SUMMARY
The microenvironment provides cues that control the behavior of epithelial stem and progenitor cells. Here, we identify matrix metalloproteinase-3 (MMP3) as a novel regulator of Wnt signaling and mammary stem cell (MaSC) activity. We show that MMP3 overexpression promotes hyperplastic epithelial growth, surprisingly, in a non-proteolytic manner via its hemopexin (HPX) domain. We demonstrate that MMP3-HPX specifically binds and inactivates Wnt5b, a non-canonical Wnt ligand that inhibits canonical Wnt signaling and mammary epithelial outgrowth in vivo. Indeed, transplants overexpressing MMP3 display increased canonical Wnt signaling, demonstrating that MMP3 is an extracellular regulator of the Wnt signaling pathway. MMP3-deficient mice exhibit decreased MaSC populations and diminished mammary reconstituting activity, while MMP3 overexpression elevates MaSC function indicating that MMP3 is necessary for the maintenance of MaSCs. Our study reveals a novel mechanism by a microenvironmental protease that regulates Wnt signaling and impacts adult epithelial stem cell function.
Keywords: Mammary stem cells, matrix metalloproteinases, Wnt signaling
INTRODUCTION
The mammary gland is composed of a highly dynamic epithelial structure that undergoes constant rounds of remodeling during puberty, pregnancy, lactation and involution (Visvader, 2009). At puberty, the mammary gland forms a branching ductal network, which connects the nipple to the milk-producing lobuloalveolar structures that arise during pregnancy (Sternlicht, 2006). Development and growth of the mammary gland depend on the function of adult mammary stem cells (MaSCs) (Visvader, 2009). These MaSCs are capable of reconstituting a complete mammary epithelial ductal structure when implanted as a single cell into a cleared fat pad in vivo (Plaks et al., 2013; Shackleton et al., 2006; Stingl et al., 2006). The morphogenetic changes of the breast epithelium are closely coordinated within the context of its microenvironment, which consists of a variety of stromal cells such as adipocytes, macrophages and fibroblasts (Lu and Werb, 2008; Wiseman and Werb, 2002). MaSCs respond to extracellular signals such as Wnt ligands provided by stromal cells of the microenvironment. For example, hyperactivation of the canonical Wnt/β-catenin signaling pathway in the mammary gland expands the MaSC population by 6-fold (Shackleton et al., 2006), and Wnt ligands are necessary for self-renewal properties of MaSCs (Zeng and Nusse, 2010). Constitutive overexpression of the canonical Wnt1 ligand in this organ ultimately gives rise to tumors, suggesting a direct link between MaSC accumulation and tumor susceptibility (Li et al., 2003; Liu et al., 2004).
Matrix metalloproteinases (MMPs) are a family of extracellular zinc-dependent endopeptidases that contribute to a wide range of both physiological and pathological processes (Page-McCaw et al., 2007). Their role in cancer has been ascribed to extracellular matrix degradation and thereby promoting tumor cell invasion and metastasis. However, recent evidence suggests that the function of MMPs in cancer are more complex, since these enzymes can regulate multiple signaling pathways and may even work in a non-proteolytic manner (Kessenbrock et al., 2010). Whether their role in cancer may involve the regulation of epithelial stem cell function remains unknown.
MMP3/stromelysin-1 is mainly produced by stromal fibroblasts and promotes epithelial branching morphogenesis during puberty (Kouros-Mehr and Werb, 2006; Wiseman et al., 2003; Witty et al., 1995). It is frequently upregulated in breast cancer, where it may also be expressed by malignant epithelial cells (Egeblad and Werb, 2002). Targeted overexpression of autoactivated MMP3 in mouse mammary glands induces hyperplasias and breast cancer, demonstrating that it can influence tumor initiation and alter neoplastic risk (Sternlicht et al., 1999). However, the cellular and molecular mechanism by which MMP3 affects epithelial homeostasis during normal mammary development or breast cancer is still elusive.
Here, we hypothesized that MMP3 is part of a signaling network that controls adult stem cell function in the mammary gland, based on previous studies showing that it affects mammary development and breast tumor formation. Our study identifies MMP3 as a novel extracellular regulator of MaSCs through modulation of the Wnt signaling pathway.
RESULTS
MMP3 Overexpression Induces Hyperplastic Epithelial Growth in Mammary Transplants
Overexpression of MMP3 in transgenic mouse models induces mammary epithelial hyperplasia (Sternlicht et al., 1999; Wiseman et al., 2003). To investigate the molecular mechanism underlying MMP3-mediated pathology, we asked whether lentivirus-mediated overexpression of MMP3 in mammary stem cells (MaSCs) could recapitulate this hyperplastic phenotype using the mammary fat pad transplantation model (Welm et al., 2008). We chose this approach because, compared to transgenic models, it facilitates rapid structure/function analysis of the various MMP3 domains.
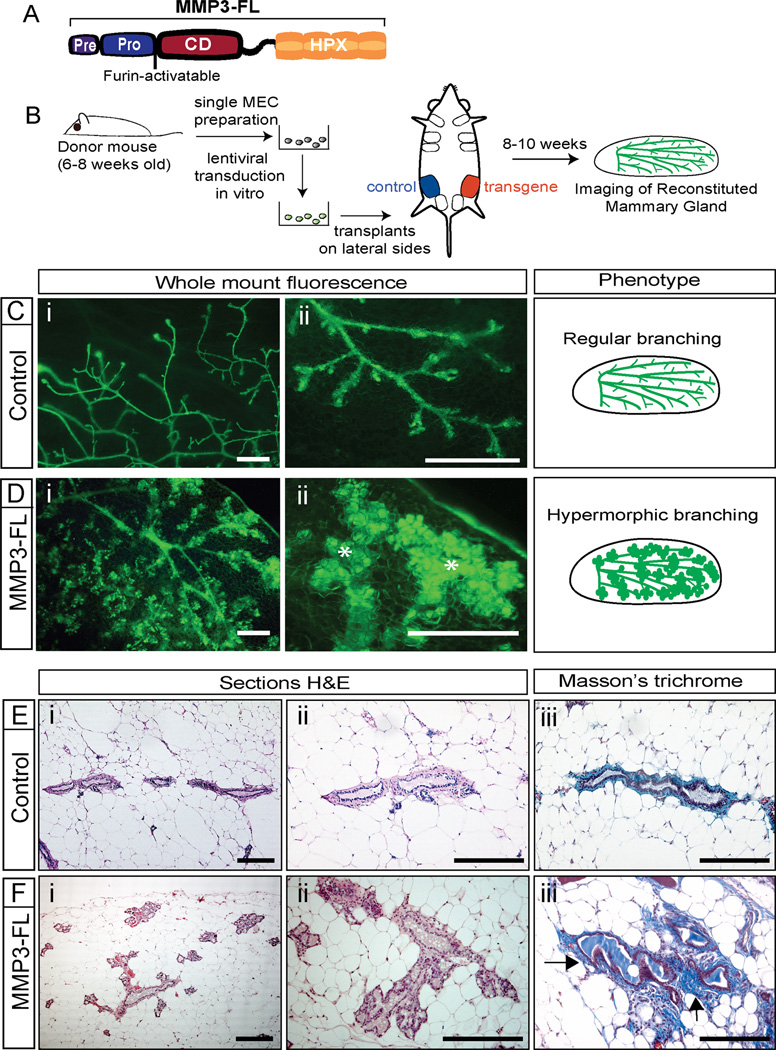
To this end, we generated lentiviral constructs to express furin-activatable full-length mouse MMP3 (MMP3-FL) (Figure 1A), and confirmed that MMP3 was processed by the ubiquitously expressed convertase furin, since it was secreted as an active proteinase (Figure S1A) and overexpressed by about 2.5 fold in primary mammary epithelial cells (MECs) (Figure S1B). These lentiviral constructs also contain an internal ribosome entry site (IRES) allowing expression of a fluorescent reporter gene, which enabled us to visualize the transduced epithelium in transplant whole mounts by fluorescence microscopy (outlined in Figure 1B).
Figure 1. MMP3 Induces Hypermorphic Mammary Epithelial Growth.
(A) Schematic representation of full length MMP3 (MMP3-FL). Pre-domain: signal sequence; Pro-domain; inhibitory peptide that was made cleavable by furin; CD: catalytic domain; HPX: hemopexin-like domain.
(B) Experimental scheme: Single MECs were isolated from donor mice and transduced with either control or transgene expressing viruses. Transduced MECs were transplanted into epithelium-cleared contralateral inguinal mammary fat pads of recipient mice. Reconstituted mammary glands were analyzed 8–10 weeks later by fluorescence microscopy.
(C–D) Representative images showing green fluorescence of whole mount transplants from control (Ci–ii) and MMP3-FL (Di–ii) transduced MECs. MMP3-FL overexpression (n=10) leads to hypermorphic mammary epithelial branching with significantly increased epithelial outgrowth (asterisk) in 9 of 10 transplants, while control transplants (n=10) showed regular epithelial growth and branching in all cases. Schematic depiction of the respective epithelial outgrowth phenotype is shown. Scale bars represent 1 mm.
(E–F) H&E (i–ii) and Masson’s trichrome (iii) stained sections of mammary transplants from control (E) and MMP3-FL (F) transduced MECs. Transplants expressing MMP3-FL displayed strongly increased epithelial branching (i–ii) and prominent deposition of collagen around ductal structures (arrows), as revealed by blue staining in Masson’s trichrome (iii). Scale bars represent 20 µm.
Also see Figure S1.
MECs from 8–16-week-old donor mice were transduced with MMP3-FL or control virus and transplanted into epithelium-cleared fat pads of pubescent recipients. Transplants were harvested 8–10 weeks after transplantation. Control outgrowths displayed normal epithelial morphology and regular branching (Figure 1C, E and Figure S1C), while transplants overexpressing MMP3-FL exhibited hypermorphic epithelial outgrowth and precocious lobuloalveolar development with excessive branching (Figure 1D, F and Figure S1D). We also observed enhanced accumulation of collagen around the ducts of MMP3 overexpressing transplants (Figure 1Fiii), as observed previously in MMP3 transgenic mice (Sternlicht et al., 2000). Therefore, MMP3 overexpression induces hyperplastic epithelial growth in mammary transplants confirming that this approach is valid for further interrogation of MMP3 function.
MMP3 Induces Hypermorphic Outgrowth via the Hemopexin Domain, Independent of its Proteolytic Activity
MMP3 contains two major functional domains, a catalytic domain that mediates proteolytic cleavage of substrate molecules, and a hemopexin domain (HPX) that binds substrates to direct MMP3 specificity (Piccard et al., 2007). To determine whether proteolytic activity is required for MMP3-induced hypermorphic outgrowth, we generated a lentiviral clone expressing a proteolytically inactive “E to A” mutant of MMP3 (MMP3-FL-E/A) (Crabbe et al., 1994), and compared this to proteolytically active MMP3-FL using our transplant approach. Surprisingly, overexpression of both MMP3-FL (Figure 2A) and non-proteolytic MMP3-FL-E/A (Figure 2B, Figure S2A) showed a comparable phenotype with increased epithelial outgrowth and hypermorphic branching. We then generated lentiviral constructs that express and secrete only the HPX domain of MMP3 (Figure S2B). Of importance, expression of the MMP3-HPX domain was sufficient to induce hypermorphic epithelial outgrowth compared to control samples (Figure 2C–D and Figure S2C), which was strikingly similar to MMP3-FL overexpression in contra-lateral transplants in the same mouse (Figure 2E–F).
Figure 2. MMP3 Promotes Hypermorphic Epithelial Outgrowth Via its Hemopexin Domain.
(A–B) Overexpression of catalytically inactive MMP3 induces hypermorphic branching. Fluorescent imaging (i) and carmine red stained (ii–iii) whole mounts of transplants from either MMP3-FL (A) or catalytically inactive MMP3-FL-E/A (B; n=6) transduced MECs both develop epithelial structures with excess branches. Scale bars represent 1 mm.
(C–D) Whole mount fluorescence imaging (i–ii) and H&E stained sections (iii) of transplants from either control (C) or MMP3-HPX (D; n=6) transduced MECs. Like MMP3-FL and MMP3-FL-E/A, MMP3-HPX strongly promotes mammary epithelial branching. Scale bars represent 1 mm (i–ii) and 100 µm (iii).
(E–H) Comparison of MMP3-HPX overexpression to MMP3-FL and MMP10-HPX in contra-lateral transplants. MMP3-FL (E) and MMP3-HPX (F) transduced MECs both promote hypermorphic branching and growth to a similar extent. MECs expressing MMP10-HPX (G, red) show regular branching and growth, while MMP3-HPX expressing MECs demonstrate hypermorphic outgrowth in the contra-lateral mammary fat pad. Schematic depiction of epithelial growth phenotype is shown. Scale bars show 1 mm.
Also see Figure S2.
MMP10/stromelysin-2 is the closest relative of MMP3, with an HPX domain that is about 67% identical to MMP3-HPX (Sirum and Brinckerhoff, 1989). Overexpression of MMP10-HPX in MEC transplants was ineffective at increasing epithelial outgrowth (Figure 2G) when compared to MMP3-HPX (Figure 2H). These results indicate that MMP3 catalytic activity is not required and that the HPX domain is sufficient to induce hyperplastic mammary growth.
MMP3-HPX Specifically Interacts with Wnt5b
To shed light on the molecular mechanism by which MMP3 promotes hypermorphic epithelial outgrowth, we used MMP3-HPX as bait in a yeast two-hybrid (Y2H) screen (Bartel and Fields, 1995; McQuibban et al., 2000) with a mouse mammary gland cDNA library (Figure 3A). We identified 98 candidates that displayed plasmid-dependent activation of both Y2H reporters. Of these, 58 clones were fused in frame to the GAL4 activation domain, representing a total of 21 different genes, mostly encoding intracellular proteins (Table S1). However, we identified two extracellular interacting proteins (Figure 3B). One of these clones contained the C-terminal 280 amino acids of ADAMTS-18 and was not further pursued in this study. The second clone contained the C-terminal 147 amino acids of Wnt5b. This candidate was promising because deregulated Wnt signaling occurs in a wide variety of cancers and has been implicated in the control of MaSC function (Reya and Clevers, 2005).
Figure 3. MMP3 Specifically Binds and Cleaves Wnt5b.
(A) Structural domains of MMP3-FL: the HPX domain with hinge region was used as bait in the Y2H screen.
(B) Summary of Y2H screen. MMP3-HPX specifically interacts with the C-terminal 147 amino acids (aa) of Wnt5b. All Y2H interactions were identified by growth in the absence of adenine and were verified by β-galactosidase activity, which was rated as strong (++), weak (+) or not detectable (−).
(C) Y2H interactions between MMPs and Wnts expressed in the MG: only Wnt5a and Wnt5b interact with MMP3-HPX. The percent identity with the C-terminus of Wnt5b is indicated between brackets.
(D) A deletional approach identified a minimal MMP3 binding domain of 55 amino acids on Wnt5b (W232-Y287; pink) that is sufficient for the Y2H interaction with MMP3-HPX.
(E) A blade swapping approach revealed that the entire HPX domain of MMP3 is necessary for Wnt5b binding. Silent point mutations were introduced in MMP3-HPX to create unique restriction sites, which were used for the blade swapping with MMP10-HPX.
(F) Recombinant mouse MMP3 catalytic domain (mM3-CD) cleaves soluble Wnt5b. Serum-free culture supernatant from NIH-3T3 cells transduced to express double tagged Wnt5b was incubated in the presence (mM3-CD) or absence (mock) of activated mM3-CD. Each of these samples was then split in half to assess the mobility of Wnt5b by anti-HA and anti-myc Western blotting before and after N-glycosidase F treatment. Molecular weight markers are indicated on the right.
(G) A structural model of Wnt5b was generated based on a recent crystal structure of Wnt8. Minimal MMP3 binding domain is highlighted (pink) and strongly overlaps with the putative co-receptor binding domain (dotted circle).
(H) Complex of MMP3 (blue) and Wnt5b (green with minimal MMP3 binding domain in pink) was generated by molecular docking using Firedock.
Also see Table S1.
We then analyzed the specificity of this interaction and found that the HPX domain of MMP3 interacted with the C-termini of both Wnt5a and Wnt5b in Y2H assays (Figure 3C), but not with the C-termini of other Wnt ligands expressed in the mammary gland [Wnt2, 4, 6, 7b or 10b (Kouros-Mehr and Werb, 2006)]. Moreover, the HPX domains of MMP2, MMP10 and MMP14, which are also expressed in the mammary gland, did not interact with any of these Wnts. The high degree of specificity of this interaction suggests that the ability to bind Wnt5b may be key the role of MMP3 in the regulation of mammary epithelial growth.
To identify the Wnt5b domains responsible for this interaction, we performed a deletional analysis of the Wnt5b Y2H clone, which revealed a minimal MMP3 binding domain of 55 amino acids (Figure 3D). We then explored the molecular determinants on MMP3-HPX necessary for the binding of Wnt5b. The HPX domain of MMP3 consists of a four-bladed propeller like domain that is connected to the catalytic domain via a linker region (Piccard et al., 2007). We used a hinge/blade swapping approach and replaced each subdomain of MMP3-HPX with the corresponding one of the MMP10-HPX and assayed for binding to Wnt5b by Y2H. We found that the hinge region and most blades of MMP3-HPX are required for binding Wnt5b (Figure 3E).
We next tested if Wnt5b is a cleavage substrate of MMP3. To this end, we produced recombinant mouse Wnt5b flanked by N-terminal Myc and C-terminal HA tags and incubated this construct with catalytically active mouse MMP3. Indeed, the MMP3 catalytic domain (Figure 3F) and recombinant full-length mouse MMP3 (data not shown) cleaved soluble Wnt5b at two sites calculated to be located within the minimal MMP3 binding domain based on the size of the cleavage fragments. We then generated a structural model of Wnt5b and the complex of MMP3 with Wnt5b using comparative protein structure modeling based on the recently published crystal structure for Wnt8 ligand (Janda et al., 2012). Our model revealed that the minimal MMP3 binding domain strongly overlaps with the putative co-receptor domain of Wnt5b (Figure 3G–H), suggesting that MMP3 may antagonize Wnt5b function by either interfering with receptor binding or by proteolytic inactivation.
Wnt5b Acts as a Non-Canonical Wnt Ligand that Inhibits MaSC Function
While Wnt5b is classified as a non-canonical Wnt ligand, its downstream signaling pathways are not well characterized. Canonical Wnts such as Wnt1 or Wnt3a signal through β-catenin, whereas non-canonical Wnt ligands such as Wnt5a activate the planar cell polarity (PCP) pathway or induce a Ca2+-response that leads to nuclear factor of activated T-cells (NFAT) transcriptional activity (Ling et al., 2009). To identify signaling pathways downstream of Wnt5b, we incubated non-tumorigenic mouse mammary epithelial cell lines or primary MECs with recombinant mouse Wnt5b. We did not detect phosphorylation of c-Jun-N-terminal kinase (JNK), which is a hallmark of the non-canonical PCP pathway (data not shown). However, addition of Wnt5b led to robust accumulation of NFAT protein in Eph4 cell lysates (Figure 4A) and in primary MECs (Figure S3A). We also observed a significant elevation of NFAT transcriptional activity (Figure 4B), consistent with Wnt5b acting as a non-canonical Wnt ligand in MECs. Addition of recombinant full-length MMP3 to Eph4 cells did not affect basal levels of NFAT signaling, but significantly reduced NFAT signaling induced by Wnt5b, demonstrating that MMP3 inactivates Wnt5b.
Figure 4. Wnt5b Activates Non-Canonical Wnt Signaling and Inhibits Mammary Outgrowth In Vitro and In Vivo.
(A) Incubation of the mammary epithelial cell line Eph4 after incubation with recombinant mouse Wnt5b for 24 h increased levels of NFAT protein as determined by Western blot.
(B) A secreted alkaline phosphatase (SEAP)-based NFAT reporter assay was used in Eph4 cells. NFAT transcriptional activity was significantly increased after incubation with recombinant mouse Wnt5b. Incubation with recombinant full-length MMP3 had no effect on basal NFAT-SEAP levels, but significantly inhibited NFAT induction by Wnt5b; *p<0.05.
(C) Eph4 cells were transfected with a TOP-SEAP reporter to monitor canonical Wnt signaling and subsequently incubated with combinations of Wnt3a, DKK1 and Wnt5b. Wnt3a promoted canonical Wnt signaling, which could be inhibited by DKK1 and to a lesser extent by Wnt5b. *p<0.05.
(D) The mammary epithelial cell line NMuMG was treated with combinations of Wnt3a, DKK1, Wnt5b and MMP3-HPX-conditioned media. Canonical Wnt signaling was monitored by Western blot analysis for LRP6 phosphorylation, with total LRP6 serving as a loading control. Canonical Wnt3a induced robust phosphorylation of LRP6, which was inhibited by both DKK1 and Wnt5b. The inhibition by Wnt5b could be partially rescued with MMP3-HPX containing supernatant.
(E) MECs (5,000 cells per well) were subjected to the mammosphere assay in the presence of Wnt5b with or without MMP3. Wnt5b significantly inhibited sphere formation, while addition of MMP3 abolished the inhibitory effect of Wnt5b. Representative images are shown with scale bar representing 100 µm. *p<0.05.
(F–H) Lentiviral overexpression of Wnt5b and DKK1 in MEC transplants was used to study their effect on mammary outgrowth in vivo. Control transplants (F) show normal mammary epithelial structures, while overexpression of both Wnt5b (G; n=10) and the Wnt inhibitor DKK1 (H; n=6) strongly inhibited mammary growth in vivo showing abrogated outgrowths and weak fluorescence.
(I) Schematic of MMP3 as a modulator of Wnt signaling. Wnt5b activates the non-canonical NFAT signaling pathway possibly through ROR and inhibits canonical Wnt signaling either by competition for co-receptor Frizzled (blue arrows) or downstream interference with β-catenin signaling. MMP3 inactivates Wnt5b and thereby indirectly enhances canonical Wnt signaling and downstream expression of Wnt regulated genes.
Also see Figure S3.
Non-canonical Wnt ligands may interfere with canonical Wnt signaling, for example by competing for Frizzled co-receptors (Grumolato et al., 2010). Indeed, we found that Wnt5b, like the known canonical Wnt antagonist Dickkopf-related protein (DKK)-1, significantly reduced Wnt3a-induced β-catenin transcriptional activity in Eph4 cells (Figure 4C). Therefore, Wnt5b can act as an inhibitor of canonical Wnt signaling.
One of the earliest events in the canonical Wnt signaling cascade after docking of extracellular Wnt ligand to low-density lipoprotein receptor-related protein 6 (LRP6) and Frizzled is phosphorylation of LRP6 (Zeng et al., 2005). Incubation of NMuMG cells with Wnt3a led to robust phosphorylation of LRP6, which could be suppressed when co-incubated with Wnt5b or DKK1. MMP3-HPX partially overcame the inhibition of LRP6 phosphorylation by Wnt5b, but did not affect the DKK1 antagonism (Figure 4D).
To address the role of Wnt5b in the regulation of mammary stem and progenitor cell function, we performed the mammosphere formation assay using single MECs that were incubated with or without exogenous Wnt5b. We found that Wnt5b interfered with the clonal proliferative capacity of MECs, which was reversible by addition of MMP3 (Figure 4E). We then used our transplant assay to determine the impact of Wnt5b overexpression on mammary epithelial outgrowth in vivo (Figure S3B). While control mammary transplants showed normal morphology (Figure 4F), overexpression of Wnt5b or the Wnt antagonist DKK1 in transplants resulted in strongly decreased branching morphogenesis and impaired epithelial outgrowth (Figure 4G–H; Figure S3C–D). These results confirm that inhibition of canonical Wnt signaling halts mammary development and suggest that Wnt5b is a negative regulator of stem cell function in the mammary gland. Taken together, these studies reveal that Wnt5b activates the non-canonical Ca2+/NFAT pathway, inhibits stem and progenitor capacity in MECs and is inactivated by MMP3 (Figure 4I).
MMP3 Overexpression Mimics Activation of Canonical Wnt Signaling In Mammary Transplants
We next asked whether the MMP3-induced phenotype mimics overexpression of canonical Wnt ligands. We used lentiviral transduction of primary MECs to express selected canonical Wnt ligands (Wnt1, Wnt2, Wnt4; Figure S4A) and determined their effects on mammary epithelial growth in vivo. Both Wnt1 and Wnt2 promoted branching and hypermorphic epithelial outgrowth comparable to overexpression of MMP3 (Figure 5A–C). Wnt4 overexpression led to dysplasia of terminal end buds in the developing mammary transplants (Figure 5D).
Figure 5. MMP3 Overexpression Enhances Canonical Wnt Signaling in Mammary Transplants.
(A–F) Comparison of MMP3-FL induced mammary phenotype to overexpression of selected Wnt ligands in mammary transplants. Overexpression of MMP3-FL (A) and canonical ligands Wnt1 (B; n=6) and Wnt2 (C; n=5) resulted in comparable phenotype showing excess branching and epithelial growth. Overexpression of Wnt4 (D; n=5) caused strong enlargement of terminal end buds. Scale bars represent 1 mm.
(E–G) Paraffin sections of 10-week-old transplants from control (E), MMP3-FL-(F) and Wnt1 (G) overexpressing transplants were immunostained for β-catenin (green). Confocal microscopy analysis revealed nuclear β-catenin by co-localization with DNA (blue). Percentage of cells presenting with nuclear β-catenin is indicated for 5 microscopic images per sample. Scale bars show 50 µm. Control vs. MMP3-FL: p<0.05; Control vs. Wnt1: p<0.05.
(H–I) MECs from Axin2-LacZ mice were transduced with control (n=2) and MMP3-FL (n=2) overexpressing lentiviruses, and transplanted into the cleared fat pads of recipient nude mice. Compared to control transplants (H), MMP3-FL overexpression (I) resulted in significantly increased Xgal staining (blue color). Sections were counterstained with nuclear fast red and percentage of LacZ positive cells per 5 fields of view was quantified. Scale bars represent 1 mm. Control vs. MMP3-FL: p<0.05.
Also see Figure S4.
MMP3 Overexpression Elevates Canonical Wnt Signaling
Based upon the above observations, we hypothesized that inactivation of Wnt5b by MMP3 may increase canonical Wnt signaling in the mammary gland. The latter involves stabilization and translocation of β-catenin into the nucleus, where it acts as a transcription factor for Wnt regulated genes such as Cyclin D1 or Axin2 (Clevers and Nusse, 2012). We therefore analyzed tissue sections from MEC transplants for nuclear localization of β-catenin and observed predominant peripheral membrane staining in control outgrowths (Figure 5E). Sections from both MMP3-FL and Wnt1 overexpressing transplants displayed a marked increase in nuclear β-catenin (Figure 5F–G), which was also observed in MMP3-HPX transplants (Figure S4B–C).
Axin2-LacZ transgenic mice express β-galactosidase under the control of the Wnt responsive Axin2 gene. This model has been used as an in vivo reporter for canonical Wnt signaling and to identify MaSCs (Lustig et al., 2002; van Amerongen et al., 2012; Zeng and Nusse, 2010). We crossed the Axin2-LacZ transgene into MMTV-Wnt1 mice and were able to validate this Wnt reporter in the mammary gland (Figure S4D–E). We then used our transplant assay to overexpress MMP3-FL in Axin2-LacZ MECs and found that these outgrowths displayed more robust β-galactosidase staining than control transplants (Figure 5H–I), consistent with MMP3 enhancing canonical Wnt signaling in the mammary gland and promoting MaSC populations.
MMP3 overexpression promotes MaSC function
We next used flow cytometry to assess luminal (CD24/CD49flo) and basal/MaSC-containing CD24/CD49fhi cell populations (Shackleton et al., 2006; Stingl et al., 2006) in transplants that overexpress MMP3. We found a significant increase in the CD24/CD49fhi MaSC-enriched population in mammary glands overexpressing MMP3 (Figure 6A). This result is in agreement with increased expression of the MaSC marker Axin2-LacZ in MMP3-overexpressing transplants (Figure 5I), and suggests that MMP3 promotes the expansion of MaSCs or blocks their differentiation.
Figure 6. MMP3 Overexpression Promotes MaSC Capacity.
(A) Flow cytometry analysis revealed an altered ratio of basal/MaSCs (CD24+/CD49fhi) versus luminal (CD24+/CD49flo) cells in MMP3-FL overexpressing transplants. The basal vs. luminal cell ratio is depicted in a bar graph and was determined from 3 independent experiments. Data is represented as mean ± SEM. *P<0.05.
(B–C) FACS sorted lentivirally transduced MECs were subjected to the mammosphere assay (5,000 cells per well). MMP3 overexpression promoted mammosphere formation compared to non-transduced control cells and control vector-transduced cells (B). Addition of the tankyrase inhibitor JW-55 blocks MMP3-mediated increase in mammosphere formation (C). *p<0.05.
(D) MMP3 overexpression promotes mammary reconstitution in vivo. FACS sorted MECs were transplanted in two dilutions (100 cells and 1,000 cells) into epithelium-cleared fat pads of recipient mice. Transplants were harvested after 6 weeks. Reconstitution efficiencies and calculated stem cell frequencies of MECs are listed in the table.
Also see Figure S5.
We next assessed MaSC function using FACS-sorted MMP3-overexpressing MECs. Using the mammosphere formation assay, we found that increased expression of MMP3 enhanced clonal proliferative capacity of MECs (Figure 6B). This increase in sphere formation was reversed to control levels by inhibition of canonical Wnt signaling using the tankyrase inhibitor JW-55 (Figure 6C), suggesting that the effect of MMP3 on sphere formation involves activation of canonical Wnt signaling. In vivo reconstitution experiments revealed a significant increase in gland reconstituting efficiency of MECs that overexpress MMP3 (Figure 6D and Figure S5A–B). Taken together, these data indicate that MMP3 may promote MaSC function.
Loss of MMP3 Results in Decreased Mammary Stem Cell Number and Function
We then used MMP3-null mice as a loss-of-function model to interrogate the importance of MMP3 in the maintenance of the MaSC pool in vivo, since decreased branching morphogenesis was observed previously in these mice (Wiseman et al., 2003). Indeed, MMP3 null glands displayed a loss of the basal/MaSC population (CD24/CD49fhi) and concomitant gain of CD24/CD49flo mature cells (Figure 7A).
Figure 7. MECs from MMP3 Null Mice have Reduced MaSC Capacity.
(A) MaSC populations were examined in primary mammary epithelial preparations from 7-week-old MMP3-sufficient and deficient mice as Lin-negative CD24-positive and CD49f–high cells by flow cytometry analysis. The ratio of basal/MaSCs (CD24+/CD49fhi; blue) versus luminal (CD24+/CD49flo; green) cells was significantly decreased in MMP3-null mice; data are represented as mean ± SEM. n=4; *p<0.05.
(B) MECs from MMP3-null mice have a reduced ability to form mammospheres. Representative DIC images are shown; scale bar represents 100 µm. Bar graph depicts the average number of spheres per well seeded with 1,000 MECs; results from 3 independent experiments are shown. Data is shown as mean ± SEM. *p<0.05.
(C–D) MECs from WT and MMP3-null mice (5,000 cells per well) were subjected to the mammosphere assay. Addition of Wnt3a (100 ng/ml) to MMP3-null MECs increased sphere forming potential to the level of WT MECs (C). Similarly, knockdown of Wnt5b (SH1 and SH2) significantly rescued mammosphere formation of MMP3-null MECs compared to Scramble control (D). Data are pooled from two independent experiments. *p<0.05.
(E) Mammary gland reconstituting activity of WT and MMP3-null MECs was determined by limiting dilution transplants (10,000/1,000/100 cells). Cut-off for positive reconstitution was 20% of mammary fat pad filling. Reconstitution efficiencies and calculated stem cell frequencies of MECs from WT and MMP3−/− mice are listed in the table. Data are pooled from 2 independent experiments.
(F) Model of MMP3 as a stroma and basal cell-derived modulator of epithelial homeostasis in the MG. Basal stem cells are stimulated by canonical Wnt ligands such as Wnt1 or Wnt2. Wnt5b functions as an inhibitor of canonical Wnt signaling and interferes with mammary epithelial outgrowth. MMP3 produced in the vicinity of mammary epithelial ducts binds and inactivates Wnt5b leading to increased canonical Wnt signaling and expansion of basal/stem-like epithelial cells.
Also see Figure S6.
Using the mammosphere formation assay, we found that MECs from MMP3 null mice produced ~50% fewer spheres than cells from WT mice (Figure 7B), with reduced size, and a predominantly luminal gene expression profile by qPCR analysis (Figure S6A). This defect in sphere formation was reversed to WT levels by the addition of exogenous canonical Wnt3a ligand (Figure 7C) and by knocking down Wnt5b in MMP3-deficient MECs using lentiviral shRNA (Figure 7D and Figure S6B–C), supporting the hypothesis that MMP3 regulates MaSC through Wnt5b.
To assess MaSC function directly, we analyzed the organ regenerative capacity of single MECs using a limiting dilution transplantation assay. MECs from MMP3 null and WT glands were injected contra-laterally into the epithelium-cleared inguinal fat pads of WT mice (i.e., MMP3 sufficient), and allowed to reconstitute for 8 weeks. MECs from MMP3 null mice possessed a 7-fold reduction in reconstituting efficiency compared to WT MECs (Figure 7E). Linear regression analysis indicated that the frequency of stem cells in MMP3 null glands was 1/3966, compared to 1/763 in WT glands. This indicates that the number of stem cells is reduced in MMP3 null mammary glands and suggests that MMP3 is a novel regulator of MaSCs (Figure 7F).
DISCUSSION
Extracellular signals released from the microenvironment strongly affect epithelial homeostasis and adult stem cell function (Wagers, 2012). In our study, we have identified the extracellular protease MMP3 as a factor provided by the microenvironment that regulates Wnt signaling and adult stem cell function in the mammary gland. Further, our results offer insights into the role of non-canonical Wnt signaling in MaSCs and show that extracellular proteolysis is a novel mechanism to specifically control non-canonical Wnt signaling. These findings have implications for the role of MMPs in general in regenerative medicine and in cancer.
Wnt signaling needs to be regulated precisely, since aberrant Wnt signaling can cause a wide range of human pathologies including cancer (Clevers and Nusse, 2012). There are several natural, endogenous Wnt inhibitors such as secreted Frizzled-related proteins (sFRP) or Wnt-inhibitory factor (WIF) that bind directly to Wnt ligands and interfere with their receptor interaction (Kawano and Kypta, 2003). Our study adds MMP3 to the list of natural Wnt inhibitors and provides unprecedented evidence for an extracellular protease as a direct regulator of Wnt signaling.
In contrast to previously described Wnt antagonists that only bind and sequester Wnt ligands, MMP3 inhibits ligand activity of Wnt5b and Wnt5a both by sequestration via its hemopexin domain, and by proteolytic cleavage of Wnt5’s C-terminal domain. Our structural analysis revealed that MMP3-mediated binding and cleavage occurs within the putative co-receptor domain of Wnt5b, which explains our result that MMP3 inactivates Wnt5b-mediated NFAT signaling. The proteolytic cleavage of Wnt5b is irreversible; thus, one MMP3 molecule may potentially inactivate multiple Wnt5b molecules, suggesting that MMP3 may be a highly efficient Wnt antagonist. Future research needs to determine the physiological relevance of proteolytic degradation of Wnt5b versus inhibition by sequestration through the MMP3 hemopexin domain, for example, by specifically mapping the cleavage sites on Wnt5b and rendering this molecule resistant to MMP3 degradation without altering its co-receptor binding capacity.
Our studies shed light on the role of Wnt5b as a non-canonical Wnt ligand that modulates mammary epithelial growth and development. We discovered that Wnt5b functions as a non-canonical Wnt ligand that activates the NFAT pathway and inhibits canonical Wnt signaling in MECs. While a receptor for Wnt5b in mammary cells remains elusive, the inhibition of canonical Wnt signaling is most likely due to competition for Frizzled co-receptors (Grumolato et al., 2010). Of note, Wnt5a signaling can induce MMP3 expression in mammary epithelial cells (Prieve and Moon, 2003). This adds another layer of complexity, as MMP3 may act as a negative feedback factor on Wnt5-mediated non-canonical Wnt signaling.
In the mammary gland, canonical Wnt ligands are key extrinsic signals of the stem cell niche that are mainly released by stromal cells to promote the self-renewal capacities of MaSCs (Zeng and Nusse, 2010). While canonical Wnt ligands strongly promote stem cell proliferation in the mammary gland, the role of non-canonical Wnt ligands remained elusive. In accordance with previous work on Wnt5a in mammary gland development (Roarty et al., 2009; Roarty and Serra, 2007), our data now establishes Wnt5b as a non-canonical Wnt ligand that inhibits mammary outgrowth and branching morphogenesis, suggesting that Wnt5 ligands may interfere with MaSC function. This notion is supported by a recent study showing that Wnt5a blocks the proliferation of intestinal epithelial stem cells (Miyoshi et al., 2012). Increased levels of Wnt5b expression in the mammary gland during mid to late pregnancy suggest that this factor may control the pregnancy-related burst of growth and proliferation of the mammary epithelium.
The localization of MMP3 expression may be key to its stem cell regulatory function and to its tumor-inducing role. Under normal conditions, MMP3 is mainly expressed by stromal cells of the microenvironment that are in direct spatial proximity to the basal cell layer of the ductal epithelium (Kouros-Mehr and Werb, 2006), and to a lesser extent by basal epithelial cells themselves, which show an enrichment of MMP3 expression compared to luminal cells (Kendrick et al., 2008). In contrast, the non-canonical ligands Wnt5a and Wnt5b are predominantly expressed in mature luminal cells (Ji et al., 2011; Kouros-Mehr and Werb, 2006). The inactivation of luminal-derived Wnt5b by MMP3 from basal or stromal cells may be crucial during phases of active epithelial reorganization such as during puberty, pregnancy or regeneration, to dampen the non-canonical Wnt axis and thereby promote canonical Wnt-driven proliferation of MaSCs to maintain tissue homeostasis.
This stem cell-promoting function of MMP3 may be the explanation for its pro-tumorigenic properties, since stem cell expansion in response to elevated Wnt signaling can lead to malignant conversion. The multistage neoplastic progression induced by MMP3 (Sternlicht et al., 2000; Sternlicht et al., 1999; Witty et al., 1995) resembles the malignant conversion that occurs in mammary glands with ectopic activation of the canonical Wnt signaling pathway by Wnt1 overexpression (Welm et al., 2008). This similarity can now be explained by MMP3’s ability to regulate Wnt signaling. Our findings are also relevant for human cancers, where MMP3 expression is frequently upregulated in the tumor cells themselves [reviewed in(Egeblad and Werb, 2002)]. In the tumor microenvironment, MMP3 might skew the balance between canonical and non-canonical Wnt signaling in favor for the promotion of cancer stem cells.
Blocking MMP3 function may help to interfere therapeutically with its pro-tumorigenic role and possibly target cancer stem cells. In previous clinical cancer trials, synthetic small molecule inhibitors designed to block MMP catalytic activity yielded disappointing results (Coussens et al., 2002). However, MMPs may also act non-proteolytically [reviewed in(Kessenbrock et al., 2010)]. Our data show that MMP3 works through its hemopexin domain, which is in line with recently published work from others (Correia et al., 2013). These findings corroborate the idea that MMPs function in a non-proteolytic manner, which would not be inhibited by the compound inhibitors used in these clinical trials. Therefore, the specific inhibition of non-proteolytic functions of MMPs could be a more effective treatment strategy for cancer patients. Indeed, small molecule inhibitors targeting the hemopexin domain of MMP14 have been successfully used to reduce mammary tumor growth in mice (Remacle et al., 2012). Further research should focus on identifying inhibitors for of the non-proteolytic functions of MMP3 and other MMPs.
In conclusion, our study unravels a novel molecular mechanism for the regulation of Wnt signaling through the extracellular proteinase MMP3, which specifically binds and inactivates the non-canonical ligand Wnt5b. We shed light on the role of Wnt5b as a negative regulator of mammary stem cells. Therefore, MMP3 is part of a regulatory network that orchestrates Wnt signaling and adult epithelial stem cell function (Figure 7F). This may affect the function of cancer stem cells during tumor initiation and metastasis in diseases such as breast cancer. The observation that MMP3 can act in a non-proteolytic manner via its hemopexin domain may pave the way for the development of novel therapeutic approaches to treat breast cancer by interfering with their non-catalytic biological properties.
EXPERIMENTAL PROCEDURES
Mice
FVB/N mice were purchased from Charles River Laboratories (Wilmington, MA). MMTV-Wnt1 mice (Tsukamoto et al., 1988), MMP3/stromelysin-1 null mice (Mudgett et al., 1998) and Axin2-LacZ mice (Lustig et al., 2002) have been described previously. All mouse strains were backcrossed to the FVB/N background. Experiments with mutant mice used littermates as controls. Mice were maintained in a pathogen-free facility. All mouse procedures were approved by the University of California, San Francisco (UCSF), Institutional Animal Care and Use Committee (IACUC).
Preparation of Primary Mouse MECs, Viral Transduction, Transplantation and Analysis of Reconstituted Mammary Glands
Primary MECs were isolated from inguinal mammary glands (#4) of 8- to 12-week-old donor mice and were prepared by differential centrifugation, transduced with lentivirus over night in ultra-low adhesion plates, and transplanted into cleared fat pads of recipient mice and the reconstituted mammary glands were analyzed as previously described (Welm et al., 2008). Details are provided in Supplemental Experimental Procedures.
Mammosphere Assay
Mammosphere assays were carried out as described previously (Shackleton et al., 2006; Stingl et al., 2006). Details are given in the Supplemental Experimental Procedures.
Measuring Mammary Reconstituting Activity
Limiting dilutions of MECs were transplanted into cleared contra-lateral inguinal mammary glands of syngeneic recipient mice. Positive reconstitution was determined at 6–8 weeks after transplantation using carmine red staining of mammary whole mounts. The MaSC frequency was calculated according to (Hu and Smyth, 2009).
Yeast Two-Hybrid (Y2H) Screen
Y2H studies were performed as previously described (Li et al., 2002) and are detailed in the Supplemental Experimental Procedures.
Cell Culture Analysis of Wnt Signaling
The non-tumorigenic mouse mammary cell lines NMuMG or Eph4 were used to study canonical Wnt by Western blot and using Wnt reporter assays that were performed essentially as described previously (Shahi et al., 2012) as detailed in Supplemental Experimental Procedures.
Structural Modeling of Wnt5b and MMP3
Comparative protein structure modeling for Wnt5b and MMP3 was carried out using the automated comparative protein structure modeling pipeline MODPIPE (Pieper et al., 2011), and is detailed in the Supplemental Experimental Procedures.
Supplementary Material
HIGHLIGHTS.
MMP3 overexpression induces hyperplastic mammary epithelial growth in a non-proteolytic manner
Wnt5b acts as a non-canonical Wnt ligand that inhibits outgrowth of mammary epithelial cells
MMP3 regulates Wnt signaling by antagonizing Wnt5b function
Loss of MMP3 impairs, while overexpression of MMP3 potentiates mammary stem cell function
ACKNOWLEDGEMENTS
We thank Dr. Howard Bussey (McGill University, Canada) for Y2H reagents and Dr. Sandra Werner (Institute of Molecular Health Sciences, Switzerland) for the murine MMP10 cDNA. We thank Markus Brown, Ying Yu and Elena Atamuniac for technical assistance. This work was supported by a grant from the National Cancer Institute (R01 CA057621 to Z.W.), postdoctoral fellowships from the Komen Foundation (K. K.), the American Cancer Society (L.E.L.) and the Congressionally Directed Breast Cancer Research Program, U.S. Department of Defense (G.J.P.D., DAMD-17-02-1-0333; D.A.L., W81XWH-11-1-0742).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Extended Experimental Procedures, 6 Figures and 1 Table.
REFERENCES
- Bartel PL, Fields S. Analyzing protein-protein interactions using two-hybrid system. Methods Enzymol. 1995;254:241–263. doi: 10.1016/0076-6879(95)54018-0. [DOI] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Correia AL, Mori H, Chen EI, Schmitt FC, Bissell MJ. The hemopexin domain of MMP3 is responsible for mammary epithelial invasion and morphogenesis through extracellular interaction with HSP90beta. Genes Dev. 2013;27:805–817. doi: 10.1101/gad.211383.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- Crabbe T, Zucker S, Cockett MI, Willenbrock F, Tickle S, O’Connell JP, Scothern JM, Murphy G, Docherty AJ. Mutation of the active site glutamic acid of human gelatinase A: effects on latency, catalysis, and the binding of tissue inhibitor of metalloproteinases-1. Biochemistry. 1994;33:6684–6690. doi: 10.1021/bi00187a039. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nature Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Grumolato L, Liu G, Mong P, Mudbhary R, Biswas R, Arroyave R, Vijayakumar S, Economides AN, Aaronson SA. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev. 2010;24:2517–2530. doi: 10.1101/gad.1957710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Smyth GK. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods. 2009;347:70–78. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC. Structural basis of Wnt recognition by Frizzled. Science. 2012;337:59–64. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Goode RJ, Vaillant F, Mathivanan S, Kapp EA, Mathias RA, Lindeman GJ, Visvader JE, Simpson RJ. Proteomic profiling of secretome and adherent plasma membranes from distinct mammary epithelial cell subpopulations. Proteomics. 2011;11:4029–4039. doi: 10.1002/pmic.201100102. [DOI] [PubMed] [Google Scholar]
- Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- Kendrick H, Regan JL, Magnay FA, Grigoriadis A, Mitsopoulos C, Zvelebil M, Smalley MJ. Transcriptome analysis of mammary epithelial subpopulations identifies novel determinants of lineage commitment and cell fate. BMC Genomics. 2008;9:591. doi: 10.1186/1471-2164-9-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouros-Mehr H, Werb Z. Candidate regulators of mammary branching morphogenesis identified by genome-wide transcript analysis. Dev Dyn. 2006;235:3404–3412. doi: 10.1002/dvdy.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Page N, Bussey H. Actin patch assembly proteins Las17p and Sla1p restrict cell wall growth to daughter cells and interact with cis-Golgi protein Kre6p. Yeast. 2002;19:1097–1112. doi: 10.1002/yea.904. [DOI] [PubMed] [Google Scholar]
- Li Y, Welm B, Podsypanina K, Huang S, Chamorro M, Zhang X, Rowlands T, Egeblad M, Cowin P, Werb Z, et al. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci U S A. 2003;100:15853–15858. doi: 10.1073/pnas.2136825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Nurcombe V, Cool SM. Wnt signaling controls the fate of mesenchymal stem cells. Gene. 2009;433:1–7. doi: 10.1016/j.gene.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Liu BY, McDermott SP, Khwaja SS, Alexander CM. The transforming activity of Wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. Proc Natl Acad Sci U S A. 2004;101:4158–4163. doi: 10.1073/pnas.0400699101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Werb Z. Patterning mechanisms of branched organs. Science. 2008;322:1506–1509. doi: 10.1126/science.1162783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuibban GA, Gong JH, Tam EM, McCulloch CA, Clark-Lewis I, Overall CM. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science. 2000;289:1202–1206. doi: 10.1126/science.289.5482.1202. [DOI] [PubMed] [Google Scholar]
- Miyoshi H, Ajima R, Luo CT, Yamaguchi TP, Stappenbeck TS. Wnt5a Potentiates TGF-beta Signaling to Promote Colonic Crypt Regeneration After Tissue Injury. Science. 2012 doi: 10.1126/science.1223821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudgett JS, Hutchinson NI, Chartrain NA, Forsyth AJ, McDonnell J, Singer II, Bayne EK, Flanagan J, Kawka D, Shen CF, et al. Susceptibility of stromelysin 1-deficient mice to collagen-induced arthritis and cartilage destruction. Arthritis Rheum. 1998;41:110–121. doi: 10.1002/1529-0131(199801)41:1<110::AID-ART14>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccard H, Van den Steen PE, Opdenakker G. Hemopexin domains as multifunctional liganding modules in matrix metalloproteinases and other proteins. J Leuk Biol. 2007;81:870–892. doi: 10.1189/jlb.1006629. [DOI] [PubMed] [Google Scholar]
- Plaks V, Brenot A, Lawson DA, Linnemann JR, Van Kappel EC, Wong KC, de Sauvage F, Klein OD, Werb Z. Lgr5-expressing cells are sufficient and necessary for postnatal mammary gland organogenesis. Cell reports. 2013;3:70–78. doi: 10.1016/j.celrep.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieve MG, Moon RT. Stromelysin-1 and mesothelin are differentially regulated by Wnt-5a and Wnt-1 in C57mg mouse mammary epithelial cells. BMC Dev Biol. 2003;3:2. doi: 10.1186/1471-213X-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remacle AG, Golubkov VS, Shiryaev SA, Dahl R, Stebbins JL, Chernov AV, Cheltsov AV, Pellecchia M, Strongin AY. Novel MT1-MMP small-molecule inhibitors based on insights into hemopexin domain function in tumor growth. Cancer Res. 2012;72:2339–2349. doi: 10.1158/0008-5472.CAN-11-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Roarty K, Baxley SE, Crowley MR, Frost AR, Serra R. Loss of TGF-beta or Wnt5a results in an increase in Wnt/beta-catenin activity and redirects mammary tumour phenotype. Breast cancer research : BCR. 2009;11:R19. doi: 10.1186/bcr2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roarty K, Serra R. Wnt5a is required for proper mammary gland development and TGF-beta-mediated inhibition of ductal growth. Development. 2007;134:3929–3939. doi: 10.1242/dev.008250. [DOI] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- Shahi P, Park D, Pond AC, Seethammagari M, Chiou SH, Cho K, Carstens JL, Decker WK, McCrea PD, Ittmann MM, et al. Activation of Wnt signaling by chemically induced dimerization of LRP5 disrupts cellular homeostasis. PloS one. 2012;7:e30814. doi: 10.1371/journal.pone.0030814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirum KL, Brinckerhoff CE. Cloning of the genes for human stromelysin and stromelysin 2: differential expression in rheumatoid synovial fibroblasts. Biochemistry. 1989;28:8691–8698. doi: 10.1021/bi00448a004. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD. Key stages in mammary gland development: the cues that regulate ductal branching morphogenesis. Breast Cancer Res. 2006;8:201. doi: 10.1186/bcr1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Bissell MJ, Werb Z. The matrix metalloproteinase stromelysin-1 acts as a natural mammary tumor promoter. Oncogene. 2000;19:1102–1113. doi: 10.1038/sj.onc.1203347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell. 1988;55:619–625. doi: 10.1016/0092-8674(88)90220-6. [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Bowman AN, Nusse R. Developmental Stage and Time Dictate the Fate of Wnt/beta-Catenin-Responsive Stem Cells in the Mammary Gland. Cell Stem Cell. 2012 doi: 10.1016/j.stem.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Visvader JE. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev. 2009;23:2563–2577. doi: 10.1101/gad.1849509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagers AJ. The stem cell niche in regenerative medicine. Cell Stem Cell. 2012;10:362–369. doi: 10.1016/j.stem.2012.02.018. [DOI] [PubMed] [Google Scholar]
- Welm BE, Dijkgraaf GJ, Bledau AS, Welm AL, Werb Z. Lentiviral transduction of mammary stem cells for analysis of gene function during development and cancer. Cell Stem Cell. 2008;2:90–102. doi: 10.1016/j.stem.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman BS, Sternlicht MD, Lund LR, Alexander CM, Mott J, Bissell MJ, Soloway P, Itohara S, Werb Z. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. J Cell Biol. 2003;162:1123–1133. doi: 10.1083/jcb.200302090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science. 2002;296:1046–1049. doi: 10.1126/science.1067431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witty JP, Wright JH, Matrisian LM. Matrix metalloproteinases are expressed during ductal and alveolar mammary morphogenesis, and misregulation of stromelysin-1 in transgenic mice induces unscheduled alveolar development. Mol Biol Cell. 1995;6:1287–1303. doi: 10.1091/mbc.6.10.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, Okamura H, Woodgett J, He X. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng YA, Nusse R. Wnt proteins are self-renewal factors for mammary stem cells and promote their long-term expansion in culture. Cell Stem Cell. 2010;6:568–577. doi: 10.1016/j.stem.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.