Summary
Peri-ictal autonomic dysregulation is best studied using a “polygraphic” approach (EEG, 3-channel EKG, pulse Oximetry, respiration and continuous non-invasive blood pressure [BP]) and may help elucidate agonal pathophysiological mechanisms leading to Sudden Unexpected Death in Epilepsy (SUDEP). A number of autonomic phenomena have been described in generalized tonic-clonic seizures (GTCS), the commonest seizure type associated with SUDEP, including decreased heart rate variability, cardiac arrhythmias and changes in skin conductance. Post-ictal generalized EEG suppression (PGES) has been identified as a potential risk marker of SUDEP and PGES has been found to correlate with post GTCS autonomic dysregulation in some patients. Here, we describe a patient with a GTCS in whom polygraphic measurements, including continuous non-invasive blood pressure recordings, were obtained. Significant post-ictal hypotension lasting >60 seconds was found which closely correlated with PGES duration. Similar EEG changes are well described in hypotensive patients with vasovagal syncope and a similar vasodepressor phenomenon and consequent cerebral hypo-perfusion may account for the PGES observed in some patients after a GTCS. This further raises the possibility that profound, prolonged and irrecoverable hypotension may comprise one potential SUDEP mechanism.
Keywords: post-ictal hypotension, autonomic dysregulation, SUDEP, seizure polygraphy
Introduction
Epileptic seizures can be associated with a variety of autonomic phenomena, including autonomic auras and partial seizures (Nagaraddi & Lüders, 2008), but also as part of the peri-ictal phenomenology of generalized tonic clonic seizures (GTCS) (Gastaut & Broughton, 1972). Recently, both GTCS and peri-ictal autonomic dysregulation have come under scrutiny in the quest for precise agonal pathophysiological mechanisms leading to Sudden Unexpected Death in Epilepsy (SUDEP). These remain unknown although there is consensus that multimodal approaches, incorporating cardiovascular, respiratory, autonomic, biochemical and genetic factors will be pivotal to success. Since most SUDEP patients die in the peri-ictal period, the Epilepsy Monitoring Unit (EMU) provides a unique study environment in a high SUDEP risk population. “Polygraphy” using several simultaneous physiological measurements (EEG, 3-channel EKG, pulse Oximetry, respiration and continuous non-invasive blood pressure [BP]) is part of the study protocol in the Prevention and Risk Identification of SUDEP Mortality (PRISM) Project (NIH/NINDS-NS076965-01) and reveals some interesting insights. Here, we describe a patient with a GTCS and another with a partial seizure in whom these measurements were obtained.
Methods
We recorded video-EEG and 3-channel EKG with a Nihon Kohden™ Neurofax™ EEG-1100A system. Oximetry was recorded with an Oximax™ N-600X machine, respiratory movements with Ambu Sleepmate® abdominal and thoracic belts and continuous, beat-to-beat non-invasive BP recordings with CNAP™ (CNSystems Medizintechnik AG, Graz, Austria). EEG files were converted to the European Data Format (EDF), output verified as identical to the original files and reviewed and analyzed in a MATLAB® based EDF viewer. After IRB approval, prior informed consent for study participation was obtained in each patient.
Results
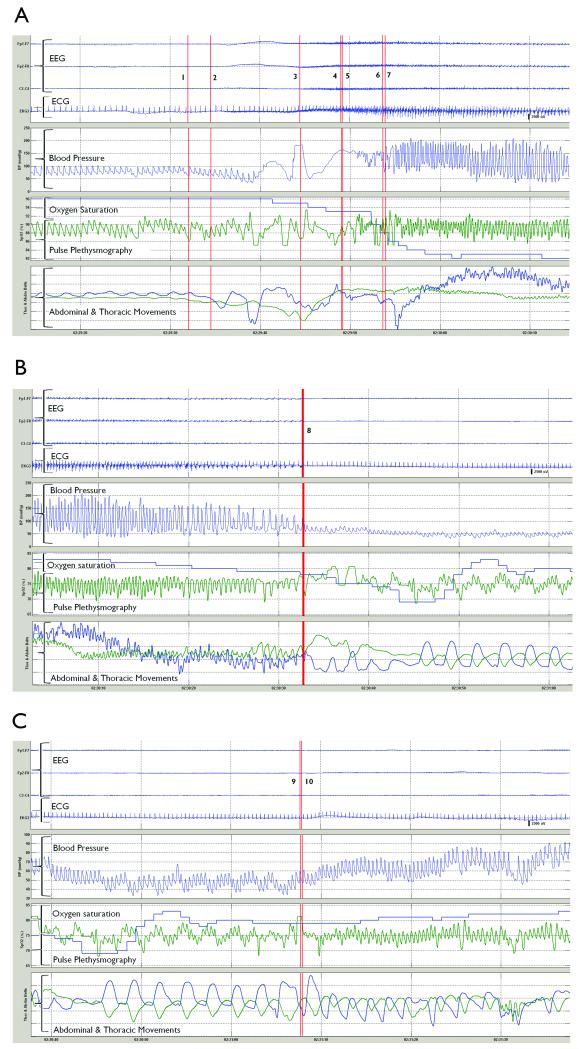
Our patient was an 18 year-old female patient with intractable, previously uncharacterized juvenile myoclonic epilepsy and with no cardiovascular, respiratory or endocrine co-morbidities. A GTCS arising from sleep was recorded whilst on a reduced dose of Depakote® 100mg BID. GTCS progression was typical with onset of generalization, tonic phase, vibratory or “jittery phase” as described by Gastaut, and a clonic phase. The post-ictal phase was characterized by stupor and the EEG showed post-ictal generalized EEG suppression (PGES) followed by gradual clinical and EEG recovery to baseline. As shown in Figures 1A to 1C, systolic (SBP), diastolic (DBP) and mean arterial BP (MAP) increased from baseline (MAP = 81 mmHg [SBP=98 mmHg, DBP=69 mmHg]) to a maximum in the middle of the clonic phase (MAP = 94 mmHg [SBP=209 mmHg, DBP=36 mmHg]). Five seconds after onset of version, BP signal was lost for 12 seconds and restored in the middle of the jittery phase. In the suppression phase, prolonged hypotension (>60 seconds) was seen with BP dropping to its lowest ebb of MAP = 41 mmHg (SBP=48 mmHg, DBP=34 mmHg) and concurrent diminution of pulse pressure signal. Onset of recovery of MAP coincided closely with the end of post-ictal generalized EEG Suppression (PGES).
Figures 1.
(a), (b) and (c) show a generalized tonic-clonic seizure. 1: EEG onset 2: Version 3: Onset of generalized tonic phase 4: End of tonic phase 5: Onset of jittery phase 6: End of jittery phase 7: Onset of clonic phase 8: End of clonic phase, clinical seizure end, EEG seizure end, onset of EEG suppression 9: End of EEG suppression 10: Onset of intermittent slow SpO2 = Oxygen saturation (green trace = pulse signal; blue trace = O2 saturation)
Discussion
Peri-ictal autonomic dysregulation has been well described, most notably by Gastaut using pulse rate, pupillometry, electrodermograms, cystometry and conventional sphygmomanometric measurements (Gastaut & Broughton, 1972). More recent technology using wrist sensors for electro-dermal activity (Poh et al., 2012) and heart rate variability algorithms (Surges et al., 2009) confirm significant sympathetic and parasympathetic changes in the ictal and post-ictal periods.
BP changes in epilepsy and seizures have been noted in several contexts. Gastaut measured BP peri-ictally but non-continuously. Continuous intra-radial invasive recordings in three acutely unwell patients with GTCS noted either a pattern of transient increase in Mean Arterial Pressure (MAP) or an M-shaped waveform where an initial increase in MAP was followed by a fall and then a secondary overshoot of BP at seizure end. The authors did not precisely correlate seizure phases with BP although an association between fall in MAP and the tonic phase was observed in one of the patients (Magnaes & Nornes, 1974). Electroconvulsive therapy induces a transient initial drop in BP as recorded by continuous, beat-to-beat BP monitoring and in pulse rate (the parasympathetic phase) followed by a sharp rise in both parameters (the sympathetic phase) (Geersing et al., 2011). Electrical stimulation of insular cortex, cingulate gyrus, prefrontal cortex and amygdalar nucleus in humans and animals have been reported to show either increase or decrease in BP without clear lateralizing value (Sevcencu & Struijk, 2010). However, continuous, beat-to-beat BP recordings in spontaneous epileptic seizures have not previously been carried out successfully because of several constraints. These include the previous absence of appropriate technology for continuous non-invasive BP, the logistics of equipment cost and relatively stringent technician oversight required in the EMU. Polygraphic correlation of BP with Oximetry, pulse rate, respiration, ECG and EEG has therefore never previously been studied. The CNAP™ machine, which is used in our study, is standard equipment in many autonomic laboratories and in the operating room where continuous non-invasive arterial BP closely correlates with simultaneous invasive arterial BP measurements (Hahn et al., 2012). The device is based on the Peñáz method which detects vascular unloading by a photo-plethysmograph. It uses beat to beat finger BP measurements and calibrates these with brachial cuff readings, correcting for hydrostatic differences (Penaz, 1973) (Chung et al., 2013). The automatic detection and correction of dispersed light reduces artifact even during motion (Fortin et al., 2006). Hand movements in a vertical plane relative to the heart can produce a physiological dip in BP recordings although this was not relevant in our patient, who was inert, supine and had her hand in the same horizontal plane as the heart in the post-ictal period. Conditions affecting peripheral perfusion such as vascular disease, cold temperature and vasoactive medications may underestimate BP. We were able to adapt use to the EMU with appropriate protocols.
In our GTCS patient, the significant hypotension observed in the post-ictal period is of major interest and differs significantly from previous BP observations in GTCS (Magnaes & Nornes, 1974). The absence of bradycardia suggests a vaso-depressor mechanism due to peripheral vasodilation rather than a vagally driven cardio-inhibitory mechanism. In contrast to the parasympathetic nervous system, the sympathetic system mediates vasoconstriction (post-synaptic α1- and α2-adrenergic receptors) and vasodilation (β-adrenergic receptors). The latter are not directly activated by sympathetic nerves but rather by circulating catecholamines (Thomas, 2011). The sudden drop in BP in this case may reflect an abrupt cessation of sympathetic drive to α1- and α2-adrenergic receptors; a β-adrenergic mechanism in the post-ictal period seems less likely. It is conceivable that in some patients, severe, prolonged and irrecoverable hypotension and failure of BP homeostasis may comprise a mechanism for SUDEP. Hypotension due to a defective neurocardiac reflex has also been observed in a parallel phenomenon, the Sudden Infant Death Syndrome (Ledwidge et al., 1998). The observation of an apparent correlation between PGES and BP in our patient (Figure 1A-C) is interesting and may explain the PGES seen in some patients after a GTCS (Lhatoo et al., 2010). Prolonged vasovagal syncope and attendant hypotension can produce a similar EEG picture due to cerebral hypo-perfusion (Brenner, 1997) where initial slowing of background rhythms is followed by high amplitude delta activity for a few seconds and then subsequent generalized flattening of the EEG, returning to normal in the reverse sequence. With the tilt-table test, neither the “flat EEG” nor loss of consciousness resolve immediately with return to the supine position, both of which occur after a further few seconds, possibly representing a reperfusion period (Ammirati et al., 1998). However, in our patient, the EEG did not return to normal baseline as rapidly as with syncope, possibly due to the post-ictal EEG slowing associated with post-ictal stupor. Nevertheless, the relationship between hypotension and PGES in our case is likely secondary to hypotensive cerebral hypo-perfusion and the BP changes do not appear artifactual. PGES has been associated with GTCS induced post-ictal autonomic dysregulation in some patients (Poh et al., 2012) and there may be other, as yet undetermined mechanisms that drive PGES. Neither respiration nor oxygenation (Figure 1) appeared to correlate closely with BP and PGES. Hypoxia and hypercapnia do not usually cause major fluctuations in BP. Acute hypoxia can produce both a generalized, dose-dependent increase in sympathetic vasoconstrictor outflow caused by activation of the carotid chemoreceptor reflex, increased levels of angiotensin II and endothelin-I, and vasodilation mediated by epinephrine, atrial natriuretic peptide and red blood cell generated ATP (Tamisier et al., 2005). The balance of these two opposing trends is slightly tipped towards the latter, resulting in a mild or no change in systemic BP (Morgan, 2007). Hypercarbia produces similar but shorter lived sympathetic activation (Xie et al., 2001). In our case, CO2 capnography was not used and the extent of hypercarbia if any, is not known.
In summary, post-ictal hypotension may occur after GTCS and appears to closely correlate with PGES suggesting significant hypotension and cerebral hypo-perfusion as a possible cause of this phenomenon. Severe hypotension and failure of BP homeostasis may comprise one potential SUDEP mechanism and PGES may identify patients at risk.
Acknowledgements
This study was supported in part by NINDS grant NS076965-01 - The Prevention and Risk Identification of SUDEP Mortality (PRISM) Project.
Footnotes
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosure: None of the authors has any conflict of interest to declare
References
- Ammirati F, Colivicchi F, Di Battista G, Garelli FF, Santini M. Electroencephalographic correlates of vasovagal syncope induced by head-up tilt testing. Stroke. 1998;29:2347–2351. doi: 10.1161/01.str.29.11.2347. [DOI] [PubMed] [Google Scholar]
- Brenner RP. Electroencephalography in syncope. J Clin Neurophysiol. 1997;14:197–209. doi: 10.1097/00004691-199705000-00004. [DOI] [PubMed] [Google Scholar]
- Chung E, Chen G, Alexander B, Cannesson M. Non-invasive continuous blood pressure monitoring: a review of current applications. Front Med. 2013;7:91–101. doi: 10.1007/s11684-013-0239-5. [DOI] [PubMed] [Google Scholar]
- Fortin J, Marte W, Grullenberger R, Hacker A, Habenbacher W, Heller A, Wagner C, Wach P, Skrabal F. Continuous non-invasive blood pressure monitoring using concentrically interlocking control loops. Comput Biol Med. 2006;36:941–957. doi: 10.1016/j.compbiomed.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Gastaut H, Broughton RJ. Epileptic Seizures: Clinical Features and Pathophysiology Epileptic Seizures: Clinical and Electrographic Features, Diagnosis and Treatment. Charles Thomas Publisher; Springfield, Illinois, USA: 1972. pp. 28–29. [Google Scholar]
- Geersing PG, Bulte CS, Viersen VA, Stek ML, Bouwman RA, Boer C, Loer SA. Beat-to- beat hemodynamic monitoring during electroconvulsive therapy. J ECT. 2011;27:189–191. doi: 10.1097/YCT.0b013e3182008de5. [DOI] [PubMed] [Google Scholar]
- Hahn R, Rinosl H, Neuner M, Kettner SC. Clinical validation of a continuous non-invasive haemodynamic monitor (CNAP™ 500) during general anaesthesia. Br J Anaesth. 2012;108:581–585. doi: 10.1093/bja/aer499. [DOI] [PubMed] [Google Scholar]
- Ledwidge M, Fox G, Matthews T. Neurocardiogenic syncope: a model for SIDS. Arch Dis Child. 1998;78:481–483. doi: 10.1136/adc.78.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhatoo SD, Faulkner HJ, Dembny K, Trippick K, Johnson C, Bird JM. An electroclinical case-control study of sudden unexpected death in epilepsy. Ann Neurol. 2010;68:787–796. doi: 10.1002/ana.22101. [DOI] [PubMed] [Google Scholar]
- Magnaes B, Nornes H. Circulatory and respiratory changes in spontaneous epileptic seizures in man. Eur Neurol. 1974;12:104–111. doi: 10.1159/000114609. [DOI] [PubMed] [Google Scholar]
- Morgan BJ. In: Vascular Consequences of Intermittent Hypoxia. Roach RC, Wagner PPD, Hackett PH, editors. Hypoxia and the Circulation; Springer London, Limited: 2007. pp. 69–84. [DOI] [PubMed] [Google Scholar]
- Nagaraddi V, Lüders HO. Autonomic seizures: localizing and lateralizing value. In: Lüders HO, editor. Textbook of Epilepsy Surgery. Informa Healthcare; London, UK: 2008. pp. 443–449. [Google Scholar]
- Penaz J. Photoelectric measurement of blood pressure, volume and flow in the finger. Dresden, Germany: 1973. pp. 104–104. [Google Scholar]
- Poh MZ, Loddenkemper T, Reinsberger C, Swenson NC, Goyal S, Madsen JR, Picard RW. Autonomic changes with seizures correlate with postictal EEG suppression. Neurology. 2012;78:1868–1876. doi: 10.1212/WNL.0b013e318258f7f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevcencu C, Struijk JJ. Autonomic alterations and cardiac changes in epilepsy. Epilepsia. 2010;51:725–737. doi: 10.1111/j.1528-1167.2009.02479.x. [DOI] [PubMed] [Google Scholar]
- Surges R, Henneberger C, Adjei P, Scott CA, Sander JW, Walker MC. Do alterations in inter-ictal heart rate variability predict sudden unexpected death in epilepsy? Epilepsy Res. 2009;87:277, 280. doi: 10.1016/j.eplepsyres.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Tamisier R, Anand A, Nieto LM, Cunnington D, Weiss JW. Arterial pressure and muscle sympathetic nerve activity are increased after two hours of sustained but not cyclic hypoxia in healthy humans. J Appl Physiol. 2005;98:343–349. doi: 10.1152/japplphysiol.00495.2004. [DOI] [PubMed] [Google Scholar]
- Thomas GD. Neural control of the circulation. Adv Physiol Educ. 2011;35:28–32. doi: 10.1152/advan.00114.2010. [DOI] [PubMed] [Google Scholar]
- Xie A, Skatrud JB, Puleo DS, Morgan BJ. Exposure to hypoxia produces long-lasting sympathetic activation in humans. J Appl Physiol. 2001;91:1555–1562. doi: 10.1152/jappl.2001.91.4.1555. [DOI] [PubMed] [Google Scholar]