Abstract
Pancreatic cancer is currently one of the deadliest of the solid malignancies. However, surgery to resect neoplasms of the pancreas is safer and less invasive than ever, novel drug combinations have been shown to improve survival, advances in radiation therapy have resulted in less toxicity, and enormous strides have been made in our understanding of the fundamental genetics of pancreatic cancer. These advances provide hope but they also increase the complexity of caring for patients. It is clear that multidisciplinary care that provides comprehensive and coordinated evaluation and treatment is the most effective way to manage patients with pancreatic cancer.
Keywords: pancreatic neoplasms, molecular biology, radiology, radiation oncology, medical oncology
Introduction
The American Cancer Society estimates that 45,220 Americans will be diagnosed with ductal adenocarcinoma of the pancreas (referred to in this review as “pancreatic cancer”) in 2013, and that 38,460 will die from the disease.1 Despite decades of effort, the five-year survival rate remains at only ~5%. There are no early detection tests and most patients with localized disease have no recognizable symptoms or signs; as a result, most patients are not diagnosed until late in their disease, after their cancer has metastasized to other organs.
Despite these grim statistics, we believe that there is real hope on the horizon. In this article we will review recent progress in pancreatic cancer, with emphasis on genetic advances and the multidisciplinary team approach to patient care.
Risk Factors (Table 1)
Table 1.
Pancreatic Cancer Risk Factors
| Risk Factor | Risk Estimate (95% CI) |
|---|---|
| Current Cigarette Smoking | OR= 2.20 (1.71–2.83) |
| Past Cigarette Smoking 1–10 years since quitting 15–20 years since quitting |
OR=1.64 (1.36–1.97) OR=1.12 (0.86–1.44) |
| Diabetes Mellitus <3 years >10 years duration |
RR=7.94 (95% CI, 4.70–12.55) OR 1.51 (95% CI=1.16–1.96) |
| BMI (>35 vs 18.9–24.9) | OR =1.55 (95%CI=1.16 – 2.07) |
| Heavy Alcohol (> 6 drinks/day) | OR 1.46 (95%CI=1.16–1.83) |
| Pancreatitits (>2 years) | 2.71 fold (95% CI 1.96–3.74) |
BMI= Body mass index; OR=odds ratio; RR=relative risk
Population and family-based studies have helped to establish that both environmental and inherited factors contribute to the development of pancreatic adenocarcinoma. The most common risk factor for pancreatic adenocarcinoma is cigarette smoking. Analyses of data from 12 case-control studies showed that current smokers have a 2.2-fold (95% Confidence Interval (CI) 1.71–2.83) increased risk of pancreatic cancer compared with never smokers.2 Approximately 25% of pancreatic cancers are attributable to cigarette smoking.3 The “finger print” of tobacco smoking can be seen in pancreatic cancers, as genetic analyses have shown that pancreatic cancers resected from smokers have more mutations than do pancreatic cancers from never-smokers.4 Importantly, smoking cessation reduces this risk.2 Risk estimates of 1.64 (Odds Ratio (OR), 95% CI 1.36–1.97) have been reported for recent quitters (1–10 years) and of 1.12 (95% CI 0.86–1.44) for individuals who quit smoking 15–20 years ago.2
Longstanding type 2 diabetes mellitus is also associated with an increased risk of pancreatic cancer, with patients with type 2 diabetes of >10 years duration having a 1.51-fold (95% CI=1.16–1.96) increased risk of pancreatic cancer compared with non-diabetics.5 In addition, new onset diabetes can be the first sign of pancreatic cancer.6 Up to 1% of new-onset adult diabetics are diagnosed with pancreatic cancer within 3 years of their diagnosis of diabetes, suggesting that new onset diabetes could be a clue to the early diagnosis of pancreatic cancer in some people.6 Thus long-standing diabetes is a risk factor for pancreatic cancer, and new onset diabetes can be an early sign of the disease.
In the past several years, a number of studies have demonstrated that increased body mass index (BMI) is also associated with an increased risk of developing pancreatic cancer.7 This risk is independent of the risk of pancreatic cancer due to diabetes. An analysis of data from 12 cohort studies and one case-control study estimated that the risk of pancreatic cancer is 1.55-fold (95% CI=1.16 – 2.07) greater for individuals with a BMI >35 compared to individuals with a BMI of 18.9 to 24.9.7 Although it has not been established, one would hope that significant weight loss in patients with an elevated BMI would help reduce some of this risk.
Other risk factors for pancreatic cancer include alcohol consumption and pancreatitis. Heavy alcohol consumption (>=6 drinks per day) has been associated with an increased risk of pancreatic cancer with an OR of 1.46 (95% CI=1.16–1.83) compared with light consumption (less than one drink per day).8 Low to moderate alcohol consumption does not appear to increase risk. Chronic pancreatitis also elevates risk of pancreatic cancer. An analysis of data from 10 case-control studies demonstrated that individuals with a history of chronic pancreatitis have a 2.71-fold (95% CI 1.96–3.74) increased risk of pancreatic cancer.9 As was true for diabetes, pancreatitis can also be caused by pancreatic cancer and new onset pancreatitis can therefore also be a sign of a pancreatic neoplasm.
Inherited Risk Factors (Table 2)
Table 2.
Pancreatic Cancer Susceptibility Genes
| Gene/Risk Group | Risk Estimate (95% CI) | Estimated Lifetime Pancreatic Cancer Risk |
|---|---|---|
| General Population | 1 | 0.96(by age 80)225 |
| Familial Pancreatic Cancer Overall 3 or more first-degree relatives with pancreatic cancer |
RR= 6.79 ( 4.54 to 9.75) RR= 17.02 (7.34 to 33.5) |
Varies with youngest age of onset |
| High Penetrance | ||
| BRCA2 | RR = 3.51(1. 87–6.58)226 | 3.36% (age 80)* |
| PALB2 | Elevated | Elevated |
| BRCA1 | OR=2.26 (1.26 to 4.06)32 |
2.16% (age 80)* |
| Mis-Match Repair (HNPCC) | RR=8.6 (4.7–15.7) 39 | 3.68%(1.45%-5.88%)(age 70)39 |
| Hereditary Pancreatitis (PRSS1) | RR=58 (23–105)47 | 30–40%(age 70) 47,48 |
| Peutz-Jeghers (STK11) | RR=132 (44, 261)227 | 11%-32%228, 229 |
| Familial Melanoma(CDKN2A) | RR=38 (10–97)230 | 17% (age 75) |
| ATM | Unknown | Unknown |
| Low-Penetrance | ||
| ABO blood group | OR=1.20 (1.12–1.28)231 | 1.15% (age 80) |
| 1q32.1(rs3790844T/C) | OR=0.77 ( 0.71–0.84)231 | 0.73% (age 80) |
| 13q22.1(rs9543325 T/C) | OR=1.26 ( 1.18–1.35)231 | 1.2% (age 80)* |
| 5p15.33(rs401681C/T) | OR=1.19 ( 1.11–1.27)231 | 1.10% (age 80)* |
Estimated based by multiplying general population risk by risk estimate. HNPCC=hereditary non-polyposis colorectal cancer syndrome; OR=odds ratio; RR=relative risk
In addition to environmental risk factors, inherited genetic changes can be important risk factors for ductal adenocarcinoma of the pancreas. Case-control and cohort studies have all demonstrated that individuals with a family history of pancreatic cancer are at an increased risk of developing pancreatic cancer themselves. Estimates from these studies range from a 1.9 to a 13-fold increased risk.10–14 Family registries, in addition to population based studies, have helped to demonstrate the clustering of ductal adenocarcinoma of the pancreas in families and these registries have been used to quantify the risk of pancreatic cancer due to familial factors. One such registry is the National Familial Pancreas Tumor Registry (NFPTR) at Johns Hopkins (www.nfptr.org). Familial pancreatic cancer is defined as at least a pair of first-degree relatives diagnosed with pancreatic cancer in a family, and prospective studies of families enrolled in the NFPTR demonstrated a 6.8-fold ( 95% CI 4.54 to 9.75) increased risk of pancreatic cancer in the first-degree relatives of familial pancreatic cancer patients compared the general United States population.15 The standardized incidence ratio reached 17 (95% CI 7.34 to 33.5) among individuals with 3 or more close relatives with pancreatic cancer.15 Data from the NFPTR also indicated that having a relative who developed pancreatic cancer at a young age is also associated with an increased risk of pancreatic cancer in familial pancreatic cancer families, however risk did not vary with age in families with just a single pancreatic cancer.15 As if having an increased risk of pancreatic cancer wasn’t bad enough, families in which there is a clustering of pancreatic cancer also have an increased risk of extra-pancreatic cancers.16 Relatives of familial pancreatic cancer patients have an increased risk of dying from breast (1.66-fold, 95% CI 1.15–2.34), ovarian (2.05-fold, 95% CI 1.10–3.49), and bile duct cancers (2.89-fold, 95% CI 1.04–6.39).16
Several germline genetic syndromes have been identified that are associated with an increased risk of ductal adenocarcinoma of the pancreas, these range from high-penetrance genes which are associated with high lifetime risk of pancreatic cancer, to low penetrance genes associated with only a slightly increased (<1.5 fold) risk of pancreatic cancer (Table 2).17 There are three important points to remember about these genetic syndromes. First, the risk of pancreatic cancer can be quantified if one knows the gene responsible for the aggregation of pancreatic cancer in a family. Quantifying risk is important for the design of clinical trials to screen at-risk patients for early curable precancerous lesions.18, 19 Second, all of these genes, with the exception of those associated with familial pancreatitis, not only increase the risk of pancreatic cancer, but they also increase the risk of extra-pancreatic malignancies.20 This suggests that lives can be saved by screening for these extra-pancreatic malignancies. Third, some of these syndromes have implications for therapy. For example, germline BRCA2 gene mutations increase the risk of pancreatic cancer, and cancers in which the BRCA2 gene has been inactivated appear to be sensitive to DNA cross-linking agents.21
BRCA2
Inherited mutations in the BRCA2 gene are associated with a significantly elevated lifetime risk of breast, ovarian, prostate and pancreatic cancer.22 The prevalence of germline BRCA2 gene mutations in pancreatic cancer patients varies among different populations and is particularly high in individuals of Ashkenazi Jewish decent.22, 23 Four to ten percent of Ashkenazi Jews with pancreatic cancer carry a germline BRCA2 mutation.23, 24 The prevalence of BRCA2 gene mutations among pancreatic cancer patients also increases as the number of relatives they have with pancreatic cancer increases; 6–12% of pancreatic cancer patients from families in which two or more relatives have pancreatic cancer carry deleterious BRCA2 mutations, and 16% of patients from families in which three or more relatives have pancreatic cancer carry germline BRCA2 mutations.25, 26 While many of the pancreatic cancer families that are found to have deleterious BRCA2 mutations also report a family history of breast and/or ovarian cancer, a sizeable proportion of pancreatic cancer patients with germline BRCA2 mutations report no breast or ovarian cancers in their family.22 Clearly, the penetrance of these genetic changes is not complete. Several clinical trials are evaluating approaches to specifically target BRCA2-deficient pancreatic cancers.27
PALB2
Germline mutations in the PALB2 (partner and localizer of BRCA2) gene have been reported in 1–3% of familial pancreatic cancer kindreds.28, 29 The first observation of PALB2 in familial pancreatic cancer kindreds was made by Jones et al using a whole-exome sequencing approach.30 Subsequent studies have replicated this finding.28, 29 As is true for its binding partner BRCA2, mutations in the PALB2 gene are also associated with an increased risk of breast cancer, yet, not all pancreatic cancer patients found to have germline PALB2 mutations report a personal or family history of breast cancer. Just as pancreatic cancers harboring BRCA2 mutations appear to be sensitive to DNA cross-linking agents, so are pancreatic cancers harboring PALB2 mutations. 19, 31
BRCA1
Unlike germline mutations in BRCA2 and PALB2 which have been consistently associated with an increased risk of pancreatic cancer, it is less clear if carriers of germline BRCA1 mutation are also at higher risk of pancreatic cancer. While several studies have reported an increased risk of pancreatic cancer in BRCA1 carriers, including a large-scale study conducted by the Breast Cancer Linkage Consortium which found a 2.26-fold (95% CI 1.26 to 4.06) increased risk of pancreatic cancer in BRCA1 mutation carriers, other studies have not reported an increased prevalence of BRCA1 gene mutations in pancreatic cancer patients.24,32 33
The associations of germline BRCA2, PALB2 and possibly BRCA1 gene mutations with pancreatic cancer make it clear that a good family history, one that specifically asks about a family history of breast cancer, is important in the evaluation of patients with pancreatic cancer.
p16/CDKN2A
Germline mutations in the p16/CDKN2A gene are associated with a high lifetime risk of melanoma and the “familial atypical multiple mole melanoma syndrome (Figure 1),” as well as an increased risk of pancreatic cancer.20, 34, 35 Individuals born with a mutation in the p16/CDKN2A gene have a 38-fold increased risk of developing pancreatic cancer.20, 34, 35 Studies of kindreds with a 19 base pair deletion in exon 2 of the p16/CDKN2A gene (the Leiden mutation) have estimated that these carriers have a 17% lifetime (by age 75) risk of developing pancreatic cancer.36 The association of germline mutations in the p16/CDKN2A gene with pancreatic cancer suggests that a careful skin examination for nevi and melanomas should be a part of the clinical evaluation of patients with pancreatic cancer. Importantly, lives can be saved by screening p16/CDKN2A gene mutation carriers and their relatives for early melanocytic lesions.37
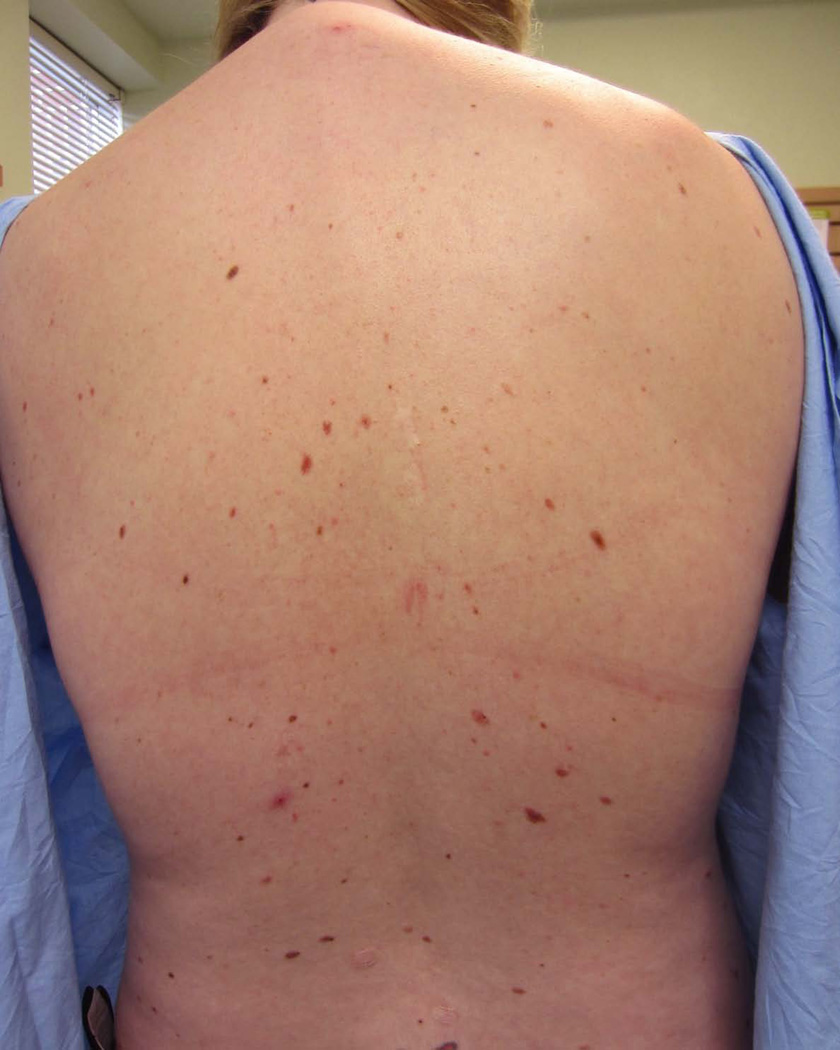
Figure 1.
Patients, such as the one shown here, with Familial Atypical Multiple Mole Melanoma syndrome have increased numbers of melanocytic nevi and an increased risk of melanoma and pancreatic cancer.
Lynch Syndrome
Lynch Syndrome is an autosomal dominant hereditary disease characterized by early onset colon cancer due to germline mutations in one of the DNA mismatch repair genes (hMSH2, hMLH1, hPMS1, hPMS2 or hMSH6/GTBP).38 Patients with Lynch Syndrome also have an increased risk of endometrial, gastric, small intestinal, ureteral and pancreatic cancer.38 A recent study of 147 families containing a mutation in a mismatch gene reported an 8.6-fold (95% CI, 4.7–15.7) increased risk of pancreatic cancer compared with the general population.39 This corresponds to a 3.68% (95% CI, 1.45–5.88%) lifetime (by age 70) risk of pancreatic cancer.39 The pancreatic cancers that occur in these kindreds frequently have microsatellite instability (MSI+) and a distinct poorly differentiated medullary histopathology as is seen in MSI+ colorectal cancers.40, 41 Of note, despite their poor differentiation, medullary cancers of the pancreas are associated with a good prognosis.41, 42
Hereditary Pancreatitis
Hereditary pancreatitis is a rare inherited form of pancreatitis in which the patients suffer repeated episodes of acute pancreatitis beginning in childhood, and which typically results in pancreatic insufficiency by early adulthood.43 Mutations in the cationic trypsinogen gene (PRSS1) cause an autosomal dominant form of hereditary pancreatitis, whereas mutations in the serine protease inhibitor gene (SPINK1) cause an autosomal recessive form of hereditary pancreatitis.44, 45,46 Patients with hereditary pancreatitis have a remarkable 58-fold (95% confidence interval [CI] = 23–105) increased risk of developing pancreatic cancer and a lifetime risk (by age 70) of pancreatic cancer of 30–40%.47,48 Cigarette smoking has been shown to further increase this risk.48 Because the risk of pancreatic cancer is high, and the risk of cancer in patients with hereditary pancreatitis is confined to the pancreas, some of these patients choose prophylactic pancreatectomy. The risks and morbidity associated with total pancreatectomy are high, and such procedures should not be undertaken lightly.
Peutz-Jeghers Syndrome
Individuals with the Peutz-Jeghers syndrome, an autosomal dominant disorder characterized by hamartomatous polyps in the gastrointestinal tract and pigmented macules of the lips, buccal mucosa, and digits, have been shown have an 11–32% lifetime risk of pancreatic cancer.49, 50 Mutations in the STK11 gene explain more than 80% of Peutz-Jeghers cases. This risk of pancreatic cancer is so high in patients with Peutz-Jeghers that they would be a natural population to benefit from screening tests for early pancreatic neoplasia as such tests become available.19, 51, 52
Susceptibility Variants
In addition to the above genetic mutations, which have been associated with a high lifetime risk of pancreatic cancer, several additional variants have been identified that are associated with a weak to modest increased risk of pancreatic cancer. For example, a single nucleotide variant in the ABO blood group gene, rs505922, has been associated with a per-allele odds ratio of 1.20-for pancreatic cancer (95% CI 1.12–1.28).53
Pathology (Table 3)
Table 3.
Pathology of the Major Neoplasms of the Pancreas
| Tumor type | Gross | Microscopy | Clinical Importance |
|---|---|---|---|
| Acinar cell carcinoma | Large soft fleshy solid masses | Pyramidal cells neoplastic cells form small lumina. Expression of digestive enzymes can be demonstrated by immunolabeling. | Rare fully malignant neoplasm. 15% associated with metastatic fat necrosis caused by the release of digestive enzymes into the blood stream. |
| Invasive ductal adenocarcinoma | Poorlydefined firm solid infiltrative masses | The neoplastic cells form glands and infiltrate tissues. Vascular and perineural invasion are common. Associated with a dense desmoplasticstroma. | Most common type of pancreatic cancer. Very poor prognosis. |
| Intraductal papillary mucinous neoplasm (IPMN) | Cystic tumors that arise in the larger pancreatic ducts. Finger-like papillae of neoplastic cells project into mucin-filled ducts | Papillae lined by mucin-producing neoplastic cells with varying degrees of dysplasia. | IPMNs are detectable and curable non-invasive precursors to invasive pancreatic cancer. The challenge is not to over treat low-grade IPMNs. |
| Mucinous cystic neoplasm (MCN) | Cystic neoplasm that almost always arises in the tail of the pancreas. Cysts filled with mucin. | Mucin-producing neoplastic epithelium resting on ovarian-type stroma. | Can progress to invasive cancer if untreated. |
| Pancreatic intraepithelial neoplasia (PanIN) | Microscopic lesions | Noninvasive epithelial proliferations in the smaller pancreatic ducts. Associated with lobulocentric atrophy. | PanINs are a curable noninvasive precursor to invasive pancreatic cancer, but most are too small to detect. |
| Pancreatoblastoma | Large soft solid masses | Similar to acinar carcinoma but also have squamoid nests. | More common in children than adults. |
| Pancreatic neuroendocrine tumor (PanNET) | Well-demarcated and soft solid masses | Nests and trabecullae of relatively uniform cells with “salt and pepper” chromatin. Expression of neuroendocrine markers and hormones can be demonstrated by immunolabeling. | Some arise in the setting of a familial genetic syndrome. Aberrant hormone production can cause clinical syndromes. Fully malignant, with a 45% 10-year survival rate |
| Serous cystadenoma | Cystic neoplasms with thin septa, and straw-colored fluid. Often have a central scar. | Clear cuboidal cells without atypia line cysts. | Virtually always benign. |
| Solid-pseudopapillary neoplasm | Solid masses that undergo cystic change caused by hemorrhage and necrosis | Poorly cohesive cells surround delicate blood vessels. | Most arise in young women. Low-grade malignant neoplasms. |
| Variants of ductal carcinoma (adenosquamous, colloid, medullary, undifferentiated, etc.) | Most are solid | Varies based on tumor type. | Can be clinically important to recognize. |
IPMN=intraductal papillary mucinous neoplasm; MCN=mucinous cystic neoplasm; PanIN=pancreatic intraepithelial neoplasia; PanNET=pancreatic neuroendocrine tumor; SCN=serous cystic neoplasm; SPN=solid-pseudopapillary neoplasm
A number of clinically and pathologically distinct neoplasms arise in the pancreas. These neoplasms can be broadly divided pathologically into those that are typically solid and those that are usually cystic. This categorization parallels the primary radiologic appearances of these neoplasms, and it helps narrow the clinical differential diagnosis. Specific pathologic diagnoses within each of these two broad categories have important implications for patient management and prognosis (Table 3). The treatment recommendations in the Treatment section of this review are specific for invasive ductal adenocarcinoma (“pancreatic cancer”), and may not apply completely to some of the other tumor types that can arise in the pancreas.
Solid Tumors
The most common solid tumor is the invasive ductal adenocarcinoma, more commonly called “pancreatic cancer.” By definition, the neoplastic cells of invasive ductal adenocarcinoma, as the name suggests, form glands and infiltrate into tissues (Figure 2A).54 Grossly, these cancers are usually solid and firm, and send tongues of neoplastic cells far beyond the main tumor. Microscopically, almost all invasive ductal adenocarcinoma invade nerves and spread along perineural spaces. These cancers also have a proclivity to invade lymphatic spaces and small veins, and in so doing to spread to regional lymph nodes and to metastasize to the liver. As a result, by the time most invasive ductal adenocarcinomas are diagnosed they have spread beyond the gland and are not amenable to surgical resection.
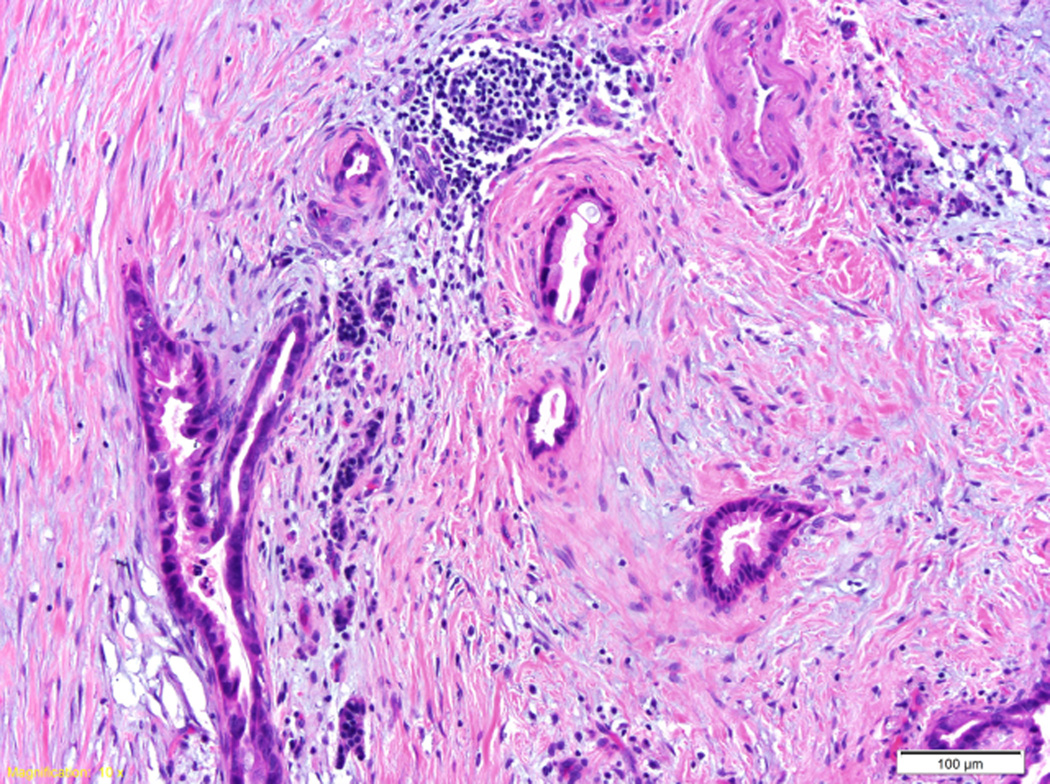
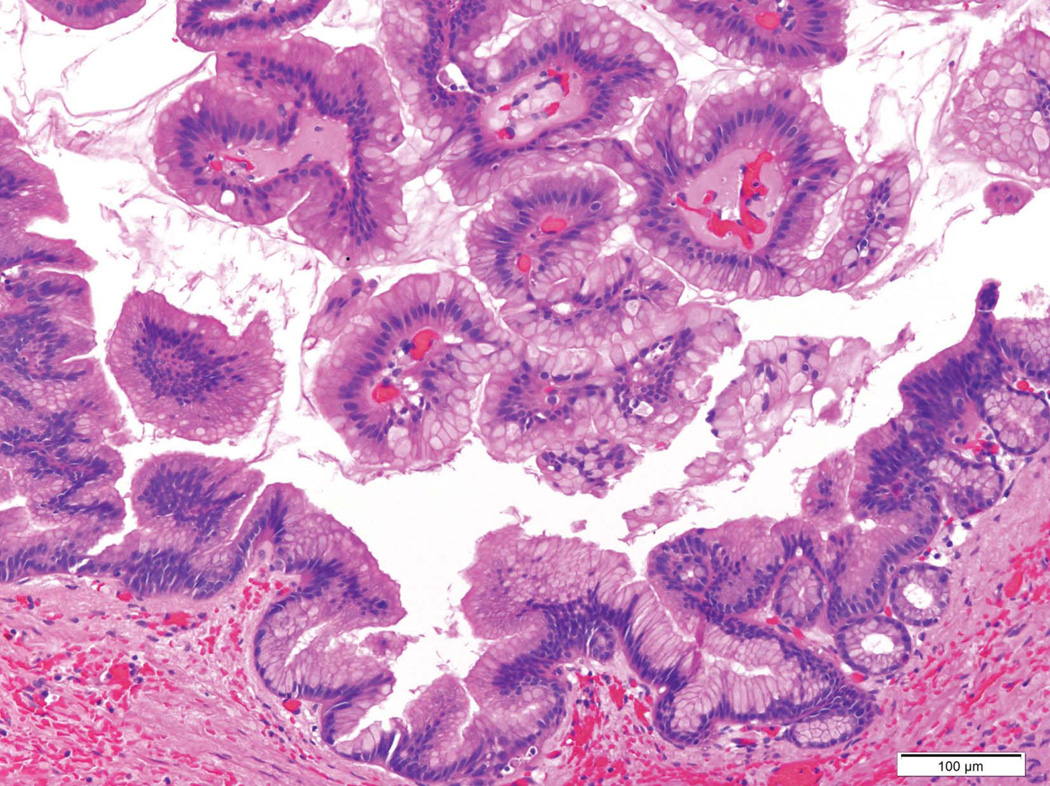
Figure 2.
A) Histopathology of a ductal adenocarcinoma of the pancreas. Note the atypical glands embedded in the desmoplastic stroma. B) Intraductal papillary mucinous neoplasms (IPMNs) are characterized by an intraductal growth of neoplastic mucin-producing cells that typically form papillae (both hematoxylin and eosin stain).
Another important histologic feature of invasive ductal adenocarcinomas of the pancreas is that these cancers elicit an intense desmoplastic reaction.54–56 This desmoplastic reaction is composed of fibroblasts, inflammatory cells, endothelial cells and a complex extracellular matrix, and is associated with significantly increased interstitial fluid pressure within the tumor. This elevated interstitial fluid pressure has been hypothesized to be an impediment to perfusion of the tumor, explaining the low attenuation seen on contrast enhanced imaging, and the elevated pressure may serve as an obstacle to the diffusion of therapeutic agents.55 The desmoplastic reaction associated with pancreatic cancer has to be taken into account when designing therapeutic regimens, as even the best agents will not be effective if they do not reach the neoplastic cells.57 Some have also suggested that the desmoplastic reaction can be exploited therapeutically. For example, the albumin in nab-paclitaxel (albumin-bound paclitaxel) may bind to SPARC, a protein expressed at high levels in the desmoplastic stroma, increasing the delivery of paclitaxel to the neoplastic cells.58, 59 Cell-matrix interactions could also be targeted therapeutically, and some investigators are testing novel approaches to remodeling the tumor microenvironment, such as enzymatic digestion of stromal hyaluronan.55
Several clinically important variants of ductal adenocarcinoma of the pancreas have been described. Adenosquamous carcinoma has, in addition to neoplastic cells with ductal differentiation, a significant component with squamous differentiation.54, 60 Adenosquamous carcinomas are particularly aggressive cancers with an extremely poor prognosis, however, patients with an adenosquamous carcinoma may still benefit from surgical resection.60, 61
Colloid carcinoma is a mucin-producing epithelial neoplasm composed of neoplastic cells “floating” in pools of mucin embedded in the stroma.54 These invasive carcinomas almost always arise in association with intraductal papillary mucinous neoplasms (IPMNs), and colloid carcinomas are associated with a better prognosis than invasive ductal adenocarcinomas.62 Some of the improved prognosis associated with colloid carcinomas may be related to their tendency to present clinically at a lower stage than invasive ductal adenocarcinomas that did not arise in association with an IPMN.63
Medullary carcinoma is composed of poorly-differentiated cells with a syncytial growth pattern, and characterized by pushing boarders, extensive necrosis, and an associated lymphocytic inflammatory cell infiltrate.41 As described earlier, some medullary cancers arise in patients with the Lynch syndrome, and not surprisingly, some medullary carcinomas are microsatellite unstable, and patients with these cancers are more likely to have a family history of cancer. 41 Patients with medullary cancers have a better prognosis than do patients with invasive ductal adenocarcinomas, despite the poor differentiation of the neoplastic cells in medullary carcinomas.41
Signet ring carcinoma is composed of individual neoplastic cells each with a prominent mucin globule, imparting a “signet ring” appearance to the cells.54 Although signet ring cell carcinomas can arise in the pancreas, clinicians should be aware that cancers with this morphology more commonly arise in the stomach or breast, and these latter two cancers can metastasize to the pancreas and mimic a pancreatic primary.
Undifferentiated carcinomas and undifferentiated carcinomas with osteoclast-like giant cells are extremely aggressive carcinomas associated with a very poor prognosis.54
Perhaps because the prognosis for invasive ductal adenocarcinoma is so poor, significant emphasis has been placed on understanding the potentially curable noninvasive precursor lesions that give rise to these cancers. Extensive histologic and genetic studies have helped establish that a small microscopic lesion, called pancreatic intraepithelial neoplasia (PanIN), can be a precursor to invasive pancreatic cancer.64–66 While most PanINs are too small to be detected using currently available imaging technologies, abnormal DNA shed from PanINs can be detected in the duodenal fluid and even in the stool, raising the possibility of a gene-based early detection test for PanINs the future.67, 68 In addition, the pancreatic parenchyma immediately surrounding PanIN lesions is often fibrotic and atrophic, a finding called “lobulocentric atrophy.”69 When PanINs are multifocal, as can be seen in individuals with a strong family history of pancreatic cancer, the associated multiple foci of lobulocentric atrophy can produce histologic changes of chronic pancreatitis, and these changes can be detected by endoscopic ultrasonography, suggesting another possible approach to the early detection of curable PanIN lesions.70
Pancreatic neuroendocrine tumors (PanNETs) are the second most common type of solid neoplasm of the pancreas. PanNETs are less aggressive than invasive ductal adenocarcinomas, but they are fully malignant with a ten-year survival rate of only 45%.54 Although not as common as ductal adenocarcinomas, these neoplasms are clinically important for two reasons. First, some arise in the setting of a genetic predisposition syndrome such as the multiple endocrine neoplasia 1 (MEN-1) and von Hippel Lindau (VHL) syndromes. PanNETs arising in these settings cannot be managed in isolation, instead the entire patient has to be considered as other neoplasms may dominate the patient’s course. Second, some PanNETs produce endocrine hormones that are released into the blood stream producing a clinical syndrome (such as insulinomas, glucagonomas, etc.). These are designated functional PanNETs, and the prognosis and management of functional PanNETs is usually determined by the specific clinical syndrome produced.54
Grossly, PanNETs are usually well-demarcated, soft and solid neoplasms. Microscopically, the neoplastic cells form nests or trabeculae, and the neoplasms are characteristically richly vascular. This rich vascularity explains the proclivity of PanNETs to enhance with contrast. Tumor grade and stage are the most important prognosticators, with grade determined by the proliferation rate of the neoplastic cells, and stage by the size and extent of the tumor.71, 72
Surgery is the treatment of choice for patients with a localized PanNET, and there are a variety of treatment options for patients with metastatic or progressive disease. For example, the tyrosine kinase inhibitor sunitinib and the mammalian target of rapamycin (mTOR) pathway inhibitor everolimus have both been reported to significantly improve progression-free survival in patients with advanced PanNETs.72, 73
Acinar carcinoma and pancreatoblastoma are rare usually solid tumors, and both are composed of neoplastic cells with differentiation along the lines of exocrine enzyme production.54 Pancreatoblastomas also have a component of squamoid nests. Unfortunately, both acinar cell carcinoma and pancreatoblastoma are associated with a poor prognosis.
Cystic Neoplasms
With recent improvements in and the increasing use of cross sectional imaging such as computed tomography (CT) scanning, more and more people are being diagnosed with cystic lesions in their pancreas. For example, in one series, pancreatic cysts were identified in 2.6% of over 2,800 CT scans performed on patients without pancreas-related symptoms.74 Many of these cysts are neoplastic cysts, and some will progress to invasive carcinoma if left untreated. Cystic neoplasms of the pancreas therefore represent a unique opportunity to treat pancreatic neoplasia before an invasive cancer develops. Unfortunately, since cystic tumors of the pancreas are so common and many are entirely benign, pancreatic cysts also present a significant risk for over treating patients.
There are four main cystic neoplasms of the pancreas.54 Intraductal papillary mucinous neoplasms (IPMNs) and mucinous cystic neoplasms (MCNs) are noninvasive mucin-producing neoplasms. IPMNs, by definition, involve the larger pancreatic ducts (Figure 2B), while MCNs usually arise in the tail of the gland and the cysts of MCNs do not communicate with the pancreatic duct system. Some IPMNs and MCNs progress over years from lesions with low-grade dysplasia and few genetic abnormalities, to lesions with high-grade dysplasia, to invasive carcinomas with complex genetic changes. The clinical goal in managing these patients is to resect high-risk lesions such as IPMNs and MCNs with high-grade dysplasia, and to observe, and not over treat, those with low-grade dysplasia. Patients who have had an IPMN resected are at risk for developing an invasive cancer in the remnant of their pancreas and should be followed carefully.75
Solid-pseudopapillary neoplasms (SPNs) are low-grade malignant neoplasms composed of poorly cohesive cells. SPNs almost always arise in younger women, and are best treated surgically.76 Serous cystic neoplasms (SCNs) are almost always benign, and are composed of uniform glycogen-rich cuboidal cells which form small cysts filled with straw-colored fluid. Most SCNs can be safely followed clinically and need not be resected unless they are large or cause symptoms.77
Molecular Biology (Table 4)
Table 4.
Genetic Alterations in Common Neoplasms of the Pancreas
| Tumor type | Gene(s) | Prevalence of the Alteration |
Comment |
|---|---|---|---|
| Acinar cell carcinoma | APC | 15% | |
| CTNNB1 (beta-catenin) | 5% | ||
| Invasive ductal adenocarcinoma | KRAS | 95% | |
| p16/CDKN2A | 95% | ||
| TP53 | 75% | ||
| SMAD4 | 55% | SMAD4 loss associated with poor prognosis and widespread disease | |
| MLL3, TGFBR2, FBXW7, ARID1A,AIRID2, and ATM | <5% | Some of these, such as ATM, may be targetable therapeutically | |
| IPMN | KRAS | 80% | |
| RNF43 | 75% | RNF43 is a marker of mucin-producing tumors as it is present in both IPMNs and MCNs | |
| GNAS | 60% | GNAS is a marker of IPMNs. GNAS and/or KRAS mutations are present in >95% of all IPMNs | |
| p16/CDKN2A | Varies dependent on histologic grade | ||
| TP53 | Varies dependent on histologic grade | Associated with higher grade lesions | |
| SMAD4 | Varies dependent on histologic grade | Associated with higher grade lesions | |
| PIK3CA | 10% | ||
| MCN | KRAS | 75% | |
| RNF43 | 40% | RNF43 is a marker of mucin-producing tumors--IPMNs and MCNs | |
| p16/CDKN2A | Varies dependent on histologic grade | ||
| TP53 | Varies dependent on histologic grade | Associated with higher grade lesions | |
| SMAD4 | Varies dependent on histologic grade | Associated with higher grade lesions | |
| Pancreatoblastoma | Imprinted region on chromosome 11 | 85% | Same region is targeted in hepatoblastoma and Wilms tumors |
| CTNNB1 (beta-catenin) | 55% | ||
| APC | 10% | ||
| PanNET | MEN1 | 45% | |
| DAXX or ATRX | 45% | ||
| TSC2, PTEN, DDIT4, and PIK3CA (mTOR Pathway genes) | 15% | Potentially targetable therapeutically with everolimus | |
| SCN | VHL | 50% | Among the cystic tumors of the pancreas, VHL loss is specific for SCN |
| SPN | CTNNB1 (beta-catenin) | 95% | Immunolabeling for beta-catenin is useful diagnostically |
IPMN=intraductal papillary mucinous neoplasm; MCN=mucinous cystic neoplasm; PanNET=pancreatic neuroendocrine tumor; SCN=serous cystic neoplasm; SPN=solid-pseudopapillary neoplasm
Cancer is fundamentally caused by inherited (germline) and acquired (somatic) mutations in cancer-causing genes. The germline changes associated with ductal adenocarcinoma of the pancreas were discussed earlier. The exomes of ductal adenocarcinoma and of all of the most common types of tumors of the pancreas have been completely sequenced, providing unprecedented insight into the somatic mutations in these neoplasms (Table 4).78–81 This insight, in turn, will almost certainly change the way these neoplasms are clinically managed, and form the basis for new approaches to the early detection and treatment of pancreatic neoplasia.
The sequencing of infiltrating ductal adenocarcinomas of the pancreas revealed that four genes, KRAS, p16/CDKN2A, TP53 and SMAD4, are each somatically altered in >50% of the cancers.79 KRAS, an oncogene on chromosome 12, is activated by point mutation in 95% of invasive ductal adenocarcinomas.79, 82 The protein coded for by the KRAS gene is a small GTPase that plays an important role in cell signaling through the mitogen-activated protein kinase (MAPK) and other pathways. The point mutations in KRAS occur early in pancreatic neoplasia, and almost exclusively target three codons (codons 12, 13 and 61), making them relatively easy to identify and, suggesting that KRAS mutations could form the basis for gene-based tests to detect early curable pancreatic neoplasia.83
The p16/CDKN2A gene, a tumor suppressor gene on chromosome 9p, is inactivated in ~95% of pancreatic cancers.79 The protein product of the p16/CDKN2A gene, p16, plays an important role in the regulation of the cell cycle and loss of p16 function in pancreatic cancer is believed to promote unrestricted cell growth.
The TP53 tumor suppressor gene on chromosome 17p is inactivated in 75% of pancreatic cancers.79 TP53 codes for the p53 protein, and p53 plays an important role in cellular stress responses, particularly by activating DNA repair, inducing growth arrest and triggering cell death (apoptosis). Loss of p53 function, through mutation of the TP53 gene, therefore promotes pancreatic neoplasia through the loss of a number of critical cell functions.
The fourth major gene that is somatically targeted in pancreatic cancer is the SMAD4 (previously designated DPC4) tumor suppressor gene on chromosome 18q.84 The protein product of the SMAD4 gene, Smad4, functions in the transforming growth factor beta (TGFβ) cell signaling pathway. SMAD4 gene mutations in pancreatic cancer are associated with poor prognosis and with more widely metastatic disease.85, 86
In addition to these four major genes, several genes are somatically mutated in pancreatic cancer at lower frequencies. They include MLL3, TGFBR2, FBXW7, ARID1A, AIRID2, and ATM.79, 87 ATM is particularly interesting because it is a possible therapeutic target as cancers which have genetically inactivated ATM may be particularly sensitive to radiation damage and to poly ADP ribose polymerase (PARP) inhibitors.88
In addition to changes in the DNA sequence, the expression of a number of genes in pancreatic cancer is altered by epigenetic mechanisms such as aberrant methylation.89 The expression of these aberrantly methylated genes is often downregulated, as can be seen with p16/CDKN2A. Other genes are hypomethylated and over-expressed in pancreatic cancer. DNA methylation in a cell can be chemically altered, and it has been suggested that the aberrant methylation in pancreatic cancer could be targeted therapeutically or used as a marker for early detection.90, 91
In addition to studies of DNA changes, a number of investigators have studied changes in gene expression in pancreatic cancer. The list of genes abnormally over-expressed in pancreatic cancer is large, and includes proteins such as mesothelin, trefoil factor 1, prostate stem cell antigen, claudin 4, and several of the S100-related proteins.92 These over-expressed genes are potentially clinically important for two reasons. First, some, such as mesothelin, are potentially targetable therapeutically.93, 94 Second, some, such as those that are secreted, could form the basis for a clinical test for the detection of pancreatic cancer.92 Of interest, Wang et al have suggested that one could integrate our understanding of the genes that are genetically altered with those that are expressed in pancreatic cancer to develop a test that detects mutant proteins shed by the cancers.95
MicroRNAs are small non-coding RNA molecules that regulate gene expression. As such, microRNAs can serve as master switches in cells, turning on and off the expression of a number of genes in concert. Several microRNAs are aberrantly expressed in pancreatic cancer, and, because microRNAs tend to be long-lived, these abnormally expressed microRNAs could serve as markers for pancreatic cancer.96–98
The molecular alterations that characterize invasive ductal adenocarcinoma can be used as tools to study other lesions in the pancreas. For example, as discussed earlier, PanINs are thought to be precursor lesions to infiltrating ductal adenocarcinomas. The genetic changes in PanINs have been extensively studied and, as one would expect in a bona fide precursor, PanINs harbor many of the same changes as have been identified in invasive cancers.65 KRAS and p16/CDKN2A mutations appear to occur early, in PanINs with low-grade dysplasia, while TP53 and SMAD4 mutations appear to be late events, occurring in PanINs with high-grade dysplasia and in invasive cancer. These findings not only help establish PanINs as noninvasive precursors to invasive pancreatic cancer, but they are also useful in designing early detection tests. For example, since KRAS mutations occur early and are present in most PanINs, they could be used as markers for the presence of a PanIN, but they would not provide information on histologic grade. By contrast, the presence of TP53 or SMAD4 gene mutations would suggest that a high-grade precursor or an invasive carcinoma is present.
The exomes (all known coding genes) of the four most common cystic neoplasms of the pancreas, IPMN, MCN, SPN and SCN, have also recently been sequenced, and each tumor type appears to have its’ own specific mutational profile (Table 4).80, 81 KRAS, TP53 and RNF43 mutations are found in the mucin-producing tumors (IPMN and MCN); GNAS mutations are relatively specific for IPMNs; VHL mutations are specific for SCN; and CTNNB1 (beta-catenin) alterations are specific for SPNs. The identification of cyst-type specific mutations is very exciting because it suggests that the genetic analysis of cyst fluid collected at the time of endoscopic ultrasound (EUS) could be used to classify cyst type.99 The challenge will be to identify markers of the degree of dysplasia.
The exomes of pancreatic neuroendocrine tumors (PanNETs) have also been sequenced and the results are dramatic.78, 100 In addition to MEN1, a known tumor suppressor gene which is inactivated in 45% of PanNETs, DAXX or ATRX are altered in 45%, and mTOR pathway genes are targeted in at least 15% of PanNETs. DAXX and ATRX mutations have been associated with “alternative lengthening of telomeres (ALT+),” establishing a new cancer pathway in PanNETs.100 The mTOR pathway gene mutations are particularly interesting because the drug everolimus targets the mTOR pathway and everolimus has been shown to be effective in some patients with PanNETs.101, 102 Although it still needs to be validated in clinical trials, it is reasonable to hypothesize that PanNETs with genetic mutations in an mTOR pathway gene may be particularly sensitive to everolimus, while patients with PanNETs that lack an mTOR pathway activation could be spared the side effects of a drug that may be less effective for them. This is an exciting potential opening of the door to individualized therapy in which the optimal therapy for a patient with a PanNET is based on the genetic changes in their tumor.
Thus, analyses of molecular alterations at the DNA, RNA and protein levels have identified alterations specific for the various tumors of the pancreas (Table 4). Some of these alterations are candidate early detection markers, and others have the potential to form the basis for the rational selection of patient specific therapies.
Signs and Symptoms
Unfortunately, most pancreatic cancers present non-specifically and are not diagnosed until late in the course of the disease, after the cancer has already spread to other organs. Common symptoms include pain, particularly epigastric pain that radiates to the back, unexplained weight loss, jaundice, clay-colored stools, nausea, and in ~10% migratory thrombophlebitis (Trousseau’s syndrome).54
As noted earlier in the section on risk factors, patients with pancreatic cancer sometimes present with new onset diabetes mellitus or with signs and symptoms of chronic pancreatitis. Of interest, depression is common in patients with pancreatic cancer, and in some instances the diagnosis of depression is established before the patient is found to have the cancer.103 This observation suggests that the cancers are producing a factor that induces the depression.
The radiologic diagnosis of pancreatic cancer has improved significantly with enhancements in the sensitivity of imaging technologies. Multi-detector computed tomography (MDCT) (Figures 3, 4A and 4B), magnetic resonance imaging (MRI), and endoscopic ultrasound (EUS) can all be used to visualize the tumors. CT and MRI have comparable sensitivities and specificities, and both have the advantage that they can also be used to stage the neoplasm, and as described below, three-dimensional reconstructions can provide detailed information on the relationship of the tumor to nearby vessels. The gold standard for establishing a diagnosis is pathology, and tissues can be sampled at the time of EUS. Positron emission tomography (PET) imaging also has a role in selected instances.104
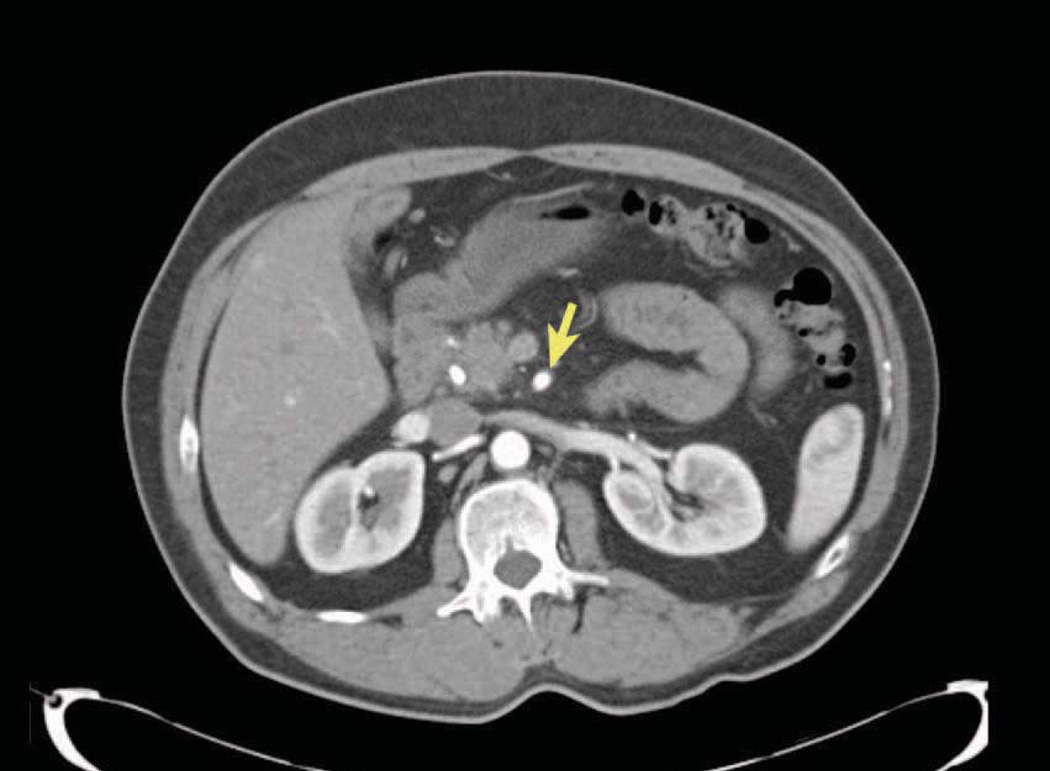
Figure 3.
CT of pancreatic cancer. A resectable pancreatic cancer with a well-defined fat plane around the artery (arrow).
Figure 4.
CT of pancreatic cancer. A) Abutment, defined as less than 180-degree involvement of the celiac axis (arrow), is considered Stage III borderline resectable. B) Greater than 180-degree involvement of the arteries (arrow) is defined as encasement and is considered Stage III locally advanced or unresectable.
Clinical Staging
Once a diagnosis of pancreatic cancer has been established, the next step is careful staging, as stage will determine treatment. The American Joint Committee on Cancer (AJCC) staging system, which includes the TNM classification, is the most widely used system to stage pancreatic cancer.105, 106 This system has undergone recent revisions to emphasize the importance of resectability of the cancer and it has been optimized to stratify survival by stage. Resectable stages include AJCC Stages I and II, and the subset of Stage III that is defined as borderline resectable. The unresectable categories include the subset of Stage III that is defined as locally advanced (unresectable), and Stage IV (metastatic). The staging workup focuses on defining the relationship of the tumor to large vessels and on identifying extra-regional metastatic disease and, when done properly, predicts the ability to perform a margin-negative resection.
The presence of clinically apparent extra-regional metastasis is classified as Stage IV and is a contraindication to an attempt at a curative resection. Frequent sites of extra-regional disease are the liver, peritoneum and lung, and these sites are not accurately assessed by physical exam. Palpable supraclavicular, periumbilical or pelvic nodes do occur, but are uncommon. Thus, the identification of extra-regional metastases relies heavily on imaging studies and the selective use of staging laparoscopy.
In the absence of metastatic disease, the relationship of the tumor to the adjacent major vascular structures defines resectability.107 These vessels include the superior mesenteric artery (SMA), the celiac axis, and the superior mesenteric and portal veins (SMV-PV). Patients who do not have vessel involvement or who have only focal involvement of the SMV-PV confluence are considered to be resectable, while patients whose cancers involve arteries or have more extensive involvement of the SMV-PV confluence are classified as clinical Stage III. As detailed below, the extent of involvement determines the further subclassification of Stage III into borderline resectable or locally advanced.107
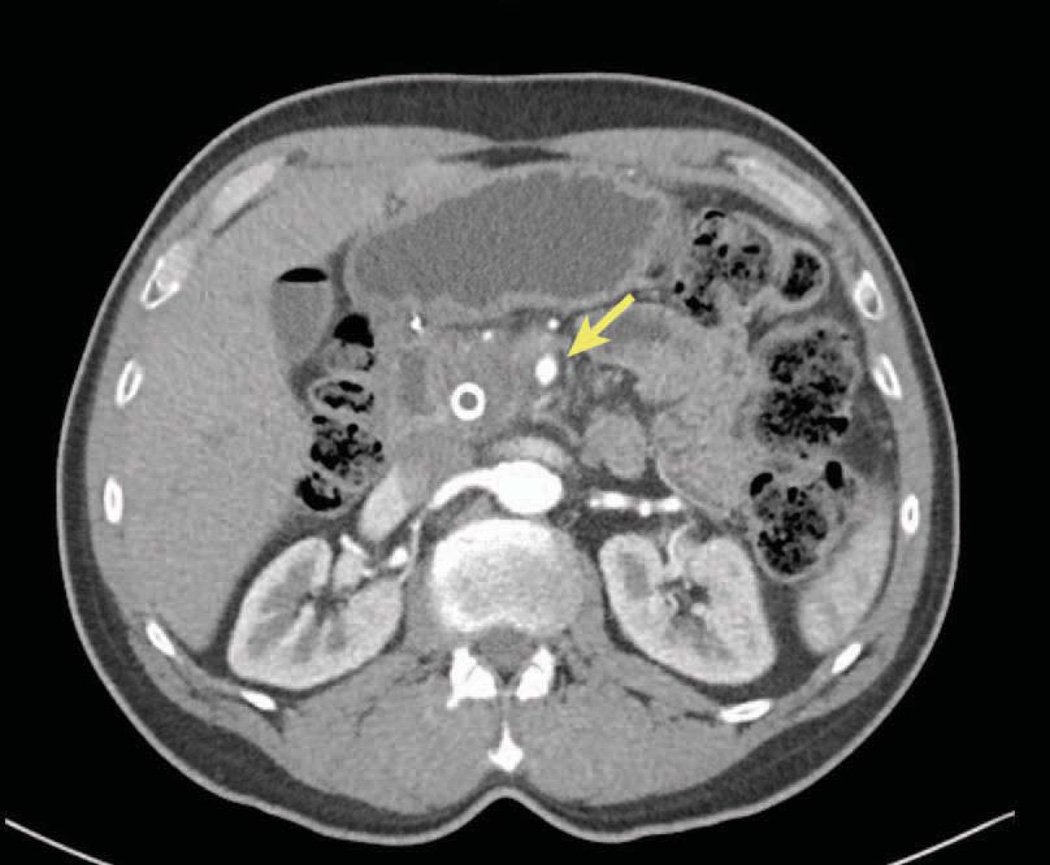
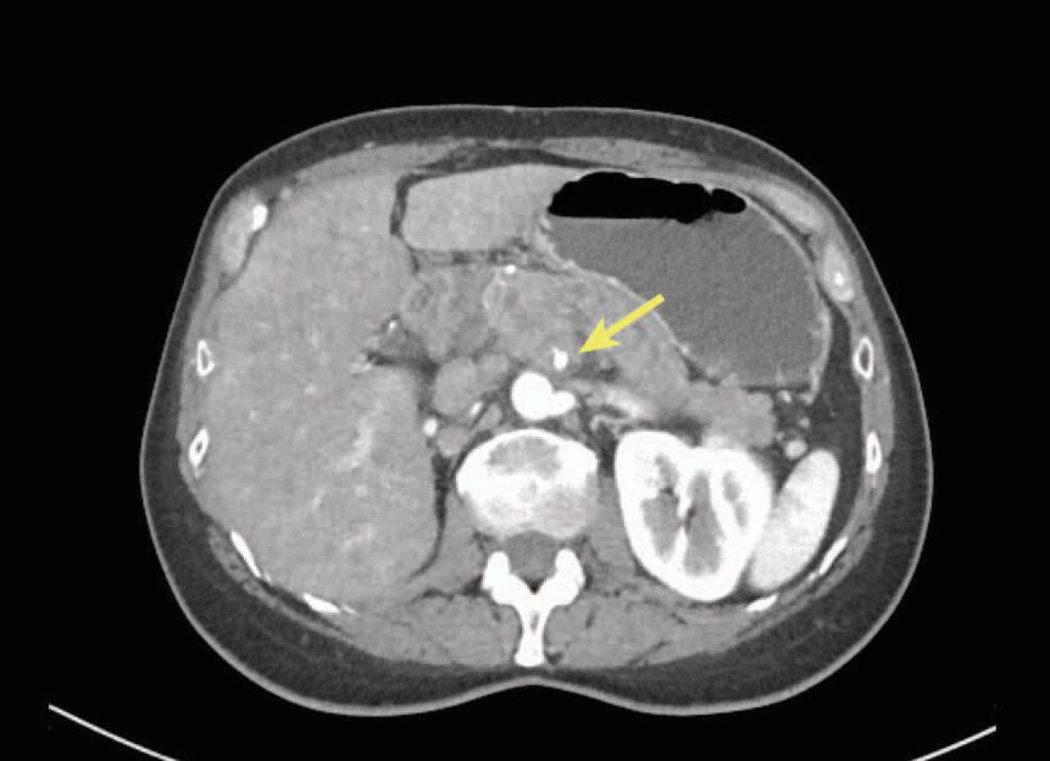
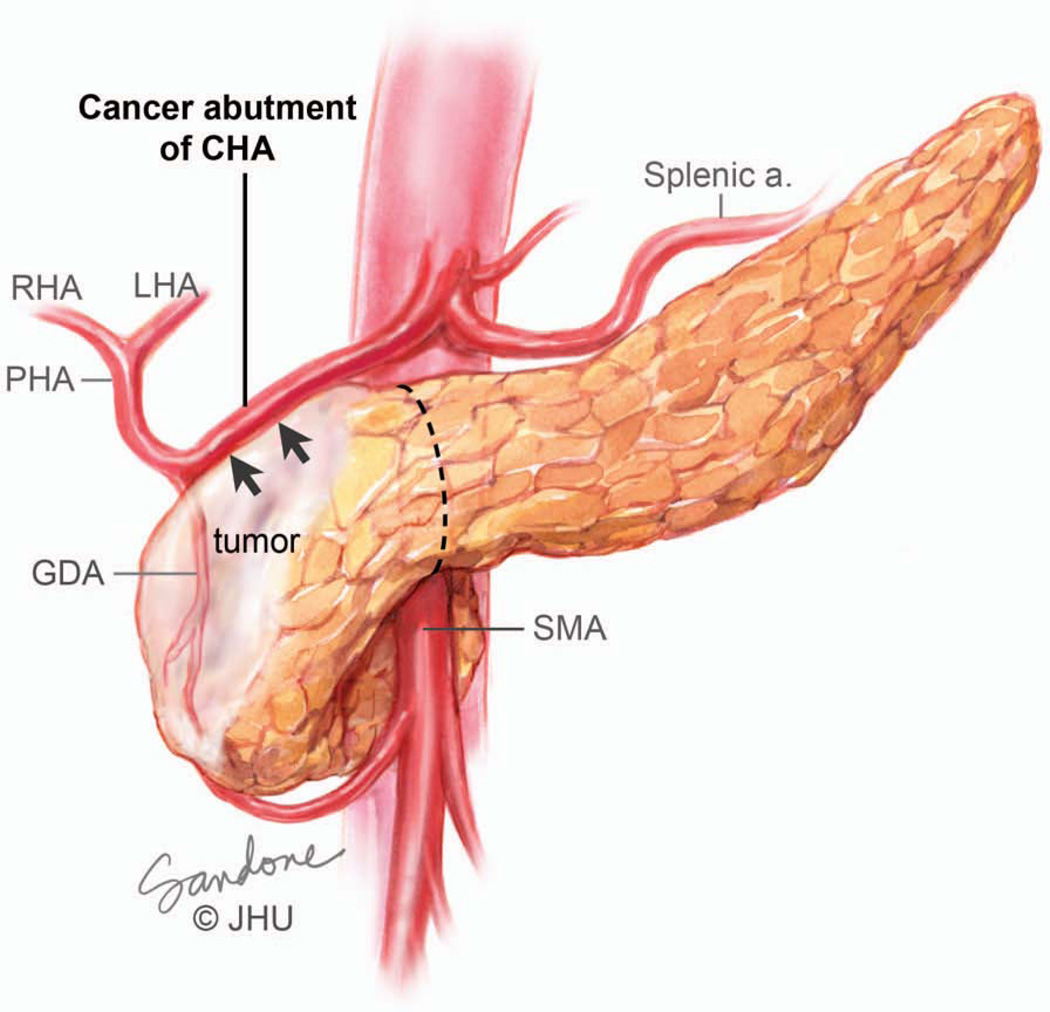
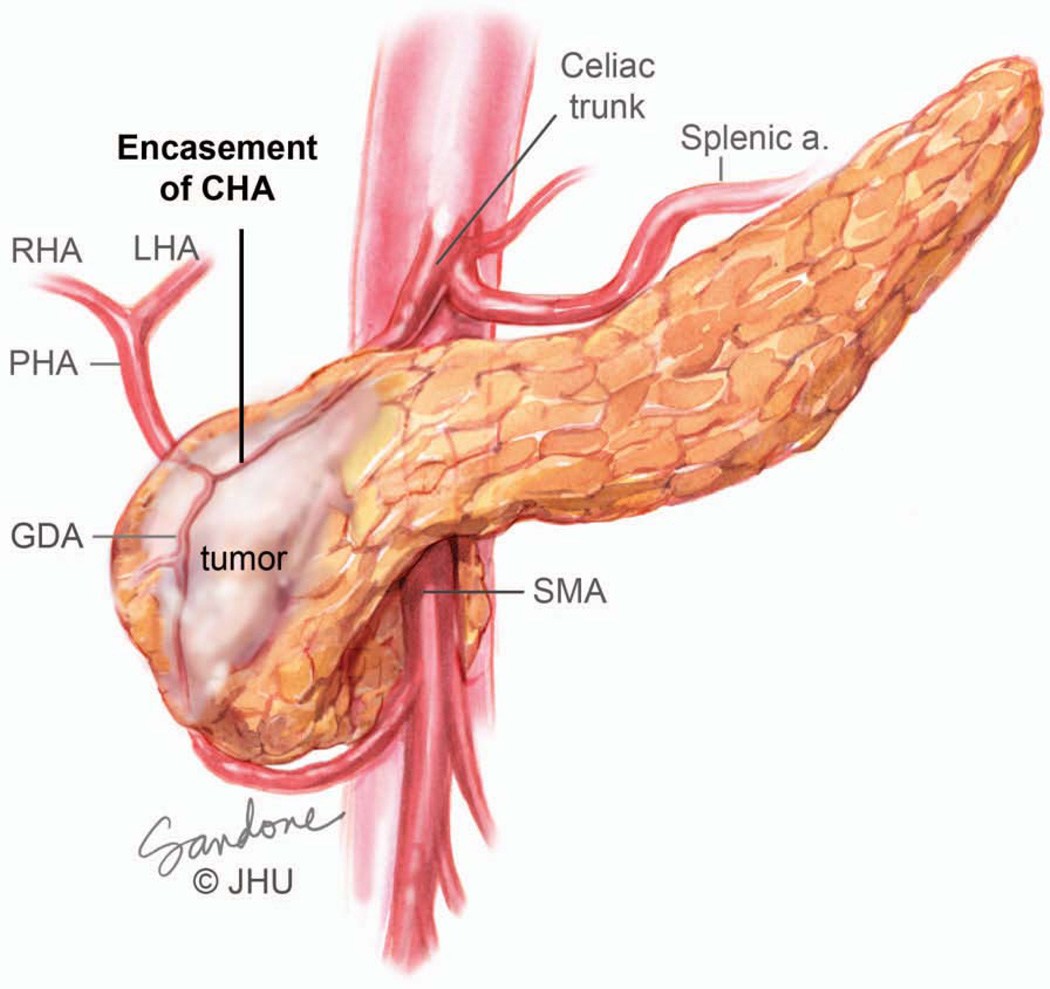
Abutment (defined as less than 180-degree involvement), of the celiac axis (celiac, common hepatic, replaced hepatic or proper hepatic arteries) or SMA is considered Stage III borderline resectable (Figures 4A and 5).106, 107 Although resection of Stage III borderline tumors is likely to be technically feasible, the probability of a positive margin is high and neoadjuvant therapy is recommended for this group of patients with the intent of sterilizing the margin of viable cancer cells. Greater than 180-degree involvement of any of these arteries is defined as encasement and is considered Stage III locally advanced or unresectable (Figure 4B and 6). Resection of these cancers is either not possible or would result in a R2 (where some visible tumor could not be removed) resection. Rarely, exceptions are made in which short-segment encasement of the common hepatic artery is resected en bloc and reconstructed or, as will be discussed in greater detail later, in which focal involvement of the celiac artery and proximal common hepatic artery is resected with an Appleby’s procedure. In these rare cases, patients are typically treated with neoadjuvant chemotherapy and radiation prior to resection.
Figure 5.
Abutment of an artery, in this case the common hepatic artery, by tumor. Note that the tumor extends to the border of the artery but does not invade or narrow the vessel. On CT scan imaging this is seen as less than 180 degree involvement of the vessel and is considered to be borderline resectable. At operation this degree of involvement would allow the artery to be separated from the tumor, although it would likely leave microscopic cancer cells at the margin. Borderline resectable patients should undergo neoadjuvant therapy. Illustration by Corinne Sandone. © Johns Hopkins University; used with permission.
Figure 6.
Encasement of an artery, in this case the common hepatic artery, by tumor. Note that the tumor surrounds and narrows the artery. On CT scan imaging this is seen as greater than 180 degree involvement of the vessel and is considered to be locally advanced unresectable. At operation this degree of involvement would not allow separation of the tumor from the artery and would likely result in an R2 resection. Illustration by Corinne Sandone. © Johns Hopkins University; used with permission.
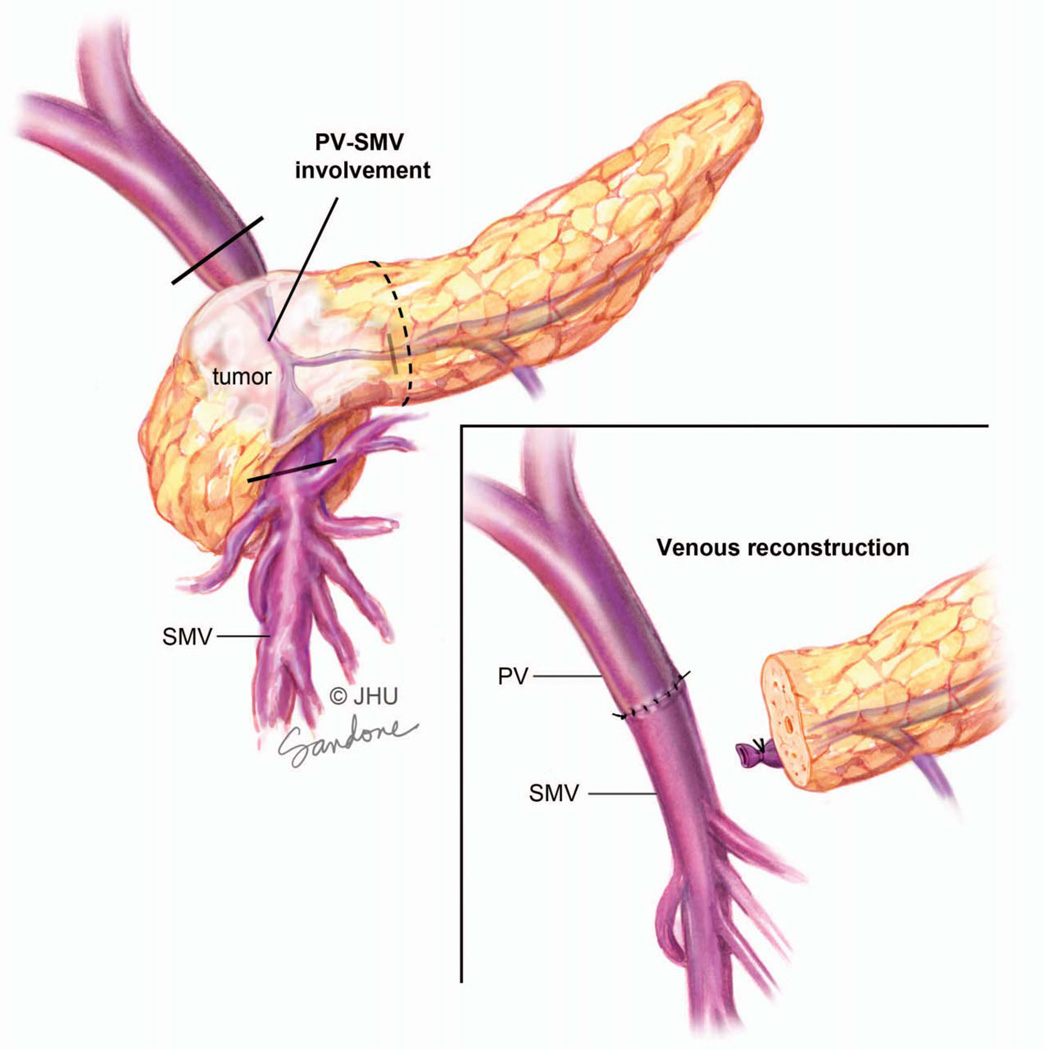
In contrast to the arterial side, resectability on the venous side depends on the prospect of resecting and reconstructing the vessel.108 Tumors that focally involve the SMV-PV confluence can be reconstructed since the PV and SMV can be reanastomosed. As a result these tumors are considered to be resectable. In contrast, tumors that involve a large segment of the portal vein high in the porta hepatis or inferior on the superior mesenteric vein in the area of confluence of multiple tributaries are not technically reconstructable and are considered to be Stage III locally advanced (Figure 7).
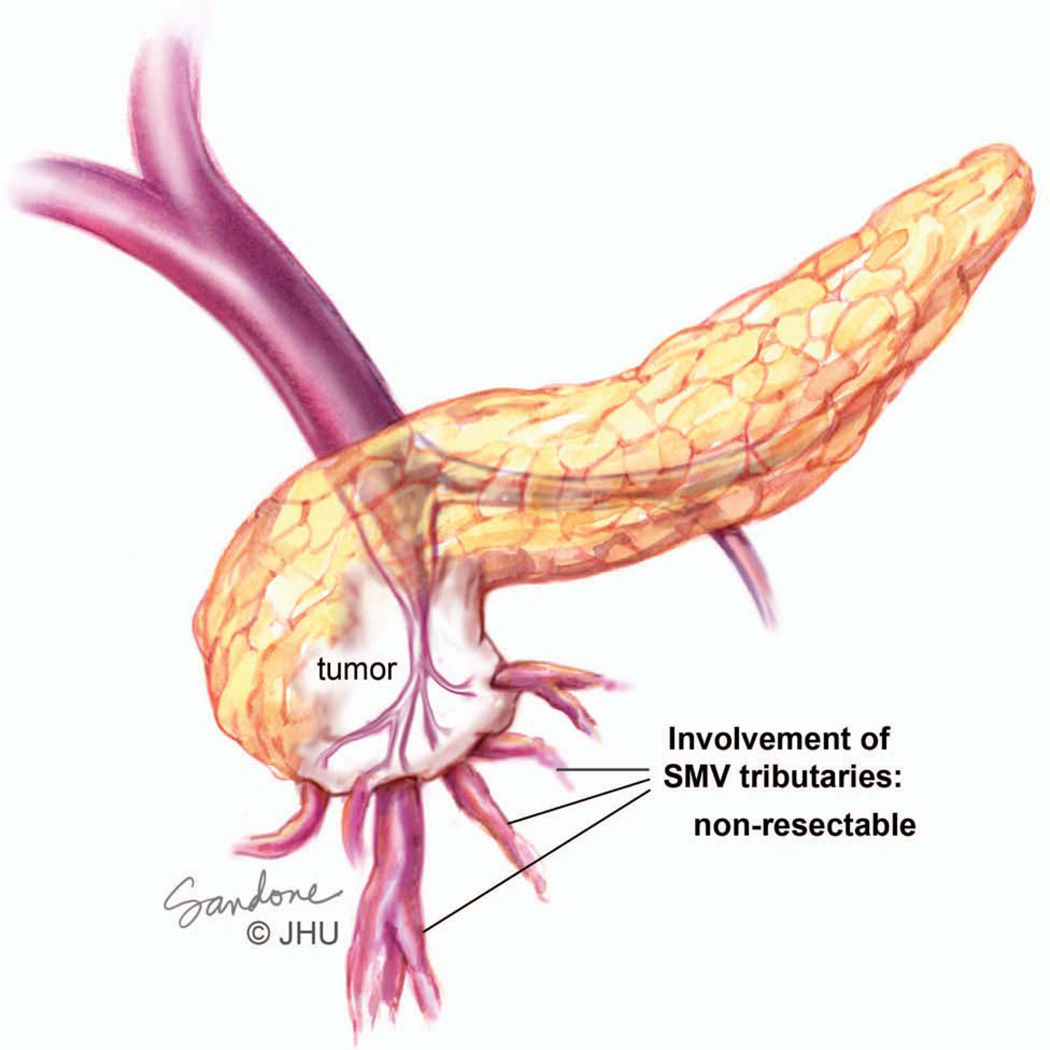
Figure 7.
Tumor involving the superior mesenteric vein (SMV), including numerous small tributaries of the vein. Since there is no single target vessel below the necessary region of resection this is considered unresectable. Illustration by Corinne Sandone. © Johns Hopkins University; used with permission.
A contrast enhanced thin slice CT scan of the chest, abdomen and pelvis is the primary modality for the clinical staging of patients with pancreatic cancer and can accurately identify metastatic disease and local tumor relationships.109, 110 Using this technology, the ability to predict a margin-negative resection (R0) is on the order of 73%. 110 Three important features should be noted on CT. The absence of vessel involvement is defined by the presence of a fat-plane separating the tumor from the vessels (Figure 3). Abutment of a vessel is defined by loss of the fat-plane with less than 180 degrees of involvement (Figures 4A and 5), while encasement is defined as greater than 180 degrees (Figures 4B and 6).
Patients who undergo R2 resection have a median survival less than 12 months which is no better than patients with locally advanced disease who do not have their tumors resected.111, 112 Patients with vascular encasement by CT should therefore not undergo surgical exploration unless they respond to neoadjuvant therapy.
Treatment
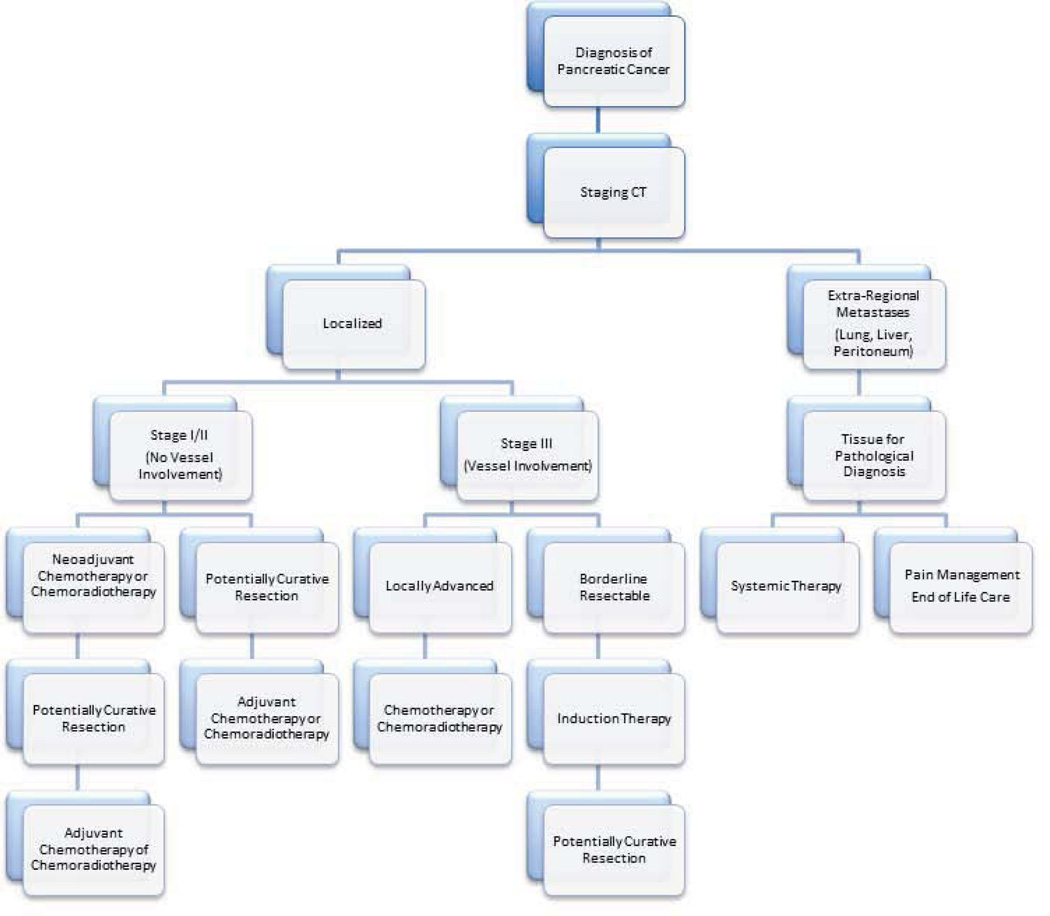
Pancreatic cancer is a complex disease, and patients with pancreatic cancer are best treated by a multi-disciplinary team (see, for example, http://pathology.jhu.edu/pc).113 As outlined in Figure 8 the optimal treatment first and foremost depends on careful accurate staging. Patients with Stage I/II disease should undergo surgical resection followed by adjuvant therapy. Neoadjuvant therapy should be considered in this patient population but is controversial, while patients with Stage III borderline resectable cancers should undergo neoadjuvant therapy prior to resection.114 Patients with stage III locally advanced disease should be treated with chemotherapy and/or chemoradiotherapy. A vast majority of these patients eventually develops metastatic disease, however select patients can still be considered for surgical resection. Patients with Stage IV and good performance status may receive systemic therapy and those with poor overall health should be given supportive therapy.115
Figure 8.
Flow chart diagraming a general approach to the treatment of pancreatic cancer. © Johns Hopkins University; used with permission.
Localized Disease: Stage I/II and Stage III Borderline Resectable
Although the best chance of long-term survival for patients with localized pancreatic cancer is through complete resection of the primary lesion, the systemic nature of pancreatic cancer at diagnosis, the impact of pancreatectomy on quality of life and the relatively low chance of long-term survival must all be taken into account when selecting patients who will most benefit from surgery. Since most patients have locally invasive and micrometastatic disease at the time of surgery, the risk of both local and systemic recurrence following a potentially curative operation is high. The operations necessary to resect pancreatic cancer are associated with significant morbidity in 40–60% of patients and mortality in the 2–3% range.111, 116 Moreover, complete recovery to a normal quality of life can take 2–3 months even in the absence of complications. In addition, long-term survival is uncommon. The median survival reported for resected pancreatic cancer ranges from 17–27 months and the 5-year survival is approximately 20%.116 Taken together, these points underscore the importance of the multidisciplinary approach in the care of patients with pancreatic cancer regardless of stage.113 The purpose of this section is to provide a surgical perspective on the management of pancreatic cancer with an emphasis on patient selection and recent advances in pancreas surgery.
Patient Selection for surgery
Several factors, in addition to stage, need to be considered in the selection of patients who will benefit from pancreatectomy.117 These include the patient’s overall health, their tumor biology and the use of neoadjuvant therapy. Often these three factors impact upon each other and should be considered collectively in formulating a management plan. It cannot be emphasized enough that this is best achieved through patient evaluation by a multidisciplinary team.113
Significant comorbidities can influence the ability of a patient to tolerate a major pancreatectomy.117 Typical comorbidities in patients presenting with pancreatic cancer include cardiac disease, chronic obstructive pulmonary disease (COPD) and dementia. Moreover, cachexia and malnutrition that result from the cancer itself are common and can be further debilitating. Patients with poor overall health as a result of comorbid conditions and advanced age (>75 years) are not likely to benefit from pancreatectomy and may be harmed by the further debilitation and immunosuppression brought on with these operations.117 A classification system proposed by Katz takes this into account and classifies patients with poor overall health as “medically borderline” regardless of tumor-vessel relationships.118
Another consideration in selecting patients for resection is “tumor biology.” This is a term used to describe the relative propensity of a patient’s tumor toward metastatic spread or locally aggressive behavior. Since no validated biomarkers are currently available to predict clinical behavior, the assessment of tumor biology is subjective. Indicators of aggressive biology include extensive metastatic disease at diagnosis, locally infiltrative tumors and rapid progression over time. Unvalidated markers include serum CA19-9 levels and tumor SMAD4 gene status.85, 86, 119 Patients with significantly elevated CA19-9 levels often do worse than those with low levels, and patients with tumors that have a wild-type SMAD4 gene have been reported to have a lower propensity for wide-spread metastases than do those with loss of SMAD4.85 In general, patients with aggressive tumor biology are unlikely to benefit from local therapy such as surgical resection even if they have an early stage tumor. Patients with localized but suspected biologically aggressive tumors may benefit from neoadjuvant therapy to control micrometastatic disease or at the very least allow assessment of biological behavior over time.
Neoadjuvant Therapy for Localized Disease
Neoadjuvant therapy remains controversial in the treatment of pancreatic cancer. It does have the advantage of down-staging some locally advanced patients and sterilizing the margin of borderline resectable patients allowing them to undergo a surgery with a higher likelihood of an R0 resection.114, 120 In fact, 15–40% of patients with initially borderline or unresectable tumors can eventually undergo surgery. Neoadjuvant therapy also has the advantage that it will spare the 15–35% of patients who develop metastatic disease the risks and stress of a major operation; as metastases develop they would no longer be considered for surgery.118, 121, 122 Neoadjuvant therapy also guarantees that almost all patients will receive some form of chemotherapy or radiation because they do not have any post-operative complications and recovery to overcome prior to starting therapy. Studies show that 73–100% of patients are able to complete the majority of their neoadjuvant regimens.114, 120, 123–126 If radiation is given as part of the neoadjuvant regimen, it can be delivered to a smaller volume (tumor plus 1–2 cm) thus allowing for escalation of radiation dose to the tumor and resulting in less toxicity by decreasing the radiation exposure of adjacent normal organs.
Although neoadjuvant therapy is attractive for the reasons described above, surgery is required for a cure and neoadjuvant therapy delays patients’ potentially curative surgery. Simply put, there are no randomized trials favoring neoadjuvant over adjuvant therapy. Therefore, at this time resectable patients are usually taken to surgery immediately and subsequently given adjuvant therapy, and neoadjuvant therapy is usually reserved for borderline resectable patients. The decision of whether to use neoadjuvant therapy followed by surgery, or surgery first followed by adjuvant therapy should be made in a multidisciplinary manner to achieve the timeliest and most coordinated treatments.113
There are no clear protocols established for neoadjuvant therapy, however most centers use similar regimens as for locally advanced/unresectable disease. The combination of 5-flurouracil (5-FU), leucovorin, irinotecan and oxaliplatin (a regimen referred to as FOLFIRINOX), the combination of gemcitabine, docetaxel and capecitabine (a regimen referred to as GTX), and gemcitabine alone are possible chemotherapy regimens that are typically followed by continuous infusion 5-FU, capecitabine, or gemcitabine-based chemoradiation to 45–54 Gy in 1.8–2.5 Gy fractions or 36 Gy in 2.4 Gy fractions.115, 120, 121, 126–128 Surgery should be performed within 6–8 weeks following completion of neoadjuvant therapy. Further delays lead to increased radiation-induced fibrosis, more challenging operations, longer operating times, and sometimes increased length of stay. An additional 2–3 cycles of adjuvant chemotherapy should be considered based on multidisciplinary evaluation of the tumor pathology.129, 130
Surgery for Localized disease
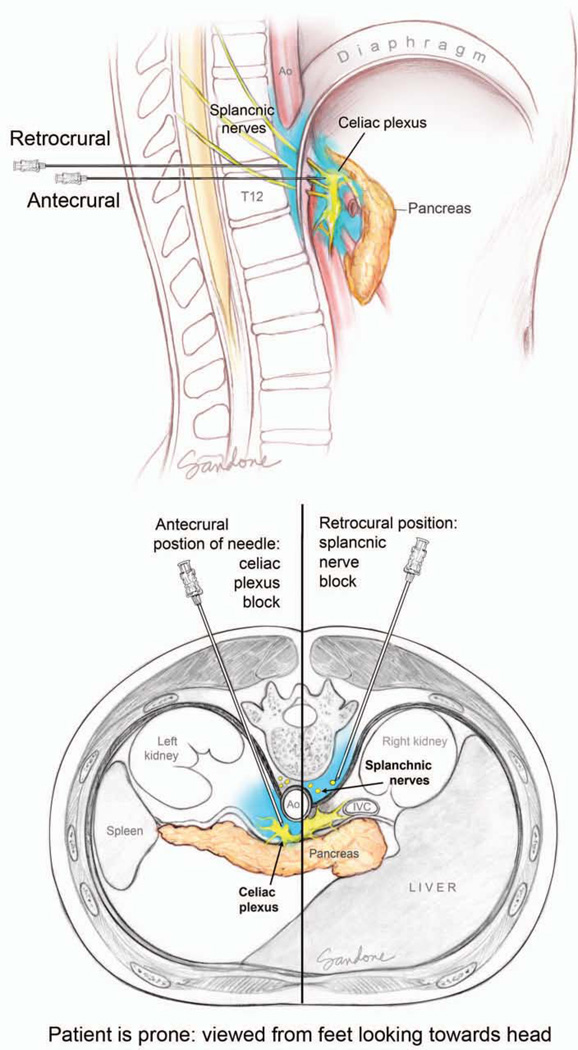
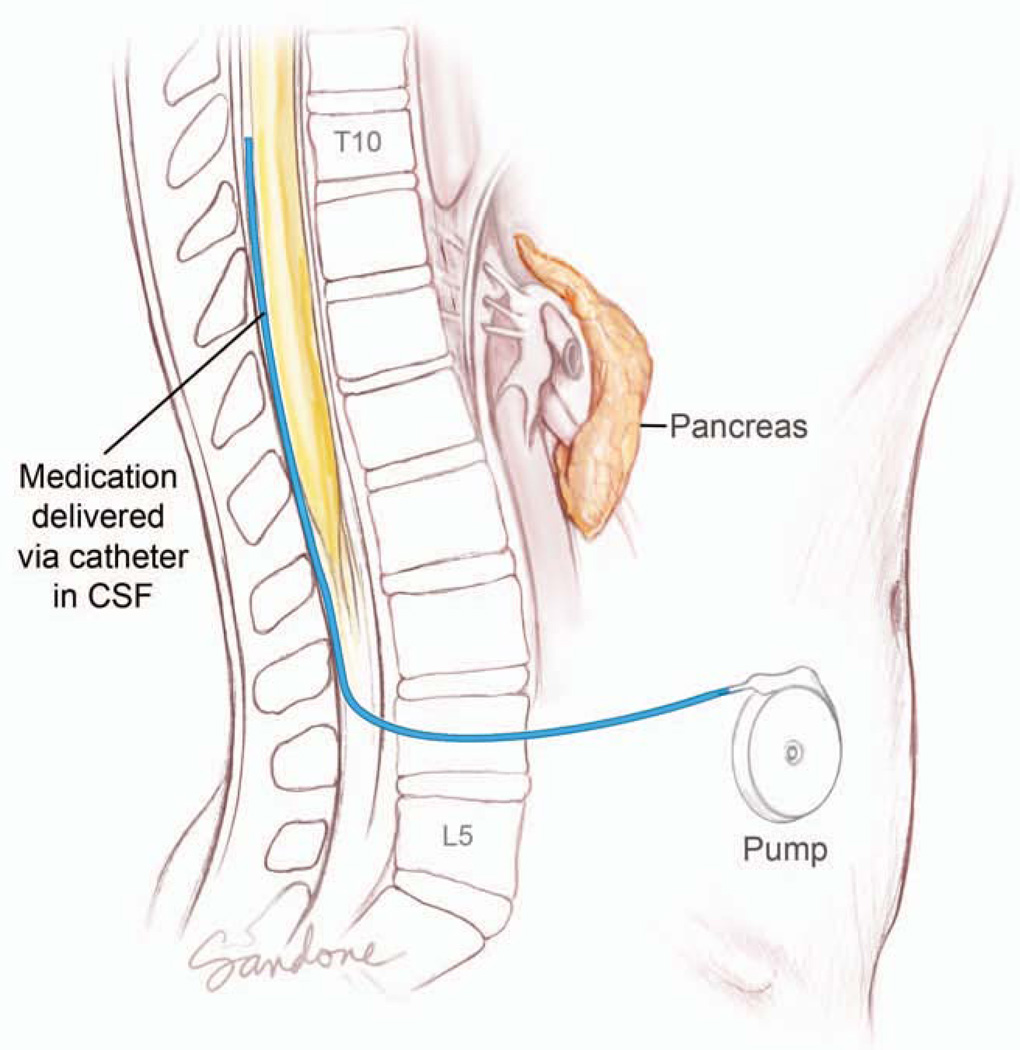
The required operation for a given patient depends on the location of their tumor. Cancers arising in the head of the pancreas require a pancreaticoduodenectomy (Whipple operation), while those in the tail require a distal pancreatectomy with an en bloc splenectomy.131 Lesions located in the neck and body may require a pancreaticoduodenectomy, distal pancreatectomy or, rarely, a total pancreatectomy.111, 132 Other partial resections, such as central pancreatectomy or enucleations do not result in an adequate lymphadenectomy and are not considered to be a potentially curative resection for pancreatic cancer. With the emergence of nonoperative biliary decompression, endoscopically directed therapies, such as duodenal wall stents and nonoperative celiac plexus blocks, the need for elective surgical palliation has dramatically decreased.
Pancreaticoduodenectomy
The pancreaticoduodectomy has three broad phases: 1) exploration to assess for occult extra-regional spread not identified on preoperative imaging; 2) resection of the tumor (Figure 9), and 3) reconstruction of the pancreaticobiliary and intestinal tracts (Figures 10A and 10B).
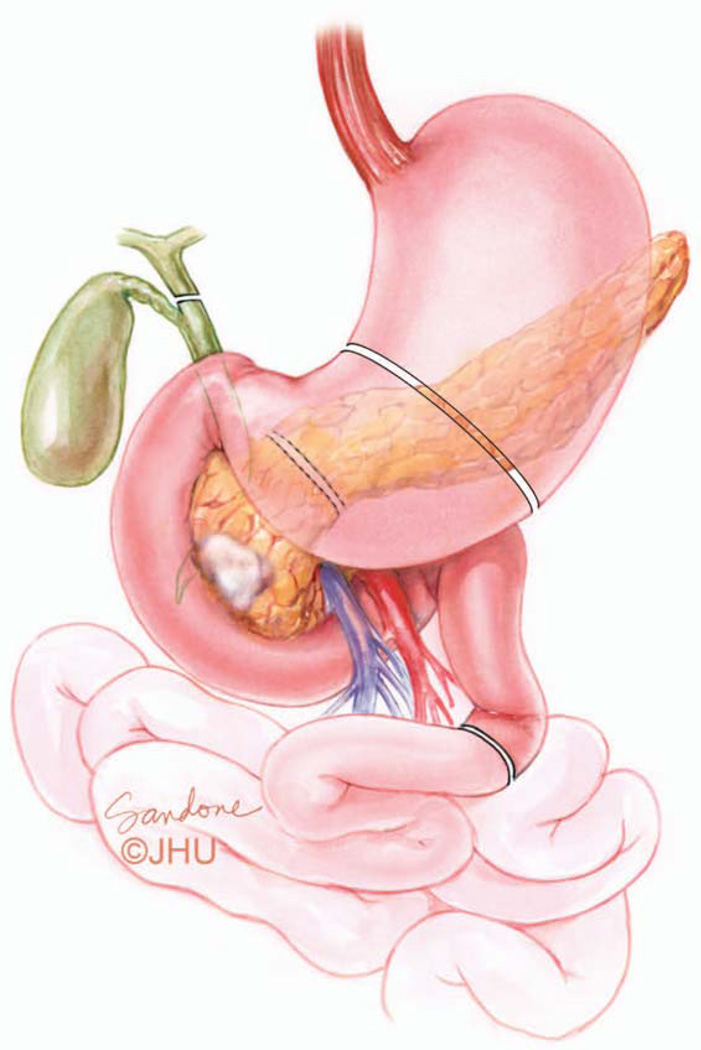
Figure 9.
The organs resected in a pancreatoduodenectomy (PD) include a portion of the stomach, the entire duodenum, the proximal 20–30 cm of jejunum, the common bile duct, gall bladder, the head and uncinate of the pancreas along with the associated regional lymph nodes. In the pylorus preserving version of the PD the duodenum is divided just beyond the first portion and the stomach and pylorus are preserved. Illustration by Corinne Sandone. © Johns Hopkins University; used with permission.
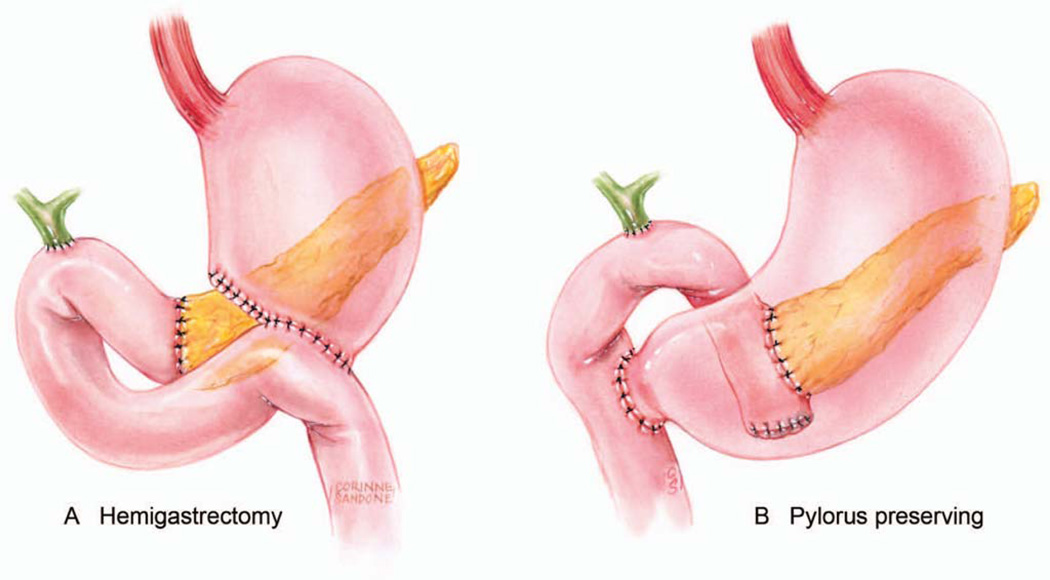
Figure 10.
Illustrations of standard (A) and pylorus-preserving (B) pancreatoduodenectomies (PD). Three anastomoses are necessary to reestablish gastrointestinal function. In the typical reconstruction shown here the end of the pancreas and end of the common hepatic duct are connected to the same limb of jejunum. Downstream from this the stomach is connected to the jejunum in a standard PD (A) or for a pylorus preserving version the duodenum is connected to the jejunum (B). Illustration by Corinne Sandone. © Johns Hopkins University; used with permission.
The operation begins with a careful inspection of the peritoneal surface for occult cancer implants. The liver is inspected both visually for surface lesions and by palpation for deeper lesions. Intraoperative ultrasonography may be used to assess the liver parenchyma. Patients at high risk for peritoneal dissemination based on CT findings or significantly elevated serum CA19-9 should undergo these initial steps laparoscopically.133–135 The second phase is the resection of the head of the pancreas, duodenum, distal common bile duct and gallbladder (Figure 9). In a standard pancreaticoduodenectomy the stomach is divided proximal to the antrum, and in a pylorus preserving pancreaticoduodenectomy the first portion the duodenum is transected distal to the pylorus (Figure 9). In tumors that involve the SMV-PV, the SMV-PV can be divided and resected en bloc with the specimen. The PV and SMV are then reconstructed (Figure 11).
Figure 11.
Focal tumor involvement of the portal vein-superior mesenteric vein confluence. Note that the tumor invades the vessel in a location that does not involve numerous small tributaries of the superior mesenteric vein (SMV) nor does it extend high on the portal vein. Since there is a single target vessel (dark lines) above and below the necessary region of resection this is considered to be resectable. The inset shows a primary anastomosis of the portal vein (PV) and SMV after en bloc resection of the vessel with the splenic vein tied off. Illustration by Corinne Sandone. © Johns Hopkins University; used with permission.
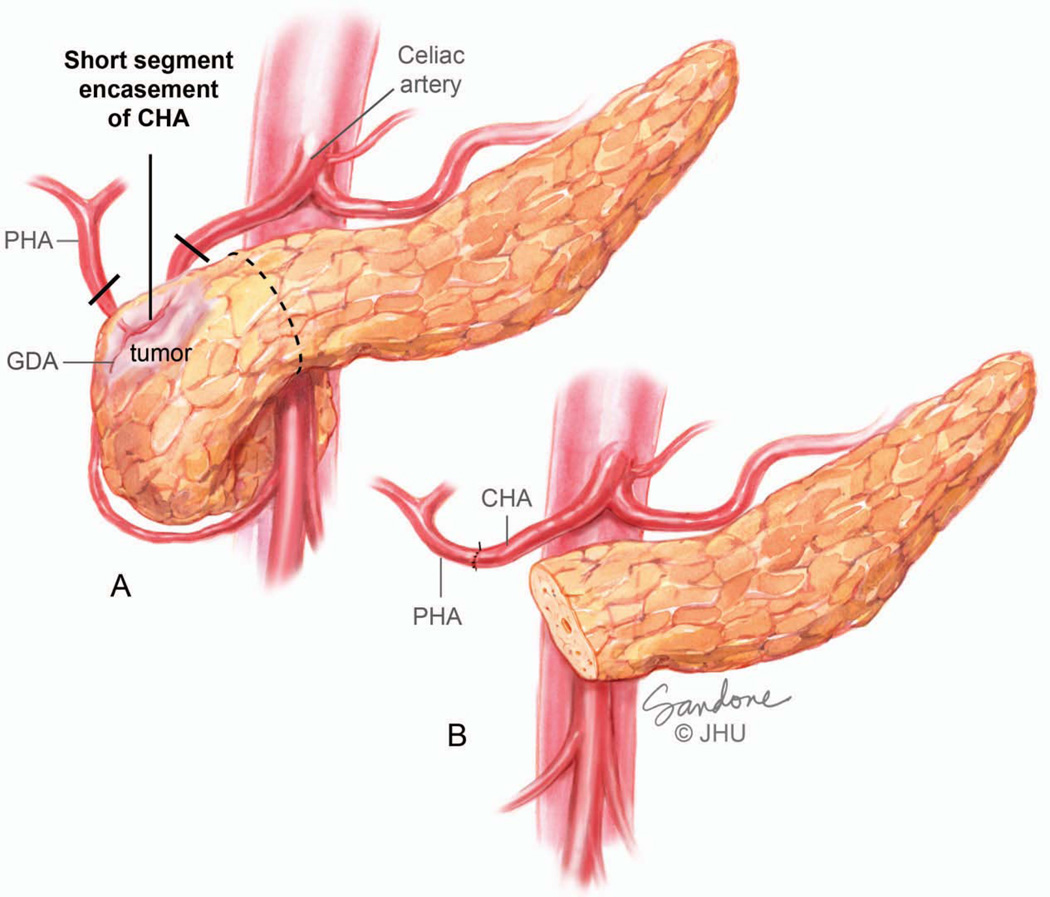
Optimal patient selection by a multidisciplinary team has made even more extensive resections possible in appropriate patients. For example, if the cancer involves the common hepatic artery in the region of the gastroduodenal artery (GDA), the cancer can be resected by removing the involved segment of artery en bloc with the specimen and performing an end-to-end reconnection of common and proper hepatic arteries (Figure 12).
Figure 12.
(A) Short-Segment encasement of the common hepatic artery. (B) Although arterial encasement is most often considered to be locally advanced, short segment encasement of the common hepatic artery is amenable to resection and primary anastomosis. Illustration by Corinne Sandone. © Johns Hopkins University; used with permission.
In the third phase of the pancreaticoduodectomy, the enteric, biliary and pancreatic continuity are re-established with three anastomoses; pancreaticojejunostomy, hepaticojejunostomy and gastrojejunostomy for a standard pancreaticoduodenectomy (Figure 10A) or pancreaticojejunostomy, hepaticojejunostomy and duodenojejunostomy for the pylorus preserving version (Figure 10B).
Distal Pancreatectomy
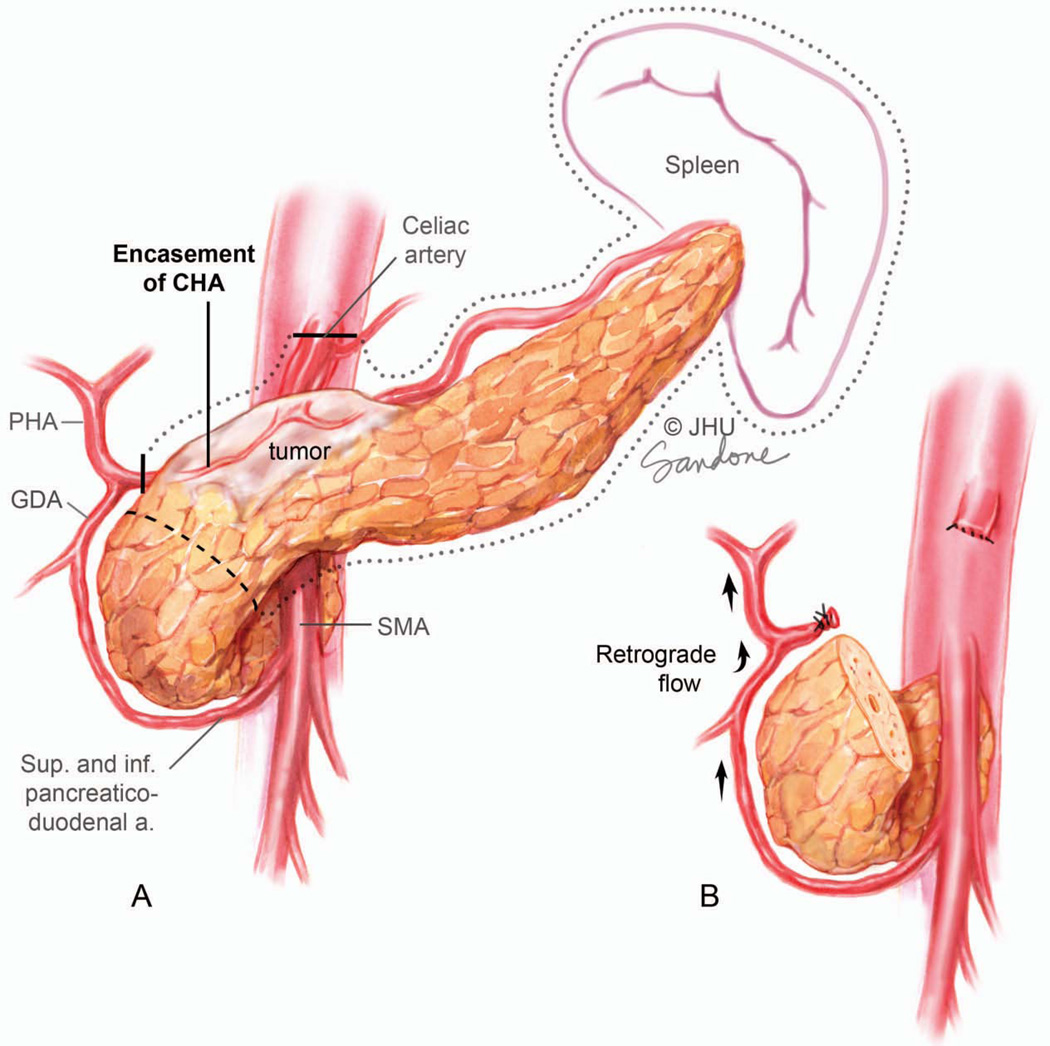
In a distal pancreatectomy the distal pancreas and spleen are removed en bloc. Cancer of the body of the pancreas can be the most difficult lesion to manage surgically and is usually resected through a distal pancreatectomy. By virtue of this location, extension of the tumor superiorly beyond the pancreas often results in involvement of the celiac trunk, common hepatic artery and base of the splenic artery at its take-off from the celiac trunk (Figure 13A). Growth slightly to the right and posteriorly will involve the medial wall of the PV or SMV and may also infiltrate the junction of the splenic vein with the PV-SMV confluence. In these patients, considerable complexity is added to a distal pancreatectomy. The determination of resectability in these patients is based on the extent of involvement of the celiac axis.
Figure 13.
Tumor encasing the celiac artery, proximal hepatic artery and splenic artery. Although arterial encasement is most often considered to be locally advanced unresectable this configuration of vessel involvement allows en bloc resection of the proximal hepatic, celiac and splenic arteries (dark line marks transection margin of artery) in combination with a distal pancreatectomy. This is called the Appleby procedure. (B) The gastroduodenal artery (GDA) must be preserved since perfusion of the liver is by retrograde flow through this vessel to the common hepatic artery. Illustration by Corinne Sandone. © Johns Hopkins University; used with permission.
In a variation of the standard distal pancreatectomy, cancers involving the celiac artery and proximal common hepatic artery can be resected using an Appleby procedure (Figure 13).136 Patients have to be carefully selected for this operation which involves the en bloc resection the body and tail of pancreas along with the celiac artery, proximal common hepatic artery and splenic artery. Hepatic arterial blood supply is dependent on retrograde flow from the GDA to the proper hepatic artery. This operation also requires a total gastrectomy since the blood supply to the stomach is compromised.
Minimally Invasive Surgery
Minimally invasive surgery is used extensively in general surgery for a variety of procedures including anti-reflux procedures, bariatric surgery, hernia repairs, colectomies and cholecystectomy. The benefits of minimally invasive approaches in these instances include less scarring, less post-operative pain, less wound complications and an earlier return to normal activity.
As a result of the complexity of most pancreatectomies, minimally invasive approaches to pancreas surgery have lagged behind other areas of general surgery in terms of wide-spread acceptance, however, in recent years minimally invasive approaches have been applied to pancreatic resections and most pancreatic cancer operations can now been performed laparoscopically or robotically.137–141 Access to the abdomen is gained through small ports placed through the abdominal wall, and early published series have shown that minimally invasive pancreatic operations can be performed safely and with similar outcomes to the standard approach.139, 140 Important oncological parameters such as margin status and lymph node counts are also comparable to standard pancreatectomy.138, 140 The benefits of minimally invasive pancreatectomy are, however, less clear than they are for other general surgical procedures. Wound complications such as surgical site infections do appear to favor minimally invasive pancreatectomy, but length of stay has not been reduced significantly.140 Moreover, in contrast to minimally invasive colectomy for colon cancer, no high quality studies exist that compare long-term disease specific outcomes between minimally invasive and open pancreatectomy. In theory, the reduction of wound complications and reduced impact on immune function seen with minimally invasive surgery does have the potential to improve disease specific survival. In particular, fewer wound complications may increase the number of patients who are able to undergo adjuvant therapy. With the ever increasing use of minimally invasive pancreatectomy the importance of this approach in the management of patients with pancreatic cancer will become clear.
Surgical Complications
Through the 1970s, the mortality rate associated with a pancreaticoduodenectomy was as high as 30%. This has been reduced to less than 2% over the subsequent three decades.116 Results have been shown to be significantly better at high-volume centers.142 However, the morbidity rate associated with the pancreaticoduodenectomy has remained between 30% and 45%, even at high volume centers.142 The most common post-operative morbid complication is delayed gastric emptying, occurring in 15% of patients, followed by wound infection (8%), pancreatic fistula (5%), cardiac morbidity (4%), abdominal abscess (4%), cholangitis (2%), sepsis (2%), bile leak (2%) and several other complications occurring in less than 2% of patients.116
Outcomes of Surgery
In one of the largest series reported of pancreaticoduodenectomies for pancreatic adenocarcinoma, the median survival was 18 months and the 5-year survival was 18%.116 Factors that negatively affected survival included tumor size of greater than 3 cm (hazard ratio [HR] 1.6; p < .001), positive resection margin (HR 1.6; p < .001), histological grade (HR 1.6; p < .001), and regional lymph node metastases (HR 1.3;p = .05).
Adjuvant Therapy
While surgery offers the only chance of cure, the majority of patients who undergo resection will still recur locally near the superior mesenteric artery margin or distantly (liver, lung, peritoneum). Adjuvant therapy is therefore indicated to decrease the risk of loco-regional and metastatic recurrence (Table 5). Adjuvant therapy is typically started 1–2 months after surgery to allow the patient to recover from the side effects or complications associated with surgery. Although no regimen has been proven substantially more effective than others, six months of adjuvant therapy with a 5-FU-based or gemcitabine-based chemotherapy is an appropriate standard option. The integration of approximately 6 weeks of 5-FU or gemcitabine based chemoradiation (CRT) (45Gy directed to the tumor bed, surgical anastomoses (pancreaticojejunostomy), and peri-pancreatic nodes with an additional 5–15 Gy boost to the tumor bed) during this six months period is an option and may be more favored for R1 (microscopically positive) resections and when the risk of loco-regional recurrence is higher (the debate surrounding radiation therapy for pancreatic cancer will be discussed in detail later in this review).143 The appropriate sequencing of chemoradiation therapy in the adjuvant setting is unclear. As over 70% of patients will recur with distant disease, systemic chemotherapy is usually given first to be followed by CRT if there are no radiographic or clinical concerns for metastatic recurrence after chemotherapy is completed.
Table 5.
Selected Key Adjuvant Studies
| Study | Treatment | 1 year survival (%) |
2 year survival (%) |
5 year survival (%) |
DFS (mo) |
Median Survival (mo) |
|---|---|---|---|---|---|---|
| GITSG (1985)232 N=43 |
Observe v. chemorad |
49 v. 63 |
15 v. 42 | 14 v. 4 | < 9 v. 16 |
11 v. 20 (p=.01) |
| EORTC 40891 (1999) N=218147 |
Observe v. Chemorad | 53 v. 65 | 23 v. 37 | 22 v. 25 | NR | 12.6 v. 17.1 (pancreas only, p=.099) |
| JHH retrospective N=452233 |
Observe v. Chemorad |
NR | 32 v 44 | 15.4 v. 20.4 | NR | 14.4 v. 21.2 P<.001 |
| ESPAC-1 (2004) N=289148 |
2 X 2 design Observe v. Chemo |
? v. 67 | ? v 38 | 11.v. 29 | chemoXRT-10.7 no chemoXRT 15.2 chemo 15.3 no chemo-9.4 |
16.9 v. 21.6 |
| CONKO-1 (2007) N=354144 |
Observe v. Chemo |
72 v 72 | 42 v. 47 | 11 v. 22 | 6.9 v. 13.4 (P<.001) | 20 v. 22 (p=.06) |
| RTOG-9704 (2008) N=538153, 234 |
Gem-5FU/XRT v. 5FU, 5FU/XRT |
69 v. 65 | 35 v. 39 | 20 v. 20 | NR | 20.6 v. 16.9 (P=.03) |
| ESPAC-3 (2009) N=1030145 |
Gem v 5FU |
70 v. 70 | 40 v. 40 | NR | 14.3 v. 14.1 | 23.6 v. 23 |
| JHH and Mayo Clinic Retrospective (2010) N=1272152 |
Observation v. chemorads | 58. v 80 | 34.6 v. 44.7 | 16.1 v. 22.3 | 15.5 v. 21.1 (P<.001) | |
| ACOSOG (2011) N=89154 |
5FU CI, IFN/cisplatin +XRT |
80 | 60 | NR | 14.1 | 25.4 |
| CapRI (2012) N=110156 |
5-FU/cis/XRT + IFN followed by 5FU v. 5FU | 85 v. 80 | 60 v. 55 | 25 v. 25 | 15.2 v. 11.5 (p=.61) | 32. 1 v. 28.5 (P=.49) |
ACOSOG=American College of Surgeons Oncology Group; CaPRI=Combined Chemoradioimmunotherapy for Pancreatic Adenocarcinoma; CI=continuous infusion; CONKO=CharitéOnkologie; DFS= disease free survival; EORTC=European Organization for Research and Treatment of Cancer; ESPAC=European Study group for Pancreatic Cancer; 5-FU=5-fluorouracil; GITSG=Gastrointestinal Tumor Study Group; IFN= interferon; JHH=Johns Hopkins Hospital; RTOG=Radiation Therapy Oncology Group; XRT=external beam radiation therapy.
The benefit of adjuvant systemic chemotherapy was evaluated in the Charité Onkologie (CONKO)-001 trial in which 354 patients were randomized to either observation or adjuvant gemcitabine given intravenously (IV) for a total of 6 cycles.144 Disease free survival (DFS) and OS were 6.9 months and 20.5 months for the observation arm, and 13.4 months and 24.2 months for the treatment arm, respectively. Similarly, the European Study Group for Pancreatic Cancer (ESPAC)-3 phase III trial randomized 1088 patients to bolus 5-FU daily with folinic acid for 5 days every 4 weeks or gemcitabine weekly for 3 weeks every 4 weeks for 6 cycles total.145 Overall survival (OS) was 23.0 months in the 5-FU group and 23.6 months in the gemcitabine group, with higher rates of stomatitis and diarrhea in the 5-FU group and higher rates of hematologic toxicity in the gemcitabine group, but without any difference in quality of life. Taken together, the CONKO and ESPAC trials establish both 5-FU and gemcitabine as effective options for adjuvant chemotherapy. Unfortunately, the median overall survival for patients with resected pancreatic cancer is still approximately 20–22 months.
The Controversy over Radiation Therapy
The role of adding radiation therapy in the adjuvant setting is controversial. The Gastrointestinal Tumor Study Group (GITSG) trial in the 1980s was the first trial to show a survival benefit for adjuvant chemoradiation.146 In this trial, patients with resected pancreatic cancer were randomized to either observation or to chemoradiation. Chemoradiation included a 40-gray (Gy) split course of radiation with a 2-week break after 20 Gy, given with concurrent bolus 5-fluorouracil (5-FU) (500 mg/m2 on days 1–3 of each 20-Gy course of RT), followed by additional weekly 5-FU for 2 years or until progression.146 The median overall survival (OS) was 20 months in the treatment arm compared to 11 months in the observation arm.146 The European Organization for Research and Treatment of Cancer (EORTC) trial randomized patients to observation or to chemoradiation with 40-Gy split course given identically to the GITSG trial, with continuous infusion 5-FU (25 mg/kg/d) during the first course of radiation therapy, and for 0, 3, or 5 days of the second course (depending on toxicities).147 Although the OS was 12.6 months in the observation arm compared to 17.1 months in the treatment arm, this difference was not statistically significant, however unlike the GITSG trial patients did not receive maintenance chemotherapy. A third trial, ESPAC-1, put the use of chemoradiation into question when it compared adjuvant chemoradiation, chemotherapy, both, or neither.148 Patients were randomized to either 20 Gy total of radiation with bolus 5-FU over 2 weeks, bolus 5-FU daily for 5 days that was repeated every 4 weeks for a total of 6 cycles, chemoradiation followed by chemotherapy, or observation. With approximately 71 patients in each arm, median overall survivals were 13.9 months, 21.6 months, 19.9 months and 16.9 months, respectively. Patients who received radiation had a median OS of 15.9 months compared to 17.9 months for those who didn’t receive radiation, suggesting that radiation was detrimental.148 However, there was a great deal of non-adherence within this trial. Only 70% of patients randomized to receive chemoradiation received the prescribed dose of radiation (50% of the violations due to patient decision not to receive assigned treatment), and only 50% of patients randomized to receive chemotherapy received the full course of chemotherapy (33% of violations due to patient decision not receive assigned treatment).149 As with trials previous to this trial, a split-course radiation regimen was utilized, and the quality assurance for the radiation therapy was lacking with no centralized review of the radiation fields. Finally, it is worth nothing that patients in the chemoradiation arm received only 2 cycles of 5-FU during radiation, while patients in the chemotherapy and chemoradiation followed by chemotherapy arms received ≥6 cycles of 5-FU. Not surprisingly, the results of this study have been criticized highly based on the perceived weaknesses in trial design and the quality of radiation delivery.150
Improvements in the delivery of radiation therapy now offer hope. The increased use of more three-dimensional (3D) conformal planning has led to more focused radiation fields (Figure 14), and it has now become feasible to deliver higher doses of continuous chemoradiation without increasing toxicities. Using these more modern approaches, two high volume surgical centers, Johns Hopkins University and the Mayo Clinic, reported on a large series of patients who had undergone surgical resection for pancreatic cancer and received post-operative CRT with a median dose of 50.4 Gy.143, 151 Independently, both studies found that chemoradiation was associated with improved survival and increased local-regional control compared to surgery alone. These two studies were combined (n=1,092) and propensity scores were created and a matched pair analysis was performed to control for patient and treatment related variables.152 Again, adjuvant chemoradiation was found to provide a statistically significant survival benefit (median survival 21.1 months) compared to surgery alone (15.5 months, p=<0.001). In the match pair analyses, the benefit of chemoradiation was seen in margin-positive or margin-negative patients and node-positive disease.
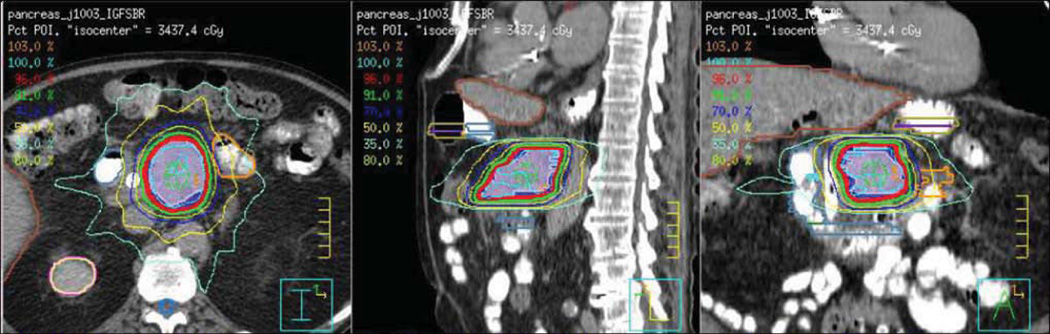
Figure 14.
Stereotactic body radiation therapy plan delivering 33 Gy to the pancreatic tumor over 5 treatments. Breath hold during treatment and pancreatic tumor markers (fiducials) allows for small margins (3 mm) and accurate targeting of the tumor. Notice the low dose of radiation (colored lines) to the bowel and adjacent normal structures.
In an effort to improve on chemoradiation therapy, several studies have examined the use of agents other than 5-FU. The benefit of adding gemcitabine to 5-FU based chemoradiation was examined in RTOG 9704, a multicenter, randomized controlled phase III study.153 The final analysis included 451 patients (388 pancreatic head tumors) and incorporated 5-FU or gemcitabine before and after 5-FU based chemoradiation. Of note, this trial utilized a continuous course of chemoradiation and prospective central quality review was instituted for the first time. A 5-year update of the trial demonstrated a trend toward improved survival (p=0.08) for those patients (pancreatic head tumors only) who received gemcitabine.153 Reported local failure rates were 25% for the gemcitabine arm and 30% for the 5-FU arm, much improved from the earlier studies which used a lower radiation dose, suggesting that an increased radiation dose is more effective in preventing local recurrence. The primary mode of failure remained distant metastasis, occurring in >70% of patients, which highlights the need for better systemic therapies.
Novel adjuvant therapy approaches
Smaller trials have also looked at other systemic therapies in the adjuvant setting (Table 5). The combination of interferon-alpha, cisplatin and 5-FU with chemoradiation had a median OS of 32 months compared to 28.5 months in the standard arm of 5-FU alone.154–156 Erlotinib is being studied in the adjuvant setting and can be safely combined with gemcitabine as well with concurrent capecitabine and chemoradiation.157 Immunotherapy is also being elavuated in the adjuvant setting. In a phase II trial of 5-FU based chemoradiation combined with a pancreatic cancer vaccine of irradiated granulocyte-macrophage colony stimulating factor (GM-CSF) transfected allogeneic whole cell tumor lines, the median OS was 24.8 months and patients who demonstrated a CD8+ T cell response to mesothelin had a higher likelihood of remaining disease free.158 K-Ras mutant vaccines and MUC1 peptide-loaded dendritic cell vaccines also have shown early promising results.159, 160
In summary, a growing body of evidence-based medicine has established a role for systemic chemotherapy in the adjuvant setting. The role of chemoradiation is less clear, but newer technologies such as intensity modulated radiation therapy (IMRT) or stereotactic body radiation therapy (SBRT) use multiple, modulated beams of radiation that are likely to be safer and more effective than older radiation techniques. IMRT and SBRT can limit the dose to normal structures (bowel, kidneys, and liver) and deliver higher doses of radiation to the tumor bed (Figure 14) than traditional methods.161 The incorporation of both modern radiation therapy and combination chemotherapies such as FOLFIRINOX (oxaliplatin, leucovorin, irinotecan, and 5-FU), which has shown promising results in the metastatic setting, is currently being tested in the adjuvant setting.162
Locally Advanced Pancreatic Adenocarcinoma
For patients with locally advanced pancreatic adenocarcinoma the median survival in most historical studies ranges from 8 to 12 months.127, 163–171 Standard therapy includes external beam radiation therapy (EBRT) (total doses of 50–54 Gy in 1.8 to 2.0 Gy fractions) with concurrent chemotherapy (often 5-FU or gemcitabine), but some radiation therapy regimens can be delivered over shorter intervals.127, 163, 172–174
More contemporary studies using aggressive induction systemic therapy and/or dose escalated radiation therapy using 3-dimensional conformal, IMRT, or SBRT have reported improvements in treatment related toxicity and survival.127, 175 Nevertheless, a majority of patients with locally advanced or metastatic pancreatic cancer are not curable, and multidisciplinary treatment that optimizes patient selection while balancing disease control, toxicity, and quality of life (QOL) is paramount.
The order in which chemo- and radiation therapy are given to patients with locally advanced disease can be important. Many patients (~20%) who receive upfront chemoradiation will be found to have metastases soon after their therapy is completed.115 Furthermore, patients who receive upfront chemoradiation are likely to experience more toxicities than patients who receive chemotherapy alone, and these toxicities may interfere with subsequent chemotherapy needed to prevent and/or delay metastatic disease. An attractive alternative approach is therefore to start with several cycles of chemotherapy (1–4 months) and then to restage the patient. Patients who have no evidence of metastatic disease on restaging, and who have a good performance status, would then be consolidated with chemoradiation. This strategy selects those patients who are more likely to benefit from chemoradiation, and has been shown to prevent or delay loco-regional progression which could result in pain (celiac plexus involvement) or duodenal obstruction. Studies have also shown that CRT-based control of local progression can prevent the development of metastatic disease and improve survival when compared to chemotherapy alone.171,176
Several modern single phase II studies have reported impressive increases in median survival by incorporating more aggressive chemotherapy (multiple agents), radiosensitizers and/or targeted agents during the induction phase of treatment and/or in conjunction with radiation therapy.169, 177–181,182 For example, S-1 is a combination of tegafur (a prodrug converted to fluorouracil), gimeracil (an inhibitor of an enzyme which degrades fluorouracil), and oteracil (an inhibitor of fluorouracil phosphorylation in the gastrointestinal tract that acts to decrease toxicities).164 In a study by Sudo et al, S-1 (oral, twice daily) was given concurrently with radiation (50.4 Gy) and followed with S-1 maintenance in patients with locally advanced pancreatic adenocarcinoma (N=−34).164 Toxicity rates were acceptable (Grade 3–4), and outcomes were promising, with a median overall survival of 16.8 months and a 1-year survival rate of 70.6%. Although the results are encouraging, S-1 is currently not available in the United States. These single arm treatment studies, however, have all been small, and future study is needed.
Agents directed against the human epidermal growth factor receptor (EGFR) are among the best studied targeted agents in pancreatic cancer.157 A phase I study examined the role of erlotinib (small molecule inhibitor of EGFR) with concurrent chemoradiation, and the results were promising, with partial responses seen in 35% of patients.170 Crane et al. reported a multicenter phase II study in which patients with locally advanced pancreatic adenocarcinoma were treated with two months of induction cetuximab (antibody targeting EGFR), gemcitabine, and oxaliplatin, followed by concurrent capecitabine and cetuximab with RT (50.4 Gy) and maintenance gemcitabine and cetuximab therapy.127 Median overall survival time was 19.2 months (95% CI, 14.2 to 24.2 months), and 1-year, 2-year, and 4-year actuarial overall survival rates were 66.0%, 25.02%, and 11.3%, respectively. Gastrointestinal toxicity was 32% and 10% for grades 2 and 3, respectively. In this study, Smad4 (Dpc4) expression was determined from pretreatment fine needle aspirations and was found to correlate with patterns of recurrence. Cancers with Smad4 inactivation were more likely to recur distantly whereas Smad4 intact cancers tended to recur locally (P = .016).
Selection of the optimal area to radiate is critical (Figure 14). The standard of care is to deliver a radiation field encompassing only the tumor plus a small margin to account for microscopic disease (clinical tumor volume=CTV) and tumor movement secondary to breathing, bowel distension, and set up error (planning treatment volume=PTV). By not treating elective nodal regions, the smaller margin around the tumor can: 1) allow for the escalation of radiation dose to the tumor above 54 Gy, 2) limit the dose delivered to the bowel and stomach in the field thus decreasing toxicity, and 3) allow for a shorter course of radiation such as can be achieved with stereotactic body radiation therapy (Figure 14).183–186 Overall, results of recent studies indicate that the use of intensity modulated radiation therapy and stereotactic body radiation therapy provide a promising means of intensifying treatment without adding excessive toxicity.104, 187, 188
Treating metastatic disease (Table 6)
Table 6.
Key metastatic pancreatic cancer studies
| Study | Chemo | RR | MS (months) | 1-year survival % |
|---|---|---|---|---|
| Burris (1997) N= 126190 |
5-FU v. Gem | NR | 4.4 v. 5.6 (P=.0025) | 2 v. 18 |
| Rocha Lima (2003) N=342235 |
Gem ± Irinotecan | 4.4 v. 16 | 6.6 v. 6.3 | 22 v. 21 |
| Heinemann (2003) N=195236 |
Gem ± Cisplatin | 8 v. 10.2 | 6 v. 7.6 (p=.12) |
22 v. 26 |
| Tempero (2003) N=92191 |
Gem ± Gem (Fixed rate) | 9.1 v. 5.9 | 5 v. 8 (p=.013) |
9 v. 29 |
| O’Reilly (2006) N=340237 |
Gem ± exatecan | 6.3 v. 8.2 | 6.2 v. 6.7 (p=.52) |
21 v. 23 |
| Richards (2004) N=565238 |
Gem ± Pemetrexed | 9.1 v 18.3 | 6.3 v. 6.2 (p=.85) | 21 v. 20 (p=.72) |
| Louvet (2005) N=313196 |
Gem v. Gem (FDR+ + Oxali | 17.3 v. 26.8 (p=.04) | 7.1 v. 9 P=.13) | 28 v. 35 |
| Cunningham (2009) N=533195 |
Gem ± Capecitabine | 14 v. 7 (p=.008) | 7.4 v. 6 (p<.05) | 26 v. 19 |
| CALGB (2009) N=259239 |
Gem/cis Gem (FDR) Gem/taxotere Gem/Irinotecan |
11 14 12 12 |
6.7 6.4 6.4 7.1 |
|
| Conroy (2011) N=343162 |
Gem. V. FOLFIRINOX | 6.8 v. 11.1 (P<.0001) |
17 v. 36 | |
| Moore (2005) N=569200 |
Gem ± erlotinib | 8.7 v. 7.9 (P=.8) |
6.4 v. 5.9 (P=.025) |
26 v. 20 |
| Kindler (2007) N=602201 |
Gem ± bevacizumab | 13.1 v. 11.3 | 6 v. 5.7 | NR |
| Philip (2007) N=735166 |
Gem ±cetuximab | 14 v. 12 | 6 v. 6.5 (p=.14) | NR |
| Von Hoff (2013) N=861199 |
Gem ±nab-paclixatel | 8.5 v. 6.7 |
Cis= cisplatin; 5-FU= 5-flurouracil; FDR=; Gem=gemcitabine; MS=median survival; NR=not reached
In the last few decades of the 20th century, 5-FU was the standard of care for the treatment of advanced pancreatic cancer. A meta-analysis of trials performed between 1970 and 2003 demonstrated that 5-FU was superior to best supportive care.189 In 1997 a randomized phase III trial demonstrated a survival benefit for gemcitabine (dose at 1000 mg/m2 weekly for 7 weeks with a 1 week break followed by cycles of 3 weeks on/1 week off) over bolus 5-FU with a median survival of 5.65 months as compared to 4.41 months, and 1-year survivals of 18% versus 2%.190 Gemcitabine also had a superior clinical benefit response described as improvement in pain, performance status or weight in 24% of the patients versus 5% in the 5-FU group.190 Both gemcitabine and 5-FU were well tolerated with similar toxicities. Based on this trial, gemcitabine was approved by the Food and Drug Administration for pancreatic cancer and gemcitabine became the standard of care. Fixed dose gemcitabine, given at a fixed rate of 10mg/m2/min, is an alternative to the standard regimen described above as the prolonged exposure with the fixed rate dosing leads to an increased intracellular gemcitabine triphosphate concentration, however it did not lead to a statistical improvement in overall survival as compared to gemcitabine standard dose and infusion.191, 192
Numerous gemcitabine-based combinations have been tested and these combinations can be considered in patients with good performance status (ECOG 0–1).193, 194 Gemcitabine combined with capecitabine in a phase III trial did not result in a statistically significant improvement in overall survival compared to gemcitabine alone.195 Capecitabine has also been studied alone and it does provide clinical benefit response, although capecitabine could be considered as an alternative to single-agent gemcitabine in patients with moderate performance status.
The combination of gemcitabine and platinums (DNA-binding alkylating agents) has not shown improved efficacy in general. Gemcitabine plus oxaliplatin or gemcitabine in combination with cisplatin showed no statistically significant improvement in survival in phase III trials, although the results may have trended in that direction.165, 196, 197 Selected subgroups of patients may, however, benefit. For example, retrospective analyses have suggested that patients with inherited BRCA1 or BRCA2 gene mutations and/or a family history of pancreatic, breast, or ovarian cancer may respond to gemcitabine-platinum combinations with longer median survivals compared to patients who were not given a platinum.198 This sensitivity to platinum is thought to be a result of the fact that the BRCA1/2 protein complex is needed to repair DNA cross-linking damage, the very type of damage that platinum containing agents can induce.27, 31
The addition of docetaxel and capecitabine to gemcitabine (in a regimen referred to as GTX) has demonstrated good activity. Phase II trials and a retrospective review have demonstrated disease control rates of 63–80% with median survival for locally advanced patients of 25 months and for metastatic patients of 11 months.128 The most common toxicities were neutropenia, diarrhea and hand/foot syndrome. Although these are smaller trials, GTX is a commonly used first-line therapy for patients with good performance status who want another option for combination therapy.
A phase I/II trial published in 2011 demonstrated exciting results when gemcitabine was combined with nab-paclitaxel (albumin-bound paclitaxel) as first-line therapy for metastatic pancreatic cancer patients. As discussed earlier, this combination makes sense as it has been suggested that nab-paclitaxel binds SPARC in the stroma of pancreatic cancer helping to deliver the paclitaxel.58, 59 Gemcitabine was given at the standard dose of 1000 mg/m2 and nab-paclitaxel (maximum tolerated dose was 125 mg/m2) weekly for 3 weeks on, repeated every 4 weeks. Median overall survival was 12.2 months with tolerable toxicity and a negative PET-CT at 3 months was a predictor for good response.59 A much anticipated phase III study of gemcitabine with or without nab-paclitaxel demonstrated only a less than two month improvement in survival from the addition of nab-paclitaxel (8.5 months vs. 6.7months) with more side effects in the nab-paclitaxel group.199 Clinicians will need to weigh the small benefit from nab-paclitaxel with its toxicity and cost.
Gemcitabine has also been tested in combination with several different targeted therapies, and the combination with erlotinib improved overall survival, albeit minimally. A phase III randomized trial demonstrated a statistically significant improvement in overall survival from 5.91 months with single-agent gemcitabine to 6.24 months with gemcitabine plus erlotinib.200 Erlotinib was approved by the Food and Drug Administration (FDA) after this trial and can be considered as first-line therapy in patients with good performance status, although again, one needs to weigh the small benefit of this agent versus the toxicity and cost. The addition of cetuximab to gemcitabine, and of bevacizumab (vascular endothelial growth factor [VEGF] inhibitor) to gemcitabine both demonstrated no survival benefit and an increase in toxicities in phase II and III trials.166, 201
The newest combination regimen for patients with metastatic pancreatic cancer is FOLFIRINOX.162 FOLFIRINOX was initially described in France as oxaliplatin 85 mg/m2, leucovorin 400 mg/m2, irinotecan 180 mg/m2, 5-FU bolus 400 mg/m2 on day 1 followed by a 46 hour continuous infusion of 5-FU (2400 mg/m2) repeated every 2 weeks. When 342 patients were randomized, disease control rate and median overall survival were 70% and 11.1 months in the FOLFIRINOX arm compared to 51% and 6.8 months in the gemcitabine arm.162 Although there were more grade 3 and 4 toxicities in the FOLFIRINOX arm, quality of life was not different. FOLFIRINOX has therefore been recommended as an option for first-line therapy in patients with a good performance status (ECOG 0–1), however the doses are usually reduced and adjusted when given in the United States because of the greater toxicity seen here.
Second-line treatment is not very well established, partially because many of the patients are not strong enough to undergo further treatment. Patients with an adequate performance status who progressed on a gemcitabine-based regimen should be treated with a 5-FU based regimen, and vice versa. Single agent capecitabine is well tolerated and can be considered in patients with moderate performance status. Capecitabine and oxaliplatin (referred to as CAPOX or XELOX), provided 28% of patients with disease control with median overall survival of 23 weeks in a second-line phase II trial.202, 203 Capecitabine plus erlotinib was reasonably tolerated and partial responses were seen in 10%.204 Modified 5-FU and oxaliplatin (FOLFOX) and 5-FU and irinotecan (FOLFIRI) regimens can also be considered as second-line options with the former demonstrating a two month survival advantage over best supportive care.
If patients recur after resection and adjuvant therapy, biopsy can be considered. If the disease relapse is locoregional only, chemoradiation can be performed if no prior radiation was given. In regards to chemotherapy, if relapse occurs > 6 months after completion of adjuvant therapy, the same regimen can be considered. If relapse occurs earlier, a different regimen should be chosen. Metastatectomy can be considered in very fit patients who have isolated metastases. Although resection of liver metastases have shown mixed results in terms of improving survival as compared to a palliative procedure only, pulmonary and brain metastatectomy have improved survival in a carefully selected group of patients.205
Future therapies
The future for the development of new treatments is promising as there has been significant progress in the understanding of the genetics and molecular biology of pancreatic cancer.69,199 Several attempts have been made to target the genetic changes specific to pancreatic cancer. The most appealing one to target is the KRAS gene since it is mutated in the vast majority of pancreatic cancers, however, inhibitors targeting the Kras protein itself have been largely unsuccessful. More recent strategies have focused on targeting downstream pathways such as MEK, ERK and AKT.206, 207 Very promising are therapies targeting the rarer genetic alterations in pancreatic cancer. As discussed earlier, cancers in which either BRCA2 or PALB2 is biallelically inactivated appear to be uniquely sensitive to DNA cross-linking agents such as mitomycin C, and to poly ADP ribose polymerase (PARP) inhibitors.21, 31 Similarly, although the evidence in pancreatic cancer is lacking, one would predict that pancreatic cancers with inactivating ATM mutations may be particularly sensitive to radiation damage and to PARP inhibitors.88
We can easily imagine a scenario in the not too distant future in which all patients with pancreatic cancer will have their tumor DNA sequenced and information gleaned from the genetic changes in their cancer will be used to individualize their care.
In addition, much has been learned about the supportive tumor-stromal cell environment that is thought to not only influence the development of pancreatic cancer progression but which also supports mechanisms for resistance to effective treatment given its inherent protective properties.208, 209 This includes the complex relationship between inflammation and the immune suppressive tumor microenvironment in pancreatic cancer. Targeting this environment is an exciting area only slowly being explored. Already there are new treatment opportunities targeting small molecules that can reverse immune suppression, as well as immunotherapy approaches that activate the immune system.158, 210
Another exciting new area is the targeting of cancer-specific cell metabolism including pathways associated with glucose/glutamine metabolism as well as mechanisms associated with the altered metabolic pathways or amino acid supply.211–216
Pain Assessment and Treatment
Patients with pancreatic cancer frequently present with pain as the initial symptom of their disease, with nearly 75% of patients suffering from pain at the time of diagnosis.217 It is important for the practitioner to manage a patient’s pain appropriately while other therapies such as chemotherapy, radiation therapy, or surgery are being carried out. Consultation with a physician with specific expertise in pain medicine is often helpful both in the outpatient clinic and multidisciplinary clinic settings. The initial assessment of pain includes evaluating the intensity, frequency, duration, exacerbating and/or alleviating factors, and a comprehensive history of current and previous pain medications along with documentation of any side effects encountered on these medications. Once obtained, this pain history and concomitant physical examination allows the health care team to make appropriately guided decisions regarding the potential implementation of pharmacologic, procedural, or surgical pain therapy.
Opioids are the mainstay of pharmacologic therapy for pancreatic cancer pain. Initial therapy may consist of a short-acting agent such as morphine or oxycodone. A sustained-release opioid, along with a short-acting opioid for breakthrough pain, may work better for those patients with constant pain or those who encounter difficulties with sleep secondary to pain. Common side effects of opioids include sedation, constipation, pruritis, nausea, and testosterone suppression in those on long-term therapy. Constipation is commonly addressed with stool softeners or bowel motility-promoting agents.
The most common and effective procedural intervention for pancreatic cancer pain is celiac plexus block218. The celiac plexus is formed by the coalescence of the greater, lesser, and least splanchnic nerves in the vicinity of the celiac trunk and provides visceral innervation from the pancreas and its surrounding structures (Figure 15). Patients who may benefit most from a celiac plexus block are those who have pain refractory to escalating doses of opioids or those who suffer debilitating opioid-mediated side effects. Patients whose pain is relieved by diagnostic celiac plexus block may undergo subsequent celiac plexus neurolysis, with careful patient selection maximizing therapeutic outcomes.219 Although initial celiac plexus neurolysis yields >3 months relief in most patients, disease progression often mitigates the efficacy of repeat procedure, and may indicate the need for more invasive therapy.220
Figure 15.
Retrocrural and antecrural nerve blocks are effective approaches to alleviating pain caused by pancreatic cancer. Top illustrates a mid-sagittal view and the bottom an axial view with the patient prone. Note the position of the needle tip in the antecrural versus retrocrural positions. Blue represents the injected anesthetic. Illustration by Corinne Sandone. © Johns Hopkins University; used with permission.
Intrathecal delivery of analgesic agents is helpful in patients whose pain is poorly controlled by less invasive means (Figure 16).221 Typically, a temporary percutaneous catheter is placed and the effectiveness of treatment is assessed and the dose optimized over several days. Subsequently, a pump and catheter system is surgically implanted to deliver opioid and/or adjuvant analgesic agents directly into the cerebrospinal fluid. Randomized, controlled studies have delineated the benefit of this therapeutic modality, not only for pain control, but particularly in minimizing the toxicities of systemically administered opioids.222
Figure 16.
Pain medication can also be delivered intrathecally into the cerebrospinal fluid (CSF) using a catheter attached to a surgically implanted pump. Blue represents the injected anesthetic. Illustration by Corinne Sandone. Illustration by Corinne Sandone. © Johns Hopkins University; used with permission.
Ongoing consultation with a pain specialist is advised to optimize an integrated approach to pharmacologic, procedural, and surgical pain care for the patient with pancreatic cancer. Reassurance given to the patient that his or her pain care is paramount during their care, regardless of the stage and severity of their disease, are essential in a process where the emphasis is often more focused on palliation rather than cure.
Depression
Ten to 20% of patients with pancreatic cancer suffer from depression, and patients who are depressed are less likely to have their cancers managed optimally than are patients who are not depressed.223, 224 Of note, while it is often assumed that this depression is caused by the psychological burden of the diagnosis of a life-threatening cancer, in fact, depression can precede the diagnosis of pancreatic cancer. This latter observation raises the possibility that pancreatic cancers provide a factor or factors that contribute to depression.103 Regardless of its etiology, the immense suffering depression causes and its prevalence and make it clear that patients should be screened for depression, and if they have it adequately treated.103
End of Life
While it is natural for health care providers to focus on prolonging life, helping a patient and their family through the difficult transitions that come all too often with pancreatic cancer are equally as important. While a detailed discussion of palliative care is beyond the scope of this review, practitioners are encouraged to include a palliative care expert as a part of their multidisciplinary team.115
The Importance of Multidisciplinary care
It should be clear from this review that pancreatic cancer is a complex disease and that this complexity is best managed clinically by a multidisciplinary team of health care providers working in concert to deliver the best individualized care for their patients.113 Indeed, a recent analysis of the Multidisciplinary Pancreatic Cancer Clinic at Johns Hopkins (http://pathology.jhu.edu/pc) showed that 25% of the patients referred to the clinic had a significant change in their diagnosis or treatment.113 In addition, a large number of patients who went through the clinic elected to participate in research studies including clinical trials and the National Familial Pancreas Tumor Registry. The advantages of multidisciplinary care go beyond this, as these clinics build cohesion and communication within the team, helping to ensure the highest quality care even after the patient leaves the clinic to pursue specific treatments.
Summary and Conclusions
Pancreatic cancer is a complex and highly lethal disease that is best treated in the multidisciplinary setting. Although the survival statics are currently bleak, there are a number of bright spots on the horizon including individualized therapies and the prevention of an invasive pancreatic cancer by improvements in the management of cystic lesions of the pancreas.
Acknowledgments
Grant support: P30 CA006973 and P50 CA62924
Footnotes
Conflict of interest: Dr. Hruban receives royalty payments from Myriad Genetics for the PALB2 invention
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Bosetti C, Lucenteforte E, Silverman DT, et al. Cigarette smoking and pancreatic cancer: an analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4) Ann Oncol. 2012;23:1880–1888. doi: 10.1093/annonc/mdr541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an update. Dig Dis. 2010;28:645–656. doi: 10.1159/000320068. [DOI] [PubMed] [Google Scholar]
- 4.Blackford A, Parmigiani G, Kensler TW, et al. Genetic mutations associated with cigarette smoking in pancreatic cancer. Cancer Res. 2009;69:3681–3688. doi: 10.1158/0008-5472.CAN-09-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. British journal of cancer. 2005;92:2076–2083. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chari ST, Leibson CL, Rabe KG, Ransom J, de AM, Petersen GM. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology. 2005;129:504–511. doi: 10.1053/j.gastro.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arslan AA, Helzlsouer KJ, Kooperberg C, et al. Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan) Arch Intern Med. 170:791–802. doi: 10.1001/archinternmed.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucenteforte E, La Vecchia C, Silverman D, et al. Alcohol consumption and pancreatic cancer: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4) Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. doi: 10.1093/annonc/mdr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duell EJ, Lucenteforte E, Olson SH, et al. Pancreatitis and pancreatic cancer risk: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4) Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23:2964–2970. doi: 10.1093/annonc/mds140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs EJ, Chanock SJ, Fuchs CS, et al. Family history of cancer and risk of pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan) Int J Cancer. 127:1421–1428. doi: 10.1002/ijc.25148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghadirian P, Boyle P, Simard A, Baillargeon J, Maisonneuve P, Perret C. Reported family aggregation of pancreatic cancer within a population-based case-control study in the Francophone community in Montreal, Canada. IntJPancreatol. 1991;10:183–196. doi: 10.1007/BF02924156. [DOI] [PubMed] [Google Scholar]
- 12.Falk RT, Pickle LW, Fontham ET, Correa P, Fraumeni JF. Life-Style Risk Factors for Pancreatic Cancer in Louisiana: A Case-Control Study. American Journal of Epidemiology. 1988;128:324–336. doi: 10.1093/oxfordjournals.aje.a114972. [DOI] [PubMed] [Google Scholar]
- 13.Price TF, Payne RL, Oberleitner MG. Familial pancreatic cancer in south Louisiana. Cancer Nurs. 1996;19:275–282. doi: 10.1097/00002820-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Schenk M, Schwartz AG, O'Neal E, et al. Familial Risk of Pancreatic Cancer. JNatlCancer Inst. 2001;93:640–644. doi: 10.1093/jnci/93.8.640. [DOI] [PubMed] [Google Scholar]
- 15.Brune KA, Lau B, Palmisano E, et al. Importance of age of onset in pancreatic cancer kindreds. J NatlCancer Inst. 2010;102:119–126. doi: 10.1093/jnci/djp466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Brune KA, Visvanathan K, et al. Elevated cancer mortality in the relatives of patients with pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:2829–2834. doi: 10.1158/1055-9965.EPI-09-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi C, Hruban RH, Klein AP. Familial pancreatic cancer. ArchPatholLab Med. 2009;133:365–374. doi: 10.5858/133.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canto MI, Hruban RH, Fishman EK, et al. Frequent Detection of Pancreatic Lesions in Asymptomatic High-Risk Individuals. Gastroenterology. 2012 doi: 10.1053/j.gastro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canto MI, Harinck F, Hruban RH, et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2012 doi: 10.1136/gutjnl-2012-303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McWilliams RR, Wieben ED, Rabe KG, et al. Prevalence of CDKN2A mutations in pancreatic cancer patients: implications for genetic counseling. European journal of human genetics : EJHG. 2011;19:472–478. doi: 10.1038/ejhg.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Heijden MS, Brody JR, Dezentje DA, et al. In vivo Therapeutic Responses Contingent on Fanconi Anemia/BRCA2 Status of the Tumor. Clin Cancer Res. 2005;11:7508–7515. doi: 10.1158/1078-0432.CCR-05-1048. [DOI] [PubMed] [Google Scholar]
- 22.Goggins M, Schutte M, Lu J, et al. Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Cancer Res. 1996;56:5360–5364. [PubMed] [Google Scholar]
- 23.Ozcelik H, Schmocker B, DiNicola N, et al. Germline BRCA2 6174delT mutations in Ashkenazi Jewish pancreatic cancer patients. Nature Genet. 1997;16:17–18. doi: 10.1038/ng0597-17. [DOI] [PubMed] [Google Scholar]
- 24.Ferrone CR, Levine DA, Tang LH, et al. BRCA germline mutations in Jewish patients with pancreatic adenocarcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:433–438. doi: 10.1200/JCO.2008.18.5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Couch FJ, Johnson MR, Rabe KG, et al. The Prevalence of BRCA2 Mutations in Familial Pancreatic Cancer. Cancer EpidemiolBiomarkers Prev. 2007;16:342–346. doi: 10.1158/1055-9965.EPI-06-0783. [DOI] [PubMed] [Google Scholar]
- 26.Murphy KM, Brune KA, Griffin CA, et al. Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: deleterious BRCA2 mutations in 17% Cancer Research. 2002;62:3789–3793. [PubMed] [Google Scholar]
- 27.Lowery MA, Kelsen DP, Stadler ZK, et al. An emerging entity: pancreatic adenocarcinoma associated with a known BRCA mutation: clinical descriptors, treatment implications, and future directions. The oncologist. 2011;16:1397–1402. doi: 10.1634/theoncologist.2011-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tischkowitz MD, Sabbaghian N, Hamel N, et al. Analysis of the gene coding for the BRCA2-interacting protein PALB2 in familial and sporadic pancreatic cancer. Gastroenterology. 2009;137:1183–1186. doi: 10.1053/j.gastro.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slater EP, Langer P, Niemczyk E, et al. PALB2 mutations in European familial pancreatic cancer families. Clin Genet. 78:490–494. doi: 10.1111/j.1399-0004.2010.01425.x. [DOI] [PubMed] [Google Scholar]
- 30.Jones S, Hruban RH, Kamiyama M, et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science. 2009;324:217. doi: 10.1126/science.1171202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villarroel MC, Rajeshkumar NV, Garrido-Laguna I, et al. Personalizing cancer treatment in the age of global genomic analyses: PALB2 gene mutations and the response to DNA damaging agents in pancreatic cancer. MolCancer Ther. 2011;10:3–8. doi: 10.1158/1535-7163.MCT-10-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson D, Easton DF. Cancer Incidence in BRCA1 mutation carriers. JNatlCancer Inst. 2002;94:1358–1365. doi: 10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- 33.Axilbund JE, Argani P, Kamiyama M, et al. Absence of germline BRCA1 mutations in familial pancreatic cancer patients. Cancer Biol Ther. 2009;8:1–5. doi: 10.4161/cbt.8.2.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moskaluk CA, Hruban RH, Lietman AS, et al. Novel germline p16 (INK4) allele (Asp145Cys) in a family with multiple pancreatic carcinomas. Mutations in brief no. 148. Online. Hum Mutat. 1998;12:70–73. doi: 10.1002/(SICI)1098-1004(1998)12:1<70::AID-HUMU13>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 35.Rutter JL, Bromley CM, Goldstein AM, et al. Heterogeneity of risk for melanoma and pancreatic and digestive malignancies: a melanoma case-control study. Cancer. 2004;101:2809–2816. doi: 10.1002/cncr.20669. [DOI] [PubMed] [Google Scholar]
- 36.Vasen HF, Gruis NA, Frants RR, van dV, Hille ET, Bergman W. Risk of developing pancreatic cancer in families with familial atypical multiple mole melanoma associated with a specific 19 deletion of p16 (p16-Leiden) Int J Cancer. 2000;87:809–811. [PubMed] [Google Scholar]
- 37.Santillan AA, Cherpelis BS, Glass LF, Sondak VK. Management of familial melanoma and nonmelanoma skin cancer syndromes. Surg Oncol Clin N Am. 2009;18:73–98. doi: 10.1016/j.soc.2008.08.003. viii. [DOI] [PubMed] [Google Scholar]
- 38.Rustgi AK. The genetics of hereditary colon cancer. Genes Dev. 2007;21:2525–2538. doi: 10.1101/gad.1593107. [DOI] [PubMed] [Google Scholar]
- 39.Kastrinos F, Mukherjee B, Tayob N, et al. Risk of pancreatic cancer in families with Lynch syndrome. JAMA. 2009;302:1790–1795. doi: 10.1001/jama.2009.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexander J, Watanabe T, Wu TT, Rashid A, Li S, Hamilton SR. Histopathological identification of colon cancer with microsatellite instability. Am J Pathol. 2001;158:527–535. doi: 10.1016/S0002-9440(10)63994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilentz RE, Goggins M, Redston M, et al. Genetic, immunohistochemical, and clinical features of medullary carcinoma of the pancreas: a newly described and characterized entity. American Journal of Pathology. 2000;156:1641–1651. doi: 10.1016/S0002-9440(10)65035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakata B, Wang YQ, Yashiro M, et al. Prognostic value of microsatellite instability in resectable pancreatic cancer. Clin Cancer Res. 2002;8:2536–2540. [PubMed] [Google Scholar]
- 43.Le Bodic L, Bignon JD, Raguenes O, et al. The hereditary pancreatitis gene maps to long arm of chromosome 7. Hum Mol Genet. 1996;5:549–554. doi: 10.1093/hmg/5.4.549. [DOI] [PubMed] [Google Scholar]
- 44.Whitcomb DC, Preston RA, Aston CE, et al. A gene for hereditary pancreatitis maps to chromosome 7q35. Gastroenterology. 1996;110:1975–1980. doi: 10.1053/gast.1996.v110.pm8964426. [DOI] [PubMed] [Google Scholar]
- 45.Whitcomb DC, Gorry MC, Preston RA, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene [see comments] NatGenet. 1996;14:141–145. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- 46.Witt H, Luck W, Hennies HC, et al. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet. 2000;25:213–216. doi: 10.1038/76088. [DOI] [PubMed] [Google Scholar]
- 47.Lowenfels AB, Maisonneuve P, DiMagno EP, et al. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst. 1997;89:442–446. doi: 10.1093/jnci/89.6.442. [DOI] [PubMed] [Google Scholar]
- 48.Lowenfels AB, Maisonneuve P, Whitcomb DC, Lerch MM, DiMagno EP. Cigarette smoking as a risk factor for pancreatic cancer in patients with hereditary pancreatitis. JAMA. 2001;286:169–170. doi: 10.1001/jama.286.2.169. [DOI] [PubMed] [Google Scholar]
- 49.Korsse SE, Harinck F, van Lier MG, et al. Pancreatic cancer risk in Peutz-Jeghers syndrome patients: a large cohort study and implications for surveillance. J Med Genet. 2013;50:59–64. doi: 10.1136/jmedgenet-2012-101277. [DOI] [PubMed] [Google Scholar]
- 50.Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers Syndrome. Gastroenterology. 2000;119:1447–1453. doi: 10.1053/gast.2000.20228. [DOI] [PubMed] [Google Scholar]
- 51.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2006;4:766–781. doi: 10.1016/j.cgh.2006.02.005. quiz 665. [DOI] [PubMed] [Google Scholar]
- 52.Sato N, Rosty C, Jansen M, et al. STK11/LKB1 Peutz-Jeghers gene inactivation in intraductal papillary-mucinous neoplasms of the pancreas. The American journal of pathology. 2001;159:2017–2022. doi: 10.1016/S0002-9440(10)63053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolpin BM, Chan AT, Hartge P, et al. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst. 2009;101:424–431. doi: 10.1093/jnci/djp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hruban RH, Pitman MB, Klimstra DS. Atlas of tumor pathology. Vol Fourth Series, Fascicle 6. Washington, DC: American Registry of Pathology and Armed Forces Institute of Pathology; 2007. Tumors of the pancreas. [Google Scholar]
- 55.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jacobetz MA, Chan DS, Neesse A, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2012 doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Infante JR, Matsubayashi H, Sato N, et al. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:319–325. doi: 10.1200/JCO.2006.07.8824. [DOI] [PubMed] [Google Scholar]
- 59.Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:4548–4554. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Voong KR, Davison J, Pawlik TM, et al. Resected pancreatic adenosquamous carcinoma: clinicopathologic review and evaluation of adjuvant chemotherapy and radiation in 38 patients. Hum Pathol. 2010;41:113–122. doi: 10.1016/j.humpath.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boyd CA, Benarroch-Gampel J, Sheffield KM, Cooksley CD, Riall TS. 415 patients with adenosquamous carcinoma of the pancreas: a population-based analysis of prognosis and survival. J Surg Res. 2012;174:12–19. doi: 10.1016/j.jss.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seidel G, Zahurak M, Iacobuzio-Donahue C, et al. Almost all infiltrating colloid carcinomas of the pancreas and periampullary region arise from in situ papillary neoplasms: a study of 39 cases. Am J Surg Pathol. 2002;26:56–63. doi: 10.1097/00000478-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 63.Poultsides GA, Reddy S, Cameron JL, et al. Histopathologic basis for the favorable survival after resection of intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma of the pancreas. Ann Surg. 2010;251:470–476. doi: 10.1097/SLA.0b013e3181cf8a19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hruban RH, Takaori K, Canto M, et al. Clinical importance of precursor lesions in the pancreas. J Hepatobiliary Pancreat Surg. 2007;14:255–263. doi: 10.1007/s00534-006-1170-9. [DOI] [PubMed] [Google Scholar]
- 65.Maitra A, Fukushima N, Takaori K, Hruban RH. Precursors to invasive pancreatic cancer. Adv Anat Pathol. 2005;12:81–91. doi: 10.1097/01.pap.0000155055.14238.25. [DOI] [PubMed] [Google Scholar]
- 66.Sipos B, Frank S, Gress T, Hahn S, Klöppel G. Pancreatic intraepithelial neoplasia revisited and updated. Pancreatology. 2009;9:45–54. doi: 10.1159/000178874. [DOI] [PubMed] [Google Scholar]
- 67.Kanda M, Sadakari Y, Borges M, et al. Mutant TP53 in Duodenal Samples of Pancreatic Juice from Patients with Pancreatic Cancer or High-Grade Dysplasia. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2012 doi: 10.1016/j.cgh.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kanda M, Knight S, Topazian M, et al. Mutant GNAS detected in duodenal collections of secretin-stimulated pancreatic juice indicates the presence or emergence of pancreatic cysts. Gut. 2012 doi: 10.1136/gutjnl-2012-302823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Detlefsen S, Sipos B, Feyerabend B, Klöppel G. Pancreatic fibrosis associated with age and ductal papillary hyperplasia. Virchows Arch. 2005;447:800–805. doi: 10.1007/s00428-005-0032-1. [DOI] [PubMed] [Google Scholar]
- 70.Brune KA, Abe T, Canto MI, et al. Multifocal neoplastic precusor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. American Journal of Surgical Pathology. 2006;30:1067–1076. [PMC free article] [PubMed] [Google Scholar]
- 71.WHO Classification of Tumours of the Digestive System. 4th ed. Lyon: IARC; 2010. [Google Scholar]
- 72.Raymond E, Hobday T, Castellano D, Reidy-Lagunes D, Garcia-Carbonero R, Carrato A. Therapy innovations: tyrosine kinase inhibitors for the treatment of pancreatic neuroendocrine tumors. Cancer Metastasis Rev. 2011;30(Suppl 1):19–26. doi: 10.1007/s10555-011-9291-2. [DOI] [PubMed] [Google Scholar]
- 73.Yao JC, Shah MH, Ito T, et al. Everolimus for Advanced Pancreatic Neuroendocrine Tumors. NEnglJ Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Laffan TA, Horton KM, Klein AP, Fishman EK, Johnson PT, Hruban RH. Prevelance of unsuspected pancreatic cysts on MDCT. AJRAmerican Journal of Roentgenology. 2008;191:802–807. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.He J, Cameron JL, Ahuja N, et al. Is It Necessary to Follow Patients after Resection of a Benign Pancreatic Intraductal Papillary Mucinous Neoplasm? J Am Coll Surg. 2013 doi: 10.1016/j.jamcollsurg.2012.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reddy S, Cameron JL, Scudiere J, et al. Surgical management of solid-pseudopapillary neoplasms of the pancreas (Franz or Hamoudi tumors): a large single-institutional series. J Am Coll Surg. 2009;208:950–957. doi: 10.1016/j.jamcollsurg.2009.01.044. discussion 957-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wargo JA, Fernandez-del-Castillo C, Warshaw AL. Management of pancreatic serous cystadenomas. Adv Surg. 2009;43:23–34. doi: 10.1016/j.yasu.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 78.Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199–1203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu J, Jiao Y, Dal Molin M, et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci U S A. 2011;108:21188–21193. doi: 10.1073/pnas.1118046108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Science translational medicine. 2011;3:92ra66. doi: 10.1126/scitranslmed.3002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hruban RH, van Mansfeld ADM, Offerhaus GJ, et al. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. American Journal of Pathology. 1993;143:545–554. [PMC free article] [PubMed] [Google Scholar]
- 83.Shi C, Fukushima N, Abe T, et al. Sensitive and quantitative detection of KRAS2 gene mutations in pancreatic duct juice differentiates patients with pancreatic cancer from chronic pancreatitis, potential for early detection. Cancer Biol Ther. 2008;7:353–360. doi: 10.4161/cbt.7.3.5362. [DOI] [PubMed] [Google Scholar]
- 84.Hahn SA, Schutte M, Hoque AT, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350–353. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- 85.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Blackford A, Serrano OK, Wolfgang CL, et al. SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer. Clin Cancer Res. 2009;15:4674–4679. doi: 10.1158/1078-0432.CCR-09-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roberts NJ, Jiao Y, Yu J, et al. ATM Mutations in Patients with Hereditary Pancreatic Cancer. Cancer Discovery. 2012;2:41–46. doi: 10.1158/2159-8290.CD-11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Williamson CT, Kubota E, Hamill JD, et al. Enhanced cytotoxicity of PARP inhibition in mantle cell lymphoma harbouring mutations in both ATM and p53. EMBO molecular medicine. 2012;4:515–527. doi: 10.1002/emmm.201200229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Omura N, Li CP, Li A, et al. Genome-wide profiling of methylated promoters in pancreatic adenocarcinoma. Cancer Biol Ther. 2008;7:1146–1156. doi: 10.4161/cbt.7.7.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Missiaglia E, Donadelli M, Palmieri M, Crnogorac-Jurcevic T, Scarpa A, Lemoine NR. Growth delay of human pancreatic cancer cells by methylase inhibitor 5-aza-2'-deoxycytidine treatment is associated with activation of the interferon signalling pathway. Oncogene. 2005;24:199–211. doi: 10.1038/sj.onc.1208018. [DOI] [PubMed] [Google Scholar]
- 91.Fukushima N, Walter KM, Uek T, et al. Diagnosing pancreatic cancer using methylation specific PCR analysis of pancreatic juice. Cancer BiolTher. 2003;2:78–83. doi: 10.4161/cbt.183. [DOI] [PubMed] [Google Scholar]
- 92.Harsha HC, Kandasamy K, Ranganathan P, et al. A compendium of potential biomarkers of pancreatic cancer. PLoS Med. 2009;6:e1000046. doi: 10.1371/journal.pmed.1000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Argani P, Iacobuzio-Donahue C, Ryu B, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin Cancer Res. 2001;7:3862–3868. [PubMed] [Google Scholar]
- 94.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. ClinCancer Res. 2004;10:3937–3942. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 95.Wang Q, Chaerkady R, Wu J, et al. Mutant proteins as cancer-specific biomarkers. Proc Natl Acad Sci U S A. 2011;108:2444–2449. doi: 10.1073/pnas.1019203108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Habbe N, Koorstra JB, Mendell JT, et al. MicroRNA miR-155 is a biomarker of early pancreatic neoplasia. Cancer Biol Ther. 2009;8 doi: 10.4161/cbt.8.4.7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Matthaei H, Wylie D, Lloyd MB, et al. miRNA Biomarkers in Cyst Fluid Augment the Diagnosis and Management of Pancreatic Cysts. Clin Cancer Res. 2012;18:4713–4724. doi: 10.1158/1078-0432.CCR-12-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wan C, Shen Y, Yang T, Wang T, Chen L, Wen F. Diagnostic value of microRNA for pancreatic cancer: a meta-analysis. Archives of medical science : AMS. 2012;8:749–755. doi: 10.5114/aoms.2012.31609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Siddiqui AA, Kowalski TE, Kedika R, et al. EUS-guided pancreatic fluid aspiration for DNA analysis of KRAS and GNAS mutations for the evaluation of pancreatic cystic neoplasia: a pilot study. Gastrointest Endosc. 2013;77:669–670. doi: 10.1016/j.gie.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 100.Heaphy CM, de Wilde RF, Jiao Y, et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science. 2011;333:425. doi: 10.1126/science.1207313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Krueger DA, Care MM, Holland K, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. NEnglJ Med. 2010;363:1801–1811. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- 102.Yao JC, Shah MH, Ito T, et al. A randomized, double-blind, placebo-controlled, multicenter Phase III trial of Everolimus in patients with advanced pancreatic neuroendorcrine tumors (PNET) (RADIANT-3) Annals of Oncology. 2010;21 [Google Scholar]
- 103.Mayr M, Schmid RM. Pancreatic cancer and depression: myth and truth. BMC cancer. 2010;10:569. doi: 10.1186/1471-2407-10-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schellenberg D, Quon A, Minn AY, et al. 18Fluorodeoxyglucose PET is prognostic of progression-free and overall survival in locally advanced pancreas cancer treated with stereotactic radiotherapy. Int J Radiat Oncol Biol Phys. 2010;77:1420–1425. doi: 10.1016/j.ijrobp.2009.06.049. [DOI] [PubMed] [Google Scholar]
- 105.American Joint Committee on C. AJCC cancer staging handbook. Vol 7th. New York: Springer-Verlag; 2010. [Google Scholar]
- 106.Tamm EP, Balachandran A, Bhosale PR, et al. Imaging of pancreatic adenocarcinoma: update on staging/resectability. Radiol Clin North Am. 2012;50:407–428. doi: 10.1016/j.rcl.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 107.Vauthey JN, Dixon E. AHPBA/SSO/SSAT Consensus Conference on Resectable and Borderline Resectable Pancreatic Cancer: rationale and overview of the conference. Ann Surg Oncol. 2009;16:1725–1726. doi: 10.1245/s10434-009-0409-5. [DOI] [PubMed] [Google Scholar]
- 108.Tseng JF, Raut CP, Lee JE, et al. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg. 2004;8:935–949. doi: 10.1016/j.gassur.2004.09.046. discussion 949–950. [DOI] [PubMed] [Google Scholar]
- 109.Qiu H, Wild AT, Wang H, et al. Comparison of conventional and 3-dimensional computed tomography against histopathologic examination in determining pancreatic adenocarcinoma tumor size: Implications for radiation therapy planning. Radiother Oncol. 2012;104:167–172. doi: 10.1016/j.radonc.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.House MG, Yeo CJ, Cameron JL, et al. Predicting resectability of periampullary cancer with three-dimensional computed tomography. J Gastrointest Surg. 2004;8:280–288. doi: 10.1016/j.gassur.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 111.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 112.Willett CG, Lewandrowski KB, Warshaw AL, Efird J, Corris PA. Resection margins in carcinoma of the head of the pancreas. Implications for radiation therapy. Annals of Surgery. 1993;217:144–148. doi: 10.1097/00000658-199302000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pawlik TM, Laheru D, Hruban RH, et al. Evaluating the impact of a single-day multidisciplinary clinic on the management of pancreatic cancer. Ann Surg Oncol. 2008;15:2081–2088. doi: 10.1245/s10434-008-9929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 115.Tempero MA, Arnoletti JP, Behrman SW, et al. Pancreatic Adenocarcinoma, version 2.2012: featured updates to the NCCN Guidelines. Journal of the National Comprehensive Cancer Network : JNCCN. 2012;10:703–713. doi: 10.6004/jnccn.2012.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single institution experience. JGastrointestSurg. 2006;10:1199–1211. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 117.Hsu CC, Wolfgang CL, Laheru DA, et al. Early mortality risk score: identification of poor outcomes following upfront surgery for resectable pancreatic cancer. J Gastrointest Surg. 2012;16:753–761. doi: 10.1007/s11605-011-1811-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833–846. doi: 10.1016/j.jamcollsurg.2007.12.020. discussion 846-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.van den Bosch RP, van Eijck CH, Mulder PG, Jeekel J. Serum CA19-9 determination in the management of pancreatic cancer. Hepatogastroenterology. 1996;43:710–713. [PubMed] [Google Scholar]
- 120.Gillen S, Schuster T, Meyer Zum Buschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Laurence JM, Tran PD, Morarji K, Eslick GD, Lam VW, Sandroussi C. A systematic review and meta-analysis of survival and surgical outcomes following neoadjuvant chemoradiotherapy for pancreatic cancer. J Gastrointest Surg. 2011;15:2059–2069. doi: 10.1007/s11605-011-1659-7. [DOI] [PubMed] [Google Scholar]
- 122.White RR, Paulson EK, Freed KS, et al. Staging of pancreatic cancer before and after neoadjuvant chemoradiation. J Gastrointest Surg. 2001;5:626–633. doi: 10.1016/s1091-255x(01)80105-0. [DOI] [PubMed] [Google Scholar]
- 123.Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:3487–3495. doi: 10.1200/JCO.2007.15.8642. [DOI] [PubMed] [Google Scholar]
- 124.Heinrich S, Pestalozzi BC, Schafer M, et al. Prospective phase II trial of neoadjuvant chemotherapy with gemcitabine and cisplatin for resectable adenocarcinoma of the pancreatic head. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:2526–2531. doi: 10.1200/JCO.2007.15.5556. [DOI] [PubMed] [Google Scholar]
- 125.Palmer DH, Stocken DD, Hewitt H, et al. A randomized phase 2 trial of neoadjuvant chemotherapy in resectable pancreatic cancer: gemcitabine alone versus gemcitabine combined with cisplatin. Ann Surg Oncol. 2007;14:2088–2096. doi: 10.1245/s10434-007-9384-x. [DOI] [PubMed] [Google Scholar]
- 126.Le Scodan R, Mornex F, Girard N, et al. Preoperative chemoradiation in potentially resectable pancreatic adenocarcinoma: feasibility, treatment effect evaluation and prognostic factors, analysis of the SFRO-FFCD 9704 trial and literature review. Ann Oncol. 2009;20:1387–1396. doi: 10.1093/annonc/mdp015. [DOI] [PubMed] [Google Scholar]
- 127.Crane CH, Varadhachary GR, Yordy JS, et al. Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: correlation of Smad4(Dpc4) immunostaining with pattern of disease progression. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:3037–3043. doi: 10.1200/JCO.2010.33.8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.De Jesus-Acosta A, Oliver GR, Blackford A, et al. A multicenter analysis of GTX chemotherapy in patients with locally advanced and metastatic pancreatic adenocarcinoma. Cancer chemotherapy and pharmacology. 2012;69:415–424. doi: 10.1007/s00280-011-1704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chatterjee D, Katz MH, Rashid A, et al. Perineural and intraneural invasion in posttherapy pancreaticoduodenectomy specimens predicts poor prognosis in patients with pancreatic ductal adenocarcinoma. Am J Surg Pathol. 2012;36:409–417. doi: 10.1097/PAS.0b013e31824104c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chatterjee D, Rashid A, Wang H, et al. Tumor invasion of muscular vessels predicts poor prognosis in patients with pancreatic ductal adenocarcinoma who have received neoadjuvant therapy and pancreaticoduodenectomy. Am J Surg Pathol. 2012;36:552–559. doi: 10.1097/PAS.0b013e318240c1c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006;10:1199–1210. doi: 10.1016/j.gassur.2006.08.018. discussion 1210-1191. [DOI] [PubMed] [Google Scholar]
- 132.Nathan H, Wolfgang CL, Edil BH, et al. Peri-operative mortality and long-term survival after total pancreatectomy for pancreatic adenocarcinoma: a population-based perspective. J Surg Oncol. 2009;99:87–92. doi: 10.1002/jso.21189. [DOI] [PubMed] [Google Scholar]
- 133.Karachristos A, Scarmeas N, Hoffman JP. CA 19-9 levels predict results of staging laparoscopy in pancreatic cancer. J Gastrointest Surg. 2005;9:1286–1292. doi: 10.1016/j.gassur.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 134.Jimenez RE, Warshaw AL, Rattner DW, Willett CG, McGrath D, Fernandez-del Castillo C. Impact of laparoscopic staging in the treatment of pancreatic cancer. Arch Surg. 2000;135:409–414. doi: 10.1001/archsurg.135.4.409. discussion 414-405. [DOI] [PubMed] [Google Scholar]
- 135.Conlon KC, Brennan MF. Laparoscopy for staging abdominal malignancies. Adv Surg. 2000;34:331–350. [PubMed] [Google Scholar]
- 136.Gagandeep S, Artinyan A, Jabbour N, et al. Extended pancreatectomy with resection of the celiac axis: the modified Appleby operation. Am J Surg. 2006;192:330–335. doi: 10.1016/j.amjsurg.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 137.Huebner M, Kendrick M, Reid-Lombardo KM, et al. Number of lymph nodes evaluated: prognostic value in pancreatic adenocarcinoma. J Gastrointest Surg. 2012;16:920–926. doi: 10.1007/s11605-012-1853-2. [DOI] [PubMed] [Google Scholar]
- 138.Kamath AS, Sarr MG, Nagorney DM, et al. Gastrointestinal stromal tumour of the duodenum: single institution experience. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2012;14:772–776. doi: 10.1111/j.1477-2574.2012.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kendrick ML, Sclabas GM. Major venous resection during total laparoscopic pancreaticoduodenectomy. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2011;13:454–458. doi: 10.1111/j.1477-2574.2011.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Venkat R, Edil BH, Schulick RD, Lidor AO, Makary MA, Wolfgang CL. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: a systematic review and meta-analysis. Ann Surg. 2012;255:1048–1059. doi: 10.1097/SLA.0b013e318251ee09. [DOI] [PubMed] [Google Scholar]
- 141.Kendrick ML, Cusati D. Total laparoscopic pancreaticoduodenectomy: feasibility and outcome in an early experience. Arch Surg. 2010;145:19–23. doi: 10.1001/archsurg.2009.243. [DOI] [PubMed] [Google Scholar]
- 142.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. New England Journal of Medicine. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 143.Herman JM, Swartz MJ, Hsu CC, et al. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: results of a large, prospectively collected database at the Johns Hopkins Hospital. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:3503–3510. doi: 10.1200/JCO.2007.15.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 145.Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2010;304:1073–1081. doi: 10.1001/jama.2010.1275. [DOI] [PubMed] [Google Scholar]
- 146.Gastrointestinal Tumor Study G. Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Cancer. 1987;59:2006–2010. doi: 10.1002/1097-0142(19870615)59:12<2006::aid-cncr2820591206>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 147.Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Annals of Surgery. 1999;230:776–782. doi: 10.1097/00000658-199912000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. New England Journal of Medicine. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 149.Abrams RA, Winter KA, Regine WF, et al. Failure to adhere to protocol specified radiation therapy guidelines was associated with decreased survival in RTOG 9704--a phase III trial of adjuvant chemotherapy and chemoradiotherapy for patients with resected adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 2012;82:809–816. doi: 10.1016/j.ijrobp.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shah AP, Strauss JB, Abrams RA. Review and commentary on the role of radiation therapy in the adjuvant management of pancreatic cancer. Am J Clin Oncol. 2010;33:101–106. doi: 10.1097/COC.0b013e31819171b9. [DOI] [PubMed] [Google Scholar]
- 151.Corsini MM, Miller RC, Haddock MG, et al. Adjuvant radiotherapy and chemotherapy for pancreatic carcinoma: the Mayo Clinic experience (1975–2005) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:3511–3516. doi: 10.1200/JCO.2007.15.8782. [DOI] [PubMed] [Google Scholar]
- 152.Hsu CC, Herman JM, Corsini MM, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma: the Johns Hopkins Hospital-Mayo Clinic collaborative study. Ann Surg Oncol. 2010;17:981–990. doi: 10.1245/s10434-009-0743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Regine WF, Winter KA, Abrams R, et al. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol. 2011;18:1319–1326. doi: 10.1245/s10434-011-1630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Picozzi VJ, Abrams RA, Decker PA, et al. Multicenter phase II trial of adjuvant therapy for resected pancreatic cancer using cisplatin, 5-fluorouracil, and interferon-alfa-2b-based chemoradiation: ACOSOG Trial Z05031. Ann Oncol. 2011;22:348–354. doi: 10.1093/annonc/mdq384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Picozzi VJ, Kozarek RA, Traverso LW. Interferon-based adjuvant chemoradiation therapy after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surgery. 2003;185:476–480. doi: 10.1016/s0002-9610(03)00051-5. [DOI] [PubMed] [Google Scholar]
- 156.Schmidt J, Abel U, Debus J, et al. Open-label, multicenter, randomized phase III trial of adjuvant chemoradiation plus interferon Alfa-2b versus fluorouracil and folinic acid for patients with resected pancreatic adenocarcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:4077–4083. doi: 10.1200/JCO.2011.38.2960. [DOI] [PubMed] [Google Scholar]
- 157.Ma WW, Herman JM, Jimeno A, et al. A tolerability and pharmacokinetic study of adjuvant erlotinib and capecitabine with concurrent radiation in resected pancreatic cancer. Translational oncology. 2010;3:373–379. doi: 10.1593/tlo.10196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lutz E, Yeo CJ, Lillemoe KD, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328–335. doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Weden S, Klemp M, Gladhaug IP, et al. Long-term follow-up of patients with resected pancreatic cancer following vaccination against mutant K-ras. International journal of cancer Journal international du cancer. 2011;128:1120–1128. doi: 10.1002/ijc.25449. [DOI] [PubMed] [Google Scholar]
- 160.Lepisto AJ, Moser AJ, Zeh H, et al. A phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer therapy. 2008;6:955–964. [PMC free article] [PubMed] [Google Scholar]
- 161.Minn AY, Koong AC, Chang DT. Stereotactic body radiation therapy for gastrointestinal malignancies. Frontiers of radiation therapy and oncology. 2011;43:412–427. doi: 10.1159/000322505. [DOI] [PubMed] [Google Scholar]
- 162.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. The New England journal of medicine. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 163.Loehrer PJ, Sr, Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:4105–4112. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sudo K, Yamaguchi T, Ishihara T, et al. Phase II study of oral S-1 and concurrent radiotherapy in patients with unresectable locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2011;80:119–125. doi: 10.1016/j.ijrobp.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 165.Colucci G, Labianca R, Di Costanzo F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:1645–1651. doi: 10.1200/JCO.2009.25.4433. [DOI] [PubMed] [Google Scholar]
- 166.Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:3605–3610. doi: 10.1200/JCO.2009.25.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sawaki A, Hoki N, Ito S, et al. Clinical impact of radiotherapy for locally advanced pancreatic cancer. J Gastroenterol. 2009;44:1209–1214. doi: 10.1007/s00535-009-0116-9. [DOI] [PubMed] [Google Scholar]
- 168.Crane CH, Winter K, Regine WF, et al. Phase II study of bevacizumab with concurrent capecitabine and radiation followed by maintenance gemcitabine and bevacizumab for locally advanced pancreatic cancer: Radiation Therapy Oncology Group RTOG 0411. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:4096–4102. doi: 10.1200/JCO.2009.21.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Moureau-Zabotto L, Phelip JM, Afchain P, et al. Concomitant administration of weekly oxaliplatin, fluorouracil continuous infusion, and radiotherapy after 2 months of gemcitabine and oxaliplatin induction in patients with locally advanced pancreatic cancer: a Groupe Coordinateur Multidisciplinaire en Oncologie phase II study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:1080–1085. doi: 10.1200/JCO.2007.12.8223. [DOI] [PubMed] [Google Scholar]
- 170.Duffy A, Kortmansky J, Schwartz GK, et al. A phase I study of erlotinib in combination with gemcitabine and radiation in locally advanced, non-operable pancreatic adenocarcinoma. Ann Oncol. 2008;19:86–91. doi: 10.1093/annonc/mdm441. [DOI] [PubMed] [Google Scholar]
- 171.Huguet F, Andre T, Hammel P, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:326–331. doi: 10.1200/JCO.2006.07.5663. [DOI] [PubMed] [Google Scholar]
- 172.Small W, Jr, Mulcahy MF, Rademaker A, et al. Phase II trial of full-dose gemcitabine and bevacizumab in combination with attenuated three-dimensional conformal radiotherapy in patients with localized pancreatic cancer. Int J Radiat Oncol Biol Phys. 2011;80:476–482. doi: 10.1016/j.ijrobp.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 173.Small W, Jr, Berlin J, Freedman GM, et al. Full-dose gemcitabine with concurrent radiation therapy in patients with nonmetastatic pancreatic cancer: a multicenter phase II trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:942–947. doi: 10.1200/JCO.2007.13.9014. [DOI] [PubMed] [Google Scholar]
- 174.Schneider BJ, Ben-Josef E, McGinn CJ, et al. Capecitabine and radiation therapy preceded and followed by combination chemotherapy in advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2005;63:1325–1330. doi: 10.1016/j.ijrobp.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 175.Katz MH, Wang H, Balachandran A, et al. Effect of neoadjuvant chemoradiation and surgical technique on recurrence of localized pancreatic cancer. J Gastrointest Surg. 2012;16:68–78. doi: 10.1007/s11605-011-1748-7. discussion 78-69. [DOI] [PubMed] [Google Scholar]
- 176.Krishnan S, Rana V, Janjan NA, et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer. 2007;110:47–55. doi: 10.1002/cncr.22735. [DOI] [PubMed] [Google Scholar]
- 177.Mishra G, Butler J, Ho C, et al. Phase II trial of induction gemcitabine/CPT-11 followed by a twice-weekly infusion of gemcitabine and concurrent external beam radiation for the treatment of locally advanced pancreatic cancer. Am J Clin Oncol. 2005;28:345–350. doi: 10.1097/01.coc.0000159559.42311.c5. [DOI] [PubMed] [Google Scholar]
- 178.Ko AH, Quivey JM, Venook AP, et al. A phase II study of fixed-dose rate gemcitabine plus low-dose cisplatin followed by consolidative chemoradiation for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2007;68:809–816. doi: 10.1016/j.ijrobp.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 179.Wahl RL, Herman JM, Ford E. The promise and pitfalls of positron emission tomography and single-photon emission computed tomography molecular imaging-guided radiation therapy. Seminars in radiation oncology. 2011;21:88–100. doi: 10.1016/j.semradonc.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Spalding AC, Jee KW, Vineberg K, et al. Potential for dose-escalation and reduction of risk in pancreatic cancer using IMRT optimization with lexicographic ordering and gEUD-based cost functions. Medical physics. 2007;34:521–529. doi: 10.1118/1.2426403. [DOI] [PubMed] [Google Scholar]
- 181.Yovino S, Maidment BW, 3rd, Herman JM, et al. Analysis of local control in patients receiving IMRT for resected pancreatic cancers. Int J Radiat Oncol Biol Phys. 2012;83:916–920. doi: 10.1016/j.ijrobp.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rich TA, Winter K, Safran H, et al. Weekly paclitaxel, gemcitabine, and external irradiation followed by randomized farnesyl transferase inhibitor R115777 for locally advanced pancreatic cancer. OncoTargets and therapy. 2012;5:161–170. doi: 10.2147/OTT.S33560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dawson LA, Eccles C, Bissonnette JP, Brock KK. Accuracy of daily image guidance for hypofractionated liver radiotherapy with active breathing control. Int J Radiat Oncol Biol Phys. 2005;62:1247–1252. doi: 10.1016/j.ijrobp.2005.03.072. [DOI] [PubMed] [Google Scholar]
- 184.Goldstein SD, Ford EC, Duhon M, McNutt T, Wong J, Herman JM. Use of respiratory-correlated four-dimensional computed tomography to determine acceptable treatment margins for locally advanced pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys. 2010;76:597–602. doi: 10.1016/j.ijrobp.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 185.Khashab MA, Kim KJ, Tryggestad EJ, et al. Comparative analysis of traditional and coiled fiducials implanted during EUS for pancreatic cancer patients receiving stereotactic body radiation therapy. Gastrointest Endosc. 2012;76:962–971. doi: 10.1016/j.gie.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Poulsen PR, Cho B, Keall PJ. A method to estimate mean position, motion magnitude, motion correlation, and trajectory of a tumor from cone-beam CT projections for image-guided radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:1587–1596. doi: 10.1016/j.ijrobp.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 187.Ben-Josef E, Schipper M, Francis IR, et al. A phase I/II trial of intensity modulated radiation (IMRT) dose escalation with concurrent fixed-dose rate gemcitabine (FDR-G) in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2012;84:1166–1171. doi: 10.1016/j.ijrobp.2012.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Murphy JD, Christman-Skieller C, Kim J, Dieterich S, Chang DT, Koong AC. A dosimetric model of duodenal toxicity after stereotactic body radiotherapy for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010;78:1420–1426. doi: 10.1016/j.ijrobp.2009.09.075. [DOI] [PubMed] [Google Scholar]
- 189.Fung MC, Takayama S, Ishiguro H, Sakata T, Adachi S, Morizane T. Chemotherapy for advanced or metastatic pancreatic cancer: analysis of 43 randomized trials in 3 decades (1974–2002) Gan To Kagaku Ryoho. 2003;30:1101–1111. [PubMed] [Google Scholar]
- 190.Burris HA, III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 191.Tempero M, Plunkett W, Ruiz VHV, et al. Randomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol. 2003;21:3402–3408. doi: 10.1200/JCO.2003.09.140. [DOI] [PubMed] [Google Scholar]
- 192.Poplin E, Feng Y, Berlin J, et al. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:3778–3785. doi: 10.1200/JCO.2008.20.9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Heinemann V, Boeck S, Hinke A, Labianca R, Louvet C. Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC cancer. 2008;8:82. doi: 10.1186/1471-2407-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sultana A, Smith CT, Cunningham D, Starling N, Neoptolemos JP, Ghaneh P. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:2607–2615. doi: 10.1200/JCO.2006.09.2551. [DOI] [PubMed] [Google Scholar]
- 195.Cunningham D, Chau I, Stocken DD, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:5513–5518. doi: 10.1200/JCO.2009.24.2446. [DOI] [PubMed] [Google Scholar]
- 196.Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:3509–3516. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 197.Heinemann V, Quietzsch D, Gieseler F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:3946–3952. doi: 10.1200/JCO.2005.05.1490. [DOI] [PubMed] [Google Scholar]
- 198.Cui Y, Brosnan JA, Blackford AL, et al. Genetically defined subsets of human pancreatic cancer demonstrate unique in vitro chemosensitivity. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-12-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Von Hoff D, E TJ, A FP. Randomized Phase III study of weekly nab-paclitacel plus gemcitabine versus gemcitabine along in patients with metastatic adenocarcinoma of the pancreas (MPACT) Journal of Clinical Oncology. 2013;30:148. [Google Scholar]
- 200.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 201.Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:3617–3622. doi: 10.1200/JCO.2010.28.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Xiong HQ, Varadhachary GR, Blais JC, Hess KR, Abbruzzese JL, Wolff RA. Phase 2 trial of oxaliplatin plus capecitabine (XELOX) as second-line therapy for patients with advanced pancreatic cancer. Cancer. 2008;113:2046–2052. doi: 10.1002/cncr.23810. [DOI] [PubMed] [Google Scholar]
- 203.Pelzer U, Schwaner I, Stieler J, et al. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer. 2011;47:1676–1681. doi: 10.1016/j.ejca.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 204.Kulke MH, Blaszkowsky LS, Ryan DP, et al. Capecitabine plus erlotinib in gemcitabine-refractory advanced pancreatic cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:4787–4792. doi: 10.1200/JCO.2007.11.8521. [DOI] [PubMed] [Google Scholar]
- 205.Arnaoutakis GJ, Rangachari D, Laheru DA, et al. Pulmonary resection for isolated pancreatic adenocarcinoma metastasis: an analysis of outcomes and survival. J Gastrointest Surg. 2011;15:1611–1617. doi: 10.1007/s11605-011-1605-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yap JL, Worlikar S, MacKerell AD, Jr, Shapiro P, Fletcher S. Small-molecule inhibitors of the ERK signaling pathway: Towards novel anticancer therapeutics. ChemMedChem. 2011;6:38–48. doi: 10.1002/cmdc.201000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Collisson EA, Trejo CL, Silva JM, et al. A central role for RAF-->MEK-->ERK signaling in the genesis of pancreatic ductal adenocarcinoma. Cancer Discov. 2012;2:685–693. doi: 10.1158/2159-8290.CD-11-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hwang RF, Moore T, Arumugam T, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Erkan M, Reiser-Erkan C, Michalski CW, Kleeff J. Tumor microenvironment and progression of pancreatic cancer. Experimental oncology. 2010;32:128–131. [PubMed] [Google Scholar]
- 210.Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yang S, Kimmelman AC. A critical role for autophagy in pancreatic cancer. Autophagy. 2011;7:912–913. doi: 10.4161/auto.7.8.15762. [DOI] [PubMed] [Google Scholar]
- 212.Yang S, Wang X, Contino G, et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25:717–729. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lim JP, Gleeson PA. Macropinocytosis: an endocytic pathway for internalising large gulps. Immunology and cell biology. 2011;89:836–843. doi: 10.1038/icb.2011.20. [DOI] [PubMed] [Google Scholar]
- 214.Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends in biochemical sciences. 2010;35:427–433. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kaelin WG, Jr, Thompson CB. Q&A: Cancer: clues from cell metabolism. Nature. 2010;465:562–564. doi: 10.1038/465562a. [DOI] [PubMed] [Google Scholar]
- 216.Le A, V RN, A M, Dang CV. Conceptual framework for cutting the pancreatic cancer fuel supply. Clinical Cancer Research. 2012;18 doi: 10.1158/1078-0432.CCR-12-0041. xx-xx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yan BM, Myers RP. Neurolytic celiac plexus block for pain control in unresectable pancreatic cancer. The American journal of gastroenterology. 2007;102:430–438. doi: 10.1111/j.1572-0241.2006.00967.x. [DOI] [PubMed] [Google Scholar]
- 218.Wong GY, Schroeder DR, Carns PE, et al. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2004;291:1092–1099. doi: 10.1001/jama.291.9.1092. [DOI] [PubMed] [Google Scholar]
- 219.Erdek MA, Halpert DE, Gonzalez Fernandez M, Cohen SP. Assessment of celiac plexus block and neurolysis outcomes and technique in the management of refractory visceral cancer pain. Pain Med. 2010;11:92–100. doi: 10.1111/j.1526-4637.2009.00756.x. [DOI] [PubMed] [Google Scholar]
- 220.McGreevy K, Hurley RW, Erdek MA, Aner MM, Li S, Cohen SP. The Effectiveness of Repeat Celiac Plexus Neurolysis for Pancreatic Cancer: A Pilot Study. Pain practice : the official journal of World Institute of Pain. 2012 doi: 10.1111/j.1533-2500.2012.00557.x. [DOI] [PubMed] [Google Scholar]
- 221.Deer TR, Prager J, Levy R, et al. Polyanalgesic Consensus Conference 2012: recommendations for the management of pain by intrathecal (intraspinal) drug delivery: report of an interdisciplinary expert panel. Neuromodulation : journal of the International Neuromodulation Society. 2012;15:436–464. doi: 10.1111/j.1525-1403.2012.00476.x. discussion 464-436. [DOI] [PubMed] [Google Scholar]
- 222.Smith TJ, Staats PS, Deer T, et al. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: impact on pain, drug-related toxicity, and survival. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:4040–4049. doi: 10.1200/JCO.2002.02.118. [DOI] [PubMed] [Google Scholar]
- 223.Boyd AD, Brown D, Henrickson C, et al. Screening for depression, sleep-related disturbances, and anxiety in patients with adenocarcinoma of the pancreas: a preliminary study. TheScientificWorldJournal. 2012;2012:650707. doi: 10.1100/2012/650707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Boyd CA, Benarroch-Gampel J, Sheffield KM, Han Y, Kuo YF, Riall TS. The effect of depression on stage at diagnosis, treatment, and survival in pancreatic adenocarcinoma. Surgery. 2012;152:403–413. doi: 10.1016/j.surg.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review. Bethesda, MD: National Cancer Institute; 1975–2008. [Google Scholar]
- 226.Cancer risks in BRCA2 mutation carriers.The Breast Cancer Linkage Consortium. JNatlCancer Inst. 1999;91:1310–1316. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- 227.Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119:1447–1453. doi: 10.1053/gast.2000.20228. [DOI] [PubMed] [Google Scholar]
- 228.van Lier MG, Wagner A, Mathus-Vliegen EM, Kuipers EJ, Steyerberg EW, van Leerdam ME. High cancer risk in Peutz-Jeghers syndrome: a systematic review and surveillance recommendations. The American journal of gastroenterology. 105:1258–1264. doi: 10.1038/ajg.2009.725. author reply 1265. [DOI] [PubMed] [Google Scholar]
- 229.Giardiello FM, Welsh SB, Hamilton SR, et al. Increased risk of cancer in the Peutz-Jeghers syndrome. NEnglJMed. 1987;316:1511–1514. doi: 10.1056/NEJM198706113162404. [DOI] [PubMed] [Google Scholar]
- 230.Goldstein AM, Struewing JP, Fraser MC, Smith MW, Tucker MA. Prospective risk of cancer in CDKN2A germline mutation carriers. Journal of medical genetics. 2004;41:421–424. doi: 10.1136/jmg.2004.019349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Petersen GM, Amundadottir L, Fuchs CS, et al. A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat Genet. 2010;42:224–228. doi: 10.1038/ng.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 233.Riall TS, Cameron JL, Lillemoe KD, et al. Resected periampullary adenocarcinoma: 5-year survivors and their 6- to 10-year follow-up. Surgery. 2006;140:764–772. doi: 10.1016/j.surg.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 234.Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2008;299:1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 235.Rocha Lima CM, Green MR, Rotche R, et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:3776–3783. doi: 10.1200/JCO.2004.12.082. [DOI] [PubMed] [Google Scholar]
- 236.Heinemann V, Quietzsch D, Gieseler M, et al. A phase III trial comparing gemcitabine plus cisplatin vs. gemcitabine alone in advanced pancreatic carcinoma. Proc Am Soc Clin Oncol. 2003;22:250. [Google Scholar]
- 237.Abou-Alfa GK, Letourneau R, Harker G, et al. Randomized phase III study of exatecan and gemcitabine compared with gemcitabine alone in untreated advanced pancreatic cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:4441–4447. doi: 10.1200/JCO.2006.07.0201. [DOI] [PubMed] [Google Scholar]
- 238.Oettle H, Richards D, Ramanathan RK, et al. A phase III trial of pemetrexed plus gemcitabine versus gemcitabine in patients with unresectable or metastatic pancreatic cancer. Ann Oncol. 2005;16:1639–1645. doi: 10.1093/annonc/mdi309. [DOI] [PubMed] [Google Scholar]
- 239.Kulke MH, Tempero MA, Niedzwiecki D, et al. Randomized phase II study of gemcitabine administered at a fixed dose rate or in combination with cisplatin, docetaxel, or irinotecan in patients with metastatic pancreatic cancer: CALGB 89904. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:5506–5512. doi: 10.1200/JCO.2009.22.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]