SUMMARY
The basidiomycete Ustilago maydis is a ubiquitous pathogen of maize (Zea mays), one of the world’s most important cereal crops. Infection by this smut fungus triggers tumor formation in aerial plant parts within which the fungus sporulates. Using confocal microscopy to track U. maydis infection on corn anthers through 7 dpi (days post-injection), we found that U. maydis is located on the epidermis on the first two days and by 3 dpi has reached all anther lobe cell types. Fungal infection can alter cell fate specification events, cell division patterns, host cell expansion, and host cell senescence depending on the developmental stage and cell type. Fungal impacts on tassel and plant growth were also quantified. Transcriptome profiling using a dual organism microarray identified thousands of anther genes affected by fungal infection 3 dpi during the cell fate specification and rapid cell proliferation phases of anther development. 4147 (17%) of anther-expressed genes were altered by infection, 2018 fungal genes were expressed in anthers, and 206 fungal secretome genes may be anther-specific. The results confirm that U. maydis deploys distinctive genes to cause disease in specific maize organs and begins to chart the mechanisms by which the host plant is manipulated to generate a tumor.
Keywords: Zea mays, anther, Ustilago maydis, development, interaction
INTRODUCTION
Infection of maize (Zea mays) by the pathogenic basidiomycete Ustilago maydis causes plant cancer, characterized by tumor formation in aerial organs. Tumors consist of mixed populations of proliferating plant cells and fungal hyphae which eventually mature into teliospores, the primary agent of fungal dispersal (Banuett, 2002). Infection is initiated by a dikaryotic filament that arises after the fusion of two compatible haploid sporidia or growth of the modified solopathogenic (SG200) fungal strain (Doehlemann et al., 2008). The invading filaments exhibit tip growth but fail to divide mitotically; during extended tip growth, empty sections are sealed off by regularly spaced septae (Steinberg et al., 1998). After appressorium formation, fungal cells proliferate and penetrate between epidermal cells, (Snetselaar and Mims, 1992) subsequently the fungal cells interact extensively with host cells, forming a biotrophic interface in which the pathogen and invaginations of the host plasma membranes are in close contact. Tumor formation is mediated, at least in part, by fungal proteins secreted into the biotrophic interface that trigger host developmental and physiological changes. The U. maydis genome was found to contain 12 gene clusters encoding three or more proposed secreted proteins whose expression in most cases was significantly up-regulated at some stage of tumor formation on seedling leaves (Kämper et al., 2006). Bioinformatics analysis estimated that there are 554 U. maydis secretome genes (Müeller et al., 2008).
Nearly all work with this host-pathogen interaction has focused on early steps in pathogen establishment, resulting in detailed knowledge of the fungal genome, the gene expression patterns during infection, and the genetic requirements for pathogenicity (Kämper et al., 2006; Doehlemann et al., 2009; Wahl et al., 2010). Cytological descriptions of tumors are restricted to electron micrographs of infected seedling leaves (Callow and Ling, 1973; Callow, 1975). More recently, infection of maize adult organs and developmental mutants established several host requirements for tumor formation such as active cell proliferation (Walbot and Skibbe, 2010), and transcriptome profiling of both host and pathogen established that both partners exhibit organ-specific gene expression during infection (Skibbe et al., 2010).
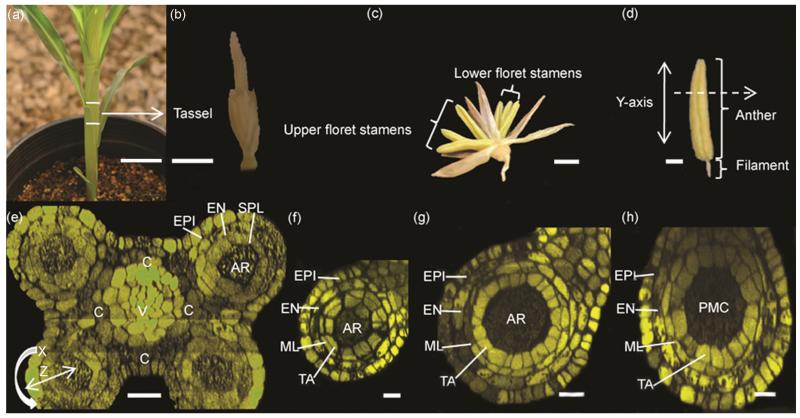
As a biotrophic pathogen, U. maydis causes tumors by reprogramming vegetative and reproductive development, causing disturbances in normal organ growth resulting in organs that grow much larger than normal (Walbot and Skibbe, 2010). Maize anthers are particularly favorable for analyzing development, because they are readily dissected and staged by length and developmental progression is highly regular within each anther and among anthers of similar size. Additionally lobe cells have well-defined patterns of cell division and growth (Ma et al., 2008; Kelliher and Walbot, 2011). Maize tassels have hundreds of paired spikelets, each containing six stamens separated into two florets (Kelliher and Walbot, 2011). Stamens consist of pollen-producing anthers subtended by filaments, which supply nutrients and water to the growing anther through a single vascular column (Figure 1a-d). An immature anther of 250 μm is composed of four lobes flanking the connective tissue and central vasculature; at this stage lobes contain the epidermis (EPI), endothecium (EN), secondary parietal layer (SPL), and the pre-meiotic archesporial (AR) cell types (Figure 1e). By 700 μm, SPL cells have divided periclinally to establish the middle layer (ML) and tapetum (TA) cells (Kelliher and Walbot, 2012), and from this stage onwards normal anther lobes contain five cell types (Figure 1f-h). Exploiting these features, we have analyzed the interaction between the maize anther and the U. maydis pathogen using confocal microscopy and transcriptome profiling to compare the developmental progression in normal and infected anthers.
Figure 1.
Orientation to maize organs and cell types. (a) Maize plant at 30 days. (b) The tassel consists of a central spike with several lateral branches, which together support hundreds of paired spikelets. This ~3 cm immature tassel contains anthers of < 100-400 μm. (c) Spikelets contain two florets of three stamens each. Note that the upper floret anthers are approximately one day more developmentally advanced that the anthers in the lower floret. (d) Stamens consist of an anther supported by a filament. In diagrams, the Y-axis represents the apical-basal axis. (e) PI (propidium iodide) stained confocal reconstruction of a transverse-section of a 250 μm anther, composed of four lobes surrounding the central connective and vascular tissue, which are continuous with the filament. In each lobe, the epidermis and three subepidermal somatic cell types surround the central reproductive cells that will undergo meiosis and ultimately form pollen. In diagrams the X, Z view represents the circumferential and the radial axis, respectively. V (vasculature), C (connective tissue), EPI (epidermal cell), EN (endothecial cell), SPL (secondary parietal layer cell), AR/PMC (archesporial cell/pollen mother cell). (f-h) Confocal reconstructions of PI stained anthers at (f) 700 μm, (g) 1000 μm, (h) 1500 μm, all locules of (f-h) are composed of EPI, EN, ML(middle layer cell), TA (tapetal cell) and AR/PMC. Scale bars: (a) = 6 cm, (b) = 1 cm, (c) = 500 μm, (d) = 250 μm, (e-h) = 50 μm.
RESULTS
Timeline of U. maydis infection on maize anthers
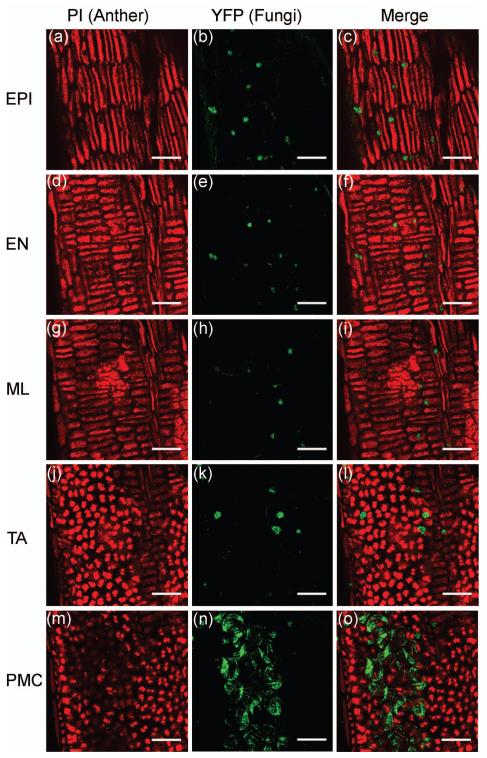
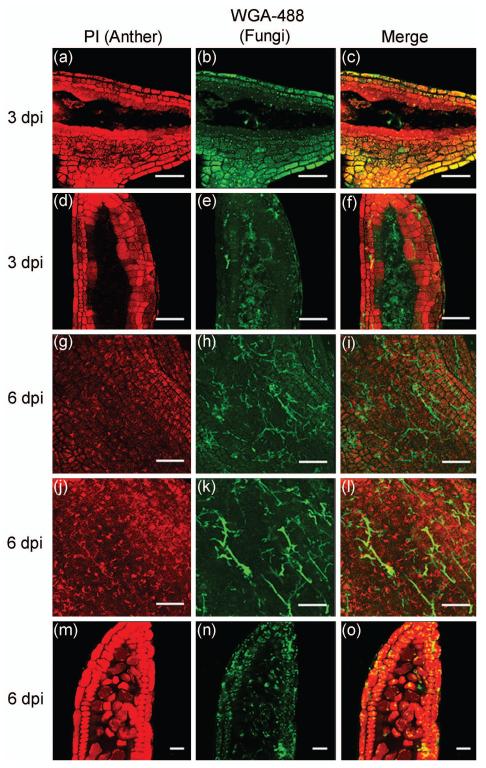
To determine appropriate times to investigate the impact of U. maydis on anther development, we tracked infection by strain SG200-YFP in three dimensions by confocal imaging. Infected and mock-infected anthers were stained with propidium iodide (PI) to clarify host cell morphologies and the YFP signal identified the tip of live fungi. The major developmental stages classified by anther length are < 100 μm before cell fate specification, ~200-500 μm during cell fate specification but before the periclinal division of the SPL, and > 700 μm when all lobe cell types are present. At all three stages, U. maydis was observed on the epidermis at 1 and 2 dpi (Table 1), and on subsequent days both epidermal and subepidermal cells could be in contact with U. maydis (Table 1, Figure 2). To confirm these observations, infected anthers were also stained with WGA-AF488 to identify both live fungal cell tips and the empty septated cell walls tracing the filaments and hyphae (Figure 3).
Table 1.
A timeline of U. maydis infection on corn anthers. Inferred length at the time of injection is based on the timeline in Kelliher and Walbot, 2012
| Dpi | Analysis length (μm) |
Inferred length (μm) at injection |
Fungal location |
|---|---|---|---|
| 1 | 750 | ~550 | EPI |
| 2 | 250 | ~90 | EPI |
| 2 | 560 | ~220 | EPI |
| 3 | 1450 | ~700 | EPI, EN, ML, TA and PMC |
| 3 | 1500 | ~700 | EPI, EN, ML, TA and PMC |
| 3 | 1700 | ~800 | EPI, EN, ML, TA and PMC |
| 3 | 2000 | ~1000 | EPI, EN, ML, TA and PMC |
| 4 | 2600 | > 1000 | EPI, EN, ML, TA and PMC |
| 4 | 3000 | > 1000 | EPI, EN, ML, TA and PMC |
| 5 | 3700 | > 1000 | EPI, EN, ML, TA and PMC |
| 6 | 2600 | > 1000 | EPI, EN, ML, TA and PMC |
| 7 | 2200 | > 1000 | EPI, EN, ML, TA and PMC |
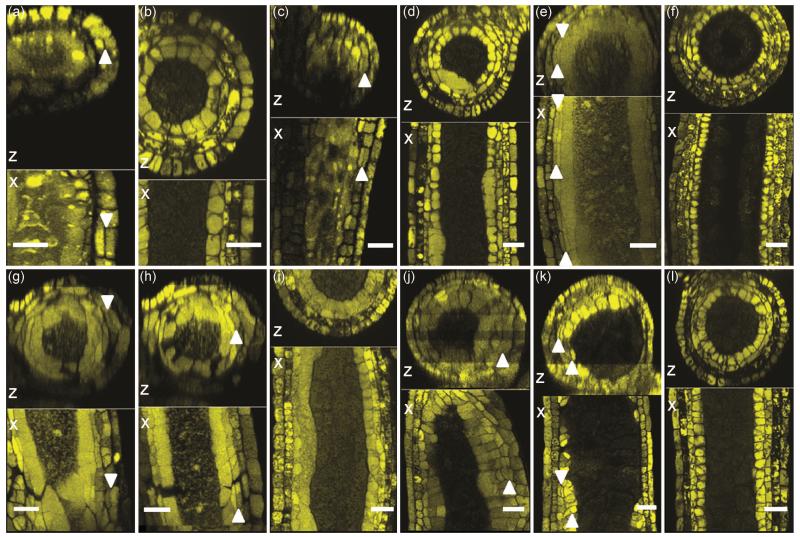
Figure 2.
U. maydis location on cell types of one maize anther (1500 μm) at 3 dpi. YFP green signal indicates live fungi tips, while PI (red) stains nuclei of all anther cell types. (a-c) Fungi located on EPI. (d-f) Fungi located at EN. (g-i) Fungi located at ML. (j-l) Fungi located at TA. (m-o) Fungi located at the PMC. Scale bars = 50 μm.
Figure 3.
Impact of U. maydis infection at 3 and 6 dpi at several anther developmental stages. Hyphal walls are stained with WGA-AF 488 (wheat germ agglutinin conjugated to Alexa Fluor 488), and PI stains anther cells. (a-c) 3 dpi, 900 μm infected anther (inferred to be ~400 μm at injection). (d-f) 3 dpi, 1400 μm infected anther (inferred to be ~700 μm at injection). (g-i) EPI of a 6 dpi, 750 μm infected anther (inferred to be ~80 μm at injection). (j-l) The same anther imaged in (g-i) was examined in deeper optical sections, however, the subepidermal maize anther cell types cannot be distinguished from each other. (m-o) The expected subepidermal cell types are distinguishable in an older anther of 6 dpi, 1850 μm (inferred to be ~200 μm at injection). Scale bars = 50 μm.
At 3 dpi, the hyphae were observed weaving around and into the central most AR cells (Figure 3a-c) of 900 μm anthers (inferred to be ~400 μm at injection); similar results were observed with 1400 μm long anthers (Figure 3d-f, inferred to be ~700 μm at injection). To reach AR/PMC cells, hyphae had made contact with all somatic subepidermal cell types. In subepidermal leaf cells the biotrophic interface is observed by 4 dpi (Doehlemann et al., 2008), and we assume that this zone is forming in anthers at 3 dpi although we have no specific marker for this structure.
Infection of young anthers prior to cell fate specification resulted in total disruption of internal lobe development: although the anthers kept growing in length and girth, subepidermal cell types were abnormal and lacked differentiated features of EN, ML, TA, or AR cells (Figure 3j-l). In addition, with anthers infected at ~80 μm, although the epidermis appeared intact (Figure 3g-i), cell length and volume were significantly smaller (p ≤ 0.001) than mock-infected anthers, and there were significantly more cells along the Y-axis (p ≤ 0.001) than in normal anthers. Anther cells infected after the initiation of cell fate specification retained aspects of normal cell identity at 6 dpi. For instance, an anther of 1850 μm (inferred to be ~200 μm at injection) contains recognizable cell types (Figure 3m-o). For deeper analysis of the impact of infection on the development of specific cell types, we therefore chose to focus on the 3 dpi infected anthers with injection after 200 μm.
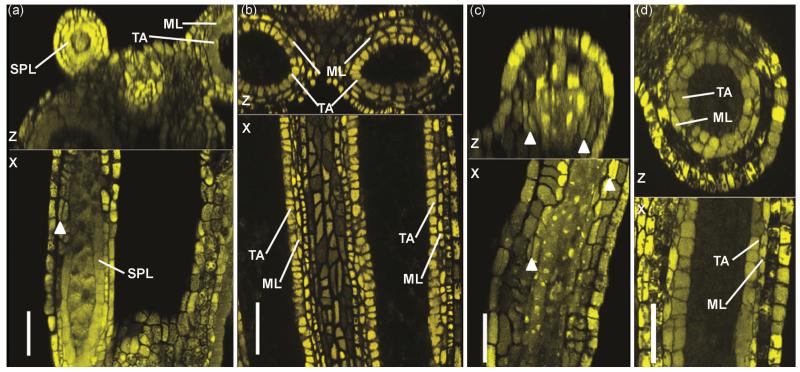
U. maydis delays the SPL cell periclinal division
Normally, the four anther lobes reach developmental landmarks coordinately; one key landmark is the periclinal division of SPL to generate the ML and TA. In infected anthers, we found variation within the same anther, with some lobes having SPL and other lobes containing ML and TA cells (Figure 4a shows a 1000 μm anther with two aberrant lobes). In mock-infected anthers all SPL have divided periclinally and terminally differentiated into ML and TA cells by the 700 μm stage (Kelliher and Walbot, 2012) (Figure 4b, d). In contrast, SPL cells were observed at 3 dpi in infected anthers of lengths > 700 μm (Figure 4c), even up to 1000 μm long (Figure 4a). We conclude that U. maydis can delay the final cell fate specification step in anthers, which requires the SPL periclinal division.
Figure 4.
Delayed periclinal cell division of SPL in anther lobes, 3 dpi. (a) Comparison of two lobes of one anther at 1000 μm: 2 lobes had finished SPL division, but 2 lobes had not finished dividing into ML and TA. Note also that the delayed lobe visible in this view is considerably smaller than the more advanced lobe of the same anther. (b) Mock-infected anthers of 1000 μm: SPL in all four lobes (two of which are visible in the image) have finished dividing into ML and TA. (c) Delay of SPL division in a 780 μm infected anther, 3 dpi. Arrowheads indicate periclinal cell divisions of SPL, a process which has just started. (d) Mock-infected 700 um anther illustrating completion of the SPL periclinal division. Scale bars = 50 μm.
U. maydis induces ectopic periclinal divisions of somatic cells
U. maydis had dramatic effects on the development of 200-700 μm anthers (Table 2), because extra periclinal cell divisions were commonly observed. Periclinal divisions are a serious developmental error, because they add an extra cell layer and hence disrupt lobe architecture. Ectopic periclinal division was observed as early as 780 μm in the EPI layer (Figure 5a), a feature never found in normal anthers (Figure 5b) (Kelliher and Walbot, 2011). Similarly, the EN at 750 μm (Figure 5c) and later stages (Figure 5g, j, see Figure 5d, i and l for size-matched control anthers) exhibited ectopic periclinal division that is never observed in normal anthers. The ML at 800 μm (Figure 5e), 950 μm (Figure 5h) and both ML and TA at 1400 μm (Figure 5k) had periclinal divisions. Excessive within-layer anticlinal divisions were also found in several layers, and in some anthers, excess proliferation occurred in all of the four somatic layers (Figure 5g, h, j, k). The most dramatic level of proliferation and abnormal cell development was found in the ML. This is surprising, because normally ML conduct only a few anticlinal cell divisions after their birth, and then these cells are greatly reduced in width and appear to be crushed between the TA and EN layers shortly after meiosis initiates. In infected anthers, ML persistence was routinely observed, these cells divided frequently both anticlinally and periclinally, and they retained a relatively cuboidal shape.
Table 2.
Disruption of cell division patterns in infected anthers of various developmental stages, 3 dpi
| Anther size (μm) |
Inferred anther size at injection (μm) |
Affected anthers (%) |
Location of extra periclinal divisions |
|---|---|---|---|
| 780 | ~200 | 50 | EPI |
| 750, 950, 1500 | ~200-700 | 80 | EN |
| 800, 950, 1400 | ~200-700 | 70 | ML |
| 1400 | ~700 | 60 | EPI, EN, ML and TA |
Figure 5.
Extra periclinal cell divisions in infected anthers, 3 dpi. (a, c, e, g, h, j, k) Infected anthers with excess periclinal divisions. (b, d, f, i, l) Mock-infected anthers at similar stages for comparison. (a) 780 μm infected anther; arrowhead points to a new periclinal cell wall in the EPI viewed in both the X and Z reconstructions. (b) Mock-infected anther of 800 μm in the X and Z reconstructions for comparison. (c, g and j) 750, 950 and 1500 μm infected anthers; arrowheads indicate new cell walls from extra periclinal divisions of EN. (d, i and l) Mock-infected anthers of similar stages, 800, 950 and 1500 μm. (e and h) Infected anthers of 800 and 950 μm in length; arrowheads indicate extra periclinal cell divisions within the ML. (f and i) Mock-infected anthers of similar stages, 800 and 950 μm. (k) 1400 μm infected anther; arrowheads indicate extra divisions of ML and TA cells. (l) Mock-infected anthers of 1500 μm. Scale bars: (a-i) = 50 μm, (j-l) = 30 μm.
Impact of U. maydis on lobe cell expansion
Tissue-specific patterns of expansion and a stereotyped pace and pattern of cell division are essential for normal anther growth and to maintain smooth contours. The combination of expansion and division contributes to final cellular dimensions in the X-, Y-, and Z-axis to fill the nested lobe rings, and there are highly consistent cell counts in these axes for each cell type at specific anther lengths (Kelliher and Walbot, 2011). In general, EPI are highly biased for length expansion (Y-axis), EN are highly biased for girth expansion (X-axis), ML are slightly biased for girth expansion, TA are rectilinear and biased for radial expansion (Z-axis), and AR/PMC are spheroid, large, and have thin walls.
We documented both cell counts and dimensions to calculate volumes from at least five mock- and five infected anthers matched by developmental stage. At least 30 regions were quantified in each anther, including the tapered basal and apical tips. Cell volumes were calculated by multiplying the X, Y, and Z lengths. We found complex, stage-specific changes in growth parameters during infection. For example, EPI cell volumes (Tables S1A-B, S2A-B) were similar to control anthers from 400 to 1600 μm. Despite this, EPI were shorter in the Y dimension and hence more epidermal cells were present along the length of the anther at specific lengths (p ≤ 0.05, Tables S1A-B, S2A-B). Thus although the final cellular volume in the infected epidermis is equivalent to mock-infected anthers this has been achieved with compensatory growth (Figure 6a).
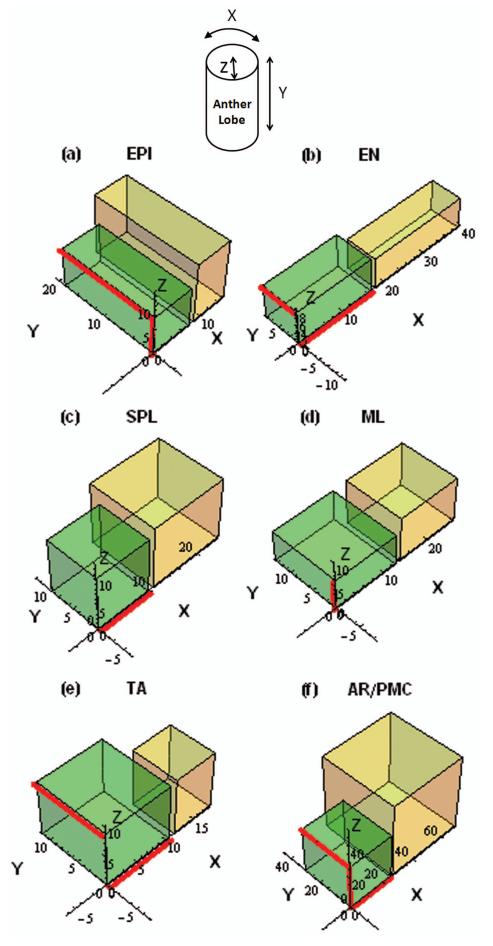
Figure 6.
Comparison of cell dimensions in infected (green) and mock-infected (yellow) anther lobe cell types with similar anther sizes. The small graphic at the top of the figure shows how the X, Y, and Z axes refer to the dimensions of the anther lobe. Dimensions significantly different in infected cells are indicated with a solid red line. (a) EPI: infected (810-870 μm), mock-infected (810-850 μm). (b) EN: infected (720-780 μm), mock-infected (710-750 μm). (c) SPL: infected (610-670 μm), mock-infected (600-620 μm). (d) ML: infected (810-870 μm), mock-infected (810-850 μm). (e) TA: infected (810-870 μm), mock-infected (810-850 μm). (f) AR/PMC: infected (1400-1600 μm), mock-infected (1480-1520 μm).
EN normally exhibit cell division and expansion patterns opposite to the EPI: elongation in the X-axis accompanies rapid cell division along the Y-axis. EN dimensions at ~700 μm are illustrated in Figure 6b. Detailed characterization at multiple stages (Table S1), determined that every stage showed significant differences in either one of the dimensions and/or cell count comparing mock-infected and infected anthers. For example, EN of infected anthers exhibit a smaller cell width and larger cell length at 750 μm, greater cell depths at 1100 and 1500 μm and larger cell volumes at 610 and 1100 μm than matched mock-infected anthers. The data from the EN confirms that compensatory growth reflecting altered patterns of cell division and of expansion occur in this cell type during infection.
As indicated earlier, persistent SPL is an abnormal feature of infected anthers (Figure 4), and SPL cells continued L- and G-anticlinal division contributing to anther elongation and girth. SPL from mock-infected anthers at ~600 μm are illustrated in Figure 6c and more detail is provided in Tables S1A-B, S2A-B.
Because the SPL periclinal division is delayed, ML are born later (in longer anthers) than in controls. Shortly after birth, the depth of ML was about half that observed in a normal anther (p ≤ 0.001, Table S1). This parameter remained relatively constant from 810 to 1600 μm in infected anthers while in the controls, ML depth steadily declines from 710-1520 μm. The ML cells were twice as long in infected anthers of 1400-1600 μm compared to mock-infected anthers (p ≤ 0.05, Tables S1A-B). The shapes of the ML at ~800 μm are compared in Figure 6d. Interestingly, despite the changes in cell dimensions, ML cell volumes were not significantly different in infected compared to mock-infected anthers at any developmental stage. In terms of cell counts, which accumulate as a result of both L-anticlinal and G-anticlinal divisions, the infected anthers had a higher circumferential cell count than normal at ~800 and ~1100 μm (Tables S2A-B); this likely reflects the delay in the periclinal division of the SPL as these cells continue to divide anticlinally. By 1500 μm the ML numbers matched the control. On the other hand, in the Y-axis, ML cell numbers were more similar to controls initially; however, there were significantly fewer ML cells along the length dimension in infected anthers by 1500 μm (Tables S2A-B, p ≤ 0.001).
TA cells are essential to support completion of meiosis by pollen mother cells (PMC); one specific role is secretion of β-1,3-glucanases to remodel callose before meiosis and later liberate microspores from the tetrad (Wang et al., 2010). Typically TA cells proliferate more rapidly than ML cells (Kelliher and Walbot, 2011). U. maydis infection greatly reduced this blistering pace and the TA grew to have a 2.5-fold greater cell volume than TA cells in mock-infected anthers at ~800 μm (Figure 6e, Tables S2A-B). Interestingly, at the next stage (1000-1200 μm), the TA cells were narrower and had a smaller volume than mock-infected anthers indicating that they had undergone multiple cell divisions then failed to expand properly; compensatory cell division increased the number of TA in the X-axis to occupy the volume of the tissue. At 1500 μm the TA were once again wider than control cells (Tables S2A-B). We interpret these results as reflecting decreased L-anticlinal division pace at ~800 μm and increased G-anticlinal division at ~1100 μm. Collectively, the measurements of the somatic anther lobe cells illustrate complex alterations of cell expansion and division patterns and hence demonstrate the impact of U. maydis on anther cell growth.
The AR cells committed to meiosis proliferate mitotically from 300-1000 μm anther length to achieve a final population of ~160 cells per lobe (Kelliher and Walbot, 2011). Early in the development of infected anthers, the AR cell sizes are consistent with controls. Beginning at ~800 μm, however, the AR fail to enlarge sufficiently in the X-dimension, and by the start of meiosis the PMC are smaller in all dimensions (Table S1A-B). AR cells at about 1500 μm are illustrated in Figure 6f. The AR continues steady proliferation to fill the internal lobe space: as a result, mock-infected anthers averaged a final count of 165 AR/PMC per lobe while the infected anthers averaged 196 (Table S2A). These results suggest that U. maydis disrupts AR development, either by increasing their rate of division or preventing them from enlarging, which indirectly permits continued cell division.
Later in infection, when tumorigenesis is evident, anthers are grossly distorted (Figure S1). In addition, U. maydis also reduces growth of maize plants, plant height, tassel length and internode elongation (Figures S2-3).
Microarray analysis of infected and mock-infected anthers at 3 dpi
To gain a comprehensive view of the impact of U. maydis on gene expression patterns, RNA was extracted from 300-600 μm infected and mock-infected anther pools at 3 dpi, and hybridized to an Agilent 44K microarray. Correlation plots of the comparisons of infected and mock samples showed the expected significant difference in hybridization to U. maydis probes for three out of the four arrays. The fourth array showed evidence of only slight infection and was therefore excluded from further analysis.
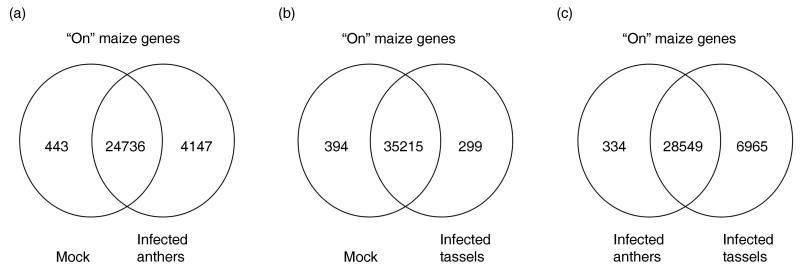
Maize anthers are notable in expressing about 25,000 genes at all stages surveyed with the microarray. As shown in Figure 7a, mock-infected anthers expressed 25179 transcript types (24736 + 443). U. maydis infection caused expression of 17% (4147/25179) additional maize anther transcripts, accompanied by loss of expression of only 443 (2%, 443/25179) anther-expressed genes (Figure 7a, Tables S3-4). Therefore, activation is nearly 10 times more prevalent than suppression of the anther gene expression program. Among the 24736 maize genes common to both control and infected anthers, 2% (534/24736) were up-regulated during infection (Table S5), 1% (188/24736) were down-regulated (Table S6), and 97% (24014/24736) were statistically similar in expression level. Therefore, the de novo activation of maize gene expression is much more extensive than differential expression of genes with persistent expression (Table S7).
Figure 7.
Maize genes expressed in infected anthers or tassels and a comparison of these samples, 3 dpi. (a) Analysis of expressed maize genes in infected and mock-infected anthers of 300-600 μm, 3 dpi. (b) Analysis of expressed maize genes in infected and mock-infected tassels of 2-3 cm containing anthers ranging from 100-700 μm, 3 dpi. (c) Co-expression analysis of the infected anther and tassel data sets.
A previous study documented fewer changes in gene expression by U. maydis infection (Skibbe et al., 2010, redrawn in Figure 7b) when whole tassels were analyzed: only 0.85% (299/(35215 + 394)) of maize genes were de novo expressed and 1.1% (394/(35215 + 394)) were no longer expressed in 3 dpi tassels. Among 35215 maize genes scored as expressed in both infected and mock-infected tassel samples (Figure 7b), 2.4% (825/35215) were up-regulated, 3% (1052/35215) were down-regulated, while 95% (33338/35215) were statistically similar in these two samples.
The tassels analyzed previously contained anthers spanning the size classes utilized in the current study of anther gene expression changes. Comparing the two data sets, there were 28549 maize genes expressed at 3 dpi in both infected anthers and tassels. Because tassels contain anthers, it is not surprising that only 334 genes were detected as uniquely expressed in infected anthers (Figure 7c, Table S8). 81% (271/334) of these were below the median in terms of intensity, suggesting that their expression was not detected in the more complex tassel organ. In contrast, 6965 genes were only expressed in tassels, likely reflecting contributions of the stem, glumes, palea, and lemma organs.
To gain insight into the anther developmental or physiological processes affected by U. maydis infection, GO term assignments with p-values for maize genes evaluated by microarray hybridization (Table S9), and GO term abundances were evaluated for all four classes of affected maize transcripts, excluding transcripts lacking any annotation (unknown function class). De novo expressed and up-regulated maize genes showed similar high-abundance GO terms (Figure S4). Transcription factor activity was more prominent in the up-regulated class at 9%, versus less than 3% of GO annotations in the “On,” “Off,” and down-regulated classes. High-abundance GO terms for down-regulated maize genes were nucleotide binding (14%), catalytic activity (10%),protein binding (10%), transferase activity (9%), transcription regulator activity (9%), and nucleic acid binding (9%). In the “Off” category, catalytic activity (12%), protein binding (11%), transporter activity (11%), nucleotide binding (11%), transferase activity (10%), and hydrolase activity (7%) accounted for 62% of all annotations. Because U. maydis causes such profound changes in maize anther cell division patterns, we investigated the DNA replication GO category in more detail (Table S10). There are 41 genes with 51 probes in the maize DNA replication categories represented on the array. Of these genes, only GRMZM5G809078 (1/41=2%) was up-regulated in infected samples, GRMZM2G044317 and GRMZM2G336879 were only expressed in infected anthers (2/41=5%), meanwhile 27 genes (27/41=65%) were down-regulated, and 8 genes (8/41=20%) were not statistically different, and 3 genes were not expressed in either mock-infected or infected anthers (3/41=7%). Therefore, most DNA replication genes were down-regulated, which could contribute to the delay in cell divisions observed in some cell types. Only 7% ((1+2)/41) had a large difference between mock and infected anthers, but these could also contribute to the abnormal morphological observations. At 3 dpi, 2018 U. maydis genes were detected as expressed in infected anthers (Figure S5). GO terms of with p-values of “On” in U. maydis were assigned (Table S11). Nearly half (45%) of the fungal genes with known functions are involved in metabolism. The other highly abundant GO categories were protein synthesis (11%), protein fate (10%), and cell cycle and DNA processing (9%).
Previous transcriptome analysis comparing U. maydis infection of seedlings, adult leaves, and tassels at 3 dpi established that the fungus expresses a unique cohort of genes in each maize organ (Skibbe et al., 2010). As shown in Figure S6a, we found that 505 U. maydis genes were expressed in both infected tassel and anther samples (Table S12), while the fungi expressed 2280 genes only in the tassels and 1513 genes only in the anthers. The fungal genes expressed uniquely in tassel samples are strong candidates for expression in floral organs other than the anthers. This analysis is supported by examination of the intensity of gene expression in infected anthers and tassels (Figure S7c): the only shared highly expressed genes are found in tassels. These patterns are also true for the U. maydis secretome genes (Figure S7b, d). Among the secretome genes, there were a high number of fungal gene expressions unique to anthers (206) or to tassels (111); as with total fungal gene expression, we expected to discover no new fungal genes by dissecting anthers, however, all the fungal secretome genes are below the median and therefore in tassel samples expression may have been below the detection limit. The unique to tassel class of U. maydis secretome genes are candidates for fungal manipulation of vegetative floral tissues such as stem, glumes, lemma, and palea.
To determine if the needle injection required to perform a mock infection altered the maize anther transcriptome, we compared mock-infected whole anthers of 300-600 μm at 3 dpi to untreated 400 or 700 μm W23 inbred whole anther datasets (GSE43982; Figure S7). 0.5% (118/21692) genes were specifically expressed in mock-injected anthers. We consider this figure insignificant. Compared to whole anther data of 400 μm, mock-injected anthers expressed 4% ((118 + 777)/24199) unique genes, while compared to 700 μm, they expressed 0.5% ((118 + 39)/29786). Given that anthers exhibit many stage-specific transcripts, the Venn analysis uncovered instances of unique expression in the pooled 300-600 μm mock-infected cohort compared to either the 400 or 700 μm normal anthers. The difference between the stages exceed the differences with the pooled mock-infected samples, therefore, we conclude that introducing water around the developing tassel does not greatly alter anther gene expression monitored at 3 dpi. By qRT-PCR, we validated 8 expression results in terms of “On”/ “Off” or up/down-regulation; several genes were selected that were not detectably expressed by microarray analysis and these exhibit extremely low values in the qRT-PCR analysis (Tables S13-14).
DISCUSSION
U. maydis effects on maize anther cells
The primary goals of studies on host-pathogen interactions have been to elucidate the sequential deployment of pathogen effectors, to analyze effector targets in the host, and to define the scope of host defense. In the case of U. maydis-maize interaction, the outcome of successful infection is redirection of maize development to form tumors within which the fungal pathogen produces diploid teliospores for dispersal (Banuett and Herskovitz, 1996). Through a combination of genetics and biochemistry, the necessity and roles of a number of fungal effectors that act early in infection have been established, such as Pep1 (Doehlemann et al., 2009). Tumor formation requires distinct suites of fungal genes, depending on the host organ infected, reflecting the discrete mechanisms governing maize organogenesis which the pathogen must subvert or co-opt to cause tumors (Skibbe et al., 2010). In the present study, we analyzed the impact of U. maydis on anther development to address the question of whether the pathogen causes cell-type specific alterations in host cell division and expansion. By monitoring host cell properties at sequential stages of anther development, we could also define the dependence of the host response on the temporal program of anther differentiation.
Fungal penetration into maize leaves is a gradual process; two days after infection, hyphal tips are just below the leaf surface (Schirawski et al., 2005). Similarly, we observed that invading U. maydis filaments are primarily on the surface of maize anthers at 1 to 2 dpi. Following penetration, the hyphae spread rapidly throughout the anther: by 3 dpi, all internal anther cell types may be in contact with the living hyphae (Table 1).
Confocal imaging permitted the measurement of host cell volumes and counts of fungal infection (Tables S1A-B, S2A-B) during the developmental period spanning anther cell fate specification (400-700 μm) and cell proliferation and expansion prior to meiosis at 1500 μm. By comparison with mock-infected anthers, we analyzed development with reference to key landmarks of cell fate specification, including the addition of new cell layers by periclinal cell division, periods of rapid cell division, and cell-type specific patterns of cell expansion. U. maydis infection triggered complete disruption of subepidermal cell development in anthers that were < 100 μm at the time of infection, such that no recognizable cell types differentiated; we conclude that the pathogen prevents the normal specification of AR cells, which in turn interrupts the production of signals that result in a periclinal division of the subepidermal layer to producing the EN and SPL cell types (Kelliher and Walbot, 2012).
In subsequent stages, anthers consist of epidermis and three subepidermal cell types, and each cell type showed significant differences in length, width, depth, and/or volume at some stages when monitored at 3 dpi (Tables S1A-B, S2A-B). Because anthers continue to elongate and increase in girth during infection, changes in cell volume are necessarily accompanied by alterations in cell numbers for specific cell types as a means of compensation (Tables S1A-B, S2A-B). It is particularly striking that mitosis continues in the pre-meiotic cell population to achieve nearly 200 AR per lobe rather than the usual 160 cells; the smaller AR proliferate to fill the central space, reinforcing the previous suggestion that spatial constraints may normally limit AR cell numbers (Wang et al., 2011). Therefore, we conclude that while overall anther growth is initially little perturbed by U. maydis infection, the cellular composition and cell type-specific volumes are substantially altered. That is, the balance of anticlinal cell division vs. expansion-which sets cell volumes is altered by U. maydis without much impact on overall anther size over 3 dpi. Despite this observation, mature tumors at 10-14 dpi are the result of enormous cell division and expansion in infected anthers compared to normal floral organs (Walbot and Skibbe, 2010). In leaf tumors, greatly enlarged cells containing endoreduplicated chromosomes are observed (Callow, 1975).
A striking observation during U. maydis infection is that SPL cell division is delayed, and this cell type can still be present at 1000 μm (Figure 4). Moreover, within a single anther, some lobes had undergone the SPL periclinal division while other lobes were arrested. These results and our collective observations indicate that there is considerable variation in anther cell response to U. maydis. We consider it likely that the timing and extent of formation of the biotrophic interface, a narrow gap between host and fungal cells, could determine host responses. The proportion of SPL cells, for example, involved in a biotrophic interaction could affect completion of the periclinal division. Periclinal divisions are precisely controlled in plant development because they generate additional layers in organs and are frequently required for the differentiation of new cell types. The somatic cell layers surrounding the germinal cells are derived by two rounds of periclinal division: the first yields the EN and SPL, and in the second the SPL generates the ML and TA (Figure 1). Depending on when infection starts, U. maydis disrupts both of these landmark events.
Wenzler and Meins (1987) demonstrated that U. maydis infection is confined to young aerial tissues of maize. Within the tassels, Walbot and Skibbe (2010) observed that all organs can form tumors, but there is a progression of which floral parts are most prominently involved that follows the chronological pattern of spikelet organ maturation (cessation of mitosis). These observations established that U. maydis can only redirect maize to tumor development in zones with actively dividing cells; thus unlike other oncogenic agents that can reactivate cell division, U. maydis can only direct the developmental outcome in proliferating floral tissues. In developing anthers there is rapid cell proliferation from ~200-800 μm with cessation of cell division in the AR, TA, and ML cells by about ~1000 μm (Kelliher and Walbot, 2011). This period of rapid cell division corresponds to the period when most anther tumors are generated (Skibbe et al., 2010; Walbot and Skibbe, 2010). In the current study we further demonstrate that there is cell type specificity in response to fungal infection, evidenced by the alterations in division and expansion in specific cell types at various developmental stages.
Microarray analysis
In the comparison of infected and mock-infected anthers, a greater number of maize genes were turned “On” or up-regulated than were turned “Off” or down-regulated; therefore, the overall response in infected anthers is activation rather than repression of gene expressions. By 3 dpi, as U. maydis subverts normal development, the anther appears to respond to fungal invasion by activating and altering the expression of developmental genes, rather than switching on host defenses to combat infection.
Comparing anther and whole tassel microarray data, we find support for the hypothesis that U. maydis tailors its gene expression to specific maize organs. There were only 505 fungal genes scored as expressed in both tassels and anthers, while 3793 (2280 + 1513) genes were found in just one of these infected samples (Figure S7a). As the tassels contain anthers, we interpret this result as indicating that anther infection requires a discrete program of fungal gene expression and that many of these genes are expressed at low levels that were undetectable in the much more complex tassel organ sample. Unlike leaf development in which there are zones of cell specification, cell proliferation, cell differentiation, and expansion in an acropetal gradient from the base of the blade, the entire anther undergoes developmental events in a chronological series. We therefore speculate that U. maydis may deploy distinctive gene expression programs reflecting the temporal series of developmental stages. This facet of host-pathogen interaction could be explored in future microarray analysis of many developmental stages of anther development. Our analysis also found that 2280 fungal genes were expressed in infected anthers that were absent (or below the detection limit) in uninfected anthers. Although U. maydis has profound impacts on the pace and pattern of host cell proliferation, only 7% (3/41) of genes in the DNA replication GO category were activated or up-regulated in expression during infection, while a large fraction of this category (27/41) was down-regulated. It appears likely, therefore, that U. maydis is modulating cell division by inducing modest changes in existing gene expression programs.
Fungal infection, growth, and spore differentiation require about 2 weeks and culminate in the release of billions of diploid teliospores (Banuett and Herskowitz, 1996). Based on the phenotype of fewer or smaller tumors after fungal gene deletion and computational analysis of the U. maydis genome, the current hypothesis to explain tumor induction is that this pathogen secretes effector proteins that cause abnormal host cell differentiation. Analysis of the U. maydis genome yielded 554 predicted secreted proteins (Müeller et al., 2008); some clusters of predicted secretome genes have been shown to be required for tumor formation in seedlings (Kämper et al., 2006) and to have differential impact on seedlings, adult leaves, and tassels (Skibbe et al., 2010). Only 25 secretome genes were detectably expressed at 3 dpi in both anther and tassel, while 206 were specifically detected in anthers, compared to 111 that were specifically expressed in tassels. These results indicate the value of anther dissection as the tassels contained anthers spanning the size range of the anther samples; it is likely that the “anther-specific” secretome genes were below the limit of detection in the more complex tassel sample and the tassel-specific secretome genes are involved in fungal interaction with non-anther tissues.
EXPERIMENTAL PROCEDURES
Plant growth
W23 bz2 inbred line of Z. mays (deficient in vacuolar anthocyanin accumulation) were greenhouse grown with supplemental lighting equivalent to 40% summer photon fluence (no UV radiation) for a 14h day/10h night period (Casati and Walbot, 2004). Light period temperature was 32°C and dark period temperature was 21°C.
Fungal growth and injections
SG200-YFP (supplied by Regine Kahmann, Max Planck Institute, Marburg, Germany) is a solopathogenic strain that can infect plants without a mating partner (Kämper et al., 2006). The strain was grown and injected following published procedures (Krüger et al., 2000; Walbot and Skibbe, 2010). Typically, 6 replicates were performed with each injection treatment (U. maydis and water-injected) for each dpi period surveyed. Three replicates of field-grown plants during summer 2012 were at tassel lengths of ~1, ~5 and ~8 cm for evaluation of growth parameters at 8-14 dpi.
Anther staining and microscopy
Spikelets were dissected to recover the anthers; one anther from each dissected floret was incubated in fixative (100% ethanol) to score for infection using light or confocal microscopy; the other 2 anthers of the same floret were snap frozen in liquid nitrogen for RNA extraction.
U. maydis was visualized using the YFP signal and/or by staining with 10 μg/ml WGA-AF488 (Molecular Probes, Invitrogen, USA) as described (Doehlemann et al., 2009). Confocal images were taken with a Leica TCS SP5 (Leica, Germany) with excitation/emission spectra of 561/590-640 nm for PI, 514/ 520-550 nm for YFP, and 488/500-520 nm for WGA-AF488. Five or more replicates were evaluated for each reported size class and treatment. Evaluation of cellular parameters and developmental landmarks utilized published protocols (Kelliher and Walbot, 2011), and cell images were processed with Volocity (6.0). The program for visualization of cell volume was Mathematica (7.0).
Microarray hybridization
For both the 3 dpi mock-infected and infected samples, there were 3 biological replicates and a technical replicate of one of the biological samples for each sample type. All eight hybridizations were performed on a single Agilent 4 × 44K dual organism array (Skibbe et al., 2010). RNA was extracted using the TRIzol® (Invitrogen, http://www.invitrogen.com) for pools of anther samples. Labeled cRNA was prepared as described previously by Ma et al. (2006). A balanced dye swap protocol was used to minimize systematic variances (Kerr and Churchill, 2001; Ma et al., 2006). After hybridization on a dual-organism according to the manufacturer’s protocols, slide scanning, feature extraction, signal criterion, statistical and graphical criteria, and GO annotation were performed as described previously (Skibbe et al., 2010). All microarray data associated with these experiments are available at GEO (http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE43544 (anther-U. maydis experiment) and GSE43982 (400 and 700 μm W23 anthers).
qRT-PCR analysis
These analyses were used to validate the microarray results for a handful of genes Tables S13-S14). Frozen anthers were pooled by size into samples of 400, 700, 1000, 1500 and 2000 μm for both infected and mock-infected anthers. RNA was extracted from 2 pools of staged infected anthers as biological replicates. Primer pairs (Table S15) were designed for selected genes, purchased from Invitrogen (http://www.invitrogen.com). qRT-PCR reactions and amplification conditions followed published protocols for maize (Ma et al., 2008) or U. maydis (Doehlemann et al., 2009; Wahl et al., 2010). The expression level of maize cyanase and fungal ppi were used as the internal controls for each species.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Carnegie Institution, Department of Plant Biology Imaging Facility, for use of the confocal microscope. John Fernandes wrote the program to visualize cell dimensions. L. G. was supported by the Scholarship Program of the Chinese Scholarship Council (2010325033). T. K. was supported by the NIH Biotechnology Training Grant (5-T32-GM008412-17). L.N. received a Stanford VPUE award to support summer undergraduate research. Manuscript preparation was supported in part by the Ministry of Science and Technology, China (Grant Nos. 2011CB100403, 2013CB127701) and by the Ministry of Agriculture, China (Grant No. 200903035).
REFERENCES
- Banuett F. Pathogenic development in Ustilago maydis. In: Osiewacz HD, editor. Molecular Biology of Fungal Development. Marcel Dekker; New York: 2002. pp. 349–398. [Google Scholar]
- Banuett F, Herskowitz I. Discrete developmental stages during teliospore formation in the corn smut fungus. Ustilago maydis. Dev. 1996;122:2965–2976. doi: 10.1242/dev.122.10.2965. [DOI] [PubMed] [Google Scholar]
- Callow JA. Endopolyploidy in maize smut neoplasms induced by maize smut fungus. Ustilago maydis. New Phytol. 1975;75:253–257. [Google Scholar]
- Callow JA, Ling IT. Histology of neoplasms and lesions in maize seedlings following the infection of sporidia of Ustilago maydis (DC) Corda. Physiol. Plant Path. 1973;3:489–494. [Google Scholar]
- Casati P, Walbot V. Rapid transcriptome responses of maize (Zea mays) to UV-B in irradiated and shielded tissues. Genome Biol. 2004;5:R16. doi: 10.1186/gb-2004-5-3-r16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehlemann G, van der Linde K, Aßmann D, Schwammbach D, Hof A, Mohanty A, Jackson D, Kahmann R. Pep1, a secreted effector protein of Ustilago maydis, is required for successful invasion of plant cells. PLoS Pathog. 2009;5(2):e1000290. doi: 10.1371/journal.ppat.1000290. doi:10.1371/journal.ppat.1000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehlemann G, Wahl R, Vranes M, de Vries RP, Kämper J, Kahmann R. Establishment of compatibility in the Ustilago maydis/maize pathosystem. J. Plant Physiol. 2008;165:29–40. doi: 10.1016/j.jplph.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Kämper J, Kahmann R, Bölker M, et al. Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature. 2006;444:97–101. doi: 10.1038/nature05248. [DOI] [PubMed] [Google Scholar]
- Kelliher T, Walbot V. Emergence and patterning of the five cell types of the Zea mays anther locule. Dev. Biol. 2011;350:32–49. doi: 10.1016/j.ydbio.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher T, Walbot V. Hypoxia triggers meiotic fate acquisition in maize. Science. 2012;337:345–348. doi: 10.1126/science.1220080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr MK, Churchill GA. Statistical design and the analysis of gene expression microarray data. Genet. Res. 2001;77:123–128. doi: 10.1017/s0016672301005055. [DOI] [PubMed] [Google Scholar]
- Krüger J, Loubradou G, Wanner G, Regenfelder E, Feldbrügge M, Kahmann R. Activation of the cAMP pathway in Ustilago maydis reduces fungal proliferation and teliospore formation in plant tumors. Mol. Plant Microbe. Interact. 2000;13:1034–1040. doi: 10.1094/MPMI.2000.13.10.1034. [DOI] [PubMed] [Google Scholar]
- Ma J, Morrow DJ, Fernandes J, Walbot V. Comparative profiling of the sense and antisense transcriptome of maize lines. Genome Biol. 2006;7 doi: 10.1186/gb-2006-7-3-r22. doi: 10.1186/gb-2006-7-3-r22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Skibbe DS, Fernandes J, Walbot V. Male reproductive development: gene expression profiling of maize anther and pollen ontogeny. Genome Biol. 2008;9:R181. doi: 10.1186/gb-2008-9-12-r181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müeller O, Kahmann R, Aguilar G, Trejo-Aguilar B, Wu A, de Vries RP. The secretome of the maize pathogen Ustilago maydis. Fungal Genet. Biol. 2008;45:S63–70. doi: 10.1016/j.fgb.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Schirawski J, Böhnert HU, Steinberg G, Snetselaar K, Adamikowa L, Kahmann R. Endoplasmic reticulum glucosidase II is required for pathogenicity of Ustilago maydis. Plant Cell. 2005;17:3532–43. doi: 10.1105/tpc.105.036285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibbe DS, Doehlemann G, Fernandes J, Walbot V. Maize tumors caused by Ustilago maydis require organ-specific genes in host and pathogen. Science. 2010;328:89–92. doi: 10.1126/science.1185775. [DOI] [PubMed] [Google Scholar]
- Snetselaar KM, Mims CW. Sporidial fusion and infection of maize seedlings by the smut fungus Ustilago maydis. Mycologia. 1992;84:193–203. [Google Scholar]
- Steinberg G, Schliwa M, Lehmler C, Bölker M, Kahmann R, McIntosh JR. Kinesin from the plant pathogenic fungus Ustilago maydis is involved in vacuole formation and cytoplasmic migration. J. Cell Sci. 1998;111:2235–46. doi: 10.1242/jcs.111.15.2235. [DOI] [PubMed] [Google Scholar]
- Wahl R, Wippel K, Goos S, Kämper J, Sauer N. A novel high-affinity sucrose transporter is required for virulence of the plant pathogen Ustilago maydis. PloS Biology. 2010;8(2):e1000303. doi: 10.1371/journal.pbio.1000303. doi:10.1371/journal.pbio.1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walbot V, Skibbe DS. Maize host requirements for Ustilago maydis tumor induction. Sex Plant Reprod. 2010;23:1–13. doi: 10.1007/s00497-009-0109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CJR, Nan GL, Kelliher T, Timofejeva L, Vernoud V, Golubovskaya IN, Harper L, Egger R, Walbot V, Cande WZ. Maize multiple archesporial cells 1 (mac1), an ortholog of rice TDL1A, modulates cell proliferation and identity in early anther development. Dev. 2012;139:2594–2603. doi: 10.1242/dev.077891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Skibbe DS, Walbot V. Maize csmd1 exhibits pre-meiotic somatic and post-meiotic microspore and somatic defects but sustains anther growth. Sex Plant Reprod. 2011;24:297–306. doi: 10.1007/s00497-011-0167-y. [DOI] [PubMed] [Google Scholar]
- Wenzler H, Meins F. Persistent changes in the proliferative capacity of maize leaf tissues induced by Ustilago infection. Physiol. Mol. Plant Pathol. 1987;30:309–319. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.