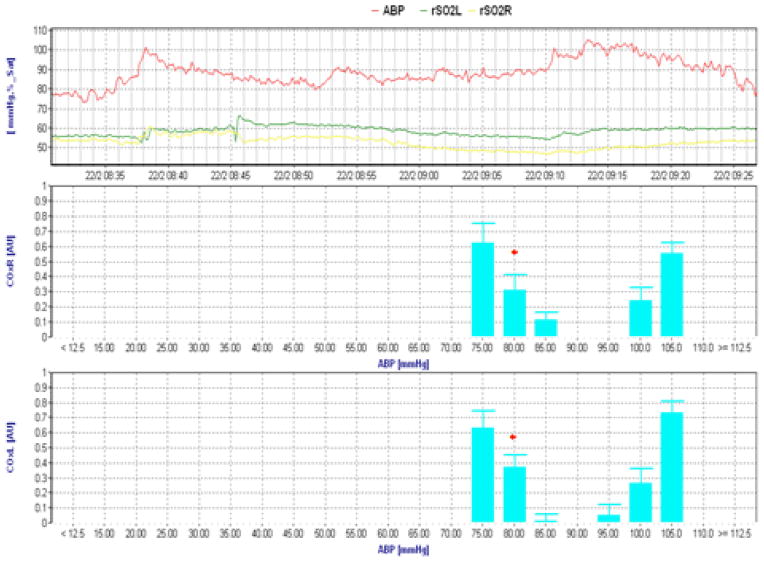
Figure 1.
Clinical cerebral oximetry index (COx) recordings during CPB illustrating a lower limit of cerebral autoregulation at a mean arterial blood pressure (ABP) of 80 mmHg (red asterisk). The top channel represents raw ABP and regional cerebral oxygen saturation (rSO2) for each cerebral hemisphere. Right and left COx recordings are shown in the other graphs. The lower limit of autoregulation is defined as the blood pressure where COx increases from < 0.3 to ≥0.3 (see text).
Figure 2.
The percentage of patients with each classification of RIFLE acute kidney injury (AKI) after surgery. The number of patients in each class is noted above each column.
Table 3.
Near infrared spectroscopy (NIRS) and cerebral autoregulation data for patients with and without acute kidney injury (AKI) after surgery.a
| AKI (n=121) | No AKI (n=227) | P-value | |
|---|---|---|---|
| Average rScO2 | 53±11 (50 to 55) | 54±11 (53 to 56) | 0.298 |
| Average COx | 0.26±0.17 (0.23 to 0.30) | 0.26±0.19 (0.23 to 0.28) | 0.820 |
| Average MAP during CPB (mmHg) | 75±7 (74 to 76) | 74±8 (73 to 75) | 0.103 |
| Lower limit of autoregulation (mmHg) | 69±16 (66 to 72) | 63±15 (61 to 65) | 0.001 |
| Magnitude of MAP ≤ lower limit of autoregulation (mmHg × min/h) | 11.2±12.4 (7.8 to 13.0) | 6.6± 7.2 (5.7 to 7.9) | 0.014 |
|
| |||
| pH | 7.39±0.03 | 7.39±0.03 | 0.9179 |
|
| |||
| PaCO2 (mmHg) | 40±3 | 41±3 | 0.2670 |
|
| |||
| PaO2 (mmHg) | 262±44 | 261±47 | 0.7746 |
|
| |||
| Hemoglobin (g/dL) | 8.9±1.2 | 9.3±1.8 | 0.0369 |
| Average Temperature (mean±SD) | 33.8±1.5°C | 33.8±2.5 °C | 0.6170 |
| Peak Temperature during rewarming | 34.5±2.0 °C | 34.5±2.0 °C | 0.8758 |
Values are given as means±SD with 95% confidence intervals in parenthesis.
rScO2, regional cerebral oxygen saturation; COx, cerebral oximetry index; MAP, mean arterial pressure; CPB, cardiopulmonary bypass.
Figure 3.
Whisker plot of duration and magnitude of blood pressure below the limit of cerebral autoregulation during cardiopulmonary bypass (mmHg x min/h) for patients with and without acute kidney injury (AKI) after surgery (p=0.014 between groups). The horizontal line represents the median value while upper and lower boarders of the shaded area represent the inter-quartile range. The error bars represent the boundaries of ±1.5xinterquartile range and the points beyond represent outliers.
Table 4.
Variables independently associated with acute kidney injury based on the generalized linear model with Poisson distribution and robust standard errors.
| Variable | Relative Risk | 95% Confidence Interval | P-Value |
|---|---|---|---|
| Magnitude of MAP ≤ lower limit of autoregulation (mmHg × min/h) | 1.02 | 1.01 to 1.03 | <0.0001 |
| Diabetes | 1.78 | 1.27 to 2.51 | 0.001 |
| Pulse pressure > 60 mmHg | 1.33 | 0.89 to 1.99 | 0.158 |
MAP, mean arterial pressure.
Variables entered into the initial general linear model included history of congestive heart failure, diabetes mellitus, current smoking, aspirin use, baseline pulse pressure > 60 mmHg, and magnitude-duration of MAP below the lower limit of autoregulation. The risk of AKI was 10% (95% confidence intervals, 7.2% to 18.8%) for each 5 mmHgxmin/hour of MAP below the limit of autoregulation. The area under the receiver operator characteristic curve for the model was 0.656 (95% confidence interval, 0.58 to 0.72). The predictive modeling was repeated without the variable normalized product of magnitude-duration of MAP below the lower limit of autoregulation during CPB. Factors independently associated with AKI in the latter analysis were current smoker (relative risk, 0.59 p= 0.040, 95% CI, 0.35 to 0.98), CHF (relative risk, 1.52 p= 0.007, 95% CI, 1.12 to 2.06), and pulse pressure > 60 mmHg (relative risk, 1.5, p=0.013, 95% CI, 1.09–2.09) with an AUC for the ROC curve of 0.613. The two ROC curves were not different (p=0.4378).
Discussion
Using a novel technique to monitor autoregulation, we found that patients who developed AKI within 7 days of surgery had a higher lower limit of CBF autoregulation than did patients who did not develop AKI. As a result, the product of the magnitude and duration of MAP below the lower limit of autoregulation during CPB was higher for patients with AKI than for those who did not develop AKI, despite a similar average MAP during surgery. The magnitude-duration spent with MAP below the lower autoregulation limit was independently identified as a risk factor for AKI. These results suggest that maintaining MAP above the real-time-determined lower limit of cerebral autoregulation may be an effective strategy for reducing AKI after cardiac surgery.
Brain and kidney injury are devastating complications after cardiac surgery, and both are associated with in-hospital and long-term mortality (1–3, 19). Normally, CBF is autoregulated to satisfy the metabolic needs of the brain tissue while renal blood flow is autoregulated to control body solute, toxin, and fluid balance and to preserve glomerular structure (13, 20). Our data suggest that obligate cerebral blood pressure is related to obligate renal blood flow and that maintaining MAP above the lower limit of CBF autoregulation may ensure renal perfusion that is adequate to prevent injury. These findings are consistent with those of Murkin et al (14) who found in a randomized trial of 200 patients undergoing CABG surgery that interventions targeted at declines in NIRS-detected regional cerebral oxygen saturation were associated with a shorter duration of hospitalization in the intensive care unit and a reduced incidence of major organ morbidity and mortality (death, lung ventilation > 48 hrs after surgery, stroke, myocardial infarction, and return to the operating room for bleeding) compared with patients who did not have clinical NIRS monitoring. In that study, COx was not monitored and no episodes of renal-only injury endpoints were reported (dialysis-dependent renal failure). Notably, in our study there was no difference in average NIRS regional cerebral oxygen saturation during surgery between patients with and without AKI. Thus, because the relationship between MAP and the limits of CBF autoregulation are assessed, monitoring COx during CPB may have value beyond that of monitoring only NIRS.
In a prior study that used transcranial Doppler methods, we noted a wide range of MAPs at the lower limit of autoregulation (40 to 90 mmHg), suggesting that when standard blood pressure management is used, many patients will have blood pressures that may predispose them to cerebral ischemia (9). Perioperative stroke occurred in 12.3% of patients who had impaired autoregulation during CPB but in only 2.2% of patients whose autoregulation was not impaired (p=0.005). In previous laboratory and clinical investigations we showed that NIRS monitoring provides a clinically suitable surrogate of CBF for autoregulation monitoring that is comparable to transcranial Doppler (7, 11, 21, 22). These findings have widespread implications for patients undergoing cardiac surgery, as NIRS overcomes many of the limitations of transcranial Doppler monitoring (e.g., inability to obtain insonating window, electrical and movement artifact, need for specialized equipment and expertise) and can be performed easily in the operating room and intensive care unit. In this study we show that basing MAP targets during CPB on COx data rather than on empiric targets has the potential to reduce the incidence of AKI and, thus, improve overall patient outcomes. Together, these data support COx monitoring as a means for more precise MAP management during CPB.
Preoperative pulse pressure has been identified previously as a risk factor for AKI after cardiac surgery with CPB (4, 23, 24). In our study, a pulse pressure > 60 mmHg was more common in those who developed AKI than in those who did not. Elevated pulse pressure indicates central vascular stiffness, which may lead to arteriolar narrowing that necessitates higher blood pressure for renal perfusion during surgery (25). Aronson et al. (4) reported that the magnitude and duration of systolic blood pressure excursions outside of predefined limits (65 to 135 mmHg intraoperatively, 75 to 145 mmHg pre- and postoperatively) predicted cardiac surgery-associated AKI. A difference in preoperative and average MAP during CPB >26 mmHg was further found to be independently associated with cardiac surgery-associated AKI (26). These results indicate that raising MAP targets during CPB might reduce the frequency of postoperative AKI. One concern, though, is that blood pressure above the upper limit of CBF autoregulation would result in cerebral hyperemia, increased cerebral embolic load, and cerebral edema that might lead to brain injury. Data from Aronson et al. (4, 5) in fact suggested that cardiac surgery-associated AKI was associated not only with hypotension but also with hypertension. Our methods will enable physicians to optimize MAP during CPB within the autoregulation limits of individual patients rather than empirically raising MAP thresholds. Such an approach would thereby prevent hypotension and hypertension.
Limitations
Our study is associated with several limitations. We did not include data on postoperative urine flow that are often considered in defining AKI based on the RIFLE criteria. Urine output data were not collected as we are unsure of the validity of this measurement obtained from the medical record particularly after the foley catheter has been removed. Failure to incorporate urine output data likely underestimates the frequency of AKI, thus reducing statistical power.(27) Nonetheless, other studies have demonstrated that RIFLE definition without urine data is associated with early and long-term mortality (3, 19, 23). Further, we do not include other factors in our analysis that might influence risk for AKI, such as timing of preoperative coronary angiography with radiocontrast dye and the concurrent use of nephrotoxic antibiotics. We did not record whether the OR table during CPB was level or what degree of Trendelenburg positioning was present. It is possible that direct blood pressure measurement at the level of the heart may overestimate renal perfusion pressure when there is Trendelenburg position. This might be particularly important in a patient whose MAP was at or below the limit of autoregulation. This and other factors not considered in our analysis may have led to an under estimation of the observed relationship between blood pressure excursions and AKI. Finally, though the magnitude-duration of MAP below the cerebral autoregulation limit was independently associated with AKI, the small magnitude of the association and the corresponding receiver operator curve data demonstrate that the presently described autoregulation algorithm may need to be improved before it can provide clinically meaningful predictive information regarding cardiac surgery-associated AKI. Nonetheless, these results provide information not previously reported suggesting that intraoperative blood pressure may contribute to AKI. Prospective evaluation of this hypothesis will be necessary.
Conclusions
Excursions of MAP below the lower limit of CBF autoregulation during CPB were independently associated with for AKI. Monitoring autoregulation with processed NIRS signals may provide a novel method for precisely determining MAP targets during CPB.
Acknowledgments
Funding Sources: Funded in part by Grant-In-Aid Number 103363 from the Mid-Atlantic Affiliate of the American Heart Association; Grant R01HL092259 from the National Institutes of Health (to Dr. Hogue
Footnotes
Reprints: There will be no reprints available.
Disclosures: Dr. Hogue has received research support from Somanetics, Inc and its parent company Covidien, Corp (Boulder, CO). Ken Brady has consulted for Somanetics, Inc. in a relationship that was managed by the committee for outside interests at the Johns Hopkins University School of Medicine.
References
- 1.Brown JR, Cochran RP, MacKenzie TA, et al. Long-term survival after cardiac surgery is predicted by estimated glomerular filtration rate. Ann Thorac Surg. 2008;86(1):4–11. doi: 10.1016/j.athoracsur.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009;119(4):495–502. doi: 10.1161/CIRCULATIONAHA.108.786913. [DOI] [PubMed] [Google Scholar]
- 3.Kuitunen A, Vento A, Suojaranta-Ylinen R, et al. Acute renal failure after cardiac surgery: evaluation of the RIFLE classification. Ann Thorac Surg. 2006;81(2):542–546. doi: 10.1016/j.athoracsur.2005.07.047. [DOI] [PubMed] [Google Scholar]
- 4.Aronson S, Dyke CM, Stierer KA, et al. The ECLIPSE trials: comparative studies of clevidipine to nitroglycerin, sodium nitroprusside, and nicardipine for acute hypertension treatment in cardiac surgery patients. Anesth Analg. 2008;107(4):1110–1121. doi: 10.1213/ane.0b013e31818240db. [DOI] [PubMed] [Google Scholar]
- 5.Weir MR, Aronson S, Avery EG, et al. Acute kidney injury following cardiac surgery: role of perioperative blood pressure control. Am J Nephrol. 2011;33(5):438–452. doi: 10.1159/000327601. [DOI] [PubMed] [Google Scholar]
- 6.Palmer BF. Renal dysfunction complicating the treatment of hypertension. N Engl J Med. 2002;347(16):1256–1261. doi: 10.1056/NEJMra020676. [DOI] [PubMed] [Google Scholar]
- 7.Brady K, Joshi B, Zweifel C, et al. Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke. 2010;41(9):1951–1956. doi: 10.1161/STROKEAHA.109.575159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joshi B, Brady K, Lee J, et al. Impaired autoregulation of cerebral blood flow during rewarming from hypothermic cardiopulmonary bypass and its potential association with stroke. Anesth Analg. 2010;110(2):321–328. doi: 10.1213/ANE.0b013e3181c6fd12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joshi B, Ono M, Brown C, et al. Anesth Analg. 2011. Predicting the limits of cerebral autoregulation during cardiopulmonary bypass. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schell RM, Kern FH, Greeley WJ, et al. Cerebral blood flow and metabolism during cardiopulmonary bypass. Anesth Analg. 1993;76(4):849–865. doi: 10.1213/00000539-199304000-00029. [DOI] [PubMed] [Google Scholar]
- 11.Brady KM, Lee JK, Kibler KK, et al. Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke. 2007;38(10):2818–2825. doi: 10.1161/STROKEAHA.107.485706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brady KM, Mytar JO, Lee JK, et al. Monitoring cerebral blood flow pressure autoregulation in pediatric patients during cardiac surgery. Stroke. 2010;41(9):1957–1962. doi: 10.1161/STROKEAHA.109.575167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cupples WA, Braam B. Assessment of renal autoregulation. Am J Physiol Renal Physiol. 2007;292(4):F1105–1123. doi: 10.1152/ajprenal.00194.2006. [DOI] [PubMed] [Google Scholar]
- 14.Murkin JM, Adams SJ, Novick RJ, et al. Monitoring brain oxygen saturation during coronary bypass surgery: a randomized, prospective study. Anesth Analg. 2007;104(1):51–58. doi: 10.1213/01.ane.0000246814.29362.f4. [DOI] [PubMed] [Google Scholar]
- 15.Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth. 2009;103 (Suppl 1):i3–13. doi: 10.1093/bja/aep299. [DOI] [PubMed] [Google Scholar]
- 16.Lassnigg A, Schmid ER, Hiesmayr M, et al. Impact of minimal increases in serum creatinine on outcome in patients after cardiothoracic surgery: do we have to revise current definitions of acute renal failure? Crit Care Med. 2008;36(4):1129–1137. doi: 10.1097/CCM.0b013e318169181a. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Greene T, Kusek JW, et al. A simplified equation to predict glomerular filtration rate from serum creatinine [Abstract] J Am Soc Nephrol. 2000;11:AO828. [Google Scholar]
- 18.Aronson S, Dyke CM, Levy JH, et al. Does perioperative systolic blood pressure variability predict mortality after cardiac surgery? An exploratory analysis of the ECLIPSE trials. Anesth Analg. 2011;113(1):19–30. doi: 10.1213/ANE.0b013e31820f9231. [DOI] [PubMed] [Google Scholar]
- 19.Robert AM, Kramer RS, Dacey LJ, et al. Cardiac surgery-associated acute kidney injury: a comparison of two consensus criteria. Ann Thorac Surg. 2010;90(6):1939–1943. doi: 10.1016/j.athoracsur.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz AE, Sandhu AA, Kaplon RJ, et al. Cerebral blood flow is determined by arterial pressure and not cardiopulmonary bypass flow rate. Ann Thorac Surg. 1995;60(1):165–169. discussion 169–170. [PubMed] [Google Scholar]
- 21.Steiner LA, Pfister D, Strebel SP, et al. Near-infrared spectroscopy can monitor dynamic cerebral autoregulation in adults. Neurocrit Care. 2009;10(1):122–128. doi: 10.1007/s12028-008-9140-5. [DOI] [PubMed] [Google Scholar]
- 22.Tsuji M, Saul JP, du Plessis A, et al. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics. 2000;106(4):625–632. doi: 10.1542/peds.106.4.625. [DOI] [PubMed] [Google Scholar]
- 23.Aronson S, Fontes ML, Miao Y, et al. Risk index for perioperative renal dysfunction/failure: critical dependence on pulse pressure hypertension. Circulation. 2007;115(6):733–742. doi: 10.1161/CIRCULATIONAHA.106.623538. [DOI] [PubMed] [Google Scholar]
- 24.Barodka VM, Joshi BL, Berkowitz DE, et al. Review article: implications of vascular aging. Anesth Analg. 2011;112(5):1048–1060. doi: 10.1213/ANE.0b013e3182147e3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanji HD, Schulze CJ, Hervas-Malo M, et al. Difference between pre-operative and cardiopulmonary bypass mean arterial pressure is independently associated with early cardiac surgery-associated acute kidney injury. J Cardiothorac Surg. 2010;5:71. doi: 10.1186/1749-8090-5-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hogue CW, Jr, Palin CA, Arrowsmith JE. Cardiopulmonary bypass management and neurologic outcomes: an evidence-based appraisal of current practices. Anesth Analg. 2006;103(1):21–37. doi: 10.1213/01.ANE.0000220035.82989.79. [DOI] [PubMed] [Google Scholar]
- 27.Bivet F. Nonuse of RIFLE classification urine output criteria: Biases for acute kidney injury biomarkers performance assessment? Crit Care Med. 2012;40:1692. doi: 10.1097/CCM.0b013e318246b72a. [Letter] [DOI] [PubMed] [Google Scholar]