Abstract
Purpose
Pelvic radiotherapy (PRT) is known to adversely affect bowel function (BF) and patient well-being. This study characterized long-term BF and evaluated quality of life (QOL) in patients receiving PRT.
Methods
Data from 252 patients were compiled from 2 North Central Cancer Treatment Group prospective studies, which included assessment of BF and QOL by the BF questionnaire (BFQ) and Uniscale QOL at baseline and 12 and 24 months after completion of radiotherapy. BFQ scores (sum of symptoms), Uniscale results, adverse-event incidence, and baseline demographic data were compared via t test, χ2, Fisher exact, Wilcoxon, and correlation methodologies.
Results
The total BFQ score was higher than baseline at 12 and 24 months (P<.001). More patients had 5 or more symptoms at 12 months (13%) and 24 months (10%) than at baseline (2%). Symptoms occurring in greater than 20% of patients at 12 and 24 months were clustering, stool-gas confusion, and urgency. Factors associated with worse BF were female sex, rectal or gynecologic primary tumors, prior anterior resection of the rectum, and 5-fluorouracil chemotherapy. Patients experiencing grade 2 or higher acute toxicity had worse 24-month BF (P values, <.001-.02). Uniscale QOL was not significantly different from baseline at 12 or 24 months, despite worse BFQ scores.
Conclusions
PRT was associated with worse long-term BF. Worse BFQ score was not associated with poorer QOL. Further research to characterize the subset of patients at risk of significant decline in BF is warranted.
Keywords: adverse events, diarrhea, large intestine, quality of life, rectum, toxicity
Introduction
Pelvic radiotherapy is used in a diverse group of patients with various malignancies. The most common adverse effects involve the gastrointestinal tract and can be categorized as acute or long-term effects.
Acute gastrointestinal adverse effects due to irradiation of the small and large bowel occur during or shortly after pelvic radiotherapy and generally resolve within 6 weeks after treatment completion (1–5). Acute effects include diarrhea, cramping, and tenesmus. Predictors include patient characteristics, tumor factors, and radiation dose (6,7). Chemotherapy and surgery can also affect the timing, severity, and duration of these acute events (8). Studies have investigated the effectiveness of cholestyramine, olsalazine, sucralfate, glutamine, and octreotide to minimize acute toxicities (9–13). To date, no agent tested in a randomized controlled trial has been shown effective with an acceptable adverse-effect profile. Cholestyramine (vs placebo) did reduce diarrhea but caused unacceptable levels of abdominal cramping (9).
Long-term effects occur and persist for weeks to months after completion of treatment, may be permanent, and may have a considerable impact on quality of life (QOL). These can involve the small and large bowel, particularly the rectum. Small-bowel effects include pain, nausea, malabsorption, stricture, obstruction, fistulae, and abscesses (14). Large-bowel effects include bleeding, frequency, urgency, stricture, fistulae, and fecal incontinence (15). Chronic gastrointestinal toxicity may be a consequence of acute damage (16). Long-term bowel function after radiotherapy has been studied for rectal, prostate, and gynecologic cancers (8,17–23).
The development of late bowel toxicity is related to the radiation dose and irradiated bowel volume (24–27), resulting in recommendations of dose constraints (26,27). This relationship between dose-volume and toxicity may be further affected by characteristics of surgery, chemotherapy, sex, age, and baseline comorbid conditions.
The current study, approved by the Mayo Clinic Institutional Review Board, aimed to characterize the long-term bowel function of patients treated in 2 completed North Central Cancer Treatment Group (NCCTG) phase III, double-blind trials whose purposes were to assess effectiveness of glutamine (NCCTG 969256) (12) and octreotide (N00CA) (13). Neither trial demonstrated efficacy in the prevention of acute diarrhea associated with pelvic radiotherapy. The goals of the current study were to explore the relationship of long-term bowel function and symptoms with baseline characteristics, treatment, recorded adverse events, and QOL.
Methods
Patient Population and Treatment
The study population consisted of 254 patients. The glutamine trial accrued 129 patients from February 1998 through October 1999. Patients received oral glutamine or placebo twice daily during radiotherapy and for 2 weeks after completion of treatment. The octreotide trial accrued 125 patients from May 2002 through October 2005. Patients received subcutaneous injections of depot octreotide acetate or placebo at the beginning of radiotherapy and on day 29 of treatment. Eligibility and study treatment criteria have been reported previously (12,13). Patients had histologic proof of cancer in the pelvis and had a planned course of definitive or adjuvant treatment to 45 to 53.5 Gy in 1.7- to 2.1-Gy fractions to the entire pelvis. The superior field border could not lie superior to the L4-5 interspace, nor inferior to the sacroiliac joints. Physicians were allowed to boost primary tumor or tumor bed as indicated.
Data Collection and Assessment of Bowel Function
Baseline characteristic data were obtained. These included patient age, sex, race, primary disease site, history of rectal resection before radiotherapy, and adjunct treatment.
Toxicity was assessed during and after treatment using the National Cancer Institute Common Toxicity Criteria version 2.0 (28). Patient-reported incidence of problematic bowel function symptoms was assessed using the bowel function questionnaire (BFQ) (12,13,29) (Appendix). Brief descriptions of evaluated symptoms are in Table 1. Each study used the single-item Uniscale measure (30) to record overall QOL, on a linear scale (glutamine trial) or numeric scale (octreotide trial). Both versions of this measure have been validated and shown to be analogous to one another (31). Normative data have been reported (32). The BFQ and Uniscale, used successfully in other clinical trials (11–13), were completed at baseline, weekly during radiotherapy, weekly for 4 weeks after treatment, and at 12 and 24 months after completion of radiotherapy.
Table 1.
Definition of Terms in the BFQ
| BFQ Symptom | Definition |
|---|---|
| Nocturnal bowel movements | Needing to get up at night for bowel movements |
| Incontinence | Loss of control of bowel movements |
| Clustering | Needing to have a bowel movement within 30 minutes of a prior bowel movement |
| Protective clothing | Need for protective clothing or a pad |
| Stool-gas confusion | Unable to differentiate between stool and gas |
| Liquid bowel movements | Having liquid bowel movements |
| Urgency | Inability to delay bowel movements |
| Cramping | Cramping with bowel movements |
| Rectal bleeding | Blood with bowel movements |
Abbreviation: BFQ, bowel frequency questionnaire.
Statistical Analysis
The primary goal of this pooled analysis was to investigate long-term bowel function. The primary end point was the BFQ score at 24 months. Secondary goals and end points included investigation of BFQ score at 12 months, Uniscale score at 12 and 24 months, and relationships of both baseline characteristics and adverse events to QOL and long-term bowel function.
Individual symptoms in the BFQ were assigned a value of 1 if the symptom was experienced and the total BFQ score (sum of values) was calculated (range 0–9). The score of the Uniscale was the number indicated by the patient (range 0–100, where 100 was best QOL). Patients were categorized as having clinically deficient QOL if the Uniscale score was less than or equal to 50 (33). Changes from baseline were calculated for the total BFQ and Uniscale scores. The adverse-event profile per patient was characterized dichotomously according to the maximum adverse-event grade (<2 vs ≥2; <3 vs ≥3).
Two-sided hypotheses tests were conducted using type I error of α=0.05. Scores and changes from baseline at 12 and 24 months were assessed using single-sample t tests. End points were compared between treatments and baseline characteristic categories. Kruskal-Wallis or Wilcoxon methodologies were applied to continuous end points and χ2 or Fisher exact methodologies were applied to discrete end points. Spearman/Pearson correlations were used to determine relationships between BFQ and Uniscale scores.
Results
Two patients were excluded because of missing QOL data; therefore, 252 patients were included in this analysis. Baseline patient characteristics are presented in Table 2. BFQ results were available for 249 patients at baseline, 178 patients at 12 months, and 148 patients at 24 months. We compared the patients who completed BFQs at baseline, 12 months, and 24 months and observed no differences in the distribution of baseline characteristics of age, race, sex, prior rectal resection, 5-FU use, or location of primary tumor. Baseline Uniscale, total BFQ score, and frequency of each BFQ symptom were balanced between investigational and placebo arms (data for BFQ symptoms not shown).
Table 2.
Baseline Characteristics (N=252)
| Characteristic | Value |
|---|---|
| Male sex, No. (%) | 161 (63.9) |
| Race, No. (%) | |
| Black | 11 (4.4) |
| Native American | 3 (1.2) |
| White | 238 (94.4) |
| Age, median (range), y | 66.5 (31.0–86.0) |
| History of rectal resection before RT, No. (%) | 18 (7.1) |
| Treatment with 5-fluorouracil, No. (%)c | |
| None | 195 (77.4) |
| Bolus | 9 (3.6) |
| Continuous infusion | 48 (19.0) |
| Primary cancer location, No. (%) | |
| Rectum | 53 (21.0) |
| Prostate | 122 (48.4) |
| Gynecologic organ | 69 (27.4) |
| Other | 8 (3.2) |
| Baseline BFQ score, meana | 1.1 |
| Baseline QOL score, meanb | 83.1 |
Abbreviations: BFQ, bowel function questionnaire; QOL, quality of life; RT, radiotherapy.
Potential scores ranged from 0–9.
Potential scores ranged from 0–100.
Of the 57 patients treated with 5-fluorouracil, 53 had rectal cancer, 2 had anal cancer, 1 had bladder cancer, and 1 had gynecologic cancer.
No statistically significant differences were identified between intervention and placebo groups regarding mean BFQ score at 12 months (2.0 vs 1.6; P=.31) and 24 months (1.7 vs 1.6; P=.97), or change from baseline in mean BFQ score at 12 months (1.1 vs 0.5; P=.12) or 24 months (0.7 vs 0.5; P=.87). Comparisons of analogous Uniscale results also showed nonsignificant differences. Thus, the data were analyzed without separation by treatment group. Results indicate a decline in bowel function over time. Primary end point results showed patients had a mean BFQ score of 1.6 at 24 months (P<.001). This significant difference was also present at 12 months (1.8; P<.001). Significant mean BFQ changes from baseline also existed at both 24 and 12 months (0.59 and 0.80, respectively; P<.001).
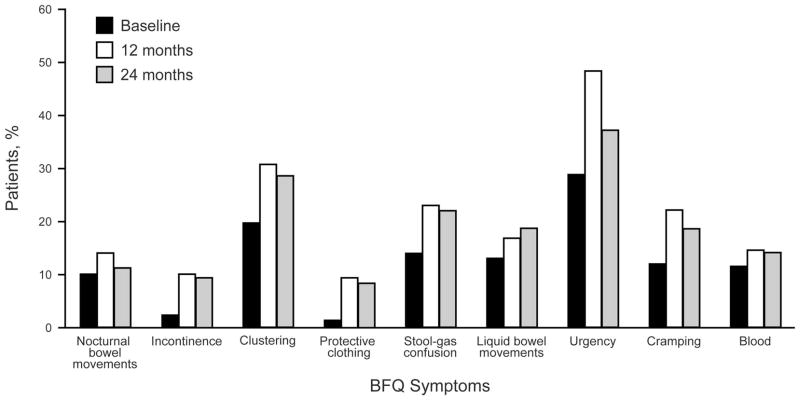
Incidence of specific symptoms of long-term bowel dysfunction increased from baseline. Figure 1 shows the percentage of patients with each individual BFQ symptom. The most common symptom was urgency, occurring in almost half of patients at 12 months after treatment. Symptoms occurring in greater than 20% of patients were urgency, stool-gas confusion, clustering, and cramping. Statistically significant increases from baseline to 12 months were observed for total BFQ score (1.1 vs 1.8; P<.001), incontinence (2.4% vs 9.6%; P=.001), protective clothing (1.2% vs 9.1%; P<.001), stool-gas confusion (14.2% vs 22.7%; P=.02), urgency (29.0% vs 48.3%; P<.001), and cramping (11.7% vs 21.7%; P=.005). Statistically significant increases from baseline to 24 months were observed for total BFQ score (1.1 vs 1.6; P<.01), incontinence (2.4% vs 9.5%; P=.002), clustering (19.5% vs 28.4%; P=.04), and the need for protective clothing (1.2% vs 8.1%; P<.001). A slight decrease in total BFQ score occurred between 12 and 24 months (mean, 1.8 vs 1.6; P=.53). The incidence frequency of each BFQ symptom also decreased, although urgency had the only statistically significant decrease (48.3% vs 36.7%; P=.04).
Figure 1.
Incidence of Bowel Function Symptoms. BFQ denotes bowel function questionnaire.
Figure 2 illustrates the percentage of affected patients with number of symptoms, stratified by time point. Many patients had no symptoms at baseline, 12 months, and 24 months (n=121 [48.6%], n=64 [36.0%], and n=57 [38.5%], respectively). More patients had 5 or more symptoms at 12 months (13%) and 24 months (10%) than at baseline (2%). At baseline, 170 patients (67.5%) had a BFQ score of 0 or 1. At 12 months, 81 (65.9%) had fewer than 2 symptoms. At 24 months, 62 (61.4%) had fewer than 2 symptoms.
Figure 2.
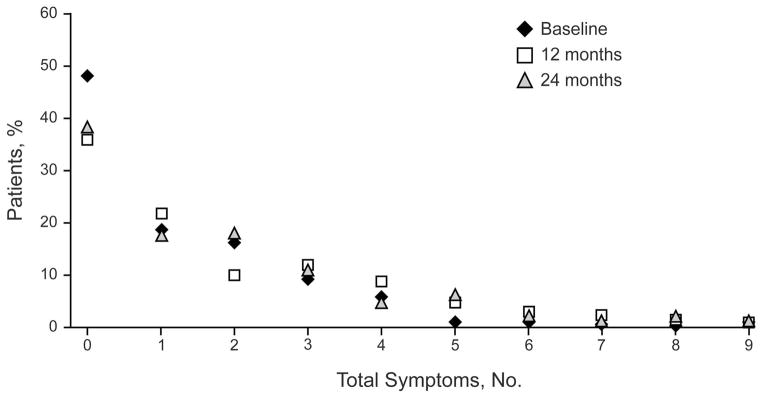
Distribution of BFQ Scores. BFQ denotes bowel function questionnaire.
Baseline characteristics were assessed to determine predictiveness. Characteristics associated with worse long-term bowel function were female sex, prior anterior resection of the rectum, use of 5-fluorouracil chemotherapy, and a primary rectal tumor (Table 3).
Table 3.
Characteristics Predictive of 24-Month Bowel Function (N=148)
| Characteristic | Value | P Value | |
|---|---|---|---|
| Patient Sex |
|||
| Female (n=56) | Male (n=92) | ||
|
| |||
| Total BFQ score, mean | 2.1 | 1.4 | .04 |
| Protective clothing, % | 14.3 | 4.3 | .03 |
| Urgency, % | 49.1 | 29.3 | .02 |
| Cramping, % | 30.9 | 10.9 | .002 |
|
|
|||
|
Anterior Resection of
the Rectum Before RT
|
|||
| Yes (n=13) | No (n=135) | ||
|
| |||
| Total BFQ score, mean | 3.2 | 1.5 | <.001 |
| Nocturnal bowel movements, % | 38.5 | 8.2 | <.001 |
| Incontinence, % | 30.8 | 7.4 | .01 |
| Clustering, % | 84.6 | 23.0 | <.001 |
| Liquid bowel movements, % | 38.5 | 16.3 | .048 |
|
|
|||
|
Chemotherapy With
5-Fluorouracil
|
|||
| Yes (n=29) | No (n=119) | ||
|
| |||
| Total BFQ score, mean | 2.5 | 1.4 | .003 |
| Nocturnal bowel movements, % | 31.0 | 5.9 | <.001 |
| Incontinence, % | 20.7 | 6.7 | .02 |
| Clustering, % | 69.0 | 18.5 | <.001 |
| Stool-gas confusion, % | 37.9 | 17.6 | .02 |
| Characteristic | Value | ||||
|---|---|---|---|---|---|
|
| |||||
| Location of Primary Cancera | 3-Group P Valueb | Rectum vs Prostate P Valuec | |||
|
|
|
||||
| Rectum (n=27) | Gynecologic Organ (n=47) | Prostate (n=72) | |||
|
|
|||||
| Total BFQ score, mean | 2.5 | 2.0 | 1.0 | <.001 | <.001 |
| Nocturnal bowel movements, % | 33.3 | 8.5 | 4.2 | <.001 | <.001 |
| Incontinence, % | 18.5 | 10.6 | 4.2 | .03 | .02 |
| Clustering, % | 70.4 | 27.7 | 11.1 | <.001 | <.001 |
| Protective clothing, % | 7.4 | 14.9 | 2.8 | .02 | .30 |
| Stool-gas confusion, % | 37.0 | 21.3 | 13.9 | .004 | .01 |
| Urgency, % | 46.2 | 46.8 | 25.0 | .01 | .04 |
| Cramping, % | 11.1 | 37.0 | 9.7 | .001 | .24 |
Abbreviation: BFQ, bowel function questionnaire; RT, radiotherapy.
Two patients had a location of “other” and were excluded from this analysis.
Significant P values indicate a difference in the incidence distributions among the 3 groups. It does not indicate pairwise differences.
P value indicates significance of rectum vs prostate scores and frequencies.
The relationship between acute toxicity and long-term bowel function was explored. Patients with grade 3 or greater acute bowel toxicity had significantly worse long-term bowel function, as measured by BFQ score at 24 months, than their lower-grade counterparts. Acute bowel toxicity grade was the maximum grade of diarrhea, abdominal cramping, constipation, rectal bleeding, or tenesmus experienced by a patient. Patients with maximum acute bowel toxicity of less than grade 3 had a mean BFQ score of 1.23 at 24 months, whereas patients with acute grade 3 or greater toxicity had a mean score of 3.09 (P<.001). Patients who had grade 2 or higher toxicity also had significantly greater incidence of clustering, stool-gas confusion, liquid stools, and cramping at 12 months (P≤.01) (data not shown).
Uniscale scores had poor correlation with BFQ scores (r=−0.26 at 12 months; r=−.17 at 24 months). Changes from baseline in Uniscale score at 12 and 24 months were not significant. Patients with clinically deficient QOL scores at baseline did not have significantly worse mean BFQ scores than those with nondeficient QOL at 12 months (2.7 vs 1.7; P=.07) or at 24 months (2.2 vs 1.6; P=.22). Table 4 contains mean Uniscale scores according to symptom incidence. At 12 months, significantly lower Uniscale scores occurred for those experiencing nocturnal bowel movements, clustering, the need for protective clothing, stool-gas confusion, liquid bowel movements, and cramping. At 24 months, significantly lower scores occurred for those experiencing clustering. Also at 24 months, significantly higher scores were reported by patients who did not experience a grade 2 or higher bowel toxicity grade (87.5 vs 80.6; P=.02).
Table 4.
Effect of BFQ Symptoms on QOL Scores
| BFQ Symptoma | QOL Score, meanb
|
|||||
|---|---|---|---|---|---|---|
| 12 Months BFQ Symptom
|
24 Months BFQ Symptom
|
|||||
| Present | Absent | P Value | Present | Absent | P Value | |
| Nocturnal bowel movements | 75.5 | 84.6 | .02 | 76.9 | 84.1 | .06 |
| Incontinence | 79.9 | 93.9 | .16 | 87.5 | 82.8 | .46 |
| Clustering | 80.3 | 84.9 | .03 | 79.7 | 84.8 | .02 |
| Protective clothing | 69.4 | 85.0 | <.01 | 86.1 | 83.0 | .65 |
| Stool-gas confusion | 75.6 | 85.9 | <.01 | 80.7 | 84.0 | .34 |
| Liquid bowel movements | 75.5 | 85.0 | <.01 | 76.8 | 84.8 | .10 |
| Urgency | 83.1 | 84.3 | .47 | 81.9 | 84.3 | .15 |
| Cramping | 80.1 | 84.6 | .04 | 81.2 | 84.0 | .53 |
| Rectal bleeding | 75.8 | 84.9 | .09 | 83.1 | 84.1 | .42 |
Abbreviations: BFQ, bowel function questionnaire; QOL, quality of life.
Complete description of symptoms is shown in Table 1.
Possible scores ranged from 0–100 (higher values indicate better QOL).
Discussion
This trial aimed to define long-term bowel function after pelvic radiotherapy and to identify factors that might predict worse bowel function. We measured patient-reported symptoms, an established, effective modality for collecting adverse effect information (29,34). For our patients, pelvic radiotherapy resulted in worse long-term bowel function. Greater acute toxicity, female sex, location of primary tumor, history of rectal resection before radiotherapy, and treatment with 5-fluorouracil were all predictive of long-term bowel dysfunction. We noted improvement in the total BFQ score and diminished frequency of symptoms between 12 months and 24 months, although differences were not statistically significant. Lastly, we observed that while many patients had no late-term bowel toxicity, a small number of patients had multisymptom dysfunction (Figure 2). Removal of BFQ scores greater than 5 resulted in a mean change from baseline at 24 months of 0.2 (P=.17). Therefore, the increase in BFQ scores in a small number of patients accounted for most of the decline in bowel function in the population.
Most reports of poor long-term bowel function after pelvic radiotherapy describe postoperative patients with primary rectal cancer. Kollmorgen and colleagues (29) reported patients were more likely to have bowel dysfunction, as measured by frequency, clustering, nocturnal bowel movements, incontinence, protective clothing, and inability to defer stooling. Univariate analysis revealed age, sex, and length of follow-up on frequency of bowel movements and incontinence had no significant effects. Lundby et al (35) found that patients had significantly worse bowel function, manifested as frequency, incontinence, urgency, protective clothing use, loose consistency, and ability to differentiate between stool and gas. Univariate analysis for possible predictors of bowel function was not reported. Similarly, Dahlberg et al (36) reported that patients enrolled in the Swedish Rectal Cancer Trial had higher stool frequency and more commonly had emptying difficulties, incontinence, urgency, and toilet dependence.
The present study is unique in that patients had various primary cancers. Data were collected prospectively, eliminating potential recall bias and allowing standardized symptom reporting. Analyses were performed to identify patterns and possible predictors of bowel dysfunction to identify at-risk populations.
Inclusion of patients with various pelvic cancers allowed assessment of the association between the type of cancer treated and subsequent toxicity. Pelvic radiotherapy parameters were broadly defined. Because few patients are treated using protocols that specify radiotherapy parameters in detail, our results likely reflect what would be observed in clinical practice. A limitation of our study is that correlation between specific radiotherapy techniques and subsequent toxicity could not be determined. Despite this limitation, our investigation provides useful data that can be applied to patients receiving pelvic radiotherapy. Further investigation into the consequences of dose and field arrangement is warranted.
Similar to prior reports (29,35,36), bowel function in our population was worse 2 years after radiotherapy than at baseline. Importantly, long-term bowel symptoms persisted only in a small subset of the population. Symptoms appeared better at 24 months than at 12 months, implying that bowel function may continue to improve for at least 2 years after treatment. This has implications for patient counseling, particularly for patients whose symptoms are severe enough to consider ostomy for symptom control.
Conclusion
Pelvic radiotherapy is associated with long-term bowel dysfunction. However, a substantial proportion of patients have no long-term symptoms and a small subset of patients have clinically significant, multisymptom dysfunction. Long-term bowel toxicity, as measured by the BFQ score, did not significantly affect patient-reported QOL. Several factors may predict long-term bowel dysfunction, including presence and severity of acute toxicity, female sex, location of primary tumor, history of rectal resection, and treatment with 5-fluorouracil. Identification of patients at high risk of long-term bowel dysfunction can direct future research toward decreasing acute rectal toxicity and provide clinicians with information relevant for patient counseling. Furthermore, we hope that this meta-analysis encourages similar research for other disease sites and treatment modalities, so that clinicians can better define and continue to improve the impact of treatment on our patients.
Acknowledgments
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grant CA-25224, CA-37404, CA-35101, CA-35103, CA-35195, CA-37417, CA-35269, CA-63848, CA-52352, CA-35415, CA-63849, CA-35119, CA-35431, CA-35267 and from the National Cancer Institute Department of Health and Human Services. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute or the National Institute of Health. Additional participating institutions include: Michigan Cancer Research Consortium (Philip J. Stella, MD), Ann Arbor, Michigan; Essentia Health Cancer Center (Daniel A. Nikcevich, MD), Duluth, Minnesota; Medcenter One Health Systems (John T. Reynolds, MD) and Mid Dakota Clinic (John T. Reynolds, MD), Bismarck, North Dakota; Missouri Valley Cancer Consortium (Gamini S. Soori, MD), Omaha, Nebraska; Sioux Community Cancer Consortium (Miroslaw Mazurczak, MD), Sioux Falls, South Dakota; Cedar Rapids Oncology Project CCOP (Deborah Weil Wilbur, MD), Cedar Rapids, Iowa; Meritcare Hospital CCOP (Preston D. Steen, MD), Fargo, North Dakota; Altru Health Systems (Grant Seeger, MD), Grand Forks, North Dakota; Mayo Clinic Arizona (Michele Y. Halyard, MD), Scottsdale, Arizona; Metro-Minnesota Community Clinical Oncology Program (Patrick J. Flynn, MD), St. Louis Park, Minnesota; Montana Cancer Consortium (Benjamin T. Marchello, MD), Billings, Montana; Upstate Carolina CCOP (James D. Bearden, III, MD) Spartanburg, South Carolina; Toledo Community Hospital Oncology Program CCOP (Rex B. Mowat, MD), Toledo, Ohio; Saskatchewan Cancer Foundation (Muhammad Salim, MD), Saskatoon, Saskatchewan, CANADA; Wichita Community Clinical Oncology Program (Shaker R. Dakhil, MD), Wichita, Kansas; Mayo Clinic Florida (Kurt A. Jaeckle, MD), Jacksonville, Florida; CentraCare Clinic (Donald J. Jurgens, MD), St. Cloud, Minnesota.
Abbreviations
- BFQ
bowel function questionnaire
- NCCTG
North Central Cancer Treatment Group
- QOL
quality of life
Appendix.
Each of these statements or questions below describes symptoms or problems which sometimes occur in patients who have had radiation therapy.
| Overall, would you say that you had problems with your bowel function in the past week? | Yes | No |
| 1. In the past week, what is the greatest number of bowel movements you have had in a day? ____________________________ | ||
| For questions 2–10, circle “yes” or “no” in response to each question. | ||
| 2. In the past week, have you had a problem causing you to get up at night to have a bowel movement? | Yes | No |
| 3. In the past week, have you had a problem causing you to lose control of your bowel movements? | Yes | No |
| 4. In the past week, have you had a problem causing you to have a bowel movement within 30 minutes of a prior bowel movement? | Yes | No |
| 5. In the past week, have you had to wear protective clothing or a pad in case you lost control of a bowel movement? | Yes | No |
| 6. In the past week, have you had a problem causing you to be unable to tell the difference between stool and gas? | Yes | No |
| 7. In the past week, have you had a problem causing you to have stools that are liquid? | Yes | No |
| 8. In the past week, have you found that once you feel the urge to have a bowel movement, you must do so within 15 minutes to avoid an accident? | Yes | No |
| 9. In the past week, have you
had cramping with a bowel movement? If yes, is your cramping: _______ Mild _______ Moderate _______ Severe |
Yes | No |
| 10. Do you ever have blood in
your bowel movement? If yes, check the description that best describes the amount of blood in your bowel movement: _______ On toilet tissue only _______ Mixed with or coating bowel movement _______ Enough to turn water in toilet bowl red |
Yes | No |
Adapted from Kozelsky et al (12). Used with permission.
Footnotes
Presented as a poster at the 52nd Annual Meeting of the American Society for Radiation Oncology, San Diego, California, November 1, 2010.
Conflict of Interest
The authors have no conflict of interest to declare.
References
- 1.Gunnlaugsson A, Kjellen E, Nilsson P, Bendahl PO, Willner J, Johnsson A. Dose-volume relationships between enteritis and irradiated bowel volumes during 5-fluorouracil and oxaliplatin based chemoradiotherapy in locally advanced rectal cancer. Acta Oncol. 2007;46(7):937–44. doi: 10.1080/02841860701317873. [DOI] [PubMed] [Google Scholar]
- 2.Baglan KL, Frazier RC, Yan D, Huang RR, Martinez AA, Robertson JM. The dose-volume relationship of acute small bowel toxicity from concurrent 5-FU-based chemotherapy and radiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2002 Jan 1;52(1):176–83. doi: 10.1016/s0360-3016(01)01820-x. [DOI] [PubMed] [Google Scholar]
- 3.Robertson JM, Lockman D, Yan D, Wallace M. The dose-volume relationship of small bowel irradiation and acute grade 3 diarrhea during chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2008 Feb 1;70(2):413–8. doi: 10.1016/j.ijrobp.2007.06.066. Epub 2007 Sep 27. [DOI] [PubMed] [Google Scholar]
- 4.Miller RC, Martenson JA, Sargent DJ, Kahn MJ, Krook JE. Acute treatment-related diarrhea during postoperative adjuvant therapy for high-risk rectal carcinoma. Int J Radiat Oncol Biol Phys. 1998 Jun 1;41(3):593–8. doi: 10.1016/s0360-3016(98)00084-4. [DOI] [PubMed] [Google Scholar]
- 5.Miller RC, Sargent DJ, Martenson JA, Macdonald JS, Haller D, Mayer RJ, et al. Acute diarrhea during adjuvant therapy for rectal cancer: a detailed analysis from a randomized intergroup trial. Int J Radiat Oncol Biol Phys. 2002 Oct 1;54(2):409–13. doi: 10.1016/s0360-3016(02)02924-3. [DOI] [PubMed] [Google Scholar]
- 6.Vavassori V, Fiorino C, Rancati T, Magli A, Fellin G, Baccolini M, et al. Predictors for rectal and intestinal acute toxicities during prostate cancer high-dose 3D-CRT: results of a prospective multicenter study. Int J Radiat Oncol Biol Phys. 2007 Apr 1;67(5):1401–10. doi: 10.1016/j.ijrobp.2006.10.040. Epub 2007 Jan 22. [DOI] [PubMed] [Google Scholar]
- 7.Valdagni R, Rancati T, Fiorino C, Fellin G, Magli A, Baccolini M, et al. Development of a set of nomograms to predict acute lower gastrointestinal toxicity for prostate cancer 3D-CRT. Int J Radiat Oncol Biol Phys. 2008 Jul 15;71(4):1065–73. doi: 10.1016/j.ijrobp.2007.11.037. Epub 2008 Jan 30. [DOI] [PubMed] [Google Scholar]
- 8.Miller AR, Martenson JA, Nelson H, Schleck CD, Ilstrup DM, Gunderson LL, et al. The incidence and clinical consequences of treatment-related bowel injury. Int J Radiat Oncol Biol Phys. 1999 Mar 1;43(4):817–25. doi: 10.1016/s0360-3016(98)00485-4. [DOI] [PubMed] [Google Scholar]
- 9.Chary S, Thomson DH. A clinical trial evaluating cholestyramine to prevent diarrhea in patients maintained on low-fat diets during pelvic radiation therapy. Int J Radiat Oncol Biol Phys. 1984 Oct;10(10):1885–90. doi: 10.1016/0360-3016(84)90267-0. [DOI] [PubMed] [Google Scholar]
- 10.Martenson JA, Jr, Hyland G, Moertel CG, Mailliard JA, O’Fallon JR, Collins RT, et al. Olsalazine is contraindicated during pelvic radiation therapy: results of a double-blind, randomized clinical trial. Int J Radiat Oncol Biol Phys. 1996 May 1;35(2):299–303. doi: 10.1016/0360-3016(96)00016-8. [DOI] [PubMed] [Google Scholar]
- 11.Martenson JA, Bollinger JW, Sloan JA, Novotny PJ, Urias RE, Michalak JC, et al. Sucralfate in the prevention of treatment-induced diarrhea in patients receiving pelvic radiation therapy: a North Central Cancer Treatment Group phase III double-blind placebo-controlled trial. J Clin Oncol. 2000 Mar;18(6):1239–45. doi: 10.1200/JCO.2000.18.6.1239. [DOI] [PubMed] [Google Scholar]
- 12.Kozelsky TF, Meyers GE, Sloan JA, Shanahan TG, Dick SJ, Moore RL, et al. North Central Cancer Treatment Group. Phase III double-blind study of glutamine versus placebo for the prevention of acute diarrhea in patients receiving pelvic radiation therapy. J Clin Oncol. 2003 May 1;21(9):1669–74. doi: 10.1200/JCO.2003.05.060. [DOI] [PubMed] [Google Scholar]
- 13.Martenson JA, Halyard MY, Sloan JA, Proulx GM, Miller RC, Deming RL, et al. Phase III, double-blind study of depot octreotide versus placebo in the prevention of acute diarrhea in patients receiving pelvic radiation therapy: results of North Central Cancer Treatment Group N00CA. J Clin Oncol. 2008 Nov 10;26(32):5248–53. doi: 10.1200/JCO.2008.17.1546. Epub 2008 Sep 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bismar MM, Sinicrope FA. Radiation enteritis. Curr Gastroenterol Rep. 2002 Oct;4(5):361–5. doi: 10.1007/s11894-002-0005-3. [DOI] [PubMed] [Google Scholar]
- 15.Babb RR. Radiation proctitis: a review. Am J Gastroenterol. 1996 Jul;91(7):1309–11. [PubMed] [Google Scholar]
- 16.Yeoh E. Radiotherapy: long-term effects on gastrointestinal function. Curr Opin Support Palliat Care. 2008 Mar;2(1):40–4. doi: 10.1097/SPC.0b013e3282f4451f. [DOI] [PubMed] [Google Scholar]
- 17.Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. EORTC Radiotherapy Group Trial 22921. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006 Sep 14;355(11):1114–23. doi: 10.1056/NEJMoa060829. Erratum in: N Engl J Med. 2007 Aug 16;357(7):728. [DOI] [PubMed] [Google Scholar]
- 18.Arnaud JP, Nordlinger B, Bosset JF, Boes GH, Sahmoud T, Schlag PM, et al. Radical surgery and postoperative radiotherapy as combined treatment in rectal cancer: final results of a phase III study of the European Organization for Research and Treatment of Cancer. Br J Surg. 1997 Mar;84(3):352–7. [PubMed] [Google Scholar]
- 19.Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004 Oct 21;351(17):1731–40. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 20.Boersma LJ, van den Brink M, Bruce AM, Shouman T, Gras L, te Velde A, et al. Estimation of the incidence of late bladder and rectum complications after high-dose (70–78 GY) conformal radiotherapy for prostate cancer, using dose-volume histograms. Int J Radiat Oncol Biol Phys. 1998 Apr 1;41(1):83–92. doi: 10.1016/s0360-3016(98)00037-6. [DOI] [PubMed] [Google Scholar]
- 21.Roach M, 3rd, DeSilvio M, Valicenti R, Grignon D, Asbell SO, Lawton C, et al. Whole-pelvis, “mini-pelvis,” or prostate-only external beam radiotherapy after neoadjuvant and concurrent hormonal therapy in patients treated in the Radiation Therapy Oncology Group 9413 trial. Int J Radiat Oncol Biol Phys. 2006 Nov 1;66(3):647–53. doi: 10.1016/j.ijrobp.2006.05.074. [DOI] [PubMed] [Google Scholar]
- 22.Yeoh E, Horowitz M, Russo A, Muecke T, Robb T, Maddox A, et al. Effect of pelvic irradiation on gastrointestinal function: a prospective longitudinal study. Am J Med. 1993 Oct;95(4):397–406. doi: 10.1016/0002-9343(93)90309-d. [DOI] [PubMed] [Google Scholar]
- 23.Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Warlam-Rodenhuis CC, et al. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicenter randomised trial. Lancet. 2000 Apr 22;355(9213):1404–11. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- 24.Potish RA. Importance of predisposing factors in the development of enteric damage. Am J Clin Oncol. 1982 Apr;5(2):189–94. doi: 10.1097/00000421-198204000-00068. [DOI] [PubMed] [Google Scholar]
- 25.Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991 May 15;21(1):109–22. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 26.Kavanagh BD, Pan CC, Dawson LA, Das SK, Li XA, Ten Haken RK, et al. Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys. 2010 Mar 1;76(3 Suppl):S101–7. doi: 10.1016/j.ijrobp.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 27.Michalski JM, Gay H, Jackson A, Tucker SL, Deasy JO. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010 Mar 1;76(3 Suppl):S123–9. doi: 10.1016/j.ijrobp.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arbuck SG, Ivy SP, Setser A, et al. The revised Common Toxicity Criteria: Version 2.0 [Internet] CTEP Website. Available from: http://ctep.info.nih.gov.
- 29.Kollmorgen CF, Meagher AP, Wolff BG, Pemberton JH, Martenson JA, Illstrup DM. The long-term effect of adjuvant postoperative chemoradiotherapy for rectal carcinoma on bowel function. Ann Surg. 1994 Nov;220(5):676–82. doi: 10.1097/00000658-199411000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sloan JA, Loprinzi CL, Kuross SA, Miser AW, O’Fallon JR, Mahoney MR, et al. Randomized comparison of four tools measuring overall quality of life in patients with advanced cancer. J Clin Oncol. 1998 Nov;16(11):3662–73. doi: 10.1200/JCO.1998.16.11.3662. [DOI] [PubMed] [Google Scholar]
- 31.Hyland ME, Sodergren SC. Development of a new type of global quality of life scale, and comparison of performance and preference for 12 global scales. Qual Life Res. 1996 Oct;5(5):469–80. doi: 10.1007/BF00540019. [DOI] [PubMed] [Google Scholar]
- 32.Sloan JA, Aaronson N, Cappelleri JC, Fairclough DL, Varricchio C Clinical Significance Consensus Meeting Group. Assessing the clinical significance of single items relative to summated scores. Mayo Clin Proc. 2002 May;77(5):479–87. [PubMed] [Google Scholar]
- 33.Sloan JA, Berk L, Roscoe J, Fisch MJ, Shaw EG, Wyatt G, et al. National Cancer Institute. Integrating patient-reported outcomes into cancer symptom management clinical trials supported by the National Cancer Institute-sponsored clinical trials networks. J Clin Oncol. 2007 Nov 10;25(32):5070–7. doi: 10.1200/JCO.2007.12.7670. [DOI] [PubMed] [Google Scholar]
- 34.Crook J, Esche B, Futter N. Effect of pelvic radiotherapy for prostate cancer on bowel, bladder, and sexual function: the patient’s perspective. Urology. 1996 Mar;47(3):387–94. doi: 10.1016/S0090-4295(99)80458-0. [DOI] [PubMed] [Google Scholar]
- 35.Lundby L, Jensen VJ, Overgaard J, Laurberg S. Long-term colorectal function after postoperative radiotherapy for colorectal cancer. Lancet. 1997 Aug 23;350(9077):564. doi: 10.1016/s0140-6736(05)63141-8. [DOI] [PubMed] [Google Scholar]
- 36.Dahlberg M, Glimelius B, Graf W, Pahlman L. Preoperative irradiation affects functional results after surgery for rectal cancer: results from a randomized study. Dis Colon Rectum. 1998 May;41(5):543–9. doi: 10.1007/BF02235256. [DOI] [PubMed] [Google Scholar]