Abstract
Introduction
Two 7-fluoroimidazobenzodiazepines (AH114726 and GEH120348), analogs of flumazenil, were labeled with fluorine-18 and evaluated as alternative radioligands for in vivo imaging of the GABAA/benzodiazepine receptor by comparing them to [11C]flumazenil in rhesus monkey.
Methods
Radiotracers were prepared from the corresponding nitro-precursors in an automated synthesis module, and primate imaging studies were conducted on a Concorde MicroPET P4 scanner. The brain was imaged for 60 (12 × 5 min frames) or 90 min (18 × 5 min frames), and data was reconstructed using the 3D MAP algorithm. Specificity of [18F]AH114726 and [18F]GEH120348 was confirmed by displacement studies using unlabeled flumazenil.
Results
[18F]GEH120348 and [18F]AH114726 were obtained in 13–24% yields (end of synthesis) with high chemical (>95%) and radiochemical (>99%) purities, and high specific activities (2061 ± 985 Ci/mmol). The in vivo pharmacokinetics of [18F]AH114726 and [18F]GEH120348 were determined in a non-human primate and directly compared with [11C]flumazenil. Both fluorine-18 radioligands showed time-dependent regional brain distributions that correlated with the distribution of [11C]flumazenil and the known concentrations of GABAA/benzodiazepine receptors in the monkey brain. [18F]AH114726 exhibited maximal brain uptake and tissue time-radioactivity curves that were most similar to [11C]flumazenil. In contrast, [18F]GEH120348 showed higher initial brain uptake but very different pharmacokinetics, with continued accumulation of radioactivity into the cortical regions of high GABA/benzodiazepine receptor concentrations and very little clearance from the regions of low receptor densities. Rapid washout of both radiotracers occurred upon treatment with unlabeled flumazenil.
Conclusion
The ease of the radiochemical synthesis, together with in vivo brain pharmacokinetics most similar to [11C]flumazenil, support that [18F]AH114726 is a suitable option for imaging the GABAA receptor.
1. Introduction
In vivo imaging of GABAA/benzodiazepine receptors in the brain using positron emission tomography (PET) has provided valuable information regarding various neurological conditions and psychiatric disorders. [11C]Flumazenil ([11C]FMZ, ethyl 8-fluoro-5-methyl-6-oxo-5,6-dihydro-4H-benzo[f]imidazo[1,5-a][1,4]diazepine-3-carboxylate, Figure 1) was the first radioligand developed for in vivo imaging of the benzodiazepine binding site of the GABAA receptor and is currently the most widely used [1, 2]. [11C]FMZ clinical use has been reported in the evaluation of patients with Alzheimer’s disease, epilepsy, panic disorders, major depression, cortical brain damage following an acute stroke, anxiety disorders, chronic alcohol dependency, and other brain disorders [3].
Figure 1.
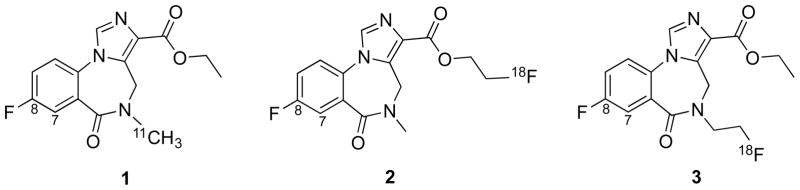
Structures of (1) [11C]FMZ; (2) [18F]FFMZ; (3) [18F]FEFMZ
There has been extensive evaluation of fluorine-18 labeled agents for GABAA imaging which would circumvent the limitations imposed for [11C]FMZ by eliminating the necessity for an on-site cyclotron, thus providing potential radiopharmaceutical distribution to remote imaging facilities. Flumazenil (Figure 1.1) contains a fluorine substituent, therefore rendering [18F]flumazenil ([18F]FMZ) an alternative to its carbon-11-labeled counterpart. The radiochemical synthesis of [18F]FMZ was first reported using 18F-for-19F exchange [4, 5], and subsequently by nucleophilic aromatic substitution of the meta-nitro precursor with [18F]fluoride ion [6–8], and via a diaryliodonium precursor [9]. When directly compared to [11C]FMZ in human studies, [18F]FMZ showed equivalent kinetic and metabolic behaviors [10]. In the search for alternative labeled benzodiazepines with improved pharmacokinetics and/or simplified radiochemical syntheses, additional analogs of flumazenil incorporating fluorine-18 have been reported. Replacement of the ethyl ester or the N-methyl group with a 2-[18F]fluoroethyl group yielded the potential radiotracers [18F]FFMZ and [18F]FEFMZ (Figure 1, compounds 2 and 3) [11, 12]. Neither of these alternative fluorine-18 benzodiazepines however exhibited satisfactory in vivo properties when compared with [18F]FMZ. More recently, Jackson et. al. reported a lengthy series of new FMZ analogs suitable for fluorine-18 labeling [13]. Of particular interest were the analogs where the fluorine substituent was moved from the 8- to the 7-position (Figure 2). This provided high affinity FMZ analogs (AH114726 (4), Ki = 5.5 nM, and GEH120348 (5), Ki = 0.76 nM, Fig 2) with simplified radiochemistry. The efficiency of nucleophilic aromatic substitution by [18F]fluoride ion can be improved by placing the leaving group in the position ortho to the carbonyl [14]. In preliminary studies, these new compounds showed good rodent brain uptake, differential binding to GABAA-rich brain regions and a lack of brain metabolites (A. Jackson, GE Healthcare, unpublished results). AH114726 was screened against >50 different brain receptors, ion channels and transporters using a single point high concentration assay, and 10 μM was found to inhibit 96% of GABAA. No other significant inhibition of other targets was noted. It is also expected that these new compounds will have comparable subunit selectivity comparable to flumazenil, [15, 16] but this has yet to be confirmed. The purpose of the present study was to evaluate the in vivo pharmacokinetics of [18F]AH114726 and [18F]GEH120348 in non-human primates, and directly compare such with [11C]FMZ.
Figure 2.
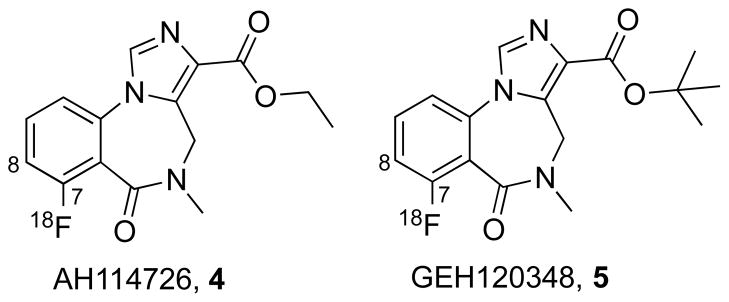
Structures of the 7-[18F]fluoro imidazobenzodiazepines
2. Materials and Methods
2.1 Materials
Chemicals and solvents were purchased from Sigma-Aldrich (Milwaukee, WI) or Fisher Scientific (Fair Lawn, NJ) and used without further purification. Unlabeled precursors and reference standards of [19F]GEH120348 and [19F]AH114726 were provided from GE Healthcare (Princeton, NJ). The quality control HPLC column: Luna C18(2) 5μ 150 × 4.6 mm, and the semi-preparative column: Luna C18 5μ 250 × 10 mm were purchased from Phenomenex (Torrance, CA). For preparation of [18F]GEH120348 and [18F]AH114726, Sep-Pak C18 1cc cartridges were purchased from Waters (Milford, MA) and conditioned with absolute ethanol (10 mL) and water (10 mL) prior to use. QMA-light Sep-Pak cartridges were purchased from Waters and conditioned with absolute ethanol (10 mL), 0.5 M aq. sodium bicarbonate (10 mL), and water (10 mL) prior to use.
2.2 Syntheses of [18F]GEH120348 and [18F]AH114726
[18F]GEH120348 and [18F]AH114726 were prepared using an automated GE TRACERLab™ FX F-N. [18F]Fluoride was produced via the 18O(p, n)18F nuclear reaction using a (GEMS) PETTrace cyclotron (40 μA beam for 15 min) to generate 33.3 GBq (900 mCi) of [18F]fluoride. The [18F]fluoride was delivered from the cyclotron (in a 2-ml bolus of [18O]water and trapped on a QMA-light Sep-Pak to remove [18O]water [18F]Fluoride was then eluted into the reaction vessel using aqueous potassium carbonate (3.5 mg in 0.5 mL of water). A solution of Kryptofix-[2.2.2] (15 mg in 1 mL of acetonitrile) was then added to the reaction vessel and the [18F]fluoride was dried by evaporating the water – acetonitrile azeotrope. Evaporation was achieved by heating the reaction vessel to 80°C and drawing full vacuum for 4 min. After this time, the reaction vessel was cooled to 60°C and subjected to both an argon stream and vacuum draw simultaneously for another 4 min.
A solution of precursor 6 or 7 (5 mg) in anhydrous DMF (1 mL) was added to the dried [18F]fluoride, and the solution was heated to 130°C with stirring for 30 min. The reaction mixture was cooled to 50°C and diluted with semi-preparative HPLC mobile phase (3 mL). The diluted reaction mixture was then purified by semi-preparative HPLC (column: Phenomonex Luna C18(2) 5μ 250 × 10 mm). For AH114726 the semi-preparative HPLC mobile phase was 75% 10 mM phosphoric acid in 25% acetonitrile (v/v), flow rate of 4.0 mL/min. For GEH120348 the semi-preparative HPLC mobile phase was 70% 10mM phosphoric acid in 30% acetonitrile, flow rate of 4.0 mL/min. The fractions corresponding to [18F]GEH120348 (typically eluting out between 15 and 17 min) or [18F]AH114726 (typically eluting between 11 and 14 min) were collected and transferred into a dilution flask containing sterile water (50 mL). The resulting solution was transferred through a Waters C18 1cc Sep-Pak to collect the desired product. The C18 Sep-Pak was then washed with sterile water (10 mL) to remove unwanted hydrophilic impurities and residual acetonitrile to waste. [18F]GEH120348 or [18F]AH114726 were eluted off into a collection vial with ethanol for injection, USP (0.5 mL) and 0.9% sodium chloride for injection, USP (9.5 mL). The final formulation (10 mL) was then passed through a 0.22 μM Millex-GV sterile filter (EMD Millipore, Billerica, MA) into a sterile vial to provide [18F]GEH120348 or [18F]AH114726 (typically 5 GBq (135 mCi), n=3) in an isotonic solution released for quality control testing.
2.3 Quality control
All doses were examined visually and were required to be clear, colorless and free of particulate matter. The pH of each dose was analyzed by applying a small amount of the dose to colorpHast pH 2.0–9.0 non-bleeding pH-indicator strips (EMD Millipore, Billerica, MA) and determined by visual comparison with the scale provided. Radionuclide identity was confirmed by determining the half-life of the dose and comparing it with the known half-life of fluorine-18 (110 min). Activities were measured using a Capintec CRC-15R Radioisotope Dose Calibrator and half-life was calculated. Residual Kryptofix-[2.2.2] levels in the [18F]GEH120348 and [18F]AH114726 doses were analyzed using the established spot test [14]. Residual Kryptofix-[2.2.2] levels of <50 μg/mL are acceptable and all doses of [18F]GEH120348 or [18F]AH114726 prepared in this study were in compliance.
HPLC analysis of radiochemical purity was conducted using a Shimadzu LC-2010AHT Liquid Chromatograph fitted with a UV detector and a Bioscan gamma-detector. Chromatographic separation was performed on a Phenomenex Luna C18(2) 5μ 150 × 4.6 mm column. The QC mobile phase conditions for [18F]GEH120348 were as follows; 25% acetonitrile: 75% H2O, oven 40°C, 254 nm, flow rate: 1.25 mL/min, RT=14.8 min, RCP ≥99.7%. The QC mobile phase conditions for [18F]AH114726 were as follows; 20% acetonitrile: 80% H2O, oven 40°C, 254 nm, flow rate: 0.9 mL/min, RT=11.8 min, RCP ≥99.7%.
2.4 Primate microPET imaging
All animal studies were performed at the University of Michigan in accordance with the standards set by the University Committee on Use and Care of Animals (UCUCA). Studies were performed in a single young mature female rhesus monkey (5.8 kg with negligible variation over the duration of the study), at intervals of not less than a week between studies, according to the study design outlined in Table 1. PET imaging was done using the Concorde Microsystems P4 tomograph. The animal was anesthetized (isoflurane) and intubated, a venous catheter was inserted into one hindlimb and the animal positioned on the bed of the MicroPET gantry. A head-holder was used to prevent motion artifacts. Isoflurane anesthesia was continued throughout the study. After completion of the transmission scan, animals were injected with [18F]GEH120348 or [18F]AH114726 (1.04–5.85 mCi in 1–3 ml isotonic saline) as a bolus over 1 min. Collection of emission data was started with radiotracer injection and continued for 60 (12 × 5 min frames) for carbon-11 labeled radiotracers or 90 min (18 × 5 min frames) for fluorine-18 labeled radiotracers. For the unlabeled FMZ displacement study, FMZ (1 mg/kg in 10% ethanol in saline ± a droplet of DMSO to aid solubility as required) was injected intravenously at 40 min after the radiotracer injection. All studies were done in duplicate.
Table 1.
Study Design
| Study | [11C]FMZ Baseline | [18F]GEH120348 Baseline | [18F]GEH120348 Blocking | [18F]AH114726 Baseline | [18F]AH114726 Blocking |
|---|---|---|---|---|---|
| n | 2 | 2 | 2 | 2 | 2 |
Emission data were corrected for attenuation and scatter, and reconstructed using the 3D maximum a priori method (3D MAP algorithm). Using a summed image of the entire data set, regions of interest (ROIs) were drawn manually on multiple planes to obtain volumetric ROIs for the striatum, thalamus, three cortical areas, cerebellum and pons. The volumetric ROIs were then applied to the full dynamic data set to obtain the regional tissue time-radioactivity data.
3. Results
3.1 Radiochemistry
The syntheses of [18F]GEH120348 and [18F]AH114726 were achieved by direct nucleophilic aromatic fluorination by substituting the nitro group on precursors 6 or 7 with [18F]fluoride (Scheme 1). [18F]GEH120348 and [18F]AH114726 were obtained in 13–24% yields (end of synthesis) with high chemical (>95%) and radiochemical (>99%) purities, and high specific activities (2061 ± 985 Ci/mmol).
Scheme 1.
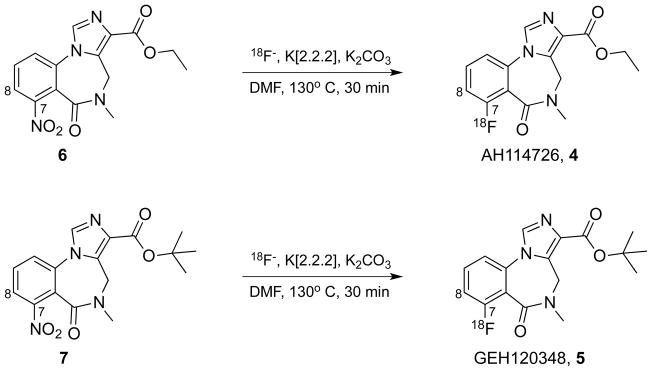
Syntheses of compounds [18F]AH114726 and [18F]GEH120348
3.2 Primate MicroPET Imaging
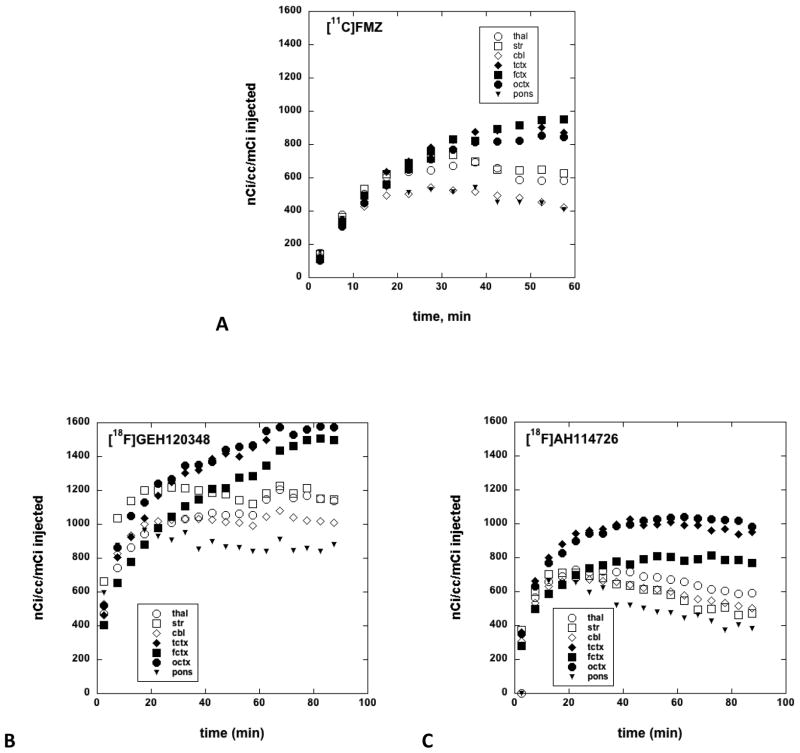
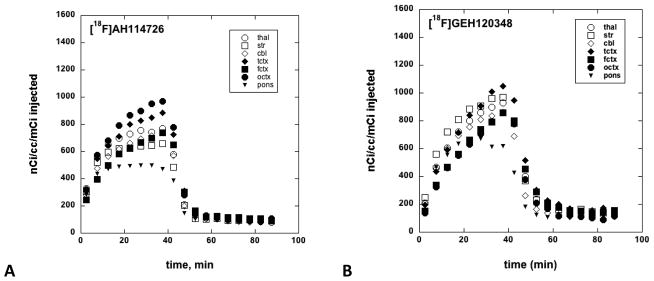
Representative coronal images of control animals (Figure 3, top row), and after displacement with unlabeled flumazenil (Figure 3, bottom row) were used to generate brain tissue time-radioactivity curves for [11C]FMZ, [18F]GEH120348, and [18F]AH114726. The time-radioactivity curves confirmed that after i.v. injection of each radioligand, the brain uptake of radioactivity was rapid (Figure 4). The initial brain uptake and pharmacokinetics of [11C]FMZ and [18F]AH114726 were most similar, with highest concentrations in the cortex and lowest in the pons. In contrast, [18F]GEH120348 also showed the expected regional distribution of radioactivity, however the tracer showed very different kinetic behavior, with significantly higher initial brain uptake and slower pharmacokinetics throughout the brain regions. Injection of a high dose of unlabeled flumazenil (1 mg/kg) at 40 minutes initiated a rapid washout of radioactivity of both [18F]-labeled radioligands, with nearly complete clearance by the end of the imaging study (Figure 3, bottom row and Figure 5).
Figure 3.
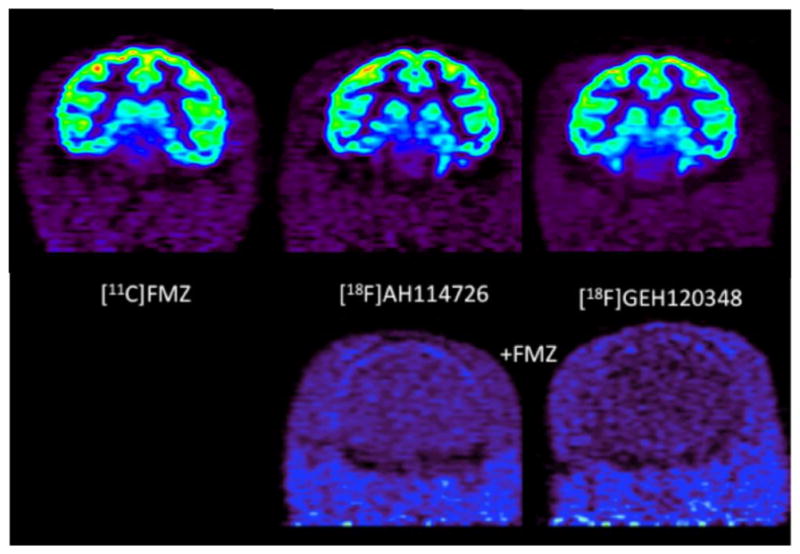
Representative coronal microPET images of control animals imaged with [11C]FMZ (summed images 40–60 minutes after i.v. injection of the radiotracer), [18F]AH114726 and [18F]GEH120348 (summed images 40–90 minutes after i.v. injection of the radiotracer) (Top Row), and after displacement with 1 mg/kg flumazenil (summed images 40–0 minutes after i.v. injection of the radiotracer (0–50 minutes after displacement with flumazenil)) (Bottom Row)
Figure 4.
Brain tissue time-radioactivity curves for [11C]FMZ (A), [18F]GEH120348 (B), and [18F]AH114726 (C). Brain regions: thal = thalamus, str = striatum, cbl = cerebellum, tctx = temporal cortex, fctx = frontal cortex, octx = occipital cortex.
Figure 5.
Brain tissue time-radioactivity curves for displacement experiments using unlabeled flumazenil (1 mg/kg, iv) with [18F]AH114726 (A) and [18F]GEH120348 (B).
4. Discussion
The 7-fluoro imidazobenzodiazepines evaluated in this study were developed as alternative structures for synthesis of fluorine-18 labeled radioligands for in vivo imaging of the GABAA/BzR system. The simplified radiochemical syntheses worked as expected, proving reliable and giving high yields of [18F]AH114726 and [18F]GEH120348 (13–24% at end of synthesis based upon starting fluoride). Amounts prepared were hundreds of millicuries, confirming the suitability of the radiosyntheses for use in future clinical production.
Evaluation of [11C]FMZ, [18F]AH114726 and [18F]GEH120348 in monkey brain showed time-dependent regional brain distributions for all radioligands that correlated with the known distribution of GABAA/Bz receptors in the monkey brain, with the highest specific binding in the cortex and the lowest in the pons. Of the two new radioligands, [18F]AH114726 exhibited brain uptake and tissue time-radioactivity curves that were most similar to those obtained with [11C]FMZ (Figure 4). Compared to [18F]AH114726 or [11C]FMZ, [18F]GEH120348 showed higher initial brain uptake but very different pharmacokinetics, with continued accumulation of radioactivity into the cortical regions of high GABA/BzR concentrations and very little clearance from the regions of low receptor densities (striatum, cerebellum) (Figure 4). It can be reasoned that the higher initial brain uptake of [18F]GEH120348 is due to increased lipophilicity (log D7.4 values: FMZ, 1.12; AH114726, 0.69; GEH120348, 1.62)[13]. The slower clearance of [18F]GEH120348 might be attributed to the higher binding affinity when compared with [18F]AH114726 (Ki values: FMZ, 1.3 nM; AH114726, 5.5 nM; GEH120348, 0.76 nM), or due to a combination of differences in affinity and lipophilicity. The pharmacokinetics of [18F]GE120348 in the monkey brain are remarkably similar to the results reported for [123I]iomazenil [17], the 7-iodo analog of flumazenil with a similar high affinity (KD = 0.5 nM, [18] and higher lipophilicity. An additional point of note is the regional differences we observed in the cortical uptake of [18F]AH114726 and [18F]GEH120348 when compared to [11C]FMZ, although there is no obvious explanation for such differences at this time.
Finally, specificity of binding for both fluorine-18 labeled radioligands was demonstrated by rapid and nearly complete washout of radioactivity from all brain regions after injection of unlabeled flumazenil (Figure 5), as has been previously demonstrated for [11C]FMZ and a comparative displacing dose of flumazenil [6].
5. Conclusion
The 7-[18F]fluoro imidazobenzodiazepine analogs of FMZ are suitable alternatives to the 8-[18F]fluoro imidazobenzodiazepine derivatives (such as [18F]FMZ, [18F]FFMZ, and [18F]FEFMZ). The ease of the radiochemical synthesis, together with in vivo brain pharmacokinetics most similar to [11C]flumazenil, support [18F]AH114726 as a suitable tracer for imaging the GABAA/benzodiazepine receptor.
Acknowledgments
Research reported in this publication was supported in part by the National Institute of Biomedical Imaging and Bioengineering, part of the National Institutes of Health, under Award Number T32-EB005172. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional financial support from GE Healthcare is also gratefully acknowledged. Finally, the authors thank Ian Wilson and Matthew S. Morrison for their support and input into study design and ethical review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Persson A, Ehrin E, Eriksson L, Farde L, Hedstroem CG, Litton JE, et al. Imaging of carbon-11-labeled Ro 15–1788 binding to benzodiazepine receptors in the human brain by positron emission tomography. J Psychiatr Res. 1985;19:609–22. doi: 10.1016/0022-3956(85)90080-9. [DOI] [PubMed] [Google Scholar]
- 2.Pike VW, Halldin C, Crouzel C, Barre L, Nutt DJ, Osman S, et al. Radioligands for PET studies of central benzodiazepine receptors and PK (peripheral benzodiazepine) binding sites-current status. Nucl Med Biol. 1993;20:503–25. doi: 10.1016/0969-8051(93)90082-6. [DOI] [PubMed] [Google Scholar]
- 3.Heiss W-D, Herholz K. Brain receptor imaging. J Nucl Med. 2006;47:302–12. [PubMed] [Google Scholar]
- 4.Krasikova RN, Ryzhikov NN, Gomzina NA, Vassiliev DA, Kostikov AP, Fedorova OS. Isotopic 18F/19F exchange in the flumazenil molecule using K18F/kryptofix complex. J. Label Compd. Radiopharm. 2003;46:S213. [Google Scholar]
- 5.Ryzhikov NN, Gomzina NA, Fedorova OS, Vasil’ev DA, Kostikov AP, Krasikova RN. Preparation of [18F]flumazenil, a potential radioligand for PET imaging of central benzodiazepine receptors, by isotope exchange. Radiochemistry. 2004;46:290–4. [Google Scholar]
- 6.Ryzhikov NN, Seneca N, Krasikova RN, Gomzina NA, Shchukin E, Fedorova OS, et al. Preparation of highly specific radioactivity [18F]flumazenil and its evaluation in cynomolgus monkey by positron emission tomography. Nucl Med Biol. 2005;32:109–16. doi: 10.1016/j.nucmedbio.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Massaweh G, Schirrmacher E, La Fougere C, Kovacevic M, Wängler C, Jolly D, et al. Improved work-up procedure for the production of [18F]flumazenil and first results of its use with a high-resolution research tomograph in human stroke. Nucl. Med. Biol. 2009;36:721–7. doi: 10.1016/j.nucmedbio.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Mandap KS, Ido T, Kiyono Y, Kobayashi M, Lohith TG, Mori T, et al. Development of microwave-based automated nucleophilic [18F]fluorination system and its application to the production of [18F]flumazenil. Nucl. Med. Biol. 2009;36:403–9. doi: 10.1016/j.nucmedbio.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Moon BS, Kil HS, Park JH, Kim JS, Park J, Chi DY, et al. Facile aromatic radiofluorination of [18F]flumazenil from diaryliodonium salts with evaluation of their stability and selectivity. Org. Biomol. Chem. 2011;9:8346–55. doi: 10.1039/c1ob06277h. [DOI] [PubMed] [Google Scholar]
- 10.Odano I, Halldin C, Karlsson P, Varrone A, Airaksinen Anu J, Krasikova Raisa N, et al. [18F]flumazenil binding to central benzodiazepine receptor studies by PET--quantitative analysis and comparisons with [11C] flumazenil. Neuroimage. 2009;45:891–902. doi: 10.1016/j.neuroimage.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Grunder G, Siessmeier T, Lange-Asschenfeldt C, Vernaleken I, Buchholz HG, Stoeter P, et al. [18F]Fluoroethylflumazenil: a novel tracer for PET imaging of human benzodiazepine receptors. Eur J Nucl Med. 2001;28:1463–70. doi: 10.1007/s002590100594. [DOI] [PubMed] [Google Scholar]
- 12.Mitterhauser M, Wadsak W, Wabnegger L, Mien L-K, Tögel S, Langer O, et al. Biological evaluation of 2′-[18F]fluoroflumazenil ([18F]FFMZ), a potential GABA receptor ligand for PET. Nucl Med Biol. 2004;31:291–5. doi: 10.1016/j.nucmedbio.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Jackson A, Guilbert BB, Plant SD, Goggi J, Battle MR, Woodcraft JL, et al. The development of potential new fluorine-18 labelled radiotracers for imaging the GABA(A) receptor. Bioorg Med Chem Lett. 2013;23:821–6. doi: 10.1016/j.bmcl.2012.11.066. [DOI] [PubMed] [Google Scholar]
- 14.Kilbourn MR. Fluorine-18 Labeling of Radiopharmaceuticals. Washington, D.C: National Academy Press; 1990. [Google Scholar]
- 15.Whitman JG, Amrein R. Pharmacology of flumazenil. Acta Anaesthesiol Scand. 1995;39 (Suppl 108):3–14. doi: 10.1111/j.1399-6576.1995.tb04374.x. [DOI] [PubMed] [Google Scholar]
- 16.Atack JR, Smith AJ, Emms F, McKernan RM. Regional differences in the inhibition of mouse in vivo [3H]Ro 15–1788 binding reflect selectivity for α1 versus α2 and α3 subunit-containing GABAA receptors. Neuropsychpharmacol. 1999;20:255–62. doi: 10.1016/S0893-133X(98)00052-9. [DOI] [PubMed] [Google Scholar]
- 17.Inoue O, Hosoi R, Kobayashi K, Itoh T, Gee A, Suzuki K. Different sensitivities to competitive inhibition of benzodiazepine receptor binding of 11C-iomazenil and 11C-flumazenil in rhesus monkey brain. Ann Nucl Med. 2001;15:137–9. doi: 10.1007/BF02988604. [DOI] [PubMed] [Google Scholar]
- 18.Johnson EW, Woods SW, Zoghbi S, McBride BJ, Baldwin RM, Innis RB. Receptor binding characterization of the benzodiazepine radioligand 125I-Ro16–0154: potential probe for SPECT brain imaging. Life Sci. 1990;47:1535–46. doi: 10.1016/0024-3205(90)90182-q. [DOI] [PubMed] [Google Scholar]