Abstract
It is well known that neurons in the rostral ventromedial medulla (RVM) are involved in descending modulation of nociceptive transmission in the spinal cord. It has been shown that activation of neurokinin-1 receptors (NK-1R) in the RVM, which are presumably located on pain facilitating ON cells, produces hyperalgesia whereas blockade of NK-1Rs attenuates hyperalgesia. To obtain a better understanding of the functions of NK-1R expressing neurons in the RVM, we selectively ablated these neurons by injecting the stable analog of substance P (SP), Sar9, Met(O2)11-Substance P, conjugated to the ribosomal toxin saporin (SSP-SAP) into the RVM. Rats received injections of SSP-SAP (1 μM) or an equal volume of 1 μM of saporin conjugated to artificial peptide (Blank-SAP). Stereological analysis of NK-1R- and NeuN-labeled neurons in the RVM was determined 21–24 days after treatment. Withdrawal responses to mechanical and heat stimuli applied to the plantar hindpaw were determined 5–28 days after treatment. Withdrawal responses were also determined before and after intraplantar injection of capsaicin (acute hyperalgesia) or complete Freund’s adjuvant (CFA) (prolonged hyperalgesia).
The proportion of NK-1R-labeled neurons in the RVM was 8.8 ± 1.3% in naïve rats and 8.1 ± 0.8% in rats treated with Blank-SAP. However, injection of SSP-SAP into the RVM resulted in a 90% decrease in NK-1R-labeled neurons. SSP-SAP did not alter withdrawal responses to mechanical or heat stimuli under normal conditions, and did not alter analgesia produced by morphine administered into the RVM. In contrast, the duration of nocifensive behaviors produced by capsaicin and mechanical and heat hyperalgesia produced by capsaicin and CFA were decreased in rats pretreated with SSP-SAP as compared to those that received Blank-SAP.
These data support our earlier studies using NK-1R antagonists in the RVM and demonstrate that RVM neurons that possess the NK-1R do not play a significant role in modulating acute pain or morphine analgesia, but rather are involved in pain facilitation and the development and maintenance of hyperalgesia.
Keywords: Capsaicin, inflammation, descending facilitation, ON cells, pain modulation
1. Introduction
It is well established that nociceptive processing in the spinal cord is modulated by descending projections from the brain stem. Neurons in the rostral ventromedial medulla (RVM), a brain stem region that includes nucleus raphe magnus, nucleus gigantocellularis pars alpha, and lateral paragigantocellular nucleus, project to the spinal cord (Watkins et al., 1980, 1981) and can inhibit (antinociception) or facilitate (pronociception) nociceptive transmission (Basbaum and Fields, 1978, 1984; Ren and Dubner, 1996, 2002; Urban and Gebhart, 1999; Porreca et al., 2002; Heinricher et al., 2009). Accumulating evidence suggests that neurons in the RVM that express neurokinin-1 receptors (NK-1Rs) (Ljungdahl et al., 1978; Nakaya et al., 1994; Budai et al., 2007) play a role in descending facilitation of nociceptive transmission. Behavioral studies showed that microinjection of substance P (SP) into the RVM produced hyperalgesia (Lagraize et al., 2010) whereas injection of NK-1R antagonists into the RVM reduced the hyperalgesia produced by intraplantar capsaicin injection (Pacharinsak et al., 2008) and hind paw inflammation (Hamity et al., 2010).
Neurons in the RVM are classified electrophysiologically as ON, OFF, NEUTRAL, and serotonergic cells (Fields et al., 1983; Fields and Heinricher, 1985; Gao and Mason, 2000). ON cells are considered to be pronociceptive because they are excited by noxious stimulation, exhibit a burst-like increase in discharge rate just prior to a withdrawal reflex, and are inhibited by morphine. OFF cells, which are believed to be antinociceptive, respond with a pause in ongoing discharge during noxious stimulation and are excited by morphine (reviewed by Mason, 2001; Heinricher et al., 2009). The role of NEUTRAL cells in nociceptive processing is unclear. These cells are not affected by noxious cutaneous stimuli but may modulate nociceptive transmission of visceral (Brink and Mason, 2003, 2004; Brink et al., 2006) and trigeminal (Ellrich et al., 2000) inputs. Serotonergic cells are a separate group of RVM neurons defined by slow, regular discharge and distinct neurochemistry that appears to modulate autonomic activities (Potrebic et al., 1994; Mason, 1997, 2012).
In earlier studies (Budai et al., 2007) we proposed that NK-1Rs are located on ON cells because iotophoretic application of the selective NK-1R agonist, Sar9, Met(O2)11-Substance P excited only ON cells and enhanced their responses to iontophoretic application of NMDA, effects that were blocked by an NK-1R antagonist. We also showed that sensitization of ON cells to cutaneous stimulation following capsaicin (Brink et al., 2012) or prolonged inflammation (Khasabov et al., 2012) was reduced by an NK-1R antagonist. Although these studies showed that activation of NK-1Rs can enhance activity of ON cells and thereby contribute to facilitation of nociceptive transmission, this approach has limitations to understanding the role of NK-1R expressing RVM neurons because NK-1Rs are only a part of neurochemical mechanisms that regulate activity of these neurons. For example, we showed that almost all RVM neurons that express NK-1Rs also express NMDA receptors (Budai et al., 2007). Also, it is possible that RVM neurons that express NK-1Rs are a subpopulation of ON cells with specific functions while other ON cells (without NK-1Rs) may have different functions, such as modulating acute nociception. To further determine the role of NK-1R expressing neurons in the RVM in pain modulation, we selectively ablated these neurons by injection of a stable analog of SP, Sar9, Met(O2)11-Substance P, conjugated to the ribosomal toxin saporin (SSP-SAP) (Wiley and Lappi, 1999, 2001, 2003; Lappi and Wiley, 2004; Wiley et al., 2007; Wiley, 2008) into the RVM. The loss of NK-1R expressing neurons following injection of SSP-SAP into the RVM was quantified using stereological methods, and the effects of loss of NK-1R expressing neurons in the RVM on withdrawal responses and on the development of hyperalgesia were determined. Our results demonstrate that a relatively small proportion of RVM neurons express NK-1Rs but these neurons play an important role in the development of hyperalgesia.
2. Experimental procedures
2.1 Animals
One hundred twenty seven adult, male, Sprague–Dawley rats (Harlan, Indianapolis, IN) weighing 250–360g were used. Rats were maintained in a climate-controlled room on a 12-h dark/light cycle, and food and water were available ad libitum. All experimental procedures were performed in accordance with the guidelines recommended by the International Association for the Study of Pain and were approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
2.2 Injection of SSP-SAP and Blank-SAP into the RVM
Rats were anesthetized with ketamine (67.5 mg/kg) and xylazine (22.5 mg/kg) and placed in a stereotaxic apparatus. Body temperature was maintained at 37°C using a feedback-controlled heating blanket (Harvard apparatus, Holliston, MA). A skin incision (~ 10 mm) was made on the top of the cranium to expose the skull and a small hole (~ 1.5 mm diameter) was made through the skull over the RVM (anterior-posterior interaural stereotaxic coordinate −2.3mm). Rats received two injections into the RVM (one each into the left and right sides) of either 1 μM of SSP-SAP in 0.3 μl or an equal volume of 1 μM of saporin conjugated to artificial peptide (Blank-SAP). Both conjugates were dissolved in phosphate buffered saline (PBS). Interaural stereotaxic coordinates for these injections were: anterior-posterior = −2.3mm; dorsal-ventral = −0.5mm; lateral = ±0.5mm according to the atlas of Paxinos and Watson (Paxinos and Watson, 1998). Injections were made over a period of 2 min each using a glass micropipette with ~ 50 μm tip diameter attached to a 1 μl Hamilton microsyringe (Hamilton Company, Reno, NV) placed in microinjection unit (KOPF, Tujunga, CA, Model 5001). After injection the micropipette was kept in place for 1 min to allow drug diffusion through the RVM. The micropipette was then removed from the brain and the skin was closed with silk ligature. Rats were returned to their home cage for recovery and were used for behavioral and immunohistochemical studies 24–30 days later.
2.3 Chronic cannula implantation into the RVM
Rats were implanted stereotaxically with a chronic stainless steel cannula into the RVM for behavioral studies of morphine analgesia. Before cannula implantation, SSP-SAP or Blank-SAP was injected into the RVM as described above. Following a 2 week recovery period, animals were anesthetized with ketamine (67.5 mg/kg) and xylazine (22.5 mg/kg) and placed in a stereotaxic apparatus. After craniotomy, a 1 mm diameter guide cannula (17.5 mm in length, 26 gauge; Plastics One, Roanoke, VA) was inserted toward the RVM. The interaural stereotaxic coordinates for implantation were: anterior-posterior = −2.3 mm; dorsal-ventral = +0.5 mm; lateral = 0 mm according to the rat brain atlas of (Paxinos and Watson, 1998). Three stainless steel screws were inserted into the skull in near proximity, and the guide cannula and screws were secured with dental resin (Duralay Dental Mfg. Co., Worth, IL). To maintain patency of the guide cannula, a dummy cannula of the same length (33 gauge; Plastics One, Roanoke, VA) was inserted into the guide cannula. Animals were allowed to recover for 7 days before the behavioral studies.
2.4 Immunohistochemistry
Rats were deeply anesthetized with ketamine (67.5 mg/kg) and xylazine (22.5 mg/kg). Perfusions were made though the ascending aorta with 100 ml of PBS at room temperature followed by 800 ml of cold 4% paraformaldehyde in PBS (pH 7.4) for 40 min. After fixation, brains were removed, post-fixed in the same fixative overnight at 4°C and transferred to 30% sucrose solution in PBS (4°C for 48 hours) for cryoprotection.
To ensure that NK-1R labeling was associated with neurons, tissue sections were co-labeled for NeuN, a marker of neuronal nuclei protein. Coronal sections (50 μm) through the RVM were made using a freezing microtome. Free-floating sections were washed in PBS three times for 10 min, blocked for one hour in 10% normal donkey serum (NDS) (Jackson ImmunoResearch Laboratories, Inc. West Grove, PA) in PBS with 0.3% of Triton X-100 at room temperature, and incubated overnight at 4°C in solutions containing two primary antibodies: Rabbit ant-NK-1R (lot# 011M4819, Sigma-Aldrich St. Louis, MO) 1:1000 (11.5 μg/ml) and Mouse anti-NeuN (lot# LV1825845, Millipore Billerica, MA) 1:500 (2 μg/ml), dissolved in PBS with 1% NDS and 0.3% Triton X-100. After the application of primary antibodies, sections were washed in PBS three times for 20 min and incubated for 2 hours in two secondary antibodies: Donkey anti-Rabbit Alexa Fluor 555 (lot# 819572) and Donkey anti-Mouse Alexa Fluor 488 (lot# 1113537) (Invitrogen, Grand Island, NY), both 1:1000 (2 μg/ml) were dissolved with 1% NDS and 0.3% Triton X-100 in PBS. After application of secondary antibodies, tissues were washed in PBS three times for 20 min, transferred on gelatinized glass slides, dried at ~37°C for 1 hour, dehydrated in graded alcohols (50–100%), and cleared in xylene. Slides were coversliped using DPX (VWR, Radnor, PA) for microscopy. To confirm the specificity of the primary antibodies, controls included pre-incubation with corresponding synthetic peptides or omission of primary antibodies.
2.5 Stereological quantification
Labeling of NK-1R-ir neurons in the RVM from rats treated with SSP-SAP or Blank-SAP was compared to labeling in naïve animals to determine the percentage of NK-1-ir neurons and their anatomical distribution in the RVM, and to ensure that Blank-SAP did not alter the number of NK-1R labeled neurons. The quantification of NeuN-immunoreactive (NeuN-ir) and NK-1R- immunoreactive (NK-1R-ir) neurons were made by conventional microscopy using a Nikon E800 epifluorescence microscope equipped with filter sets designed to allow selective visualization of Donkey anti-Rabbit Alexa Fluor 555 and Donkey anti-Mouse Alexa Fluor 488. Microscopic images were collected with a Microfire digital camera (Optronics, Goleta, CA) and analyzed with the stereological software, Stereoinvestigator (Microbrightfield, Colchester, VT).
The rostro-caudal dimension of RVM ranged from the caudal margins of the trapezoid body to the rostral edge of inferior olives. In coronal sections, an area of the RVM used for stereological analysis was located within the shape of a rectangle. The ventral border of this rectangle was located at the dorsal margins of the pyramids, and the dorsal border was at the level of most dorsal margins of facial nuclei. The lateral borders of the rectangle were located at the middle distance between the lateral edge of the pyramid and the most medial edge of the facial nucleus (Figure 1). The planar rectangular shape of the area used for stereological analyses differed from the triangular shape of the RVM area, which includes primarily the nucleus raphe magnus, that was used in earlier studies (Gu and Wessendorf, 2007; Leong et al., 2011). This rectangular area of the RVM, as used earlier (Parra et al., 2002; Hurley et al., 2003), ensured quantification of NK-1R neurons not only in nucleus raphe magnus but also in nuclei gigantocellularis pars alpha, and lateral paragigantocellular, which are components of the RVM (Millan, 2002). Neuronal counts were based on NeuN-ir and quantification of NK-1R-ir neurons was done only on cells that exhibited double labeling. Since no differences were found between left and right sides of the RVM, the labeled neurons on both sides were pooled for estimation. The brain stem was sectioned from caudal to rostral direction and 50-μm sections were arranged in the same order. A section from which stereological counting began was randomly chosen from five consecutive sections starting from the first section in which the inferior olives were not present. Every fifth section thereafter that did not include the trapezoid body was analyzed. Thus, the interval between sampled sections was 250 μm. Five sections from each RVM were sampled for stereological analysis.
Figure 1.
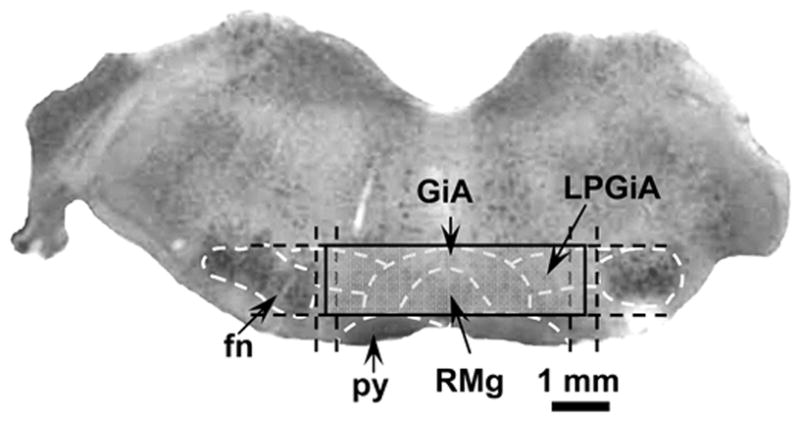
An example from a single coronal section (50 μm thick) of the area of the RVM used for stereological quantifications. This section of the brainstem was obtained at −2.16 mm interaural coordinate and stained with neutral red. Structures in the RVM that were used to define the analyzed area are indicated by the white dashed lines according an atlas of the rat brain (Paxinos and Watson, 1998). Levels that were used to define position of the area are indicated by black dashed lines. The region where numbers and proportions of neurons were evaluated is indicated by solid lines. Abbreviations: fn – facial nucleus; py – pyramidal tract; RMg – raphe magnus nucleus; GiA – gigantocellular reticular nucleus pars alpha; LPGiA – lateralis paragigantocellular reticular nucleus pars alpha.
For each section that was used for quantification under a low magnification (4×), the borders of the rectangle within RVM were drawn using stereological software. When the area to be analyzed was identified, a grid and counting frames were superimposed over the RVM by the software. The optical fractionator was used to estimate the number of neurons in the RVM of each treated group together with absolute numbers and proportion of NK-1R-ir neurons among all neuronal populations that were NeuN-ir. Quantification was conducted under a Nikon 40× Planapoachromat lens (N.A. 1.3). The stereological software allowed an automated scan of the entire RVM area in the X-Y plane with 140 × 140 μm counting frames and an optical dissector of 20 μm of depth with 3-μm guarding zones at the top and the bottom of the section. This resulted in an average of 25 counting sites per a section or ~125 per entire RVM. Examination with confocal microscopy demonstrated that the antibodies labeled tissues uniformly throughout all tissue sections. It was determined that immunohistochemical processing resulted in shrinkage of the thickness of tissue sections to about 30 μm.
2.6 Behavioral studies
Mechanical hyperalgesia was determined by measuring withdrawal response threshold and the frequency of withdrawal evoked by von Frey monofilaments. Rats were placed under a clear plastic box on an elevated platform with a mesh plastic floor (1 cm2 perforations) and were allowed to acclimate for 15 min before testing. Von Frey monofilaments with different bending forces were applied to the plantar surface of both hind paws. Monofilaments were each applied for durations of 2–3 s with an inter-stimulus interval of approximately 10 s. Withdrawal response threshold (g) was determined for each hind paw using the up–down method described previously (Chaplan et al., 1994). Care was taken to stimulate random locations on the plantar surface. Only robust withdrawal responses were counted.
We next determined the frequency of withdrawal from a standard von Frey monofilament (26 g) applied to the plantar surface of each hind paw 10 times, each for 1–2 s with an inter-stimulus interval of approximately 5–10 s. The number of withdrawal responses was expressed as a percentage of the 10 stimulus applications. Mechanical hyperalgesia was defined as a withdrawal response frequency >50%.
Sensitivity to heat was determined by measuring withdrawal latency of the hind paws as well as tail flick latency. Rats were placed on a transparent 3-mm thick glass platform and allowed to acclimate for at least 15 min. Radiant heat was applied to the plantar surface of each hind paw and latencies to withdrawal were determined according to a method similar to that described previously (Pacharinsak et al., 2008). The intensity of the radiant heat was adjusted to maintain stable withdrawal responses with latencies approximately 10–14 s. A 19 s cutoff was imposed to prevent tissue damage. During testing each hind paw was stimulated four times, alternating between each paw. The time interval between stimulation of the same paw was at least 1 min. The withdrawal latency for each hind paw was defined as mean latency of the last three trials. Heat hyperalgesia was defined as a significant decrease in withdrawal response latency.
Tail flick latencies were determined by applying radiant heat to the distal third of the tail four times with at least a 1 min inter-stimulus interval. The intensity of heat was adjusted to produce stable tail flick latencies of ~3 s. The tail flick latency was defined as the mean latency of the last three trials.
For all behavioral studies, the experimenter was blinded to the treatment.
2.7 Drug preparation
Stock solutions of SSP-SAP and Blank-SAP (Advanced Targeting Systems, San Diego, CA) at 5 μM concentration were prepared with deionized water and stored at −80°C. Just before injections, 1 μM solutions were prepared in PBS. Morphine (Gallipot Inc., St. Paul MN) was dissolved in PBS prior to injections at concentrations of 6 or 60 mg/ml. Capsaicin (Sigma-Aldrich St. Louis, MO) was dissolved in 5% Tween-80 and saline at a concentration of 10 μg in 10 μl (0.1%). Rats received an intraplantar injection of 10 μg using an insulin syringe with 28-guage needle. For studies of prolonged inflammation, rats were anesthetized with 2–4% isoflurane and received an intraplantar injection of 100 μl of undiluted complete Freund’s adjuvant (CFA; Sigma-Aldrich St. Louis, MO).
2.8 Experimental design
Acute pain
Mechanical withdrawal thresholds, the frequencies of withdrawal to the standard von Frey monofilament, paw withdrawal latencies to heat, and tail flick latencies were determined for three consecutive days before and at 5–7, 12–14, 19–21, and 26–28 days after injection of SSP-SAP or Blank-SAP into the RVM to determine whether injection into the RVM altered withdrawal responses to acute nociceptive stimuli. Since there were no differences in withdrawal responses to mechanical or heat stimuli between the hind paws at any time, data from each paw were pooled.
Morphine analgesia
Rats pretreated with either SSP-SAP or Blank-SAP received one injection of either 3 or 30 μg of morphine, or an equal volume (0.5 μl) of vehicle (PBS) into the RVM. The injection cannula was attached to PE-10 tubing with a 1-μl microsyringe placed in microinjection unit. Rats were placed under a clear plastic box and injections were given slowly over a period of 2 min. The injection cannula was left in position for 60 s after injection to allow maximum diffusion. Following injection, the injection cannula was removed and replaced with the dummy cannula. Mean tail flick latencies were obtained before and at 15, 30, 60, 90, and 120 min after RVM injection.
Capsaicin-evoked pain and hyperalgesia
The duration of nocifensive behaviors (lifting, guarding and licking the injected paw) was determined over a 5-min period immediately following capsaicin injection in rats pretreated with SSP-SAP or Blank-SAP. Mechanical response threshold, paw withdrawal frequency, and heat withdrawal latency were determined for each paw before and at 15, 30, 60, and 90 min after the capsaicin injection. Responses from the injected and contralateral paws were averaged separately.
Prolonged inflammatory hyperalgesia
Mechanical response threshold, paw withdrawal frequency, and heat withdrawal latency were determined for each hind paw before and daily for four consecutive days after injection of CFA into one hind paw.
2.9 Histological verification of injection sites in the RVM
At the end of all behavioral experiments, rats were deeply anesthetized with Nembutal (100 mg/kg) and perfused with cold 4% paraformaldehyde in PBS through ascending aorta. The position of the glass pipette used for injections of SSP-SAP and Blank-SAP was verified histologically. Only rats with minimal damage at the injection site (less than 100 μm diameter) were used. For morphine experiments, the location of the tip of the chronic cannula used was also verified histologically. Animals with cannula placement outside the RVM were not used.
2.10 Data analyses
For stereological analyses and behavioral measures, one- and two-way analyses of variance (ANOVA) with repeated measures were used to determine differences between treatment groups. Post-hoc analyses were conducted using Bonferroni t-tests. Differences with a p<0.05 were considered significant. All data are expressed as the mean (±SEM).
3. RESULTS
3.1 Stereological analysis of NK-1R expressing neurons in the RVM
In naïve rats, neurons in the RVM that express the NK-1R were located mainly above the pyramids and in the middle region of the RVM. Injection of SSP-SAP, but not inactive Blank-SAP, into the RVM almost completely eliminated NK-1R labeling (Figure 2). Stereological analyses of these areas 21–24 days after injection revealed that there were 65,785 ± 5,443 NeuN-ir nuclei in naïve rats (n = 4) and 65,181 ± 6,270 in rats treated with Blank-SAP (n = 4). The number of neurons with NeuN-ir nuclei in rats pretreated with SSP-SAP into the RVM (n = 4) was lower (60,670 ± 1,851) but this decrease was not different from naïve rats or rats pretreated with Blank-SAP (one-way ANOVA, p = 0.73) (Figure 3A).
Figure 2.
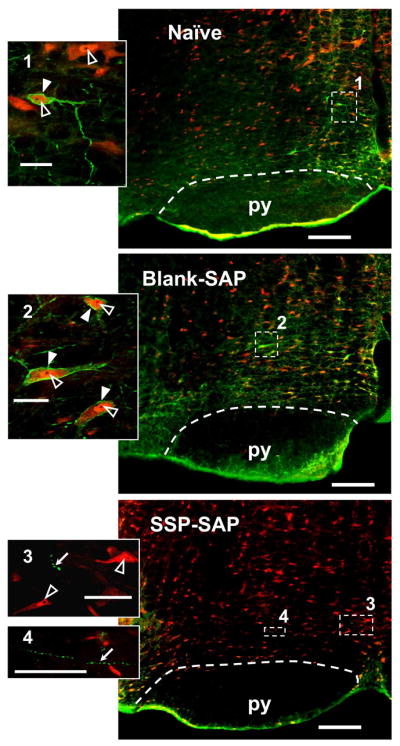
Examples of NK-1R labeling in single RVM sections (left side) from a naïve rat (top), and from rats pretreated with Blank-SAP (middle) and SSP-SAP (bottom). These single virtual tissue images were taken with a 10× objective. NK-1R-ir appears green and NeuN-ir appears red. In the RVM of naïve and Blank-SAP treated rats, labeling of NK-1R-ir neurons are similar. Pretreatment with SSP-SAP nearly abolished NK-1R-ir and few NK-1R-ire axons with varicosities could be found as a result of internalization of SP receptors (Mantyh et al., 1997). Inserts are single optical sections taken at 40× objective of the areas marked on the corresponding RVM by the numbered white dashed rectangles. NeuN-ir cell bodies with negative nucleoli (black) and NK-1R-ir plasma membrane are indicated by white arrowheads. NK-1R negative neurons with NeuN-ir nuclei with nucleoli are indicated by open arrowheads. NK-1R-ir varicosities induced by SSP-SAP are indicated by white arrows. Scale bars for the RVM and the inserts are 200 and 20 μm, respectively. Pyramidal tracts (py) are indicated by white dashed lines.
Figure 3.
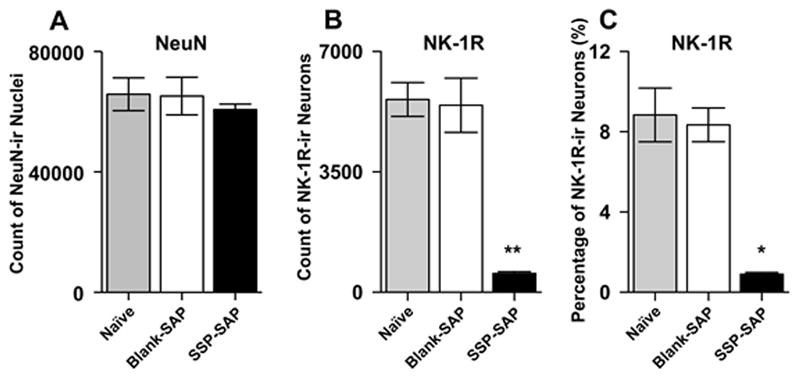
Stereological quantification of the total neuronal population (NeuN-ir), NK-1R labeled neurons, and their proportions in the RVM of naïve, Blank-SAP, and SSP-SAP treated rats. A) Mean (±SEM) number of neurons in the RVM of naïve and Blank-SAP and SSP-SAP treated rats were similar. B) Mean (±SEM) number of NK-1R-ir neurons was lower in rats pretreated with SSP-SAP as compared to rats treated with Blank-SAP and naïve rats. C) Mean (±SEM) percentage of NK-1R-ir neurons in SSP-SAP injected rats was lower than in naïve and Blank-SAP rats. Asterisks indicate significant differences between SSP-SAP group and the naïve and Blank-SAP treated groups; * p < 0.01, **p < 0.001.
Animals pretreated with SSP-SAP exhibited fewer NK-1R expressing neurons in the RVM. In naïve rats, among all RVM neurons that were NeuN-ir, 5,603 ± 492 cells exhibited NK-1R-ir, and this number was similar to that in rats pretreated with Blank-SAP (5,436 ± 788) (one-way ANOVA, p = 0.86). In contrast, rats pretreated with SSP-SAP had only 543 ± 53 NK-1R-ir neurons and this was less than the number observed in naïve rats and in rats pretreated with and Blank-SAP rats (one-way ANOVA, p < 0.001) (Figure 3B). These data demonstrate that treatment with SSP-SAP decreased the number of NK-1R-ir neurons by ~90%.
The proportion of all neurons in the RVM that exhibited NK-1R-ir was 8.8 ± 1.3% in naïve rats and 8.1 ± 0.8% in rats pretreated with Blank-SAP (one-way ANOVA, p = 0.83). However, as shown in Figure 3C, rats pretreated with SSP-SAP exhibited a lower proportion (0.9 ± 0.09%) of NK-1R-ir neurons in the RVM (one-way ANOVA, p < 0.01).
Neurons in the RVM that exhibited NeuN-ir were evenly distributed though the rostrocaudal direction and did not differ between the groups (two-way ANOVA with repeated measures, p = 0.77) (Figure 4A). The percentage of NK-1R-ir neurons was also evenly distributed along the rostrocaudal length of the RVM. The distribution of NK-1R-ir neurons in the RVM of naïve and Blank-SAP rats did not differ between the groups. As shown in Figure 4B, the proportion of NK-1R-ir neurons in rats pretreated with SSP-SAP was constant along the entire length of the RVM but the number of neurons decreased compared to naïve and Blank-SAP groups (two-way ANOVA with repeated measures, p < 0.001). This indicates that SSP-SAP did not target neurons located in specific areas of the RVM but rather ablated NK-1R expressing neurons throughout the RVM.
Figure 4.
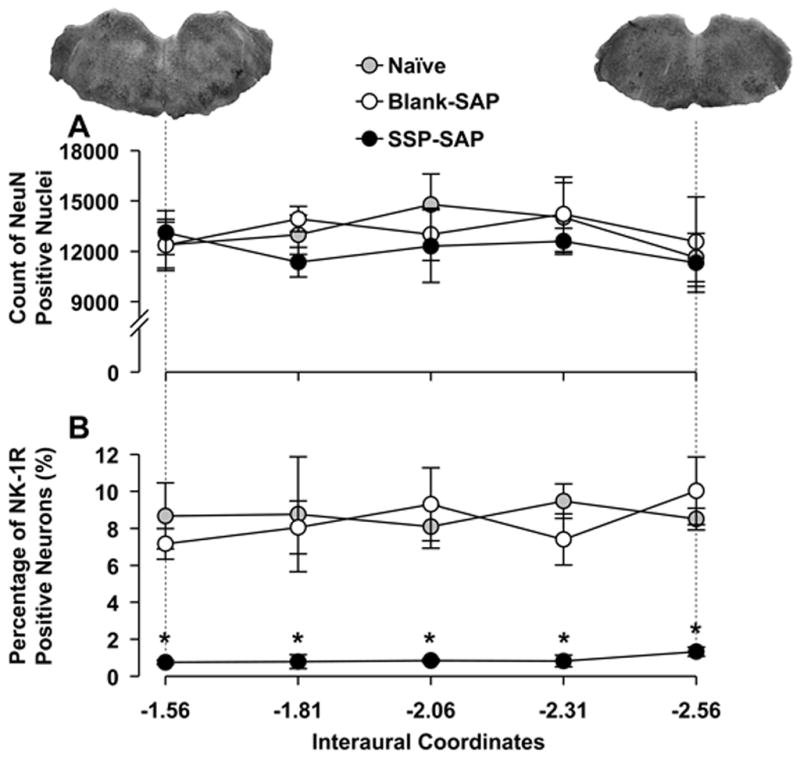
Distributions of neurons in rostrocaudal plane of the RVM. A) Numbers of RVM neurons in the three groups did not differ. B) Percentages of NK-1R-ir neurons in rats treated with SSP-SAP were lower than those in naïve and Blank-SAP treated rats along the length of the RVM. Horizontal axes indicate interaural coordinates of the RVM. Images above the graphs are s stained with neutral red and show representative sections at the most rostral and caudal areas of the RVM. Asterisks indicate statistically significant differences between SSP-SAP group and naïve and Blank-SAP groups at the corresponding rostrocaudal positions (p < 0.01).
3.2 Depletion of NK-1R expressing neurons in the RVM and responses to acute pain
To evaluate the contribution of NK-1R expressing RVM neurons in modulating withdrawal responses to acute noxious stimuli under normal conditions, we determined hind paw mechanical withdrawal thresholds (Blank-SAP n = 9, SSP-SAP n = 8), paw withdrawal latencies to heat (Blank-SAP n = 8, SSP-SAP n = 15), and tail flick latencies (Blank-SAP n = 9, SSP-SAP n = 9) over a period of 4 weeks following injection. As shown in Figure 5A–C, neither Blank-SAP nor SSP-SAP altered paw withdrawal thresholds (one-way ANOVA, p = 0.919), paw withdrawal latencies to heat (one-way ANOVA, p = 0.89), and tail flick latencies (one-way ANOVA, p = 0.58) at any time throughout the period of testing. These data indicate that RVM neurons expressing NK-1Rs do not modulate withdrawal responses to mechanical and heat stimuli under normal conditions.
Figure 5.
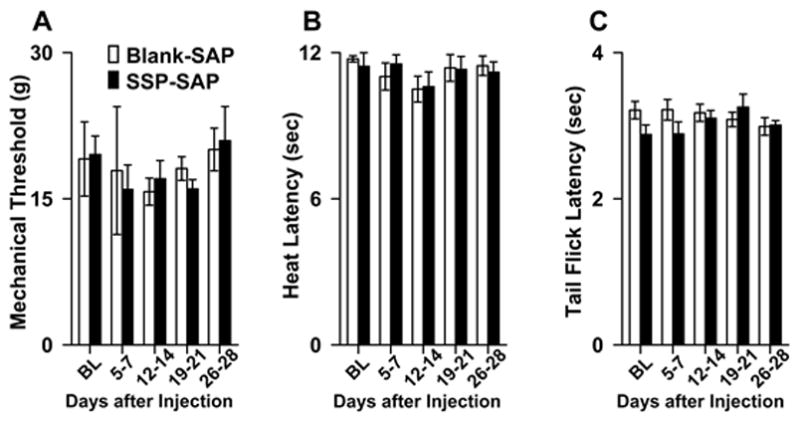
A) Mean (±SEM) hind paw mechanical withdrawal thresholds, B) Hind paw withdrawal latencies to heat, and C) Tail flick latencies in rats treated with Blank-SAP and SSP-SAP before injection (BL) and at 5–7, 12–14, 19–21, and 26–28 days after treatment. Withdrawal responses did not differ between the groups at any time.
3.3 The contribution of NK-1R expressing neurons to morphine analgesia
To evaluate the contribution of NK-1R expressing RVM neurons to opioid analgesia, we compared changes in tail flick latency following injection of morphine into the RVM in rats pretreated with SSP-SAP (n = 6) or Blank-SAP (n = 6). As illustrated in Figure 6, tail flick latencies before morphine administration did not differ between the groups (one-way ANOVA, p = 0.66). Injections of 3 or 30 μg morphine into the RVM produced similar dose-dependent increases in tail flick latencies that did not differ between SSP-SAP and Blank-SAP treated groups (one-way ANOVA, p = 0.86 and p = 0.87, respectively). Injection of vehicle into the RVM did not alter tail flick latency in any of the treatment groups (n = 4 per group; data not shown).
Figure 6.
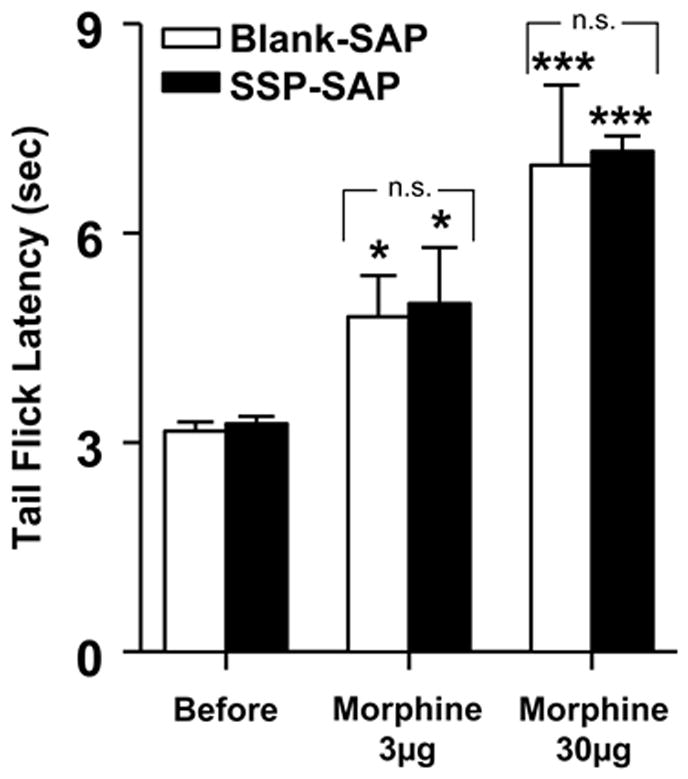
Morphine analgesia was not altered in rats pretreated with SSP-SAP. Morphine at doses of 3 and 30 μg into the RVM produced similar increases in mean (±SEM) tail flick latency in Blank-SAP and SSP-SAP treated rats. Asterisks indicate a significant difference from tail flick latency before morphine; *p < 0.05, **p < 0.001.
3.4 Ablation of NK-1R expressing neurons in the RVM and the development of capsaicin-evoked hyperalgesia
To evaluate the contribution of NK-1R expressing neurons in the RVM to the development of acute hyperalgesia, we used a model of hyperalgesia produced by intraplantar injection of capsaicin. We compared the duration of nocifensive behaviors, mechanical paw withdrawal thresholds, paw withdrawal response frequencies to mechanical stimuli, and paw withdrawal latencies to heat before and after intraplantar injection in rats pretreated with Blank-SAP (n = 21) or SSP-SAP (n = 23). As shown in Figure 7, rats pretreated with SSP-SAP exhibited a 43% reduction in nocifensive behavior following capsaicin (one-way ANOVA, p < 0.001).
Figure 7.
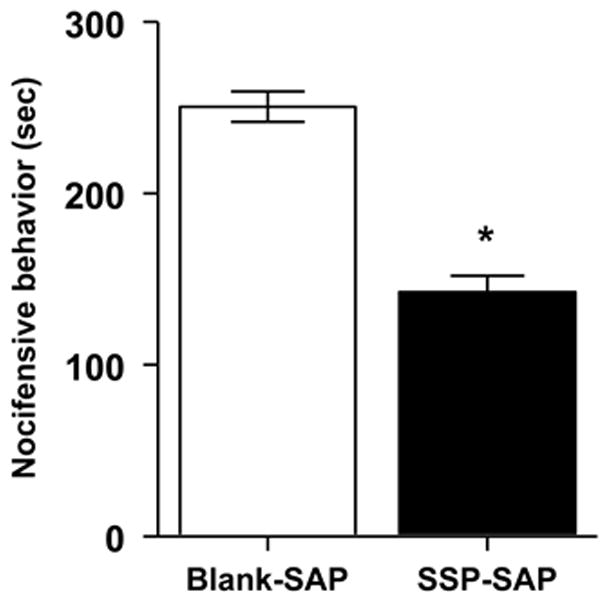
Effect of SSP-SAP pretreatment on nocifensive behavior produced by capsaicin. Rats pretreated with SSP-SAP exhibited a decrease in the duration of nocifensive behavior produced by intraplantar injection of capsaicin. Asterisks indicate difference between groups; *p < 0.001.
Loss of NK-1R expressing neurons in the RVM decreased mechanical hyperalgesia produced by capsaicin. In rats pretreated with Blank-SAP (n = 13), capsaicin produced a 76% decrease in the withdrawal response threshold at 15 min after injection, the time of maximum hyperalgesia (one-way ANOVA, p < 0.001,). However, as shown in Figure 8A, rats pretreated with SSP-SAP (n = 10) exhibited a 56% decrease in withdrawal response threshold (one-way ANOVA, p < 0.001) which differed from rats pretreated with Blank-SAP (two-way repeated measures ANOVA, p < 0.05). Figure 8B shows that the capsaicin-evoked increase in the frequency of withdrawal to the standard Frey monofilament (26 g) was also diminished in rats pretreated with SSP-SAP (two-way ANOVA with repeated measures). Mechanical response threshold and withdrawal response frequencies were not altered in the contralateral, non-injected paw at any time (data not shown).
Figure 8.
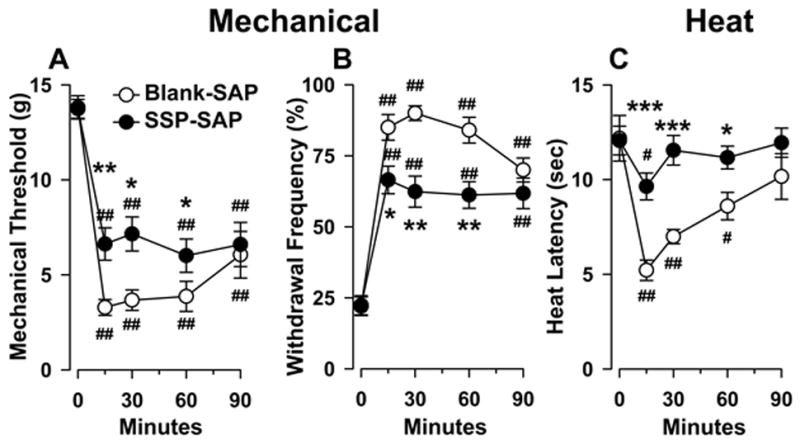
Loss of NK-1R-ir neurons in the RVM decreased hyperalgesia produced by capsaicin. A) Mechanical paw withdrawal threshold; B) Paw withdrawal response frequencies to mechanical stimuli; and C) Paw withdrawal latencies to heat before and up to 90 min following intraplantar injection of capsaicin in rats pretreated with Blank-SAP and SSP-SAP. #p < 0.05, ##p < 0.001 refer to differences from before the capsaicin injection (at time = 0); *p < 0.05, **p < 0.01, ***p < 0.001 indicate differences between groups at corresponding time points.
Hyperalgesia to heat following intraplantar injection capsaicin was also attenuated in rats pretreated with SSP-SAP (Figure 8C). In rats pretreated with Blank-SAP (n = 10) into the RVM, paw withdrawal latency decreased 57% at 15 min after injection (one-way ANOVA, p < 0.001). However, rats pretreated with SSP-SAP (n = 15) exhibited only a 20% decrease in latency after capsaicin (one-way ANOVA, p < 0.05), and this differed from the Blank-SAP-treated group (two-way ANOVA with repeated measures, p<0.005). Also, the duration of hyperalgesia to heat was shorter in rats pretreated with SSP-SAP. In these animals, hyperalgesia to heat was present at 15 min after capsaicin, whereas hyperalgesia persisted for 60 min in rats pretreated with Blank-SAP. Withdrawal response latencies for the contralateral, non-injected paw were not different between the groups and were not affected by capsaicin injection at any time (data not shown).
3.5 The contribution of NK-1R expressing neurons in the RVM to prolonged inflammatory hyperalgesia
To determine whether NK-1R-ir neurons in the RVM play a role in the maintenance of prolonged hyperalgesia, we studied the effects of SSP-SAP on inflammatory hyperalgesia produced by intraplantar injection of CFA. Figure 9A shows that in rats pretreated with Blank-SAP into the RVM, withdrawal response thresholds were reduced during all 4 days following CFA. Response thresholds in Blank-SAP (n = 8) treated rats decreased a maximum of 74% at one day after injection (one-way ANOVA, p < 0.001). Withdrawal response thresholds in rats pretreated with SSP-SAP (n = 11) decreased a maximum of 51% and this maximum decrease in withdrawal threshold occurred 2 days after CFA (one-way ANOVA, p < 0.001), and the decreases in withdrawal threshold following CFA differed between the groups (two-way ANOVA with repeated measures, p < 0.05). As illustrated in Figure 9B, rats pretreated with SSP-SAP into the RVM also exhibited smaller increases in paw withdrawal frequency during 4 days following intraplantar injection of CFA as compared to rats pretreated with Blank-SAP (two-way ANOVA with repeated measures, p < 0.01).
Figure 9.
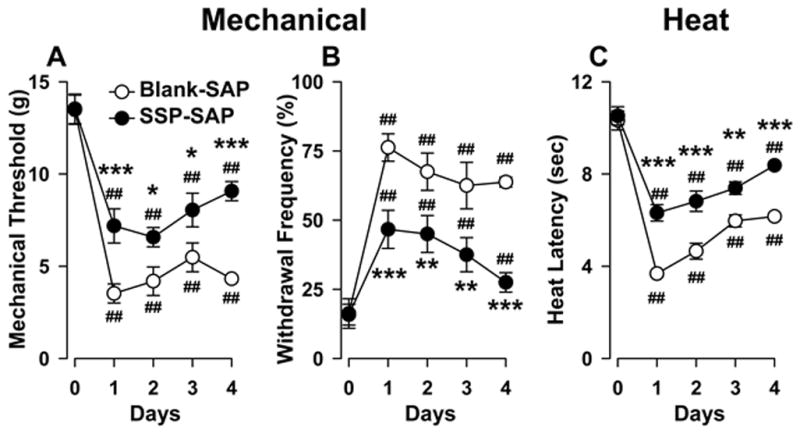
Loss of NK-1R-ir neurons in the RVM decreased hyperalgesia produced by prolonged inflammation. A) Mechanical paw withdrawal threshold; B) Paw withdrawal response frequencies to mechanical stimuli; and C) Paw withdrawal latencies to heat before (0) and up to 4 days following CFA in animals pretreated with Blank-SAP and SSP-SAP. ##p< 0.001 refers to differences from before CFA (day 0); *p < 0.05, **p < 0.01, ***p < 0.001 indicate differences between groups at corresponding days after CFA.
Rats pretreated with SSP-SAP also exhibited a reduced magnitude of heat hyperalgesia during prolonged inflammation as compared to the Blank-SAP-treated group (two-way ANOVA with repeated measures, p < 0.005). Figure 9C shows that rats treated with Blank-SAP (n = 23) exhibited a maximum decrease in withdrawal latency of 64% whereas rats given SSP-SAP (n = 11) exhibited a maximum decrease in withdrawal latency of 40% decrease. As for capsaicin, withdrawal responses of the contralateral, non-inflamed hind paw evoked by mechanical and heat stimuli were not altered at any time (data not shown).
4. Discussion
To examine the contribution of NK-1R expressing neurons in the RVM to hyperalgesia, we injected SSP-SAP into the RVM to selectively ablate NK-1R expressing neurons. This produced approximately a 90% loss of neurons that express NK-1Rs. Our behavioral studies showed that loss of these neurons in the RVM did not alter withdrawal responses to acute mechanical or thermal stimuli but decreased hyperalgesia produced by intraplantar injection of capsaicin or prolonged hind paw inflammation. These findings are consistent with earlier studies and provide additional evidence that neurons in the RVM that express NK-1Rs are part of descending circuitry that facilitates nociception (Pacharinsak et al., 2008; Hamity et al., 2010; Lagraize et al., 2010; Brink et al., 2012; Khasabov et al., 2012).
4.1 Stereological analysis of NK-1R expressing neuron in the RVM
Our stereological studies showed that neurons in the RVM that have NK-1Rs are evenly distributed in the rostral-caudal plane and comprise a relatively small proportion of the neurons in this region of the brain stem. Approximately 8% of neurons in the RVM expressed NK-1Rs, as confirmed by co-labeling with the neuron-specific marker Neu-N. Injection of 1 μM of SSP-SAP, but not Blank-SAP, into the RVM ablated approximately 90% of KN-1R-ir neurons. The number of NK-1R-ir neurons lost following SSP-SAP was similar to the decrease in the number of NeuN-ir neurons, suggesting that SSP-SAP was selective for NK-1R expressing neurons. Interestingly, we were not able to eliminate all NK-1R expressing neurons with 1 μM of SSP-SAP. Similarly, intrathecal application of SP-SAP produced a ~ 80% loss of NK-1R-ir neurons in the dorsal horn (Mantyh et al., 1997; Vierck et al., 2003; Wiley et al., 2007) and this was enough to produce significant physiological effects on the modulation of remaining nociceptive dorsal horn neurons (Khasabov et al., 2002, 2005; Suzuki et al., 2002).
4.2 NK-1R expressing neurons in the RVM and acute pain
Elimination of NK-1R-ir neurons in the RVM did not alter tail flick latencies or withdrawal responses of the hind paw to mechanical or heat stimuli. The tail flick reflex is mediated by spinal segmental mechanisms (Irwin et al., 1951; Bonnycastle et al., 1953; Sinclair et al., 1988) and is under strong descending modulation (Mitchell and Hellon, 1977). Thus, the absence of an effect on the tail flick latency supports the notion that descending projections from NK-1R expressing neurons in the RVM are not involved in modulation of acute pain. Moreover, it was shown previously that NK-1R antagonists injected into the RVM did not alter withdrawal responses to acute stimuli (Pacharinsak et al., 2008; Hamity et al., 2010), indicating that SP is not released tonically in the RVM and does not modulate nociceptive transmission under normal conditions.
Pronociceptive and antinociceptive descending modulation from the RVM is mediated by activity of ON and OFF neurons, respectively (Fields et al., 1983; Fields and Heinricher, 1985; Mason, 2012). We have previously demonstrated that only ON cells were activated by iontophoretic application of an NK-1R agonist (Budai et al., 2007) suggesting that NK-1Rs are located mainly on subset of these pronociceptive neurons. Thus, it is likely that SSP-SAP deleted ON cells. We also showed that microinjection of an NK-1R antagonist into the RVM did not alter responses of ON neurons to noxious stimuli under normal conditions (Brink et al., 2012). These findings are consistent with results of the present study showing that removal of NK-1R expressing ON cells did not modulate acute pain behavior under normal conditions.
Similarities in the magnitude of morphine analgesia between rats pretreated with Blank-SAP and SSP-SAP also support the suggestion that NK-1Rs in the RVM are located on ON and not OFF cells. It was proposed that morphine produces descending analgesia through direct inhibition of ON cells. Opioids, including morphine, injected into the RVM inhibit tail flick responses (Akaike et al., 1978; Kuraishi et al., 1978; Takagi et al., 1978; Aimone and Gebhart, 1986). At the neuronal level, morphine inhibits ON neurons and blocks activity of interneurons that tonically decrease the excitability of OFF cells, causing disinhibition and activation of OFF cells (see review, Fields, 2004). It has been shown that disinhibition of OFF cells is a key element in the analgesic response following opioids into the RVM under normal conditions (Heinricher et al., 1994). In the present study, analgesia produced by morphine injected into the RVM was similar in rats that received Blank-SAP and SSP-SAP, further suggesting that OFF cells were not affected by SSP-SAP and that activation of OFF cells alone can produce opioid analgesia. It is noteworthy that our earlier studies (Brink et al., 2012) showed that injection of SSP into the RVM not only increased evoked responses of ON cells, but enhanced the stimulus-evoked inhibition of OFF cells. Since NK-1Rs appear to be located on ON cells (Budai et al., 2007), it is likely that NK-1R expressing ON cells can modulate activity of OFF cells by enhancing their evoked inhibitory responses. If ON cells exert direct, tonic inhibitory influences on OFF cells, acute antinociception and an enhancement of morphine analgesia would be expected following ablation of NK-1R expressing ON cells. Since this was not the case, it is likely that ON cells do not provide ongoing modulation of OFF cell activity during acute nociception but rather influence OFF cell activity during hyperalgesia. These results also suggest that ON cells influence the activity of OFF cells indirectly, perhaps by exciting inhibitory interneurons, that will enhance the stimulus-evoked inhibitory responses of OFF cells.
4.3 The contribution of RVM neurons with NK-1Rs to hyperalgesia
Intraplantar injection of capsaicin in rats produces nocifensive behaviors and hyperalgesia to mechanical and heat stimuli (Gilchrist et al., 1996) and appears to model the pain and hyperalgesia observed in humans produced by intradermal capsaicin injection (Simone et al., 1987, 1989; LaMotte et al., 1991). Here we show that rats pretreated with SSP-SAP exhibited less capsaicin-evoked nocifensive behaviors and hyperalgesia, results similar to our previous studies in which capsaicin-evoked nocifensive behaviors and hyperalgesia were attenuated following administration of NK-1R antagonists into the RVM (Pacharinsak et al., 2008). Since iontophoretic application of SP and intraplantar injection of capsaicin excited and sensitized only ON cells in the RVM via NK-1Rs (Budai et al., 2007; Brink et al., 2012), it appears that intense activation of ascending nociceptive spinal neurons, such as following intracutaneous injection of capsaicin (Simone et al., 1991; Khasabov et al., 2002), causes the release of SP in the RVM and activation of descending pathways that facilitate nociceptive transmission in the spinal cord. Thus, the activation of NK-1R in the RVM is an important step in the development of central sensitization and hyperalgesia following capsaicin.
Ablation of NK-1R-ir neurons in the RVM also reduced long-lasting hyperalgesia. CFA produces prolonged inflammation (Iadarola et al., 1988) that is accompanied by persistent mechanical (Ma and Woolf, 1996a, b) and heat hyperalgesia (Hargreaves et al., 1988; Iadarola et al., 1988; Ren et al., 1992; Bellavance and Beitz, 1996; Hamity et al., 2010) lasting up to several weeks. Peripheral inflammation leads to sensitization of nociceptors (Andrew and Greenspan, 1999; Djouhri et al., 2006; Potenzieri et al., 2008), increases neuropeptide mediators in primary afferent neurons (Noguchi et al., 1988; Donaldson et al., 1992; Mapp et al., 1993), and augments the proportion of afferents that contain these neuropeptides (Hanesch et al., 1993a, b; Leslie et al., 1995). These changes lead to sensitization of spinal nociceptive neurons (Tal, 1999; Hama et al., 2003; Seybold et al., 2003; Vikman et al., 2003;) that is mediated, at least in part, by increased afferent input to the RVM, and enhanced release of SP and activation of NK-1R-ir neurons (ON cells) in the RVM that facilitate nociceptive transmission in the spinal cord to contribute to hyperalgesia. However, we suggest that prolonged inflammation does not increase tonic release of SP in the brain stem since blockade of NK-1Rs did not alter ongoing spontaneous activity of RVM neurons in inflamed rats (Khasabov et al., 2012). Indeed, descending facilitation from the RVM, and involvement of NK-1Rs, has been demonstrated in other models of prolonged hyperalgesia including nerve injury (Carlson et al., 2007; Sanoja et al., 2008; Guo et al., 2012), headache-related pain (Edelmayer et al., 2009), postoperative pain (Rivat et al., 2009), and bilateral hyperalgesia produced by inflammation of the masseter muscle (Sugiyo et al., 2005; Rivat et al., 2009; Chai et al., 2012).
4.4 Mechanisms underlying NK-1R-dependent descending facilitation
In addition to NK-1Rs, additional cellular mechanisms are likely to contribute to descending facilitation of nociceptive transmission. For example, we showed that almost all NK-1R-ir neurons in the RVM also posses NMDA receptors (Budai et al., 2007), suggesting that glutamate-induced excitation in the RVM is decreased following SSP-SAP injection. Importantly, activation of NMDA receptors in the RVM can produce pronociceptive and antinociceptive effects. Iontophoretic application of NMDA activated both ON and OFF cells in the RVM (Budai et al., 2007) and, when injected into the RVM, NMDA was able to facilitate or inhibit spinal nociceptive reflexes (Terayama et al., 2000, 2002; Guan et al., 2002). Thus, depletion of NK-1R-ir neurons decreases facilitatory modulation that is dependent on NMDA receptors.
It has been established that serotonin, acting through 5-HT3 receptors in the spinal cord, contributes to descending facilitation of nociceptive transmission and hyperalgesia (Suzuki et al., 2002, 2004a, b, 2005; Rahman et al., 2004; Donovan-Rodriguez et al., 2006; Dogrul et al., 2009; Gu et al., 2011; Sikandar et al., 2012). Importantly, descending serotonergic mechanisms and spinal 5-HT3 receptors contribute to descending facilitation produced by activation of NK-1Rs in the RVM (Lagraize et al., 2010). Thus, ablation of NK-1R expressing neurons in the RVM likely decreased serotonin-dependent descending facilitation. The relationship between NK-1R expressing neurons in the RVM and serotonin is unclear since there is no expression of NK-1Rs in serotonin-containing neurons (Leger et al., 2002; Zhang and Hammond, 2009; Hahm et al., 2011). Therefore, ON cells that possess NK-1Rs may excite serotonin-containing neurons in the RVM indirectly through interneurons that activate descending serotonergic neurons.
4.5 Additional mechanisms underlying descending facilitation of nociceptive transmission
In the present study, elimination of about 90% of the RVM neurons that possess NK-1Rs did not completely eliminate hyperalgesia produced by capsaicin or prolonged inflammation. The residual hyperalgesia is probably not due to the remaining NK-1R expressing neurons since injections of different NK-1R antagonists also did not completely abolish hyperalgesia (Pacharinsak et al., 2008; Hamity et al., 2010; Lagraize et al., 2010). It is possible that the remaining hyperalgesia is mediated by other descending facilitatory systems. For example, even though NK-1R-ir neurons appear to engage descending facilitatory serotonergic pathways, these pathways might also be activated by mechanisms other than NK-1Rs.
Another possible source of remaining descending facilitation after SSP-SAP treatment is cholecystokinin (CCK) receptor expressing ON cells (Urban et al., 1996; Urban and Gebhart, 1997; Kovelowski et al., 2000; Ossipov et al., 2003; Xie et al., 2005; Dogrul et al., 2009; Zhang et al., 2009). It is not known if CCK receptors they are located on neurons that possess NK-1Rs or on separate neurons. The same is true for other neurotransmitter systems that contribute to descending facilitation from the RVM, including neurotensin (Fields et al., 1991; Smith et al., 1997; Urban and Gebhart, 1997; Vanegas, 2004), brain-derived neurotrophic factor (Guo et al., 2006), and tumor necrosis factor-alpha (Wei et al., 2008). Further studies of anatomical and neurochemical mechanisms of multiple descending facilitatory pathways and their interactions are needed.
Another possible explanation for the hyperalgesia that remained after SSP-SAP treatment is local segmental sensitization in the spinal cord. The ability of the spinal neurons to become sensitized without involvement of supraspinal modulation has been shown in spinalized animals (Woolf and King, 1990; Wang et al., 2001; Seybold et al., 2003; Molina and Herrero, 2006; Yamamoto et al., 2013) as well as in the isolated spinal cord in vitro (Woolf and King, 1987; King et al., 1988a, b; King et al., 1990; Woolf et al., 1988). It was suggested that the RVM plays a modulating rather than a permissive role in its influence on responsiveness of nociceptive spinal neurons (Foo and Mason, 2003; Jinks et al., 2004). Ablation of NK-1R expressing neurons in the RVM did not alter withdrawal responses of the paw contralateral to the capsaicin-injected or inflamed paws, consistent with previous studies (Pacharinsak et al., 2008; Hamity et al., 2010; Lagraize et al., 2010). These findings suggest that NK-1R-dependent descending facilitation from the RVM amplifies the sensitization that occurs at the spinal level via local mechanisms. However, it should be noted that activation of NK-1Rs in the RVM alone is able to produce hyperalgesia (Lagraize et al., 2010) and sensitization of spinal dorsal horn neurons to mechanical and heat stimuli (Khasabov et al., 2012).
4.6 Conclusions
Neurons in the RVM that express NK-1Rs are part of a descending pain facilitatory pronociceptive system that contributes to hyperalgesia. The functions of NK-1R expressing neurons in the RVM resemble those in the spinal cord. The ablation of superficial spinal dorsal horn neurons that possess NK-1Rs prevented the development of hyperalgesia (Mantyh et al., 1997; Nichols et al., 1999) and sensitization of remaining dorsal horn neurons (Khasabov et al., 2002; Suzuki et al., 2002) without altering withdrawal responses to acute stimuli. The majority of spinal NK-1R expressing neurons in the spinal cord send projections to the brain (Todd et al., 2005; Al-Khater and Todd, 2008, 2009) and likely drive descending facilitation (Khasabov et al., 2002, 2005; Suzuki et al., 2002; Rahman et al., 2008). A greater understanding of the mechanisms that initiate and maintain descending facilitation of nociceptive transmission may lead to novel approaches to disrupt this circuitry and thereby reduce central sensitization and hyperalgesia.
Highlights.
Neurons in the RVM that express NK-1 receptors were ablated using SSP-SAP.
Withdrawal responses to acute stimuli applied to the hind paw were unchanged.
Inflammatory hyperalgesia was attenuated.
Further evidence that RVM neurons with NK-1R contribute to hyperalgesia.
Acknowledgments
The authors want to thank Drs. Donna L. Hammond and Marta V. Hamity for critically reading an earlier version of the manuscript, and Mr. Kody Zalowski for technical assistance. This work was supported by NIH grants DA011471 and CA091007 (D.A. Simone) and a subcontract from DA023576 (D.L. Hammond).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aimone LD, Gebhart GF. Stimulation-produced spinal inhibition from the midbrain in the rat is mediated by an excitatory amino acid neurotransmitter in the medial medulla. J Neurosci. 1986;6:1803–1813. doi: 10.1523/JNEUROSCI.06-06-01803.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike A, Shibata T, Satoh M, Takagi H. Analgesia induced by microinjection of morphine into, and electrical stimulation of, the nucleus reticularis paragigantocellularis of rat medulla oblongata. Neuropharmacology. 1978;17:775–778. doi: 10.1016/0028-3908(78)90093-x. [DOI] [PubMed] [Google Scholar]
- Al-Khater KM, Kerr R, Todd AJ. A quantitative study of spinothalamic neurons in laminae I, III, and IV in lumbar and cervical segments of the rat spinal cord. J Comp Neurol. 2008;511:1–18. doi: 10.1002/cne.21811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Khater KM, Todd AJ. Collateral projections of neurons in laminae I, III, and IV of rat spinal cord to thalamus, periaqueductal gray matter, and lateral parabrachial area. J Comp Neurol. 2009;515:629–646. doi: 10.1002/cne.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew D, Greenspan JD. Mechanical and heat sensitization of cutaneous nociceptors after peripheral inflammation in the rat. Journal of Neurophysiology. 1999;82:2649–2656. doi: 10.1152/jn.1999.82.5.2649. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol. 1978;4:451–462. doi: 10.1002/ana.410040511. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Bellavance LL, Beitz AJ. Altered c-fos expression in the parabrachial nucleus in a rodent model of CFA-induced peripheral inflammation. Journal of Comparative Neurology. 1996;366:431–47. doi: 10.1002/(SICI)1096-9861(19960311)366:3<431::AID-CNE5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Bonnycastle DD, Cook L, Ipsen J. The action of some analgesic drugs in intact and chronic spinal rats. Acta Pharmacol Toxicol (Copenh) 1953;9:332–336. doi: 10.1111/j.1600-0773.1953.tb02962.x. [DOI] [PubMed] [Google Scholar]
- Brink TS, Mason P. Raphe magnus neurons respond to noxious colorectal distension. J Neurophysiol. 2003;89:2506–2515. doi: 10.1152/jn.00825.2002. [DOI] [PubMed] [Google Scholar]
- Brink TS, Mason P. Role for raphe magnus neuronal responses in the behavioral reactions to colorectal distension. J Neurophysiol. 2004;92:2302–2311. doi: 10.1152/jn.00374.2004. [DOI] [PubMed] [Google Scholar]
- Brink TS, Hellman KM, Lambert AM, Mason P. Raphe magnus neurons help protect reactions to visceral pain from interruption by cutaneous pain. J Neurophysiol. 2006;96:3423–3432. doi: 10.1152/jn.00793.2006. [DOI] [PubMed] [Google Scholar]
- Brink TS, Pacharinsak C, Khasabov SG, Beitz AJ, Simone DA. Differential modulation of neurons in the rostral ventromedial medulla by neurokinin-1 receptors. J Neurophysiol. 2012;107:1210–1221. doi: 10.1152/jn.00678.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budai D, Khasabov SG, Mantyh PW, Simone DA. NK-1 receptors modulate the excitability of ON cells in the rostral ventromedial medulla. J Neurophysiol. 2007;97:1388–1395. doi: 10.1152/jn.00450.2006. [DOI] [PubMed] [Google Scholar]
- Carlson JD, Maire JJ, Martenson ME, Heinricher MM. Sensitization of pain-modulating neurons in the rostral ventromedial medulla after peripheral nerve injury. J Neurosci. 2007;27:13222–13231. doi: 10.1523/JNEUROSCI.3715-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai B, Guo W, Wei F, Dubner R, Ren K. Trigeminal-rostral ventromedial medulla circuitry is involved in orofacial hyperalgesia contralateral to tissue injury. Mol Pain. 2012;8:78. doi: 10.1186/1744-8069-8-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci. 2006;26:1281–1292. doi: 10.1523/JNEUROSCI.3388-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogrul A, Ossipov MH, Porreca F. Differential mediation of descending pain facilitation and inhibition by spinal 5HT-3 and 5HT-7 receptors. Brain Res. 2009;1280:52–59. doi: 10.1016/j.brainres.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Donaldson LF, Harmar AJ, McQueen DS, Seckl JR. Increased expression of preprotachykinin, calcitonin gene-related peptide, but not vasoactive intestinal peptide messenger RNA in dorsal root ganglia during the development of adjuvant monoarthritis in the rat. Brain Res Mol Brain Res. 1992;16:143–149. doi: 10.1016/0169-328x(92)90204-o. [DOI] [PubMed] [Google Scholar]
- Donovan-Rodriguez T, Urch CE, Dickenson AH. Evidence of a role for descending serotonergic facilitation in a rat model of cancer-induced bone pain. Neurosci Lett. 2006;393:237–242. doi: 10.1016/j.neulet.2005.09.073. [DOI] [PubMed] [Google Scholar]
- Edelmayer RM, Vanderah TW, Majuta L, Zhang ET, Fioravanti B, De Felice M, Chichorro JG, Ossipov MH, King T, Lai J, Kori SH, Nelsen AC, Cannon KE, Heinricher MM, Porreca F. Medullary pain facilitating neurons mediate allodynia in headache-related pain. Ann Neurol. 2009;65:184–193. doi: 10.1002/ana.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellrich J, Ulucan C, Schnell C. Are ‘neutral cells’ in the rostral ventro-medial medulla subtypes of on- and off-cells? Neuroscience Research - Supplement. 2000;38:419–423. doi: 10.1016/s0168-0102(00)00190-5. [DOI] [PubMed] [Google Scholar]
- Fields H. State-dependent opioid control of pain. Nat Rev Neurosci. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- Fields HL, Bry J, Hentall I, Zorman G. The activity of neurons in the rostral medulla of the rat during withdrawal from noxious heat. J Neurosci. 1983;3:2545–2552. doi: 10.1523/JNEUROSCI.03-12-02545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Heinricher MM. Anatomy and physiology of a nociceptive modulatory system. Philos Trans R Soc Lond B Biol Sci. 1985;308:361–374. doi: 10.1098/rstb.1985.0037. [DOI] [PubMed] [Google Scholar]
- Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219–245. doi: 10.1146/annurev.ne.14.030191.001251. [DOI] [PubMed] [Google Scholar]
- Foo H, Mason P. Discharge of raphe magnus ON and OFF cells is predictive of the motor facilitation evoked by repeated laser stimulation. J Neurosci. 2003;23:1933–1940. doi: 10.1523/JNEUROSCI.23-05-01933.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao K, Mason P. Serotonergic Raphe magnus cells that respond to noxious tail heat are not ON or OFF cells. J Neurophysiol. 2000;84:1719–1725. doi: 10.1152/jn.2000.84.4.1719. [DOI] [PubMed] [Google Scholar]
- Gilchrist HD, Allard BL, Simone DA. Enhanced withdrawal responses to heat and mechanical stimuli following intraplantar injection of capsaicin in rats. Pain. 1996;67:179–188. doi: 10.1016/0304-3959(96)03104-1. [DOI] [PubMed] [Google Scholar]
- Gu M, Wessendorf M. Endomorphin-2-immunoreactive fibers selectively appose serotonergic neuronal somata in the rostral ventral medial medulla. J Comp Neurol. 2007;502:701–713. doi: 10.1002/cne.21343. [DOI] [PubMed] [Google Scholar]
- Gu M, Miyoshi K, Dubner R, Guo W, Zou S, Ren K, Noguchi K, Wei F. Spinal 5-HT(3) receptor activation induces behavioral hypersensitivity via a neuronal-glial-neuronal signaling cascade. J Neurosci. 2011;31:12823–12836. doi: 10.1523/JNEUROSCI.1564-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Guan Y, Terayama R, Dubner R, Ren K. Plasticity in excitatory amino acid receptor-mediated descending pain modulation after inflammation. Journal of Pharmacology & Experimental Therapeutics. 2002;300:513–520. doi: 10.1124/jpet.300.2.513. [DOI] [PubMed] [Google Scholar]
- Guo W, Robbins MT, Wei F, Zou S, Dubner R, Ren K. Supraspinal brain-derived neurotrophic factor signaling: a novel mechanism for descending pain facilitation. J Neurosci. 2006;26:126–137. doi: 10.1523/JNEUROSCI.3686-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Wang H, Zou S, Dubner R, Ren K. Chemokine signaling involving chemokine (C-C motif) ligand 2 plays a role in descending pain facilitation. Neurosci Bull. 2012;28:193–207. doi: 10.1007/s12264-012-1218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm ET, Hammond DL, Proudfit HK. Substance P induces the reversible formation of varicosities in the dendrites of rat brainstem neurons. Brain Res. 2011;1369:36–45. doi: 10.1016/j.brainres.2010.10.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama A, Woon Lee J, Sagen J. Differential efficacy of intrathecal NMDA receptor antagonists on inflammatory mechanical and thermal hyperalgesia in rats. Eur J Pharmacol. 2003;459:49–58. doi: 10.1016/s0014-2999(02)02828-5. [DOI] [PubMed] [Google Scholar]
- Hamity MV, White SR, Hammond DL. Effects of neurokinin-1 receptor agonism and antagonism in the rostral ventromedial medulla of rats with acute or persistent inflammatory nociception. Neuroscience. 2010;165:902–913. doi: 10.1016/j.neuroscience.2009.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanesch U, Pfrommer U, Grubb BD, Heppelmann B, Schaible HG. The proportion of CGRP-immunoreactive and SP-mRNA containing dorsal root ganglion cells is increased by a unilateral inflammation of the ankle joint of the rat. Regul Pept. 1993a;46:202–203. doi: 10.1016/0167-0115(93)90033-5. [DOI] [PubMed] [Google Scholar]
- Hanesch U, Pfrommer U, Grubb BD, Schaible HG. Acute and chronic phases of unilateral inflammation in rat’s ankle are associated with an increase in the proportion of calcitonin gene-related peptide-immunoreactive dorsal root ganglion cells. Eur J Neurosci. 1993b;5:154–161. doi: 10.1111/j.1460-9568.1993.tb00481.x. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Morgan MM, Tortorici V, Fields HL. Disinhibition of off-cells and antinociception produced by an opioid action within the rostral ventromedial medulla. Neuroscience. 1994;63:279–288. doi: 10.1016/0306-4522(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res Rev. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RW, Banfor P, Hammond DL. Spinal pharmacology of antinociception produced by microinjection of mu or delta opioid receptor agonists in the ventromedial medulla of the rat. Neuroscience. 2003;118:789–796. doi: 10.1016/s0306-4522(03)00041-1. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Brady LS, Draisci G, Dubner R. Enhancement of dynorphin gene expression in spinal cord following experimental inflammation: stimulus specificity, behavioral parameters and opioid receptor binding. Pain. 1988;35:313–326. doi: 10.1016/0304-3959(88)90141-8. [DOI] [PubMed] [Google Scholar]
- Irwin S, Houde RW, Bennett DR, Hendershot LC, Seevers MH. The effects of morphine methadone and meperidine on some reflex responses of spinal animals to nociceptive stimulation. J Pharmacol Exp Ther. 1951;101:132–143. [PubMed] [Google Scholar]
- Jinks SL, Carstens E, Antognini JF. Isoflurane differentially modulates medullary on and off neurons while suppressing hind-limb motor withdrawals. Anesthesiology. 2004;100:1224–1234. doi: 10.1097/00000542-200405000-00026. [DOI] [PubMed] [Google Scholar]
- Khasabov SG, Brink TS, Schupp M, Noack J, Simone DA. Changes in response properties of rostral ventromedial medulla neurons during prolonged inflammation: Modulation by neurokinin-1 receptors. Neuroscience. 2012;224:235–248. doi: 10.1016/j.neuroscience.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasabov SG, Ghilardi JR, Mantyh PW, Simone DA. Spinal neurons that express NK-1 receptors modulate descending controls that project through the dorsolateral funiculus. J Neurophysiol. 2005;93:998–1006. doi: 10.1152/jn.01160.2003. [DOI] [PubMed] [Google Scholar]
- Khasabov SG, Malecha P, Simone DA. Activation of Neurokinin-1 receptors in the brain stem contributes to spinal sensitization and hyperalgesia. Neuroscience Meeting Planner Program No 17814/GG5 2012 [Google Scholar]
- Khasabov SG, Rogers SD, Ghilardi JR, Peters CM, Mantyh PW, Simone DA. Spinal neurons that possess the substance P receptor are required for the development of central sensitization. J Neurosci. 2002;22:9086–9098. doi: 10.1523/JNEUROSCI.22-20-09086.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AE, Thompson SW, Urban L, Woolf CJ. An intracellular analysis of amino acid induced excitations of deep dorsal horn neurones in the rat spinal cord slice. Neurosci Lett. 1988a;89:286–292. doi: 10.1016/0304-3940(88)90541-1. [DOI] [PubMed] [Google Scholar]
- King AE, Thompson SW, Urban L, Woolf CJ. The responses recorded in vitro of deep dorsal horn neurons to direct and orthodromic stimulation in the young rat spinal cord. Neuroscience. 1988b;27:231–242. doi: 10.1016/0306-4522(88)90233-3. [DOI] [PubMed] [Google Scholar]
- King AE, Thompson SW, Woolf CJ. Characterization of the cutaneous input to the ventral horn in vitro using the isolated spinal cord-hind limb preparation. J Neurosci Methods. 1990;35:39–46. doi: 10.1016/0165-0270(90)90092-t. [DOI] [PubMed] [Google Scholar]
- Kovelowski CJ, Ossipov MH, Sun H, Lai J, Malan TP, Porreca F. Supraspinal cholecystokinin may drive tonic descending facilitation mechanisms to maintain neuropathic pain in the rat. Pain. 2000;87:265–273. doi: 10.1016/S0304-3959(00)00290-6. [DOI] [PubMed] [Google Scholar]
- Kuraishi Y, Fukui K, Shiomi H, Akaike A, Takagi H. Microinjection of opioids into the nucleus reticularis gigantocellularis of the rat: analgesia and increase in the normetanephrine level in the spinal cord. Biochem Pharmacol. 1978;27:2756–2758. doi: 10.1016/0006-2952(78)90054-0. [DOI] [PubMed] [Google Scholar]
- Lagraize SC, Guo W, Yang K, Wei F, Ren K, Dubner R. Spinal cord mechanisms mediating behavioral hyperalgesia induced by neurokinin-1 tachykinin receptor activation in the rostral ventromedial medulla. Neuroscience. 2010;171:1341–1356. doi: 10.1016/j.neuroscience.2010.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte RH, Shain CN, Simone DA, Tsai EF. Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. J Neurophysiol. 1991;66:190–211. doi: 10.1152/jn.1991.66.1.190. [DOI] [PubMed] [Google Scholar]
- Lappi DA, Wiley RG. Immunotoxins and neuropeptide-toxin conjugates experimental applications. Mini Rev Med Chem. 2004;4:585–595. doi: 10.2174/1389557043403882. [DOI] [PubMed] [Google Scholar]
- Leger L, Gay N, Cespuglio R. Neurokinin NK1- and NK3-immunoreactive neurons in serotonergic cell groups in the rat brain. Neurosci Lett. 2002;323:146–150. doi: 10.1016/s0304-3940(01)02543-5. [DOI] [PubMed] [Google Scholar]
- Leong ML, Gu M, Speltz-Paiz R, Stahura EI, Mottey N, Steer CJ, Wessendorf M. Neuronal loss in the rostral ventromedial medulla in a rat model of neuropathic pain. J Neurosci. 2011;31:17028–17039. doi: 10.1523/JNEUROSCI.1268-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie TA, Emson PC, Dowd PM, Woolf CJ. Nerve growth factor contributes to the up-regulation of growth-associated protein 43 and preprotachykinin A messenger RNAs in primary sensory neurons following peripheral inflammation. Neuroscience. 1995;67:753–761. doi: 10.1016/0306-4522(95)00101-n. [DOI] [PubMed] [Google Scholar]
- Ljungdahl A, Hokfelt T, Nilsson G. Distribution of substance P-like immunoreactivity in the central nervous system of the rat--I. Cell bodies and nerve terminals. Neuroscience. 1978;3:861–943. doi: 10.1016/0306-4522(78)90116-1. [DOI] [PubMed] [Google Scholar]
- Ma QP, Woolf CJ. Basal and touch-evoked fos-like immunoreactivity during experimental inflammation in the rat. Pain. 1996a;67:307–316. doi: 10.1016/0304-3959(96)03132-6. [DOI] [PubMed] [Google Scholar]
- Ma QP, Woolf CJ. Progressive tactile hypersensitivity: an inflammation-induced incremental increase in the excitability of the spinal cord. Pain. 1996b;67:97–106. doi: 10.1016/0304-3959(96)03105-3. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- Mapp PI, Terenghi G, Walsh DA, Chen ST, Cruwys SC, Garrett N, Kidd BL, Polak JM, Blake DR. Monoarthritis in the rat knee induces bilateral and time-dependent changes in substance P and calcitonin gene-related peptide immunoreactivity in the spinal cord. Neuroscience. 1993;57:1091–1096. doi: 10.1016/0306-4522(93)90051-g. [DOI] [PubMed] [Google Scholar]
- Mason P. Physiological identification of pontomedullary serotonergic neurons in the rat. J Neurophysiol. 1997;77:1087–1098. doi: 10.1152/jn.1997.77.3.1087. [DOI] [PubMed] [Google Scholar]
- Mason P. Contributions of the medullary raphe and ventromedial reticular region to pain modulation and other homeostatic functions. Annu Rev Neurosci. 2001;24:737–777. doi: 10.1146/annurev.neuro.24.1.737. [DOI] [PubMed] [Google Scholar]
- Mason P. Medullary circuits for nociceptive modulation. Curr Opin Neurobiol. 2012;4:640–645. doi: 10.1016/j.conb.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Mitchell D, Hellon RF. Neuronal and behavioural responses in rats during noxious stimulation of the tail. Proceedings of the Royal Society of London - Series B: Biological Sciences. 1977;197:169–194. doi: 10.1098/rspb.1977.0064. [DOI] [PubMed] [Google Scholar]
- Molina C, Herrero JF. The influence of the time course of inflammation and spinalization on the antinociceptive activity of the alpha2-adrenoceptor agonist medetomidine. Eur J Pharmacol. 2006;532:50–60. doi: 10.1016/j.ejphar.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Nakaya Y, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N. Immunohistochemical localization of substance P receptor in the central nervous system of the adult rat. J Comp Neurol. 1994;347:249–274. doi: 10.1002/cne.903470208. [DOI] [PubMed] [Google Scholar]
- Nichols ML, Allen BJ, Rogers SD, Ghilardi JR, Honore P, Luger NM, Finke MP, Li J, Lappi DA, Simone DA, Mantyh PW. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science. 1999;286:1558–1561. doi: 10.1126/science.286.5444.1558. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Morita Y, Kiyama H, Ono K, Tohyama M. A noxious stimulus induces the preprotachykinin-A gene expression in the rat dorsal root ganglion: a quantitative study using in situ hybridization histochemistry. Brain Res. 1988;464:31–35. doi: 10.1016/0169-328x(88)90015-0. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lai J, Vanderah TW, Porreca F. Induction of pain facilitation by sustained opioid exposure: relationship to opioid antinociceptive tolerance. Life Sci. 2003;73:783–800. doi: 10.1016/s0024-3205(03)00410-7. [DOI] [PubMed] [Google Scholar]
- Pacharinsak C, Khasabov SG, Beitz AJ, Simone DA. NK-1 receptors in the rostral ventromedial medulla contribute to hyperalgesia produced by intraplantar injection of capsaicin. Pain. 2008;139:34–46. doi: 10.1016/j.pain.2008.02.032. [DOI] [PubMed] [Google Scholar]
- Parra MC, Nguyen TN, Hurley RW, Hammond DL. Persistent inflammatory nociception increases levels of dynorphin 1–17 in the spinal cord, but not in supraspinal nuclei involved in pain modulation. J Pain. 2002;3:330–336. doi: 10.1054/jpai.2002.125185. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. New York, NY: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends in Neurosciences. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Potenzieri C, Brink TS, Pacharinsak C, Simone DA. Cannabinoid modulation of cutaneous Adelta nociceptors during inflammation. J Neurophysiol. 2008;100:2794–2806. doi: 10.1152/jn.90809.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potrebic SB, Fields HL, Mason P. Serotonin immunoreactivity is contained in one physiological cell class in the rat rostral ventromedial medulla. J Neurosci. 1994;14:1655–1665. doi: 10.1523/JNEUROSCI.14-03-01655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman W, Suzuki R, Hunt SP, Dickenson AH. Selective ablation of dorsal horn NK1 expressing cells reveals a modulation of spinal alpha2-adrenergic inhibition of dorsal horn neurones. Neuropharmacology. 2008;54:1208–1214. doi: 10.1016/j.neuropharm.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Rahman W, Suzuki R, Rygh LJ, Dickenson AH. Descending serotonergic facilitation mediated through rat spinal 5HT3 receptors is unaltered following carrageenan inflammation. Neurosci Lett. 2004;361:229–231. doi: 10.1016/j.neulet.2003.12.069. [DOI] [PubMed] [Google Scholar]
- Ren K, Hylden JL, Williams GM, Ruda MA, Dubner R. The effects of a non-competitive NMDA receptor antagonist, MK-801, on behavioral hyperalgesia and dorsal horn neuronal activity in rats with unilateral inflammation. Pain. 1992;50:331–344. doi: 10.1016/0304-3959(92)90039-E. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Enhanced descending modulation of nociception in rats with persistent hindpaw inflammation. J Neurophysiol. 1996;76:3025–3037. doi: 10.1152/jn.1996.76.5.3025. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Descending modulation in persistent pain: an update. Pain. 2002;100:1–6. doi: 10.1016/s0304-3959(02)00368-8. [DOI] [PubMed] [Google Scholar]
- Rivat C, Vera-Portocarrero LP, Ibrahim MM, Mata HP, Stagg NJ, De Felice M, Porreca F, Malan TP. Spinal NK-1 receptor-expressing neurons and descending pathways support fentanyl-induced pain hypersensitivity in a rat model of postoperative pain. Eur J Neurosci. 2009;29:727–737. doi: 10.1111/j.1460-9568.2009.06616.x. [DOI] [PubMed] [Google Scholar]
- Sanoja R, Vanegas H, Tortorici V. Critical role of the rostral ventromedial medulla in early spinal events leading to chronic constriction injury neuropathy in rats. J Pain. 2008;9:532–542. doi: 10.1016/j.jpain.2008.01.332. [DOI] [PubMed] [Google Scholar]
- Seybold VS, Jia YP, Abrahams LG. Cyclo-oxygenase-2 contributes to central sensitization in rats with peripheral inflammation. Pain. 2003;105:47–55. doi: 10.1016/s0304-3959(03)00254-9. [DOI] [PubMed] [Google Scholar]
- Sikandar S, Bannister K, Dickenson AH. Brainstem facilitations and descending serotonergic controls contribute to visceral nociception but not pregabalin analgesia in rats. Neurosci Lett. 2012;519:31–36. doi: 10.1016/j.neulet.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone DA, Ngeow JY, Putterman GJ, LaMotte RH. Hyperalgesia to heat after intradermal injection of capsaicin. Brain Research. 1987;418:201–203. doi: 10.1016/0006-8993(87)90982-6. [DOI] [PubMed] [Google Scholar]
- Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989;38:99–107. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- Simone DA, Sorkin LS, Oh U, Chung JM, Owens C, LaMotte RH, Willis WD. Neurogenic hyperalgesia: central neural correlates in responses of spinothalamic tract neurons. J Neurophysiol. 1991;66:228–246. doi: 10.1152/jn.1991.66.1.228. [DOI] [PubMed] [Google Scholar]
- Sinclair JG, Main CD, Lo GF. Spinal vs. supraspinal actions of morphine on the rat tail-flick reflex. Pain. 1988;33:357–362. doi: 10.1016/0304-3959(88)90296-5. [DOI] [PubMed] [Google Scholar]
- Smith DJ, Hawranko AA, Monroe PJ, Gully D, Urban MO, Craig CR, Smith JP, Smith DL. Dose-dependent pain-facilitatory and -inhibitory actions of neurotensin are revealed by SR 48692, a nonpeptide neurotensin antagonist: influence on the antinociceptive effect of morphine. J Pharmacol Exp Ther. 1997;282:899–908. [PubMed] [Google Scholar]
- Sugiyo S, Takemura M, Dubner R, Ren K. Trigeminal transition zone/rostral ventromedial medulla connections and facilitation of orofacial hyperalgesia after masseter inflammation in rats. J Comp Neurol. 2005;493:510–523. doi: 10.1002/cne.20797. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Morcuende S, Webber M, Hunt SP, Dickenson AH. Superficial NK1-expressing neurons control spinal excitability through activation of descending pathways. Nature Neuroscience. 2002;5:1319–1326. doi: 10.1038/nn966. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Rahman W, Hunt SP, Dickenson AH. Descending facilitatory control of mechanically evoked responses is enhanced in deep dorsal horn neurones following peripheral nerve injury. Brain Res. 2004a;1019:68–76. doi: 10.1016/j.brainres.2004.05.108. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci. 2004b;25:613–617. doi: 10.1016/j.tips.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Rahman W, Rygh LJ, Webber M, Hunt SP, Dickenson AH. Spinal-supraspinal serotonergic circuits regulating neuropathic pain and its treatment with gabapentin. Pain. 2005;117:292–303. doi: 10.1016/j.pain.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Takagi H, Satoh M, Akaike A, Shibata T, Yajima H, Ogawa H. Analgesia by enkephalins injected into the nucleus reticularis gigantocellularis of rat medulla oblongata. Eur J Pharmacol. 1978;49:113–116. doi: 10.1016/0014-2999(78)90229-7. [DOI] [PubMed] [Google Scholar]
- Tal M. A Role for Inflammation in Chronic Pain. Curr Rev Pain. 1999;3:440–446. doi: 10.1007/s11916-999-0071-4. [DOI] [PubMed] [Google Scholar]
- Terayama R, Guan Y, Dubner R, Ren K. Activity-induced plasticity in brain stem pain modulatory circuitry after inflammation. Neuroreport. 2000;11:1915–1919. doi: 10.1097/00001756-200006260-00022. [DOI] [PubMed] [Google Scholar]
- Terayama R, Dubner R, Ren K. The roles of NMDA receptor activation and nucleus reticularis gigantocellularis in the time-dependent changes in descending inhibition after inflammation. Pain. 2002;97:171–181. doi: 10.1016/s0304-3959(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Spike RC, Young S, Puskar Z. Fos induction in lamina I projection neurons in response to noxious thermal stimuli. Neuroscience. 2005;131:209–217. doi: 10.1016/j.neuroscience.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Urban MO, Jiang MC, Gebhart GF. Participation of central descending nociceptive facilitatory systems in secondary hyperalgesia produced by mustard oil. Brain Res. 1996;737:83–91. doi: 10.1016/0006-8993(96)00631-2. [DOI] [PubMed] [Google Scholar]
- Urban MO, Gebhart GF. Characterization of biphasic modulation of spinal nociceptive transmission by neurotensin in the rat rostral ventromedial medulla. J Neurophysiol. 1997;78:1550–1562. doi: 10.1152/jn.1997.78.3.1550. [DOI] [PubMed] [Google Scholar]
- Urban MO, Gebhart GF. Supraspinal contributions to hyperalgesia. Proc Natl Acad Sci U S A. 1999;96:7687–7692. doi: 10.1073/pnas.96.14.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanegas H. To the descending pain-control system in rats, inflammation-induced primary and secondary hyperalgesia are two different things. Neurosci Lett. 2004;361:225–228. doi: 10.1016/j.neulet.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Kline RH, Wiley RG. Intrathecal substance p-saporin attenuates operant escape from nociceptive thermal stimuli. Neuroscience. 2003;119:223–232. doi: 10.1016/s0306-4522(03)00125-8. [DOI] [PubMed] [Google Scholar]
- Vikman KS, Duggan AW, Siddall PJ. Increased ability to induce long-term potentiation of spinal dorsal horn neurones in monoarthritic rats. Brain Res. 2003;990:51–57. doi: 10.1016/s0006-8993(03)03385-7. [DOI] [PubMed] [Google Scholar]
- Wang C, Sholas MG, Berde CB, DiCanzio J, Zurakowski D, Wilder RT. Evidence that spinal segmental nitric oxide mediates tachyphylaxis to peripheral local anesthetic nerve block. Acta Anaesthesiol Scand. 2001;45:945–953. doi: 10.1034/j.1399-6576.2001.450805.x. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Griffin G, Leichnetz GR, Mayer DJ. The somatotopic organization of the nucleus raphe magnus and surrounding brain stem structures as revealed by HRP slow-release gels. Brain Res. 1980;181:1–15. doi: 10.1016/0006-8993(80)91255-x. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Griffin G, Leichnetz GR, Mayer DJ. Identification and somatotopic organization of nuclei projecting via the dorsolateral funiculus in rats: a retrograde tracing study using HRP slow-release gels. Brain Res. 1981;223:237–255. doi: 10.1016/0006-8993(81)91139-2. [DOI] [PubMed] [Google Scholar]
- Wei F, Guo W, Zou S, Ren K, Dubner R. Supraspinal glial-neuronal interactions contribute to descending pain facilitation. J Neurosci. 2008;28:10482–10495. doi: 10.1523/JNEUROSCI.3593-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley RG. Substance P receptor-expressing dorsal horn neurons: lessons from the targeted cytotoxin, substance P-saporin. Pain. 2008;136:7–10. doi: 10.1016/j.pain.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Wiley RG, Lappi DA. Targeting neurokinin-1 receptor-expressing neurons with [Sar9, Met(O2)11 substance P-saporin. Neurosci Lett. 1999;277:1–4. doi: 10.1016/s0304-3940(99)00846-0. [DOI] [PubMed] [Google Scholar]
- Wiley RG, Lappi DA. Targeted toxins. Curr Protoc Neurosci. 2001;Chapter 1(Unit1.7) doi: 10.1002/0471142301.ns0107s14. [DOI] [PubMed] [Google Scholar]
- Wiley RG, Lappi DA. Targeted toxins in pain. Adv Drug Deliv Rev. 2003;55:1043–1054. doi: 10.1016/s0169-409x(03)00102-9. [DOI] [PubMed] [Google Scholar]
- Wiley RG, Kline RH, 4th, Vierck CJ., Jr Anti-nociceptive effects of selectively destroying substance P receptor-expressing dorsal horn neurons using [Sar9, Met(O2)11]-substance P-saporin: behavioral and anatomical analyses. Neuroscience. 2007;146:1333–1345. doi: 10.1016/j.neuroscience.2007.01.066. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, King AE. Physiology and morphology of multireceptive neurons with C-afferent fiber inputs in the deep dorsal horn of the rat lumbar spinal cord. J Neurophysiol. 1987;58:460–479. doi: 10.1152/jn.1987.58.3.460. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Thompson SW, King AE. Prolonged primary afferent induced alterations in dorsal horn neurones, an intracellular analysis in vivo and in vitro. J Physiol (Paris) 1988;83:255–266. [PubMed] [Google Scholar]
- Woolf CJ, King AE. Dynamic alterations in the cutaneous mechanoreceptive fields of dorsal horn neurons in the rat spinal cord. J Neurosci. 1990;10:2717–2726. doi: 10.1523/JNEUROSCI.10-08-02717.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie JY, Herman DS, Stiller CO, Gardell LR, Ossipov MH, Lai J, Porreca F, Vanderah TW. Cholecystokinin in the rostral ventromedial medulla mediates opioid-induced hyperalgesia and antinociceptive tolerance. J Neurosci. 2005;25:409–416. doi: 10.1523/JNEUROSCI.4054-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Ohsawa M, Ono H. Contribution of TRPV1 Receptor-Expressing Fibers to Spinal Ventral Root After-Discharges and Mechanical Hyperalgesia in a Spared Nerve Injury (SNI) Rat Model. J Pharmacol Sci. 2013;121:9–16. doi: 10.1254/jphs.12213fp. [DOI] [PubMed] [Google Scholar]
- Zhang L, Hammond DL. Substance P enhances excitatory synaptic transmission on spinally projecting neurons in the rostral ventromedial medulla after inflammatory injury. J Neurophysiol. 2009;102:1139–1151. doi: 10.1152/jn.91337.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Gardell S, Zhang D, Xie JY, Agnes RS, Badghisi H, Hruby VJ, Rance N, Ossipov MH, Vanderah TW, Porreca F, Lai J. Neuropathic pain is maintained by brainstem neurons co-expressing opioid and cholecystokinin receptors. Brain. 2009;132:778–787. doi: 10.1093/brain/awn330. [DOI] [PMC free article] [PubMed] [Google Scholar]


