Abstract
Background
Antibiotic use may be associated with higher breast cancer risk and breast cancer mortality, but no study has evaluated the relation between antibiotic use and second breast cancer events (SBCE).
Methods
We conducted a retrospective cohort study among women ≥18 years, diagnosed with incident stage I/II breast cancer during 1990–2008. Antibiotic use and covariates were obtained from health plan administrative databases and medical record review. Frequent antibiotic use was defined as ≥4 antibiotic dispensings in any moving 12-month period after diagnosis. Our outcome was SBCE defined as recurrence or second primary breast cancer. We used multivariable Cox proportional hazards models to estimate hazard ratios (HR) and 95% confidence intervals (CI), accounting for competing risks.
Results
4,216 women were followed for a median of 6.7 years. Forty percent were frequent antibiotic users and 558 (13%) had a SBCE. Results are suggestive of a modest increased risk of SBCE (HR 1.15, 95% CI 0.88–1.50) among frequent antibiotic users compared to nonusers. Any potential increased risk was not supported when we evaluated recent use and past use. We observed no dose-response trends for SBCE with increasing duration of antibiotic use nor did we find evidence for altered SBCE risk in the antibiotic classes studied.
Conclusions
Frequent antibiotic use may be associated with modestly elevated risk of SBCEs, but the association was not significant.
Impact
Additional investigation by antibiotic class and underlying indication are important next steps given the high prevalence of frequent antibiotic use and growing number of breast cancer survivors.
Keywords: antibiotics, breast cancer, recurrence, second primary, prognosis
INTRODUCTION
Each year in the United States more than 200,000 women are diagnosed with breast cancer (1), and currently there are an estimated 2.6 million breast cancer survivors (2). Breast cancer survivors are at risk for recurrence and second primary breast cancers (3).
Antibiotics were proposed as a risk factor for cancer over two decades ago (4). Biological mechanisms have not been clearly established but suggest antibiotics could have different effects on cancer risk (5). Antibiotics may promote cancer by disrupting the ability of intestinal microflora to metabolize phytochemicals into biologically active compounds hypothesized to protect against cancer (4). Antibiotic-induced disturbance of intestinal microflora may also decrease the risk of some hormonal cancers by disrupting estrogen metabolism, resulting in lower levels of circulating estrogens (5).
Several epidemiologic studies report on antibiotic use and risk of incident breast cancer with conflicting results, as some studies demonstrated no association (6–10) and others have suggested antibiotic use increases risk (11–15). Notably, one of these studies found a dose-response trend for both incident breast cancer risk and breast cancer related mortality compared to non-users; as much as a two-fold higher risk of incident breast cancer was found among women with >500 cumulative days of antibiotic use (15). To our knowledge, no study to date has evaluated antibiotic use and risk of recurrence and second primary breast cancers among women with a history of breast cancer.
The risk-benefit of antibiotic use in relation to breast cancer outcomes is most relevant for chronic or frequent use. Antibiotics are widely prescribed and frequent antibiotic use is common (15), such as for the management of acne and/or rosacea, recurrent urinary tract infections, or chronic obstructive pulmonary disease. Antibiotics are often overused or prescribed inappropriately (16); therefore a better understanding of risks associated with frequent use could bolster measures to improve antibiotic prescribing. We therefore evaluated the association between frequent antibiotic use and risk of recurrence and second primary breast cancer among women diagnosed with early stage invasive breast cancer.
MATERIALS AND METHODS
Study Population
This retrospective cohort study was conducted within Group Health Cooperative (GH), a nonprofit integrated health care delivery system that provides comprehensive health care on a pre-paid basis to approximately 620,000 individuals throughout Washington State and parts of Idaho. GH is located within the geographic reporting region of the western Washington Cancer Surveillance System, a population-based cancer Surveillance, Epidemiology, and End Results (SEER) registry (2). Women were included if they were: 1) ≥18 years of age; 2) diagnosed with an incident, histologically confirmed stage I or II (17) breast cancer between January 1, 1990 and December 31, 2008 via the SEER registry; 3) had no evidence of bilateral disease per the SEER registry; and 4) enrolled in GH’s integrated group practice for at least 1 year before and 1 year after the breast cancer diagnosis (unless they died). A total of 4,426 subjects were identified and sent to chart review, of which a subset (1,268 women diagnosed 1990–1999) was already partially abstracted (first 5 years post diagnosis) as part of two previous studies (18–20). We verified the eligibility of 4,225 women per chart review (women were excluded for no medical record (n=72), bilateral disease (n=6), breast cancers that were not first primaries (n=79), and no definitive surgery (n=44)). We required that women be alive and recurrence free for 120 days after completing definitive surgery for the incident breast cancer, yielding a final cohort of 4,216 women (excluding 5 deaths and 4 metastases prior to 120 days). The GH Institutional Review Board approved this study.
Data Collection
Information on patient and tumor characteristics, breast cancer treatment, outcomes (i.e., recurrence and second primaries), other cancers, co-morbid conditions of interest, and breast cancer surveillance were obtained from GH automated databases, review of medical records by trained abstractors, and SEER. Data were collected on all eligible women from one year before diagnosis through the earliest of death, disenrollment from GH (a lapse in membership of 90+ days), or end of study (i.e., date of chart abstraction). GH’s automated databases include demographics, enrollment, inpatient and outpatient diagnoses and procedures, breast imaging (e.g., mammography) results, pharmacy dispensings, laboratory results, vital signs, and death (21). Charlson co-morbidity index scores (22) were collected annually from medical charts and automated databases. Deaths are determined through GH’s link to Washington State death tapes (23). SEER was the gold standard for incident breast cancer characteristics including year of diagnosis, American Joint Committee on Cancer (AJCC) stage (17), lymph node status, hormone receptor status, and tumor size. Education and menopausal status were collected from a self-administered questionnaire on breast cancer risk factors completed at each screening mammogram (24).
Antibiotic Exposure
We identified all outpatient prescription medication use dispensed at any GH pharmacy as well as claims received from outside contracting pharmacies. The database contains one record per dispensing that includes: drug name, date of dispensing, quantity dispensed, route, strength, days supply, prescriber, and National Drug Code number. We identified all antibiotics dispensed in the year before each woman’s diagnosis through the end of follow-up.
We included the following antibiotic classes in our exposure ascertainment: penicillins, cephalosporins, sulfonamides, fluoroquinolones, tetracyclines, nitrofurans, macrolides, ketolides, carbapenems, oxazolidinones (linezolid), glycopeptides (vancomycin), folate antagonists (trimethoprim), nitroimidazoles (metronidazole), and methenamine. A dispensing was defined as a prescription with ≤30 days supply. If a dispensing had >30 days supply, we split the dispensing into multiple dispensings reflective of days supply ≤30 days. For example, a dispensing of 90 days supply was counted as three 30 day dispensings. Based on consensus of a group of prescribers and pharmacists, frequent antibiotic use was defined as having 4+ dispensings for any antibiotic within any moving 12-month period after breast cancer diagnosis. Women were categorized as frequent users at the time of the fourth dispensing. Infrequent antibiotic use was defined as having 1–3 antibiotic dispensings within any moving 12-month period. Nonusers were defined as women with no dispensings for an antibiotic. Exposure was time-varying. Women were only allowed to transition from non-user, to infrequent user, to frequent user over time. Once a woman was classified as a frequent user, she remained a frequent user throughout the rest of the follow-up period. We established a priori that frequent antibiotic use was the most clinically meaningful exposure so we only report frequent users versus non-users in our study. Dispensings before breast cancer diagnosis were not included even though its use could potentially last past diagnosis.
Additionally, we evaluated several secondary exposures. To investigate the influence of recency of use on SBCE risk, we stratified frequent use according to the following mutually exclusive categories: non-use, recent use (became frequent user <3 years prior to event date), and past use (became frequent user ≥3 years prior to event date). We restricted this analysis to person-time and events ≥3 years since diagnosis (N=3,475). We further explored frequent use for each of the 5 most commonly dispensed antibiotic classes where a woman had to fill 4+ prescriptions from the antibiotic class of interest within any moving 12-month period after diagnosis. Women could contribute to multiple antibiotic classes.
Duration of use was estimated by summing the days supply for each antibiotic prescription to obtain the cumulative days of antibiotic dispensings (15). Duration of use was time-varying. We restricted this analysis to women with >500 days of follow-up (N=3,996) to include only those who had the opportunity to be in any of the exposure categories (1–50 days, 51–100 days, 101–500 days and >500 days of antibiotic use). We also estimated duration of use by the top 5 most commonly dispensed antibiotic classes during follow-up. Women could contribute to multiple antibiotic classes if she filled at least one prescription for that class.
Outcome Ascertainment
The primary outcome was second breast cancer event (SBCE), defined as the first ductal carcinoma in situ (DCIS) or an invasive cancer of the ipsilateral (recurrence) or contralateral (second primary) breast or in any regional or distant sites (25). A woman was at risk for a SBCE starting 120 days after completing definitive surgery for the incident breast cancer (index date) (20).
Data Analysis
We used multivariable Cox proportional hazards models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) to assess whether frequent antibiotic use was associated with SBCEs while accounting for competing risks. We also stratified the primary analysis by tumor histology of the incident breast cancer (ductal, lobular, mixed/other). We modeled time from the incident breast cancer with a delayed entry at 120 days post-surgery (19, 20) to the composite and individual outcomes as a function of frequent antibiotic use while adjusting for precision variables (i.e., variables associated with the outcome) and potential confounders. Individual events (i.e., recurrence and second primaries) that make up the composite outcome were also modeled to obtain a comprehensive assessment of the medication effects (26). Women were followed from their index date until the first of SBCE, death, disenrollment, or end of study. In analyses of individual events (e.g., recurrence), women were censored at the earliest of disenrollment, end of follow-up, and other competing events (e.g., death and second primary). We evaluated secondary exposures including frequent antibiotic use by antibiotic class and duration of use (all antibiotics and by antibiotic class). Nonusers were the reference category in all analyses. All exposures were time-varying.
All models were adjusted for confounders we selected a priori, including diagnosis year (1990–1994, 1995–1999, 2000–2004, 2005–2008), age at diagnosis (18–49, 50–59, 60–69, 70–79, 80+ years), menopausal status at diagnosis (peri- or pre-menopausal, post-menopausal), body mass index (BMI) at diagnosis (<18.5, 18.5–24.9, 25.0–29.9, 30.0–34.9, 35+ kg/m2), smoking status at diagnosis (current, past, never/unknown), Charlson comorbidity score (0, 1, 2+, time-varying), conditions commonly managed with chronic antibiotic use (yes/no, time-varying for acne and/or rosacea, chronic obstructive pulmonary disease (COPD), urinary tract infection (UTI)), and surveillance mammography receipt in the prior 12 months (yes/no, time-varying). All models were further adjusted for precision variables predictive of SBCEs, including AJCC stage (I, IIA, IIB) hormone receptor status (estrogen receptor [ER] negative/progesterone receptor [PR] negative, ER+/PR−, ER−/PR+, ER+/PR+), primary treatment for the incident breast cancer (mastectomy, breast conserving surgery with radiation, breast conserving surgery without radiation), and endocrine therapy (yes/no, time-varying).
We evaluated the proportional hazards assumption by testing for an interaction between the exposure variable and the logarithm of time since diagnosis. There was no evidence suggesting a violation of the proportional hazards assumption. All analyses were performed using SAS statistical software version 9.2 (SAS Institute Inc, Cary, North Carolina).
Sensitivity Analyses
We assessed the influence of assumptions about the antibiotic exposure on our results. We varied the definition of frequent antibiotic use to 5+ and 6+ dispensings in any 12-month period post-diagnosis. We excluded antibiotic use in the year immediately preceding the event to rule out protopathic bias. We also excluded antibiotic use during the first year post-diagnosis since antibiotic use during this time may be related to immunosuppression from the treatment regimen. Last, we considered frequent antibiotic use during the 12 months pre-diagnosis as a potential confounder.
RESULTS
The median age of the cohort at initial breast cancer diagnosis was 63 years. The majority of women were post-menopausal (72.9%), White (88.5%), had some college or higher education (76.6%), were not smokers (85.6%), and had a Charlson comorbidity score of zero (76.5%).
During the study period, 1,678 (40%) were frequent antibiotic users with 9,112 person-years of follow-up. Penicillins and cephalosporins were the most commonly prescribed classes, and this was consistent throughout the study period. The average time between the first and fourth antibiotic dispensings for frequent users was approximately 6 months and the average cumulative duration of use was 235 days. Characteristics of the study cohort by antibiotic use are described in Table 1. Women who became frequent antibiotic users during follow-up were more likely to have received an antibiotic prescription and to have been frequent antibiotic users in the 12 months pre-diagnosis compared to nonusers. Frequent antibiotic users were diagnosed in earlier years, had longer duration of follow-up, higher Charlson scores, higher BMI, and were more likely to be older, White, and have acne and/or rosacea, COPD, UTIs, and other cancers compared to nonusers. Frequent antibiotic users were also more likely to initial tumors that were smaller, non ductal carcinomas tested for HER2 and treated with mastectomy and endocrine therapy. During follow-up, frequent antibiotic users were less likely to receive surveillance mammography compared to nonusers.
Table 1.
Descriptive characteristics of women, by antibiotic medication use after early stage breast cancer diagnosis
| Nonuser (n=525) | Frequent Antibiotic User (n=1,678) | P valuea | |
|---|---|---|---|
| Characteristics at diagnosis of initial breast cancer | |||
| Year of diagnosis | |||
| 1990–1994 | 87 (16.6) | 468 (27.9) | <0.001 |
| 1995–1999 | 110 (21.0) | 539 (32.1) | |
| 2000–2004 | 161 (30.7) | 427 (25.4) | |
| 2005–2008 | 167 (31.8) | 244 (14.5) | |
| Years of follow-up | |||
| Median (IQR) | 3.7 (0.5–16.8) | 7.6 (0.5–19.2) | <0.001 |
| Age, years | |||
| Median (IQR) | 61 (34–91) | 64 (27–93) | <0.01 |
| 18–39 | 18 (3.4) | 56 (3.3) | 0.03 |
| 40–49 | 94 (17.9) | 227 (13.5) | |
| 50–59 | 131 (25.0) | 386 (23.0) | |
| 60–69 | 122 (23.2) | 431 (25.7) | |
| 70–79 | 93 (17.7) | 384 (22.9) | |
| 80+ | 67 (12.8) | 194 (11.6) | |
| Menopausal status | |||
| Peri- or Pre-menopausal | 158 (30.1) | 442 (26.3) | 0.09 |
| Post-menopausal | 367 (69.9) | 1236 (73.7) | |
| Race | |||
| White | 436 (83.7) | 1527 (91.2) | <0.001 |
| African American | 28 (5.4) | 39 (2.3) | |
| American Indian/Alaska Native | 8 (1.5) | 53 (3.2) | |
| Asian/Pacific Islander | 49 (9.4) | 55 (3.3) | |
| Unknown | 4 | 4 | |
| Education | |||
| High school or less | 51 (21.2) | 158 (25.5) | 0.18 |
| At least some college | 190 (78.8) | 461 (74.5) | |
| Unknown | 284 | 1059 | |
| Body mass index (kg/m2) | |||
| <18.5 | 13 (2.5) | 27 (1.6) | <0.001 |
| 18.5–24.9 | 209 (40.2) | 519 (31.1) | |
| 25.0–29.9 | 157 (30.2) | 546 (32.7) | |
| 30.0–34.9 | 85 (16.3) | 316 (18.9) | |
| 35+ | 56 (10.8) | 261 (15.6) | |
| Unknown | 5 | 9 | |
| Smoking status | |||
| Current | 33 (6.3) | 91 (5.4) | 0.10 |
| Past | 51 (9.7) | 119 (7.1) | |
| Never | 441 (84.0) | 1468 (87.5) | |
| Charlson score | |||
| 0 | 425 (81.0) | 1183 (70.5) | <0.001 |
| 1 | 75 (14.3) | 363 (21.6) | |
| 2+ | 25 (4.8) | 132 (7.9) | |
| Medication use in year prior to diagnosis | |||
| Antibiotic use, ever | 132 (25.1) | 974 (58.0) | <0.001 |
| Frequent antibiotic user | 8 (1.5) | 256 (15.3) | <0.001 |
| AJCC stage(17) | |||
| I | 308 (58.7) | 1049 (62.5) | 0.06 |
| IIA | 163 (31.0) | 433 (25.8) | |
| IIB | 54 (10.3) | 196 (11.7) | |
| Lymph node status | |||
| Negative | 363 (78.1) | 1095 (73.9) | 0.20 |
| Positive | 102 (21.9) | 386 (26.1) | |
| Unknown | 60 | 197 | |
| ER/PR status | |||
| ER−/PR− | 90 (17.1) | 255 (15.2) | 0.73 |
| ER+/PR− | 47 (9.0) | 167 (10.0) | |
| ER−/PR+ | 8 (1.5) | 35 (2.1) | |
| ER+/PR+ | 350 (66.7) | 1123 (66.9) | |
| ER & PR unknown | 30 (5.7) | 98 (5.8) | |
| Tumor size | |||
| ≤ 2 cm | 362 (69.0) | 1257 (74.9) | <0.01 |
| > 2 cm | 163 (31.0) | 421 (25.1) | |
| Tumor histology | |||
| Ductal | 435 (82.9) | 1290 (76.9) | <0.01 |
| Lobular | 31 (5.9) | 168 (10.0) | |
| Mixed/other | 59 (11.2) | 220 (13.1) | |
| HER2 test result | |||
| Test done | 322 (83.0) | 678 (76.1) | <0.01 |
| Positive/Borderline | 57 (17.7) | 119 (17.6) | 0.74 |
| Negative | 263 (81.7) | 557 (82.2) | |
| No result | 2 (0.6) | 2 (0.3) | |
| Surgical procedure | |||
| Mastectomy +/− radiation | 176 (33.5) | 681 (40.6) | 0.001 |
| BCS + radiation | 262 (49.9) | 802 (47.8) | |
| BCS | 87 (16.6) | 195 (11.6) | |
| Other treatment | |||
| Chemotherapy | 164 (31.2) | 545 (32.5) | 0.59 |
| Completed course | 147 (89.6) | 472 (86.6) | 0.59 |
| Endocrine therapy | 269 (51.2) | 969 (57.7) | <0.01 |
| Characteristics throughout study period | |||
| Co-morbidities | |||
| Acne and/or rosacea | 28 (5.3) | 191 (11.4) | <0.001 |
| COPD | 47 (9.0) | 358 (21.3) | <0.001 |
| UTI | 53 (10.1) | 912 (54.4) | <0.001 |
| Other cancers | 22 (4.2) | 166 (9.9) | <0.001 |
| % follow-up years with yearly surveillance mammogram | |||
| <50% | 146 (27.8) | 391 (23.3) | <0.001 |
| 50%–80% | 130 (24.8) | 627 (37.4) | |
| >80% | 249 (47.4) | 660 (39.3) | |
Abbreviations: IQR=interquartile range; BCS=breast conserving surgery; HER2= Human Epidermal Growth Factor Receptor 2.
Data are represented as number (percentage) unless otherwise noted. Frequent antibiotic users included women with 4+ dispensings in any moving 12-month period post-diagnosis. Infrequent antibiotic users are not presented here as this is not a clinically meaningful exposure.
To test for differences of the characteristics between the two groups, we used chi-square test for categorical variables and Wilcoxon rank-sum test for medians.
Among the 4,216 women in our cohort, 432 (10%) women had a recurrence and 153 (4%) had a second primary breast cancer, a total of 558 having a SBCE (n=415 recurrences and n=143 second primary breast cancers). Among recurrences, 67% were distant, 32% were local or regional, and 1% was DCIS. Among second primary cancers, 21% were DCIS, 49% were stage I, 21% were stage II, 4% were stage III/IV, and staging was unavailable for 5%. Histological types of the SBCEs were 35% ductal, 3% lobular, and 62% mixed/other. The distribution did not differ by exposure status. The median follow-up was 6.7 years and the median time to first SBCE was 3.3 years.
Characteristics of the study cohort by SBCE status are described in Table 2. SBCE cases were diagnosed in earlier years, had shorter duration of follow-up, and were more likely to have initial cancers that were not tested for HER2 and were higher stage, larger tumor size, lymph node positive, and hormone receptor negative compared to non-cases. Consistent with these clinical characteristics, SBCE cases were more likely to receive mastectomy and chemotherapy but less likely to receive endocrine therapy. Women experiencing a SBCE were also more likely to be younger, non White, pre- or peri-menopausal, less likely to be smokers and less likely to have acne and/or rosacea, COPD, UTIs, and other cancers compared to women who did not experience a SBCE. During follow-up, SBCE cases had shorter duration of antibiotic use, and were less likely to be frequent antibiotic users and to receive surveillance mammography.
Table 2.
Descriptive characteristics of women, by second breast cancer event status
| SBCE (N=4,216) | P valuea | ||
|---|---|---|---|
| No (n=3,658) | Yes (n=558) | ||
| Characteristics at diagnosis of initial breast cancer | |||
| Year of diagnosis | |||
| 1990–1994 | 755 (20.6) | 195 (34.9) | <0.001 |
| 1995–1999 | 1020 (27.9) | 171 (30.6) | |
| 2000–2004 | 1073 (29.3) | 128 (22.9) | |
| 2005–2008 | 810 (22.1) | 64 (11.5) | |
| Years of follow-up | |||
| Median (IQR) | 6.7 (0.4–19.4) | 3.3 (0.5–16.5) | <0.001 |
| Age, years | |||
| Median (IQR) | 63 (28–95) | 62 (29–89) | <0.01 |
| 18–39 | 112 (3.1) | 27 (4.8) | 0.06 |
| 40–49 | 544 (14.9) | 102 (18.3) | |
| 50–59 | 866 (23.7) | 129 (23.1) | |
| 60–69 | 889 (24.3) | 129 (23.1) | |
| 70–79 | 824 (22.5) | 116 (20.8) | |
| 80+ | 423 (11.6) | 55 (9.9) | |
| Menopausal status | |||
| Peri- or Pre-menopausal | 956 (26.1) | 189 (33.9) | <0.001 |
| Post-menopausal | 2702 (73.9) | 369 (66.1) | |
| Race | |||
| White | 3232 (88.7) | 487 (87.3) | <0.01 |
| African American | 104 (2.9) | 32 (5.7) | |
| American Indian/Alaska Native | 104 (2.9) | 9 (1.6) | |
| Asian/Pacific Islander | 203 (5.6) | 30 (5.4) | |
| Unknown | 15 | 0 | |
| Education | |||
| High school or less | 393 (23.5) | 25 (21.4) | 0.83 |
| At least some college | 1279 (76.5) | 92 (78.6) | |
| Unknown | 1986 | 441 | |
| Body mass index (kg/m2) | |||
| <18.5 | 55 (1.5) | 14 (2.5) | 0.37 |
| 18.5–24.9 | 1269 (34.8) | 184 (33.3) | |
| 25.0–29.9 | 1186 (32.6) | 176 (31.8) | |
| 30.0–34.9 | 666 (18.3) | 100 (18.1) | |
| 35+ | 467 (12.8) | 79 (14.3) | |
| Unknown | 15 | 5 | |
| Smoking status | |||
| Current | 230 (6.3) | 23 (4.1) | 0.01 |
| Past | 318 (8.7) | 34 (6.1) | |
| Never | 3110 (85.0) | 501 (89.8) | |
| Charlson score | |||
| 0 | 2784 (76.1) | 445 (79.7) | 0.16 |
| 1 | 625 (17.1) | 79 (14.2) | |
| 2+ | 249 (6.8) | 34 (6.1) | |
| Medication use in year prior to diagnosis | |||
| Antibiotic use, ever | 1621 (44.3) | 251 (45.0) | 0.77 |
| Frequent antibiotic user | 277 (7.6) | 45 (8.1) | 0.68 |
| AJCC stage(17) | |||
| I | 2384 (65.2) | 264 (47.3) | <0.001 |
| IIA | 906 (24.8) | 172 (30.8) | |
| IIB | 368 (10.1) | 122 (21.9) | |
| Lymph node status | |||
| Negative | 2525 (77.4) | 322 (64.3) | <0.001 |
| Positive | 739 (22.6) | 179 (35.7) | |
| Unknown | 394 | 57 | |
| ER/PR status | |||
| ER−/PR− | 531 (14.5) | 136 (24.4) | <0.001 |
| ER+/PR− | 319 (8.7) | 64 (11.5) | |
| ER−/PR+ | 47 (1.3) | 14 (2.5) | |
| ER+/PR+ | 2572 (70.3) | 316 (56.6) | |
| ER & PR unknown | 189 (5.2) | 28 (5.0) | |
| Tumor size | |||
| ≤ 2 cm | 2785 (76.1) | 325 (58.5) | <0.001 |
| > 2 cm | 873 (23.9) | 231 (41.5) | |
| Unknown | 0 | 2 | |
| HER2 test result | |||
| Test done | 1874 (80.5) | 200 (73.0) | <0.01 |
| Positive/Borderline | 311 (16.6) | 42 (21.0) | 0.21 |
| Negative | 1556 (83.0) | 158 (79.0) | |
| No result | 7 (0.4) | 0 | |
| Surgical procedure | |||
| Mastectomy +/− radiation | 1289 (35.2) | 232 (41.6) | <0.001 |
| BCS + radiation | 1927 (52.7) | 245 (43.9) | |
| BCS | 442 (12.1) | 81 (14.5) | |
| Other treatment | |||
| Chemotherapy | 1142 (31.2) | 234 (41.9) | <0.001 |
| Completed course | 1003 (87.8) | 209 (89.3) | 0.74 |
| Endocrine therapy | 2101 (57.4) | 262 (47.0) | <0.001 |
| Characteristics throughout study period | |||
| Co-morbidities | |||
| Acne and/or rosacea | 329 (9.0) | 31 (5.6) | <0.01 |
| COPD | 574 (15.7) | 58 (10.4) | <0.001 |
| UTI | 1392 (38.1) | 146 (26.2) | <0.001 |
| Other cancers | 303 (8.3) | 24 (4.3) | <0.001 |
| Antibiotic use post-diagnosis | |||
| Frequent user | 1476 (40.3) | 202 (36.2) | <0.001 |
| Infrequent user | 1762 (48.2) | 251 (45.0) | |
| Nonuser | 420 (11.5) | 105 (18.8) | |
| Max cumulative days supply | |||
| 0 | 420 (11.5) | 105 (18.8) | <0.001 |
| 1–50 | 1527 (41.7) | 250 (44.8) | |
| 51–100 | 723 (19.8) | 89 (15.9) | |
| 101–500 | 838 (22.9) | 104 (18.6) | |
| ≥501 | 150 (4.1) | 10 (1.8) | |
| % of follow-up years with yearly surveillance mammogram | |||
| <50% | 793 (21.7) | 146 (26.2) | <0.01 |
| 50%–80% | 1284 (35.1) | 155 (27.8) | |
| >80% | 1581 (43.2) | 257 (46.1) | |
Abbreviations: IQR=interquartile range; BCS=breast conserving surgery; HER2= Human Epidermal Growth Factor Receptor 2.
Data are represented as number (percentage) unless otherwise noted. Frequent antibiotic users included women with 4+ dispensings in any moving 12-month period post-diagnosis. Infrequent antibiotic users included women with 1–3 dispensings in any moving 12-month period post-diagnosis.
To test for differences of the characteristics between the two groups, we used chi-square test for categorical variables and Wilcoxon rank-sum test for medians.
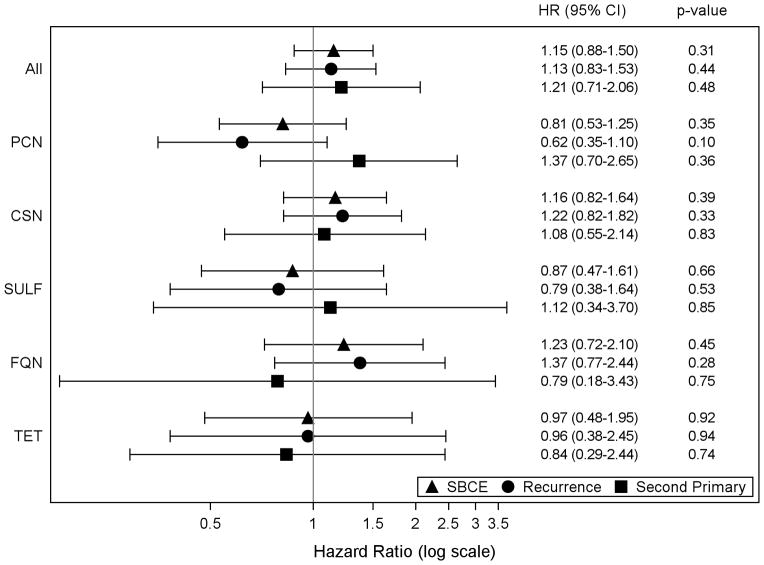
The unadjusted hazard ratios for frequent use were 1.31 (95% CI 1.02–1.68) for SBCE, 1.38 (95% CI 1.03–1.84) for recurrences, and 1.11 (95% CI 0.67–1.84) for second primaries. Hazard ratios from the multivariable-adjusted models suggest a modestly elevated risk of SBCE, including both recurrences and second primaries, with frequent antibiotic use versus no-use but the differences were not statistically significant: 1.15 (95% CI 0.88–1.50) for SBCE, 1.13 (95% CI 0.83–1.53) for recurrences, and 1.21 (0.71–2.06) for second primaries (Figure 1). The potential association with SBCE was not supported when frequent antibiotic use was stratified by recent use (<3 years) (HR 0.78, 95% CI 0.53–1.17) and past use (≥3 years) (HR 0.91, 95% CI 0.48–1.72), or duration of use compared to no-use (Table 3). In the subgroup analysis by histological type of the incident breast cancer, the adjusted hazard ratios for frequent antibiotic use and risk of SBCEs were: 1.15 (95% CI 0.86–1.54) for ductal, 2.99 (95% CI 0.95–9.40) for lobular, and 0.62 (95% CI 0.25–1.55) for mixed/other histologies. Findings for the individual outcomes of SBCE (i.e., recurrence and second primaries) were similar to those observed for SBCEs (data not shown). The associations for frequent use and SBCE (or the individual outcomes of recurrence and second primaries) by antibiotic class varied from slightly reduced to slightly elevated risk but none were statistically significant and most of the CIs were wide (Figure 1). We observed some significant associations when we examined duration of use by antibiotic class, though there are no clear trends overall (Table 3).
Figure 1.
Adjusted hazard ratios and 95% confidence intervals (CI) for frequent antibiotic use and the risk of second breast cancer events. Abbreviations: CSN=cephalosporins; FQN=fluoroquinolones; PCN=penicillins; SULF=sulfonamides; TET=tetracyclines. Frequent antibiotic users included women with 4+ dispensings in any moving 12-month period post-diagnosis. Nonusers were the reference category for all analyses. A woman could contribute to multiple antibiotic classes if she met the frequent use definition for that class. We excluded 20 women from analyses due to missing BMI information. All models adjusted for age, incident breast cancer diagnosis year, AJCC stage, hormone receptor status, primary treatment for the initial breast cancer, endocrine therapy, body mass index, smoking status, menopausal status, Charlson co-morbidity score, acne and/or rosacea, COPD, UTI, and receipt of surveillance mammography.
Table 3.
Adjusted hazard ratios and 95% confidence intervals for duration of antibiotic use and the risk of second breast cancer events.
| Antibiotic Exposure | Adjusted Hazard Ratios and 95% Confidence Intervalsa | ||
|---|---|---|---|
| SBCE (n cases=558) | Recurrence (n cases= 415) | Second Primary Breast Cancer (n cases=143) | |
| Any antibiotic | |||
| 1–50 days | 0.82 (0.63–1.08) | 0.79 (0.58–1.07) | 0.94 (0.52–1.71) |
| 51–100 days | 0.98 (0.71–1.37) | 0.82 (0.56–1.20) | 1.56 (0.81–3.02) |
| 101–500 days | 1.14 (0.81–1.61) | 1.11 (0.75–1.65) | 1.37 (0.68–2.76) |
| >=501 days | 0.83 (0.42–1.66) | 0.96 (0.44–2.08) | 0.59 (0.13–2.71) |
| Trend test p-value | 0.25 | 0.41 | 0.33 |
| Penicillin | |||
| 1–50 days | 1.25 (1.04–1.50) | 1.23 (1.00–1.53) | 1.28 (0.89–1.84) |
| 51–100 days | 1.14 (0.74–1.76) | 1.06 (0.61–1.83) | 1.37 (0.65–2.85) |
| >=101 days | 1.22 (0.71–2.11) | 1.19 (0.61–2.33) | 1.31 (0.50–3.44) |
| Trend test p-value | 0.07 | 0.16 | 0.22 |
| Cephalosporin | |||
| 1–50 days | 1.20 (1.00–1.44) | 1.13 (0.92–1.40) | 1.39 (0.97–2.01) |
| 51–100 days | 1.12 (0.73–1.72) | 1.00 (0.59–1.69) | 1.48 (0.70–3.15) |
| >=101 days | 1.18 (0.65–2.14) | 1.13 (0.55–2.32) | 1.52 (0.52–4.50) |
| Trend test p-value | 0.13 | 0.42 | 0.10 |
| Sulfonamide | |||
| 1–50 days | 0.90 (0.73–1.11) | 0.99 (0.78–1.25) | 0.71 (0.47–1.07) |
| 51–100 days | 0.74 (0.43–1.27) | 1.08 (0.62–1.90) | 0.12 (0.02–0.91) |
| >=101 days | 1.14 (0.55–2.36) | 1.20 (0.48–2.98) | 1.11 (0.33–3.69) |
| Trend test p-value | 0.35 | 0.82 | 0.04 |
| Fluoroquinolone | |||
| 1–50 days | 1.03 (0.82–1.29) | 1.13 (0.87–1.46) | 0.70 (0.43–1.13) |
| 51–100 days | 1.09 (0.56–2.10) | 1.32 (0.63–2.75) | 0.65 (0.15–2.79) |
| >=101 days | 1.79 (0.77–4.15) | 2.12 (0.84–5.34) | 1.02 (0.13–7.72) |
| Trend test p-value | 0.40 | 0.17 | 0.21 |
| Tetracycline | |||
| 1–50 days | 0.88 (0.68–1.14) | 0.73 (0.52–1.00) | 1.40 (0.90–2.16) |
| 51–100 days | 0.92 (0.43–1.96) | 0.73 (0.27–2.00) | 1.69 (0.51–5.56) |
| >=101 days | 0.90 (0.45–1.79) | 0.93 (0.40–2.14) | 0.85 (0.26–2.86) |
| Trend test p-value | 0.42 | 0.14 | 0.41 |
Duration of antibiotic use is defined as cumulative days of dispensings. Nonusers were the reference category for all analyses. A woman could contribute to multiple antibiotic classes if she filled at least one prescription for that class. We excluded 20 women from analyses due to missing BMI information.
All models adjusted for age, incident breast cancer diagnosis year, AJCC stage, hormone receptor status, primary treatment for the initial breast cancer, endocrine therapy, body mass index, smoking status, menopausal status, Charlson co-morbidity score, acne and/or rosacea, COPD, UTI, and receipt of surveillance mammography.
Study findings were robust to sensitivity analyses where we varied definitions of frequent antibiotic use and evaluated risk of SBCE and its individual outcomes. Consideration of frequent antibiotic use pre-diagnosis did not change our results. We also did not observe appreciable changes in risk estimates when we excluded antibiotic dispensings during the first year post-diagnosis or the year immediately prior to the SBCE event date.
DISCUSSION
This study addresses an important gap in the literature as no prior studies have examined post-diagnosis antibiotic use among women with an early stage breast cancer diagnosis. There was suggestion that risk of SBCEs and its individual outcomes of recurrence and second breast primaries may be modestly elevated with frequent antibiotic use following diagnosis, though confidence intervals included 1.0. The least detectable HRs for SBCEs by frequent antibiotic use vs. no-use was 1.20 for increased risk – 2 sided test; α=0.05; β=0.80. The potential association with SBCE was not supported when we compared duration of antibiotic use to no-use and evaluated dose-response trends. Mechanistic studies suggest antibiotics may alter the risk of cancer primarily through direct effects on intestinal microflora. We hypothesized, therefore, that recency of use would influence the risk of SBCEs, yet we found no differences in risk when stratified by recent use and past use. We did, however, observe differences in the association between frequent antibiotic use and risk of SBCEs by histological subtype of the incident breast cancer. Women with ductal and lobular tumors demonstrated elevated risk of SBCEs with frequent antibiotic use and mixed/other tumors demonstrated reduced risk, though confidence intervals were wide and included 1.0.
Although we found no strong evidence of increased risk of SBCEs by antibiotic class, we lacked sufficient power for this secondary aim (least detectable HRs for SBCEs varied by class but all were less than 0.60 for reduced risk and greater than 1.50 for increased risk). We observed some statistically significant associations when we examined duration of use by antibiotic class (e.g. sulfonamide use). However, these results should be interpreted cautiously with multiple testing performed and in the absence of any significant trends.
Because this is the first study on post-diagnosis antibiotic use and SBCEs, we are unable to directly compare our findings with others. A previous study, conducted at Group Health, evaluated pre-diagnosis antibiotic use relative to incident breast cancer (15). In that case-control study of 2,266 women diagnosed 1993–2001, pre-diagnosis antibiotic use was associated with higher risk of incident breast cancer compared to nonusers and the association was stronger with increased duration of use. Odds ratios (95% CIs) for breast cancer were 1.45 (1.24–1.69) for 1–50 days use, 1.53 (1.28–1.83) for 51–100 days use, 1.68 (1.42–2.00) for 101–500 days use, 2.14 (1.60–2.88) for 501–1000 days use, and 2.07 (1.48–2.89) for ≥1001 days use (15). Although our findings among Group Health enrollees also suggest a higher risk of SBCEs, the magnitude of association is lower in our study, had wide confidence intervals, and demonstrated no trend with increasing duration of use. The inconsistent results may be attributed to different exposures since Velicer et al. only evaluated antibiotic use pre incident breast cancer diagnosis. We also evaluated different outcomes. As such, the risk of second primary breast cancers in our study is most comparable to the outcome of incident breast cancer in Velicer et al. Our findings suggest frequent antibiotic use after diagnosis may increase risk of second breast primaries but CIs included 1.0. Our study was not powered to evaluate the development of new cancers and we only observed 143 second breast primaries (least detectable HRs for second primaries by frequent antibiotic use vs. no-use was 0.58 for reduced risk and 1.55 for increased risk).
We developed the definition of frequent antibiotic use de novo based on clinician input and coupled this with sensitivity analyses where different thresholds for frequent antibiotic use provided consistent results with the primary analysis. Compared to nonusers, frequent antibiotic users were diagnosed in earlier years and had longer duration of follow-up which gives more opportunity to observe a SBCE. Pre-diagnosis antibiotic exposure may confound the association between post-diagnosis frequent antibiotic use and risk of SBCEs. Including frequent antibiotic use during the 12 months pre-diagnosis did not change our results but it is possible that this was not a sufficient duration of pre-diagnosis exposure. Subjects were from a single health plan which may not represent other care settings or populations. However, being enrolled in a health plan mitigates disparities in care that may exist among women without health coverage. There is always the potential for exposure misclassification for patients who receive a dispensing, but fail to take the drug. We required multiple dispensings in a year in order for a woman to be classified as a frequent user, which minimizes exposure misclassification since multiple dispensings in a defined period of time increase the possibility women actually took the medication. Women who received medications at non-GH pharmacies without submitting a claim to GH may be erroneously classified as nonusers. This is likely minor since previous studies have found GH enrollees obtained 97% of their medications at GH owned or contracting pharmacies (27, 28). Residual confounding from unknown factors we did not adjust for and from known factors we did adjust for (e.g., diagnosis year, Charlson score, BMI) is always possible. Of importance, we adjusted for conditions associated with chronic antibiotic use. Other studies found no association between UTIs and breast cancer risk (13) and no differences in breast cancer risk comparing different indications for antibiotic use (15). We also found no association between any of the co-morbidities of interest and SBCE.
Major strengths of our study include: a large sample of population-based breast cancer cases with extensive follow-up; complete ascertainment of incident cancer cases and tumor characteristics through a validated registry; detailed information on incident breast cancer characteristics and treatment; availability of medical charts to identify recurrences and details on recurrent cancer characteristics; unbiased information on medications dispensed; detailed information on co-morbidities and mammographic exams that enabled us to examine confounding in a robust manner; and application of robust analytic methods to address potential competing risks and informative censoring.
Although not statistically significant, our study provides a weak signal that frequent antibiotic use may be associated with a modestly elevated risk of SBCEs among women with a history of early stage invasive breast cancer. This study is an important part of ongoing efforts to improve cancer prognosis and address drug safety. Given the public health importance of learning more about survivorship care and factors that influence breast cancer prognosis, further investigation into this common exposure among women with a history of breast cancer is warranted. Since our findings provide only an initial signal, additional studies to better understand the role of frequent antibiotic use and SBCEs would be helpful. Further evaluations in different study populations and investigation of frequent use by antibiotic class and underlying indication would also be a next step in understanding such an association. This would allow breast cancer survivors and their providers to make more informed decisions about antibiotic selection for managing medical conditions where frequent use is necessary.
Acknowledgments
Financial support: Supported by a grant from the National Cancer Institute (CA120562, PI: Boudreau). Part of the data collection was supported by a grant from the National Cancer Institute: U01CA63731, PI: Buist). The collection of cancer incidence data used in this study was supported by the Cancer Surveillance System of the Fred Hutchinson Cancer Research Center, which is funded by Contract No. N01-CN-67009 and N01-PC-35142 from the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute with additional support from the Fred Hutchinson Cancer Research Center and the State of Washington.
Footnotes
Potential Conflicts of Interest: Heidi Wirtz reports owning stock in Teva Pharmaceuticals. All remaining authors have no potential conflicts of interest to disclose.
References
- 1.National Cancer Institute. Breast Cancer. US National Institutes of Health; [Accessed June 11, 2011]. http://www.cancer.gov/cancertopics/types/breast. [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975–2008. National Cancer Institute; Bethesda, MD: 2011. [Accessed June 11, 2011]. http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site. [Google Scholar]
- 3.Houssami N, Abraham LA, Miglioretti DL, Sickles EA, Kerlikowske K, Buist DS, et al. Accuracy and outcomes of screening mammography in women with a personal history of early-stage breast cancer. JAMA. 2011;305:790–9. doi: 10.1001/jama.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Setchell KD, Lawson AM, Borriello SP, Harkness R, Gordon H, Morgan DM, et al. Lignan formation in man--microbial involvement and possible roles in relation to cancer. Lancet. 1981;2:4–7. doi: 10.1016/s0140-6736(81)90250-6. [DOI] [PubMed] [Google Scholar]
- 5.Velicer CM, Lampe JW, Heckbert SR, Potter JD, Taplin SH. Hypothesis: is antibiotic use associated with breast cancer? Cancer Causes Control. 2003;14:739–47. doi: 10.1023/a:1026323424792. [DOI] [PubMed] [Google Scholar]
- 6.Didham RC, Reith DM, McConnell DW, Harrison KS. Antibiotic exposure and breast cancer in New Zealand. Breast Cancer Res Treat. 2005;92:163–7. doi: 10.1007/s10549-005-2115-8. [DOI] [PubMed] [Google Scholar]
- 7.Garcia Rodriguez LA, Gonzalez-Perez A. Use of antibiotics and risk of breast cancer. Am J Epidemiol. 2005;161:616–9. doi: 10.1093/aje/kwi087. [DOI] [PubMed] [Google Scholar]
- 8.Kaye JA, Jick H. Antibiotics and the risk of breast cancer. Epidemiology. 2005;16:688–90. doi: 10.1097/01.ede.0000172131.84877.42. [DOI] [PubMed] [Google Scholar]
- 9.Sergentanis TN, Zagouri F, Zografos GC. Is antibiotic use a risk factor for breast cancer? A meta-analysis. Pharmacoepidemiol Drug Saf. 2010;19:1101–7. doi: 10.1002/pds.1986. [DOI] [PubMed] [Google Scholar]
- 10.Sorensen HT, Skriver MV, Friis S, McLaughlin JK, Blot WJ, Baron JA. Use of antibiotics and risk of breast cancer: a population-based case-control study. Br J Cancer. 2005;92:594–6. doi: 10.1038/sj.bjc.6602313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman GD, Oestreicher N, Chan J, Quesenberry CP, Jr, Udaltsova N, Habel LA. Antibiotics and risk of breast cancer: up to 9 years of follow-up of 2. 1 million women. Cancer Epidemiol Biomarkers Prev. 2006;15:2102–6. doi: 10.1158/1055-9965.EPI-06-0401. [DOI] [PubMed] [Google Scholar]
- 12.Kilkkinen A, Rissanen H, Klaukka T, Pukkala E, Heliovaara M, Huovinen P, et al. Antibiotic use predicts an increased risk of cancer. Int J Cancer. 2008;123:2152–5. doi: 10.1002/ijc.23622. [DOI] [PubMed] [Google Scholar]
- 13.Knekt P, Adlercreutz H, Rissanen H, Aromaa A, Teppo L, Heliovaara M. Does antibacterial treatment for urinary tract infection contribute to the risk of breast cancer? Br J Cancer. 2000;82:1107–10. doi: 10.1054/bjoc.1999.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamim HM, Hajeer AH, Boivin JF, Collet JP. Association between antibiotic use and risk of prostate cancer. Int J Cancer. 2010;127:952–60. doi: 10.1002/ijc.25139. [DOI] [PubMed] [Google Scholar]
- 15.Velicer CM, Heckbert SR, Lampe JW, Potter JD, Robertson CA, Taplin SH. Antibiotic use in relation to the risk of breast cancer. JAMA. 2004;291:827–35. doi: 10.1001/jama.291.7.827. [DOI] [PubMed] [Google Scholar]
- 16.Avorn J, Solomon DH. Cultural and economic factors that (mis)shape antibiotic use: the nonpharmacologic basis of therapeutics. Ann Intern Med. 2000;133:128–35. doi: 10.7326/0003-4819-133-2-200007180-00012. [DOI] [PubMed] [Google Scholar]
- 17.Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, et al. AJCC Cancer Staging Manual. New York: Springer; 2002. [Google Scholar]
- 18.Buist DS, Ichikawa L, Prout MN, Yood MU, Field TS, Owusu C, et al. Receipt of appropriate primary breast cancer therapy and adjuvant therapy are not associated with obesity in older women with access to health care. J Clin Oncol. 2007;25:3428–36. doi: 10.1200/JCO.2007.11.4918. [DOI] [PubMed] [Google Scholar]
- 19.Enger SM, Thwin SS, Buist DS, Field T, Frost F, Geiger AM, et al. Breast cancer treatment of older women in integrated health care settings. J Clin Oncol. 2006;24:4377–83. doi: 10.1200/JCO.2006.06.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geiger AM, Thwin SS, Lash TL, Buist DS, Prout MN, Wei F, et al. Recurrences and second primary breast cancers in older women with initial early-stage disease. Cancer. 2007;109:966–74. doi: 10.1002/cncr.22472. [DOI] [PubMed] [Google Scholar]
- 21.Saunders KW, Davis RL, Stergachis A. Group Health Cooperative. In: Strom BL, editor. Pharmacoepidemiology. West Sussex, England: John Wiley and Sons; 2005. pp. 223–39. [Google Scholar]
- 22.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 23.Washington State Department of Health, Center for Health Statistics. [Accessed September 15, 2012];Death Data. http://www.doh.wa.gov/ehsphl/CHS/chs-data/death/deatmain.htm.
- 24.Group Health Breast Cancer Surveillance Registry. [Accessed March 20, 2013];Data Sources: Background. http://www.grouphealthresearch.org/surveillanceproject/data/data-sources.html.
- 25.Hudis CA, Barlow WE, Costantino JP, Gray RJ, Pritchard KI, Chapman JA, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25:2127–32. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 26.Varadhan R, Weiss CO, Segal JB, Wu AW, Scharfstein D, Boyd C. Evaluating health outcomes in the presence of competing risks: a review of statistical methods and clinical applications. Med Care. 2010;48:S96–105. doi: 10.1097/MLR.0b013e3181d99107. [DOI] [PubMed] [Google Scholar]
- 27.Boudreau DM, Doescher MP, Jackson JE, Fishman PA, Saver BG. Impact of healthcare delivery system on where HMO-enrolled seniors purchase medications. Ann Pharmacother. 2004;38:1317–8. doi: 10.1345/aph.1D569. [DOI] [PubMed] [Google Scholar]
- 28.Buist DS, LaCroix AZ, Brenneman SK, Abbott T., 3rd A population-based osteoporosis screening program: who does not participate, and what are the consequences? J Am Geriatr Soc. 2004;52:1130–7. doi: 10.1111/j.1532-5415.2004.52311.x. [DOI] [PubMed] [Google Scholar]