Abstract
It has been widely recognized that the understanding of the brain code would require large-scale recording and decoding of brain activity patterns. In 2007 with support from Georgia Research Alliance, we have launched the Brain Decoding Project Initiative with the basic idea which is now similarly advocated by BRAIN project or Brain Activity Map proposal. As the planning of the BRAIN project is currently underway, we share our insights and lessons from our efforts in mapping real-time episodic memory traces in the hippocampus of freely behaving mice. We show that appropriate large-scale statistical methods are essential to decipher and measure real-time memory traces and neural dynamics. We also provide an example of how the carefully designed, sometime thinking-outside-the-box, behavioral paradigms can be highly instrumental to the unraveling of memory-coding cell assembly organizing principle in the hippocampus. Our observations to date have led us to conclude that the specific-to-general categorical and combinatorial feature-coding cell assembly mechanism represents an emergent property for enabling the neural networks to generate and organize not only episodic memory, but also semantic knowledge and imagination.
Aristotle has once pondered the concept of sensation and memory, and how they are produced in the mind. But it wasn’t until the end of 19th century neuroscientists, such as Ramon Y. Cajal, had begun to look into how this process may occur at the cellular level. Fifty years after Cajal’s observations Donald Hebb postulated that information processing in the brain may involve the coordinated activity of large numbers of neurons, or cell assemblies (Hebb, 1949). This notion, although beautifully vague, makes a good sense both from the computational and cellular perspective (Abbott and Sejnowski, 1999; Bi and Poo, 2001; Bliss and Collingridge, 1993; Malenka and Nicoll, 1999; Sanger, 2003; Shamir and Sompolinsky, 2004; Tsien, 2000; Wigstrom and Gustafsson, 1985). The major challenge to date has been to identify the real-time brain activity patterns and their corresponding cell assemblies, and to understand how such cell assemblies, if any, are organized to generate real-time perception, memory, and behavior.
As early as 1920s, neuroscientists try to decipher the brain codes by searching for reliable correlation between firing patterns of neurons and behavioral functions for many decades (Adrian, 1926; Fuster, 1973; Gross, Rocha-Miranda, and Bender, 1972; Thompson, 2005; Zhou and Fuster, 1996). Edgar Adrian in his pioneering recording showed that the firing rate of a frog muscle’s stretch receptor increases as a function of the weights on the muscle (Adrian, 1926), suggesting that information is conveyed by specific firing patterns of neurons. However, due to a large amount of response-variability at the single neuron level in the brain even in response to identical stimulus (Bialek and Rieke, 1992; Lestienne, 2001), single neuron-based decoding schemes often produce significant errors in predictions about the stimulus identities or external information. The traditional way to deal with the response variability of single neurons is to average spike discharge of the neurons over repeated trials. Although the data averaging across trials permits the identification of response properties of the individual neurons, unfortunately, this practice invariably loses crucial information regarding real-time encoding process in the brain (Lin, Osan, and Tsien, 2006b).
Early efforts in examining population-level mechanisms relied on the “reconstructed” ensembles of neurons from serially recorded single neuron data. Such “reconstructed population codes” can improve the classification and prediction of datasets (Eskandar, Richmond, and Optican, 1992; Gochin, Colombo, Dorfman, Gerstein, and Gross, 1994; Miller, Li, and Desimone, 1993). With technical developments over the past decades, simultaneous monitoring of activities of many neurons has become more feasible (Buzsaki, 2004; Harris, Henze, Csicsvari, Hirase, and Buzsaki, 2000; McNaughton, O’Keefe, and Barnes, 1983; Schmidt, 1999). For example, Georgopoulos and his colleagues were among the first to apply a population-vector method to analyze ensemble firing patterns corresponding to arm movements of monkeys (Georgopoulos, Schwartz, and Kettner, 1986). By calculating the mean firing rates for each neuron corresponding to arm movement, a set of population vectors can be obtained that correspond to specific angles of arm rotation and movement (Musallam, Corneil, Greger, Scherberger, and Andersen, 2004; Nicolelis and Ribeiro, 2006; Velliste, Perel, Spalding, Whitford, and Schwartz, 2008). Similarly, the discovery of place cells in 1970s has prompted many researchers to examine how the hippocampus encodes space (O’Keefe and Dostrovsky, 1971; O’Keefe and Nadel, 1978). Multiple tetrodes techniques have been successfully applied to the study of several dozens of place cells in the rat hippocampus (Wilson and McNaughton, 1993). This has led to extensive knowledge of how the hippocampal system may generate perceptual representation of the animal’s self-location during spatial navigation (Buzsaki and Moser, 2013; Kentros, 2006; Lisman and Redish, 2009; McNaughton, Battaglia, Jensen, Moser, and Moser, 2006; Mizumori, 2006; Oler, Penley, Sava, and Markus, 2008; Redish, 2001; Smith and Mizumori, 2006). Yet it remains unclear as to whether motion-sensitive place cell firing would represent part of long-term episodic memory for which the hippocampus is known.
In parallel, development of region- and cell type-specific cre/loxP conditional transgenic methods in mid 1990s has opened a new door to studying gene, neural circuits, and behavior (Tsien, Chen, Gerber, Tom, Mercer, Anderson, Mayford, Kandel, and Tonegawa, 1996a; Tsien, Huerta, and Tonegawa, 1996b). This Cre/loxP method has also provided a useful platform for opsin-based optogenetics to restrict its manipulation to a given cell type within a given region. We have provided some of the earliest evidence that memory in mice can be impaired, enhanced, or rapidly erased by genetic means (Cao, Wang, Mei, An, Yin, Wang, and Tsien, 2008; Cui, Wang, Tan, Zaia, Zhang, and Tsien, 2004; Shimizu, Tang, Rampon, and Tsien, 2000; Tang, Shimizu, Dube, Rampon, Kerchner, Zhuo, Liu, and Tsien, 1999; Tsien et al., 1996b; Wang, Li, Wang, Xie, Shen, and Tsien, 2011). Because the hippocampus is widely known for creating long-term memory of what event, when it happened, and at where, this has led us to focus on the following questions: what are real-time memory engrams underlying dramatic events or emotional experiences? Can real-time memory traces be mathematically described and decoded at any given moment? What are the organizing principles for memory-coding cell assemblies in the hippocampus? How does the memory circuit generate not only episodic memories but also semantic knowledge and imagination?
Brain Decoding Project Initiative for creating Brain Activity Map of Memory Engrams
To approach the above fundamental questions, it is obvious that it would require large-scale decoding of brain activity patterns. Over the course of past several years, we have focused our initial efforts on three different but coherently linked aspects: 1) To employ large-scale neural recording techniques to collect large datasets on memory process in the mouse hippocampus; 2) To use a set of innovative behavioral paradigms to facilitate the discovery of memory organizing principles; 3) To develop and apply mathematical tools that are suitable for identification of neural ensembles activity patterns and uncovering its underlying cell assembly structures.
Based on our initial success in decoding event-related neural patterns in the mouse hippocampus (Lin, Osan, Shoham, Jin, Zuo, and Tsien, 2005; Lin et al., 2006b; Tsien, 2007), in 2008 we have obtained strong support from Georgia Research Alliance and launched the Brain Decoding Project Initiative to identify neural dynamics in the memory circuits (http://gra.org/Stories/StoryDetail/tabid/622/xmid/632/Default.aspx). The basic idea of our Brain Decoding Project, now similarly expressed by Brain Activity Map proposal (Alivisatos, Chun, Church, Greenspan, Roukes, and Yuste, 2012), is to investigate and discover the underlying organizing principles by which the brain generates real-time perception, emotion, memory, knowledge, and behavior. Here, we share some of the insights and lessons from our brain decoding project effort which we believe may be useful to the planning of the BRAIN project that is currently underway:
Large-scale neural recording capacity: how large is large enough to get started?
Any brain decoding or activity mapping effort will face the question of how many neurons should be recorded in order to decipher the real-time brain code and more importantly to understand the basic designing principles. One of the grand claims in the Brain Activity Map proposal is to measure every spike from every neuron (Alivisatos et al., 2012). This has raised some theoretical questions as to whether the brain’s “emergent properties” can only be studied by recording all spikes from all neurons in the brain (Mitra, 2013). While collecting such complete information would be ideal, it may take more than fifteen years (the presumed time frame of the BRAIN project) before every spike of every neuron from a brain region of mammal species, say the hippocampus of freely behaving mice, can be achieved. Because the ultimate goal of the BRAIN project is to crack the brain code and establish its organizing principles, researchers may approach it with more practical question as to what the sizes of the recorded neurons should be recorded to get this decoding problem going.
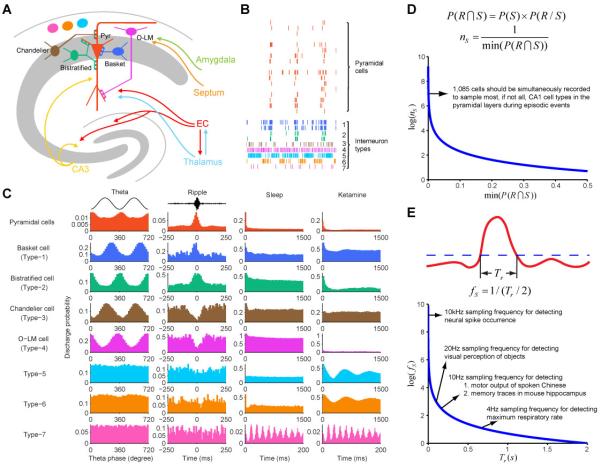
In the case of the CA1 region of the hippocampus, it is known that pyramidal cells and diverse interneurons compose the intricate hippocampal circuits and are involved in various firing patterns. Much of current knowledge has been obtained from studies of in vitro brain slices (Freund and Buzsaki, 1996; Klausberger and Somogyi, 2008; Somogyi and Klausberger, 2005). Little is known about its detailed action on dynamic patterns of hippocampal cells during learning and memory. By taking the advantage of 96- or 128-channel in vivo neural recording technique, we are allowed to monitor many pyramidal cells and interneurons from the CA1 of freely behaving mice. Although the interneuron types identified in vitro or anesthetized state may not map clearly to those in freely behaving state, for simplicity we used these classification terms and identified at least seven major interneurons types, including known and unknown types of interneurons, based on their distinct firing patterns and compare with the in vitro results (Fig. 1A).
Fig. 1. Diverse neuron types in the hippocampus and theoretical consideration for brain activity mapping.
(A) Illustration of diverse neuron types in the CA1, which include Pyramidal cells (pyr), basket cells, bistratified cells, Chandelier cells, O-LM cells, and other unidentified interneuron types. O-LM cell, Type-5, type-6, and type-7 interneurons are located in the str. oriens. Firing temporal rhythms of pyramidal cells and seven distinct interneuron types under ketamine-induced anesthesia. (C) Distinct profiles of distinct CA1 cells in relationship with theta oscillations (the first columns of plots from left), ripples (second column), and their autocorrelograms during sleep (third column) and ketamine-induced anesthesia (right column). The figure is partially adopted from Kuang et al. 2010. (D) Joint probability distribution for estimating cell numbers for covering basic CA1 cell types involved in processing fear memory using the Chandelier cell as a low end for estimation. Approximately 1,085 neurons in hippocampus CA1 should be ideally recorded simultaneously so that the recorded dataset will contain all most likely responsive neurons of most types, if not all, for the study of encoding of fearful experiences. This is just an example of sketchy estimation, more accurate calculations with confidence levels can be implemented using bootstrapping method. (E) Nyquist–Shannon sampling theorem for estimating the sample speed for detecting various network-level dynamics.
Type-1 and type-2 interneurons were putative basket cells and bistratified cells according the characteristics of these cells (Buzsaki and Eidelberg, 1983; Klausberger and Somogyi, 2008; Somogyi and Klausberger, 2005). They were made of nearly half of recorded interneurons. These cells innervate pyramidal cell somas and dendrites. Type-3 and type-4 interneurons matched well with firing characteristics of Chandelier cells and O-LM cells, respectively. These putative Chandelier cells and O-LM cells interneurons tended to fire during the period when pyramidal cells were silent. Cross-correlation analyses confirmed their negative dynamic correlation with pyramidal cells. These four types of interneurons all exhibited dynamic relationships with the theta and ripple episodes which provided the characteristic classifications to their putative identities (Klausberger, Magill, Marton, Roberts, Cobden, Buzsaki, and Somogyi, 2003; Klausberger and Somogyi, 2008; Somogyi and Klausberger, 2005). The type-5, type-6, and type-7 interneurons were recorded slightly above the pyramidal cell body layer, namely, in the str. Oriens (often together with O-LM cells), they may correspond to the trilaminar cells, back-projection cells, hippocampo-septal cells (Buzsaki and Eidelberg, 1983; Klausberger and Somogyi, 2008; Somogyi and Klausberger, 2005; Tukker, Fuentealba, Hartwich, Somogyi, and Klausberger, 2007). But their firm identifications remain to be determined (Fig. 1A).
Using the above neuron types as an example, we can ask how many neurons should be recorded simultaneously in order to obtain the first activity map of a CA1 circuit-processing unit (we term it as CPU) that would contain all of the above cell types in conjunction with pyramidal cells in memory processing. In statistics, sampling is concerned with the selection of a subset of individuals from a statistical population to estimate characteristics of the whole population. If assuming small numbers of interneurons have broad control or regulation over large numbers of pyramidal cells (as a basic CPU), one can use parameter estimation method to first calculate joint probability of responsive neurons for classified cell types by maximum likelihood estimate, and then obtain minimum joint probability among all classified cell types for estimating minimum size of recorded unit number. This is similar to the question how to assess all the fish species in a lake. Instead of counting all fish after draining the water from the lake, one use subgroup-sampling methods at multiple locations and depths to obtain the meaningful estimation. This same principle can be applied to Brain Activity Map project, that is, instead of measuring every spike from every neuron, one may reveal the fundamental properties of the neural circuit by performing well designed sampling.
Here we illustrate that minimum size of feature-coding neurons may be estimated from neurons’ distribution in a network population involved in memory processing. As shown in Fig. 1B, P(R∩S)=P(S)×P(R/S), where P(R∩S)is the joint probability of events that recorded neurons pertain to certain neural types and would be also responding to or encoding a set of given stimuli, P(S)is the probability of the events that recorded neuron pertains to certain neural type(s) (i.e. pyramidal cells, ~63%; basket cell, ~1.3%, etc) and P(R/S) is the probability of the events that recording certain type neuron responding to stimulus (i.e. on average ~20% of pyramidal cells reacted to fearful stimuli, etc). Minimum size of recording neurons nS is defined as the inverse of the minimum (P(R∩S) among all types of neuron, with the relationship of log(nS) and min(P(RȩS)) shown in Fig. 1B.
Based on our recording in the mouse CA1 region during behavior, the joint probability P(R∩S) for recorded pyramidal cells, basket cells, bistratified cells, and chandelier cells, responding to fearful stimuli are 6.8%, 1.3%, 1.9%, 0.092%. Using Chandelier cells as the lower end of the population samples (because these cells are more or less located in the same layer with the pyramidal cells where the recording electrodes are inserted), we estimated that approximately ~1,085 neurons in hippocampus CA1 should be ideally recorded simultaneously to cover most, if not all, response types for the study of memory encoding. With additional rare types of interneurons to be identified and characterized, the estimations of the size of CA1 neurons within a minimal CPU can be updated correspondingly. It is noteworthy to point out that the size of recorded units will increase significantly for estimating cross-region interactions (i.e. DG-to CA3-to-CA1).
Temporal resolutions of large-scale activity mapping: how fast is fast enough?
The second issue relevant to Brain Activity Mapping is the consideration of temporal resolution for any types of new imaging tools to be developed. The gold standard of neural activity measurement is a variety of in vivo microelectrodes (i.e. in stereotrode or tetrode format) that can offer the state-of-the-art in terms of robust signal quality and fine temporal resolution. In theory, to detect occurrence of the event and avoid signal aliasing between events, minimum sampling frequency of activity mapping techniques can be predicted based on Nyquist–Shannon sampling theorem. In Fig. 1C (the upper drawing), the red curve denote the states in neural population and an event occur beyond the threshold (blue dashed line), and the length of this event is Tr. To detect this hypothetical event or stimulus, the minimum temporal resolution of calcium imaging or other recording methods is the inverse of half Tr, fS = 1/(Tr / 2). The relationship between log(fS) and Tr, and five examples are shown (Fig. 1C, bottom plot). In the case of detecting two individual neural spikes, because the wave crest of a spike can last ~0.2ms, a minimum 10kHz sampling frequency is needed for detecting spike. In this calculation, we only assume to distinguish the occurrence of spikes. But a much higher sampling frequency is required if one wants to reconstruct the all waveform of neural spike (i.e. 40 kHz at 16-bit resolution in Plexon OmniPlex neural data acquisition system). For detecting rapid object categorization from complex natural scenes (which can be achieved ~100ms in the visual cortex), a minimum 20Hz sampling frequency is required to measure detailed dynamics. For assessing motor output control of spoken Chinese (the fastest speaking speed for Chinese is ~300 words per minute), a minimum 10Hz sampling frequency will then be needed to discriminate. Similarly, our memory decoding shows that the shortest time duration of CA1 memory traces is ~0.2s, thus, sampling frequency should be minimally at or higher than 10Hz.
At the moment, calcium imaging techniques based on GCaMPs have been promised to study neural activity associated with animal behaviors (Harvey, Coen, and Tank, 2012; Ziv, Burns, Cocker, Hamel, Ghosh, Kitch, El Gamal, and Schnitzer, 2013). Yet, the temporal resolution of calcium imaging is mostly at the sampling frequency of 0.1-0.25 Hz, inherently due to variable durations of calcium transient wave which can range from ~4 to 10 sec (presumably triggered by multiple action potentials). It will need 40- to 100-fold of improvement in order to reflect many details of memory circuit dynamics. In addition, calcium buffering and potential interferences of intracellular signaling process represents other concerns that will need to be addressed. An alternative is to develop voltage-based imaging methods. Another issue for imaging-based methods is how to simultaneously identify a variety of interneurons types from the imaging view field and how to take advantages of the currently available information in the literature (such as shown in Fig. 1A using microelectrodes based on spike discharge probability and theta or ripples phases).
Decoding real-time memory traces of fearful events in the mouse hippocampus
The hippocampus is well known for its role in the formation of emotionally charged episodic memories, such as fear conditioning memories (Clark and Squire, 1998; Davis, Hitchcock, and Rosen, 1988; Kim and Jung, 2006; LeDoux, 1994; Maren, 2001). To investigate what real-time fear memory traces look like in the brain, we use trace fear conditioning protocol which involves a neutral tone followed by a mild foot-shock with a time interval of 20 seconds in-between. This classical associative memory task produces trace-fear memory as well as contextual fear memory (Biedenkapp and Rudy, 2007; Chowdhury, Quinn, and Fanselow, 2005; Clark and Squire, 1998; Clark and Zola, 1998; Knight, Cheng, Smith, Stein, and Helmstetter, 2004; Matus-Amat, Higgins, Barrientos, and Rudy, 2004; McEchron, Bouwmeester, Tseng, Weiss, and Disterhoft, 1998), and offers an ideal opportunity to study real-time memory engram.
Toward this end, we have employed 128-channel electrode array recording techniques to monitor 200~300 CA1 units simultaneously in mice (Lin, Chen, Xie, Zaia, Zhang, and Tsien, 2006a; Lin et al., 2005). More importantly, we have systematically explored and compared various multi-variant statistics and were able to optimize multi-discriminant analysis-(MDA)/sliding window methods to quantitatively measure and intuitively visualize dynamic activity patterns from the recorded large datasets related to episodic memory traces (Osan, Zhu, Shoham, and Tsien, 2007; Tsien, 2007). As a result, we were able to measure and decode, for the first time, real-time memory traces in the hippocampus as mice underwent the acquisition and retrieval of fear conditioning memories (Chen, Wang, and Tsien, 2009). For example, we found that conditioned tone trace can emerge quickly during learning. Moreover, foot shock-triggered ensemble responses, which originally evoked only US-specific simple traces, would turn into the US-to-CS association traces as CS/US pairing was repeated over trial. The emergence of such associative traces suggests that circuitry-level dynamics have captured nicely the CS-US causal relationship. We also found that these CS and US traces reverberated during the learning phase, and more interestingly, the numbers of reverberations increase in proportion to CS/US pairing trial numbers.
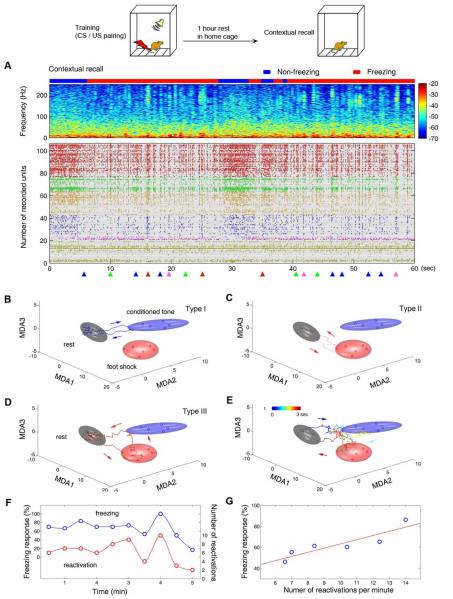
To examine whether these CA1 traces observed during learning represent true memory traces, we asked whether those patterns will re-emerge upon the recall cues during memory retention tests and whether they would precede and correlate with behavioral performances. Indeed, we observed that the first recalled memory trace consistently precedes freezing behavior on the average of 1.4 seconds (Fig. 2A). On average, various CS and/or US memory traces (Fig. 2B-F) were retrieved at a rate of 8-14 times per minute in the mouse hippocampus during the fear memory retention tests (Chen et al., 2009). Importantly, the numbers of retrieved memory traces in the retention tests were tightly correlated with the amount of freezing at both the individual and group levels (Fig. 2H & 2I). In trace retention test, we further found that upon hearing the conditioned tone, various memory traces re-emerged over the 60 second period, but it was the US memory traces consistently reappeared at the time point of 20 seconds after the tone (Chen et al., 2009), thereby demonstrating that the animals formed the real-time memory trace of time for accurately predicting the anticipated arrival of foot shock, a hallmark feature of real-time hippocampal memory traces for “what” and “when”. Therefore, by using 128-channel recording techniques and appropriate decoding methods, we have revealed, for the first time, that the identity and quantity of various fear memory traces during learning and recall in the CA1 region, including working memory traces for time and events.
Fig. 2. Real-time fear memory traces during contextual fear memory recall.
(A) At contextual recall test, the mouse entered the freezing state (illustrated by red bar above the local field potential power spectrum) 8 seconds after entered the conditioning chamber. The observed first retrieved pattern emerged 360 milli-second before the animal froze. During the first freezing epoch (~23 seconds), eight memory traces were detected (see triangles at the bottom of spike raster, only 105 CA1 units out of 208 simultaneously recorded units were shown here). In the second freezing epoch that lasted about 20 seconds, another set of trace retrievals (8 trajectories) was observed. These dynamic ensemble traces included CS memory trace (Fig. 2B), US memory trace (Fig. 2C), US-to-CS associative trace (Fig. 2D), and CS-to-US associative trace (Fig. 2E). (F) Correlation between the retrieved memory traces and freezing within the mouse during the contextual retention test. (G) Correlation between the retrieved memory traces and freezing behavior during recall as a group. The figure is adopted from Chen et al. 2009.
Uncovering of specific-to-general and categorical feature-coding cell assembly organization
The hippocampus is widely known to be crucial for the formation of declarative memory which can be further divided into episodic memory and semantic memory (Squire and Zola, 1998; Tulving, 1972). The essence of episodic memory is in its specificity in term of representing a specific event in a given time and context (Tulving, 1972), whereas semantic memory is the memory of the personal semantics and world knowledge of facts that are no longer ascribable to any particular occasion in life (Cohen and Eichenbaum, 1993; Squire and Zola, 1998; Tulving, 1972). fMRI studies in healthy humans have shown that the hippocampus is activated during the encoding and retrieval of both episodic memory and semantic memories (Burianova and Grady, 2007; Duzel, Cabeza, Picton, Yonelinas, Scheich, Heinze, and Tulving, 1999; Kapur, Friston, Young, Frith, and Frackowiak, 1995; Maguire, Frith, Rudge, and Cipolotti, 2005; McIntosh, Harrison, Forrester, Lawrie, and Johnstone, 2005; Ryan, Cox, Hayes, and Nadel, 2008).
To seek the understanding of the memory organizing principles how the hippocampus encodes and organizes episodic and semantic memories, we designed a set of novel categorical behavioral paradigms to mimic how human would acquire long-term memories (i.e. roller coaster rides, earthquakes, etc.) (Tsien, 2007). By subjecting the mice to these distinct fearful episodic events (such as Drop, Quake, Air-blow), we found diverse changes in the firing of CA1 neuron population (Lin et al., 2005). We show that these episodic events resulted in distinct CA1 ensemble encoding patterns that can be reliably classified (Chen et al., 2009; Lin et al., 2005). Similar, those ensemble traces were found to reverberate within seconds after the episodic stimulation (Chen et al., 2009; Lin et al., 2005; Lin et al., 2006b).
To provide an overall view of how CA1 cell populations are organized to process and represent diverse episodic memories, we have employed a pattern classification method known as agglomerative hierarchical clustering which led to the discovery of various cell groups in the CA1 region, invariantly ranging from specific to general coding responsiveness (Chen et al., 2009; Lin et al., 2005; Lin et al., 2006b; Osan et al., 2007) (Fig. 3A). That is, each group of cells that respond similarly to a select event or feature and thus operate collectively as a robust functional coding unit, termed as neural clique. For example, under the experimental paradigms of subjecting the mice to drop, earthquake, and air-blow, some of CA1 cells exhibit an increase in firing rate to all three types of emotionally charged events, and these cells were termed as general neural clique (Fig. 3A). Other CA1 cells responded to a subset of multiple events (i.e. two events such as Drop/Quake clique, Drop/Air-blow clique, etc), and they were termed as sub-general neural cliques. Many cells showed firing changes specific to one type of event (i.e. “Air-blow clique”, “Drop clique”, and “Quake clique”) and acted as event-specific neural cliques. Moreover, we found a small portion of the cells which exhibited not only event-specificity, but also context-specific firing changes (e.g. responding only to the earthquake happened in environment-A but not in environment-B) (Fig. 3A). These cells are known as event/context-specific cliques. These event/context-specific cliques encode and integrate specific information about both “what” and “where”, another hallmark feature of episodic memory. Therefore, by designing novel behavioral paradigms coupled with mathematic analyses, we have discovered that each episodic event is actually represented by a set of neural cliques in the CA1 that are invariantly organized from the general to the specific manner (Fig. 3B).
Fig. 3. Categorical and hierarchical organization of the memory coding neural clique assembly.

(A) The hierarchical clustering analysis of responses of a total of 757 CA1 neurons from four mice to the three different types of startling episodes reveals the existence of 7 major neural cliques (Panel A): General responsive clique, sub-general cliques (Drop-Shake clique, Air blow-Drop cliques, Shake-Air blow clique), event-specific cliques (Drop-specific clique, Shake-specific clique, and Air blow-specific clique), and event/context-specific clique (Air-blow in context A-specific clique, Air-blow in context B-specific clique, Drop in Elevator A-specific clique, and Drop in Elevator B-specific clique). Non-responsive units are grouped in the bottom half. The color scale bar indicates the normalized response magnitude (1 to 7). (B) A given episodic event activates a neural clique assembly invariantly organized from specific-to-general. (C) Categorical and hierarchical representation of episodic and semantic information by the specific-to-general feature-coding neural clique assemblies.
This specific-to-general feature coding neural clique assemblies suggest a number of emergent organizing principles that govern memory organization in the brain (Tsien, 2007) (Fig. 3C): First, members of a given clique that share the similar response property and selectivity exhibit collective co-spiking dynamics that enables them to overcome the trial-to-trial response variability of individual neurons as an emergent network-level property. This allows the memory coding units to achieve not only real-time encoding robustness but also be much less vulnerable to the deaths of one or a few member neurons during the ageing process or under disease states.
Second, various neural clique assemblies are further organized in a categorical manner, thereby providing the network-level mechanism for efficiently organizing various memories. Because the memory coding is categorically and hierarchically organized, representing new episodic experiences might simply involve substituting the specific cliques that form the bottoms of the memory pyramids to indicate, for example, that the earthquake took place in Los Angles rather than in Kyoto.
Third, the hippocampus relies on memory-coding neural cliques to not only record and extract specific details, but also to extract subcommon or common features from different events via these general and subgeneral neural cliques. The general clique may encode abstract and generalized knowledge indicating that “the events such as drop, earthquake and sudden air blow are all scary events”, whereas the earthquake/drop-subgeneral clique may encode the semantic knowledge that “those events involve motion disturbances (Fig. 3C). It would be of great interest to define from which brain subregions these cells received the common or subcommon input (i.e. amygdala and/or VTA dopamine neurons) (Wang and Tsien, 2011).
For example, Frey and her colleagues described the requirement of specific neuromodulatory inputs to hippocampal neurons to transform a short-term into a long-term memory by means of ‘synaptic tagging’ (Frey and Frey, 2008; Frey and Morris, 1998). Efferent associations to hippocampal neurons - for instance from the amygdala or the VTA within a distinct effective time window - are necessary processes to make a transient memory trace permanent. It was shown that each of these neuromodulators may act as an associative evaluation tool required for the long-term memory formed. A given neuromodulator system is thereby specifically activated in response to and if, for instance, a reward- or novelty- associated stimulus. These brain subregions may thus contribute to evaluate the meaningfulness of an afferent stimulus to a particular glutamatergic synapse population and transform the transient into a permanent memory trace (Frey, Bergado-Rosado, Seidenbecher, Pape, and Frey, 2001; Frey and Frey, 2008; Frey and Morris, 1998). Using more sophisticated techniques - such as VTA-specific optogenetic stimulation - one could study now more specifically the role of a single modulator on hippocampal clique behavior and what “flavor” of memory is encoded by a given neuromodulator within a specific set of neurons.
Thus, the notion that the hippocampus encodes generalized semantic knowledge is supported by our recent finding for the existence of hippocampal cells in encoding of the abstract concepts for nest (Lin, Chen, Kuang, Wang, and Tsien, 2007). These ‘nest cells’ exhibited invariant coding properties during episodic exploration of nest-like objects, over many variations in nest’s shape, material type, color, odor, or locations. Our have shown that these nest cells reply on episodic encounters or experiences to determine the object’s functionality as nest (Lin et al., 2007).
Parametric analysis of CA1 episode cell assemblies underlying memory consolidation
While our brains can recall a great amount of detail immediately after the event (within the time domain of short-term memory), there appears to be a gradual loss of many specific details over the long-term memory time domain. To investigate the neural network mechanism underlying this biased consolidation process, we used the same set of fearful events (drop, earthquake, air-blow, etc) but varied these events’ intensities or durations as a way of introducing additional details about these episodes (Osan, Chen, Feng, and Tsien, 2011). For example, we varied drop heights at 5 cm, 13 cm and 30 cm, or air-blow with 200 ms, 400ms and 800 ms durations. We found that about many hippocampal cells (51.3% of all responsive cells) exhibited intensity-sensitive responsive, termed as event intensity-sensitive neurons (Fig. 4A). In contrast, other CA1 cells (48.7% of all responsive cells) showed similar changes in their firing rates irrespective of the magnitude of the stimulus inputs, and they were termed as intensity-invariant cells (Fig. 4B). Interestingly, our detailed examinations using shuffling techniques suggested that post-learning pattern reverberations were primarily driven by event intensity-invariant cell groups (Fig. 4D-E), not by the intensity-sensitive cells (Fig. 4G-I). Reduced participation by event intensity-coding cells in post-learning period may provide a mechanism for explaining why some parametric details may not be equally retained in the long-term memory domain (Osan et al., 2011).
Fig. 4.
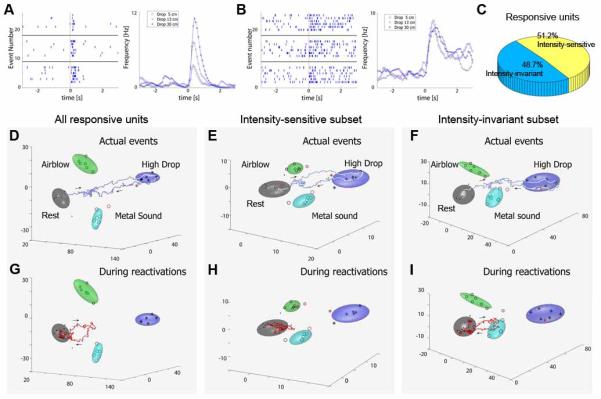
Pattern reverberations are mainly driven by the fearful event intensity invariant cell subpopulation, but by intensity-sensitive cell subpopulation. (A) A representative CA1 unit encoded event-intensities through its increased firing changes to different drop heights from 5, 13 and 30 cm (upper, middle and lower raster, respectively). Time is represented on the horizontal X-axis (-3 to 3 seconds) and the trial number is listed on the vertical Y-axis. The vertical red line indicates t = 0. (B) A representative CA1 unit did not encodes event intensity as it maintained firing rate monotonically in response to changes in drop heights. (C) Percentage of event intensity-sensitive and intensity-invariant cells in the simultaneously recorded CA1 cell population. (D) A typical trajectory during a drop event from 30 cm is plotted in MDA subspaces. (E) Activation dynamics can be also observed in the MDA encoding subspaces which used only the event intensity-sensitive subpopulation of cells. (F) Activation dynamics can be further observed in the MDA encoding subspaces constructed from the intensity-invariant subpopulation only. (G) A typical reactivation trajectory is detected in whole population activity. (H) However, at this time point little reactivation is observed in the intensity-sensitive subpopulation. (I) In contrast, the intensity-invariant responsive subpopulation exhibits a significant reactivation. Please note that the directionality of trajectory towards the drop cluster and away from the air-blow cluster or acoustic metal sound is confirmed in other rotated 3-D dimensions. The figure was adopted from Osan et al. 2011.
How can we close the knowledge gap between episodic event cells with place cells and time cells?
One of the fundamental questions in the field of learning and memory is to understand how the hippocampus generates long-term memories of what, when, and where. The studies on place cells and grid cells have provided crucial lights on how the where information such as self-location is represented (Buzsaki and Moser, 2013; Kentros, 2006; Lisman and Redish, 2009; McNaughton, Battaglia, Jensen, Moser, and Moser, 2006; Mizumori, 2006; Oler, Penley, Sava, and Markus, 2008; Redish, 2001; Smith and Mizumori, 2006). Emerging studies have indicated that some of the hippocampal cells encode time information (Chen et al., 2009; Kraus, Robinson, White, Eichenbaum, and Hasselmo, 2013; MacDonald, Lepage, Eden, and Eichenbaum, 2011). Our recent work has shown that the existence of real-time working memory trace for time in the CA1 that predicts the arrival time of foot shock upon hearing the recall tone during trace fear conditioning retention test (Chen et al., 2009). These working memory traces intrinsically captured both the organization of time and event. In addition to these event-related time cells, Eichenbaum and his colleagues have reported the existence of place-related “time cells” in the CA1. Those cells fire at particular moments during periods when running behavior and location are relatively constant (MacDonald et al., 2011). They recently showed that hippocampal neurons were strongly influenced by time and distance, and less so by minor variations in location (Kraus et al., 2013). They further suggest that hippocampal neuronal networks captured both the organization of time and distance in a situation where these dimensions dominated an ongoing experience. In light of these observations, our studies collectively suggest that time cells in the CA1 are highly integrated, containing information on either the event and/or distance information.
As to the representation of “what information” in the hippocampus, our recent studies of episode cells responding to earthquake, free fall, and air blast have uncovered how the dramatic events are encoded and organized in the hippocampus. In addition to study the relationship between episodic memory and semantic memory, one of the major future directions should be to establish protocols and design new experiments that can allow researchers to link these event cells with place cells, two separate yet intrinsically coherent aspects of hippocampal memory engrams.
A recent study from Schnitzer’s laboratory used a calcium imaging technique to track activity from more than a thousand CA1 units over the course of a month during repeated exposures to a familiar environment (Ziv et al., 2013). They reported only limited place field overlap between repeated sessions in the same context, and interestingly, almost no cells showed truly stable place fields across more than two exposures. While there are many potential explanations from the purely place cell perspective (i.e. stable place fields), one alternative consideration or interpretation is that activity patterns of many place cells during these daily running sessions may be easily influenced by episodic cells which are designed to respond to both specific (distinct) and discover generalized episodic information over a month. This is similar to our own experiences when driving to work every day, there is always something different or new to notice or pay attention to along the way.
Can the mouse brain study inform us about the human brain?
Our discovery of specific-to-general and categorical feature-coding neural clique assembly in the mouse hippocampus offers a crucial insight how the memory circuit generates both episodic memory and semantic knowledge. This explains why humans with hippocampal damage show profound deficits in the acquisition and retrieval of both new semantic and episodic memory (Hodges and Graham, 2001; Messas, Mansur, and Castro, 2008; Tranel, Damasio, and Damasio, 1997; Warrington and Shallice, 1984). Moreover, the significant percentage of general and subgeneral neural cliques in the hippocampus fits well with various anatomical observations that the human hippocampus also receives higher-order, multimodal cortical and subcortical inputs, and is well suited to process abstract memories.
Shortly after our large-scale recording revealed the specific-to-general categorical organization of neural cliques in the mouse hippocampus (Lin et al., 2005), Fried and his colleagues has reported that the existence of cells in the human hippocampus responding to abstract recognition of people identity (Quiroga, Reddy, Kreiman, Koch, and Fried, 2005). For example, they showed that a hippocampal cell from a patient was selectively activated by pictures of the actress Halle Berry, a drawing of her, several pictures of Halle Berry dressed as Cat woman, and even the letter string ‘Halle Berry’, but not by images of other people. This Halle Berry-specific cell apparently encodes the identity about Halle Berry. The same research team later also found another neuron in yet another patient’s hippocampus was activated by pictures of Jennifer Aniston and Lisa Kudrow, both actresses in the TV series ‘Friends’ (Quiroga, Kreiman, Koch, and Fried, 2008), which are clearly related to the sub-common themes. These human neurophysiological data, although obtained from serial recording from different human subjects, support our conclusion for the existence of the specific-to-general and categorical feature-coding cell assembly organization in the memory system.
Moreover, emerging data from human neuroimaging study on cortical connections show the strikingly common structural and functional cortical architecture across individuals and populations. Using diffusion tensor imaging (DTI) techniques, Tianming Liu and his colleagues have reported a dense and consistent map of 358 cortical landmarks, named Dense Individualized and Common Connectivity-based Cortical Landmarks (DICCCOL) (Fig. 5). Each DICCCOL is defined by group-wise consistent DTI-derived white-matter fiber connection patterns (Zhu, Li, Guo, Jiang, Zhang, Zhang, Chen, Deng, Faraco, Jin, Wee, Yuan, Lv, Yin, Hu, Duan, Han, Wang, Shen, Miller, Li, and Liu, 2013). Remarkably, these 358 landmarks are reproducible over more than two hundred human brains, and possess accurate intrinsically-established structural and functional cross-subject correspondences. For example, the fear network in the human brain contains 14 nodes (including the amygdala and insular cortex, etc) in the atlas space that were activated by task-based fMRI (Fig. 5A). These DTI-derived fiber connections show diverse yet distinct convergent patterns across these corresponding brain regions (Yuan, Jiang, Zhu, Chen, Li, Lv, Yu, Li, Zhang, Zhang, Hu, Han, Guo, and Liu, 2013; Zhu et al., 2013) (Fig. 5B). Such conserved structural and functional architecture would provide neural substrates for the general-to-specific feature processing cell assemblies at each node (Fig. 5C). Encouragingly, our finding of specific-to-general cliques seem to map on nicely to the recently published fMRI findings from Schacter group in linking hippocampal activation with recombination of episodic elements using an ‘experimental recombination task (Addis, Pan, Vu, Laiser, and Schacter, 2009). This human fMRI study provides some fairly direct and striking empirical evidence in support of our proposed cellular organizing mechanism.
Fig. 5. Specific-to-general cell assembly architecture for building other high cognitions in the brain.
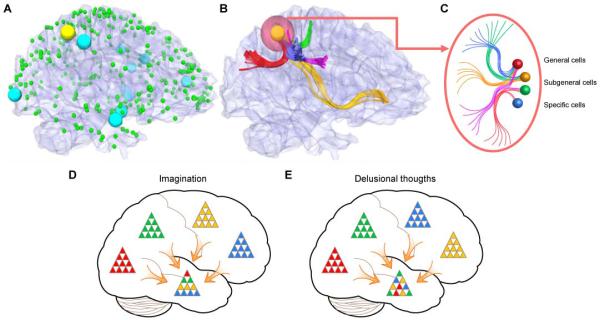
(A) Fear network in the human brain mapped by fMRI. The cyan spheres represent the fear network activated by task-based fMRI (a total of 14 nodes is identified). The green spheres are 358 DICCCOL (dense individualized and common connectivity-based cortical landmarks) landmarks. The cyan and yellow landmarks represent the fear network activated by task-based fMRI. They are located in the Brodmann areas 2, 7, 9, 10, 19, 21 and 43. The green spheres are other DICCCOL landmarks. Additional information is referred to Zhu et al., 2013. (B) The consistent DTI-derived fiber connections to the yellow cortical landmark are shown in color curves. (C) Schematic illustration of general, subgeneral and specific cells that compose the yellow cortical landmark in B. Additional details of the fMRI task design and landmark mapping are in Zhu et al., 2013, Cerebral Cortex. (D) Imagination and creative thoughts can be generated from coherent combinatorial co-activation of various neural cliques from different cell assemblies. (E) Delusional thoughts or nightmares can be produced by inappropriate combinatorial co-activation of various neural cliques from various cell assemblies.
With this general-to-specific feature cell assembly architecture across many different cortical sites, the brain can use a combinatorial activation strategy to generate an almost unlimited number of global patterns representing both specific memory and generalized knowledge of events, object, people, and environments. More importantly, the same mechanism can be employed to create the infinite number of fictitious or future events, actions, or experiences during the imagination (Fig. 5D). This combinatorial strategy is similar to the way that DNA uses combinations of four deoxynucleotides (A, T, G, C) to encode diverse genetic information or the immune system uses combinatorial rearrangement of immunoglobulin gene segments to generate diverse antibodies for dealing with various antigens that the animals may encounter in life. Likewise, under the abnormal conditions (i.e. genetic mutations affecting connectivity patterns in schizophrenic patients), incorrect combinatorial activations of the neural clique assemblies would lead to delusional thoughts or nightmares (Fig. 5E).
Conclusion
Innovative behavioral paradigms and appropriate mathematical analyses of large datasets obtained from neural ensemble recordings have enabled us to decode real-time associative memory traces in the hippocampus. It also led to the discovery of specific-to-general feature-coding and categorical cell assembly organization in the memory system which can explain how the brain generates not only episodic memory, but also semantic knowledge and imagination. By mapping neural activity in a brain-wide fashion, scientists should uncover many more emergent properties of the neural networks that generate real-time perception, memory, knowledge, and behaviors. Moreover, by comparing the brain activity patterns between the normal and the mutant mouse models, scientists can further discover aberrant circuitry dynamic patterns underlying various brain diseases including schizophrenia and posttraumatic stress disorder. Such knowledge should lead to better and more efficient development of novel treatment for these brain diseases in future.
Acknowledgement
We would like to express our deep gratitude to Georgia Research Alliance for the Brain Decoding Initiative (2007-present), and Yunnan Province Department of Science and Technology for the support of our work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott LF, Sejnowski TJ. Neural codes and distributed representations: foundations of neural computation. Mit Press; 1999. [Google Scholar]
- Addis DR, Pan L, Vu MA, Laiser N, Schacter DL. Constructive episodic simulation of the future and the past: distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia. 2009;47:2222–2238. doi: 10.1016/j.neuropsychologia.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Adrian ED. The impulses produced by sensory nerve endings Part I. The Journal of physiology. 1926;61:49–72. doi: 10.1113/jphysiol.1926.sp002273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alivisatos AP, Chun M, Church GM, Greenspan RJ, Roukes ML, Yuste R. The brain activity map project and the challenge of functional connectomics. Neuron. 2012;74:970–974. doi: 10.1016/j.neuron.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi G, Poo M. Synaptic modification by correlated activity: Hebb’s postulate revisited. Annu Rev Neurosci. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- Bialek W, Rieke F. Reliability and information transmission in spiking neurons. Trends Neurosci. 1992;15:428–434. doi: 10.1016/0166-2236(92)90005-s. [DOI] [PubMed] [Google Scholar]
- Biedenkapp JC, Rudy JW. Context preexposure prevents forgetting of a contextual fear memory:implication for regional changes in brain activation patterns associated with recent and remote memory tests. Learn Mem. 2007;14:200–203. doi: 10.1101/lm.499407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Burianova H, Grady CL. Common and unique neural activations in autobiographical, episodic, and semantic retrieval. J Cogn Neurosci. 2007;19:1520–1534. doi: 10.1162/jocn.2007.19.9.1520. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Large-scale recording of neuronal ensembles. Nat Neurosci. 2004;7:446–451. doi: 10.1038/nn1233. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Eidelberg E. Phase relations of hippocampal projection cells and interneurons to theta activity in the anesthetized rat. Brain Res. 1983;266:334–339. doi: 10.1016/0006-8993(83)90665-0. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Wang H, Mei B, An S, Yin L, Wang LP, Tsien JZ. Inducible and selective erasure of memories in the mouse brain via chemical-genetic manipulation. Neuron. 2008;60:353–366. doi: 10.1016/j.neuron.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Wang LP, Tsien JZ. Neural population-level memory traces in the mouse hippocampus. PLoS ONE. 2009;4:e8256. doi: 10.1371/journal.pone.0008256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury N, Quinn JJ, Fanselow MS. Dorsal hippocampus involvement in trace fear conditioning with long, but not short, trace intervals in mice. Behav Neurosci. 2005;119:1396–1402. doi: 10.1037/0735-7044.119.5.1396. [DOI] [PubMed] [Google Scholar]
- Clark RE, Squire LR. Classical conditioning and brain systems: the role of awareness. Science. 1998;280:77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola S. Trace eyeblink classical conditioning in the monkey: a nonsurgical method and behavioral analysis. Behav Neurosci. 1998;112:1062–1068. doi: 10.1037//0735-7044.112.5.1062. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, amnesia, and the hippocampal system. MIT Press; Cambridge, Mass: 1993. [Google Scholar]
- Cui Z, Wang H, Tan Y, Zaia KA, Zhang S, Tsien JZ. Inducible and reversible NR1 knockout reveals crucial role of the NMDA receptor in preserving remote memories in the brain. Neuron. 2004;41:781–793. doi: 10.1016/s0896-6273(04)00072-8. [DOI] [PubMed] [Google Scholar]
- Davis M, Hitchcock JM, Rosen JB. Anxiety and the amygdala: Pharmacological and anatomical analysis of the fear-potentiated startle paradigm. Psychology of learning and motivation. 1988;21:263–305. [Google Scholar]
- Duzel E, Cabeza R, Picton TW, Yonelinas AP, Scheich H, Heinze HJ, Tulving E. Task-related and item-related brain processes of memory retrieval. Proc Natl Acad Sci U S A. 1999;96:1794–1799. doi: 10.1073/pnas.96.4.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskandar EN, Richmond BJ, Optican LM. Role of inferior temporal neurons in visual memory. I. Temporal encoding of information about visual images, recalled images, and behavioral context. J Neurophysiol. 1992;68:1277–1295. doi: 10.1152/jn.1992.68.4.1277. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Frey S, Bergado-Rosado J, Seidenbecher T, Pape HC, Frey JU. Reinforcement of early long-term potentiation (early-LTP) in dentate gyrus by stimulation of the basolateral amygdala: heterosynaptic induction mechanisms of late-LTP. J Neurosci. 2001;21:3697–3703. doi: 10.1523/JNEUROSCI.21-10-03697.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, Frey JU. ‘Synaptic tagging’ and ‘cross-tagging’ and related associative reinforcement processes of functional plasticity as the cellular basis for memory formation. Prog Brain Res. 2008;169:117–143. doi: 10.1016/S0079-6123(07)00007-6. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging: implications for late maintenance of hippocampal long-term potentiation. Trends Neurosci. 1998;21:181–188. doi: 10.1016/s0166-2236(97)01189-2. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Unit activity in prefrontal cortex during delayed-response performance: neuronal correlates of transient memory. Journal of Neurophysiology. 1973 doi: 10.1152/jn.1973.36.1.61. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Schwartz AB, Kettner RE. Neuronal population coding of movement direction. Science. 1986;233:1416–1419. doi: 10.1126/science.3749885. [DOI] [PubMed] [Google Scholar]
- Gochin PM, Colombo M, Dorfman GA, Gerstein GL, Gross CG. Neural ensemble coding in inferior temporal cortex. J Neurophysiol. 1994;71:2325–2337. doi: 10.1152/jn.1994.71.6.2325. [DOI] [PubMed] [Google Scholar]
- Gross CG, Rocha-Miranda CE, Bender DB. Visual properties of neurons in inferotemporal cortex of the Macaque. J Neurophysiol. 1972;35:96–111. doi: 10.1152/jn.1972.35.1.96. [DOI] [PubMed] [Google Scholar]
- Harris KD, Henze DA, Csicsvari J, Hirase H, Buzsaki G. Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J Neurophysiol. 2000;84:401–414. doi: 10.1152/jn.2000.84.1.401. [DOI] [PubMed] [Google Scholar]
- Harvey CD, Coen P, Tank DW. Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature. 2012;484:62–68. doi: 10.1038/nature10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior. Wiley; New York: 1949. [Google Scholar]
- Hodges JR, Graham KS. Episodic memory: insights from semantic dementia. Philos Trans R Soc Lond B Biol Sci. 2001;356:1423–1434. doi: 10.1098/rstb.2001.0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur N, Friston KJ, Young A, Frith CD, Frackowiak RS. Activation of human hippocampal formation during memory for faces: a PET study. Cortex. 1995;31:99–108. doi: 10.1016/s0010-9452(13)80108-6. [DOI] [PubMed] [Google Scholar]
- Kentros C. Hippocampal place cells: the “where” of episodic memory? Hippocampus. 2006;16:743–754. doi: 10.1002/hipo.20199. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neurosci Biobehav Rev. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, Buzsaki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Cheng DT, Smith CN, Stein EA, Helmstetter FJ. Neural substrates mediating human delay and trace fear conditioning. J Neurosci. 2004;24:218–228. doi: 10.1523/JNEUROSCI.0433-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus BJ, Robinson RJ, 2nd, White JA, Eichenbaum H, Hasselmo ME. Hippocampal “Time Cells”: Time versus Path Integration. Neuron. 2013;78:1090–1101. doi: 10.1016/j.neuron.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion, memory and the brain. Sci Am. 1994;270:50–57. doi: 10.1038/scientificamerican0694-50. [DOI] [PubMed] [Google Scholar]
- Lestienne R. Spike timing, synchronization and information processing on the sensory side of the central nervous system. Prog Neurobiol. 2001;65:545–591. doi: 10.1016/s0301-0082(01)00019-3. [DOI] [PubMed] [Google Scholar]
- Lin L, Chen G, Kuang H, Wang D, Tsien JZ. Neural encoding of the concept of nest in the mouse brain. Proc Natl Acad Sci U S A. 2007;104:6066–6071. doi: 10.1073/pnas.0701106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Chen G, Xie K, Zaia KA, Zhang S, Tsien JZ. Large-scale neural ensemble recording in the brains of freely behaving mice. J Neurosci Methods. 2006a;155:28–38. doi: 10.1016/j.jneumeth.2005.12.032. [DOI] [PubMed] [Google Scholar]
- Lin L, Osan R, Shoham S, Jin W, Zuo W, Tsien JZ. Identification of network-level coding units for real-time representation of episodic experiences in the hippocampus. Proc Natl Acad Sci U S A. 2005;102:6125–6130. doi: 10.1073/pnas.0408233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Osan R, Tsien JZ. Organizing principles of real-time memory encoding: neural clique assemblies and universal neural codes. Trends Neurosci. 2006b;29:48–57. doi: 10.1016/j.tins.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Lisman J, Redish AD. Prediction, sequences and the hippocampus. Philos Trans R Soc Lond B Biol Sci. 2009;364:1193–1201. doi: 10.1098/rstb.2008.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron. 2011;71:737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Frith CD, Rudge P, Cipolotti L. The effect of adult-acquired hippocampal damage on memory retrieval: an fMRI study. Neuroimage. 2005;27:146–152. doi: 10.1016/j.neuroimage.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J Neurosci. 2004;24:2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8:638–646. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Harrison LK, Forrester K, Lawrie SM, Johnstone EC. Neuropsychological impairments in people with schizophrenia or bipolar disorder and their unaffected relatives. Br J Psychiatry. 2005;186:378–385. doi: 10.1192/bjp.186.5.378. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the ‘cognitive map’. Nat Rev Neurosci. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, O’Keefe J, Barnes CA. The stereotrode: a new technique for simultaneous isolation of several single units in the central nervous system from multiple unit records. J Neurosci Methods. 1983;8:391–397. doi: 10.1016/0165-0270(83)90097-3. [DOI] [PubMed] [Google Scholar]
- Messas CS, Mansur LL, Castro LH. Semantic memory impairment in temporal lobe epilepsy associated with hippocampal sclerosis. Epilepsy Behav. 2008;12:311–316. doi: 10.1016/j.yebeh.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Miller EK, Li L, Desimone R. Activity of neurons in anterior inferior temporal cortex during a short-term memory task. J Neurosci. 1993;13:1460–1478. doi: 10.1523/JNEUROSCI.13-04-01460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra P. What’s wrong with the brain activity map proposal. Sci Am. 2013 [Google Scholar]
- Mizumori SJ. Hippocampal place fields: a neural code for episodic memory? Hippocampus. 2006;16:685–690. doi: 10.1002/hipo.20209. [DOI] [PubMed] [Google Scholar]
- Musallam S, Corneil BD, Greger B, Scherberger H, Andersen RA. Cognitive control signals for neural prosthetics. Science. 2004;305:258–262. doi: 10.1126/science.1097938. [DOI] [PubMed] [Google Scholar]
- Nicolelis MA, Ribeiro S. Seeking the neural code. Sci Am. 2006;295:70–77. doi: 10.1038/scientificamerican1206-70. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. Clarendon Press; Oxford: 1978. [Google Scholar]
- Oler JA, Penley SC, Sava S, Markus EJ. Does the dorsal hippocampus process navigational routes or behavioral context? A single-unit analysis. Eur J Neurosci. 2008;28:802–812. doi: 10.1111/j.1460-9568.2008.06375.x. [DOI] [PubMed] [Google Scholar]
- Osan R, Chen G, Feng R, Tsien JZ. Differential Consolidation and Pattern Reverberations within Episodic Cell Assemblies in the Mouse Hippocampus. PLoS ONE. 2011;6:e16507. doi: 10.1371/journal.pone.0016507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osan R, Zhu L, Shoham S, Tsien JZ. Subspace projection approaches to classification and visualization of neural network-level encoding patterns. PLoS ONE. 2007;2:e404. doi: 10.1371/journal.pone.0000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroga RQ, Kreiman G, Koch C, Fried I. Sparse but not ‘grandmother-cell’ coding in the medial temporal lobe. Trends Cogn Sci. 2008;12:87–91. doi: 10.1016/j.tics.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Quiroga RQ, Reddy L, Kreiman G, Koch C, Fried I. Invariant visual representation by single neurons in the human brain. Nature. 2005;435:1102–1107. doi: 10.1038/nature03687. [DOI] [PubMed] [Google Scholar]
- Redish AD. The hippocampal debate: are we asking the right questions? Behav Brain Res. 2001;127:81–98. doi: 10.1016/s0166-4328(01)00356-4. [DOI] [PubMed] [Google Scholar]
- Ryan L, Cox C, Hayes SM, Nadel L. Hippocampal activation during episodic and semantic memory retrieval: comparing category production and category cued recall. Neuropsychologia. 2008;46:2109–2121. doi: 10.1016/j.neuropsychologia.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger TD. Neural population codes. Current Opinion in Neurobiology. 2003;13:238. doi: 10.1016/s0959-4388(03)00034-5. [DOI] [PubMed] [Google Scholar]
- Schmidt EM. Electrodes for many single neuron recordings. Methods for Neural Ensemble Recordings. 1999:1–23. [Google Scholar]
- Shamir M, Sompolinsky H. Nonlinear population codes. Neural computation. 2004;16:1105–1136. doi: 10.1162/089976604773717559. [DOI] [PubMed] [Google Scholar]
- Shimizu E, Tang YP, Rampon C, Tsien JZ. NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation. Science. 2000;290:1170–1174. doi: 10.1126/science.290.5494.1170. [DOI] [PubMed] [Google Scholar]
- Smith DM, Mizumori SJ. Hippocampal place cells, context, and episodic memory. Hippocampus. 2006;16:716–729. doi: 10.1002/hipo.20208. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Zola SM. Episodic memory, semantic memory, and amnesia. Hippocampus. 1998;8:205–211. doi: 10.1002/(SICI)1098-1063(1998)8:3<205::AID-HIPO3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Thompson RF. In search of memory traces. Annu Rev Psychol. 2005;56:1–23. doi: 10.1146/annurev.psych.56.091103.070239. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Damasio AR. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia. 1997;35:1319–1327. doi: 10.1016/s0028-3932(97)00085-7. [DOI] [PubMed] [Google Scholar]
- Tsien JZ. Building a brainier mouse. Sci Am. 2000;282:62–68. doi: 10.1038/scientificamerican0400-62. [DOI] [PubMed] [Google Scholar]
- Tsien JZ. The memory code. Sci Am. 2007;297:52–59. doi: 10.1038/scientificamerican0707-52. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER, Tonegawa S. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996a;87:1317–1326. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996b;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- Tukker JJ, Fuentealba P, Hartwich K, Somogyi P, Klausberger T. Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. J Neurosci. 2007;27:8184–8189. doi: 10.1523/JNEUROSCI.1685-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of memory. Academic Press; New York: 1972. pp. 381–403. [Google Scholar]
- Velliste M, Perel S, Spalding MC, Whitford AS, Schwartz AB. Cortical control of a prosthetic arm for self-feeding. Nature. 2008;453:1098–1101. doi: 10.1038/nature06996. [DOI] [PubMed] [Google Scholar]
- Wang DV, Tsien JZ. Convergent Processing of Both Positive and Negative Motivational Signals by the VTA Dopamine Neuronal Populations. PLoS ONE. 2011;6:e17047. doi: 10.1371/journal.pone.0017047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LP, Li F, Wang D, Xie K, Shen X, Tsien JZ. NMDA Receptors in Dopaminergic Neurons Are Crucial for Habit Learning. Neuron. 2011;72:1055–1066. doi: 10.1016/j.neuron.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK, Shallice T. Category specific semantic impairments. Brain. 1984;107(Pt 3):829–854. doi: 10.1093/brain/107.3.829. [DOI] [PubMed] [Google Scholar]
- Wigstrom H, Gustafsson B. On long-lasting potentiation in the hippocampus: a proposed mechanism for its dependence on coincident pre- and postsynaptic activity. Acta Physiol Scand. 1985;123:519–522. doi: 10.1111/j.1748-1716.1985.tb07621.x. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Jiang X, Zhu D, Chen H, Li K, Lv P, Yu X, Li X, Zhang S, Zhang T, Hu X, Han J, Guo L, Liu T. Meta-analysis of functional roles of DICCCOLs. Neuroinformatics. 2013;11:47–63. doi: 10.1007/s12021-012-9165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YD, Fuster JM. Mnemonic neuronal activity in somatosensory cortex. Proc Natl Acad Sci U S A. 1996;93:10533–10537. doi: 10.1073/pnas.93.19.10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Li K, Guo L, Jiang X, Zhang T, Zhang D, Chen H, Deng F, Faraco C, Jin C, Wee CY, Yuan Y, Lv P, Yin Y, Hu X, Duan L, Han J, Wang L, Shen D, Miller LS, Li L, Liu T. DICCCOL: dense individualized and common connectivity-based cortical landmarks. Cereb Cortex. 2013;23:786–800. doi: 10.1093/cercor/bhs072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Burns LD, Cocker ED, Hamel EO, Ghosh KK, Kitch LJ, El Gamal A, Schnitzer MJ. Long-term dynamics of CA1 hippocampal place codes. Nat Neurosci. 2013;16:264–266. doi: 10.1038/nn.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]