Abstract
The environmental pollutants polychlorinated biphenyls (PCBs), especially dioxin-like PCBs, cause oxidative stress and associated toxic effects, including cancer and possibly atherosclerosis. We previously reported that PCB 126, the most potent dioxin-like PCB congener, decreases antioxidants such as hepatic selenium (Se), selenium-dependent glutathione peroxidase and glutathione (GSH), but also increases levels of the anti-atherosclerosis enzyme paraoxonase 1 (PON1) in liver and serum. To probe the interconnection of these three antioxidant systems, Se, GSH, and PON1, we examined the influence of varying levels of dietary Se and N-acetylcysteine (NAC), a scavenger of reactive oxygen species (ROS) and precursor for GSH synthesis, on PON1 in the absence and presence of PCB 126 exposure. Male Sprague Dawley rats, fed diets with differing Se levels (0.02, 0.2, or 2 ppm) or NAC (1%), were treated with a single intraperitoneal injection of corn oil or various doses of PCB 126 and euthanized 2 weeks later. PCB126 significantly increased liver PON1 mRNA, protein level and activity and serum PON1 activity in all dietary groups, but did not consistently increase thiobarbituric acid levels (TBARS), an indicator for lipid oxidation and oxidative stress, in liver or serum. Inadequate (high or low) dietary Se decreased baseline and PCB 126-induced aryl hydrocarbon receptor expression but further increased PCB 126-induced cytochrome P450 1A1 expression, the enzyme believed to be the cause for PCB 126-induced oxidative stress. In addition, a significant inverse relationship was observed between dietary Se levels and PON1 mRNA and PON1 activity, but also with TBARS levels in the liver, suggesting significant antioxidant protection from dietary Se. NAC lowered serum baseline TBARS levels in the controls and increased serum PON1 activity but lowered liver PON1 activities in animals treated with 1 μmol/kg PCB 126, suggesting antioxidant activity by NAC primarily in serum. These results also show an unexpectedly predominantly inverse relationship between Se or NAC and PON1 during normal and PCB 126 exposure conditions. These interactions should to be further explored in the development of dietary protection regimens.
Keywords: polychlorinated biphenyls, PCB 126, paraoxonase 1, PON 1, selenium, N-acetylcysteine, lipid peroxidation, rat, dioxin
Introduction
PCBs are ubiquitous environmental and human contaminants. The 209 different congeners are often grouped according to their chemical structure and/or biological activity. Of these the most prominent group is dioxin-like PCBs, congeners with no or one chlorine substitution in ortho position which, like dioxin, interact with the arylhydrocarbon receptor (AhR). PCB 126 is the most potent AhR agonist among them (Bandiera et al. 1982) and was the first PCB congener classified by the International Agency for Research on Cancer (IARC) as a human carcinogen (Cogliano et al. 2011). A more recent review of PCBs by IARC classified PCBs as carcinogenic to humans (Group 1) and additionally dioxin-like PCBs as human carcinogens based on extensive evidence of an AhR-mediated mechanism of carcinogenesis (Lauby-Secretan et al. 2013). PCB 126 significantly alters the expression of numerous genes resulting in altered enzyme activities, particularly of cytochrome P450 1A1 (CYP1A1) and antioxidant enzymes, resulting in oxidative stress in the liver (Hassoun et al. 2002; Parkinson et al. 1983). Oxidative stress is believed to be an important factor in all stages of carcinogenesis (Klaunig et al. 2011) as well as in many chronic diseases, including atherosclerosis (Vogiatzi et al. 2009).
PON1, an antioxidant enzyme, is believed to have preventive properties against atherosclerosis and cardiovascular diseases, mainly through removal of oxidized lipids in low density lipoproteins (LDL) and cell membranes (Furlong et al. 2010). However, the prevalence of atherosclerosis indicates that PON1 may not be sufficient to protect against endogenous and exogenous sources of reactive oxygen species. Various nutrients or dietary supplements may be needed to provide additional protection against oxidative stress.
In 1979 selenium (Se) was recognized as an essential human nutrient (Navarro-Alarcon et al. 2002). Se plays an important role in various biological pathways, particularly in the oxidative stress defense system due to the formation of selenocysteine, a fundamental component of the active site of the glutathione peroxidase enzyme (SeGPx) (Papp et al. 2007). SeGPx reduces hydrogen peroxide and lipid hydroperoxides in the cytosol and mitochondrial matrix (Flohe et al. 1973). Se was reported to influence the pathogenesis of various diseases, among them cancer, cardiovascular and neurological diseases and immune function (Brigelius-Flohe and Maiorino 2012; Darvesh and Bishayee 2010; Rayman 2012; Sanmartin et al. 2011). Nutritional uptake of Se may vary considerably among individuals and thereby their ability to prevent adverse health effects due to oxidative stress from endogenous and exogenous factors.
The most important intracellular antioxidant is GSH. However, poor absorption when taken orally is an impediment to the use of dietary glutathione as supplement. N-acetylcysteine (NAC) has been widely used in replacement of direct glutathione administration (Witschi et al. 1992). NAC, a thiol-containing amino acid, has been in clinical use for over 30 years, primarily as an antidote for acetaminophen overdose (Baumgardner et al. 2008). NAC is a precursor in the GSH synthesis pathway and NAC's potential protective function has been linked to the prevention of chronic diseases characterized by decreased GSH and/or increased oxidative stress such as alcoholic liver disease (Thong-Ngam et al. 2007). Being a source of sulfhydryl groups, NAC supports glutathione-S-transferase (GST) activity, an important phase II detoxification enzyme. In addition, NAC is believed to promote detoxification and to act as a scavenger of free radicals due to its direct interaction with reactive oxygen species (ROS) (Aruoma et al. 1989; De Vries and De Flora 1993; Dodd et al. 2008).
An often overlooked factor in public health is that different contaminants may modulate our endogenous antioxidant enzyme activities. Our previous studies showed that PCB 126 significantly increased PON1 gene transcription in rat livers in a time- and dose-dependent manner (Shen et al. 2012). Although this change in PON1 should enhance protection against cardiovascular diseases, PCB 126 was linked to increased risk for atherosclerosis (Hennig et al. 2002a; Hennig et al. 2002b). PCB 126 also disrupted the homeostasis of antioxidants such as selenium (Hassan et al. 1985; Lai et al. 2011; Stemm et al. 2008), an element that appears to be protective for high-density lipoprotein (HDL)-associated enzymes like PON1 (Kaur and Bansal 2009). In addition, PCB 126 depleted GSH (Hassoun and Periandri-Steinberg 2010) which may place the free –SH group in PON1 at increased risk of oxidation. Thus the increase in PON1 transcription was possibly counteracted by changes in other antioxidants that could lead to enhanced inactivation of the enzyme and/or cancellation of its protective effect.
Based on these recent findings, we designed in vivo studies to elucidate the interactions of the two dietary antioxidants Se and NAC, who are expected to act through different mechanisms (gene regulation and radical scavenging, respectively) on PON1 regulation and activity in the presence and absence of PCB 126, an oxidative stress producing contaminant, to understand the potential consequent for lipid peroxidation levels and human health.
Materials and Methods
Chemicals and Reagents
PCB 126 was synthesized and purified using a modified Suzuki-coupling method of 3,4,5-trichlorobromobenzene with 3,4-dichlorophenyl boronic acid utilizing a palladium-catalyzed cross coupling reaction as previously reported (Luthe et al. 2009). All reagents were obtained from Fisher Scientific (Pittsburgh, PA) if not stated otherwise. The AIN-93 diets modified with three different sodium selenate and NAC levels (Supplemental Table 1.) were purchased from Harland Teklad (Madison, WI).
Animals and Treatment Protocol
For the Se study, male Sprague Dawley rats, weighing around 100 g, were purchased from Harlan Laboratories, Inc (Indianapolis, IN). Rats were fed with three AIN-93 diets (Supplemental Table 1A) containing 0.02 ppm (low), 0.2 ppm (adequate) and 2 ppm (supplemented) sodium selenate and water ad libitum. After three weeks on the diet, a time chosen to allow Se equilibration in the tissues, rats from each dietary group were administered a single i.p. injection of corn oil (5 mL/kg body weight; Acros Chemical Company, Pittsburgh, PA), 0.2 ,1, or 5 μmol/kg body weight of PCB 126 (n=6 per group). For the NAC study, male Sprague Dawley rats, weighing 75-100 grams from Harlan Sprague Dawley (Indianapolis, IN) were randomly divided into two dietary groups, and were fed with an AIN-93G diet or an AIN-93G based diet supplemented with 1.0% NAC (Supplemental Table 1B) and water ad libitum. After one week, a time expected to be sufficient for the equilibration of NAC loading in tissues, animals in each diet group were given a single i.p. injection of corn oil alone or, 1 or 5 μmol/kg body weight of PCB 126 in corn oil. All animals in both studies were housed in individual wire cages in a controlled environment maintained at 22°C with a 12 h light-dark cycle and were euthanized 2 weeks later by carbon dioxide asphyxiation and cervical dislocation. Livers and other organs were excised, weighed, and further processed as described below. The animal protocols used were conducted with approval from the Institutional Animal Care and Use Committee of the University of Iowa.
Liver and Blood Sample Process
Liver samples were homogenated in ice-cold 0.25 M sucrose solution (pH 7.4) and centrifuged at 10,000g for 20 min. The supernatants were then centrifuged at 100,000g for 1hr and the resulting microsomal pellets were washed and resuspended with the same sucrose solution. Serum was separated from whole blood after centrifugation at 3,000g for 15 min at 4°C. All sample aliquots were stored at -80°C until use.
Measurement of Liver and Serum PON1 Activity
PON1 enzyme activities were determined in liver microsomes (Se study) of homogenate (NAC study) and serum using two substrates, paraoxon and phenyl acetate. Briefly, the enzyme activities were determined spectrophotometrically by following the initial rate of substrate hydrolysis to p-nitrophenyl (412nm) or phenol (270nm), respectively, in 1000 mL assay mixtures (100 mM Tris-HCl, 2.0 mM CaCl2, 2.0 mM paraoxon or 4.0 mM phenylacetate, pH 8.0) at 25°C. A blank sample containing the assay buffer without enzyme was run simultaneously to correct for spontaneous substrate hydrolysis. The units (U) of enzyme activity were calculated from the molar extinction coefficients for p-nitrophenol and phenol, E412 (18,290 M-1cm-1) and E270 (1310 M-1cm-1), respectively, and expressed as U/mL serum or U/mg protein in liver homogenate. Each unit of enzyme is defined as that hydrolyzing 1 nmol of paraoxon or 1 μmol of phenylacetate per min (Beltowski et al. 2005). The protein concentration in liver homogenates and microsomes was determined using the Bradford protein assay (Bio-Rad Laboratories, Inc CA).
Analysis of a Direct Effect of NAC on Rat Serum PON1
Serum from an untreated rat was incubated with 0, 10 or 40 mM of NAC for 0, 10, 20 or 30 min at 37°C. The serum PON 1 activities were determined using paraoxon and phenylacetate spectrophotometrically as described above. Experiments were repeated twice.
Liver Gene Expression Profile
Total RNA was isolated from rat liver samples using the RNeasy Mini Kit from Qiagen, Inc (Valencia, CA) according to the manufacturer's protocol. RNA concentration and purity was determined by spectrophotometric measurement of A260/A280. cDNA templates were generated using 1 μg of total RNA per 20-μL reaction and a High-Capacity cDNA Reverse Transcription Kit from Applied Biosystems, Inc (Foster City, CA) according to the manufacturer's protocol. Real-time PCR was performed in a 20-μL reaction with 4 ng of cDNA template and 900 nM primer using a SYBR Green Master Mix kit from Applied Biosystems, Inc. (Foster City, CA) according to the manufacturer's protocol. Primers used were synthesized by Integrated DNA Technologies, Inc. (Coralville,IA). The primers were selected from the indicated publications and the specificity of these primers was further verified by the Primer-BLAST online program provided by National Library of Medicine. The primer pairs for the rat genes are listed in Supplemental Table 2 and were designed according to previously published reports (Boesch-Saadatmandi et al. 2009; Gaub et al. 2010; Romani et al. 2009; Shipley and Waxman 2006; Varatharajalu et al. 2009; Vondracek et al. 2006).
The PCR program used started with 95° for 10 min followed by 40 cycles at 95°C for 30 s, 55°C for 30 s and 72°C for 1 min performed on an Eppendorf RealPlex2 Mastercycler® (Hamburg, Germany). A melting curve analysis was carried out after the reaction to check for false amplification caused by primer dimers, non-specific binding or other contamination. The relative gene expression levels were calculated using the relative standard curve method. The target gene expression levels were adjusted to a housekeeping gene coding for ribosomal protein L13a (RPL13a).
Sodium Dodecyl Sulfate (SDS) Polyacrylamide Gel Electrophoresis and Western Blot Analyses
To measure liver PON1 protein levels, Western blotting was performed. Briefly, liver microsome samples (10 μg protein per sample, loaded on 1 gel in the order 3 controls, 1 empty slot, 3 samples of each PCB 126 dose) were electrophoretically resolved and separated in 4% to 15% denaturing ployacrylamide gel (PAGE) and then transferred to the polyvinylidene fluoride (PVDF) membrane using Bio-Rad Mini-PROTEAN and Mini-Trans Blot systems, Bio-Rad Laboratories, Inc. (Hercules, CA). The membranes then were immunoblotted using antibodies against PON1 and glyceraldehyde-3-phosphate dehydrogenase (GRPDH; internal reference protein) from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The protein signal was determined using enhanced chemiluminescence. Data were analyzed by IMAGE J software (V1.43, National Institute of Health, US).
Serum Lipid Profile
Lipid assay kits from BioVision Inc. (Mountain View, CA) were used. Briefly, total cholesterol (TC) was measured directly in serum samples according to the manufacturer's protocol. Rat serum was separated into high density lipoprotein (HDL) and Low density lipoprotein (LDL) fractions by centrifugation under 2000g for 10 min with the precipitation buffer supplied in the kit. The fractions were used for analysis of HDL associated cholesterol (HDL-C) and LDL associated cholesterol (LDL-C), respectively, according to the manufacturer's protocol.
HDL Associated PON1 Activity and LDL Associated Lipid Hydroperoxides
PON1 is assumed to be primarily associated with HDL in the serum. Since PCBs change lipid profiles we investigated the specific HDL-associated PON1 activity besides the overall PON1 activity in serum (Se study only). The HDL-PON1 activity was determined against phenylacetate as described above.
PON1 is known to specifically prevent LDL oxidation. The ferrous oxidation-xylenol orange (FOX) assay was used to detect the lipid hydroperoxide level in the LDL fraction (Jiang et al. 1992). Lipid hydroperoxides are linked to oxidative stress. The FOX assay is a simple and sensitive method. It is based on the oxidation of Fe+2 to Fe+3 by simple-oxidizing agents, which in turn binds to a sensitive dye, xylenol orange. The complex then can be measured spectrophotometrically at 560 nm. The standard curve with hydrogen peroxide was used for the quantification (Banerjee et al. 2003; DeLong et al. 2002; Nourooz-Zadeh et al. 1994). Briefly, 10 μL LDL fraction sample was incubated with 90 μL FOX working solution for 30 min at room temperature and absorbance was monitored at 560 nm. Aqueous solutions of known H2O2 concentration were used for calibration and results were expressed as nmol/L H2O2 equivalents.
Liver and Serum Lipid Peroxidation Analysis
To obtain an overall indication of oxidative stress the thiobarbituric acid reactive substances (TBARS) assay was used. This method determines the total lipid peroxidation status although with a limited specificity and sensitivity. TBARS level, expressed in terms of malondialdehyde (MDA), was used as the index of lipid peroxidation in liver and serum according to the methods described previously (Ohkawa et al. 1979; Yagi 1998). Briefly, standard solutions (MDA), liver homogenate or serum lipid fraction were incubated with 0.8% or 0.67%, respectively of thiobarbituric acid at 95°C for 60 minutes and the red pigment produced in the reaction was extracted by n-butanol-pyridine mixture (liver homogenate) or n-butanol (serum). The pigment concentration was determined spectrophotometrically (at 535 nm for liver samples) or spectrofluorometrically (at excitation 515 nm and emission 553 nm for serum samples). TBARS level is expressed as nmol/L of malondialdehyde (MDA) equivalents.
Determination of Total Serum Antioxidant Capacity
The total serum antioxidant capacity is a commonly used parameter in nutritional studies. NAC and other dietary components were shown to increase the total serum antioxidant capacity, while pathological conditions lower it. The ferric reducing ability of plasma (FRAP) assay is a simple and reproducible method to assess the combined antioxidant effects of non-enzymatic defense factors in biological fluids in addition to plasma (Benzie and Strain 1996). At low pH, the ferrictripyridyltriazine (Fe3+-TPTZ) complex can be reduced to the ferrous form (Fe2+-TPTZ) which has an intense blue color. This complex can be monitored at 593 nm and used as the putative index of antioxidant or any other reducing potential in the samples. Aqueous solutions of known Fe2+ concentration were used for calibration and results were expressed as μmol/L Fe2+ equivalents.
Statistics
All data are expressed as means ± SEM, interaction analysis was performed by two-way ANOVA and One-way ANOVA was used within each factor (diet or PCB 126). All analyses were carried out by General Linear Models (GLM) in SAS 9.2, P<0.01) followed by Dunnett's T multiple comparison tests. A P value of <0.05 was considered to be statistically significant. Pearson correlation analysis was used to establish the relationship between various endpoints measured in this study.
Results
Liver Gene Expression Profile of CYP1A1, AhR, and ApoA1
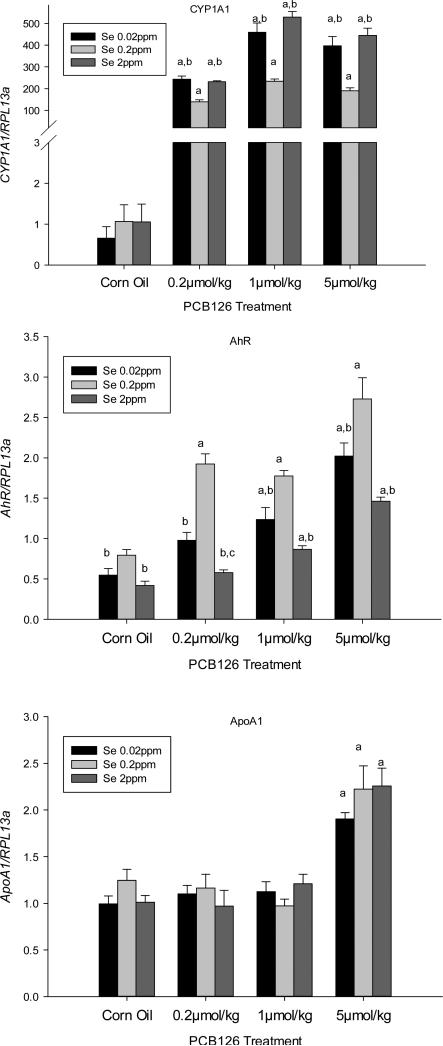
Liver gene expressions of CYP1A1, a classic AhR target gene, and AhR gene itself were significantly increased by all PCB 126 doses in a dose-dependent manner (Figs 1). This effect of PCB 126 was, however, significantly more pronounced in low and supplemented Se diet groups for the CYP1A1 gene while the opposite, significantly less induction in low and supplemental Se diet groups, was found for the AhR gene. Reduced AhR gene expression in the low and high Se diet groups was also seen in the corn oil control animals, but no diet-related difference was seen in the control animals for CYP1A1 gene. ApoA1 expression was significantly increased in all three Se diets by 5 μmol/kg PCB 126 exposure (P<0.05), but no Se-effect was observed.
Figure 1. Effects of PCB 126 and Se on CYP1A1 (top), AhR (middle), and APOA1 (bottom) gene expression in the liver.
mRNA levels were adjusted to the level of the housekeeping gene RPL13a. Results are expressed as mean ± SEM (n=6). Statistical significant (p<0.05) differences between (a) a PCB 126 level and the corn oil treatment, (b) 0.02 or 2 ppm Se and 0.2 ppm Se diet, (c) 0.02ppm and 2ppm Se diet.
Se and PCB 126 Effects on Liver PON1 Activities, Protein Levels, and Gene Expression
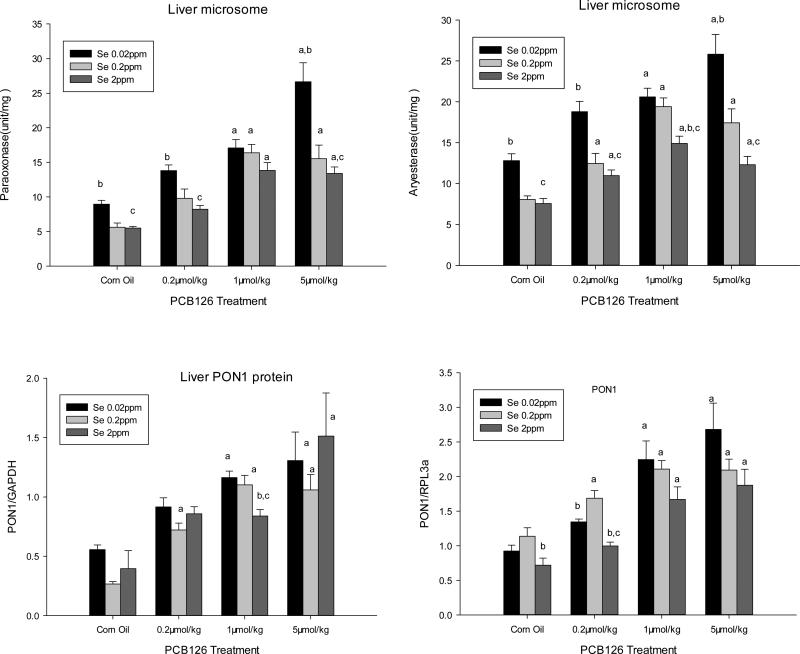
Liver PON1 activities were significantly and dose-dependently increased by PCB 126 treatments to up to 198% and 140% of the corn oil treated controls in all three Se diet groups when assayed with paraoxon and phenylacetate, respectively (Fig 2 top). Compared to the adequate level of Se (0.2 ppm), feed with low (0.02 ppm) Se resulted in increased liver PON1 activities in both, the untreated controls and across all PCB 126 treatments. The supplemented Se (2 ppm) diet group had slightly lower PON1 activity levels than the adequate (0.2 ppm) Se group, but this was statically significant only for the arylesterase activity in the 1 μmol/kg PCB 126 group. Thus dietary Se levels were inversely related to liver PON1 activity and Se reduced the magnitude of the response of PON1 to the PCB 126 exposure in liver.
Figure 2. Effects of PCB 126 and Dietary Se Levels on PON1 Activity (top), Protein Level (bottom left), and Gene Expression (bottom right) in the Liver of Male Rats.
Liver PON1 activity was determined with paraoxon (top left) and phenylacetate (top right) as substrate. Results are expressed as mean ± SEM (n=3-6). Statistical significance (p<0.05) between: (a) each PCB 126 dose vs the corn oil treatment, (b) low (0.02) or supplemented (2 ppm) vs the adequate (0.2ppm) Se diet, and (c) low and supplemented (0.02ppm and 2ppm) Se diets.
Western blot analysis shows significantly higher liver PON1 protein levels with the medium and high doses of PCB 126 across all the Se levels (Fig 2 bottom left, Supplemental Figure 1). Se had no overall significant effect on PON1 protein level with the exception of the medium (1 μmol/kg) PCB 126 treatment group where a significantly lower PON1 protein level was visible in the supplemented (2 ppm) Se diet group compared to the 0.02 ppm and 0.2 ppm Se levels. These findings are consistent with our PON1 gene expression and activity data in this study.
PCB 126 treatments significantly increased PON1 gene expression (Fig 2, bottom right) at all doses in a dose-dependent manner. Supplemental Se (2 ppm) reduced PON1 gene expression which was significant in the control and low PCB 126 treatment group. These two groups also showed lower PON1 mRNA levels with low (0.02 ppm) dietary Se compared to adequate (0.2 ppm) Se, but with medium and high PCB 126 doses the PON1 gene expression was highest in the low Se groups, although not significantly.
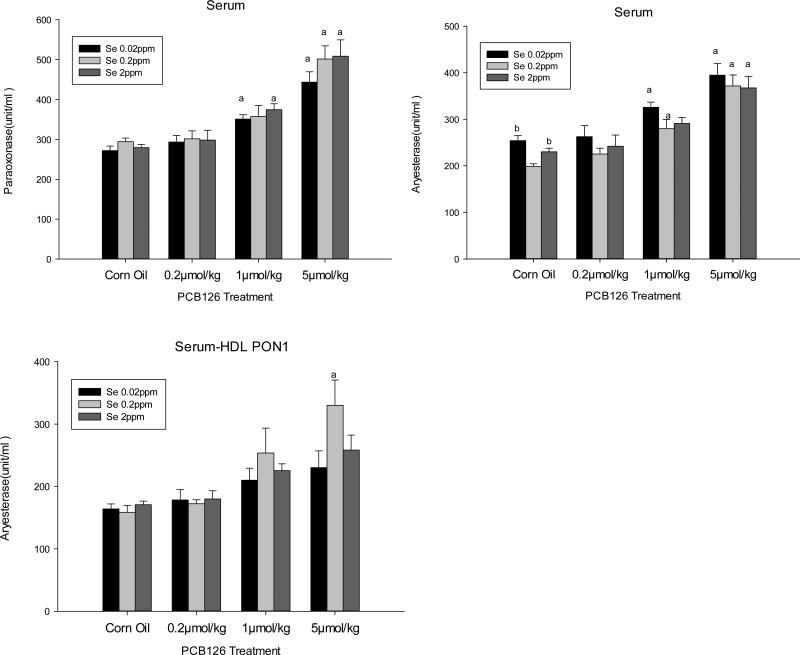
Se and PCB 126 Effects on PON1 Activities in the Serum
Serum PON1 activity with both substrates was significantly increased by medium (1μmol/kg) and high (5μmol/kg) doses of PCB 126. No significant effect of different Se diets was found (Fig 3). It should be noted, however, that serum PON1 activity expressed as per mg protein was about 30-50 times higher than liver PON1 activity.
Figure 3. Effects of PCB 126 and Se on PON1 Activity in Serum.
PON1 activity in serum was determined with paraoxon (top left) and phenylacetate (top right), and in serum-HDL with phenylacetate (bottom left). Results are expressed as mean ± SEM. (n=3-6). Statistically significant differences (p<0.05) between (a) a PCB 126 level and the corn oil treatment, (b) 0.02 or 2 ppm Se and 0.2ppm Se diet, (c) 0.02ppm and 2ppm Se diet.
About 72% of serum PON1 activity was associated with HDL. PCB 126 produced the same trend of increased PON1 activity in serum HDL-associated PON1 activity compared to total serum PON1 activity, but significant PON1 increase was detected only in the 5 μmol/kg PCB 126 and 0.2 ppm Se treatment group.
Serum Lipid Profile Changes due to Se diet and/or PCB 126 exposure
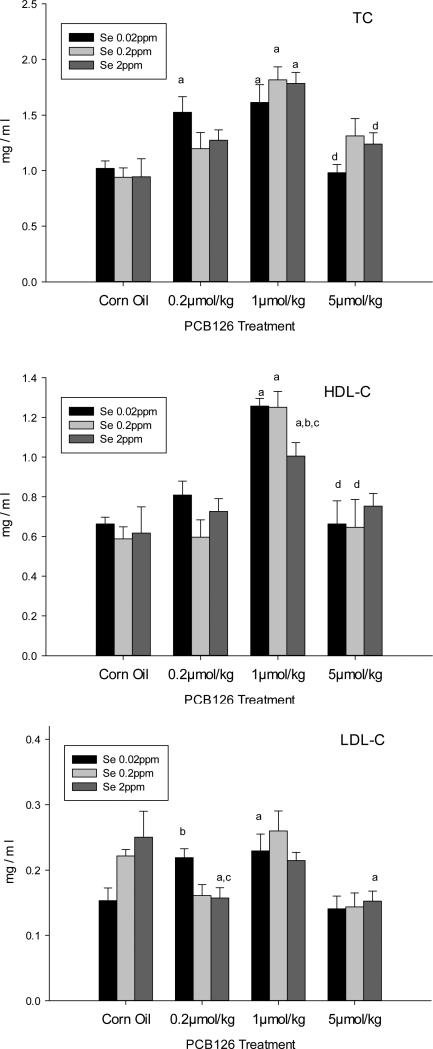
Serum TC and HDL-C were significantly increased by treatment with 1 μmol/kg PCB 126 (Figs 4). A significant drop back to the levels of corn oil treated rats was found at the 5 μmol/kg PCB 126 in TC and HDL-C compared to the 1 μmol/kg. These effects were seen in all three Se diet groups, but the increase in HDL-C caused by 1 μmol/kg PCB 126 was significantly smaller with the supplemented (2 ppm) Se diet.
Figure 4. Serum Lipid Profile in animals treated with PCB 126 and different Se diets.
TC (top), HDL-C (middle) and LDL-C (bottom) results are expressed as mean ± SEM (n=6). One-way ANOVA followed by Dunnett's test found statistical differences (0.05) between (a) a PCB 126 level and the corn oil treatment, (b) 0.02 or 2 ppm Se and 0.2 ppm Se diet, (c) 0.02 ppm and 2 ppm Se diet, (d) 1 and 5 μmol/kg PCB 126.
PCB 126 had an inconsistent effect on LDL-C (Fig 4 bottom). No changes were seen in the adequate (0.2 ppm) Se diet group. With low (0.02 ppm) Se in the diet, the untreated animals seemed to have lower LDL-C, although this trend was not significant, while the LDH-C level was higher with 0.2 and 1 μmol/kg PCB 126 treatment which was significant compared to the PCB 126-treated control and to the corn oil control, respectively. In the supplemental (2 ppm) Se diet group LDL-C was lower in the 1 and 5 μmol/kg PCB 126 groups compared to the untreated control
PCB 126 had no effect on the HDL-C/TC ratio, while the HDL-C/LDL-C ratio was significantly increased by all doses of PCB 126 (Supplemental Table 3). Se had no major effect on these ratios.
Serum Antioxidant Capacity and LDL Associated Lipid Hydroperoxides
As shown in Table 1, serum antioxidant capacity was increased in response to all three doses of PCB 126 at all Se levels with significance (P<0.05) detected with the adequate 0.2 ppm Se diet.
Table 1.
Serum antioxidant capacity and LDL-hydroperoxide levels in rats on different Se diets treated with 1 ip injection of PCB 126
| PCB 126 Treatment | Serum antioxidant capacity (μmol /L) |
LDL-hydroperoxide (nmol/mg protein) |
||||
|---|---|---|---|---|---|---|
| Low (0.02 ppm Se) | Adequate (0.2 ppm Se) | Supplemental (2 ppm Se) | Low (0.02 ppm Se) | Adequate (0.2 ppm Se) | Supplemental (2 ppm Se) | |
| Corn Oil | 633 ± 66 | 598 ± 28 | 765 ± 79 | 159 ± 5.8 | 179 ± 12 | 139 ± 6.0b |
| 0.2 μmol/kg | 757 ± 71 | 847 ± 37a | 781 ± 52 | 178 ± 9.3b | 147 ± 7.2 | 143 ± 7.4c |
| 1 μmol/kg | 701 ± 32 | 761 ± 45 | 904 ± 40b,c | 168 ± 10 | 148 ± 3.5 | 132 ± 6.8c |
| 5 μmol/kg | 742 ± 61 | 848 ± 55a | 871 ± 49 | 195 ± 11a | 179 ± 15 | 144 ± 6.2c |
Results are expressed as mean ± SEM. Each group contained 6 animals. Indicated significant differences (p<0.05) are between:
a PCB 126 dose and the corn oil treatment
0.02 or 2 ppm Se compared to 0.2 ppm Se
0.02 ppm and 2 ppm Se.
PCB 126 had no significant impact on the LDL-lipid hydroperoxides except for the high dose exposure (5μmol/kg) with low Se level (0.02 ppm), but Se overall significantly decreased LDL-associated lipid hydroperoxide levels in a Se-concentration-dependent manner (P<0.05).
Liver and Serum Lipid Peroxidation Analyses (TBARS) in the Se study groups
The overall liver and serum lipid peroxidation levels (TBARS level) are shown in Table 2. PCB 126 increased liver TBARS level statistical significantly only with the high dose (5μmol/kg) in the low (0.02 ppm) Se diet group. Different dietary Se levels did not change liver TBARS in any treatment group. Serum TBARSs levels on the other hand were significantly (P<0.05) affected by Se. Animals on the adequate (0.2 ppm) Se diet had significantly higher TBARS values than those in the low (0.02 ppm) and supplemental (2 ppm) Se diet groups. In addition, a significant increase of serum TBARS occurred in the groups exposed by 1 and 5 μmol/kg PCB 126 in the adequate (0.2 ppm) Se diet group.
Table 2.
Liver and Serum TBARS levels 2 weeks after 1 ip injection of PCB 126 in animals on different Se diets
| PCB 126 Treatment | Liver TBARS (nmol/100mg protein) |
Serum TBARS (nmol/L) |
||||
|---|---|---|---|---|---|---|
| Low (0.02 ppm Se) | Adequate (0.2 ppm Se) | Supplemental (2 ppm Se) | Low (0.02 ppm Se) | Adequate (0.2 ppm Se) | Supplemental (2 ppm Se) | |
| Corn Oil | 405 ± 18 | 403 ± 12 | 361 ± 14 | 1.79± 0.11b | 3.30 ± 0.23 | 2.47 ± 0.24b |
| 0.2 μmol/kg | 390 ± 14 | 374 ± 16 | 420 ± 25 | 1.39 ± 0.18b | 3.47 ± 0.20 | 2.06 ± 0.23b |
| 1 μmol/kg | 426 ± 10 | 415 ± 30 | 367 ± 12 | 1.98 ± 0.20b | 4.18 ± 0.28a | 3.03 ± 0.43b |
| 5 μmol/kg | 470 ± 16a | 413 ± 24 | 399 ± 22 | 2.04 ± 0.16b | 4.32 ± 0.19a | 2.84 ± 0.43b |
Results are expressed as mean ± SEM. Each group contained 6 animals. Indicated significant differences (p<0.05) are between:
a PCB 126 dose and the corn oil treatment
0.02 or 2 ppm Se compared to 0.2 ppm Se.
Effects of NAC on Liver and Serum PON1 Activities in Untreated and PCB 126-Exposed Rats
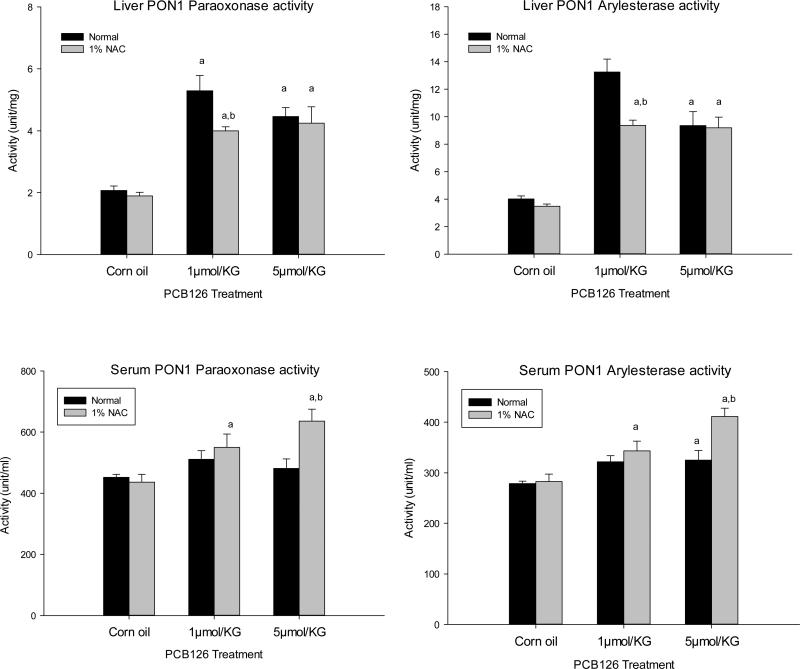
As shown in Fig. 6 top, both doses of PCB 126 significantly increased liver PON1 activities measured with both substrates by up to 5.1- and 4.8-fold in normal and NAC diet groups, respectively. Serum PON1 activities (Fig. 6 bottom) were also significantly increased in both dietary groups with increases of up to 46% compared to the untreated corn oil control. Interestingly, NAC reduced the magnitude of induction of liver PON1 activity at 1 μmol/kg of PCB 126. In contrast, NAC caused significant increases (P<0.05) in serum PON 1 activity compared to the normal diet group at the 5 μmol/kg PCB 126 dose. No effect of NAC on either liver or serum PON1 activity was found in control groups, indicating a potential interaction between PCB 126 and NAC treatments (Table 4).
Figure 6. Effects of PCB 126 and NAC on liver (above) and serum (below) PON1 activity.
Animals on a normal or NAC supplemented diet received 1 ip injection of PCB 126. PON 1 activity was determined 2 weeks after injection using paraoxonase (left) or phenylacetate (right) as substrate. Results are expressed as mean ± SEM (n=6). One-way ANOVA followed by Dunnett's test was used to examine the difference between each PCB 126 level and the corn oil treatment (significance indicated by ‘a’ p<0.05) and between the normal and NAC diet (significance indicated by ‘b’, p<0.05).
Table 4.
Two –Way ANOVA Analysis of the Effects of PCB 126 and Diets with or without NAC Supplementation
| PCB126 |
NAC |
Interaction Effect |
Model Overall |
||
|---|---|---|---|---|---|
| P | P | P | R2 | P | |
| PON1-LPX | <0.0001 | 0.05 | 0.14 | 0.84 | <0.0001 |
| PON1-LPH | <0.0001 | 0.01 | 0.03 | 0.80 | <0.0001 |
| PON1-SPX | 0.003 | 0.02 | 0.03 | 0.48 | 0.001 |
| PON1-SPH | <0.0001 | 0.005 | 0.02 | 0.62 | <0.0001 |
| L-TBARS | <0.0001 | 0.01 | 0.36 | 0.53 | 0.0001 |
| S-TBARS | <0.0001 | 0.13 | 0.06 | 0.52 | 0.003 |
Abbreviations: PON1-LPX (PON1 in liver with paraoxon), PON1-LPH (PON1 in liver with phenylacetate), PON1-SPX (PON1 in serum with paraoxon), PON1-SPH (PON1 in serum with phenylacetate), L-TBARS (liver TBARS level), S-TBARS (serum TBARS level).
Liver and Serum Lipid Peroxidation in the NAC study
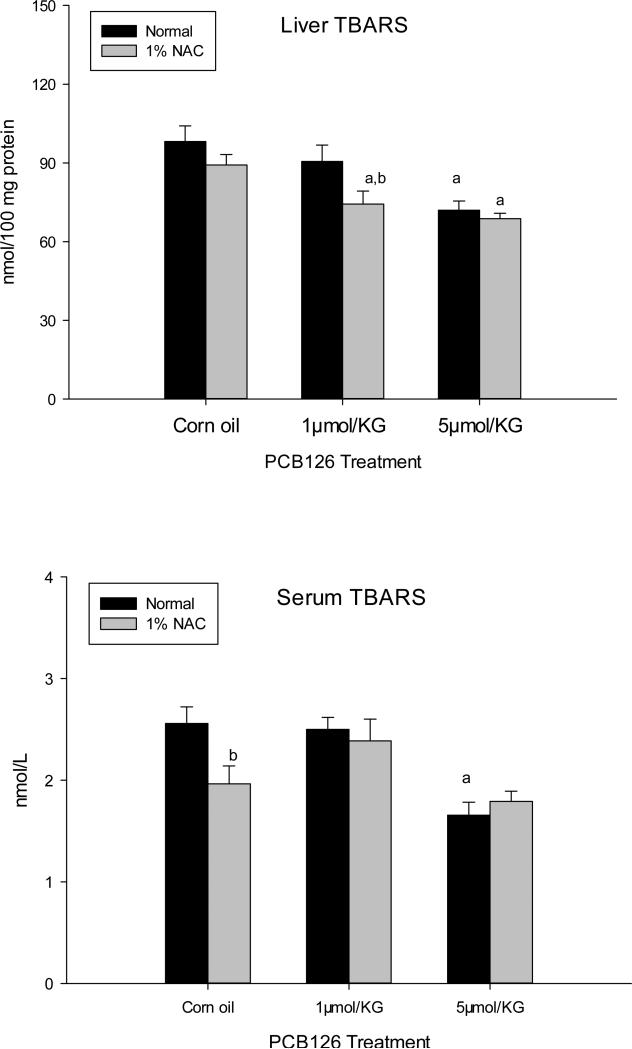
Liver TBARS levels (Fig 7A) were significantly lower in the 5 μmol/kg dose of PCB 126 in both NAC and normal diet groups compared to controls. Lower levels of TBARS were also noticed with 1 μmol/kg PCB 126 but significance (P<0.05) was only observed in NAC diet group. Serum TBARS were decreased in 5 μmol/kg PCB 126 treated animals, but significantly (P<0.05) only in the normal diet group (Fig 7B) Nevertheless, NAC had a noticeable influence on both the liver and serum TBARS, causing a significant reductions in liver TBARS in 1 μmol/kg PCB 126 treated group and in serum TBARS in the control group.
Figure 7. Liver and serum TBARS level in the NAC study.
TBARS levels in liver homogenate (above) and serum (below) of animals on a normal or NAC supplemented diet 2 weeks after ip injection of corn oil or PCB 126. Results are expressed as mean ± SEM (n=6). One-way ANOVA followed by Dunnett's test was used to examine the difference between each PCB 126 level and the corn oil treatment (significance indicated by ‘a’ p<0.05) and between the normal and NAC diet (significance indicated by ‘b’, p<0.05).
Direct Effect of NAC on Serum PON1 Activity
In order to investigate whether NAC as a reducing agent has a direct effect on the serum PON1 enzyme, NAC was added to serum from untreated rats and PON1 activities were determined. No effect was observed with 10 and 20 mM NAC and only a non-significant reduction was seen with 40 mM NAC after up to 30 min of incubation at 37°C compared to the untreated serum (Supplemental Figure 2).
Discussion
Polychlorinated biphenyls (PCBs), particularly dioxin-like congeners like PCB 126, can cause increased production of reactive oxygen species (ROS) leading to a condition of oxidative stress (Jin et al. 2001; Rodriguez-Ariza et al. 2003) and increased lipid peroxidation (Borlakoglu et al. 1990; Fadhel et al. 2002) which are major risk factors for cancer and atherosclerosis. Recently we reported that the gene expression in rat livers and hepatic and serum activities of PON1, an antioxidant and anti-atherosclerotic enzyme, were significantly increased by PCB 126 in a dose- and time-dependent pattern (Shen et al. 2012). Nevertheless, oxidative stress in the form of increased oxidated GSH levels was visible as was the reduction of a second antioxidant factor, hepatic Se (Lai et al. 2010). Se was reported to reduce lipid peroxidation (Dillard et al. 1978; Hafeman and Hoekstra 1977; Kutluhan et al. 2009) and hepatocellular damage (Bulucu et al. 2009; Chen et al. 1993). A reduction of Se and GSH indicate a weakening of the antioxidant defense concurrently with an enhanced defense due to increased PON1 activities. We were therefore interested in the interaction among Se, GSH, PON1 and PCB 126 in oxidative stress production and prevention. Such knowledge may allow the development of dietary intervention against the toxic effects of dioxin-like compounds, like PCB 126.
We used diets with varying amounts of Se or supplemented with NAC, a precursor in the glutathione synthesis pathway and sulfhydryl-group containing radical scavenger which is already used in antioxidant therapy (Dodd et al. 2008) and analyzed parameters of oxidative stress in animals without and with exposure to PCB 126. We already reported that the low Se diet strongly reduced liver Se, SeGPx and thioredoxin reductase activity while diets with supplemental Se increased these factors to or above the level of the adequate Se diet (Lai et al. 2011). NAC reduced the severity of PCB 126-induced fatty liver (Lai et al. 2012). These were first indications that both, dietary Se and NAC provide some antioxidant protection to PCB 126-exposed rats.
Table 3 shows the two-way ANOVA analysis for the overall effects of Se, PCB 126 and their potential interaction on various responses in this study. PCB 126 significantly affected most endpoints except liver TBARS and the HDL/TC ratio, confirming the expected changes in lipid profile, increased in gene transcription of CYP1A1, PON1 and others, and increased oxidative stress by this dioxin-like PCB congener. Not expected was the significant effect of dietary Se on the gene expression of CYP1A1, AhR and PON1 and an interaction effect between Se and PCB126 for gene expression of CYP1A1 and AhR. An overall significant effect of Se and interactive effect of Se plus PCB 126 was also apparent for liver PON1 activities but not for serum PON1 activities. We also observed a significant interaction effect of Se and PCB 126 for LDL-C, known as ‘bad cholesterol’. LDL-C is closely related to lipid peroxidation, arthrosclerosis and cardiovascular diseases (Shao and Heinecke 2009). In addition, Se influenced serum and liver TBARS, liver LDL-peroxides and serum total antioxidant capacity, confirming the expected effect of Se on oxidative stress levels (Table 3). These overall effects will be discussed below in detail.
Table 3.
Two-Way ANOVA Analysis of the Effects of PCB 126 and Different Selenium Diets
| PCB126 |
Se |
PCB126xSe |
Model Overall |
||
|---|---|---|---|---|---|
| P | P | P | R2 | P | |
| PON1 | <0.0001 | 0.0004 | 0.2112 | 0.6752 | <0.0001 |
| CYP1A1 | <0.0001 | <0.0001 | <0.0001 | 0.9270 | <0.0001 |
| AhR | <0.0001 | <0.0001 | 0.0030 | 0.8707 | <0.0001 |
| ApoA1 | <0.0001 | 0.4292 | 0.2934 | 0.7087 | <0.0001 |
| PON1-LPX | <0.0001 | <0.0001 | 0.0024 | 0.7752 | <0.0001 |
| PON1-LPH | <0.0001 | <0.0001 | 0.0151 | 0.7665 | <0.0001 |
| PON1-SPX | <0.0001 | 0.1945 | 0.8227 | 0.7152 | <0.0001 |
| PON1-SPH | <0.0001 | 0.0171 | 0.9726 | 0.6891 | <0.0001 |
| PON1-LPRO | <0.0001 | 0.1830 | 0.3712 | 0.7437 | <0.0001 |
| L-TBARS | 0.0822 | 0.0310 | 0.0705 | 0.3087 | 0.0139 |
| S-TBARS | 0.0006 | <0.0001 | 0.7558 | 0.7147 | <0.0001 |
| TC | <0.0001 | 0.8196 | 0.1380 | 0.5729 | <0.0001 |
| HDL-C | <0.0001 | 0.4346 | 0.2381 | 0.6048 | <0.0001 |
| LDL-C | <0.0001 | 0.8058 | 0.0099 | 0.4628 | <0.0001 |
| HDL-C/TC | 0.3339 | 0.2144 | 0.6347 | 0.1637 | 0.4609 |
| HDL-C/LDL-C | 0.0020 | 0.3692 | 0.1831 | 0.3329 | 0.0111 |
| SANTIOX | 0.0062 | 0.0086 | 0.3260 | 0.3493 | 0.0050 |
| LDL-FOX | 0.0205 | <0.0001 | 0.1045 | 0.4791 | <0.0001 |
| HDL-PON1 | <0.0001 | 0.1757 | 0.3575 | 0.5755 | 0.0006 |
Abbreviation: PON1-LPX (PON1 in liver with paraoxon), PON1-LPH (liver PON1 in liver with phenylacetate), PON1-SPX (PON1 in serum with paraoxon), PON1-SPH (PON1 in serum with phenylacetate), PON1-LPRO (PON1 liver protein level), HDLPON1 (serum HDL-PON1 with phenylacetate), SANTIOX (serum antioxidant capacity), LDL-FOX (LDL hydroperoxide).
PCB 126 dose dependently increased in PON1 mRNA (Fig 2) and no overall effect of Se on PON1 gene expression was observed, but high Se significantly lowered the baseline and PCB 126 induced PON1 levels while low Se lowered PON1 expression in controls and low PCB 126 exposure. Genes reportedly up regulated by selenium supplementation include TNF, IL1B, IL8, SOD2, CXCL2 and several other immunological and oxidative stress-related genes (Kibriya et al. 2007) while Se deficiency has been associated with down regulation of antioxidant defense genes (Fischer et al. 2002). At the gene expression level, PON1 seems to display an inverted U shaped response during normal or low stress situation, but an inverse correlation to Se levels during high stress situation. Protein and activity levels, however, display a strictly inverse relationship between PON1 and Se, as discussed further below.
Interesting is the significant individual and interactive effects of Se on AhR gene expression, resulting in lower AhR transcription with the low and high Se diets compared to adequate diet in all PCB 126 and the control groups. More AhR transcription in the adequate Se group did not result in more CYP1A1 transcription: the opposite occurred: CYP1A1 displayed a U-shaped response with all samples from PCB 126 exposed animals in the low and high Se groups displaying increased CYP1A1 transcription compared to the adequate Se groups. The AhR transcription pattern resembled more the PON1 than the CYP1A1 transcription pattern (Fig 1,2). The mechanism of these Se effects are not clear, but inadequate dietary Se levels reportedly may have caused negative effects in therapeutic settings (Rayman 2012). These (inverted) U-shaped dose-response curves with Se with or without PCB 126 exposure need to be understood before any therapeutic application of Se diets.
PON1 binds to Apo A1 in HDL-C and Apo A1 has some antioxidant capacity (Hine et al. 2012). Some microarray studies reported a decrease in liver ApoA1 gene expression after treatments with PCB 126 or TCDD, but those data were not verified by RT-PCR (Boverhof et al. 2005; Kopec et al. 2008). Our observation of increased Apo A1 transcription with the high dose of PCB 126 may be a consequence of the visible liver toxicity in our animals and seems unrelated to PON1 and oxidative protection.
Despite less transcription of PON1 in low dietary Se controls/low PCB 126 exposure, liver protein data presented a non-significant opposite effect, possibly due to the low sensitivity of Western blots. However, liver PON1 activity was significantly higher in these animals compared to the adequate Se groups, supporting the protein data. A strong Se and Se x PCB 126 interactive effect was confirmed by ANOVA analysis. We could hypothesize that the mechanism may be, at least in part, through protein stabilization in low Se conditions, which could result in PON1 compensating in part for the loss of protection in low Se situations.
PCB 126 also dose-dependently increased the PON1 activity in the serum, but significantly only at the higher doses of PCB 126, most likely a consequence of the increased production of PON1 in the liver and/or liver toxicity at the high PCB 126 doses. A significant Se effect was only seen for arylesterase activity in the controls on low/high Se diets. This difference between paraoxonase and arylesterase activity raises the question whether another, minor enzyme may contribute to the total arylesterase activity in serum compared to the paraoxonase activity.
PON1 binds to HDL-C and removes peroxides from LDL-C in serum. Thus a change in the lipid profile may have an influence on serum PON1 level and activity. Dioxin-like compounds are known to elevate serum total cholesterol and HDL cholesterol (Carter 1985; Quazi et al. 1983), which may be due to the increment in liver biosynthesis of cholesterol (Horio et al. 1987). We observed a similar increase in serum TC and HDL-C with the median dose of PCB 126. The high dose (5 μmol/kg) produced dramatically lower elevations of these cholesterols, probably as a result of acute liver toxicity. The marginal effect of PCB 126 on LDL-C, consistent with other reports (Lind et al. 2004), resulted in an increase in the HDL-C/LDL-C ratio which should be protective against cardiovascular disease if not disturbed otherwise, for example by oxidative stress. Se had no consistent effect on serum lipids, which is in agreement (Chen et al. 1986; Crespo et al. 1995; Panczenko-Kresowska and Ziemlanski 1987) or disagreement (Bleys et al. 2008; Kaur and Bansal 2009; Qu et al. 2000; Stranges et al. 2010) with other reports. Differences in Se concentration and duration of feeding may be accountable for these discrepancies, since only a low Se level may increase the ‘good cholesterol’, while too much Se will elevate TC, LDL-C, and HDL-C (Laclaustra et al. 2009b), and increase the risk of cardiovascular diseases (Laclaustra et al. 2009a; Stranges et al. 2010). The interaction effect of PCB 126 with Se on LDL-C (Table 3) might hint towards a slight protective effect of Se during low dose exposure to xenobiotics, but this may be offset by an increased LDL-C level during no-exposure periods (Fig 4). Overall, effects of Se on lipid profiles seem to be delicate, possibly biphasic, and an unlikely parameter for PON1 activity modulations.
The ultimate goal of these dietary studies is to develop interventions of chemoprevention through reduction of oxidative stress, which we determined in our samples with three methods: TBARS indicate total lipid peroxidation, serum LDL-hydroperoxide the specific oxidation of LDL-C lipids, and total antioxidant capacity the remaining non-enzymatic reducing potential in serum. Comparing the control groups for all three parameters reveals that the supplemented and low Se groups have lower liver (supplemented only) and serum TBARS, serum LDL-hydroperoxide levels, and higher serum antioxidant capacity. Apparently high Se provides antioxidant protection. The slightly higher PON1 levels in the low Se groups possibly were sufficient to compensate for the lower Se levels.
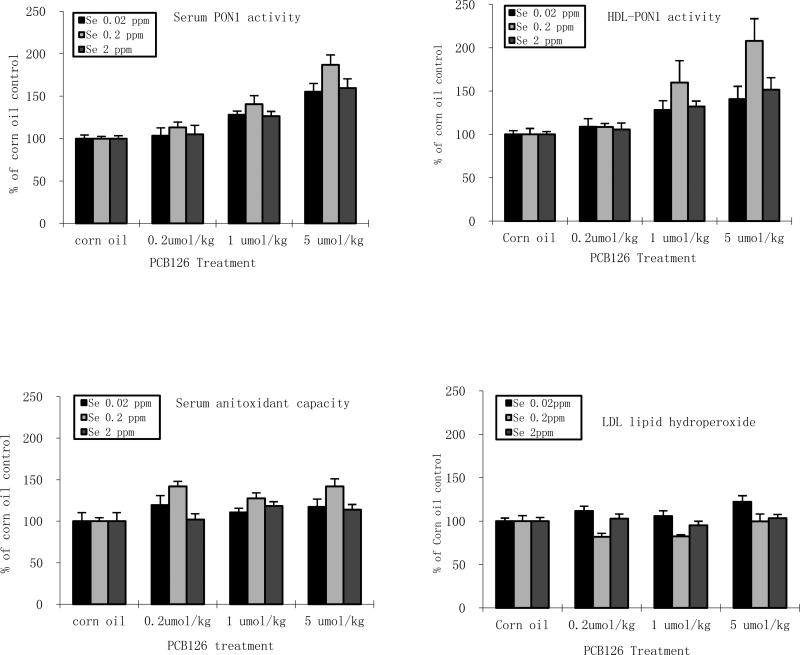
PCB 126 only consistently increased TBARS in serum and only in the adequate Se groups. The serum antioxidant capacity was increased with PCB 126, opposite to what was expected, and cannot be explained at this time. In the PCB 126 samples, supplemented and low Se significantly decreased serum TBARS, supporting the hypothesis that high Se and compensation of low Se by increased PON1 are efficient antioxidant. To analyze possible cinteractions between antioxidants and PCB 126 we normalized the data (Figs 5). These figures suggest that the strongest induction of PON1 activity by PCB 126 but also the best reduction in oxidative stress occur with adequate dietary Se. The reduced oxidative stress may be attributed to increased PON1 activities, but a definite cause-effect correlation can only be made by employing PON1 knock-out animals, which is beyond the scope of this study. More importantly, supplemented or low dietary Se levels reduced the baseline oxidative stress level to an extend that even during increased ROS production by xenobiotic the oxidative damage remained often below the level in control rats with adequate Se diet.
Figure 5. Effects of PCB 126 and Se on normalized level of serum endpoints.
Serum PON1 activity (top left), serum HDL PON1 activity (top right), serum antioxidant capacity level (bottom left), serum LDL hydroperoxide (bottom right). Results are expressed as % of corresponding corn oil control value.
For the NAC study, the two-way ANOVA analysis indicates a significant effect of dietary NAC on PON1 activity in liver and serum and also an interaction effect between PCB 126 and NAC (Table 4). Closer analysis reveals that NAC did not affect basal PON1 activities, but modified the PCB 126 effect, i.e. lessened the PCB 126-induced increase in PON1 activity in the liver (1 μmol/kg dose only) while boosting the increase in serum (both doses). Possible mechanisms for these effects are i. increased excretion of PON1 from liver into blood, ii. a direct interaction of NAC with sulfhydryl groups in PON1, or iii. an indirect effect due to NAC's ROS scavenging and GSH supplementing activity. We have no data to support or disprove the first mechanism, the enhanced export from the liver. Comparing the dose-responses in liver and serum, this mechanism appears unlikely. Our in vitro study confirmed that NAC alone has no detectable direct effects on the basal level of serum PON1 activities (Supplemental Fig 1), suggesting that the second mechanism is unlikely. PON1's anti-atherogenic activity is attributed to its ability to break down oxidized lipids of serum lipoproteins (Aviram et al. 2000; Aviram 2006). This is, however, accompanied by a partial inactivation of the enzyme through an interaction of oxidized lipids with the enzyme's free sulfhydryl group (Aviram et al. 1999). PCB 126 is known to increase oxidative stress and lipid peroxidation. NAC may have reduced lipid peroxidation in serum by scavenging radicals or replenishing GSH, thereby preventing PON1 inactivation in the PCB 126 treatment groups. Since NAC did not increase total liver GSH, but reduced the level of oxidized GSH in PCB 126 exposed and control animals (Lai et al. 2012), and serum TBARS were significantly lower in the NAC controls compared to normal controls. Similar protective effects of NAC were reported in other animal and cell culture studies (Grosicka-Maciag et al. 2011; Sciuto et al. 1995; Vendemiale et al. 2001). Quercetin, another dietary antioxidant supplement, also preserved PON1 activity, which was attributed to either prevention of GSH depletion (Varatharajalu et al. 2009) or induction of PON1 gene expression (Aviram 2006). For NAC, however, its radical scavenging activity may have resulted in lower lipid peroxides and thereby less PON1 suicide inactivation and higher PON1 activities in serum of PCB 126 exposed rats.
Conclusions
Our results demonstrate that dietary antioxidants like Se and NAC have protective effects against oxidative stress, but are no easy quick-fix solution. PCB 126 was still able to increase oxidative stress, probably by inducing CYP enzymes, depleting liver selenium and reducing activities of Se-dependent anti-oxidant enzymes such as thioredoxin reductase and SeGPx (Lai et al. 2011). Thus avoidance of exposure to dioxin-like compounds needs to be the first line of public health protection. Supplemental dietary Se reduced baseline and induced oxidative stress levels in serum, but caution should be used in choosing a dietary Se level, since very high doses are known to have negative effects. The effect of Se on AhR and CYP 1A1 transcription should be further explored to elucidate the underlying mechanisms. The significant interaction effects between dietary Se and PON1 suggest complementation which needs to be understood to avoid unexpected negative consequences of dietary interventions. The Se effect on PON1 and ultimately on oxidative stress was probably primarily mediated through gene induction in the liver, but the gene regulation pathway Se unknown and other mechanisms, like PON1 protein stabilizing, cannot be excluded. NAC on the other hand probably worked by scavenging radicals, thereby preventing PON1 inactivation in serum. Overall, increased PON1 activities, by whatever mechanism, may have a protective effect, counteracting PCB 126-induced oxidative stress in the form of lipid peroxidation thereby reducing the risk of atherosclerosis, cancer and other diseases. Further studies should focus on the mechanisms of Se induced gene regulation and PON1 regulation, the interaction between different endogenous and exogenous antioxidants, and their combined effects in prevention of oxidative damage by various contaminants.
Supplementary Material
Acknowledgements
The authors would like to express their appreciation to all members of the Ludewig and Robertson labs for help with the animal experiments, and we would like to particularly acknowledge Susanne Flor for managing the Ludewig and Robertson labs. The study and HS, BW, ML, and IL were funded by project 1 and the Training Core of the Iowa Superfund Research Program ES 013661. This work is part of the doctoral thesis of HS.
References
- Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of n-acetylcysteine: Its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989;6:593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- Aviram M, Rosenblat M, Billecke S, Erogul J, Sorenson R, Bisgaier CL, et al. Human serum paraoxonase (pon 1) is inactivated by oxidized low density lipoprotein and preserved by antioxidants. Free Radic Biol Med. 1999;26:892–904. doi: 10.1016/s0891-5849(98)00272-x. [DOI] [PubMed] [Google Scholar]
- Aviram M, Hardak E, Vaya J, Mahmood S, Milo S, Hoffman A, et al. Human serum paraoxonases (pon1) q and r selectively decrease lipid peroxides in human coronary and carotid atherosclerotic lesions: Pon1 esterase and peroxidase-like activities. Circulation. 2000;101:2510–2517. doi: 10.1161/01.cir.101.21.2510. [DOI] [PubMed] [Google Scholar]
- Aviram M. Hdl--associated paraoxonase 1 (pon1) and dietary antioxidants attenuate lipoprotein oxidation, macrophage foam cells formation and atherosclerosis development. Pathophysiol Haemost Thromb. 2006;35:146–151. doi: 10.1159/000093558. [DOI] [PubMed] [Google Scholar]
- Bandiera S, Safe S, Okey AB. Binding of polychlorinated biphenyls classified as either phenobarbitone-, 3-methylcholanthrene- or mixed-type inducers to cytosolic ah receptor. Chem Biol Interact. 1982;39:259–277. doi: 10.1016/0009-2797(82)90045-x. [DOI] [PubMed] [Google Scholar]
- Banerjee D, Madhusoodanan UK, Sharanabasappa M, Ghosh S, Jacob J. Measurement of plasma hydroperoxide concentration by fox-1 assay in conjunction with triphenylphosphine. Clin Chim Acta. 2003;337:147–152. doi: 10.1016/j.cccn.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Baumgardner JN, Shankar K, Hennings L, Albano E, Badger TM, Ronis MJ. N-acetylcysteine attenuates progression of liver pathology in a rat model of nonalcoholic steatohepatitis. J Nutr. 2008;138:1872–1879. doi: 10.1093/jn/138.10.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltowski J, Jamroz-Wisniewska A, Borkowska E, Wojcicka G. Differential effect of antioxidant treatment on plasma and tissue paraoxonase activity in hyperleptinemic rats. Pharmacol Res. 2005;51:523–532. doi: 10.1016/j.phrs.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (frap) as a measure of “antioxidant power”: The frap assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bleys J, Navas-Acien A, Stranges S, Menke A, Miller ER, 3rd, Guallar E. Serum selenium and serum lipids in us adults. Am J Clin Nutr. 2008;88:416–423. doi: 10.1093/ajcn/88.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesch-Saadatmandi C, Pospissil RT, Graeser AC, Canali R, Boomgaarden I, Doering F, et al. Effect of quercetin on paraoxonase 2 levels in raw264.7 macrophages and in human monocytes--role of quercetin metabolism. Int J Mol Sci. 2009;10:4168–4177. doi: 10.3390/ijms10094168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlakoglu JT, Welch VA, Edwards-Webb JD, Dils RR. Transport and cellular uptake of polychlorinated biphenyls (pcbs)--ii. Changes in vivo in plasma lipoproteins and proteins of pigeons in response to pcbs, and a proposed model for the transport and cellular uptake of pcbs. Biochem Pharmacol. 1990;40:273–281. doi: 10.1016/0006-2952(90)90688-h. [DOI] [PubMed] [Google Scholar]
- Boverhof DR, Burgoon LD, Tashiro C, Chittim B, Harkema JR, Jump DB, et al. Temporal and dose-dependent hepatic gene expression patterns in mice provide new insights into tcdd-mediated hepatotoxicity. Toxicol Sci. 2005;85:1048–1063. doi: 10.1093/toxsci/kfi162. [DOI] [PubMed] [Google Scholar]
- Brigelius-Flohe R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta. 2012 doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- Bulucu F, Ocal R, Karadurmus N, Sahin M, Kenar L, Aydin A, et al. Effects of n-acetylcysteine, deferoxamine and selenium on doxorubicin-induced hepatotoxicity. Biol Trace Elem Res. 2009 doi: 10.1007/s12011-009-8377-y. [DOI] [PubMed] [Google Scholar]
- Carter JW. Effects of dietary pcbs (aroclor 1254) on serum levels of lipoprotein cholesterol in fischer rats. Bull Environ Contam Toxicol. 1985;34:427–431. doi: 10.1007/BF01609756. [DOI] [PubMed] [Google Scholar]
- Chen H, Pellett LJ, Andersen HJ, Tappel AL. Protection by vitamin e, selenium, and beta-carotene against oxidative damage in rat liver slices and homogenate. Free Radic Biol Med. 1993;14:473–482. doi: 10.1016/0891-5849(93)90104-3. [DOI] [PubMed] [Google Scholar]
- Chen XS, Xue A, Morris VC, Ferrans VJ, Herman EH, el-Hage A, et al. Effect of selenium deficiency on the chronic toxicity of adriamycin in rats. J Nutr. 1986;116:2453–2465. doi: 10.1093/jn/116.12.2453. [DOI] [PubMed] [Google Scholar]
- Cogliano VJ, Baan R, Straif K, Grosse Y, Lauby-Secretan B, El Ghissassi F, et al. Preventable exposures associated with human cancers. J Natl Cancer Inst. 2011;103:1827–1839. doi: 10.1093/jnci/djr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo AM, Lanca MJ, Vasconcelos S, Andrade V, Rodrigues H, Santos MC. Effect of selenium supplementation on some blood biochemical parameters in male rats. Biol Trace Elem Res. 1995;47:343–347. doi: 10.1007/BF02790136. [DOI] [PubMed] [Google Scholar]
- Darvesh AS, Bishayee A. Selenium in the prevention and treatment of hepatocellular carcinoma. Anti-cancer agents in medicinal chemistry. 2010;10:338–345. doi: 10.2174/187152010791162252. [DOI] [PubMed] [Google Scholar]
- De Vries N, De Flora S. N-acetyl-l-cysteine. J Cell Biochem Suppl. 1993;17F:270–277. doi: 10.1002/jcb.240531040. [DOI] [PubMed] [Google Scholar]
- DeLong JM, Prange RK, Hodges DM, Forney CF, Bishop MC, Quilliam M. Using a modified ferrous oxidation-xylenol orange (fox) assay for detection of lipid hydroperoxides in plant tissue. J Agric Food Chem. 2002;50:248–254. doi: 10.1021/jf0106695. [DOI] [PubMed] [Google Scholar]
- Dillard CJ, Litov RE, Tappel AL. Effects of dietary vitamin e, selenium, and polyunsaturated fats on in vivo lipid peroxidation in the rat as measured by pentane production. Lipids. 1978;13:396–402. doi: 10.1007/BF02533708. [DOI] [PubMed] [Google Scholar]
- Dodd S, Dean O, Copolov DL, Malhi GS, Berk M. N-acetylcysteine for antioxidant therapy: Pharmacology and clinical utility. Expert Opin Biol Ther. 2008;8:1955–1962. doi: 10.1517/14728220802517901. [DOI] [PubMed] [Google Scholar]
- Fadhel Z, Lu Z, Robertson LW, Glauert HP. Effect of 3,3′,4,4′-tetrachlorobiphenyl and 2,2′,4,4′,5,5′-hexachlorobiphenyl on the induction of hepatic lipid peroxidation and cytochrome p-450 associated enzyme activities in rats. Toxicology. 2002;175:15–25. doi: 10.1016/s0300-483x(02)00086-0. [DOI] [PubMed] [Google Scholar]
- Fischer A, Pallauf J, Rimbach G. Selenium- and vitamin e-dependent gene expression in rats: Analysis of differentially expressed mrnas. Methods in enzymology. 2002;347:267–276. doi: 10.1016/s0076-6879(02)47026-7. [DOI] [PubMed] [Google Scholar]
- Flohe L, Gunzler WA, Schock HH. Glutathione peroxidase: A selenoenzyme. FEBS Lett. 1973;32:132–134. doi: 10.1016/0014-5793(73)80755-0. [DOI] [PubMed] [Google Scholar]
- Furlong CE, Suzuki SM, Stevens RC, Marsillach J, Richter RJ, Jarvik GP, et al. Human pon1, a biomarker of risk of disease and exposure. Chem Biol Interact. 2010;187:355–361. doi: 10.1016/j.cbi.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaub P, Tedeschi A, Puttagunta R, Nguyen T, Schmandke A, Di Giovanni S. Hdac inhibition promotes neuronal outgrowth and counteracts growth cone collapse through cbp/p300 and p/caf-dependent p53 acetylation. Cell Death Differ. 2010;17:1392–1408. doi: 10.1038/cdd.2009.216. [DOI] [PubMed] [Google Scholar]
- Grosicka-Maciag E, Kurpios-Piec D, Szumilo M, Grzela T, Rahden-Staron I. Protective effect of n-acetyl-l-cysteine against maneb induced oxidative and apoptotic injury in chinese hamster v79 cells. Food Chem Toxicol. 2011;49:1020–1025. doi: 10.1016/j.fct.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Hafeman DG, Hoekstra WG. Protection against carbon tetrachloride-induced lipid peroxidation in the rat by dietary vitamin e, selenium, and methionine as measured by ethane evolution. J Nutr. 1977;107:656–665. doi: 10.1093/jn/107.4.656. [DOI] [PubMed] [Google Scholar]
- Hassan MQ, Stohs SJ, Murray WJ, Birt DF. Dietary selenium, glutathione peroxidase activity, and toxicity of 2,3,7,8-tetrachloro-dibenzo-p-dioxin. J Toxicol Environ Health. 1985;15:405–415. doi: 10.1080/15287398509530668. [DOI] [PubMed] [Google Scholar]
- Hassoun EA, Wang H, Abushaban A, Stohs SJ. Induction of oxidative stress in the tissues of rats after chronic exposure to tcdd, 2,3,4,7,8-pentachlorodibenzofuran, and 3,3′,4,4′,5-pentachlorobiphenyl. J Toxicol Environ Health A. 2002;65:825–842. doi: 10.1080/00984100290071054. [DOI] [PubMed] [Google Scholar]
- Hassoun EA, Periandri-Steinberg S. Assessment of the roles of antioxidant enzymes and glutathione in 3,3′,4,4′,5-pentachlorobiphenyl (pcb 126)-induced oxidative stress in the brain tissues of rats after subchronic exposure. Toxicol Environ Chem. 2010;92:301. doi: 10.1080/02772240902846660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig B, Hammock BD, Slim R, Toborek M, Saraswathi V, Robertson LW. Pcb-induced oxidative stress in endothelial cells: Modulation by nutrients. Int J Hyg Environ Health. 2002a;205:95–102. doi: 10.1078/1438-4639-00134. [DOI] [PubMed] [Google Scholar]
- Hennig B, Meerarani P, Slim R, Toborek M, Daugherty A, Silverstone AE, et al. Proinflammatory properties of coplanar pcbs: In vitro and in vivo evidence. Toxicol Appl Pharmacol. 2002b;181:174–183. doi: 10.1006/taap.2002.9408. [DOI] [PubMed] [Google Scholar]
- Hine D, Mackness B, Mackness M. Coincubation of pon1, apo a1, and lcat increases the time hdl is able to prevent ldl oxidation. IUBMB Life. 2012;64:157–161. doi: 10.1002/iub.588. [DOI] [PubMed] [Google Scholar]
- Horio F, Ozaki K, Oda H, Makino S, Hayashi Y, Yoshida A. Effect of dietary ascorbic acid, cholesterol and pcb on cholesterol concentrations in serum and liver in a rat mutant unable to synthesize ascorbic acid. J Nutr. 1987;117:1036–1044. doi: 10.1093/jn/117.6.1036. [DOI] [PubMed] [Google Scholar]
- Jiang ZY, Hunt JV, Wolff SP. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem. 1992;202:384–389. doi: 10.1016/0003-2697(92)90122-n. [DOI] [PubMed] [Google Scholar]
- Jin X, Kennedy SW, Di Muccio T, Moon TW. Role of oxidative stress and antioxidant defense in 3,3′,4,4′,5-pentachlorobiphenyl-induced toxicity and species-differential sensitivity in chicken and duck embryos. Toxicol Appl Pharmacol. 2001;172:241–248. doi: 10.1006/taap.2001.9150. [DOI] [PubMed] [Google Scholar]
- Kaur HD, Bansal MP. Studies on hdl associated enzymes under experimental hypercholesterolemia: Possible modulation on selenium supplementation. Lipids Health Dis. 2009;8:55. doi: 10.1186/1476-511X-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibriya MG, Jasmine F, Argos M, Verret WJ, Rakibuz-Zaman M, Ahmed A, et al. Changes in gene expression profiles in response to selenium supplementation among individuals with arsenic-induced pre-malignant skin lesions. Toxicol Lett. 2007;169:162–176. doi: 10.1016/j.toxlet.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaunig JE, Wang Z, Pu X, Zhou S. Oxidative stress and oxidative damage in chemical carcinogenesis. Toxicol Appl Pharmacol. 2011;254:86–99. doi: 10.1016/j.taap.2009.11.028. [DOI] [PubMed] [Google Scholar]
- Kopec AK, Boverhof DR, Burgoon LD, Ibrahim-Aibo D, Harkema JR, Tashiro C, et al. Comparative toxicogenomic examination of the hepatic effects of pcb126 and tcdd in immature, ovariectomized c57bl/6 mice. Toxicol Sci. 2008;102:61–75. doi: 10.1093/toxsci/kfm289. [DOI] [PubMed] [Google Scholar]
- Kutluhan S, Naziroglu M, Celik O, Yilmaz M. Effects of selenium and topiramate on lipid peroxidation and antioxidant vitamin levels in blood of pentylentetrazol-induced epileptic rats. Biol Trace Elem Res. 2009;129:181–189. doi: 10.1007/s12011-008-8287-4. [DOI] [PubMed] [Google Scholar]
- Laclaustra M, Navas-Acien A, Stranges S, Ordovas JM, Guallar E. Serum selenium concentrations and hypertension in the us population. Circ Cardiovasc Qual Outcomes. 2009a;2:369–376. doi: 10.1161/CIRCOUTCOMES.108.831552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laclaustra M, Stranges S, Navas-Acien A, Ordovas JM, Guallar E. Serum selenium and serum lipids in us adults: National health and nutrition examination survey (nhanes) 2003-2004. Atherosclerosis. 2009b;210:643–648. doi: 10.1016/j.atherosclerosis.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai I, Chai Y, Simmons D, Luthe G, Coleman MC, Spitz D, et al. Acute toxicity of 3,3′,4,4′,5-pentachlorobiphenyl (pcb 126) in male sprague-dawley rats: Effects on hepatic oxidative stress, glutathione and metals status. Environ Int. 2010;36:918–923. doi: 10.1016/j.envint.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai IK, Chai Y, Simmons D, Watson WH, Tan R, Haschek WM, et al. Dietary selenium as a modulator of pcb 126-induced hepatotoxicity in male sprague-dawley rats. Toxicol Sci. 2011;124:202–214. doi: 10.1093/toxsci/kfr215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai IK, Dhakal K, Gadupudi GS, Li M, Ludewig G, Robertson LW, et al. N-acetylcysteine (nac) diminishes the severity of pcb 126-induced fatty liver in male rodents. Toxicology. 2012;302:25–33. doi: 10.1016/j.tox.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauby-Secretan B, Loomis D, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, et al. Carcinogenicity of polychlorinated and polybromiated biphenyls. Lancet. 2013 [Google Scholar]
- Lind PM, Orberg J, Edlund UB, Sjoblom L, Lind L. The dioxin-like pollutant pcb 126 (3,3′,4,4′,5-pentachlorobiphenyl) affects risk factors for cardiovascular disease in female rats. Toxicol Lett. 2004;150:293–299. doi: 10.1016/j.toxlet.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Luthe GM, Schut BG, Aaseng JE. Monofluorinated analogues of polychlorinated biphenyls (f-pcbs): Synthesis using the suzuki-coupling, characterization, specific properties and intended use. Chemosphere. 2009;77:1242–1248. doi: 10.1016/j.chemosphere.2006.02.029. [DOI] [PubMed] [Google Scholar]
- Navarro-Alarcon M, Lopez-Ga de la Serrana H, Perez-Valero V, Lopez-Martinez MC. Selenium concentrations in serum of individuals with liver diseases (cirrhosis or hepatitis): Relationship with some nutritional and biochemical markers. Sci Total Environ. 2002;291:135–141. doi: 10.1016/s0048-9697(01)01088-9. [DOI] [PubMed] [Google Scholar]
- Nourooz-Zadeh J, Tajaddini-Sarmadi J, Wolff SP. Measurement of plasma hydroperoxide concentrations by the ferrous oxidation-xylenol orange assay in conjunction with triphenylphosphine. Anal Biochem. 1994;220:403–409. doi: 10.1006/abio.1994.1357. [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Panczenko-Kresowska B, Ziemlanski S. The effect of long-term selenium and vitamin e-enriched diet on the content of lipid peroxides and cholesterol in rats. Acta Physiol Pol. 1987;38:346–352. [PubMed] [Google Scholar]
- Papp LV, Lu J, Holmgren A, Khanna KK. From selenium to selenoproteins: Synthesis, identity, and their role in human health. Antioxid Redox Signal. 2007;9:775–806. doi: 10.1089/ars.2007.1528. [DOI] [PubMed] [Google Scholar]
- Parkinson A, Safe SH, Robertson LW, Thomas PE, Ryan DE, Reik LM, et al. Immunochemical quantitation of cytochrome p-450 isozymes and epoxide hydrolase in liver microsomes from polychlorinated or polybrominated biphenyl-treated rats. A study of structure-activity relationships. J Biol Chem. 1983;258:5967–5976. [PubMed] [Google Scholar]
- Qu X, Huang K, Deng L, Xu H. Selenium deficiency-induced alterations in the vascular system of the rat. Biol Trace Elem Res. 2000;75:119–128. doi: 10.1385/BTER:75:1-3:119. [DOI] [PubMed] [Google Scholar]
- Quazi S, Yokogoshi H, Yoshida A. Effect of dietary fiber on hypercholesterolemia induced by dietary pcb or cholesterol in rats. J Nutr. 1983;113:1109–1118. doi: 10.1093/jn/113.6.1109. [DOI] [PubMed] [Google Scholar]
- Rayman MP. Selenium and human health. Lancet. 2012;379:1256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Ariza A, Rodriguez-Ortega MJ, Marenco JL, Amezcua O, Alhama J, Lopez-Barea J. Uptake and clearance of pcb congeners in chamaelea gallina: Response of oxidative stress biomarkers. Comp Biochem Physiol C Toxicol Pharmacol. 2003;134:57–67. doi: 10.1016/s1532-0456(02)00207-7. [DOI] [PubMed] [Google Scholar]
- Romani R, De Medio GE, di Tullio S, Lapalombella R, Pirisinu I, Margonato V, et al. Modulation of paraoxonase 1 and 3 expression after moderate exercise training in the rat. J Lipid Res. 2009;50:2036–2045. doi: 10.1194/jlr.M800493-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanmartin C, Plano D, Font M, Palop JA. Selenium and clinical trials: New therapeutic evidence for multiple diseases. Current medicinal chemistry. 2011;18:4635–4650. doi: 10.2174/092986711797379249. [DOI] [PubMed] [Google Scholar]
- Sciuto AM, Strickland PT, Kennedy TP, Gurtner GH. Protective effects of n-acetylcysteine treatment after phosgene exposure in rabbits. Am J Respir Crit Care Med. 1995;151:768–772. doi: 10.1164/ajrccm.151.3.7881668. [DOI] [PubMed] [Google Scholar]
- Shao B, Heinecke JW. Hdl, lipid peroxidation, and atherosclerosis. J Lipid Res. 2009;50:599–601. doi: 10.1194/jlr.E900001-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Robertson LW, Ludewig G. Regulation of paraoxonase 1 (pon1) in pcb 126-exposed male sprague dawley rats. Toxicol Lett. 2012;209:291–298. doi: 10.1016/j.toxlet.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley JM, Waxman DJ. Aryl hydrocarbon receptor-independent activation of estrogen receptor-dependent transcription by 3-methylcholanthrene. Toxicol Appl Pharmacol. 2006;213:87–97. doi: 10.1016/j.taap.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Stemm DN, Tharappel JC, Lehmler HJ, Srinivasan C, Morris JS, Spate VL, et al. Effect of dietary selenium on the promotion of hepatocarcinogenesis by 3,3′, 4,4′-tetrachlorobiphenyl and 2,2′, 4,4′, 5,5′-hexachlorobiphenyl. Exp Biol Med (Maywood) 2008;233:366–376. doi: 10.3181/0708-RM-211. [DOI] [PubMed] [Google Scholar]
- Stranges S, Laclaustra M, Ji C, Cappuccio FP, Navas-Acien A, Ordovas JM, et al. Higher selenium status is associated with adverse blood lipid profile in british adults. J Nutr. 2010;140:81–87. doi: 10.3945/jn.109.111252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thong-Ngam D, Samuhasaneeto S, Kulaputana O, Klaikeaw N. N-acetylcysteine attenuates oxidative stress and liver pathology in rats with non-alcoholic steatohepatitis. World J Gastroenterol. 2007;13:5127–5132. doi: 10.3748/wjg.v13.i38.5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varatharajalu R, Garige M, Leckey LC, Gong M, Lakshman MR. Betaine protects chronic alcohol and omega-3 pufa-mediated down-regulations of pon1 gene, serum pon1 and homocysteine thiolactonase activities with restoration of liver gsh. Alcohol Clin Exp Res. 2009;34:424–431. doi: 10.1111/j.1530-0277.2009.01107.x. [DOI] [PubMed] [Google Scholar]
- Vendemiale G, Grattagliano I, Caruso ML, Serviddio G, Valentini AM, Pirrelli M, et al. Increased oxidative stress in dimethylnitrosamine-induced liver fibrosis in the rat: Effect of n-acetylcysteine and interferon-alpha. Toxicol Appl Pharmacol. 2001;175:130–139. doi: 10.1006/taap.2001.9234. [DOI] [PubMed] [Google Scholar]
- Vogiatzi G, Tousoulis D, Stefanadis C. The role of oxidative stress in atherosclerosis. Hellenic J Cardiol. 2009;50:402–409. [PubMed] [Google Scholar]
- Vondracek J, Svihalkova-Sindlerova L, Pencikova K, Krcmar P, Andrysik Z, Chramostova K, et al. 7h-dibenzo[c,g]carbazole and 5,9-dimethyldibenzo[c,g]carbazole exert multiple toxic events contributing to tumor promotion in rat liver epithelial ‘stem-like’ cells. Mutat Res. 2006;596:43–56. doi: 10.1016/j.mrfmmm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Witschi A, Reddy S, Stofer B, Lauterburg BH. The systemic availability of oral glutathione. Eur J Clin Pharmacol. 1992;43:667–669. doi: 10.1007/BF02284971. [DOI] [PubMed] [Google Scholar]
- Yagi K. Simple assay for the level of total lipid peroxides in serum or plasma. Methods Mol Biol. 1998;108:101–106. doi: 10.1385/0-89603-472-0:101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.