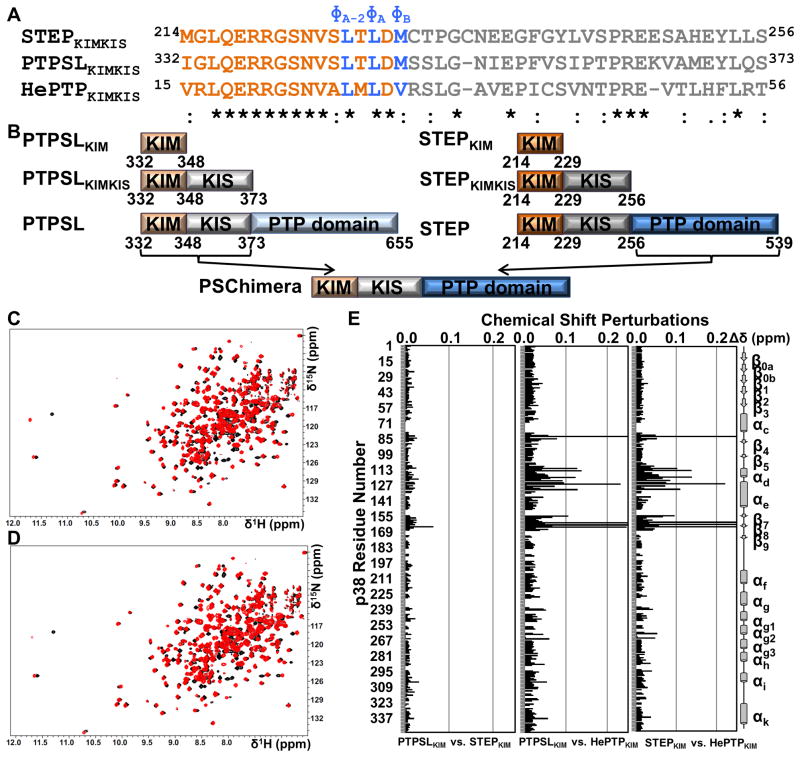
Figure 1. The mode of KIM binding is conserved within the KIM-PTP family.
(A) Sequences of STEPKIMKIS, PTPSLKIMKIS, and HePTPKIMKIS and conservation as determined using ClustalW (Larkin et al., 2007). KIM residues are colored in orange and blue. KIS residues are colored in gray. (B) Constructs used in this study. (C) 2D [1H,15N] TROSY spectrum of [2H,15N]-p38α (black) overlaid with the 2D [1H,15N] TROSY spectrum of [2H,15N]-p38α:PTPSLKIM (red; 1:8 molar ratio p38α:PTPSLKIM) (D) 2D [1H,15N] TROSY spectrum of [2H,15N]-p38α (black) overlaid with the 2D [1H,15N] TROSY spectrum of [2H,15N]-p38α:STEPKIM (red; 1:8 molar ratio p38α:STEPKIM) (E) Histograms which show the combined 1H/15N CSPs vs. p38α residue for the final titration points of each KIM-PTPKIM peptide with [2H,15N]-p38α. See also Figure S1.