Abstract
Measurement of neuropeptides in the brain through in vivo microdialysis sampling provides direct correlation between neuropeptide concentration and brain function. Capillary liquid chromatography-multistage mass spectrometry (CLC-MSn) has proven effective at measuring endogenous neuropeptides in microdialysis samples. In the method, microliter samples are concentrated onto nanoliter volume packed beds before ionization and mass spectrometry analysis. The long times required for extensive preconcentration present a barrier to routine use because of the many samples that must be analyzed and instability of neuropeptides. In this study, we evaluated the capacity of 75 µm inner diameter (I.D.) capillary column packed with 10 µm reversed phase particles for increasing the throughput in CLC-MSn based neuropeptide measurement. Coupling a high injection flow rate for fast sample loading/desalting with a low elution flow rate to maintain detection sensitivity, this column was reduced analysis time from ~30 min to 3.8 min for 5 µl sample, with 3 pM limit of detection (LOD) for enkephalins and 10 pM LOD for dynorphin A1–8 in 5 µl sample. The use of isotopic labeled internal standard lowered peptide signal variation to less than 5%. This method was validated for in vivo detection of Leu and Met Enkephalin with microdialysate collected from rat globus pallidus (GP). The improvement in speed and stability makes capillary LC-MSn measurement of neuropeptides in vivo more practical.
1. Introduction
Neuropeptides act as neurotransmitters, neuromodulators, growth factors and hormones in the central nervous system. They are involved in physiological and pathological pathways such as learning, appetite control, depression, addiction, and reproduction [1–5]. Monitoring extracellular concentrations of neuropeptides can provide insights into their dynamics and physiological roles within the brain [6–8]. Such measurements are performed by microdialysis sampling followed by assay for peptides of interest in collected fractions. Measurement of neuropeptides is challenging due to their low extracellular concentration (usually pM) and tendency to degrade during storage [9]. The difficulty is further exacerbated by low recovery by microdialysis sampling [10]; although recent developments have illustrated routes to improved recovery [11–13]. Measurement is also hampered by slow analysis times. Thus, despite their importance, neuropeptides are infrequently measured in vivo relative to other neurotransmitters. In this work, we describe an approach to increase the throughput of neuropeptide measurements while maintaining sufficient sensitivity.
Capillary zone electrophoresis [14,15], high performance liquid chromatography (HPLC) [16–21] and radioimmunoassay [22,23] have been applied for determining the in vivo concentration of neuropeptides. Since seminal work by Caprioli [24], electrospray ionization (ESI) multistage mass spectrometry (MSn) has gained popularity for such measurements due to low amol detection limit for microliter samples, high specificity, and multi-analyte capacity [17,25–32]. Although ESI-MSn is a useful method, the high salt content and low concentration of samples mean that they must be preconcentrated, desalted, and separated, typically by LC or solid phase extraction, for analysis [33]. Use of capillary LC columns is critical because it allows the microliter samples to be concentrated on packed beds with nanoliter volumes to improve sensitivity [24]. This method has achieved limits of detection (LOD) as low as 0.5 pM for 4 µL volume samples of opioid neuropeptides [26], making it suitable for in vivo measurements.
A limitation of capillary LC-ESI-MSn for high sensitivity neuropeptide measurements is its low throughput. When using columns with small bore (25–50 µm) packed with 5 µm reversed phase particles, loading a few microliter sample can take as long as 15 min, even at high pressures (4000 psi), and yield overall analysis time of 20–30 min/sample [26,31]. Narrow bore columns may also be prone to clogging, especially when repeatedly loading large volume samples, which can further reduce throughput due to frequent column changes. Low throughput is a significant concern because microdialysis generally produces many sample fractions over the course of a single experiment. Neuropeptide degradation, which is frequently observed for trace concentrations found in brain samples [26,34], places a further premium on rapid analysis.
To accelerate analysis in capillary LC-MSn, flow rate during preconcentration and column rinsing must be increased since these are rate limiting steps; however, it is unclear if more rapid preconcentration can be achieved while maintaining the low LOD needed. At a given pressure (e.g., the maximal pressure of the system) flow rate is directly proportional to the square of both column I.D. and particle diameter [35]. In this study we improved throughput of neuropeptide analyses by: (1) using larger bore capillary columns (75 µm I.D.) packed with larger diameter (10 µm) reversed phase particles for lower pressure loading and rinsing; (2) determining the flow rate limits for operation; and (3) developing a periodic column washing scheme to maintain column stability under repeated injections. As a demonstration of the method, leu-enkephalin (LE) and met-enkephalin (ME) were detected in microdialysis samples collected in vivo from rat globus pallidus (GP). The enkephalins are endogenous opioid ligands with important roles in many processes such as pain inhibition [36], addiction [37], feeding [8], and movement [38]. Rapid assays for these and related peptides will facilitate studies of their role in normal and pathological brain chemistry.
2. Materials and methods
2.1 chemicals and materials
Fused silica capillary was from Polymicro Technologies (Phoenix, AZ). Solvents for capillary LC were Burdick and Jackson from Honeywell (Muskegon, MI). Alltima™ C18 packing materials (10 µm, 5 µm) were from Grace Davison (Deerfield, IL). Formic acid, hydrofluoric acid and iso-propanol were from Fisher Scientific. Inc. (Ann Arbor, MI). Enkephalins, high purity acetic acid and formamide were purchased from Sigma-Aldrich (Saint Louis, MO). Dynorphin A1–8 (DynA1–8) was from Phoenix Pharmaceutical Inc (Belmont, CA), Kasil™ 1624 and K™ sodium silicates were from PQ Corporation (Malvern, PA). Artificial cerebrospinal fluid (aCSF) used for making neuropeptide standards and microdialysis perfusion was made to a final concentration of 145 mM NaCl, 2.68 mM KCl, 1.10 mM MgSO4, 1.22 mM CaCl2, 0.50 mM NaH2PO4, and 1.55 mM Na2HPO4 with MiliQ™ water (EMD, MiliporeBillerica, MA), and pH was adjusted to 7.4 using 1 M NaOH. High potassium aCSF solution has the same composition as regular aCSF except a combination of 75 mM KCl and 70 mM NaCl was used to substitute the 145 mM NaCl in regular aCSF. aCSF was stored at 4 °C and was filtered with 0.2 µm pore filters (GE, Piscataway, NJ) to remove particulates prior to experiment.
2.2 Capillary LC
Capillary columns were prepared in house using 75 µm inner diameter (I.D.)/ 360 µm outer diameter (O.D.) fused silica capillary. A 1 mm silica sol-gel frit was placed at one end of the column using a slightly modified version of a method previously described [31]. In brief, a 20 cm long capillary was filled with a mixture of 3:1:1 (v/v/v) Kasil™ 1624: K™ sodium silicate: formamide up to 5 cm by capillary force, and then placed in an oven (100 °C) overnight for polycondensation of the silica sol-gel network. The capillary was cut by a ceramic capillary cutter (Polymicro Technologies) to leave a ~1 mm section of the sol-gel network acting as a single-ended frit. The capillary frit was flushed with water and methanol prior to packing. The column was packed with a slurry of 10 µm Alltima™ C18 reversed phase particles (5 mg/ml particles in acetone) at 200 psi to 3.6 cm. The open end of the capillary was also cut to leave only 0.4 cm void capillary to minimize dead volume, resulting in 4 cm total column length. The electrospray emitter was prepared in house using 20 cm of 40 µm I.D./360 µm O.D. fused silica capillary. A 1 cm section of polyimide coating 10 cm away from one end was removed with flame to expose bare silica that was pulled into two separate tips by a P-2000 CO2 laser puller (Sutter Instruments, Novato, CA). The tip was etched with 49% hydrofluoric acid for 3 minutes to create the electrospray emitter, and the emitter was cut at the open capillary side to a length of 1.3 cm. The column and emitter tip were joined by a 2 cm long PTFE tubing (1/16”X0.010”, Grace Davison, Deerfield, IL).
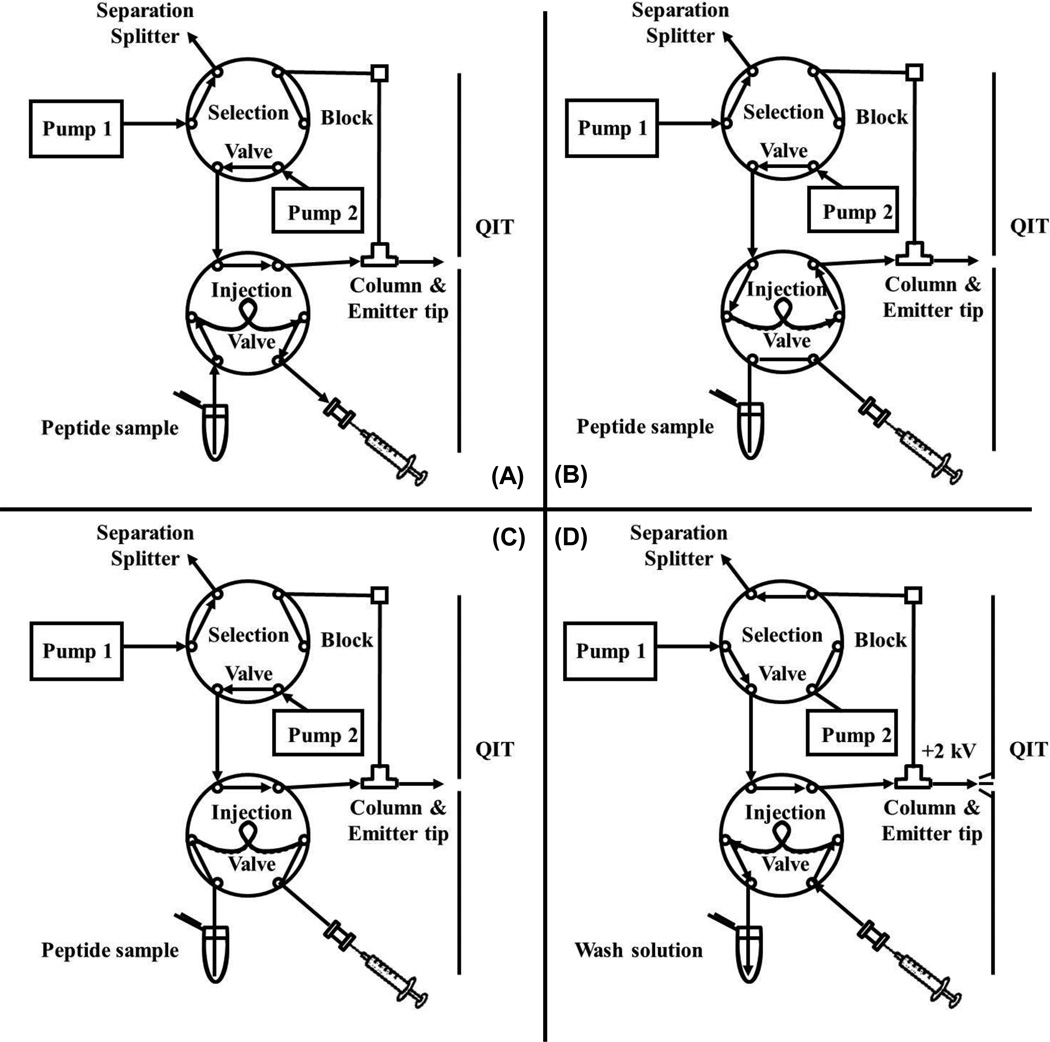
Injection and LC separation were performed semi-automatically using the system depicted in Fig. 1 as follows: (A) manually load 5 µl sample loop with sample using syringe and while (re)equilibrating column with loading solvent (0.1% formic acid) under 3600 psi driven by a Isco 100D high pressure syringe pump (Teledyne Isco Inc., Lincoln, NE); (B) switch injection valve (Nanovolume, VICI, Poulsbo, WA) and inject peptide standard under 3600 psi plus rinse with additional 5 µl loading solvent; (C) switch injection valve out of line and rinse column with another 5 µl loading solvent to remove remaining salt from column; and (D) switch selection valve (Nanovolume, VICI) to a HPLC pump (Waters 626, Waters Corporation, Milford, MA) with mobile phase to elute peptide(s) for detection.
Figure 1.
Diagram and operation scheme of the dual valve dual pump LC-MSn system. Valve positions shown for filling sample loop (A), sample loading onto column (B), rinsing column (C), and peptide elution and detection (D). Detailed description is given in text.
2.3 MS detection
The LC system was coupled to a quadrupole ion trap (QIT) mass spectrometer (LCQ Deca XP Plus, Thermo Fisher, Waltham, MA) operating at positive mode with a Finnigan nanospray ionization source (Thermo Fisher Scientific, Waltham, MA). All measurements were made with the following setting: automatic gain control (AGC) on, collisional induced dissociation (CID) q = 0.25, isolation width m/z = 3, activation time 0.25 ms, number of micro scan = 2, maximum injection time = 400 ms. Normalized collision energies were 38%, 33% for LE (MS3), 36%, 33% for ME (MS3) and 34% for DynA1–8 (MS2). Optimization of optics for best sensitivity was done monthly with constant infusion of 2 µM ME into the mass spectrometer with a flow rate of 100 nl/min. The MSn pathways were: 556→397→278+323+380 for LE, 574→397→278+323+380 for ME which provide the best sensitivity as discussed in our previous work [26,27]. For DynA1–8 we used the following transition as it was found to offer the best sensitivity for this peptide 491→435. The MS was set to scan for each peptide in sequence throughout the chromatogram.
2.4 In vivo Microdialysis
Adult male Sprague–Dawley rats (Harlan, Indianapolis, IN) weighing between 250 and 350 g were used. Rats were housed in a temperature and humidity controlled room with 12 h light/dark cycles with food and water available ad libitum. All animals were treated as approved by the University of Michigan Unit for Laboratory Animal Medicine (ULAM) and in accordance with the National Institute of Health (NIH) Guidelines for the Care and Use of Laboratory Animals.
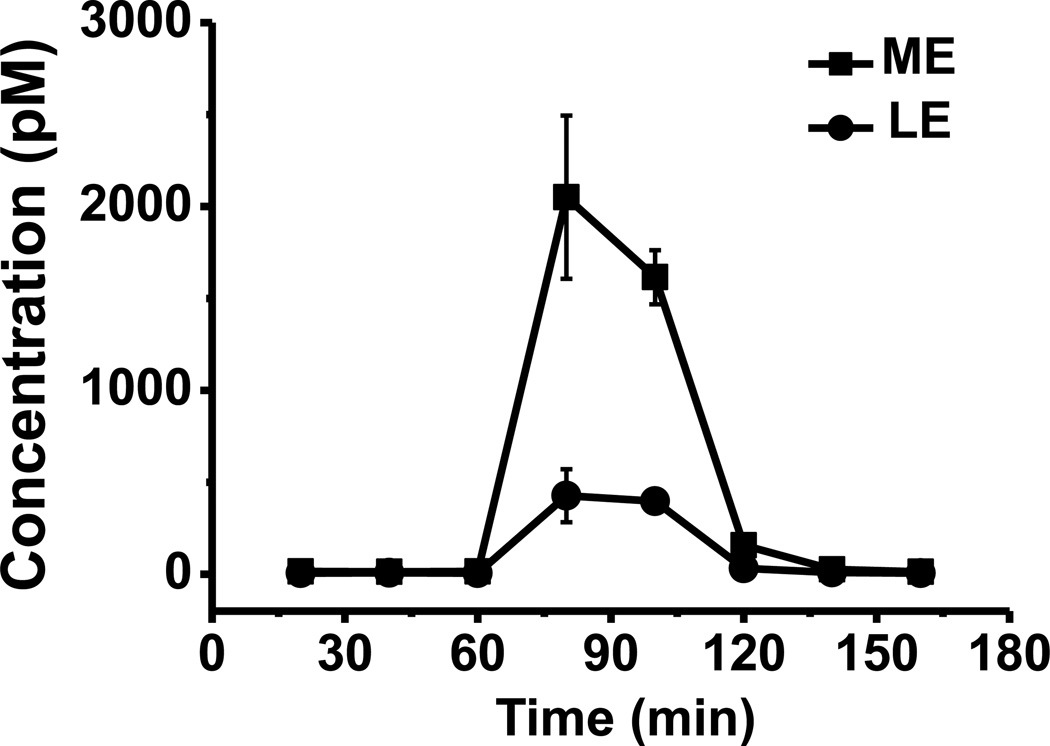
Prior to surgery, rats were anesthetized with an intraperitoneal (i.p.) injection of a ketamine (65 mg/kg) and dexdormitor (0.25 mg/kg) mixture prepared in an isotonic salt solution. Concentric microdialysis probes with 1.5 mm long polyacrylonitrile active membrane (AN69 from Hospal, Bologna, Italy) with recoveries calibrated ex vivo of 9.6% for LE and 8.3% for ME were implanted under anesthesia into the GP according to the following coordinates from bregma and top of the skull: AP −1.3, ML ±3.3, DV −7.0 [39]. Probes were secured to the skull by acrylic dental cement and metallic screws. Following surgery, rats were allowed to recover and experiments were run 24 h after probe implantation. Microdialysis probes were flushed at a flow rate of 1.5 µL/min with aCSF for 2 h using a Chemyx Fusion 400 syringe pump (Chemyx, Stafford, TX). Perfusion flow rate was then reduced to 0.6 µL/min and samples were collected every 20 minutes into vials containing 0.5 µL acetic acid to preserve peptide stability as previously described [26]. Samples were immediately injected on the LC-MSn system following collection. High K+ aCSF (75 mM) was perfused for 40 min through the probe after 3 baseline samples were collected. Lines were then switched back to standard aCSF and 3 more samples were collected (Fig. 6).
Figure 6.
K+ stimulation profile of ME and LE. Potassium concentration: 75 mM. Stimulation time: 40 minutes. Number of replicates: 3. Error bar represents standard error of measurement (SEM).
3. Results and discussion
3.1 Influence of column bore size and particle size on sensitivity
The procedure for analysis by on-column preconcentration LC-MSn is based on previous work which has been successful for several neuropeptides [17,26,27]. The dual pump system with selection valve allows sample to be loaded onto the column at high flow rate but then eluted at a lower flow rate that gives better ESI sensitivity [40,41] and LC performance without requiring an extensive pressure equilibration [26]. Despite these steps, the rate limiting step of analysis is preconcentration and rinsing [17,26,27]. In prior work, when using a 25 µm I.D. by 4 cm long column packed with 5 µm reversed phase particles, 15 min was required to inject a 5 µl sample and rinse the column with 10 µl loading solvent (i.e. ~1 µl/min). The total analysis time was 30 min when including gradient dwell time (i.e. time for the elution gradient to reach the column), gradient elution, and column re-equilibration [26].
Injection flow rate could potentially be increased by increasing applied pressure; however, without specialized fittings and valves, the system is prone to leakage at pressures over 5000 psi. (Pumps with significantly higher pressure are also available but much more expensive.) Another solution is to use shorter columns; however, preliminary experiments revealed that shorter columns tended lose sample during preconcentration, perhaps due to elution during the long injection step. To facilitate faster loading, we therefore explored larger bore (75 µm I.D.) capillary columns packed with larger diameter (10 µm) reversed phase particles to reduce column back pressure. Potential issues associated with this method are: (1) worse separation efficiency of larger particles [42] might result in broader elution band that affects detection sensitivity; (2) when the same volumetric flow rate is applied for elution, 75 µm I.D. capillary columns have a 9-fold decrease in linear flow velocity compared to 25 µm I.D. column. The change in linear velocity also changes separation efficiency [42], further complicating system performance determination.
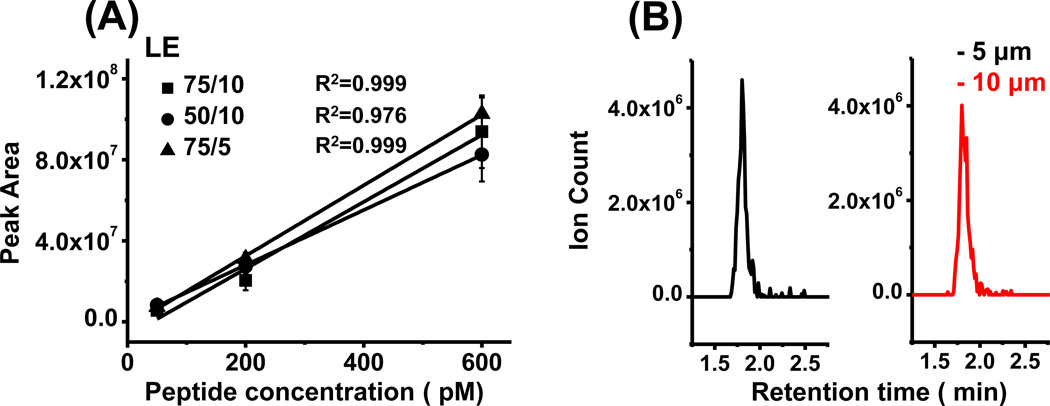
To explore these effects, we made LE calibration curves comparing columns with different I.D.s and particle sizes under the same loading and elution flow rate. As shown in Fig. 2A, increasing column diameter from 50 µm to 75 µm resulted in an 18% increase in calibration curve slope while increasing particle size from 5 µm to 10 µm resulted in a 6% decrease in calibration curve slope. Because the column-to-column calibration curve slope variation was already 10% between columns with the same I.D. and particle size, these data show that column and particle size had minor effects on detection sensitivity. The full width at half maximum (FWHM) of the LE peak using 10 µm particles was 7.4% ±6.2% (n = 3) wider than with 5 µm particles (Fig. 2B). This small change is likely due to the strong dependence of peak width on gradient slope for peptides. (Peptides were eluted with a fixed mobile phase of 53% methanol; however, because the column was flushed with aqueous solution prior to elution, an “step” gradient was generated in the column.) Although 75 µm I.D. columns packed with 10 µm particles gave similar performance as the other two tested columns, the flow resistance on this column was much smaller, resulting in 15 µl/min injection flow rate at 4000 psi and potential to load and desalt 5 µl sample in one minute. Under the same applied pressure, the injection flow rate was 6 µl/min on 50 µm I.D. columns packed with 10 µm particles and 4 µl/min on 75 µm I.D. columns packed with 5 µm particle, requiring at least 2.5 min and 3.8 min to load and desalt the same 5 µl sample, respectively. Previous studies [26,31] required 10–15 minutes to load and desalt 5–10 µl samples using smaller bore (25–50 µm) column with 5 µm column packing.
Figure 2.
Influence of column I.D. and particle size on detection sensitivity. (A) Calibration curve showed column I.D. and particle size had little influence on sensitivity under the same volumetric elution flow rate. (B) Chromatogram of LE (5 µl 200 pM injected) showed no obvious peak broadening using column packed with 10 µm particle compared to column packed with 5 µm particle. All measurements were done with 3 replicates.
3.2 Influence of loading flow rate and elution flow rate on sensitivity
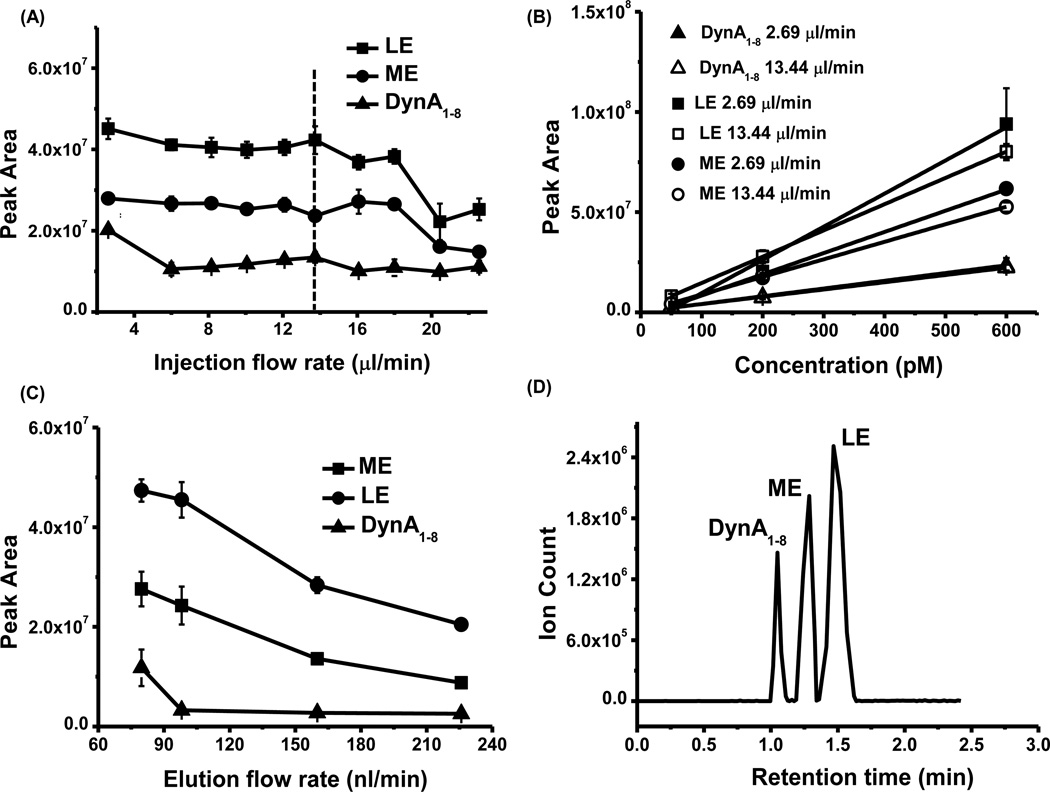
After establishing that 10 µm particles in 75 µm I.D. columns could be used with little effect on sensitivity but at lower pressures, we investigated the relationship between peptide signal and sample injection/rinsing flow rates to determine how rapidly the assay could be performed. For these experiments we used a sample volume of 5 µl and 10 µl of loading solvent to rinse the column. (The volume of rinsing solution needed was determined in separate experiments. If lower volumes are used, less stable signals are achieved, presumably due to interference from salts and other sample constituents that are not fully washed away.) The experiments revealed that peptide peak area remained stable for loading flow rate from 2.6 to 14 µl/min (Fig 3A). Interestingly, above 14 µl/min signal began to decrease for at least one of the peptides. The reason for this effect of higher flow rates is not clear. It seems unlikely to be a kinetic limitation to binding since the residence time of an unretained species was ~0.5 s under these conditions. Calibration curve of all three peptides also showed no sensitivity change when comparing injection under low (2.7 µl/min) and high (14 µl/min) flow rates (Fig 3 B).
Figure 3.
Influence of flow rate on detection sensitivity. (A) Peptide signal remained stable within the injection/rinsing flow rate range of 2.6 µl/min to 13.7 µl/min. (B) Calibration curve under different injection/rinsing flow rate (2.69 µl/min v.s. 13.44 µl/min) suggested no major sensitivity decrease when injecting peptide under high flow rate. (C). Increasing elution flow rate decreased peptide signal. Peptide standard: 200 pM LE, ME and DynA1–8 dissolved in aCSF with 5% HAc. Injection volume: 5 µl. (D). Reconstructed ion chromatogram (RIC) of 5 µl 60 pM neuropeptide being injected onto the column. All measurements were done with 3 replicates.
Another way to increase analysis speed is to increase separation flow rate; however, increasing volumetric flow rate may also cause dilution of the eluted peptide band and worsen ESI efficiency [40,41]. We found that peptide signal from a 200 pM standard decreased 68%, 57% and 78% for ME, LE and DynA1–8, respectively, when elution flow rate increased from 80 nl/min to 225 nl/min (Fig. 3C). In theory, higher sensitivity could be obtained by pushing the electrospray flow rate lower [40,41]; however, in practice, further lowering flow rates will require an extended time period for elution, thus lowering throughput. An elution flow rate of 100 nl/min was selected as a compromise between time and sensitivity [26,27]. This flow rate allowed good separation of 3 peptides in 2 min (Fig. 3D) while maintaining a LOD of 3 pM for enkephalins, and 10 pM for DynA1–8.
The total analysis time under these conditions was 3.8 min/sample (0.4 min for sample loading and column re-equilibration, 0.4 min for injection/preconcentration, 0.8 min for rinsing, and 2.2 min for elution). In previous work using a 25 µm I.D. column with 5 µm packing, a total analysis time of 30 min was needed for analyzing one sample with 4–5 µl volume, with approximately the same LOD when using the same quadrupole ion trap MS as used in this work [26]. (Better LODs were obtained by using newer MS, e.g. linear ion trap MS that has higher sensitivity [26].) Thus this approach achieves similar sensitivity compared to previous results, but in 13% of the time. For an experiment which results in collection of 100 samples, analysis could potentially be completed in 6.3 h rather than 50 h, a substantial time savings.
The time savings found here comes at the cost of using larger particles which reduce column efficiency and therefore the potential to resolve multiple peptides. However, as shown above, because short columns and step gradients were used here, there was little loss in resolving power with the larger particles. We demonstrate detection of 3 peptides simultaneously with this approach. In principle the best way to allow assay of multiple peptides at high speed would be to use ultra-high pressures with smaller particles; however, this approach adds significant cost.
Chromatographic peaks were 5 to 10 s wide. When using this model of ion trap, 3.0 and 1.8 s were required to acquire each MS3 and MS2 spectrum respectively meaning that we typically obtained 4–6 points across a chromatographic peak when scanning for all three peptides and 10–12 points across a peak when scanning for just two peptides. More points could be obtained by using MS2 instead of MS3; however, better sensitivity by MS3 over MS2 in detecting enkephalins [27] was considered important for this experiment. In principle more points per peak can be obtained by using time segmented scanning where scans are focused on one peptide during its elution time and switched for each peptide; however, this requires excellent timing when peaks are closely spaced as found here. In principle a faster scanning instrument, such as a triple quadruople, may also allow better multiplexed detection [43].
3.3. Improved stability during serial injections
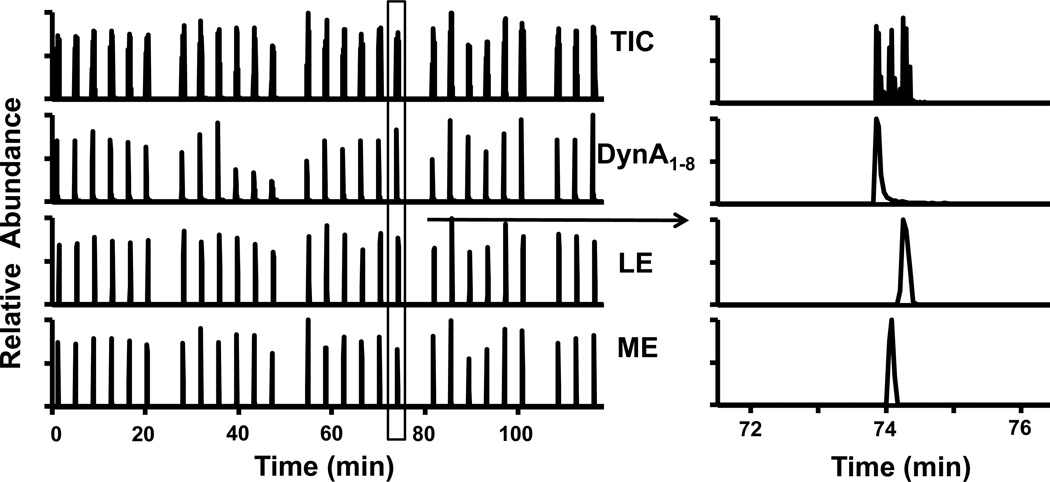
To test system stability and injection reproducibility, we performed serial injections of 30 peptide standards containing 60 pM ME, LE and DynA1–8. The relative standard deviation (RSD) was higher than 10% for all three peptides, and the RSD for ME was higher than 40% (Table 1). We considered several possibilities for this large RSD including: (1) loss of particles from the column due to the sudden pressure drop between sample injection and elution causing backflow of particles to the inlet side; (2) partial clogging causing decreased amount of sample injection and rinsing; (3) build-up of impurities from peptide sample and solvent on the column causing a change in system response (e.g. by eluting with peptide or altering preconcentration capacity). By inspecting the column and tip assembly under a light microscope, explanations (1) and (2) were eliminated since no clogging or bed length reduction was found.
Table 1.
Peak area RSDs of tested neuropeptides under different injection protocols. Adding wash injection and injecting peptide with internal standard significantly improves injection reproducibility.
| Injection type | Peak Area RSD (DynA1–8) | Peak Area RSD (LE) | Peak Area RSD (ME) |
| No column rinsing (n = 30) | 20.2% | 18.5% | 46.0% |
| Wash injection every 6 injections (n = 27) | 15.4% | 8.4% | 9.1% |
| Peak Area RSD (dLE) | Peak Area RSD (LE) | Peak Area ratio RSD (LE/dLE) | |
| Injection of LE and internal standard (with wash injection) (n = 27) | 9.2% | 9.3% | 4.3% |
Supporting the idea that accumulation of impurities affected the RSD, we found that in a similar series of injections in which a “wash” was applied (injection of 20 µl of wash solvent containing 60% (v/v) isopropanol, 30% (v/v) acetonitrile and 10% (v/v) water) after every 6 assays the peak area RSD was reduced to less than 10% for the enkephalins and less than 20% for DynA1–8 (see Fig. 4A and Table 1). Peak area RSD of DynA1–8 still remained > 10% could be the result from using SRM while enkephalins are detected by CRM. The reason for using SRM to detect DynA1–8 is that it produced an easily identifiable daughter ion (x7, m/zz = 435), while the MS3 spectrum showed no major granddaughter ions for quantification. Previous paper published by us also showed mediocre detection limit for DynA1–8 using MS3 [26], urging us to use the SRM method as alternative for higher sensitivity. However, since SRM is essentially MS2, whose’s specificity is lower than MSn based CRM method, the higher variation in DynA1–8 peak area is not unexpected. Although adding the wash step decreased system throughput from 3.8 to 4.4 min per sample, it proved to be effective in improving long term performance. Improvement of reproducibility could also be obtained through adding internal standards. As a demonstration, we monitored the peak area of 60 pM LE and deuterated LE we have in lab (dLE, m/z 560) under serial injection. Although deuterated internal standard has the potential for chromatographic isotope effect, we found negligible retention time difference between LE and dLE. This lack of effect is likely due to the relatively low chromatographic resolution achieved. These experiments showed that the RSD of peak area ratio of LE versus dLE was reduced to <5%, while peak area RSD of individual peptide was still around 8–9% (Table 1). Based on these results, we presume that use of stable isotopes of other peptides would also aid detection, but this was not tested for cost savings.
Figure 4.
Chromatogram of serial injections of 60 pM peptide standards in series. A wash injection with 20 µl 60:30:10 (v/v/v) isopropanol:acetonitrile:H2O injected onto the column was carried out every 6 injections. The right image shows a zoomed-in view of one injection where 5 µl peptide standard was preconcentrated, rinsed, and separated for detection within 4 minutes.
The larger columns with rinsing were also found to be resistant to clogging. Under these conditions, one column could be operated stably during two 2-hour long series injections with 60 samples injected onto the column. After 60 injections, reduction in both injection and elution flow rate and retention time shift was frequently observed, indicating partial clogging. Therefore one column was typically used within one day to analyze 60 samples. While potentially cumbersome, the ease and low cost of packing one such column (~10 min after frit preparation) mean that columns are “disposable”. Although we found low column-to-column retention time and calibration curve slope (~10%) differences, we performed daily calibration to allow quantitative comparison from different columns. Previous work found that 25 µm columns could only survive 20–40 injections, with column-to-column peak area variation up to 50% [26].
3.4 Detecting enkephalins in microdialysate
To demonstrate the feasibility of this method for in vivo peptide measurements, we used it to monitor enkephalins in microdialysis fractions collected at 20 min intervals under basal conditions and while perfusing 75 mM K+ aCSF through a probe implanted in the GP brain of freely moving rats (n = 3) as summarized in Table 2. Microdialysis flow rate was set to be 0.6 µl/min. Five µl of the 12 µl dialysate collected over 20 min was actually injected onto the column. (Excess dialysate was required to overfill the loop. More efficient use of such precious samples may be achieved by partial loop and “microliter pickup” mode using commercial autosamplers [26].) The concentration of peptides in dialysate samples was determined by external calibration using standard solutions of peptide dissolved in aCSF (5% HAc added) with the following concentrations: 5 pM, 50 pM, 800 pM, and 3 nM. Calibrations were linear with correlation coefficients of 0.99.
Table 2.
ME and LE’s basal and stimulated concentration in in vivo dialysate. In vivo concentration was corrected by the relative recovery of the microdialysis probe. Values are dialysate concentration and not corrected for recovery. Average relative recovery of probes was 8.3 ± 1.8% for ME and 9.6 ± 0.4% for LE.
| Collection condition | [ME] ± SEM (pM) (n = 3 animals) |
[LE] ± SEM (pM) (n = 3 animals) |
|---|---|---|
| Basal, dialysate | 14 ± 1 | 7 ± 1 |
| Stimulated, dialysate | 1800 ± 230 | 410 ± 66 |
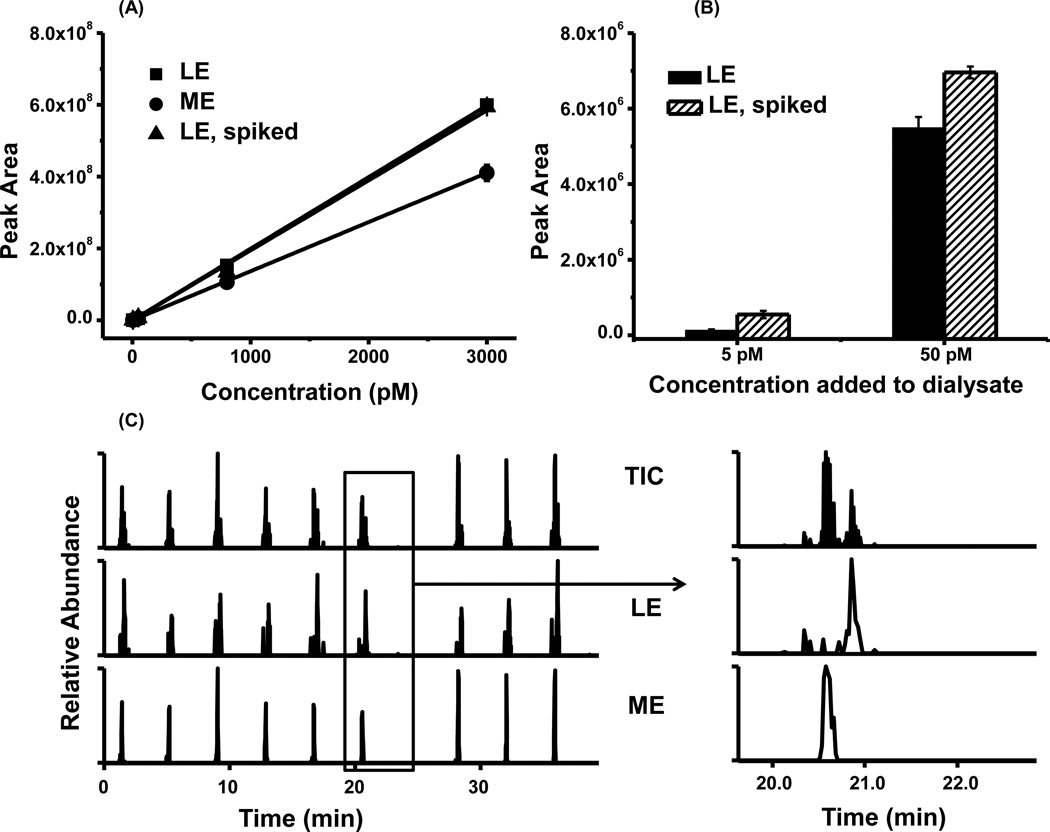
To determine if concentration determined by external calibration was accurate, we also performed a study of matrix effect. Spiking 5 pM, 50 pM, 800 pM, 3000 nM LE into dialysate gave a similar calibration curve as the same concentration in aCSF (Fig. 5A). This suggests that matrix effects were negligible in the calibration of peptide concentrations. To better examine this effect at low concentrations, we compared peak area of 5 and 50 pM LE standards to that found for spiking the same concentrations into dialysate. The difference in peak area between the aCSF and spiked dialysate corresponded to 5.0 ± 1.6 (n = 3, SEM) pM (Fig. 5 B), while LE’s concentration in dialysate was determined to be 7.1 ± 1.0 (n = 3, SEM) pM by external calibration, which was not significantly different. This result further suggests minimal matrix effect on signal and that external calibration can be used.
Figure 5.
In vivo measurement of LE and ME from rat brain dialysate. (A). Calibration curve of LE and ME from 5 pM to 3 nM (n=3) (B) LE’s peak area in 5 pM/50 pM standard and dialysate spiked with 5 pM/50 pM LE. (C). Serial injection of 9 basal dialysate sample in 37 minutes. Injection volume: 5 µl.
Stability of the dialysate sample signal was examined under serial injection conditions. 9 basal dialysate fractions collected over 3 h were serially injected over 37 min (9 samples plus 1 wash injection). These samples showed 21% and 22% peak area RSD for LE and ME, respectively (Fig.5 C). The RSD was higher than standards possibly because of natural variation of brain peptide concentration over this period. Average concentrations of LE and ME in serial injection were determined to be 6.0 ± 0.4 (SEM) pM and 14.4 ± 1.0 (SEM) pM, respectively.
K+ stimulation causes depolarization of the neuronal cell membrane and subsequent neuropeptide level into the extracellular space [44,45]. Perfusion of 75 mM K+ aCSF into the brain caused 129 fold and 58 fold increase in ME and LE’s in vivo concentration, respectively to over 2 nM total enkephalins (Fig. 6). This appears to be a larger response than previously reported for microdialysis sampling with K+ stimulation in this brain region; however, the differences are likely due to experimental protocols. Using RIA for analysis of opioid peptides, it was reported that applying K+ stimulation for 2 min in a 30-min long collection period caused collected opioid peptide (mostly enkephalins) to increase 29-fold from 1.5 fmol to 43.9 fmol per fraction [9]. Because the stimulation was only applied for 2 of 30 min, we may expect a substantially lower fold-increase. Another study using microdialysis coupled online to capillary LC-MS2 reported K+ stimulation over 30 minutes caused 32 and 19 fold increase in ME and LE concentration, respectively. In this case though, the K+ was twice as high, which may have other effects, and the dialysis probe was over twice as long which means it is less likely to be contained completely in the field of opioid peptide neuron terminals and therefore might detect smaller changes.
Smaller increases in enkephalin levels upon K+ stimulation have been reported in the striatum. For example, enkephalins increase in rat striatum was less than 10-fold under K+ stimulation [26,27]. Besides possible experimental differences mentioned above, these lower fold changes in the striatum may be expected because the GP is the primary target of striatopallidal neurons which express high levels of enkephalins suggesting release from the terminals than other portions of these neurons [46,47].
4. Conclusions
The method described in this paper has demonstrated fast neuropeptide analysis with low pM detection limits. With ~4 min cycle time per sample, the throughput is improved 5–8 fold over previous work. This method was also validated for in vivo measurement where lower amol of enkephalins could be detected from rat brain dialysate. The enhanced analysis speed and column stability suggest the possibility of routine processing of many in vivo samples. Stable isotope labeled peptides will likely be needed for cases where small changes in peptides are to be measured. Further, as characteristic peptides are increasingly used to quantify proteins, the general approach of rapid loading and rinsing may be of utility for trace analysis in other applications as well.
Acknowledgement
This work was supported by NIH Grant R37 EB003320.
References
- 1.Ogren SO, Kuteeva E, Elvander-Tottie E, Hokfelt T. Neuropeptides in Learning and Memory Processes with Focus on Galanin. Eur. J. Pharmacol. 2010;626(1):9–17. doi: 10.1016/j.ejphar.2009.09.070. [DOI] [PubMed] [Google Scholar]
- 2.Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To Eat or to Sleep? Orexin in the Regulation of Feeding and Wakefulness. Annu. Rev. Neurosci. 2001;24:429–458. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- 3.Heilig M. The Npy System in Stress, Anxiety and Depression. Neuropeptides. 2004;38(4):213–224. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 4.DiLeone RJ, Georgescu D, Nestler EJ. Lateral Hypothalamic Neuropeptides in Reward and Drug Addiction. Life Sci. 2003;73(6):759–768. doi: 10.1016/s0024-3205(03)00408-9. [DOI] [PubMed] [Google Scholar]
- 5.Lambert RC, Moos FC, Richard P. Action of Endogenous Oxytocin within the Paraventricular or Supraoptic Nuclei - a Powerful Link in the Regulation of the Bursting Pattern of Oxytocin Neurons During the Milk-Ejection Reflex in Rats. Neuroscience. 1993;57(4):1027–1038. doi: 10.1016/0306-4522(93)90046-i. [DOI] [PubMed] [Google Scholar]
- 6.Olive MF, Maidment NT. Repeated Heroin Administration Increases Extracellular Opioid Peptide-Like Immunoreactivity in the Globus Pallidus Ventral Pallidum of Freely Moving Rats. Psychopharmacology (Berl) 1998;139(3):251–254. doi: 10.1007/s002130050712. [DOI] [PubMed] [Google Scholar]
- 7.Nieto MM, Wilson J, Cupo A, Roques BP, Noble F. Chronic Morphine Treatment Modulates the Extracellular Levels of Endogenous Enkephalins in Rat Brain Structures Involved in Opiate Dependence: A Microdialysis Study. J. Neurosci. 2002;22(3):1034–1041. doi: 10.1523/JNEUROSCI.22-03-01034.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Difeliceantonio AG, Mabrouk OS, Kennedy RT, Berridge KC. Enkephalin Surges in Dorsal Neostriatum as a Signal to Eat. Curr. Biol. 2012;22(20):1918–1924. doi: 10.1016/j.cub.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maidment NT, Brumbaugh DR, Rudolph VD, Erdelyi E, Evans CJ. Microdialysis of Extracellular Endogenous Opioid-Peptides from Rat-Brain Invivo. Neuroscience. 1989;33(3):549–557. doi: 10.1016/0306-4522(89)90407-7. [DOI] [PubMed] [Google Scholar]
- 10.Kendrick KM. Microdialysis Measurement of Invivo Neuropeptide Release. J. Neurosci. Methods. 1990;34(1–3):35–46. doi: 10.1016/0165-0270(90)90040-m. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher HJ, Stenken JA. An in Vitro Comparison of Microdialysis Relative Recovery of Met- and Leu-Enkephalin Using Cyclodextrins and Antibodies as Affinity Agents. Anal. Chim. Acta. 2008;620(1–2):170–175. doi: 10.1016/j.aca.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbaugh AW, Stenken JA. Antibody-Enhanced Microdialysis Collection of Ccl2 from Rat Brain. J. Neurosci. Methods. 2011;202(2):124–127. doi: 10.1016/j.jneumeth.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pettersson A, Amirkhani A, Arvidsson B, Markides K, Bergquist J. A Feasibility Study of Solid Supported Enhanced Microdialysis. Anal. Chem. 2004;76(6):1678–1682. doi: 10.1021/ac035305l. [DOI] [PubMed] [Google Scholar]
- 14.Freed AL, Cooper JD, Davies MI, Lunte SM. Investigation of the Metabolism of Substance P in Rat Striatum by Microdialysis Sampling and Capillary Electrophoresis with Laser-Induced Fluorescence Detection. J. Neurosci. Methods. 2001;109(1):23–29. doi: 10.1016/s0165-0270(01)00397-1. [DOI] [PubMed] [Google Scholar]
- 15.Kostel KL, Lunte SM. Evaluation of Capillary Electrophoresis with Post-Column Derivatization and Laser-Induced Fluorescence Detection for the Determination of Substance P and Its Metabolites. J. Chromatogr. B. Biomed. Sci. App. 1997;695(1):27–38. doi: 10.1016/s0378-4347(97)00173-4. [DOI] [PubMed] [Google Scholar]
- 16.Desiderio DM, Zhu XG. Quantitative Analysis of Methionine Enkephalin and Beta-Endorphin in the Pituitary by Liquid Secondary Ion Mass Spectrometry and Tandem Mass Spectrometry. J. Chromatogr. A. 1998;794(1–2):85–96. doi: 10.1016/s0021-9673(97)00670-5. [DOI] [PubMed] [Google Scholar]
- 17.Haskins WE, Wang ZQ, Watson CJ, Rostand RR, Witowski SR, Powell DH, Kennedy RT. Capillary Lc-Ms2 at the Attomole Level for Monitoring and Discovering Endogenous Peptides in Microdialysis Samples Collected in Vivo. Anal. Chem. 2001;73(21):5005–5014. doi: 10.1021/ac010774d. [DOI] [PubMed] [Google Scholar]
- 18.Xu N, Qiu C, Wang W, Wang Y, Chai C, Yan Y, Zhu D. Hplc/Ms/Ms for Quantification of Two Types of Neurotransmitters in Rat Brain and Application: Myocardial Ischemia and Protection of Sheng-Mai-San. J. Pharm. Biomed. Anal. 2011;55(1):101–108. doi: 10.1016/j.jpba.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 19.Sinnaeve BA, Storme ML, Van Bocxlaer JF. Capillary Liquid Chromatography and Tandem Mass Spectrometry for the Quantification of Enkephalins in Cerebrospinal Fluid. J. Sep. Sci. 2005;28(14):1779–1784. doi: 10.1002/jssc.200500114. [DOI] [PubMed] [Google Scholar]
- 20.Dawson R, Steves JP, Lorden JF, Oparil S. Reverse-Phase Separation and Electrochemical Detection of Neuropeptides. Peptides. 1985;6(6):1173–1178. doi: 10.1016/0196-9781(85)90446-2. [DOI] [PubMed] [Google Scholar]
- 21.Shen H, Lada MW, Kennedy RT. Monitoring of Met-Enkephalin in Vivo with 5-Min Temporal Resolution Using Microdialysis Sampling and Capillary Liquid Chromatography with Electrochemical Detection. J. Chromatogr. B. 1997;704(1–2):43–52. doi: 10.1016/s0378-4347(97)00436-2. [DOI] [PubMed] [Google Scholar]
- 22.Marinelli PW, Lam M, Bai L, Quirion R, Gianoulakis C. A Microdialysis Profile of Dynorphin a (1–8) Release in the Rat Nucleus Accumbens Following Alcohol Administration. Alcohol. Clin. Exp. Res. 2006;30(6):982–990. doi: 10.1111/j.1530-0277.2006.00112.x. [DOI] [PubMed] [Google Scholar]
- 23.Liu ZR, Welin M, Bragee B, Nyberg F. A High-Recovery Extraction Procedure for Quantitative Analysis of Substance P and Opioid Peptides in Human Cerebrospinal Fluid. Peptides. 2000;21(6):853–860. doi: 10.1016/s0196-9781(00)00219-9. [DOI] [PubMed] [Google Scholar]
- 24.Andren PE, Emmett MR, Caprioli RM. Micro-Electrospray - Zeptomole-Attomole Per Microliter Sensitivity for Peptides. J. Am. Soc. Mass Spectrom. 1994;5(9):867–869. doi: 10.1016/1044-0305(94)87010-1. [DOI] [PubMed] [Google Scholar]
- 25.Lanckmans K, Stragier B, Sarre S, Smolders I, Michotte Y. Nano-Lc-Ms/Ms for the Monitoring of Angiotensin Iv in Rat Brain Microdialysates: Limitations and Possibilities. J. Sep. Sci. 2007;30(14):2217–2224. doi: 10.1002/jssc.200700159. [DOI] [PubMed] [Google Scholar]
- 26.Li Q, Zubieta J-K, Kennedy RT. Practical Aspects of in Vivo Detection of Neuropeptides by Microdialysis Coupled Off-Line to Capillary Lc with Multistage Ms. Anal. Chem. 2009;81(6):2242–2250. doi: 10.1021/ac802391b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baseski HM, Watson CJ, Cellar NA, Shackman JG, Kennedy RT. Capillary Liquid Chromatography with Ms3 for the Determination of Enkephalins in Microdialysis Samples from the Striatum of Anesthetized and Freely-Moving Rats. J. Mass Spectrom. 2005;40(2):146–153. doi: 10.1002/jms.733. [DOI] [PubMed] [Google Scholar]
- 28.Emmett MR, Andren PE, Caprioli RM. Specific Molecular Mass Detection of Endogenously Released Neuropeptides Using in Vivo Microdialysis Mass Spectrometry. J. Neurosci. Methods. 1995;62(1–2):141–147. doi: 10.1016/0165-0270(95)00070-4. [DOI] [PubMed] [Google Scholar]
- 29.Andren PE, Caprioli RM. Determination of Extracellular Release of Neurotensin in Discrete Rat Brain Regions Utilizing in Vivo Microdialysis/Electrospray Mass Spectrometry. Brain Res. 1999;845(2):123–129. doi: 10.1016/s0006-8993(99)01751-5. [DOI] [PubMed] [Google Scholar]
- 30.Mabrouk OS, Li Q, Song P, Kennedy RT. Microdialysis and Mass Spectrometric Monitoring of Dopamine and Enkephalins in the Globus Pallidus Reveal Reciprocal Interactions That Regulate Movement. J. Neurochem. 2011;118(1):24–33. doi: 10.1111/j.1471-4159.2011.07293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mabrouk OS, Kennedy RT. Simultaneous Oxytocin and Arg-Vasopressin Measurements in Microdialysates Using Capillary Liquid Chromatography-Mass Spectrometry. J. Neurosci. Methods. 2012;209(1):127–133. doi: 10.1016/j.jneumeth.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Behrens HL, Chen R, Li L. Combining Microdialysis, Nanolc-Ms, and Maldi-Tof/Tof to Detect Neuropeptides Secreted in the Crab, Cancer Borealis. Anal. Chem. 2008;80(18):6949–6958. doi: 10.1021/ac800798h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Constantopoulos TL, Jackson GS, Enke CG. Effects of Salt Concentration on Analyte Response Using Electrospray Ionization Mass Spectrometry. J. Am. Soc. Mass Spectrom. 1999;10(7):625–634. doi: 10.1016/S1044-0305(99)00031-8. [DOI] [PubMed] [Google Scholar]
- 34.Reed B, Bidlack JM, Chait BT, Kreek MJ. Extracellular Biotransformation of Beta-Endorphin in Rat Striatum and Cerebrospinal Fluid. J. Neuroendocrinol. 2008;20(5):606–616. doi: 10.1111/j.1365-2826.2008.01705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giddings JC. Unified Separation Science. New York: Wiley; 1991. [Google Scholar]
- 36.Hokfelt T, Ljungdahl A, Terenius L, Elde R, Nilsson G. Immunohistochemical Analysis of Peptide Pathways Possibly Related to Pain and Analgesia - Enkephalin and Substance-P. Proc. Natl. Acad. Sci. U. S. A. 1977;74(7):3081–3085. doi: 10.1073/pnas.74.7.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whistler JL, Chuang HH, Chu P, Jan LY, von Zastrow M. Functional Dissociation of Mu Opioid Receptor Signaling and Endocytosis: Implications for the Biology of Opiate Tolerance and Addiction. Neuron. 1999;23(4):737–746. doi: 10.1016/s0896-6273(01)80032-5. [DOI] [PubMed] [Google Scholar]
- 38.Angulo JA, McEwen BS. Molecular Aspects of Neuropeptide Regulation and Function in the Corpus Striatum and Nucleus-Accumbens. Brain Res. Rev. 1994;19(1):1–28. doi: 10.1016/0165-0173(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 39.Paxinos G, Watson CJ. The Rat Brain in Stereotaxic Coordinates. Elsevier; 2007. [DOI] [PubMed] [Google Scholar]
- 40.Heemskerk AAM, Busnel J-M, Schoenmaker B, Derks RJE, Klychnikov O, Hensbergen PJ, Deelder AM, Mayboroda OA. Ultra-Low Flow Electrospray Ionization-Mass Spectrometry for Improved Ionization Efficiency in Phosphoproteomics. Anal. Chem. 2012;84(10):4552–4559. doi: 10.1021/ac300641x. [DOI] [PubMed] [Google Scholar]
- 41.Wilm M, Mann M. Analytical Properties of the Nanoelectrospray Ion Source. Anal. Chem. 1996;68(1):1–8. doi: 10.1021/ac9509519. [DOI] [PubMed] [Google Scholar]
- 42.Vandeemter JJ, Zuiderweg FJ, Klinkenberg A. Longitudinal Diffusion and Resistance to Mass Transfer as Causes of Nonideality in Chromatography. Chem. Eng. Sci. 1956;5(6):271–289. [Google Scholar]
- 43.Song P, Mabrouk OS, Hershey ND, Kennedy RT. In Vivo Neurochemical Monitoring Using Benzoyl Chloride Derivatization and Liquid Chromatography-Mass Spectrometry. Anal. Chem. 2012;84(1):412–419. doi: 10.1021/ac202794q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siegel RE, Eiden LE, Affolter HU. Elevated Potassium Stimulates Enkephalin Biosynthesis in Bovine Chromaffin Cells. Neuropeptides. 1985;6(6):543–552. doi: 10.1016/0143-4179(85)90117-9. [DOI] [PubMed] [Google Scholar]
- 45.Richter JA, Wesche DL, Frederickson RCA. K-Stimulated Release of Leu-Enkephalin and Met-Enkephalin from Rat Striatal Slices - Lack of Effect of Morphine and Naloxone. Eur. J. Pharmacol. 1979;56(1–2):105–113. doi: 10.1016/0014-2999(79)90439-4. [DOI] [PubMed] [Google Scholar]
- 46.Simantov R, Kuhar MJ, Uhl GR, Snyder SH. Opioid Peptide Enkephalin - Immunohistochemical Mapping in Rat Central Nervous-System. Proc. Natl. Acad. Sci. U. S. A. 1977;74(5):2167–2171. doi: 10.1073/pnas.74.5.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wamsley JK, Young WS, Kuhar MJ. Immunohistochemical Localization of Enkephalin in Rat Forebrain. Brain Res. 1980;190(1):153–174. doi: 10.1016/0006-8993(80)91166-x. [DOI] [PubMed] [Google Scholar]