Abstract
The poly(A)-binding protein PAB1 from the yeast Saccharomyces cerevisiae plays an important role in controlling mRNA deadenylation rates. Deletion of either its RRM1 or proline-rich domain (P domain) severely restricts deadenylation and slows mRNA degradation. Because these large deletions could be having unknown effects on the structure of PAB1, different strategies were used to determine the importance of the RRM1 and P domains to deadenylation. Since the P domain is quite variable in size and sequence among eukaryotes, P domains from two human PABPCs and from Xenopus were substituted for that of PAB1. The resultant PAB1 hybrid proteins, however, displayed limited or no difference in mRNA deadenylation as compared to PAB1. In contrast to the P domain, the RRM1 domain is highly conserved across species, and a systematic mutagenesis of the RRM1 domain was undertaken to identify its functional regions. Several mutations along the RNA binding surface of RRM1 inhibited deadenylation whereas one set of mutations on its exterior non-RNA binding surface shifted deadenylation from a slow distributive process to a rapid processive deadenylation. These results suggest that the RRM1 domain is the more critical region of PAB1 for controlling deadenylation and consists of at least two distinguishable functional regions.
Introduction
In the yeast, Saccharomyces cerevisiae, the regulation of mRNA turnover is governed principally by the rate at which the mRNA poly(A) tail is deadenylated (Cao and Parker 2001). Once the poly(A) tail length reaches an oligo(A) size (about 8-12 A’s) decapping of the mRNA occurs followed by exonuclease destruction of the mRNA (Coller and Parker 2003). Control of the removal of the poly(A) tail by the principal deadenylase, CCR4 (Tucker et al 2001, 2002; Chen et al 2002), is influenced greatly by the state of the mRNP structure involving the mRNA and the poly(A)-binding protein (PAB1) (Yao et al 2007; Lee et al 2010; Simon and Seraphin 2007).
The N-terminus of the PAB1 protein consists of four consecutive RNA binding domains (termed RRM1 to RRM4) that are linked to a C-terminal globular domain (see Figure 1A) important for binding translation termination factor eRF3 and other regulatory proteins (Deo et al 1999; Hosoda et al 2003; Hoshino et al 1999). Connecting the RRM domains to the C-terminus is an apparently unstructured domain rich in proline and alanine residues that we have designated the P domain (Yao et al 2007). Previous analyses concerning the importance of each of these PAB1 domains to deadenylation have demonstrated that deletion of either the RRM1 or the P domain severely restricted normal deadenylation (Yao et al 2007; Lee et al 2010; Simon and Seraphin 2007). The RRM1 domain may be having the more important role in general deadenylation processes, for it has also been shown that deleting the RRM1 domain but not the P domain severely blocks the rapid inducement of deadenylation of the COX17 mRNA upon binding the PUF3 regulatory protein to 3′ sequences present in the COX17 (Lee et al 2010). Differences in how the PAB1 proteins self-associate into a circular form as influenced by these RRM1 and P domain deletions was also linked to differences in mRNA deadenylation (Yao et al 2007).
Figure 1.
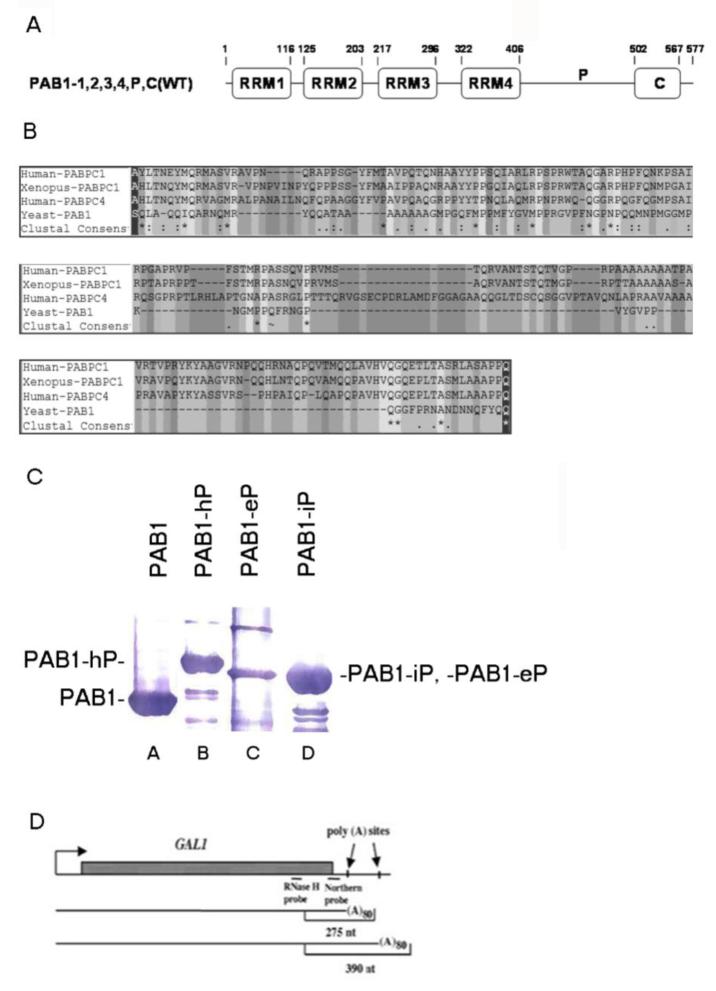
P domain sequence alignments and GAL1 mRNA species. A. Schematic for the structure of the yeast PAB1 protein. Residues for each domain are indicated. Higher eukaryotic P domain substitutions were constructed by removing residues 406 to 502 of PAB1 and substituting the corresponding sequence from PABPC1, ePABP and iPABP for that of the yeast P domain. B. Sequence alignment of PABPC1 protein (16358990, Homo sapiens) (human PABPC1 in the text), PABPC1 protein (30353795, Xenopus laevis) (ePABP in the text), PABPC4 protein (66267552, Homo sapiens) (iPABP in the text), and PAB1 protein (603406, Saccharomyces cerevisiae) using Bioedit software. The P domain within the PAB1 protein is from 406 to 502, showing as gray residues between a black residue at each end. “−” represents gap position in the alignment. The consensus symbol “*” means that the residues are identical in all sequences in the alignment, “:” means that conserved substitutions have been observed and “.” means that semi-conserved substitutions are observed. C. Relative levels of PAB1 hybrid proteins were observed following Western analysis using anti-Flag antibody. Lane A- PAB1; lane B- PAB1-hP; lane C- PAB1-eP; and lane D- PAB1-iP. D. Diagram of GAL1 gene mRNA. The two poly(A) sites located 50 and 160 bp downstream of the stop codon are indicated, as are the RNase H probe and the Northern probe used in our analyses (Yao et al 2007).
While similar studies on the PAB1 protein in higher eukaryotes have not been conducted, a wide variety of PAB1 proteins have been identified and the overall rate of deadenylation has been studied in specific cell types. The RRM1-4 regions of these higher eukaryotic PAB1 proteins are very conserved, whereas the P domain shows extreme variability in size and sequence. Based on the yeast PAB1 results, we hypothesized that certain differences in deadenylation rates for higher eukaryotic cell types could result from differences in the PABPC protein expressed in that cell type. For example, human PABPC4, also known as iPABP (which is nearly identical to human PABPC1 in its RRM1-4 domains but contains a very distinct P domain) is preferentially expressed in T lymphocyte cells and T cell mRNA undergoes slow deadenylation (Lindstein et al 1989; Yang et al 1995). Similarly, in Xenopus somatic cells, slowed deadenylation is accompanied with expression of the ePABP protein whose P domain is different from all other P domains (Voeltz et al 2001).
Because our previous analyses of the importance of PAB1 to deadenylation relied principally on large deletions that could adversely affect PAB1 function through unknown structural effects, in this paper we address the importance of the RRM1 and P domains of yeast PAB1 to deadenylation by other types of mutation analyses. In order to ascertain the importance of the P domain to deadenylation without constructing large deletions, we chose to substitute different P domains from higher eukaryotic PAB proteins for the P domain of PAB1. By this means, the presumed overall linear structure of the PAB1 protein would be maintained and only the effect of different P domains on deadenylation would be determined. In contrast, because the RRM1 domain is highly conserved amongst all PAB proteins, we chose to conduct a systematic mutagenesis of the RRM1 domain to determine which regions were important to deadenylation. Our results indicate that vastly different sizes and sequences of P domains when substituted for the P domain of PAB1 make no real difference on the deadenylation process. On the other hand, we have identified certain residues in RRM1 that are critical to deadenylation both for restricting it and for accelerating it.
Materials and Methods
Yeast strains and growth conditions
All yeast strains were isogenic to AS319/YC504 (Mat ura3 trp1 his3 leu2 pab1::HIS3 YC504 [Flag-PAB1-TRP1]), except each contained a different PAB1 variant as indicated in the text. All PAB1 variants were shown to express equivalent amounts of the PAB1 protein as determined by Western analysis (Yao et al 2007). Yeast growth conditions have been previously described (Yao et al 2007; Lee et al 2010). All RRM1 mutations were constructed by directed PCR mutagenesis (Yao et al 2007). The P domain substitutions were constructed by cloning the representative P domain sections depicted in Figure 1B and substituting them for the P domain of PAB1 (residues 406 to 502 of PAB1 were removed).
mRNA deadenylation analysis and other analyses
Pulse-chase analyses for the GAL1 mRNA were conducted as previously described (Yao et al 2007; Lee et al 2010). Briefly, after growth of cells in non-inducing medium containing 2% sucrose, the mRNA were induced for 10 min with 2% galactose and mRNA expression was shut off with 4% glucose. At the time points indicated, the RNA was isolated and subjected to Northern analysis. All pulse-chase experiments were conducted at least in duplicate. BMH (bis-maleimidohexane) crosslinking was conducted following purification of PAB1 protein on Flag-agarose beads exactly as described (Yao et al 2007). The 77S monosomal translation complex was detected following analytical ultracentrifugation (AU) as previously reported (Wang et al 2012). Briefly, Flag agarose-purified yeast extracts obtained from cells grown to equivalent cell densities were subjected to AU analysis (conducted at 20°C) using A260 absorption optics using a rotor speed of 15,000 rpm. Data were analyzed by SEDFIT software.
Results
P domain substitutions and their effect on deadenylation
Because the deletion of the P domain that restricts deadenylation may be having unknown effects on PAB1 structure, another approach to assaying P domain importance to deadenylation was used. However, the construction of mutations across the P domain did not seem to be a reasonable approach to analyzing P domain function because of the highly variable nature of the P domain across species. For example, higher eukaryotic P domains, such as human PABPC1 and ePABP (for embryonic poly(A)-binding protein) from the frog (Xenopus), are very distinct from that of the yeast P domain (Figure 1B). Possible excessive redundancy of residues might obviate any particular mutation analysis. To surmount this apparent limitation in conducting mutation analysis on the P domain, we created three yeast PAB1 variants with P domain substitutions: PAB1-eP in which the P domain from ePABP was substituted for that of PAB1, PAB1-iP replaced the P domain of wild type PAB1 with that of iPABP (human PABPC4), and the PAB1-hP in which the P domain of human PABPC1 replaced that of PAB1. The choice of these three P domain substitutions was based on the following additional hypothesis. The general deadenylation rate of mRNA poly(A) tails has been followed in cells expressing ePABP and in iPABP and have been compared to cells containing the normal PABP (PABPC1 in the case of humans) (Lindstein et al 1989; Yang et al 1995; Voeltz et al 2001). The results from these studies indicated that deadenylation was dramatically slowed in cells expressing ePABP or iPABP. Because the P domains of these two proteins differ dramatically from that of other PABP molecules (Figure 1B), we hypothesized, based on our yeast analysis demonstrating the importance of the P domain to yeast mRNA deadenylation, that it was the distinct nature of these ePABP and iPABP P domains that led to the decrease in rates of deadenylation.
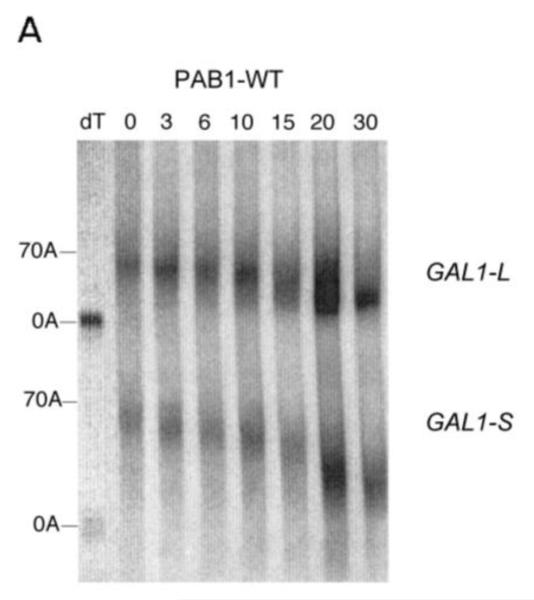
Each of these hybrid PAB1 proteins was expressed in yeast containing a deletion of the endogenous PAB1 gene (Yao et al 2007). No phenotypic differences were observed for any of these three hybrid PAB1 proteins when compared to wild-type PAB1, suggesting that the resultant PAB1 proteins were generally functional, as they were expressed to similar levels in the cell (Figure 1C). The phenotypes that were monitored were slow growth at 30°C or 37°C, increased sensitivity to the presence of 8 mM caffeine, and lethality with a caf1 deletion. Pulse-chase analysis on the GAL1 mRNA was used subsequently for determining the rates of mRNA deadenylation with each of these strains. Two GAL1 mRNA species are produced in vivo that result from differential poly(A) site usage and differ by 110 nt in their 3′ UTR (Cui and Denis 2003) (Figure 1D). Following a brief induction of GAL1 mRNA synthesis with the addition of galactose to the medium (the pulse), mRNA synthesis was shut off with glucose and RNA was isolated at various times (the chase). The 3′ ends of GAL1 mRNA were detected by using an RNase H assay and a DNA probe that was complementary to sequences present in both species (Yao et al 2007) (Figure 1D). In wild-type PAB1, two polyadenylated species migrating at about 390 and 275 nucleotides (nt) that corresponded to poly(A) sites at about 160 bp and 50 bp, respectively, downstream of the GAL1 stop codon were identified (Figure 1D). Each mRNA species contained about 80 nt of poly(A), as determined previously (Yao et al 2007).
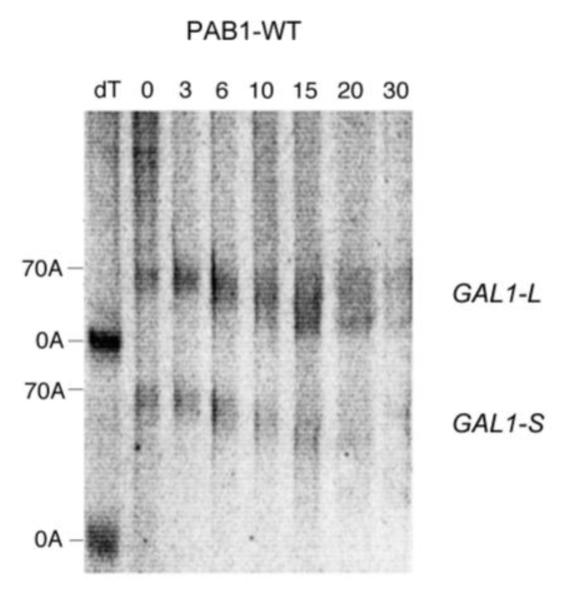
As shown in Figure 2A, for wild type yeast PAB1, the poly(A) tails for GAL1-L mRNA from 0 min to 6 min become progressively shorter, indicative of distributive deadenylation in agreement with our previous results (Yao et al 2007). At around 10 min, however, a spread of poly(A) tail lengths begins to be visible in which the oligo(A) species (about 10-12 A’s) for GAL1-L begins to be apparent. This spread is presumed to be due to increased processive deadenylation by CCR4, as removal of the PAB1 protein from the poly(A) tail enhances CCR4 processive activity (Viswanathan et al 2003; Tucker et al 2002). By 15 min, in agreement with our previous results, a large percentage of the poly(A) tail lengths have reached this oligo(A) stage (Yao et al 2007), which has been shown to coincide with mRNA decapping (Decker and Parker 1993). In contrast to GAL1-L mRNA, the GAL1-S mRNA is deadenylated much more slowly and remains in the distributive mode until late times (around 20 to 30 min) (Figure 2A and 5A) (Yao et al 2007). For the distributive phases of deadenylation, in the wild-type PAB1 background, GAL1-L is deadenylated at a rate of 3.7 A’s/min whereas GAL1-S is deadenylated at the rate of 2.8 A’s/min (Yao et al 2007). This should be contrasted with the effect of deleting the P domain that severely reduced both the distributive and processive phases of GAL1-L deadenylation (distributive deadenylation rate reduced to 1.5A’s/min) and GAL1-S (1.1 A’s/min) (Yao et al 2007).
Figure 2.
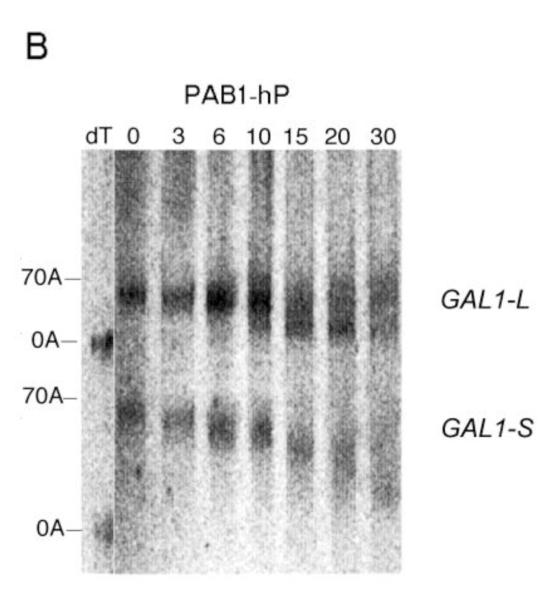
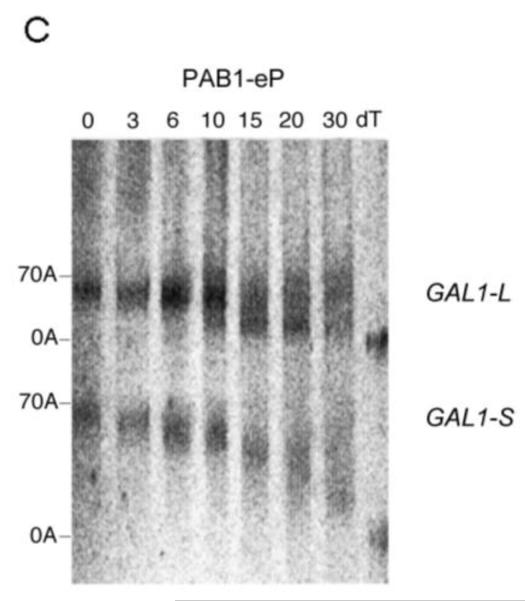
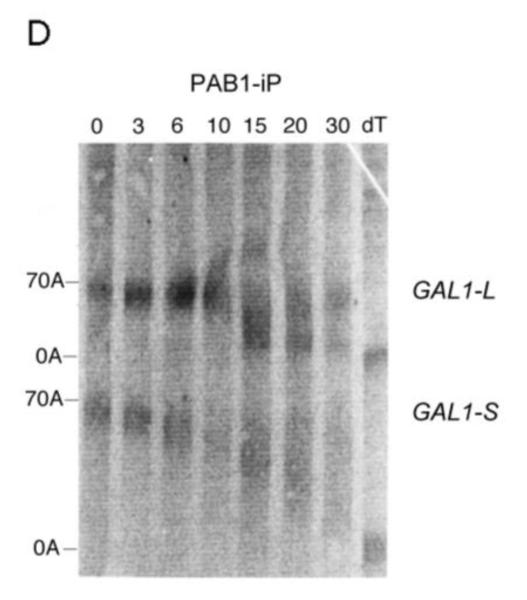
Pulse-chase deadenylation analysis of P substitution PAB1 variants. A. Northern analysis of GAL1 mRNA in yeast expressing wild-type PAB1. Yeast was induced by growth in galactose-containing medium for 8 min, followed by glucose addition, and samples were collected at different time points (times are in minutes), upon which total RNA was extracted. The poly(A) tail lengths are indicated. The dT-RNA sample was pretreated with oligo d(T) and RNase to remove the poly(A) tail prior to Northern analysis. B. Same as ‘A’ above, except PAB1-hP was expressed instead of yeast PAB1. C. Same as ‘A’ above, except PAB1-eP was expressed. D. Same as ‘A’ above, except PAB1-iP was expressed.
Figure 5.
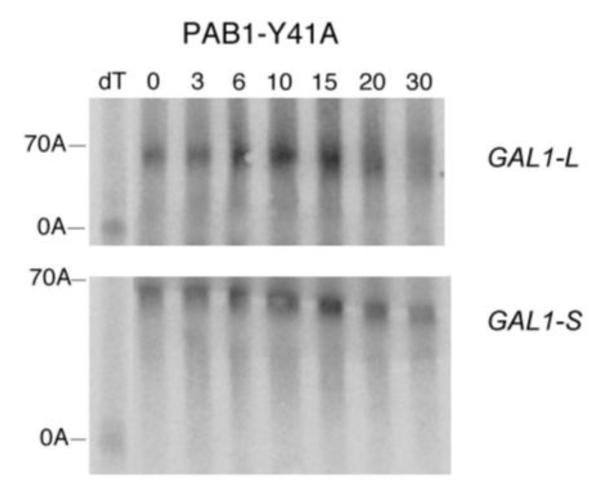
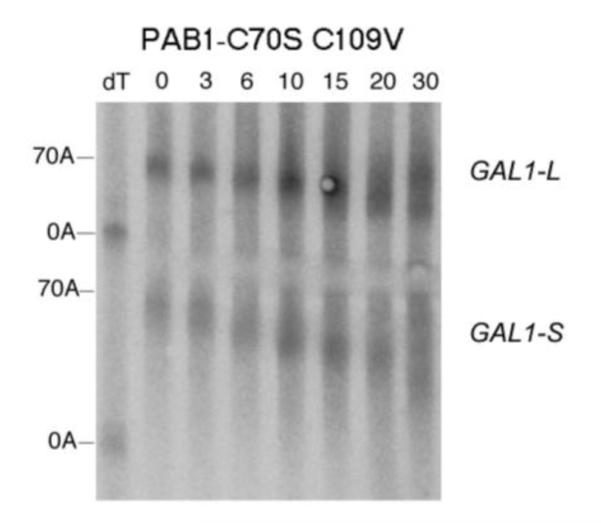
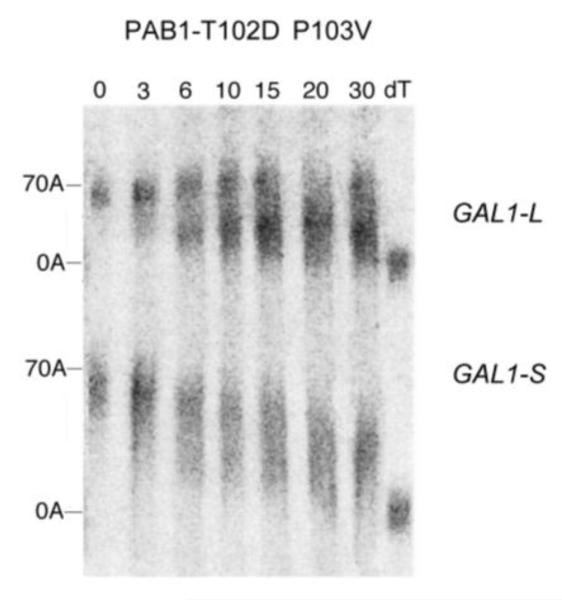
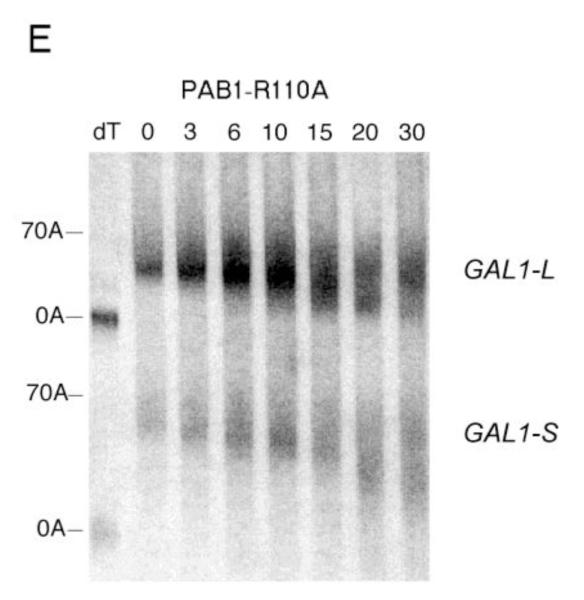
Deadenylation assays for RRM1 mutations. A. Wild-type PAB1, and the analysis was conducted as described in Figure 2A. B. Same as ‘A’ above, except PAB1-Y41A. C. Same as ‘A’ above, except PAB1-C70S C109V. D. Same as ‘A’ above, except PAB1-T102D P103V; E. Same as ‘A’ above, except PAB1-R110A.
For the PAB1-hP hybrid protein, however, very similar results were obtained as were observed with PAB1. Some oligo(A) species for GAL1-L was visible at 10 min and it was definitely present at 15 min. In regards to GAL1-S, in the PAB1-hP background, the oligo(A) species began to be displayed at 20 to 30 min (Figure 2B). The rates of distributive deadenylation for PAB1-hP were calculated to be 3.9 A’s/min for GAL1-L and 2.2 A’s/min for GAL1-S. These results suggest that although human PABPC1 and yeast PAB1 display very different sequences within their P domains, these differences do not severely affect yeast mRNA deadenylation. PAB1-eP showed a deadenylation pattern that was similar to PAB1-hP in its effect on both GAL1-L and GAL1-S (Figure 2C). For PAB1-eP the oligo(A) species did not appear for GAL1-L until about 10 min and was abundant at 15 min. The rates of deadenylation in the PAB1-eP background were calculated to be slightly slower than that observed with PAB1 (3.4 A’s/min for GAL1-L and 2.2 A’s/min for GAL1-S). Although PAB1-eP concentration in yeast was always significantly lower than that of PAB1 (Figure 1C), the resultant deadenylation rates did not appear greatly impeded and no phenotypes were observed as a consequence of the lower PAB1-eP abundance. PAB1-iP displayed very slow initial distributive deadenylation of GAL1-L (0.8 A’s/min), as the oligo(A) form was not apparent at 10 min, but it rapidly transitioned to normal processive deadenylation (5.0 A’s/min), for at 15 min the oligo(A) form was quite apparent (Figure 2D). For the GAL1-S mRNA in the PAB1-iP background no significant decrease in deadenylation rate was observed (2.6 A’s/min) when compared to that observed in the PAB1 background (2.8 A’s/min). Overall, in contrast to deleting the P domain and its blockage of both distributive and processive deadenylation for both GAL1 mRNA species, these results indicate that either the PAB1 P domain is not critical to mRNA deadenylation or that its important elements may be not highly dependent on a particular sequence. Moreover, our results do not support the hypothesis that the different P domain sequences found in ePABP or iPABP as compared to that of PABPC1 are solely responsible for slowing mRNA deadenylation when these PAB proteins are expressed in their respective species.
Rationale for constructing RRM1 point mutations
RRM1 and RRM2 share similar structures in which these two RRMs form a continuous RNA-binding trough, lined by an antiparallel β sheet backed by four α helices (shown in Figure 3). Because deletion of the RRM1 domain slowed deadenylation in vivo, we were interested in identifying particular residues important to this protein, especially since RRM1 is highly conserved across species (Figure 4A). The N-terminal regions that include RRM1-4 of the human PABPC1, ePABP, and iPABP proteins are at least 72% identical to PAB1 (see Figure 4A for a comparison of RRM1 domains). Previously, we had constructed the mutation Y83V of RRM1 and shown that this mutation slowed deadenylation (Yao et al 2007). The Y83V alteration takes place on the RNA binding surface of RRM1 in which Y83 is critical to contacting the poly(A) tail (Deardorff and Sachs 1997), suggesting that lack of PAB1 association with the poly(A) tail was correlated with slowing deadenylation. However, the ability of PAB1 variants to bind poly(A) was found not to correlate at all with effects on deadenylation (Yao et al 2007).
Figure 3.
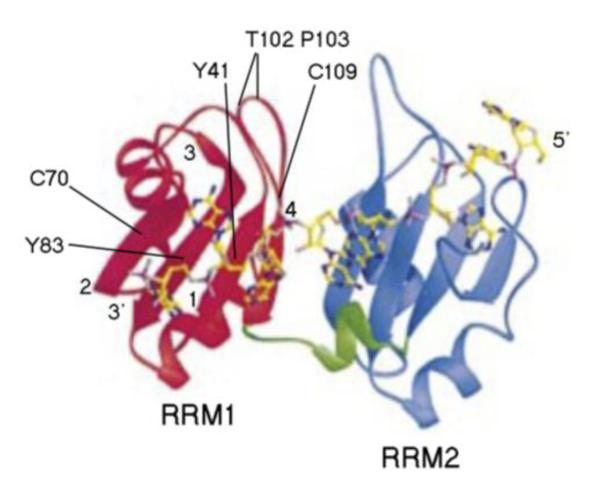
Three dimensional structure of the RRM1 and RRM2 domains of human PAB1. Structure of the Human PABPC1 RRM1/2-RNA complex (Deo et al 1999) showing the extended RNA-binding surface created by RRM1 (red-left) and RRM2 (blue-right). The 3′ and 5′ ends of the poly(A) segment are indicated. Residues of RRM1 are indicated that when mutated gave altered deadenylation rates. The -strands of RRM1 are numbered 1 to 4.
Figure 4.
RRM1 mutations and sequence alignments. A. The four PAB proteins whose alignments are represented are described in Figure 1. The RRM1 domains are gray color residues between the black color residues as shown. The RRM1 domains of first three proteins are from 12 to 86. RRM1 within PAB1 protein is from 40 to 113. The consensus symbol “*” means that the residues are identical in all sequences in the alignment, “:” means that conserved substitutions have been observed and “.” means that semi-conserved substitutions are observed. B. RRM1 sequence alignment of PABPC1 protein with PAB1 protein. RRM1 contains four β-sheets, showing as β1-β4 (Deo et al 1999). The amino acid residues numbers of PAB1 are showed above the sequences. The mutations used for deadenylation assays are identified by vertical linesand the grouped mutations that were created are linked together as shown.
To take a more systematic mutation analysis to identifying residues we scanned the RRM1 domain for homology among dissimilar PAB proteins (Figure 4A). PAB1 homology analysis showed many conserved amino acid residues in the RRM1 domain, most of them in the β sheet RNA binding surface of RRM1. These conserved residues in the RNA binding surface are probably important for the function of PAB1 for binding mRNA but may possibly be important to other processes. Targeting these residues would allow us to generalize the importance of the RNA binding domain surface to deadenylation based on the Y83V result. However, we did not wish to mutate only highly conserved residues, as many of these might be structural in nature and mutating them might disrupt the total function of the RRM1 domain.
A second group of mutations were constructed that targeted those residues on the external surface of RRM1 that are not used in RNA binding. Previously, it had been shown that residues on the surface of RRM2 are important to its function (Otero et al 1999; Ohn et al 2007). These residues were identified by lack of conservation between human and yeast RRM1 sequences but retention of conservation when a number of yeast RRM1 sequences were compared (not shown). As displayed in Figure 4B, and listed in Table 1, we found nine individual or clusters of residues in the non-RNA binding surface of RRM1 that displayed differences between human and yeast sequences but which are conserved amongst yeast species. We presumed that such differences might represent residues involved in key protein contacts in which the protein binding residues had changed between yeast and human PAB proteins, although the actual protein contacted might have remained constant across eukaryotes (Otero et al 1999). If this were the case, we would be identifying regions on the RRM1 external non-RNA binding surface that were making contact to other proteins, possibly those involved in mRNA deadenylation.
Table 1. Summary of RRM1 mutations.
The listed RRM1 mutations include those previous constructed (C70S C109V and Y83V; Yao et al 2007). The conservation of the yeast residue relative to the corresponding human residue is indicated (see Figure 4) as well as whether the alteration involved converting the yeast residue to that of the human.The location of the mutation on the RNA binding surface is also indicated (Deo et al 1999).
| Residue/Alteration | Conserved Yeast to Human |
Change to Human Residue |
RNA Binding Surface |
|---|---|---|---|
| Y41A | Y | N | Y |
| D44A | Y | N | Y |
| E46H S48D | N/N | Y/Y | N/N |
| I57K | N | Y | N |
| I61A | N | Y | N |
| S65L | N | Y | Y |
| C70A | Y | N | Y |
| C70S C109V | Y/N | N/Y | Y/Y |
| R71A | Y | N | Y |
| A73N | N | N | N |
| T77R | N | N | N |
| L79K | N | N | Y |
| Y83V | Y | N | Y |
| D88A E90A | N/N | N/N | N/N |
| A91A R93E | N/N | Y/Y | N/N |
| Q98T | N | Y | N |
| T102D P103V | N/N | Y/Y | N/N |
| R110A | Y | N | Y |
| M112A | Y | N | Y |
| S114A | Y | N | Y |
Phenotypic effects of RRM1 mutations
PAB1 proteins containing each of the RRM1 mutations depicted in Figure 4B were constructed and substituted for endogenous PAB1, as described above. Table 1 lists all of the new RRM1 mutations we constructed and includes two that we had previously constructed (PAB1-Y83V and –C70S C109V) (Yao et al 2007). Table 1 also summarizes on which RRM1 surface the mutations occur, whether the substitutions mimic the human RRM1 PABPC1 RRM1 domain, and whether the residue is conserved between human and yeast PAB proteins. All PAB1 RRM1 mutations were expressed to equivalent levels as that of the wild-type PAB1 protein as assayed by Western analysis (see, for example, PAB1 as compared to PAB1-Y41A in Figure 6; Yao et al 2007; data not shown). Because PAB1-RRM1 and PAB1-Y83V, which slow deadenylation, result in several phenotypic differences when compared with wild-type PAB1, we initially assayed each of our new PAB1 variants for effects on a variety of processes and synthetic lethalities. These processes included growth at 41°C, lethality at 34°C under glycerol growth conditions when the PAB1 allele was combined with the cdc33-1 allele (a temperature sensitive defect in translation initiation factor eIF4E resulting in an eIF4E protein defective in binding the mRNA cap), and synthetic lethality with caf1 (CAF1 encodes a component of the CCR4-NOT deadenylation complex) (Draper et al 1995; Liu et al 1998). The few phenotypic differences that were observed between different PAB1 alleles are summarized in Table 2. The effect of these RRM1 alterations on other phenotypes associated with possible deadenylation phenotypes (increased sensitivity to 8 mM caffeine and synthetic lethalities with defects in decapping factors DCP1, DCP2, or LSM6) was also monitored but no effects were observed for any of the new RRM1 alterations. Therefore, for this large group of alterations, only three displayed aberrant phenotypes: PAB1-C70S C109V, -R110A, and -Y41A. Because some of these phenotypic effects were similar to that of deleting the entire RRM1 domain, these mutations could be affecting deadenylation processes.
Figure 6.
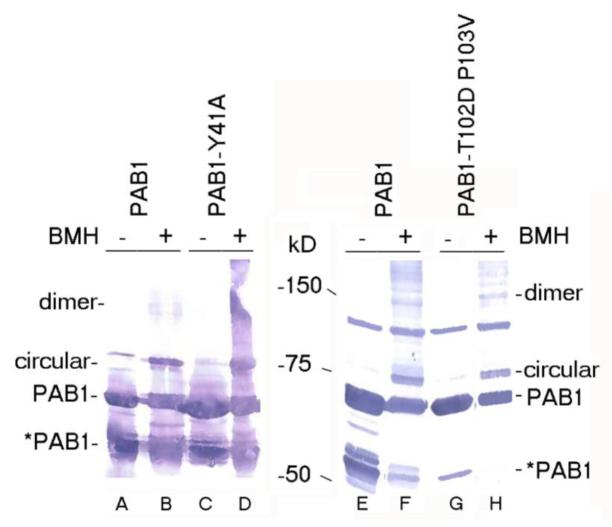
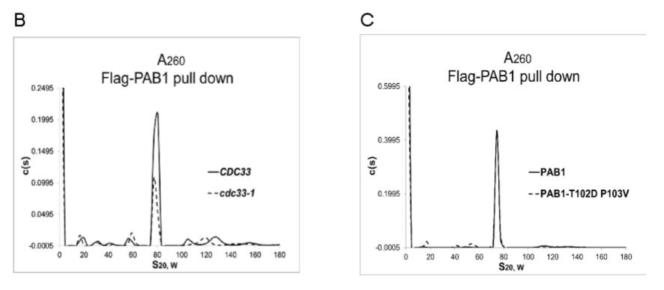
PAB1-Y41A and –T102D P103V effects on protein interactions. A. BMH cross-linking (+) was compared to mock-treated samples (−). Lanes A and E- PAB1 (mock-treated); lanes B and F- PAB1 (BMH treated); lane C- PAB1-Y41A (mock-treated); lane D- PAB1-Y41A- (BMH treated); lane G- PAB1-T102D P103V (mock-treated); and lane H- PAB1-T102D P103V- (BMH treated). PAB1 proteins were identified by Western analysis using anti-Flag antibody. The circular and linear species of PAB1 are indicated. In lanes A and C, the slower migrating species in the mock-treated samples that nearly co-migrates with the circular form is a non-specific protein detected with the anti-Flag antibody (Yao et al 2007). *PAB1 refers to the C-terminally truncated form of PAB1 (Yao et al 2007). B. AU analysis of Flag-agarose purified yeast extracts containing wild-type PAB1 (CDC33 is strain AS319/YC504 (PAB1) and cdc33-1 is strain AS1881/YC504 that is isogenic to AS319/YC504 except for carrying the cdc33-1 allele). C. Same as ‘B’ above, except PAB1 versus PAB1-T102D P103V are depicted.
Table 2.
Phenotypic analysis of RRM1 mutations
Strain YC504 containing the YC360 plasmid (PAB1-URA3) instead of theYC504 plasmid (Flag-PAB1-TRP1) was transformed with each Flag-PAB1-TRP1 variants as described in the Table. The ability to subsequently lose the YC360 plasmid was assayed on 5-fluoroorotic acid-containing plates (Yao et al 2007). Transformed cells incapable of losing YC360 were considered displaying a synthetic lethality with caf1. cdc33-1 carries mutations in elF4E rendering it unable to grow at 37°C under glucose growth conditions (Lee et al 2010). Growth at 41°C glucose and 34°C glycerol was monitored for cells containing only the RRM1 variant as indicated following loss of the YC360 plasmid. All other RRM1 alterations listed in Table 1 elicited no discernable phenotypic effects. ‘++’ indicates good growth and ‘−‘ indicates no growth.
| RRM1 mutation | Growth, 41°C glucose |
Growth in cafl background, 30°C glucose |
Growth in cdc33-1 background, 34°C glycerol |
|---|---|---|---|
| PAB1-wt | ++ | ++ | ++ |
| PAB1-Y83V | ++ | − | − |
| PAB1 Y41A | ++ | − | − |
| PAB1-R110A | ++ | − | ++ |
| PAB1-C70S C109V | − | ++ | ++ |
| PAB1-T102D P103V |
++ | ++ | ++ |
| PAB1-RRM1 | − | − | − |
Effect of RRM1 mutations on mRNA deadenylation
Because of the large number of mutations we had constructed and the lack of phenotypes for almost all of them, we conducted a rapid snapshot screening procedure to determine if any of these mutations were affecting deadenylation. This snapshot involved assaying the effect of each of these PAB1 variants on GAL-L and GAL1-S mRNA deadenylation using the above described pulse chase analysis in which only the 0 min and 8 min time points were compared. By this means, any mutation that was slowing deadenylation or speeding it up could be rapidly identified and a more comprehensive pulse-chase analysis could be conducted. Through this analysis, only three mutations were identified that either reduced or sped up the deadenylation rate (PAB1-Y41A, -C70S C109V, and T102D P103V).
Each of the variants that affected deadenylation using our snapshot screening procedure (as well as PAB1-R110A which displayed a synthetic lethality, Table 2) was assayed for effects on GAL1 deadenylation. For PAB1-Y41A, deadenylation was slowed considerably for both GAL1 mRNA species (Figure 5B) as compared to that of wild-type (Figure 5A). For PAB1-Y41A, little oligo(A) species is formed even at late times for either GAL1-L or GAL1-S, indicative of very slow deadenylation. The rates of poly(A) removal for PAB1-Y41A compared to PAB1 are summarized in Table 3. When we analyzed PAB1-C70S C109V, which contains mutations also along the RNA binding surface of RRM1, only a slight delay in deadenylation was observed (Figure 5C). In the case of GAL1-L, significant abundance of the oligo(A) species is observed in the PAB1-C70S C109V background at 15 min as compared to 10 min as observed in the PAB1 wild-type strain. However, no significant effect on GAL1-S deadenylation was observed (summarized in Table 3). These results confirm that not all highly conserved residues on the RNA binding surface of the RRM1 domain (such as C70) are critical to deadenylation.
Table 3. Representative PAB1 variant effects on deadenylation.
PAB1 RRM1 mutations and their effect on deadenylation. Deadenylation rates were conducted as described (Yao et al 2007). GAL1-L (long) and GAL1-S (short) refer to the two GAL1 mRNA that result from differential poly(A) site usage and differ by 110 nt in their 3’ UTR end (Yao et al 2007; Cui and Denis 2003). Deadenylation rates were the average of two to three determinations and are given with Standard Errors of the Means in parentheses . Deadenylation rate values in bold differ significantly from wild-type.
| PAB1 variant | Deadenylation rate GAL1-L, A’s/min (SEM) |
Deadenylation rate, GAL1-S, A’s/min (SEM) |
|---|---|---|
| PAB1 (wt) | 3.7 (0.12) | 2.8 (0.034) |
| PAB1-Y83V | 2.0 (0.10) | 1.8 (0.050) |
| PAB1-Y41A | 1.3 (0.25) | 1.4 (0.10) |
| PAB1-R110A | 3.1 (0.50) | 2.4 (0.25) |
| PAB1-C70S C109V |
2.6 (0.25) | 2.4 (0.35) |
| PAB1-T102D P103V |
7.0 (0.54) | 5.6 (0.10) |
| PAB1- RRM1 | 2.0 (0.10) | 2.0 (0.10) |
Most noteworthy, when we analyzed PAB1-T102D P103V, it displayed significantly faster deadenylation (Figure 5D). By 6 min the mRNA was already present in two distinct bands (long poly(A) tails with average length 60 A’s and a short poly(A) pool with average length about 13 A’s as determined by densitometric analysis, not shown). The presence of two bands is characteristic of rapid processive deadenylation in which long poly(A) tails co-exist with short poly(A) tail lengths (Decker and Parker 1993; Olivas and Parker 2001; Viswanathan et al 2003, 2004; Lee et al 2010; Richardson et al 2012). These results were supported by the observation that GAL1-S deadenylation was also noticeably faster in the PAB1-T102D P103V background. For GAL1-S, which is usually very slowly distributively deadenylated (Figure 5A), a very significant spreading of the poly(A) species is evident with PAB1-T102D P103V at very early times (6 min) and this spreading becomes more dramatic and shifts to shorter poly(A) tail lengths at later times (Figure 5D). This pattern is again indicative of processive deadenylation. In contrast to the above mutations, PAB1-R110A showed no large differences in deadenylation from that of wild type PAB1 for either GAL1-L or GAL1-S (Figure 5E). For GAL1-L, at the 10 min time point, the oligo(A) form becomes present and by 15 min a large amount of the oligo(A) form is present. Similarly for GAL1-S deadenylation, no particular differences were observed between PAB1-R110A (Figure 5E, lower panel) and PAB1-wt (Figure 2A or 5A, lower panels). In that this particular mutation displayed synthetic lethality with caf1 but had no effect on deadenylation suggests that another function of PAB1 is being affected by this alteration.
Our previous data on the role of PAB1 in mRNA deadenylation had suggested a correlation between CCR4 deadenylation and the ability of PAB1 to form a circular, intramolecular association between the RNA binding surface of RRM1 and RRM4 (Yao et al 2007). The model suggested that the ability of PAB1 to self-associate would preclude it from binding the mRNA and thereby allow CCR4 to access the poly(A) tail. Because the PAB1-Y41A mutation was also on the RNA binding surface of RRM1 and also severely restricted CCR4 deadenylation, we subsequently tested whether this alteration affected PAB1 ability to circularize. The PAB1 intramolecular interaction can be readily followed by determining if the RRM1 and RRM4 domains (each containing cysteine residues) are in close proximity to each other by the ability of the crosslinking agent BMH to cross-link the respective cysteine residues in the RRM1 and RRM4 domains. The resultant cross-linked protein results in a circular species of PAB1 that migrates slower than non-cross-linked PAB1 following SDS-PAGE (Yao et al 2007). However, as shown in Figure 6A, PAB1-Y41A, following BMH cross-linking, displayed the same degree of circular form (lane D) as that displayed by PAB1 following BMH cross-linking (lane B), implying that PAB1-Y41A is not deficient in forming the circular form. These results do not support the hypothesis that PAB1-Y41A is deficient in deadenylation because it self-associates less well than the unmutated PAB1. We similarly analyzed PAB1-T102D P103V self-association, but there was no effect of this alteration that causes more rapid deadenylation on PAB1 circularization (Figure 6A, lanes E-H).
Finally, in regards to the PAB1-T102D P103V alteration that enhanced deadenylation, it has previously been shown that defects in translation initiation (such as represented by the cdc33-1 allele or a defect in eIF3b) could also cause enhanced CCR4 deadenylation (Schwartz and Parker 1999; Lee et al 2010). Because the RRM1 domain of PAB1 makes contacts to eIF4G that is involved in forming translational complexes (Richardson et al 2012), it is possible that PAB1-T102D P103V is accelerating deadenylation by disrupting the formation of translating ribosomes. We have shown in other research that the cdc33-1 allele reduces by two-fold the ability of the 77S monosomal translation complex to be formed (Figure 6B) (Wang et al 2012). Similar effects on the 77S translation complex were obtained for an eIF3b defect, the prt1-1 allele (Wang et al 2012). The 77S monosomal translation complex contains eIF4E, eIF4G, PAB1, mRNA, and a single 80S translating ribosome. Consequently, we tested the effect of the PAB1-T102D P103V protein on the ability of the 77S translation complex to be formed. As shown, however, in Figure 6C, there was no difference in 77S complex formation between PAB1 and PAB1-T102D P103V (PAB1-T102D P103V expressed 108% of the abundance of the 77S complex as compared to PAB1, average of two determinations, SEM of 4%). These results indicate that PAB1-T102D P103V is not enhancing deadenylation by disrupting the translational complex.
Discussion
Two domains of PAB1 had previously been implicated in being most important to controlling deadenylation: the RRM1 and P domains. Deletion of the RRM1 domain has been shown to severely block normal deadenylation for slowly and rapidly deadenylated mRNA species (GAL1-S and MFA2 mRNA, respectively), for induced deadenylation (PUF3 induction of COX17 mRNA), and for accelerated deadenylation that occurs upon induction of nonsense mediated decay (Yao et al 2007; Lee et al 2010; Richardson et al 2012). Deletion of the P domain, in contrast, while severely reducing GAL1 and MFA2 RNA deadenylation, had only limited effect on the COX17 mRNA rapid deadenylation induced by PUF3 and no effect on nonsense mediated deadenylation. These results suggested that the RRM1 domain played a more important role in deadenylation.
To overcome the limitation of these previous analyses that utilized large deletions in these domains that might grossly affect the structure or other functional roles of PAB1, in the current research, we constructed much more modest modifications in these two domains. For RRM1, a series of point mutations were constructed in across its RNA binding domain as well throughout its exterior non-RNA binding region presumed to interact with other proteins. Both of these regions were found to be important to mRNA deadenylation. In addition to the mutation Y83V that we had shown previously to inhibit CCR4 deadenylation (Yao et al 2007), we identified Y41A and C70S C109V as slowing deadenylation. On the non-RNA binding surface, one set of mutations was also identified that accelerated CCR4 deadenylation. These combined data indicate that the RRM1 domain is critical to the deadenylation process.
For the P domain, however, our analyses did not support its principal role in deadenylation. Using three different P domains from higher eukaryotic species, we created PAB1 hybrid domains in which the yeast P domain had been replaced by the orthologous sequence from these other PAB proteins. The sequence identity between any of these domains is extremely limited, but in each case deadenylation proceeded relatively normally, although some small effects were observed. Our conclusion is that the P domain deletion that we had studied previously may have been affecting the overall structure and function of the PAB1 protein in respect to deadenylation and not necessarily demonstrating a critical role for the P domain by itself in the deadenylation process. The fact that vastly different sequences in the P domain (albeit all enriched in an excess of proline and alanine residues) result in similar deadenylation phenotypes further suggests that if the P domain were to be important to deadenylation, then some feature of its three-dimensional structure is important and not the exact specific contiguous amino acid arrangement. The preponderance of proline and alanine residues in the P domain suggests a flexibility to this region, perhaps keeping the C domain at a certain distance from the four N-terminal RRM binding domains. Alternatively, the high number of alanine (12% of the P domain for PAB1) and proline (14%) residues may be having direct functional contacts other than simply providing a linker region.
The mutations in the RRM1 that we showed that affected mRNA deadenyation in vivo also displayed specific phenotypes and synthetic lethalities. The correlation of strong defects in deadenylation for the PAB1-Y83V, -Y41A, and RRM1 variants and the synthetic lethality with the cdc33-1 mutation suggests a linkage of these effects. However, the exact meaning of this correlation is not clear, as the cdc33-1 mutation accelerates deadenylation (Schwartz and Parker 1999; Lee et al 2010) and the PAB1 mutations slow deadenylation. Moreover, this phenotype was observed only under non-fermentative conditions, implying that the lethality was related to only a specific subset of genes being expressed and not necessarily related to the normal pattern of deadenylation that takes place under glucose growth conditions. The other phenotypes that we detected do not correlate with the PAB1 variants’ effects on deadenylation, implying instead that they result from aberration in other processes.
Both Y83V and Y41A alterations in PAB1 lie adjacent on the RNA binding surface of RRM1 (Figure 3), and both are presumed to make significant contacts to the poly(A) tail (Deardorff and Sachs 1997; Yao et al 2007; Deo et al 1999). A number of other mutations on the RNA binding surface of RRM1 were mutated and did not elicit effects on deadenylation. How the Y83V and Y41A alterations are blocking deadenylation is not clear. We have previously shown that differences in PAB1 binding to the poly(A) tail clearly do not correspond to effects on deadenylation (Yao et al 2007). We had also suggested previously that PAB1 self-association into a circular could be affecting deadenylation, but we were unable to provide any additional support for this hypothesis as demonstrated in Figure 6A. Because the PAB1 protein does not make direct contact to the CCR4-NOT complex and the proteins known to which the PAB1 RRM1 binds are not likely to cause large decreases in the deadenylation rate (Richardson et al 2012), it is unclear how these PAB1 alterations interfere with deadenylation.
Similarly, we found that the PAB1-T102D P103V changes accelerated deadenylation, but we have no clear idea on how this is accomplished. Both of these mutations lie in a loop between -strands 1 and 4 of the RRM1 domain (Figure 3). The substituted residues in this case derive from the human sequence, implying that the residues are not critically interfering with PAB1 overall structure and are rather altering some specific contact. As this loop is close to RRM2, it could be affecting RRM1-RRM2 contacts or RRM1 contacts to another protein. While acceleration of deadenylation is known to accompany disruption of the translation complex (Wang et al 2012; Lee et al 2010), we found no difference in forming the 77S monosomal translation complex between a strain carrying the wild-type PAB1 as compared to a strain with PAB1-T102D P103V.
Based on the above considerations, it is clear that the RRM1 domain of PAB1 plays an important role in allowing deadenylation to proceed. While it is not yet clear how the structure of the PAB1 protein, as controlled by the RRM1 domain, helps aid deadenylation, more complete structural analysis of the complete PAB1 protein associated with poly(A) might further elucidate this process.
Acknowledgments
This research was supported by NIH grants GM78087 and GM82048 and from the ARRA initiative of 2009. Partial funding was provided by the New Hampshire Agricultural Experiment Station to C.L.D. This is Scientific Contribution Number 2514.
References
- Cao D, Parker R. Computational modeling of eukaryotic mRNA turnover. RNA. 2001;7:1192–1212. doi: 10.1017/s1355838201010330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Chiang Y-C, Denis CL. CCR4, a 3′-5′ poly (A) RNA and ssDNA exonuclease, is the catalytic component of the cytoplasmic deadenylase. EMBO J. 2002;21:1414–1426. doi: 10.1093/emboj/21.6.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Denis CL. In vivo evidence that defects in transcription elongational factors, RPB2, TFIIS, and SPT5 enhance upstream poly (A) site utilization. Mol. Cell. Biol. 2003;23:7887–7901. doi: 10.1128/MCB.23.21.7887-7901.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker CJ, Parker R. A turnover pathway for both stable and unstable mRNAs in yeast: evidence for a requirement for deadenylation. Genes Dev. 1993;7:1632–1643. doi: 10.1101/gad.7.8.1632. [DOI] [PubMed] [Google Scholar]
- Deardorff JA, Sachs AB. Differential effects of aromatic and charged residue substitutions in the RNA binding domains of the yeast poly(A)-binding protein. J. Mol. Biol. 1997;269:67–81. doi: 10.1006/jmbi.1997.1013. [DOI] [PubMed] [Google Scholar]
- Deo RC, Bonanno JB, Sonenberg N, Burley SK. Recognition of polyadenylate RNA by the poly(A)-binding protein. Cell. 1999;98:835–845. doi: 10.1016/s0092-8674(00)81517-2. [DOI] [PubMed] [Google Scholar]
- Draper MP, Salvadore C, Denis CL. Identification of a mouse protein whose homolog in yeast is a component of the CCR4 transcriptional regulatory complex. Mol. Cell. Biol. 1995;15:3487–95. doi: 10.1128/mcb.15.7.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino S, Imai M, Kobayashi T, Uchida N, Katada T. The eukaryotic polypeptide chain releasing factor (eRF3/GSPT) carrying the translation termination signal to the 3′-poly(A) tail of mRNA. Direct association of erf3/GSPT with polyadenylate-binding protein. J. Biol. Chem. 1999;274:16677–16680. doi: 10.1074/jbc.274.24.16677. [DOI] [PubMed] [Google Scholar]
- Hosoda N, Kobayashi, Uchida N, Funakoshi Y, Kikuchi Y, Hoshino S, Katada T. Translation termination factor eRF3 mediates mRNA decay through the regulation of deadenylation. J. Biol. Chem. 2003;278:38287–38291. doi: 10.1074/jbc.C300300200. [DOI] [PubMed] [Google Scholar]
- Lee D, Ohn T, Chiang Y-C, Liu Y, Quigley G, Yao G, Denis CL. PUF3 acceleration of deadenylation in vivo can operate independently of CCR4 activity, possibly involving effects on the PAB1-mRNP structure. J. Mol. Biol. 2010;399:562–575. doi: 10.1016/j.jmb.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstein T, June CH, Ledbetter JA, Stella G, Thompson CB. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science. 1989;244:339–343. doi: 10.1126/science.2540528. [DOI] [PubMed] [Google Scholar]
- Liu H-Y, Badarinarayana V, Audino DC, Rappsilber J, Mann M, Denis CL. The NOT proteins are part of the CCR4 transcriptional complex and affect gene expression both positively and negatively. EMBO J. 1998;17:1096–1106. doi: 10.1093/emboj/17.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlrad D, Decker CJ, Parker R. Deadenylation of the unstable mRNA encoded by the yeast MFA2 gene leads to decapping followed by 5′-3′ digestion of the transcript. Genes Dev. 1994;8:855–866. doi: 10.1101/gad.8.7.855. [DOI] [PubMed] [Google Scholar]
- Ohn T, Chiang Y-C, Lee DJ, Yao G, Zhang C, Denis CL. CAF1 plays an important role in mRNA deadenylation separate from its contact to CCR4. Nucl. Acids Res. 2007;35:3002–3015. doi: 10.1093/nar/gkm196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivas W, Parker R. The Puf3 protein is a transcript-specific regulator of mRNA degradation in yeast. EMBO J. 2000;19:6602–661. doi: 10.1093/emboj/19.23.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero LJ, Ashe MP, Sachs AB. The yeast poly (A)-binding protein Pab1p stimulates in vitro poly (A)-dependent and cap-dependent translation by distinct mechanisms. EMBO J. 1999;18:3153–3163. doi: 10.1093/emboj/18.11.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson R, Denis CL, Zhang C, Nielsen MO, Chiang YC, Kierkegaard M, Wang X, Lee D,J, Andersen J,S, Yao G. Mass spectrometric identification of proteins that interact with specific domains of the poly(A) binding protein. Mol. Genet. Genomics. 2012;271:711–730. doi: 10.1007/s00438-012-0709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DC, Parker R. Mutations in translation initiation factors lead to increased rates of deadenylation and decapping of mRNAs in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:5247–5256. doi: 10.1128/mcb.19.8.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon E, Seraphin B. A specific role for the C-terminal region of the poly(A)-binding protein in mRNA decay. Nucl Acids Res. 2007;35:6017–6028. doi: 10.1093/nar/gkm452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker M, Staples RR, Valencia-Sanchez MA, Muhlrad D, Parker R. CCR4p is the catalytic sub-unit of Ccr4p/Pop2p/Notp mRNA deadenylase ecomplex in Saccharomyces cerevisiae. EMBO J. 2002;21:1427–1436. doi: 10.1093/emboj/21.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker M, Valencia-Sanchez MA, Staples RR, Chen J, Denis CL, Parker R. The transcription factor associated proteins, Ccr4p and Caf1p, are components of the major cytoplasmic mRNA deadenylase in Saccharomyces cerevisiae. Cell. 2001;104:377–386. doi: 10.1016/s0092-8674(01)00225-2. [DOI] [PubMed] [Google Scholar]
- Viswanathan P, Chen J, Chiang Y-C, Denis CL. Identification of multiple RNA features that influence CCR4 deadenylation activity. J. Biol. Chem. 2003;278:14949–14955. doi: 10.1074/jbc.M211794200. [DOI] [PubMed] [Google Scholar]
- Viswanathan P, Ohn T, Chiang Y-C, Chen J, Denis CL. Mouse CAF1 can function as a processive deadenylase 3′-5′-exonuclease in vitro but in yeast the deadenylase function of CAF1 is not required for mRNA poly(A) removal. J. Biol. Chem. 2004;279:23988–23995. doi: 10.1074/jbc.M402803200. [DOI] [PubMed] [Google Scholar]
- Voeltz GK, Ongkasuwan J, Standart N, Steitz JA. A novel embryonic poly(A) binding protein, ePAB, regulates mRNA deadenylation in Xenopus egg extracts. Genes Dev. 2001;15:774–778. doi: 10.1101/gad.872201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Duckett CS, Lindstein T. iPABP, an inducible poly(A)-binding protein detected in activated human T cells. Mol. Cell. Biol. 1995;15:6770–6776. doi: 10.1128/mcb.15.12.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao G, Chiang Y-C-, Zhang C, Lee D, Denis CL. PAB1 self-association precludes its binding to poly (A), thereby accelerating CCR4 deadenylation in vivo. Mol. Cell. Biol. 2007;27:6243–6253. doi: 10.1128/MCB.00734-07. [DOI] [PMC free article] [PubMed] [Google Scholar]