Abstract
Efficient transfer of sound by the middle ear ossicles is essential for hearing. Various pathologies can impede the transmission of sound and thereby cause conductive hearing loss. Differential diagnosis of ossicular disorders can be challenging since the ossicles are normally hidden behind the tympanic membrane (TM). Here we describe the use of a technique termed optical coherence tomography (OCT) vibrography to view the sound-induced motion of the TM and ossicles simultaneously. With this method, we were able to capture three-dimensional motion of the intact TM and ossicles of the chinchilla ear with nanometer-scale sensitivity at sound frequencies from 0.5 to 5 kHz. The vibration patterns of the TM were complex and highly frequency dependent with mean amplitudes of 70–120 nm at 100 dB sound pressure level. The TM motion was only marginally sensitive to stapes fixation and incus-stapes joint interruption; however, when additional information derived from the simultaneous measurement of ossicular motion was added, it was possible to clearly distinguish these different simulated pathologies. The technique may be applicable to clinical diagnosis in Otology and to basic research in audition and acoustics.
The tympanic membrane (TM) and the middle ear ossicles (malleus, incus and stapes) play a pivotal role in hearing by gathering and transmitting airborne sound waves to the cochlea. In the most sensitive frequency range, between 1 to 5 kHz, a normal human can detect sound pressure levels (SPL) as low as 20 µP (0 dB SPL), corresponding to TM vibration amplitudes of less than 10 pm (Merchant and Rosowski, 2010). Conversational speech (40 to 60 dB SPL) produces motions that are 10–100 fold larger. Middle-ear injuries (e.g. perforations of the TM or dislocation of the ossicles) or disease (e.g. otosclerosis causing joint fixation) can attenuate sound conduction by as much as 50 to 60 dB, seriously compromising communication.
It is estimated that worldwide over 12 million adults have moderate to severe conductive hearing loss (Holley, 2005). In current clinical practice, no diagnostic methods can reliably and noninvasively differentiate between different ossicular disorders. Otoscopy is commonly used to determine the presence of middle-ear infection and perforations of the TM. However, ossicular disorders are not visible through the intact TM. Standard audiometry quantifies the degree of conductive hearing loss, but it cannot provide a differential diagnosis. Tympanometry, which measures acoustic reflections from the whole TM at different static pressures, allows a diagnosis of middle-ear fluid (Dempster and Mackenzie, 1991), but cannot reliably differentiate ossicular pathologies because of the variability in the coupling of the TM to the ossicles (Jerger, 1975; Nakajima et al., 2012; Shanks and Schohet, 2009). Presently, the definitive diagnosis of ossicular pathologies requires invasive surgical exploration that involves incising the TM around its periphery and lifting the TM to expose the ossicles (Athanasiadis-Sismanis and Poe, 2010). A noninvasive method capable of identifying the cause of conductive hearing loss without having to lift the TM would be highly useful for differential diagnosis and to improve the pre-surgical planning and outcomes of ossicular reconstructive procedures (Fisch et al., 2001; Rosowski et al., 2008).
Scanning laser vibrometry (LDV) (Huber et al., 2001; Rosowski et al., 2008) and laser holography (Cheng et al., 2010; Rosowski et al., 2009; Wada et al., 2002) provide quantitative information about the amplitude and phase of vibration. However, these methods are surface measurements and therefore require invasive surgical procedures to access the incus and stapes (Chien et al., 2009). In contrast, optical coherence tomography (OCT) with infrared light can penetrate tissues and provide cross-sectional imaging at various depths (Heermann et al., 2002; Just et al., 2009; Lin et al., 2008; Pitris et al., 2001). While use of OCT for retinal imaging is valuable and widespread, applications to hearing, where tissues may be in vibratory motion, have just begun to be explored. OCT has recently been used to measure the vibrational amplitudes produced by 0.5 kHz stimulation of the malleus and incus through the TM in cadaveric human ears (Subhash et al., 2012) and motion of the Organ of Corti in guinea pigs has been measured through the round window (Chen et al., 2011).
In this paper, we describe a novel technique termed OCT vibrography that simultaneously captures the three-dimensional shape and sound-induced motion of the TM and substantial parts of the ossicular chain with high spatial and dynamic resolution. The vibrography system uses synchronized data acquisition, beam scanning and sound stimulus generation (Chang et al., 2012). We tested the system on cadaver chinchilla temporal bones at frequencies ranging from 0.5 to 5 kHz. Surgical models of ossicular fixation and dislocation were used to assess the potential of the method to detect and differentiate various pathologies in the middle ear.
Methods
Imaging system
The OCT system employed a wavelength-swept laser source with a tuning range from 1220 to 1350 nm and an average output power of 30 mW at the sample (Yun et al., 2003; Yun et al., 2006). The laser was operated at a sweeping repetition rate between 15–20 kHz. The sample arm employed a pair of XY galvanometer-mounted mirror scanners (Cambridge Technologies) and a 35 mm focusing lens. The focus of the infrared laser light was placed approximately at the mid-plane of the middle ear space between the TM and cochlear wall. The system controlled a loudspeaker (Auvio 3-way speaker) to generate pure tone sound stimuli and synchronously measured the amplitude and phase of the light scattered from middle-ear tissues (Fig. 1a and Fig. S1). Monotone sinusoidal signals from a synthesizer were amplified with an audio amplifier and directed to the loudspeaker placed above the specimen (Figs. S2). A calibrated probe microphone was placed near the edge of the TM to record the sound pressure level (SPL) applied to the TM surface. A two-channel 10 MS/s data acquisition board (National Instruments) was used to acquire the interferometric signals. A single clock generated from the data acquisition board served as a master clock that synchronized the XY galvanometers and loudspeaker with the laser sweeps. The transverse and axial resolutions of the OCT system were 25 and 12 µm, respectively.
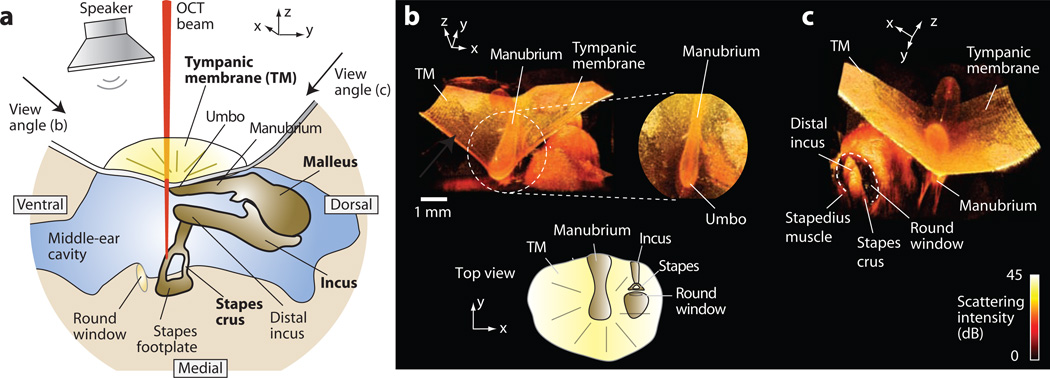
Figure 1.
Views of ear anatomy. (a) A schematic representation of the TM and the middle ear. (b) 3D OCT images of the chinchilla middle ear. The view angle of rendering is indicated in (a). The left figure shows a fraction of the TM surface as well as some of the structure medial to the TM looking from ventral to dorsal. The manubrium of the malleus is oriented along the ventral-dorsal projection (−y to +y). Positive x is anterior. The expanded view shows the manubrium in the center of the TM and umbo near the bottom. The bottom inset is a schematic of corresponding anatomy (top view). (c) A view looking from dorsal to ventral, as indicated in (a). The image shows the round window in the cochlear base, a segment of the distal incus (the ventral most projection of the incus), and one crus of the stapes. The prong-like structures below the manubrium are shadows in the reconstruction.
Vibrography data acquisition
At a given position of the optical beam, a series of axial lines (A-lines), locked in time to selected phases of the acoustic stimulus, were acquired during a single (or a few) cycle(s) of sound stimulation. The scanning mirrors steered the OCT beam to measure reflectance from a column of 500 points along an A-line (z-axis) at a specific XY grid locations. After this was repeated for one cycle, the beam was moved to a neighboring location, and a new series of A-lines was gathered. Repeating this step, the probe beam was scanned laterally across the sample to acquire four-dimensional data containing the amplitude and phase of the scattered light at three-dimensional spatial coordinates (x, y, z) as a function of time or motion phase (φ) (Fig. S3). The number of motion phases acquired per cycle was determined by the ratio of the A-line rate and vibration frequency. For example, for an A-line rate of 18,000 Hz and sound frequency of 1,500 Hz, 12 (=18,000/1,500) motion phases were captured (Kobler et al., 2010). A subsequent binning of the acquired data with respect to the motion phase generated a series of “snapshot” images over the vibration cycle. To cancel inherent deterministic motion jitter from the scanner and normalize the measurements for deterministic sound-induced bulk motion of the specimen, a reference measurement was made on a quasi stationary object within the scan volume (usually the bony wall of the cochlea). The measured jitter was then subtracted from all measurements. The sensitivity to vibration amplitude, which depends on the magnitude of light scattering, was about 0.5 nm for the TM and about 5 nm for the stapes (where the reflected signal was more attenuated). For more details of data processing, see Supplementary Information.
Tissue preparation
Three cadaver chinchillas were used. The cartilaginous and bony external ear canals were removed from the 3 chinchilla specimens to expose most of the TM while maintaining the integrity of the bony TM annulus. The middle ear was maintained widely open to allow surgical manipulation of the ossicular chain. Stapes immobilization was achieved by applying a drop of cyanoacrylic cement between the stapes footplate and surrounding bone. A surgical hook was used to interrupt the incudo-stapedial joint. In one case we also removed one crus of the stapes. The cadaveric specimens were kept moist between measurements by applying saline.
Results
Optical coherence vibrography of the chinchilla middle ear
In the chinchilla ear, the superior (dorsal) malleus head and incus body are obscured by the opaque bony structures around the TM (Fig. 1a). However, the manubrium (the handle of the malleus embedded in the TM) and parts of the incus, stapes and cochlear wall are readily visualized. Figure 1b shows representative renderings of the imaged volume (7 mm × 7 mm × 3 mm in xyz). The images show the topography of the intact, cone-shaped TM, the manubrium, the umbo near the center of the TM, the inferior or distal portion of the incus, the lateral portion of the stapes crura, the tendon of the stapedius muscle, and a partial view of the cochlea including the bone surrounding the round window (Vrettakos et al., 1988).
Digitally reconstructed snapshot vibrational images
Vibrography produced a series of images corresponding to evenly spaced phases (φs) of motion over one vibratory cycle (Fig. S5). A large number of cycles were used to create the final sequence since each cycle contributes a single axial line in each image. The number of phases equals the A-line acquisition rate (15–20 kHz) divided by the sound frequency (0.5–5 kHz). In these experiments the number of phases ranged from 4 to 30. The displacement in z was visualized using a standard color map overlaid on the structural images (Fig. S6, Videos S1, S2).
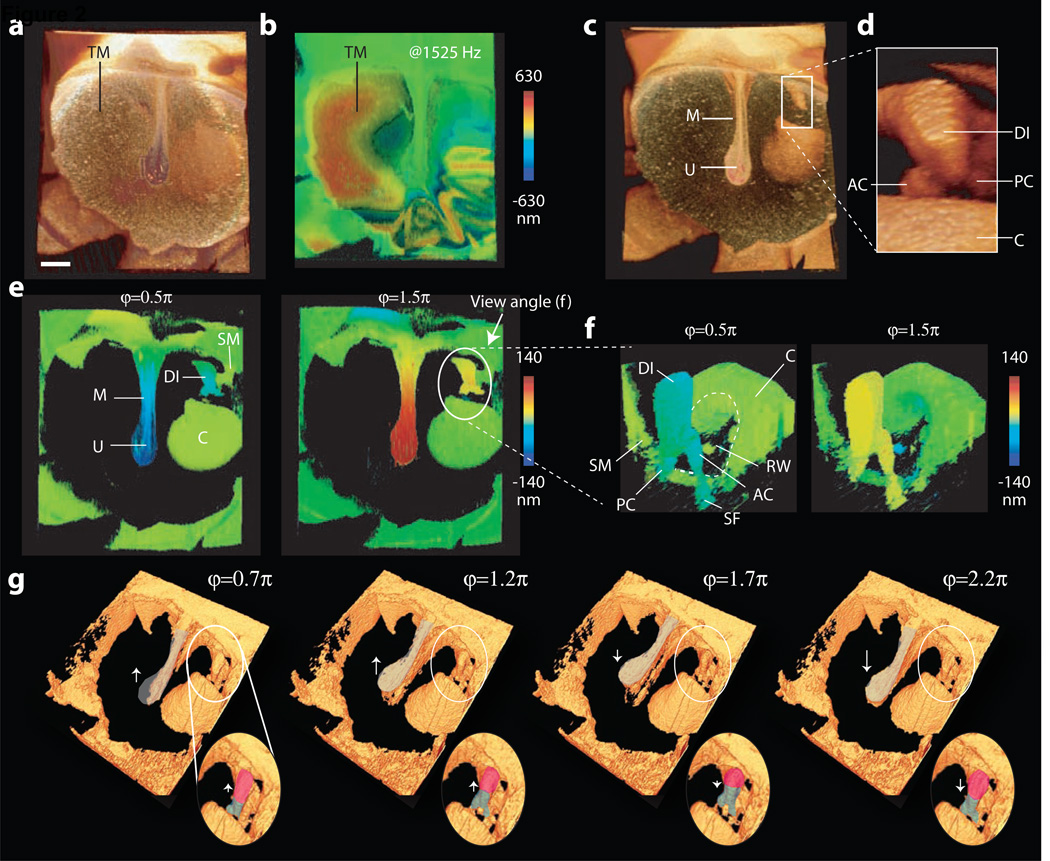
Figure 2a shows a top view of the exposed chinchilla ear, and Figure 2b shows the corresponding vibration pattern at the maximum overall upward displacement of the TM. The sound frequency was 1,525 Hz, with an SPL of 104 dB. With these stimulus parameters the pattern of vibration of the TM was complex. The TM was next digitally segmented and removed to view motion of the underlying ossicles (Fig. 2c). The manubrium, distal incus and the head of the stapes were clearly visible (Fig. 2d). Figure 2e shows corresponding vibrography images at two phases where there were maximal inward (positive) and outward (negative) displacements of the umbo. The images show an amplitude gradient along the manubrium, and in-phase motion of the manubrium, distal incus, and stapes. The tympanic ring and cochlea also show a relatively small amount of motion to the intense stimulus. The 3D dataset allows the ossicular chain to be viewed from different angles. In Fig. 2f, a portion of the distal incus was digitally removed to view the most ventral portion of the incus, the posterior and anterior crura of the stapes, the petrous bone surrounding the round window, and the fluid-filled scala tympani of the cochlea. The ventral incus, stapes crura, and stapes footplate were oserved to vibrate nearly in phase. The vibration of the round window membrane may also be detectable as small spots in the round window area that changed from pale green to light blue in opposite phase to stapes motion. The sound-induced motion of ossicles was also visualized by digitally exaggerating the motion (Fig. 2g and Video S3).
Figure 2.
Structural and functional imaging of the chinchilla ear. (a) Structural image, and (b) a color-coded instantaneous vibrographic map of the outer surface of the TM for stimulation of a 1,525 Hz, 104 dB SPL tone. The motions are relative to the position in the no-stimulus condition. (c–e) A view of the middle ear after digital subtraction of the TM. (c) Structural image showing the manubrium (M) of the malleus and the umbo (U). (d) A magnified and slightly rotated view of the cochlear base (C), the distal incus (DI) and the anterior and posterior crura of the stapes (AC & PC). (e) Vibrographic maps at two phases of the tonal stimulus that are one-half-cycle apart. (f) Vibrographic maps of the stapes and incus at the corresponding phases. The view angle from dorsal to ventral is indicated in (e). The vertical motion of the distal incus (DI) and stapes crura (AC and PC) are of similar magnitude at both phases. There are small islands of reflected light that appear to move out of phase with the ossicles. The location of these islands is consistent with the location of the round window membrane. (g) Reconstructions of the computationally 500× magnified motion of the manubrium, incus, and stapes at four phases of vibration.
Vibration of the TM at various frequencies
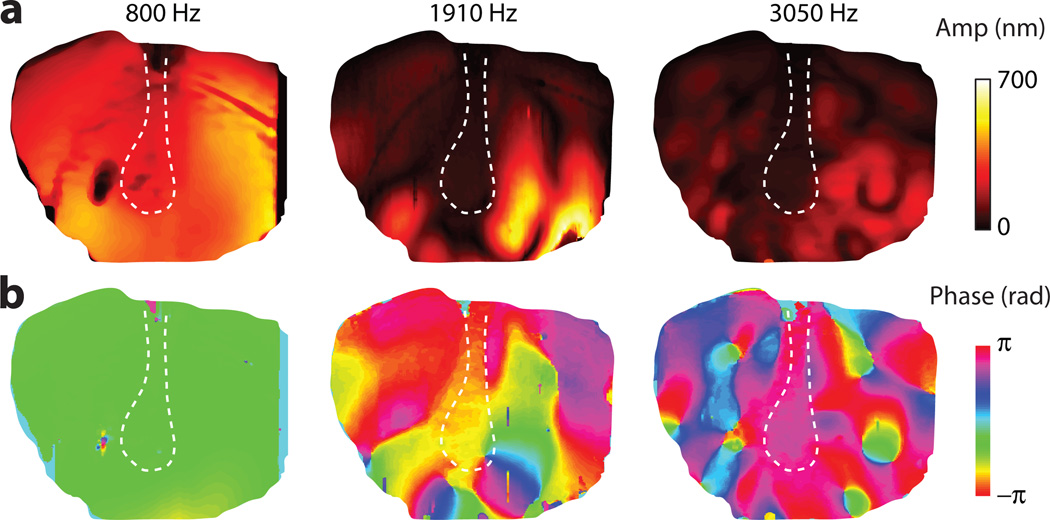
Figure 3a shows amplitude and phase maps of the TM for the stimuli of near 100 dB SPL at 800, 1910, and 3050 Hz. As expected, the number and complexity of the vibration modes increased with frequency. At 800 Hz the entire TM moved relatively in-phase, while at 1910 Hz and 3050 Hz there were multiple sharply defined nodes as well as more gradual phase transitions that suggest either the presence of traveling waves or damped modal motions on the TM surface. The root-mean-square (RMS) amplitude, averaged over the TM surface, was approximately 170 nm at 800 Hz (104 dB), 85 nm at 1910 Hz (102 dB), and 75 nm at 3050 Hz (96 dB). From the series of vibrography images, the average displacement over the TM surface was calculated. Assuming a typical area of 60 mm2 for the chinchilla TM, the RMS volume displacements of the TM normalized by the sound pressure were calculated to be 2×10−3, 0.3×10−3, and 1×10−3 mm3/Pa for 800, 1910, and 3050 Hz, respectively.
Figure 3.
Vibrographs of the magnitude (a) and phase (b) of the motions of the lateral surface of the TM at three frequencies and SPL levels: 800 Hz and 104 dB, 1,910 Hz and 102 dB, and 3,050 Hz and 96 dB. Dotted lines, outline of the manubrium.
Effects of simulated ossicular disorders on TM motion
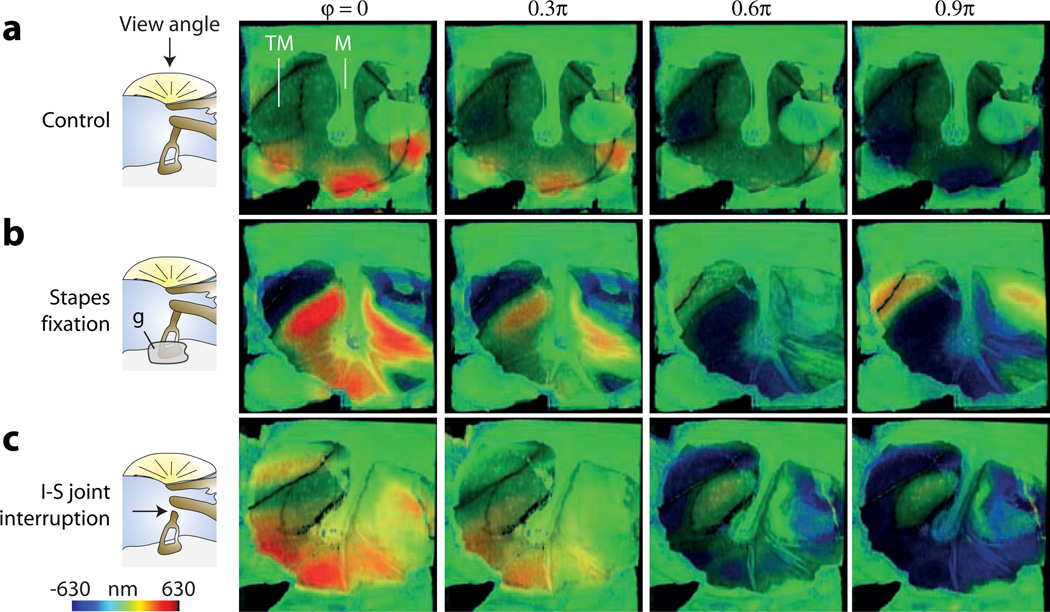
To investigate the potential of this method for identifying middle ear pathologies that would affect the relative motion of the TM and ossicles, we simulated two prevalent disorders: otosclerosis and ossicular joint interruption (Nakajima et al., 2005a; Nakajima et al., 2005b). Clinically, otosclerosis is an inflammatory process that produces abnormal bony growth of the cochlear wall invading the stapes footplate and impeding its motion. This condition was simulated by immobilizing the stapes footplate with cyanoacrylic glue. Joint interruption was produced using a surgical hook. Figure 4 shows vibrography images of the TM (top view) before and after the fixation of the stapes and then following interruption of the incudo-stapedial (I-S) joint at 1,525 Hz frequency and SPL of 104 dB (See also Videos S4a–c). While the vibration patterns of the TM changed after ossicular manipulations, we failed to draw simple relationships between the TM motion and specific ossicular disorders over the 0.5–5 kHz frequency range tested. For example, the RMS vibration amplitudes of the TM are altered by stapes fixation or joint interruption, but these values were frequency dependent (Fig. S7) and the differences between ossicular disorders were not statistically significant.
Figure 4.
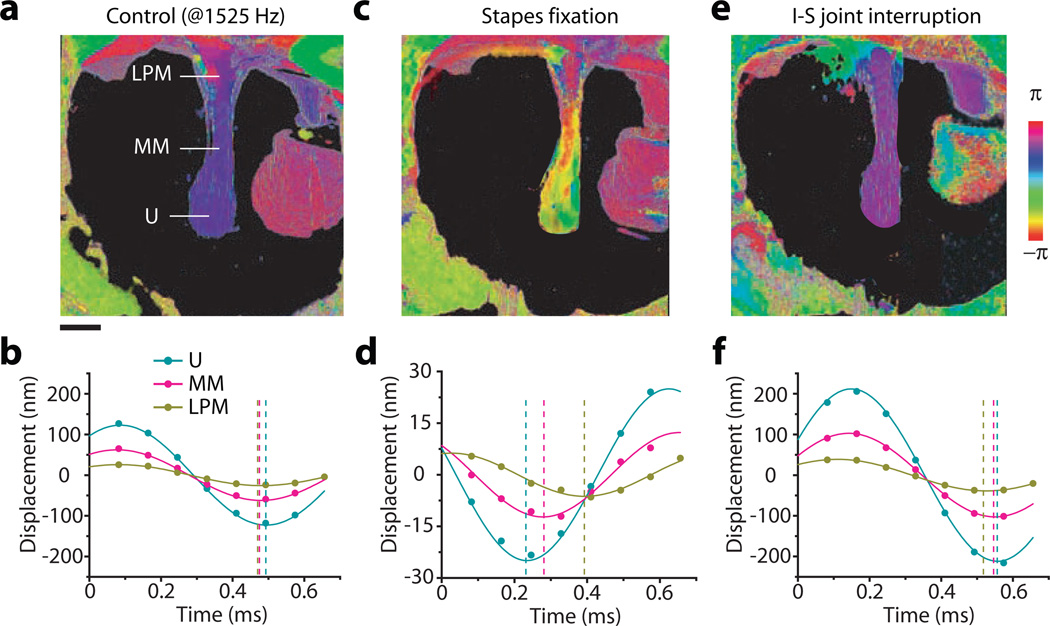
The effects of ossicular manipulations on the motion of the lateral surface of the TM. For each schematized measurement condition, the images show the position of the TM surface relative to the rest position at four motion phases of 0, 0.3, 0.6 and 0.9π, respectively. The stimulus was a 1,525 Hz, 104 dB SPL tone. (a) The control case with intact ossicular chain and mobile stapes. (b) After stapes fixation with cyanoacrylic glue. (c) After interrupting the I-S joint.
Effects of simulated ossicular disorders on mallear motion
The part of the malleus attached to the TM is the manubrium and the distal, spoon-like tip of the manubrium near the center of the TM is the umbo. The manubrium joins the lateral process of the malleus (LPM) near the edge of the TM. The manubrium is relatively thin near the umbo and thickens towards the LPM. The vibration amplitude of the manubrium was highest at the umbo and decreased toward the LPM (see Fig. 2e). In the normal control state, the entire manubrium oscillated nearly in phase at 1,525 Hz (104 dB SPL) (Fig. 5a, b) and at other frequencies up to 5 kHz. This is consistent with simple lever-action of the manubrium with a fulcrum dorsal to the LPM. Stapes fixation decreased the amplitude of manubrial motion and introduced a phase gradient along the length of the manubrium (Fig. 5c, d). Interruption of the I-S joint led to an increase in the vibration amplitude of the entire manubrium above that of the control condition, with a phase of vibration that was largely uniform across the manubrium but slightly lagging with respect to the control (Fig. 5e, f).
Figure 5.
Magnitude and phase of the motion of the manubrium in the control (a, b), stapes-fixed (c, d) and I-S ioint-interrupted (e, f) conditions. The stimulus was a 1,525 Hz, 104 dB SPL tone. (a, c & e) Vibrographic maps showing the phase of motion measured along the lateral surface of the manubrium of the malleus: (U) umbo, (MM) a mid-manubrium position, (LPM) the lateral (dorsal most process) process of the manubrium. (b, d & f) Time waveforms of the motion of the three manubrial locations.
Effects of simulated ossicular disorders on incus and stapes motion
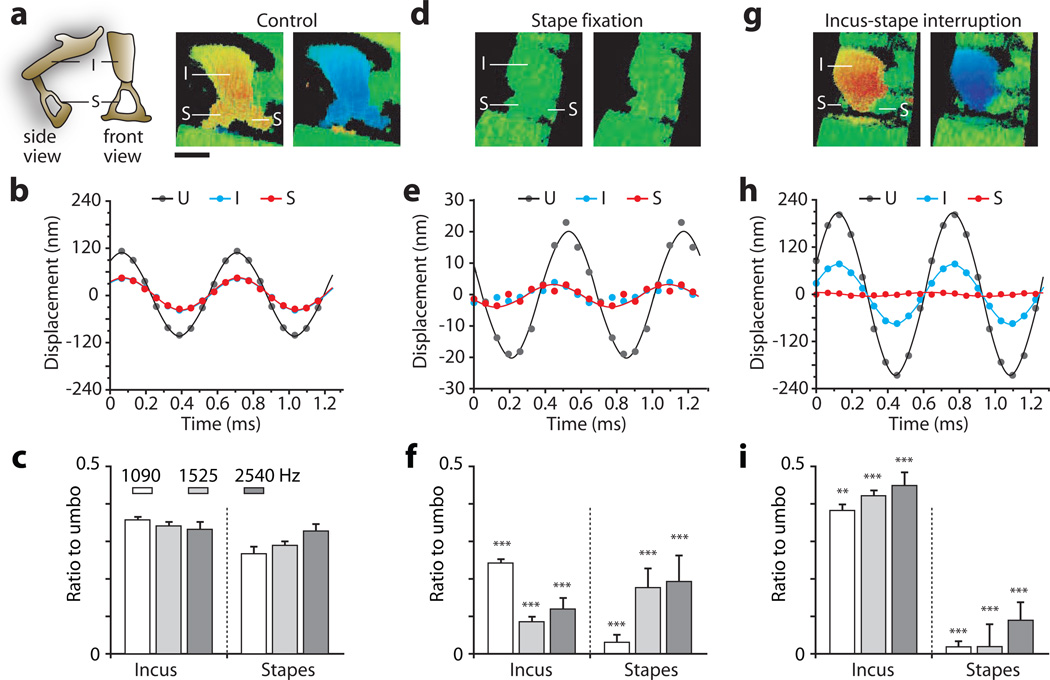
In the control condition, the incus and stapes vibrated with similar magnitudes of 50 nm (1,525 Hz, 104 dB) (Fig. 6a, b and Video S5a), and the incus-to-umbo (I-U) and stapes-to-umbo (S-U) amplitude ratios were measured to be about 0.3 (Fig. 6c). When the stapes footplate was fixed, the motion of the incus and the stapes head were reduced from 50 nm to 3 nm (a 24 dB loss; Fig. 6d, e and Video S5b), while the umbo motion was reduced from 120 nm to 20 nm (a 16 dB loss). Stapes fixation reduced both I-U and S-U ratios significantly in a frequency dependent manner (P<0.005) (Fig. 6f). When the I-S joint was interrupted, stapes motion was reduced to the same low-level motion as the cochlear wall (Fig. 6g and Video S5c). In contrast, incus motion increased to 80 nm (4 dB above control), and umbo motion increased to 210 nm (5 dB above control, Fig. 6h). Thus with I-S joint interruption, the S-U ratio was significantly reduced (P<0.005) and I-U ratio was increased (P<0.01) (Fig. 6i).
Figure 6.
Vibrographic measurements of the magnitude and phase of the motion of the umbo, incus and stapes in the control (a–c), stapes-fixed (d–f) and I-S joint-interrupted (g–i) conditions. The stimulus was a 1,525 Hz, 104 dB SPL tone. (a, d & g) Vibrographic maps showing the displacement relative to rest condition of the incus and stapes. The views are not precisely the same in the three conditions, and the stapes crura are most distinguishable in the control state. (b, e & h) Time waveforms of the displacement of the umbo, incus and stapes in the three conditions. (c, f & i) Measurements of the ratio of the displacement amplitudes of the incus and stapes to the displacement amplitude of the umbo in the three conditions at three frequencies: 1,090, 1,525, and 2,540 Hz. The amplitudes were captured at the umbo, the distal portion of incus and from one stapes crus. Error bars represent standard deviation over multiple (N>20) measurements made at adjacent locations. P-values (two-sided t test) were calculated with respect to the control: ***, <0.005, **, <0.01
Lissajous visuatlization
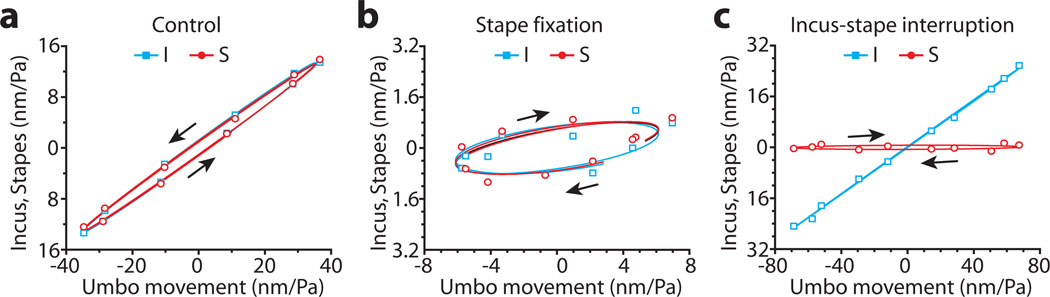
Lissajous plots were useful for visualizing the effects of ossicular manipulations on the relative phase of motion of the three ossicles. A Lissajous plot of the relative motions of the incus and the stapes with respect to the umbo traces a highly eccentric ellipse, indicating a small phase lag (delay) between the distal incus and umbo (Fig. 7a) (Ravicz et al., 2010). After stapes fixation, this Lissajous plot became less eccentric (more round) with clockwise rotation (Fig. 7c). This indicates that the motion of both the incus and stapes were now leading the umbo. With the stapes decoupled from the ossicular chain, the Lissajous plot between the incus and umbo become a straight line without a phase lag (Fig. 7c). The stapes versus umbo plot exhibits a highly eccentric ellipse with an apparent π/2 phase advance.
Figure 7.
Lissajous curves of the motion of the incus and stapes relative to that of the umbo in the control (a), stapes-fixed (b) and I-S ioint-interrupted (c) conditions. The stimulus was a 1,525 Hz, 104 dB SPL tone.
Discussion
We showed that OCT vibrography could simultaneous resolve both the structure and motion of the TM and parts of the ossicular chain. Therefore, this technique should be highly useful for investigating how sound energy is coupled from the TM to the ossicular system in normal and diseased ears (Tuck-Lee et al., 2008).
The observed TM vibration patterns at various sound frequencies were consistent with observations made by laser holography (Cheng et al., 2013; Rosowski et al., 2009; Rosowski et al., 2013). Several models of the TM emphasize the modal (standing wave) motion of the TM (Funnell, 1983), while other models assume that only the waves traveling along the TM surface are important to ossicular coupling (Goll and Dalhoff, 2011; Parent and Allen, 2010). Consistent with holographic measurements (Cheng et al., 2010; Cheng et al., 2013), OCT vibrography images revealed spatial variations in the motion of the TM surface that are consistent with the presence of both modal motions and traveling waves. Further analysis in conjunction with measures of ossicular motion may provide new insight into this issue.
Perhaps most significantly, the simultaneous capture of TM and ossicular motion offers novel diagnostic opportunities. The large variation in the sound-induced motion of the TM alone in normal-hearing humans has precluded diagnosis of ossicular problems based solely on TM measures (Nakajima et al., 2012; Rosowski et al., 2008). OCT vibrography increases the diagnostic information available for detecting and discriminating different ossicular disorders, which it may be possible to boil down to some simple clinical metrics. Stapes fixation reduces the motion of all three ossicles and incus-to-umbo and the stapes-to-umbo amplitude ratios (Figs. 5–7). There is also a reversal of the phase relationship between the umbo and the other ossicles. For I-S joint interruption stapes motion is greatly decreased, while umbo and incus motions are increased. Phase relations change such that the incus and umbo move in-phase rather than the incus lagging the umbo. Other types of disorders, such as fixation of malleus and incus or interruption of malleus-incus joint, are expected to produce different characteristic alterations in the amplitude and phase responses of the ossicles (Nakajima et al., 2005b).
Some features of middle ear function may be more difficult to image in humans than in chinchilla. For example, in chinchilla we observed the opposite-phased motions of the round window membrane through the TM, a motion that will be more difficult to see in humans with its partially sequestered round window. The greater thickness of the human TM may also reduce the measurement sensitivity of ossicular motion, particularly if the TM is inflamed.
An additional issue in the translation of these findings to the otology clinic is the time required to acquire vibrography images. Measurement duration depends on the sampling resolution in xy and is also proportional to the inverse of the frequency of the sound. In our experiments, the 3-dimensional OCT images typically consisted of 500 × 500 × 250 pixels in x, y, and z. With the current system operated at an A-line rate of 20 kHz, 12.5 seconds are needed per motion phase. Thus at 2 kHz, ten volumetric vibrography images per stimulus cycle can be acquired in about 2 minutes. This measurement time is acceptable for ex vivo studies or preclinical experiments with anesthetized animals, but in a clinic it would be highly desirable for stability and patient comfort reasons to shorten the acquisition time. One strategy would be to reduce the number of x and y scan points. OCT systems with A-line rates of 400–1000 kHz are also becoming readily available (Klein et al., 2011; Oh et al., 2010).
Since OCT measures back-scattered light, only the motion parallel to the optical beam is measured. As a result, the vibrography measurement underestimates the true vibration amplitude by the cosine of the angle between the optical beam and actual vibrational axis. This error (e.g. 14% for an angular mismatch of 30°) would likely be tolerable for diagnostic applications. Greater accuracy could be achieved using multiple probe beams at different view angles to determine full 3D vector components, including the transverse and shear movements (Ahn et al., 2007; Makita et al., 2008). This enhancement would be useful in measuring the degree of bending and rocking of the ossicles (or prostheses) and the significance of such motions in hearing (Huber and Eiber, 2011).
A clinical instrument can be imagined where TM and middle ear vibrography is performed via the ear canal using a flexible fiber-optic catheter (Nguyen et al., 2012; Yun et al., 2006) or a small-diameter endoscope (Kim et al., 2010), in conjunction with an earphone. With continued development, OCT vibrography should prove useful in basic research, preclinical and clinical settings for noninvasively evaluating the structure and function of the TM and middle ear ossicles.
Supplementary Material
Highlights.
Introducing a novel OCT technique for visualizing the sound-induced motion of the TM and ossicles with three-dimensional resolution.
Imaging sound-induced motion of stapes through the intact TM.
Optical vibrography distinguishes different pathologies in the middle ear.
Acknowledgements
This work was supported by the Center for Biomedical OCT Research and Translation funded by National Institute of Health (P41EB015903, R01DC00194). E.W.C. acknowledges the Wellman Center Graduate Student Scholarship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
E.W.C., J.J.R., and S.H.Y designed research; E.W.C., J.T.C., C.R., J.B.K performed research;
E.W.C., J.J.R., S.H.Y. analyzed data; and E.W.C., J.J.R., and S.H.Y. wrote the paper.
References
- Ahn Y-C, Jung W, Chen Z. Quantification of a three-dimensional velocity vector using spectral-domain Doppler optical coherence tomography. Optics Letters. 2007;32:1587–1589. doi: 10.1364/ol.32.001587. [DOI] [PubMed] [Google Scholar]
- Athanasiadis-Sismanis A, Poe DS. Ossicular Chain Reconstruction. In: Gulya AJ, Minor LB, Poe DS, editors. Glasscock-Shambaugh surgery of the ear. Shelton, Ct.: People's Medical Publishing House-USA; 2010. pp. 489–500. [Google Scholar]
- Chang EW, Kobler JB, Yun SH. Subnanometer optical coherence tomographic vibrography. Optics Letters. 2012;37:3678–3680. doi: 10.1364/OL.37.003678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FY, Zha DJ, Fridberger A, Zheng JF, Choudhury N, Jacques SL, Wang RK, Shi XR, Nuttall AL. A differentially amplified motion in the ear for near-threshold sound detection. Nat Neurosci. 2011;14:770-U366. doi: 10.1038/nn.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JT, Aarnisalo AA, Harrington E, Hernandez-Montes MD, Furlong C, Merchant SN, Rosowski JJ. Motion of the surface of the human tympanic membrane measured with stroboscopic holography. Hearing Research. 2010;263:66–77. doi: 10.1016/j.heares.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JT, Hamade M, Harrington E, Furlong C, Merchant SN, Rosowski JJ. Wave motion on the surface of the human tympanic membrane: holographic measurement and modeling analysis. Journal of the Acoustic Society of America. 2013;133:918–937. doi: 10.1121/1.4773263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien W, Rosowski JJ, Ravicz ME, Rauch SD, Smullen J, Merchant SN. Measurements of stapes velocity in live human ears. Hearing Research. 2009;249:54–61. doi: 10.1016/j.heares.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster JH, Mackenzie K. Tympanometry in the detection of hearing impairments associated with otitis-media with effusion. Clinical Otolaryngology. 1991;16:157–159. doi: 10.1111/j.1365-2273.1991.tb01967.x. [DOI] [PubMed] [Google Scholar]
- Fisch U, Acar GO, Huber AM. Malleostapedotomy in revision surgery for otosclerosis. Otol Neurotol. 2001;22:776–785. doi: 10.1097/00129492-200111000-00011. [DOI] [PubMed] [Google Scholar]
- Funnell WRJ. On the undamped natural frequencies and mode shapes of a finite element model of the cat eardrum. Journal of the Acoustical Society of America. 1983;73:1657–1661. doi: 10.1121/1.389386. [DOI] [PubMed] [Google Scholar]
- Goll E, Dalhoff E. Modeling the eardrum as a string with distributed force. Journal of the Acoustical Society of America. 2011;130:1452–1462. doi: 10.1121/1.3613934. [DOI] [PubMed] [Google Scholar]
- Heermann R, Hauger C, Issing PR, Lenarz T. Application of optical coherence tomography (OCT) in middle ear surgery. Laryngo-Rhino-Otologie. 2002;81:400–405. doi: 10.1055/s-2002-32213. [DOI] [PubMed] [Google Scholar]
- Holley MC. The auditory system, hearing loss and potential targets for drug development. Drug Discovery Today. 2005;10:1269–1282. doi: 10.1016/S1359-6446(05)03595-6. [DOI] [PubMed] [Google Scholar]
- Huber AM, Eiber A. Vibration properties of the ossicle and cochlea and their importance for our hearing system. Hno. 2011;59:255–260. doi: 10.1007/s00106-011-2271-6. [DOI] [PubMed] [Google Scholar]
- Huber AM, Schwab C, Linder T, Stoeckli SJ, Ferrazzini M, Dillier N, Fisch U. Evaluation of eardrum laser Doppler interferometry as a diagnostic tool. Laryngoscope. 2001;111:501–507. doi: 10.1097/00005537-200103000-00022. [DOI] [PubMed] [Google Scholar]
- Jerger J. Impedance terminology. Archives of Otolaryngology-Head & Neck Surgery. 1975;101:589–590. doi: 10.1001/archotol.1975.00780390003001. [DOI] [PubMed] [Google Scholar]
- Just T, Lankenau E, Huttmann G, Pau HW. Optical coherence tomography in middle ear surgery. Hno. 2009;57:421–427. doi: 10.1007/s00106-009-1907-2. [DOI] [PubMed] [Google Scholar]
- Kim P, Chung E, Yamashita H, Hung KE, Mizoguchi A, Kucherlapati R, Fukumura D, Jain RK, Yun SH. In vivo wide-area cellular imaging by side-view endomicroscopy. Nature Methods. 2010;7:303–305. doi: 10.1038/nmeth.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T, Wieser W, Eigenwillig CM, Biedermann BR, Huber R. Megahertz OCT for ultrawide-field retinal imaging with a 1050nm Fourier domain mode-locked laser. Optics Express. 2011;19:3044–3062. doi: 10.1364/OE.19.003044. [DOI] [PubMed] [Google Scholar]
- Kobler JB, Chang EW, Zeitels SM, Yun SH. Dynamic imaging of vocal fold oscillation with four-dimensional optical coherence tomography. Laryngoscope. 2010;120:1354–1362. doi: 10.1002/lary.20938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Staecker H, Jafri MS. Optical coherence tomography imaging of the inner ear: A feasibility study with implications for cochlear implantation. Annals of Otology Rhinology and Laryngology. 2008;117:341–346. doi: 10.1177/000348940811700503. [DOI] [PubMed] [Google Scholar]
- Makita S, Fabritius T, Yasuno Y. Quantitative retinal-blood flow measurement with three-dimensional vessel geometry determination using ultrahigh-resolution Doppler optical coherence angiography. Optics Letters. 2008;33:836–838. doi: 10.1364/ol.33.000836. [DOI] [PubMed] [Google Scholar]
- Merchant SN, Rosowski JJ. Acoustic and mechanics of the middle ear. In: Gulya AJ, Minor LB, Poe DS, editors. Glasscock-Shambaugh surgery of the ear. Shelton, Ct.: People's Medical Publishing House-USA; 2010. pp. 49–72. [Google Scholar]
- Nakajima HH, Pisano DV, Roosli C, Hamade MA, Merchant GR, Mahfoud L, Halpin CF, Rosowski JJ, Merchant SN. Comparison of ear-canal reflectance and umbo velocity in patients with conductive hearing loss: A preliminary study. Ear and Hearing. 2012;33:35–43. doi: 10.1097/AUD.0b013e31822ccba0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima HH, Ravicz ME, Merchant SN, Peake WT, Rosowski JJ. Experimental ossicular fixations and the middle ear's response to sound: Evidence for a flexible ossicular chain. Hearing Research. 2005a;204:60–77. doi: 10.1016/j.heares.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Nakajima HH, Ravicz ME, Rosowski JJ, Peake WT, Merchant SN. Experimental and clinical studies of malleus fixation. Laryngoscope. 2005b;115:147–154. doi: 10.1097/01.mlg.0000150692.23506.b7. [DOI] [PubMed] [Google Scholar]
- Nguyen CT, Jung W, Kim J, Chaney EJ, Novak M, Stewart CN, Boppart SA. Noninvasive in vivo optical detection of biofilm in the human middle ear. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9529–9534. doi: 10.1073/pnas.1201592109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh WY, Vakoc BJ, Shishkov M, Tearney GJ, Bouma BE. > 400 kHz repetition rate wavelength-swept laser and application to high-speed optical frequency domain imaging. Optics Letters. 2010;35:2919–2921. doi: 10.1364/OL.35.002919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent P, Allen JB. Time-domain "wave" model of the human tympanic membrane. Hearing Research. 2010;263:152–167. doi: 10.1016/j.heares.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Pitris C, Saunders KT, Fujimoto JG, Brezinski ME. High-resolution imaging of the middle ear with optical coherence tomography - A feasibility study. Archives of Otolaryngology-Head & Neck Surgery. 2001;127:637–642. doi: 10.1001/archotol.127.6.637. [DOI] [PubMed] [Google Scholar]
- Ravicz ME, Slama MCC, Rosowski JJ. Middle-ear pressure gain and cochlear partition differential pressure in chinchilla. Hearing Research. 2010;263:16–25. doi: 10.1016/j.heares.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosowski JJ, Cheng JT, Ravicz ME, Hulli N, Hernandez-Montes M, Harrington E, Furlong C. Computer-assisted time-averaged holograms of the motion of the surface of the mammalian tympanic membrane with sound stimuli of 0.4–25 kHz. Hearing Research. 2009;253:83–96. doi: 10.1016/j.heares.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosowski JJ, Dobrev I, Khaleghi M, Lu W, Cheng JT, Harrington E, Furlong C. Measurements of three-dimensional shape and sound-induced motion of the chinchilla tympanic membrane. Hearing Research. 2013 doi: 10.1016/j.heares.2012.11.022. accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosowski JJ, Nakajima HH, Merchant SN. Clinical utility of laser-Doppler vibrometer measurements in live normal and pathologic human ears. Ear and Hearing. 2008;29:3–19. doi: 10.1097/AUD.0b013e31815d63a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks J, Schohet J. Tympanometry in Clinical Practice. In: Katz J, editor. Handbook of clinical audiology. Philadelphia: Lippincott Williams & Wilkins; 2009. pp. 157–188. [Google Scholar]
- Subhash HM, Anh NH, Wang RKK, Jacques SL, Choudhury N, Nuttall AL. Feasibility of spectral-domain phase-sensitive optical coherence tomography for middle ear vibrometry. Journal of Biomedical Optics. 2012;17:060505. doi: 10.1117/1.JBO.17.6.060505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuck-Lee JP, Pinsky PM, Steele CR, Puria S. Finite element modeling of acousto-mechanical coupling in the cat middle ear. Journal of the Acoustical Society of America. 2008;124:348–362. doi: 10.1121/1.2912438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrettakos PA, Dear SP, Saunders JC. Middle-ear structure in the chinchilla - a quantitative study. American Journal of Otolaryngology. 1988;9:58–67. doi: 10.1016/s0196-0709(88)80009-7. [DOI] [PubMed] [Google Scholar]
- Wada H, Ando M, Takeuchi M, Sugawara H, Koike T, Kobayashi T, Hozawa K, Gemma R. Vibration measurement of the tympanic membrane of guinea pig temporal bones using time-averaged speckle pattern interferometry. Journal of the Acoustical Society of America. 2002;111:2189–2199. doi: 10.1121/1.1467671. [DOI] [PubMed] [Google Scholar]
- Yun SH, Tearney GJ, de Boer JF, Iftimia N, Bouma BE. High-speed optical frequency-domain imaging. Optics Express. 2003;11:2953–2963. doi: 10.1364/oe.11.002953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun SH, Tearney GJ, Vakoc BJ, Shishkov M, Oh WY, Desjardins AE, Suter MJ, Chan RC, Evans JA, Jang IK, et al. Comprehensive volumetric optical microscopy in vivo. Nature Medicine. 2006;12:1429–1433. doi: 10.1038/nm1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.