Abstract
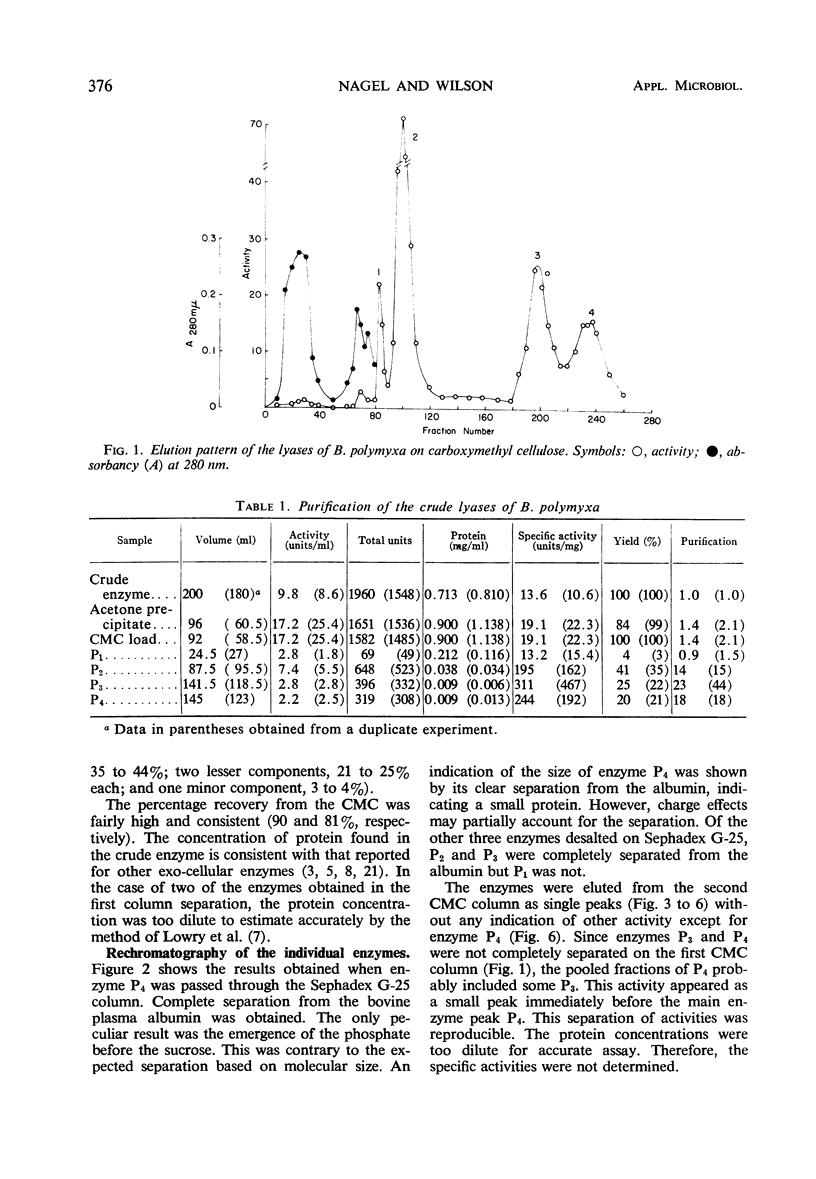
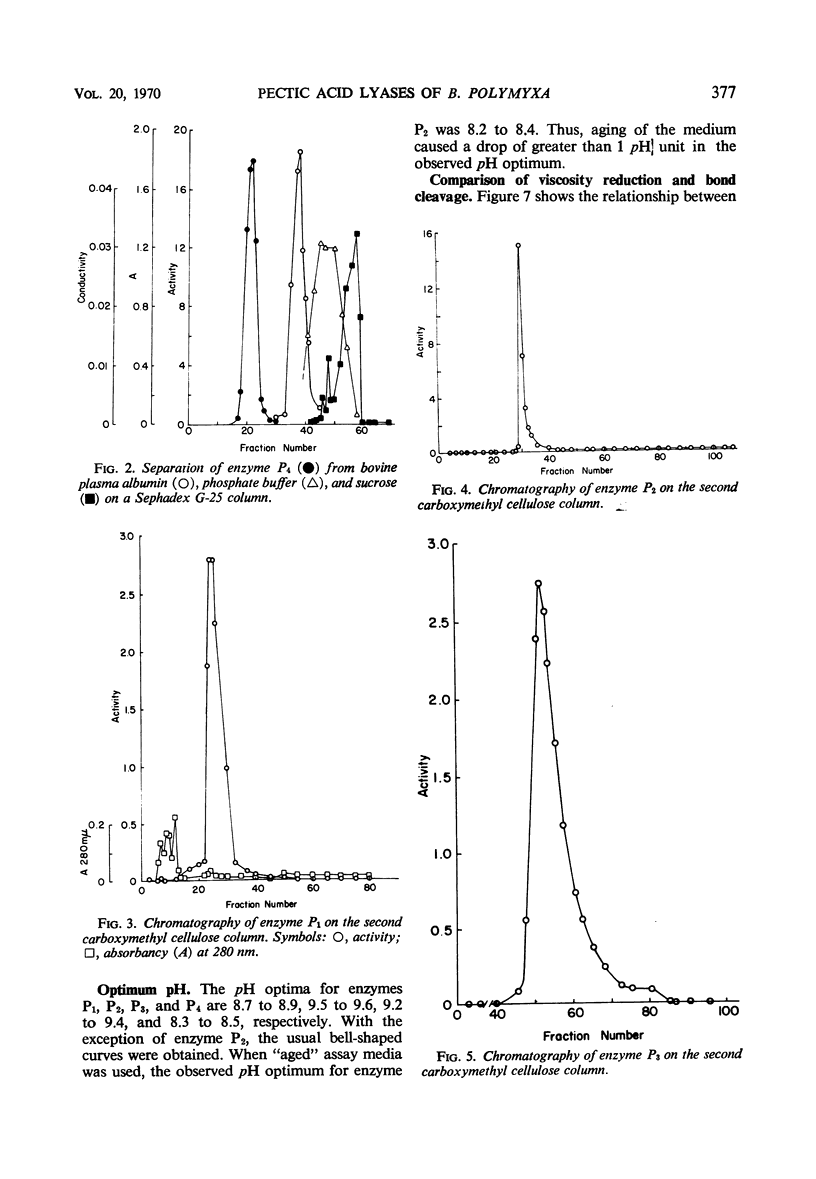
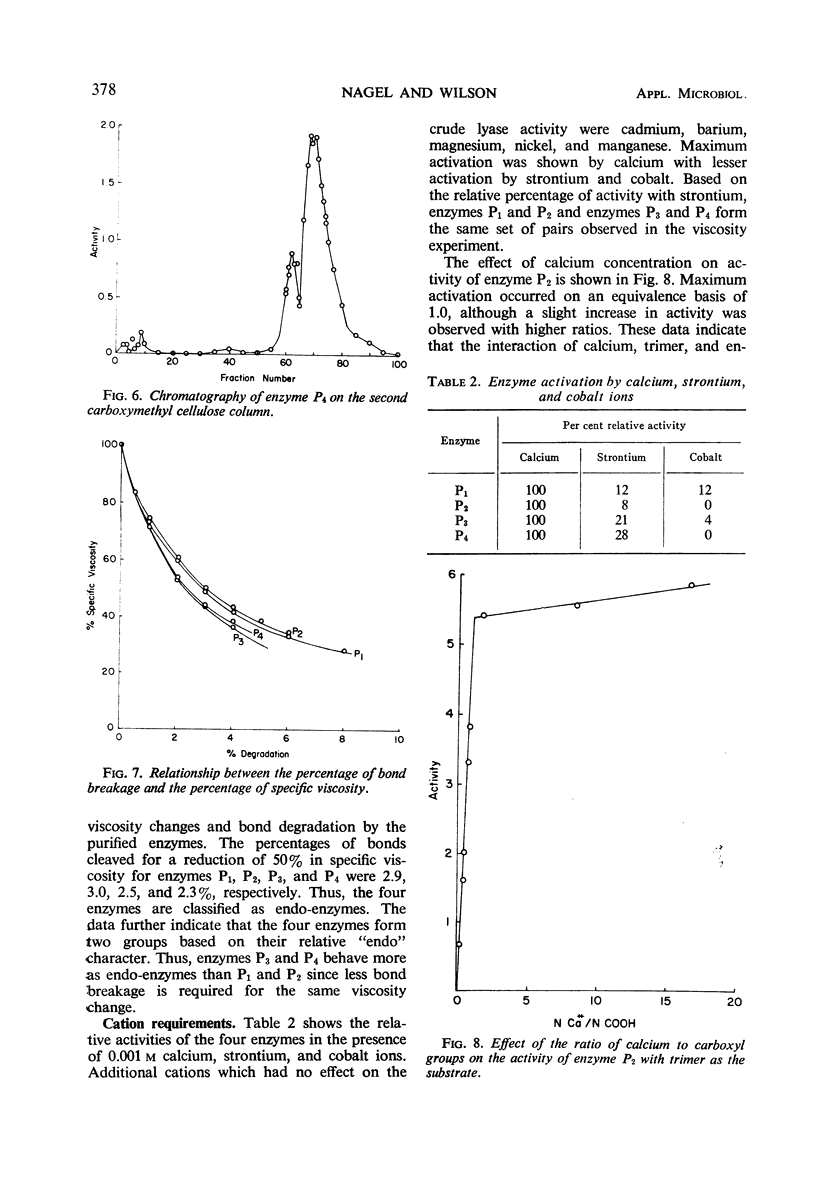
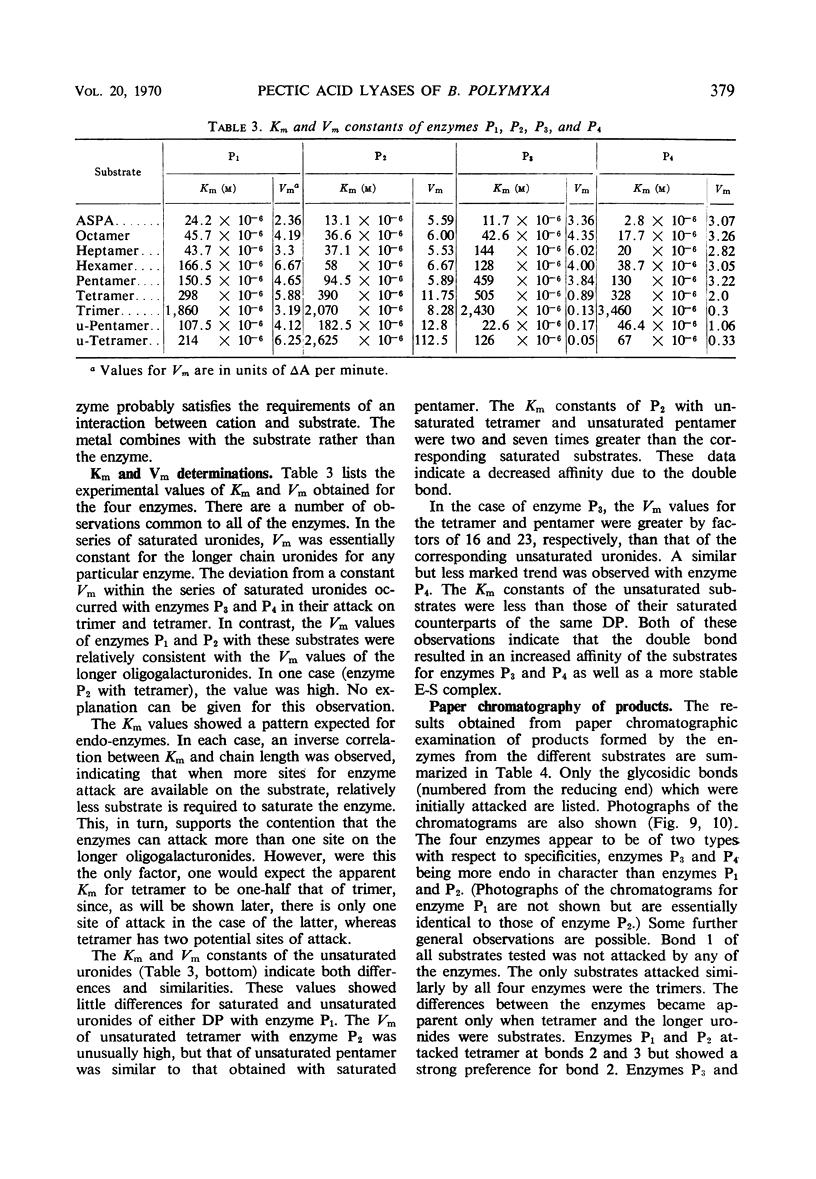
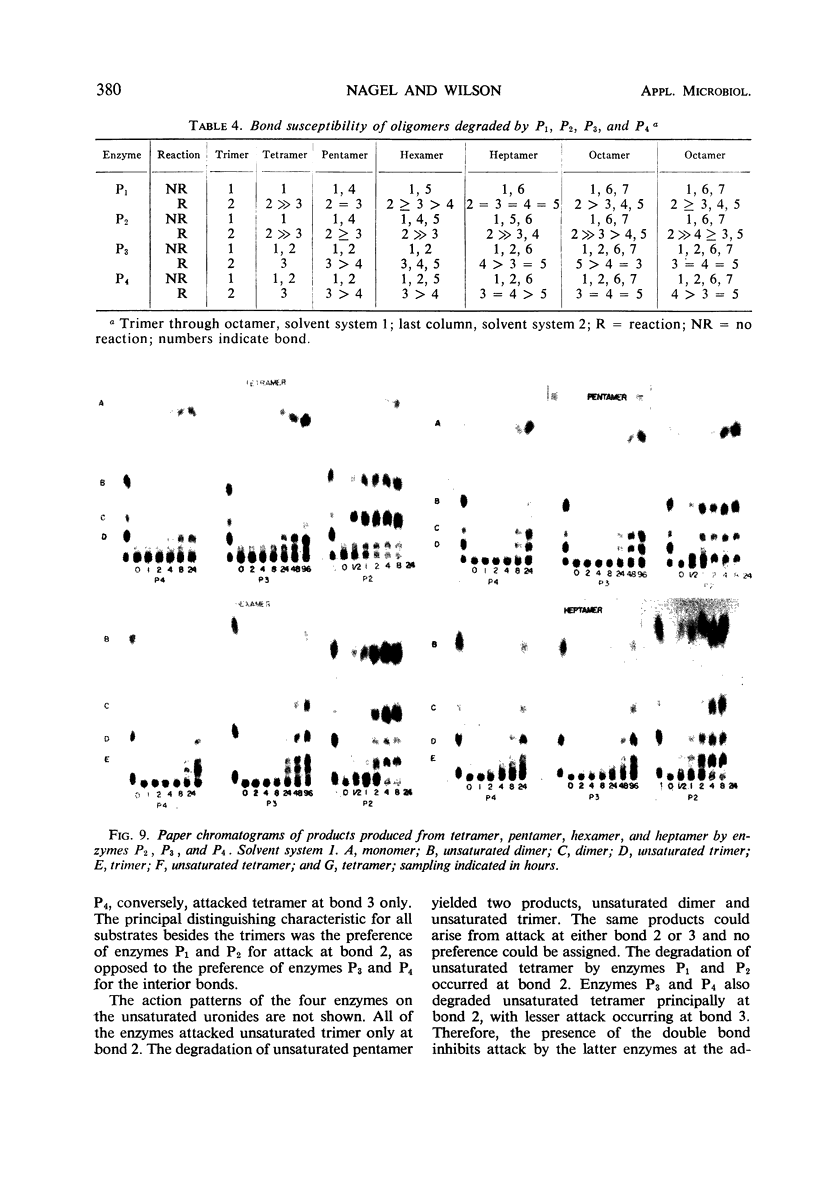
Four enzymes were separated from an extracellular preparation of Bacillus polymyxa by carboxymethylcellulose column chromatography. The pH optima were 8.3 to 8.5, 8.7 to 8.9, 9.2 to 9.4, and 9.5 to 9.6. All of the enzymes required calcium ion for maximum activity, whereas strontium ion was only partially effective in stimulating activity. Cobalt was the only other cation tested which was effective in two of the enzymes. The lyases seem to attack a calcium salt-bridged substrate. Km and Vm data of the four enzymes on various oligomers are presented as well as paper chromatographic evidence of preferred sites of attack. All of the enzymes are endo-enzymes which, based upon their characteristics, were classed into two types.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EDSTROM R. D., PHAFF H. J. PURIFICATION AND CERTAIN PROPERTIES OF PECTIN TRANS-ELIMINASE FROM ASPERGILLUS FONSECAEUS. J Biol Chem. 1964 Aug;239:2403–2408. [PubMed] [Google Scholar]
- Fuchs A. The trans-eliminative breakdown of Na-polygalacturonate by Pseudomonas fluorescens. Antonie Van Leeuwenhoek. 1965;31(3):323–340. doi: 10.1007/BF02045912. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MACMILLAN J. D., PHAFF H. J., VAUGHN R. H. THE PATTERN OF ACTION OF AN EXOPOLYGALACTURONIC ACID-TRANS-ELIMINASE FROM CLOSTRIDIUM MULTIFERMENTANS. Biochemistry. 1964 Apr;3:572–578. doi: 10.1021/bi00892a017. [DOI] [PubMed] [Google Scholar]
- MACMILLAN J. D., VAUGHN R. H. PURIFICATION AND PROPERTIES OF A POLYGALACTURONIC ACID-TRANS-ELIMINASE PRODUCED BY CLOSTRIDIUM MULTIFERMENTANS. Biochemistry. 1964 Apr;3:564–572. doi: 10.1021/bi00892a016. [DOI] [PubMed] [Google Scholar]
- McCREADY R. M., SEEGMILLER C. G. Action of pectic enzymes on oligogalacturonic acids and some of their derivatives. Arch Biochem Biophys. 1954 Jun;50(2):440–450. doi: 10.1016/0003-9861(54)90060-0. [DOI] [PubMed] [Google Scholar]
- NAGEL C. W., VAUGHN R. H. Comparison of growth and pectolytic enzyme production by Bacillus polymyxa. J Bacteriol. 1962 Jan;83:1–5. doi: 10.1128/jb.83.1.1-5.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGEL C. W., VAUGHN R. H. The characteristics of a polygalacturonase produced by Bacillus polymyxa. Arch Biochem Biophys. 1961 May;93:344–352. doi: 10.1016/0003-9861(61)90277-6. [DOI] [PubMed] [Google Scholar]
- NAGEL C. W., VAUGHN R. H. The degradation of oligogalacturonides by the polygalacturonase of Bacillus polymyxa. Arch Biochem Biophys. 1961 Aug;94:328–332. doi: 10.1016/0003-9861(61)90047-9. [DOI] [PubMed] [Google Scholar]
- Nasuno S., Starr M. P. Polygalacturonase of Erwinia carotovora. J Biol Chem. 1966 Nov 25;241(22):5298–5306. [PubMed] [Google Scholar]
- Nasuno S., Starr M. P. Polygalacturonic acid trans-eliminase of Xanthomonas campestris. Biochem J. 1967 Jul;104(1):178–185. doi: 10.1042/bj1040178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAZUR J. H., BUDOVICH T. Preferential hydrolysis of glucosidic bonds of maltooligosaccharides by salivary amylase. J Biol Chem. 1956 May;220(1):25–31. [PubMed] [Google Scholar]
- PAZUR J. H. The hydrolysis of amylotriose and amylotetraose by salivary amylase. J Biol Chem. 1953 Nov;205(1):75–80. [PubMed] [Google Scholar]
- PHAFF H. J., DEMAIN A. L. The unienzymatic nature of yeast polygalacturonase. J Biol Chem. 1956 Feb;218(2):875–884. [PubMed] [Google Scholar]
- STARR M. P., MORAN F. Eliminative split of pectic substances by phytopathogenic soft-rot bacteria. Science. 1962 Mar 16;135(3507):920–921. doi: 10.1126/science.135.3507.920. [DOI] [PubMed] [Google Scholar]