Summary
Purpose
The role of granule cell axon (mossy fiber) sprouting in temporal lobe epileptogenesis is unclear and controversial. Rapamycin suppresses mossy fiber sprouting, but its reported effects on seizure frequency are mixed. The present study used high-dose rapamycin to more completely block mossy fiber sprouting and measure the effect on seizure frequency.
Methods
Mice were treated with pilocarpine to induce status epilepticus. Beginning 24 h later and continuing for 2 months, vehicle or rapamycin (10 mg/kg/d) was administered. Starting 1 month after status epilepticus, mice were video-monitored 9 h/d every day for 1 month to measure the frequency of spontaneous motor seizures. At the end of seizure monitoring, a subset of mice was prepared for anatomical analysis. Mossy fiber sprouting was measured as the proportion of the granule cell layer and molecular layer that displayed black labeling in Timm-stained sections.
Key findings
Extensive mossy fiber sprouting developed in mice that experienced status epilepticus and were treated with vehicle. In rapamycin-treated mice, mossy fiber sprouting was blocked almost to the level of naïve controls. Seizure frequency was similar in vehicle- and rapamycin-treated mice.
Significance
These findings suggest mossy fiber sprouting is not necessary for epileptogenesis in the mouse pilocarpine model. They also reveal that rapamycin does not have anti-seizure or anti-epileptogenic effects in this model.
Keywords: dentate gyrus, granule cells, hilar neurons, pilocarpine, axon sprouting, Timm stain
Introduction
Aberrant sprouting of granule cell axons (mossy fibers) into the molecular layer of the dentate gyrus is a common neuropathological finding in patients with temporal lobe epilepsy (de Lanerolle et al., 1989; Sutula et al., 1989; Houser et al., 1990; Babb et al., 1991). Mossy fiber sprouting establishes an aberrant positive-feedback circuit among granule cells (Wuarin & Dudek, 1996; Molnár & Nadler, 1999; Lynch & Sutula, 2000; Buckmaster et al., 2002; Scharfman et al., 2003), which has been proposed to be epileptogenic (Tauck & Nadler, 1985; Feng et al., 2003). In contrast, some have argued that mossy fiber sprouting restores excitatory synaptic input to inhibitory interneurons and is anti-epileptogenic (Sloviter, 1992; Sloviter et al., 2006).
We previously identified a drug, rapamycin, that suppresses mossy fiber sprouting (Buckmaster et al., 2009) and evaluated its effect on seizure frequency in a mouse model of temporal lobe epilepsy (Buckmaster & Lew, 2011). Beginning 24 h after pilocarpine-induced status epilepticus, mice were treated systemically with up to 3 mg/kg/d of rapamycin for at least 2 months. Seizure frequency was not affected despite significant reductions in mossy fiber sprouting, suggesting sprouting is neither anti- nor pro-epileptogenic in the mouse pilocarpine model. However, computer simulations suggest even modest levels of mossy fiber sprouting are sufficient to generate seizure activity (Santhakumar et al., 2005). Furthermore, rapamycin reduces seizure frequency in some rat models of temporal lobe epilepsy (Zeng et al., 2009; Huang et al., 2010; van Vliet et al., 2012) but not all (Sliwa et al., 2012). In previous rat studies, rapamycin was administered at doses up to 6 mg/kg/d, which raises the possibility that the 3 mg/kg/d dose used in mice was insufficient to reduce seizures.
The present study used a higher dose of rapamycin (10 mg/kg/d) to more rigorously evaluate the role of mossy fiber sprouting in epileptogenesis. The main questions were whether sprouting could be blocked more completely and whether seizure frequency would be affected.
Methods
Animals
All animal procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by an institutional animal care and use committee at Stanford University. GIN mice (FVB-Tg(GadGFP)4570Swn/J, The Jackson Laboratory) of both sexes (66% female) at 54 ± 2 d old (mean ± sem; range, 28–220 d) were treated with pilocarpine. There were no significant differences in sex or age between mice in the vehicle- and rapamycin-treated groups. Pilocarpine (300 mg/kg, i.p.) was administered 44 ± 1 min (range, 19–76 min) after atropine methyl bromide (5 mg/kg, i.p.). Diazepam (10 mg/kg, i.p.) was administered 2 h after onset of motor seizures and repeated as necessary to suppress convulsions. After status epilepticus, mice were kept warm and received lactated Ringer’s solution with dextrose. Beginning 24 h after status epilepticus, daily treatment began with 10 mg/kg rapamycin (LC Laboratories) in vehicle (5% Tween 80, 5% polyethylene glycol 400, and 4% ethanol) or with vehicle alone.
Seizure monitoring
Seizure monitoring began 1 month after status epilepticus. Mice were placed in individual slots in a terrarium and video-recorded 9 h/d every day for 1 month. Recording began at approximately 7:30 am. Recordings were manually reviewed by investigators who were blind to experimental groups. Only motor seizures of stage 3 or greater (Racine, 1972) were counted.
Anatomy
After video-recording was complete (2 months after status epilepticus), a subset of mice in each group was prepared for anatomical analysis, as described previously (Buckmaster & Lew, 2011). A 1-in-12 series of sections from the hippocampus was developed for Timm staining. An adjacent series of sections was Nissl-stained with 0.25% thionin. All analyses were done by investigators who were blind to experimental groups. Mossy fiber sprouting was measured as the percentage of the volume of granule cell layer plus molecular layer that was black in Timm-stained sections. NIH ImageJ was used to outline and measure the area of the granule cell layer plus molecular layer. Timm-positive areas were selected by adjusting a darkness threshold tool. Volumes were calculated by summing the area from each hippocampus and multiplying for section sampling (12) and section thickness (40 μm). The optical fractionator method was used to estimate the number of hilar neurons per dentate gyrus. Borders of the hilus extended 25 μm from the granule cell layer into the hilus. An average of 197 cells was counted per dentate gyrus. The mean coefficient of error (0.113) was much less than the coefficient of variation (0.487), suggesting within-animal sampling was sufficient (West et al., 1991).
Results
High-dose rapamycin blocks mossy fiber sprouting
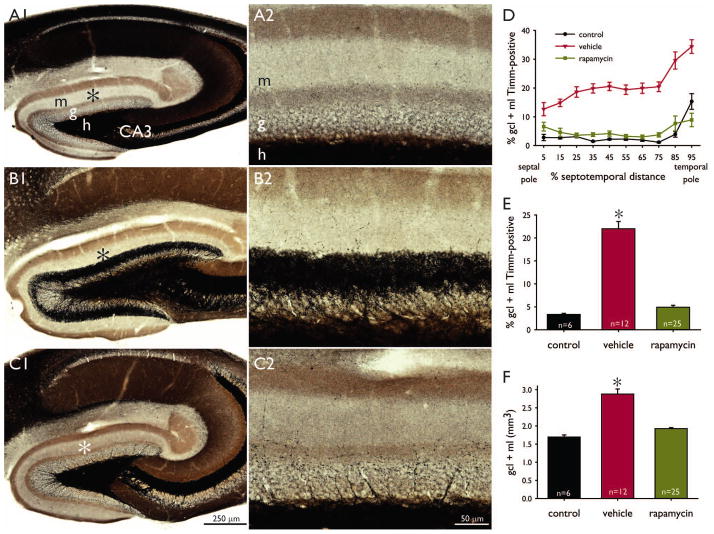
Similar to previous reports, rapamycin qualitatively appeared to stunt growth (Zeng et al., 2008) and attenuate aggressiveness (Huang et al., 2012). The high dose did not have any obvious ill health effects. Naïve control mice displayed little, if any, black Timm staining in the granule cell or molecular layer (Figure 1A1,2), except at the temporal pole of the hippocampus (Figure 1D). In contrast, mice that had experienced status epilepticus and were treated with vehicle for 2 months displayed a dense, black band of Timm staining in the inner molecular layer (Figure 1B1,2). In the vehicle-treated group, mossy fiber sprouting occurred at all septotemporal levels of the hippocampus. On the other hand, mice that had experienced status epilepticus and were treated with 10 mg/kg rapamycin for 2 months displayed scattered small punctae of black Timm staining in the granule cell layer and inner molecular layer (Figure 1C1,2).
Figure 1.
High-dose rapamycin blocks mossy fiber sprouting and suppresses dentate gyrus hypertrophy. Timm-stained sections of the dentate gyrus of a naïve control mouse (A), a mouse that experienced pilocarpine-induced status epilepticus and was treated with vehicle (B), and a mouse that experienced status epilepticus and was treated with 10 mg/kg rapamycin (C). m = molecular layer, g = granule cell layer, h = hilus, CA3 = CA3 pyramidal cell layer. Asterisks in A1–C1 indicate areas shown at higher magnification in A2–C2. D Mossy fiber sprouting along the septotemporal axis of the hippocampus. Values indicate mean ± s.e.m. E Average levels of mossy fiber sprouting. *p < 0.05 compared to other groups (ANOVA on ranks with Dunn’s method). Control and rapamycin groups are not significantly different. F Average volumes of the dentate gyrus. *p < 0.05 compared to other groups (ANOVA on ranks with Dunn’s method). Control and rapamycin groups are not significantly different.
The extent of mossy fiber sprouting was measured as the proportion of the granule cell layer plus molecular layer that was labeled black. In naïve control mice, only 3.3 ± 0.2% (mean ± s.e.m., n = 6) of the granule cell layer plus molecular layer was Timm-positive (Figure 1E). In vehicle-treated mice, that value was over 6-fold higher (22.0 ± 1.6%, n = 12, p < 0.05, ANOVA on ranks with Dunn’s method). In rapamycin-treated mice, only 4.9 ± 0.4% of the granule cell layer plus molecular layer was Timm-positive (n = 25), which was not significantly different from controls but significantly less than vehicle-treated mice (p < 0.05).
At all septotemporal levels but one, the average extent of Timm staining in the granule cell layer plus molecular layer was slightly higher in rapamycin-treated mice that had experienced status epilepticus than in naïve controls (Figure 1D). At all septotemporal levels, the average extent of mossy fiber sprouting in rapamycin-treated mice was substantially less than that of vehicle-treated mice. Together, these findings reveal that high-dose rapamycin blocks mossy fiber sprouting to levels slightly higher but close to those of control mice.
High-dose rapamycin suppresses dentate gyrus hypertrophy
The dentate gyrus appeared larger in sections from mice that had experienced status epilepticus (Figure 1B1). In vehicle-treated mice the dentate gyrus was 1.7-fold larger (2.88 ± 0.14 mm3) than that of controls (1.70 ± 0.06 mm3, p < 0.05, ANOVA on ranks with Dunn’s method) (Figure 1F). In rapamycin-treated mice, dentate gyrus volume was 1.1-fold (1.93 ± 0.03 mm3) that of controls (not significantly different) but only 67% of vehicle-treated mice (p < 0.05). These findings show that high-dose rapamycin blocks dentate gyrus hypertrophy.
High-dose rapamycin does not prevent hilar neuron loss
Mossy fiber sprouting correlates with hilar neuron loss in patients with temporal lobe epilepsy (Houser et al., 1990; Babb et al., 1991; Masukawa et al., 1992) and in animal models (Cavazos & Sutula, 1990; Buckmaster & Dudek, 1997; Gorter et al., 2001; Nissinen et al., 2001). Therefore, it was important to determine whether rapamycin blocked mossy fiber sprouting indirectly by preventing hilar neuron loss. Mossy fiber sprouting correlates most closely with the loss of mossy cells (Jiao & Nadler, 2007). To avoid GABAergic interneurons at the hilar border with the granule cell layer and ectopic granule cells in the hilus and instead focus on mossy cells (albeit not exclusively), only large hilar neurons (soma diameter >10 μm) positioned beyond 25 μm of the border with the granule cell layer were counted. Control mice (n = 6) had 10,500 ± 300 hilar neurons per hippocampus (Figure 2A,D). Hilar neuron loss was evident in sections from all mice that experienced status epilepticus (Figure 2B,C). In vehicle- and rapamycin-treated mice hilar neurons were reduced to 51% and 41% of controls, respectively (p < 0.05, ANOVA on ranks with Dunn’s method). These findings reveal that hilar neurons were not protected by rapamycin. Therefore, reduced mossy fiber sprouting was not attributable to hilar neuron survival in rapamycin-treated mice.
Figure 2.
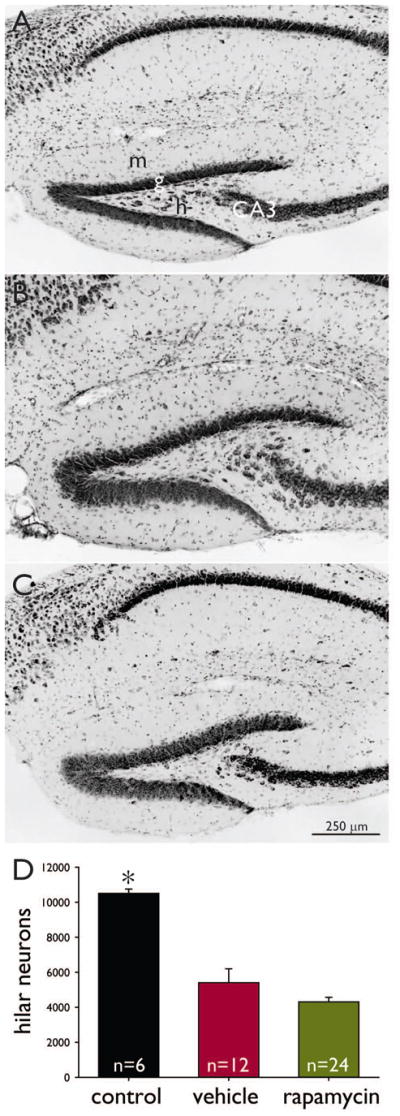
High-dose rapamycin does not prevent hilar neuron loss. Nissl-stained sections of the dentate gyrus of a naïve control mouse (A), a mouse that experienced pilocarpine-induced status epilepticus and was treated with vehicle (B), and a mouse that experienced status epilepticus and was treated with 10 mg/kg rapamycin (C). m = molecular layer, g = granule cell layer, h = hilus, CA3 = CA3 pyramidal cell layer. D Average number of hilar neurons per hippocampus. *p < 0.05 compared to other groups (ANOVA on ranks with Dunn’s method). Vehicle and rapamycin groups are not significantly different. Error bars indicate s.e.m.
Rapamycin pretreatment can be neuroprotective (Zeng et al., 2009) or it can exacerbate neuron loss (Chen et al., 2012) depending on timing relative to status epilepticus in rats. To avoid confounding pretreatment effects in the present study, rapamycin administration did not begin until 24 h after status epilepticus. Rapamycin-treated mice had fewer hilar neurons (4300 ± 300, n = 24) than vehicle-treated mice (5400 ± 800, n = 12), but the difference was not significant (p = 0.11, t test). These findings suggest that high-dose rapamycin treatment did not significantly exacerbate neuron loss.
High-dose rapamycin does not affect seizure frequency
Seizure frequency was measured by video-recording mice 9 h/d beginning 1 month after status epilepticus and continuing for 1 month. Over 99.9% of identified seizures were stage 4 or greater (Racine, 1972). Mice were recorded in groups of up to 10. Many individuals displayed seizure clusters (Figure 3A). Over 29,000 mouse-hours were evaluated, and 3881 seizures were identified. In the vehicle- and rapamycin-treated groups 29.7 ± 2.9 (range, 0–125) and 30.9 ± 2.9 (range, 3–86) seizures/mouse were counted, respectively. Seizure frequency was averaged over the total recording period for each mouse, and averages ranged from zero in one mouse up to 0.606 seizures/h. The coefficient of variation for the entire sample of mice was 0.72. Seizure frequency was similar in vehicle- (0.137 ± 0.013 seizures/h, n = 64) and rapamycin-treated mice (0.133 ± 0.012 seizures/h, n = 64, p = 0.845, t test) (Figure 3B). Statistical power was sufficient to detect a difference as small as 10%. These findings reveal that rapamycin did not affect seizure frequency in pilocarpine-treated mice.
Figure 3.
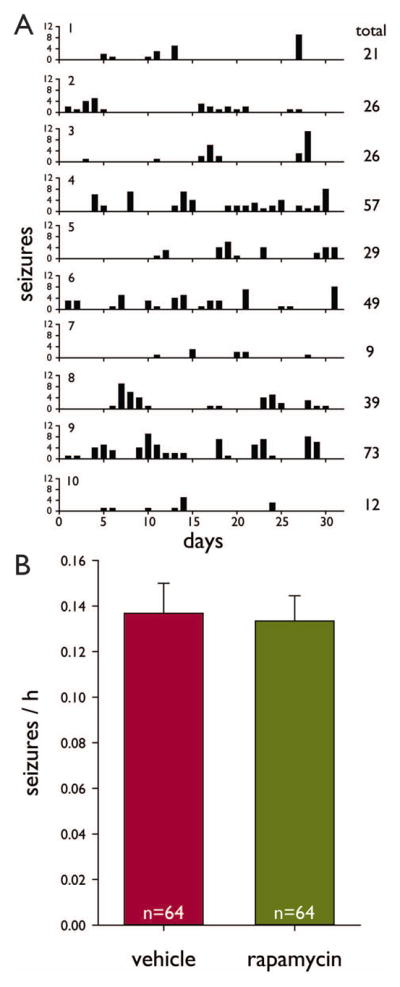
High-dose rapamycin does not affect seizure frequency. A Histograms of seizures versus days of recording in a group of mice (1–10) that were video-monitored together in a terrarium with slots separating individuals. Recordings began 1 month after status epilepticus. Only seizures of grade 3 or greater (Racine, 1972) were counted. Plotted values indicate number of seizures observed per 9 h recording period. B Average seizure frequency. There was no significant difference (p = 0.845, t test). Error bars indicate s.e.m.
Discussion
The principal finding of this study is that high-dose rapamycin blocked mossy fiber sprouting without affecting seizure frequency. This finding suggests rapamycin is neither anti-seizure nor anti-epileptogenic and that mossy fiber sprouting is neither anti- nor pro-epileptogenic in the mouse pilocarpine model.
Mossy fiber sprouting is neither anti- nor pro-epileptogenic in pilocarpine-treated mice
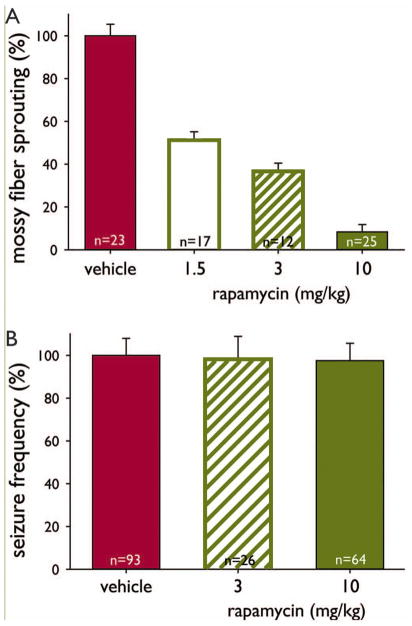
The present study and previous experiments in pilocarpine-treated mice (Buckmaster & Lew, 2011) used a range of rapamycin doses (1.5–10 mg/kg/d) that suppressed mossy fiber sprouting to increasing degrees, but seizure frequency was not affected (Figure 4). If sprouted mossy fibers excited interneurons to inhibit seizures (Sloviter, 1992; Sloviter et al., 2006), one would expect increased seizure frequency in rapamycin-treated mice with less sprouting. On the other hand, if mossy fiber sprouting caused seizures (Tauck & Nadler, 1985; Feng et al., 2003), one would expect reduced seizure frequency in rapamycin-treated mice with reduced sprouting. Neither was the case. Instead, the present findings suggest mossy fiber is an epiphenomenon unrelated to epileptogenesis (Gloor, 1997).
Figure 4.
Dose-dependent effect of rapamycin on mossy fiber sprouting but not seizure frequency in pilocarpine-treated mice. Data from Buckmaster and Lew (2011) and the present study. A Percent mossy fiber sprouting was calculated by subtracting the average percentage of the molecular layer plus granule cell layer that was Timm-positive in naïve control mice and normalizing by the average value of mice that had experienced status epilepticus and were treated with vehicle for 2 months. Averages of all groups are significantly different from others (p < 0.05, ANOVA with Student-Newman-Keuls method). Error bars indicate s.e.m. B Percent seizure frequency was calculated by normalizing by the average of the vehicle-treated group.
Many previous studies have considered whether mossy fiber sprouting correlates with seizure frequency (reviewed in Buckmaster, 2012). Most reported no correlation, but some did. In those cases, it remains unclear whether seizures were caused by mossy fiber sprouting or other changes that occurred in parallel, of which there are many possibilities. The present study used rapamycin to experimentally suppress mossy fiber sprouting and test its effect on seizure frequency. However, rapamycin has many side-effects (Swiech et al., 2008), including suppression of axon sprouting by surviving GABAergic interneurons (Buckmaster & Wen, 2011). It is possible (but not parsimonious) that rapamycin had multiple pro- and anti-epileptogenic effects that cancelled out equally, resulting in no net change in seizure frequency.
Rapamycin inhibits the mammalian (or mechanistic) target of rapamycin (mTOR) signaling pathway (Sabatini et al., 1994). Experimentally increasing mTOR activity in subsets of granule cells causes epilepsy and morphological abnormalities, including mossy fiber sprouting, but some mice develop epilepsy without mossy fiber sprouting, suggesting other changes were responsible for epileptogenesis in that model (Pun et al., 2012). Rapamycin suppresses dentate gyrus hypertrophy without affecting seizure frequency, suggesting granule cell enlargement is neither pro- nor anti-epileptogenic (Buckmaster & Lew, 2011; present study).
Rapamycin (10 mg/kg/d) blocked mossy fiber sprouting substantially but incompletely. The remaining low level of mossy fiber sprouting in rapamycin-treated mice appeared comparable to that of kindled animals, which do not have spontaneous seizures (Sutula et al., 1988). In the dentate gyrus of pilocarpine-treated animals, other recurrent excitatory circuits develop (Zhang et al., 2012) and might persist in rapamycin-treated mice. However, rapamycin blocks the functional development of abnormal recurrent excitation of granule cells in pilocarpine-treated mice (Tang et al., 2012). Together, these findings suggest mossy fiber sprouting and recurrent excitation of granule cells is not epileptogenic in pilocarpine-treated mice.
Rapamycin is neither anti-seizure nor anti-epileptogenic in pilocarpine-treated mice
Rapamycin does not affect seizure frequency in pilocarpine-treated mice (Buckmaster and Lew, 2011; present study). In contrast, epileptic pilocarpine-treated rats display reduced seizure frequency within 5 d of rapamycin administration, and seizure frequency increases soon after rapamycin treatment ends (Huang et al., 2010). The rapamycin treatment protocols of the studies differ: 10 mg/kg beginning 24 h after status epilepticus and continuing daily in mice versus 5 mg/kg/d for 3 d followed by every other day in rats that had already developed epilepsy. Nevertheless, these findings suggest rapamycin has an anti-seizure effect in rats but not mice. The rapamycin dose used in the present study exceeded what is needed to block mTOR activity in the hippocampus of mice (Zeng et al., 2008) and was approximately twice that used to suppress seizures in rats. Underlying mechanisms of the species-specific difference in anti-seizure effects of rapamycin are unclear. Mice are not generally resistant to the effects of rapamycin. In other mouse models with mutations that cause over-activation of the mTOR pathway, seizures are reduced by rapamycin and related drugs (Kwon et al., 2003; Zeng et al., 2008; Ljungberg et al., 2009; Zhou et al., 2009; Sunnen et al., 2011; McMahon et al., 2012). However, in control mice, rapamycin has limited acute anticonvulsant effects (Hartman et al., 2012).
In summary, rapamycin blocks mossy fiber sprouting (Buckmaster et al., 2009; Lew & Buckmaster, 2011; Tang et al., 2012), but its effect on seizure frequency is mixed, with either no effect (Buckmaster & Lew, 2011; Sliwa et al., 2012) or seizure suppression in some rat models (Zeng et al., 2009; Huang et al., 2010; van Vliet et al., 2012). It is important to determine whether rapamycin’s effect is truly anti-epileptogenic in rats. If not, available evidence suggests mossy fiber sprouting is not a cause of temporal lobe epilepsy.
Acknowledgments
Supported by NINDS and ORIP/OD of the NIH.
Footnotes
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Babb TL, Kupfer WR, Pretorius JK, Crandall PH, Levesque MF. Synaptic reorganization by mossy fibers in human epileptic fascia dentata. Neuroscience. 1991;42:351–363. doi: 10.1016/0306-4522(91)90380-7. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS. Mossy fiber sprouting in the dentate gyrus. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Japser’s basic mechanisms of the epilepsies. 4. Oxford University Press; New York: 2012. pp. 416–431. [PubMed] [Google Scholar]
- Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol. 1997;385:385–404. [PubMed] [Google Scholar]
- Buckmaster PS, Ingram EA, Wen X. Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy. J Neurosci. 2009;29:8259–8269. doi: 10.1523/JNEUROSCI.4179-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Lew FH. Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy. J Neurosci. 2011;31:2337–2347. doi: 10.1523/JNEUROSCI.4852-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Wen X. Rapamycin suppresses axon sprouting by somatostatin interneurons in a mouse model of temporal lobe epilepsy. Epilepsia. 2011;52:2057–2064. doi: 10.1111/j.1528-1167.2011.03253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Zhang GF, Yamawaki R. Axon sprouting in a model of temporal lobe epilepsy creates a predominantly excitatory feedback circuit. J Neurosci. 2002;22:6650–6658. doi: 10.1523/JNEUROSCI.22-15-06650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazos JE, Sutula TP. Progressive neuronal loss induced by kindling: a possible mechanism for mossy fiber synaptic reorganization and hippocampal sclerosis. Brain Res. 1990;527:1–6. doi: 10.1016/0006-8993(90)91054-k. [DOI] [PubMed] [Google Scholar]
- de Lanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989;495:387–395. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- Feng L, Molnár P, Nadler JV. Short-term frequency-dependent plasticity at recurrent mossy fiber synapses of the epileptic brain. J Neurosci. 2003;23:5381–5390. doi: 10.1523/JNEUROSCI.23-12-05381.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor P. The temporal lobe and limbic system. Oxford University Press; New York: 1997. pp. 677–691. [Google Scholar]
- Gorter JA, van Vliet EA, Aronica E, Lopes da Silva FH. Progression of spontaneous seizures after status epilepticus is associated with mossy fibre sprouting and extensive bilateral loss of hilar parvalbumin and somatostatin-immunoreactive neurons. Eur J Neurosci. 2001;13:657–669. doi: 10.1046/j.1460-9568.2001.01428.x. [DOI] [PubMed] [Google Scholar]
- Hartman AL, Santos P, Dolce A, Hardwick JM. The mTOR inhibitor rapamycin has limited acute anticonvulsant effects in mice. PLOS One. 2012;7:e45156. doi: 10.1371/journal.pone.0045156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser CR, Miyashiro JE, Swartz BE, Walsh GO, Rich JR, Delgado-Escueta AV. Altered patterns of dynorphin immunoreactivity suggest mossy fiber reorganization in human hippocampal epilepsy. J Neurosci. 1990;10:267–282. doi: 10.1523/JNEUROSCI.10-01-00267.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, McMahon J, Huang Y. Rapamycin attenuates aggressive behavior in a rat model of pilocarpine-induced epilepsy. Neuroscience. 2012;215:90–97. doi: 10.1016/j.neuroscience.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Zhang H, Yang J, Wu J, McMahon J, Lin Y, Cao Z, Gruenthal M, Huang Y. Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiol Dis. 2010;40:193–199. doi: 10.1016/j.nbd.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Nadler JV. Stereological analysis of GluR2-immunoreactive hilar neurons in the pilocarpine model of temporal lobe epilepsy: correlation of cell loss with mossy fiber sprouting. Exp Neurol. 2007;205:569–582. doi: 10.1016/j.expneurol.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C-H, Zhu X, Zhang J, Baker SJ. mTor is required for hypertrophy of Pten-deficient neuronal soma in vivo. Proc Natl Acad Sci USA. 2003;100:12923–12928. doi: 10.1073/pnas.2132711100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew FH, Buckmaster PS. Is there a critical period for mossy fiber sprouting in a mouse model of temporal lobe epilepsy? Epilepsia. 2011;52:2326–2332. doi: 10.1111/j.1528-1167.2011.03315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungberg MC, Sunnen CN, Lugo JN, Anderson AE, D’Arcangelo G. Rapamycin suppresses seizures and neuronal hypertrophy in a mouse model of cortical dysplasia. Dis Model Mech. 2009;2:389–398. doi: 10.1242/dmm.002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M, Sutula T. Recurrent excitatory connectivity in the dentate gyrus of kindled and kainic acid-treated rats. J Neurophysiol. 2000;83:693–704. doi: 10.1152/jn.2000.83.2.693. [DOI] [PubMed] [Google Scholar]
- Masukawa LM, Uruno K, Sperling M, O’Connor MJ, Burdette LJ. The functional relationship between antidromically evoked field responses of the dentate gyrus and mossy fiber reorganization in temporal lobe epileptic patients. Brain Res. 1992;579:119–127. doi: 10.1016/0006-8993(92)90750-4. [DOI] [PubMed] [Google Scholar]
- McMahon J, Huang X, Yang J, Komatsu M, Yue Z, Qian J, Zhu X, Huang Y. Impaired autophagy in neurons after disinhibition of mammalian target of rapamycin and its contribution to epileptogenesis. J Neurosci. 2012;32:15704–15714. doi: 10.1523/JNEUROSCI.2392-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár P, Nadler JV. Mossy fiber - granule cell synapses in the normal and epileptic rat dentate gyrus studied with minimal laser photostimulation. J Neurophysiol. 1999;82:1883–1894. doi: 10.1152/jn.1999.82.4.1883. [DOI] [PubMed] [Google Scholar]
- Nissinen J, Lukasiuk K, Pitkänen A. Is mossy fiber sprouting present at the time of the first spontaneous seizures in rat experimental temporal lobe epilepsy? Hippocampus. 2001;11:299–310. doi: 10.1002/hipo.1044. [DOI] [PubMed] [Google Scholar]
- Pun RYK, Roll IJ, LaSarge CL, Hosford BE, Rosen JM, Uhl JD, Schmeltzer SN, Faulkner C, Bronson SL, Murphy BL, Richards DA, Holland KD, Danzer SC. Excessive activation of mTOR in postnatally generated granule cells is sufficient to cause epilepsy. Neuron. 2012;75:1022–1034. doi: 10.1016/j.neuron.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78:35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- Santhakumar V, Aradi I, Soltesz I. Role of mossy fiber sprouting and mossy cell loss in hyperexcitability: a network model of the dentate gyrus incorporating cell types and axonal topography. J Neurophysiol. 2005;93:437–453. doi: 10.1152/jn.00777.2004. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Sollas AL, Berger RE, Goodman JH. Electrophysiological evidence of monosynaptic excitatory transmission between granule cells after seizure-induced mossy fiber sprouting. J Neurophysiol. 2003;90:2536–2547. doi: 10.1152/jn.00251.2003. [DOI] [PubMed] [Google Scholar]
- Sliwa A, Plucinska G, Bednarczyk J, Lukasiuk K. Post-treatment with rapamycin does not prevent epileptogenesis in the amygdala stimulation model of temporal lobe epilepsy. Neurosci Lett. 2012;509:105–109. doi: 10.1016/j.neulet.2011.12.051. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Possible functional consequences of synaptic reorganization in the dentate gyrus of kainate-treated rats. Neurosci Lett. 1992;137:91–96. doi: 10.1016/0304-3940(92)90306-r. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Zappone CA, Harvey BD, Frotscher M. Kainic acid induced recurrent mossy fiber innervation of dentate gyrus inhibitory interneurons: possible anatomical substrate of granule cell hyperinhibition in chronically epileptic rats. J Comp Neurol. 2006;494:944–960. doi: 10.1002/cne.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunnen CN, Brewster AL, Lugo JN, Vanegas F, Turcios E, Mukhi S, Parghi D, D’Arcangelo G, Anderson AE. Inhibition of the mammalian target of rapamycin blocks epilepsy progression in NS-Pten conditional knockout mice. Epilepsia. 2011;52:2065–2075. doi: 10.1111/j.1528-1167.2011.03280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutula T, Xiao-Xian H, Cavazos J, Scott G. Synaptic reorganization in the hippocampus induced by abnormal functional activity. Science. 1988;239:1147–1150. doi: 10.1126/science.2449733. [DOI] [PubMed] [Google Scholar]
- Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol. 1989;26:321–330. doi: 10.1002/ana.410260303. [DOI] [PubMed] [Google Scholar]
- Swiech L, Perycz M, Malik A, Jaworski J. Role of mTOR in physiology and pathology of the nervous system. Biochim Biophys Acta. 2008;1784:116–132. doi: 10.1016/j.bbapap.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Tang H, Long H, Zeng C, Li Y, Bi F, Wang J, Qian H, Xiao B. Rapamycin suppresses the recurrent excitatory circuits of dentate gyrus in a mouse model of temporal lobe epilepsy. Biochem Biophys Res Comm. 2012;420:199–204. doi: 10.1016/j.bbrc.2012.02.143. [DOI] [PubMed] [Google Scholar]
- Tauck DL, Nadler JV. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J Neurosci. 1985;5:1016–1022. doi: 10.1523/JNEUROSCI.05-04-01016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet EA, Forte G, Holtman L, den Buerger JCG, Sinjewel A, de Vries HE, Aronica E, Gorter JA. Inhibition of mammalian target of rapamycin reduces epileptogenesis and blood-brain barrier leakage but not microglia activation. Epielpsia. 2012;43:1254–1263. doi: 10.1111/j.1528-1167.2012.03513.x. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJG. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wuarin J-P, Dudek FE. Electrographic seizures and new recurrent excitatory circuits in the dentate gyrus of hippocampal slices from kainate-treated rats. J Neurosci. 1996;16:4438–4448. doi: 10.1523/JNEUROSCI.16-14-04438.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L-H, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci. 2009;29:6964–6972. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L-H, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63:444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Huguenard JR, Buckmaster PS. Increased positive-feedback from hilar and CA3 neurons to granule cells in a rat model of temporal lobe epilepsy. J Neurosci. 2012;32:1183–1196. doi: 10.1523/JNEUROSCI.5342-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Blundell J, Ogawa S, Kwon C-H, Zhang W, Sinton C, Powell CM, Parada LF. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knockout mice. J Neurosci. 2009;29:1773–1783. doi: 10.1523/JNEUROSCI.5685-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]