Abstract
Objectives
To examine the associations of stressful experiences and social support with cognitive function in a sample of middle-aged adults with a family history of Alzheimer’s disease (AD).
Methods
Using data from the Wisconsin Registry for Alzheimer’s Prevention (WRAP; N=623), we evaluated relationships between stressful events experienced in the past year, as well as social support, and cognitive performance in four domains: speed and flexibility, immediate memory, verbal learning and memory, and working memory. We assessed interactions between psychosocial predictors, and with APOE ε4 status.
Results
Greater number of stressful events was associated with poorer performance on tests of speed and flexibility. Greater social support was associated with better performance in the same domain; this relationship was diminished by presence of the ε4 allele. No associations were seen in the remaining three domains.
Discussion
Psychosocial factors may influence cognition in at-risk individuals; influence varies by cognitive domain and ε4 status.
Keywords: Cognitive function, geriatrics, social factors, stressful events, gene-environment interaction
Introduction
Cognitive health and decline over the course of aging are determined by a complex set of risk factors as diverse as genetic profile, early life experiences and education, physical health status, and, purportedly, psychosocial resources. Stressful experiences, feelings of isolation, and depression appear to increase risk for poorer cognitive functioning and even incidence of Alzheimer’s disease (AD), the most common type of age-related dementia (Bassuk, Glass, & Berkman, 1999; Johansson et al., 2010; Lakey, Tardiff, & Drew, 1994; McEwen & Sapolsky, 1995). Coping with stressful situations requires attention and may compete for cognitive resources; it may be this diversion of resources that results in the processing deficits associated with higher stress levels (Stawski, Sliwinski, & Smyth, 2006). Additionally, stress may be linked with dysregulation of cortisol and inflammatory cytokines (McEwen, 1998), which at elevated levels have been associated with a host of negative health outcomes including cognitive impairment (Lupien et al., 2005; Marsland et al., 2006).
Stronger social networks and greater levels of social support have been shown to be associated with better immediate cognitive function (Seeman, Lusignolo, Albert, & Berkman, 2001), less cognitive decline over time (Barnes, Mendes de Leon, Wilson, Bienias, & Evans, 2004; Seeman et al., 2001; Seeman et al., 2011), and reduced risk for incident dementia (Fratiglioni, Wang, Ericsson, Maytan, & Winblad, 2000). The relationship is a plausible one, with several potential bidirectional pathways supporting relationships between psychosocial and cognitive health. For instance, perceived support and a bigger social network may also be associated with greater levels of social engagement and more frequent interaction, requiring cognitive stimulation and activity (Fillit et al., 2002). Social resources may also “buffer” the effects of stressful events, and facilitate coping efforts (Cohen & Wills, 1985). The buffering hypothesis has been applied to the association between social relationships, stress, and a number of health conditions (Cohen, 2004; Uchino, Cacioppo, & Kiecolt-Glaser, 1996), though only rarely with cognitive outcomes. Relatedly, support and social network size and strength may also protect against depression, which has been shown to be associated with reduced cognitive function across the lifespan (Brown, Scott, Bench, & Dolan, 1994; Butters et al., 2008; Dean, Kolody, & Wood, 1990; Gualtieri, Johnson, & Benedict, 2006; Nebes et al., 2000; Saczynski et al., 2010; Thomas et al., 2009).
Previous literature on psychosocial factors and cognitive function has focused on the general population; to our knowledge, no studies have been done in individuals with a family history of AD who are likely at heightened genetic risk for earlier cognitive decline (La Rue, Matsuyama, McPherson, Sherman, & Jarvik, 1992; La Rue, O’Hara, Matsuyama, & Jarvik, 1995), as well as increased stress reactivity (Peskind, Wilkinson, Petrie, Schellenberg, & Raskind, 2001). Research findings in this high-risk group are important for targeted prevention strategies. Therefore, the purpose of this preliminary study was to examine the association of stressful experiences and different kinds of social support on cognition, using cross-sectional data from a sample of adult children with a parent diagnosed with AD (N=623) taken from the Wisconsin Registry for Alzheimer’s Prevention study (WRAP). We hypothesized that greater levels of recent life stress would be associated with poorer cognitive performance, and that greater levels of perceived social support of any kind would be associated with better performance. We further hypothesized, in accordance with the buffering theory, that perceived social support would moderate the effect of stress on cognitive function, diminishing the negative impact. Finally, we assessed the potential interaction between the psychosocial factors and a known genetic risk factor for AD, the APOE ε4 allele. We focused on selected aspects cognitive functioning, namely memory and executive function. These are the first cognitive domains to show change in early stages of AD (Backman, Jones, Berger, Laukka, & Small, 2005; Twamley, Ropacki, & Bondi, 2006), which is particularly relevant in WRAP, where participants are at increased risk for cognitive changes due to their parental family history of AD. In addition, memory and executive function are cognitive domains for which associations with social factors have been reported in prior studies (e.g., Seeman et al., 2011).
Methods
Participants
Data were drawn from WRAP, a longitudinal study of cognitive function in middle-aged and older adults who have a family history of AD (Sager, Hermann, & La Rue, 2005). WRAP’s study design and assessment protocols are described in detail elsewhere (La Rue et al., 2008; Sager et al., 2005). In brief, participants in the family history group have at least one biological parent with autopsy-confirmed or probable AD as defined by NINCDS-ADRDA research criteria (McKhann et al., 1984). Many were recruited while accompanying a parent diagnosed with dementia to an evaluative visit in the Memory Assessment Clinic at the University of Wisconsin – Madison or in one of 16 satellite memory clinics affiliated with the University of Wisconsin Alzheimer’s Institute; others learned of the study via educational presentations or word of mouth. Participants were English speaking and cognitively intact at baseline; most were between the ages of 40 and 65 (mean=54) at the start of the study. Baseline enrollment began in late 2001 and is ongoing. The first round of follow-up assessments (“Wave 2”) began in 2006, with a test-retest interval of 4 years. The assessment battery was expanded from baseline to Wave 2 to include the life stress and social activities questionnaires. Thus, the data presented in this study were collected during Wave 2 visits. Exclusion criteria for the current study include history of multiple sclerosis, Parkinson’s disease, stroke, epilepsy, or meningitis. Additionally, although WRAP’s baseline sample includes just over 7% underrepresented ethnic groups, most of these participants were not yet eligible for Wave 2 testing and the small number of ethnic minorities (N=11) in the sample with Wave 2 data led to exclusion of those who did not self-identify their ethnicity as non-Hispanic Caucasian, resulting in a final sample of 623 participants. This study was conducted with the approval of the University of Wisconsin Institutional Review Board and all subjects provided signed informed consent before participation.
Measures
Wave 2 visits were approximately three hours in duration and included a battery of commonly used clinical neuropsychological tests (see Sager et al, 2005, for a description of the cognitive battery); completion of questionnaires about health history, psychosocial factors, and lifestyle; laboratory tests; and APOE genotyping.
Neuropsychological assessment
Factor analysis using promax rotation and maximum likelihood estimation (Grice, 2001) was used to reduce the set of cognitive measures to a smaller number of factors and obtain weights used to combine the measures within each factor. The resulting six weighted factor scores were then standardized [~N (0, 1)] into z-scores, using means and standard deviations obtained from the whole baseline sample. Factors of interest in the present analyses included two factors representing new learning and recall (immediate memory, verbal learning and memory), both derived from the Rey Auditory Verbal Learning Test (Lezak, Howieson, Loring, Hannay, & Fischer, 2004). There are also two factors reflecting components of executive function (working memory, derived from the Digits Forward, Digits Backward, and Letter-Number Sequence subtests of the Weschler Adult Intelligence Scale-III; speed and flexibility, derived from Trails A, Trails B, and Stroop Color-Word; additional details can be found elsewhere (Dowling, Hermann, La Rue, & Sager, 2010). Additional tests comprising two general ability factors were dropped from the WRAP battery after Wave 2, and as a result, these factors were omitted from current analyses.
Stressful events
Stressful experiences were assessed using a 12-item questionnaire adapted from the Women’s Health Initiative (Matthews et al., 1997). Participants indicated whether or not they had experienced common potentially stressful events (e.g., “Did your spouse or partner die?” and “Did you have any major problems with money?”) in the past 12 months. Responses from all 12 items were summed to create a stress index score (possible range, 0–12). Prior to analyses, the stress index score was standardized across all participants [~N (0, 1)].
Social support
Perceived social support was assessed using a questionnaire incorporating nine items, taken from the Medical Outcomes Survey (MOS) (Sherbourne & Stewart, 1991). Participants were asked how often the various examples of support were available to them if they needed it (e.g., “Someone you can count on to listen to you when you need to talk” and “Someone to help with daily chores if you are sick”). Response options ranged from 0 (none of the time) to 4 (all of the time) (Sherbourne & Stewart, 1991). Responses from all nine items were summed to create a support index score (possible range, 0–36). Prior to analyses, the support index score was standardized across all participants [~N (0, 1)].
APOE Genotyping
DNA for the APOE genotyping was extracted from whole blood samples using PUREGENE® DNA Isolation Kit (Gentra Systems, Inc., Minneapolis, MN). DNA concentrations were quantified using UV spectrophotometry (DU® 530 Spectrophotometer, Beckman Coulter, Fullerton, CA). Genotyping for the two APOE single nucleotide polymorphisms that determine the ε2, ε3, and ε4 alleles, rs429358 and rs7412, was done previously by WRAP and has been described in detail (Johnson et al, 2011).
Other potential covariates
A health history questionnaire assessing demographic factors and medical and psychiatric history, as well as selected lifestyle factors such as physical activity; caregiving for a sick or limited friend or relative; and use of tobacco, caffeine, and alcohol, was completed by all participants. Participants were coded as never, ever, or current smokers and as abstinent, moderate, or heavy drinkers (abstinent=0 drinks in the past month, moderate=<1 or 1–2 drinks per day, heavy=3–5 or ≥6 drinks per day). Physical activity and caffeine consumption were coded as dichotomous: participants were categorized as active (engaging in physical activities or exercise more than four times per month) or non-active, and heavy or not-heavy users of caffeine (heavy caffeine use = 3 or more caffeinated beverages per day). Height and weight were measured by WRAP personnel and body mass index (BMI) was calculated (kg/m2).
Statistical analysis
In preliminary regression analyses, associations between potential covariates and the four outcome variables were assessed in a partial base model and a fuller second model including health and lifestyle covariates that could confound the basic demographic variables of the base model. Covariates which were not associated at the p <0.05 level with at least one outcome variable and which did not change the coefficients of any other covariate by more than 10% were excluded from final full models. Consequently, physical activity, alcohol use, and caffeine use were excluded from the final full models. After covariate effects were assessed, the two key predictor variables were individually added to both the partial and the full model in order to better examine the role of health and behavioral confounders in the relationship between psychosocial predictors and cognitive function. In sum, the first, partial model regressed the four cognitive composite scores of interest, SF, IM, VLM, and WM, on each distinct psychosocial predictor plus age, gender, education, and number of APOE ε4 alleles. The second, fuller model also adjusted for three additional health and lifestyle factors (BMI, marital/partner status, and smoking status) potentially associated with both cognitive function and the stress and support predictors. Finally, to test the buffering hypothesis, we examined the interaction between the stress and social support variables, and to test our hypothesis that presence of a known genetic risk factor could modify the relationship between psychosocial factors and cognitive function, we examined the interaction between individual psychosocial factors and the number of APOE ε4 alleles. Each interaction term was assessed separately, after being added to the second, full covariate model. Preliminary model diagnostics established that basic regression assumptions including linearity and homoscedasticity were met. A high degree of internal consistency was observed for the items in our social support index (Cronbach’s alpha = 0.92). Alpha value was lower (0.36) for our stressful events index; this is unsurprising, since there is little reason that the recent experience of one stressful event measured by the index should be associated with the recent experience of any other measured stressful events. Potential multicollinearity was assessed at a basic level by running preliminary Pearson correlation analyses (not shown) of variables included in the full model; additionally, tolerance values indicated that collinearity was not a concern. All analyses were performed with SAS, version 9.2 for Linux.
Results
Descriptive statistics on WRAP participant characteristics at Wave 2 are presented in Table 1. Participant age ranged from 40 to 73 years old and the majority was female. The sample was highly educated; nearly 40% had at least some post-college education. Pre-standardization psychosocial predictor scores are also presented in Table 1. Reported stress scores were relatively low, with a mean of 1.7 out of 12 possible points. Reported total social support was high, with a mean score of 30.5 out of 36 possible points. Means for the standardized cognitive outcome variables vary slightly from zero, indicating that average performance in the sample as a whole has improved very slightly from Wave 1, consistent with a practice effect.
Table 1.
Descriptive statistics for study participants (N=623).
| Variable | Percent or Mean (SD) | Range |
|---|---|---|
| Age, years | 56.7 (6.5) | 40 – 73 |
| Gender, female | 71% | |
| Education | ||
| High school/GED | 10% | |
| Some college | 31% | |
| College graduate | 20% | |
| Post-college | 39% | |
| BMI, kg/m2 | 28.8 (6.3) | 17.5 – 56.5 |
| APOE ε4 alleles | ||
| 0 | 54% | |
| 1 | 41% | |
| 2 | 5% | |
| Smoking | ||
| Never | 56% | |
| Past | 37% | |
| Current | 7% | |
| Alcohol use | ||
| Abstinent | 16% | |
| Moderate | 69% | |
| Heavy | 15% | |
| Caffeine consumption, heavy | 32% | |
| Physical activity, >4 times/mo. | 76% | |
| Partner, yes | 80% | |
| Stress index | 1.7 (1.4) | 0 – 7 |
| Support index | 30.5 (6.0) | 5 – 36 |
| Speed and flexibility | .25 (.98) | −4.64 – 2.97 |
| Immediate memory | .02 (1.06) | −2.74 – 2.86 |
| Verbal learning and memory | .08 (1.05) | −3.39 – 1.83 |
| Working memory | .11 (.99) | −2.62 – 2.79 |
Main Effects
Table 2 presents a summary of results from preliminarily regressing SF, IM, VLM, and WM composite scores on a number of covariates. Of the health and lifestyle covariates considered, physical activity, alcohol use, and caffeine use were excluded from the final full models because they were not associated with at least one outcome variable at the p <0.05 level and they did not change the coefficients of any other covariate by more than 10%. Only education showed any change from Model 1 when health and lifestyle variables were added (Model 2); the association between education and test performance in the SF domain was no longer significant in the full model. This was not true for any of the other cognitive domains.
Table 2.
Regression coefficients (standard errors) for the included covariates by cognitive domain.
| Speed and Flexibility (SF) | Immediate Memory (IM) | Verbal Learning and Memory (VLM) | Working Memory (WM) | |
|---|---|---|---|---|
| Model 1: Key Covariates | ||||
| Age | −.058 (.006)** | −.032 (.006)** | −.038 (.006)** | −.008 (.006) |
| Female | .323 (.080)** | .587 (.089)** | .769 (.085)** | −.005 (.087) |
| Education | .069 (.035)* | .068 (.039) | .153 (.037)** | .136 (.038)** |
| APOE ε4 count | −.091 (.061) | −.012 (.068) | −.005 (.065) | −.073 (.066) |
| Model 2: Model 1 + Health and Lifestyle Covariates | ||||
| Age | −.059 (.006)** | −.034 (.006)** | −.038 (.006)** | −.010 (.006) |
| Female | .320 (.080)** | .613 (.090)** | .796 (.087)** | .029 (.087) |
| Education | .046 (.035) | .050 (.040) | .145 (.038)** | .145 (.039)** |
| APOE ε4 count | −.085 (.060) | −.010 (.068) | −.003 (.065) | −.005 (.006) |
| BMI | −.015 (.006)* | −.003 (.007) | −.004 (.006) | −.004 (.006) |
| Past smoking | −.124 (.077) | .029 (.086) | .080 (.083) | .238 (.084)* |
| Current smoking | −.432 (.148)* | −.496 (.166)* | −.260 (.160) | .113 (.161) |
| Partner | .170 (.091) | .010 (.102) | .039 (.099) | .287 (.099)* |
Table 3 shows coefficients for the key psychosocial predictors in both base and full models. In a model adjusting for age, gender, education, and number of APOE ε4 alleles (Table 3, Model 1), higher stress index score was significantly associated with lower SF score (β = −.117, p = .002). This relationship remained significant following the introduction of several health and lifestyle factors (β = −.094, p = .01; Table 3, Model 2). Higher social support index score was significantly associated with higher SF score (β = .106, p = .005); this association remained in the full model (β = .084, p = .03). Neither of the psychosocial predictors was significantly associated with IM, VLM or WM.
Table 3.
Coefficients (standard errors) for each psychosocial index by cognitive domain.
| Speed and Flexibility (SF) | Immediate Memory (IM) | Verbal Learning and Memory (VLM) | Working Memory (WM) | |
|---|---|---|---|---|
| Model 1 | ||||
| Stress index | −.117 (.037)* | .039 (.041) | .057 (.040) | −.041 (.041) |
| Social support index | .106 (.037)* | .009 (.042) | .044 (.040) | .022 (.041) |
| Model 2 | ||||
| Stress index | −.094 (.037)* | .057 (.042) | .070 (.041) | −.031 (.041) |
| Social support index | .084 (.039)* | .006 (.045) | .037 (.043) | −.024 (.043) |
| Model 3: Interactions | ||||
| (3a) Stress index* social support | .011 (.036) | .017 (.041) | −.017 (.039) | .026 (.040) |
| (3b) Stress index*APOE | −.004 (.059) | −.039 (.067) | −.058 (.100) | .117 (.065) |
| (3c) Social support*APOE | −.118 (.058)* | .053 (.067) | .073 (.064) | .035 (.065) |
Model 1 includes age, gender, education, and number of APOE ε4 alleles. Model 2 adds BMI, smoking status, and marital/partner status.
p ≤ .05;
p ≤ .001.
Interactions
As seen at the bottom of Table 3, the hypothesized interaction between stress index and social support was not significant for any of the cognitive outcome measures, nor was the interaction between stress index and APOE ε4 allele count. However, for SF, there was a modestly significant negative interaction between social support and APOE ε4 allele count (β = −.118, p = .04); each additional copy of the ε4 allele appears to significantly weaken the positive relationship between social support and SF score. This relationship was not seen for the remaining three cognitive domains.
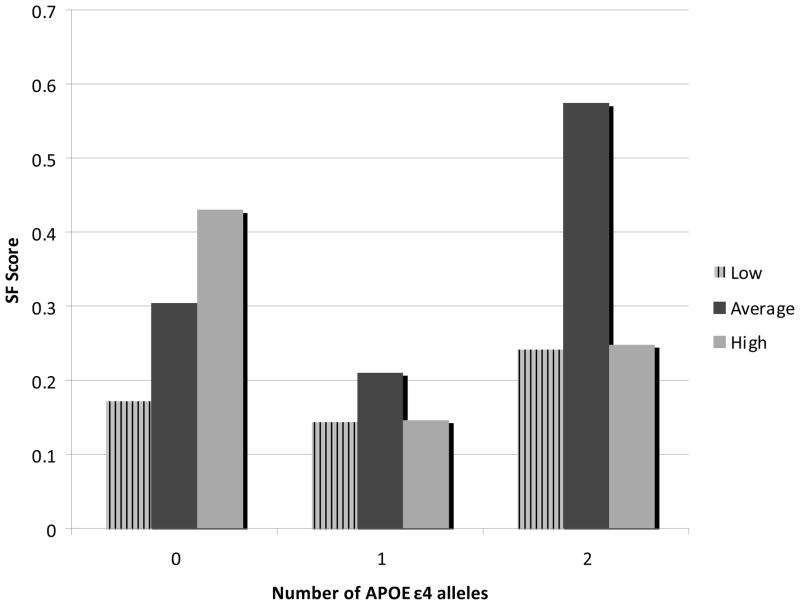
In order to help visualize the significant interaction, adjusted mean SF scores were calculated, stratified by tertile of psychosocial risk factor and number of APOE ε4 alleles (Figure 1). A positive linear relationship was seen only in the group with no APOE ε4 alleles (N=338). Among participants with one APOE ε4 allele (N=253) or two alleles (N=32), it appears that those reporting average levels of social support score highest on tests of speed and flexibility; both low and high perceived support is associated with lower adjusted mean cognitive scores.
Figure 1.
Adjusted mean standardized speed and flexibility scores for low, average, and high social support by number of APOE e4 alleles.
Discussion
We examined the impact and interplay of two distinct psychosocial risk factors on the cognitive domain of speed and flexibility, which plays a significant role in independent living and daily activities, as well as three domains of memory: immediate memory, verbal learning and working memory; these have been shown to be the domains of cognition most vulnerable to change in early AD (Backman et al., 2005; Twamley et al., 2006). Our hypotheses, that life stress would be associated with poorer cognitive function, and that higher levels of perceived social support would be associated with better cognitive function, appear to be supported by cross-sectional data from the WRAP sample. On the other hand, we did not see the expected stress-support buffering effect, in the form of an interaction between the psychosocial factors. To our knowledge, this is the first time a study of psychosocial risk factors has focused on a large sample of persons at increased risk for AD and earlier cognitive changes due to their family history of AD.
The association between recent stressful events and cognitive function varied depending on the cognitive domain in question. Stressful events were significantly associated with speed and flexibility, even after adjustment for health and lifestyle factors. This relationship was seen despite generally low scores on our index of reported stressful events. No association was observed between stressful events and memory. Previous studies examining relationships between reported stressful experiences and cognitive function have generally shown mixed results; several have found that positive or negative impact varies by type of event, and that the types of events experienced may be more important than a cumulative score (Comijs, van den Kommer, Minnaar, Penninx, & Deeg, 2011; Rosnick, Small, McEvoy, Borenstein, & Mortimer, 2007). Although we were not able to examine the type of stressful events experienced, our finding that potentially stressful experiences are negatively associated with cognitive performance supports the underlying hypothesis that stress has a detrimental effect on brain health and cognition (McEwen & Sapolsky, 1995).
We also examined the presumably positive impact that social support might have on all four domains of cognitive function. We found a positive relationship between perceived social support and speed and flexibility, but no association between support and memory. As with stressful experiences, the association remained significant when additional health and lifestyle covariates were added to regression models. Higher reported social support has previously been positively associated with cognitive function, especially executive function, in middle-aged adults (Seeman et al., 2011) and with global cognitive function in older adults (Seeman et al., 2001). Another recent study examining longitudinal relationships between social support and cognition found that the effect of social support was dependent upon type of support represented; decreased instrumental but not subjective support was associated with diminishing cognitive function over time (Dickinson, Potter, Hybels, McQuoid, & Steffens, 2011). The lack of association seen between our psychosocial predictors and memory is surprising given these previous findings. Possibly contributing to our null findings is our WRAP sample’s higher-than-expected (with regard to age norms) performance on the AVLT, a key component of both the Immediate Memory and Verbal Learning and Memory factors.
Due to an a priori hypothesis that, based on buffering models, perceived social support would modify the negative effect of stress, we ultimately included a stress-support interaction term in the model. Contrary to our expectations, the hypothesized interaction between social support and stressful experiences was not seen. One factor that cannot be discounted is the lack of variance in the stress index score; scores were quite low overall (mean = 1.7 out of 12 possible points). Moreover, because some pertinent and potentially chronic stressors, such as caregiving activity, were not included in our stressful events scale, the scores may not represent all of the psychological stress participants were experiencing, especially in this population of individuals with at least one parent with AD. It is also possible that our sample was simply not large enough to detect an interaction between relatively subtle psychosocial risk factors.
We also examined the potential interaction between individual psychosocial predictors and the number of APOE ε4 alleles present. Relatively few researchers have examined gene-social environment interactions in cognitive function and decline; however, there is reason to believe that such interactions exist. Comijs et al. recently found that the presence of at least one APOE ε4 allele amplified the association between negative life events and cognitive decline in older adults (Comijs et al., 2011). And, providing clues for a pathway for such effect modification, Noble et al. found an interaction between inflammatory biomarker C-reactive protein and the APOE ε4 allele, showing that detrimental effects of stress-related inflammation are strongest when both environmental and genetic risk factors are present (Noble et al., 2010). While we did not see a significant interaction between genetic risk and stressful events, we did find that the presence of one or two APOE ε4 alleles diminished the positive relationship between social support and speed and flexibility. To further illuminate this interaction, we calculated covariate-adjusted mean speed and flexibility scores for each subgroup (tertile of reported social support by number of risk alleles) and it became clear that the relationship between social support and speed and flexibility becomes non-linear in the presence of the ε4 risk allele, with those reporting in the second, or average, tertile performing better than those reporting either low or high levels of social support. While the sample size in the group with two risk alleles, generally considered at the highest risk for cognitive decline and AD, is too small to draw any conclusions about the data presented in Figure 1, it does seem clear when looking at both the one- and two-allele groups that presence of the APOE ε4 allele changes the relationship between social support and speed and flexibility. This is particularly notable given that our population is limited to participants with at least one parent diagnosed with AD, so both carriers and non-carriers of the ε4 risk allele remain likely to share some general risk factors, such as genetic vulnerability to cognitive decline as well as caregiving stress and potential anxiety surrounding their own perceived cognitive health. It is possible that individuals in the highest tertile of social support who have 1 or 2 copies of the ε4 risk allele may either need or perceive higher levels of social support due to declining cognition or comorbidities resulting from the ε4 risk allele.
One limitation of the current study is our inability to assess the causal nature of our relationships. The use of cross-sectional data makes parsing the likely bidirectional relationship between psychosocial risk factors and cognitive decline somewhat challenging. Another limitation is that the stressful events questionnaire does not capture chronic everyday stress, which may be important in cognitive function. Finally, it must be acknowledged that the majority of cross-sectional variance in cognition scores, particularly in a sample of middle-aged adults, arises not from cognitive decline but from differences in peak levels of cognitive performance. It is therefore possible that the associations seen here represent associations with peak cognitive ability rather than early cognitive aging. Despite any limitations, however, explorations of risk factors for AD in a genetically vulnerable sample have been rare, and WRAP’s comprehensive psychosocial and neuropsychological inventory provides a unique opportunity to examine relationships that are unlikely to be simple or unidirectional. Future longitudinal analyses with the WRAP cohort will allow us to begin untangling the cause-and-effect relationships among these variables.
Acknowledgments
Funding
The Wisconsin Registry for Alzheimer’s Prevention is supported by a National Institutes of Health, National Institute on Aging grant, (R01AG27161) as well as grants from the Helen Bader Foundation, the Northwestern Mutual Foundation, and the Extendicare Foundation. The current study received further funding support from the University of Wisconsin – Madison Graduate School.
Footnotes
Declaration of Conflicting Interests
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Backman L, Jones S, Berger AK, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer’s disease: a meta-analysis. Neuropsychology. 2005;19(4):520–531. doi: 10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- Barnes LL, Mendes de Leon CF, Wilson RS, Bienias JL, Evans DA. Social resources and cognitive decline in a population of older African Americans and whites. Neurology. 2004;63(12):2322–2326. doi: 10.1212/01.wnl.0000147473.04043.b3. [DOI] [PubMed] [Google Scholar]
- Bassuk SS, Glass TA, Berkman LF. Social disengagement and incident cognitive decline in community-dwelling elderly persons. Annals of Internal Medicine. 1999;131(3):165–173. doi: 10.7326/0003-4819-131-3-199908030-00002. [DOI] [PubMed] [Google Scholar]
- Brown RG, Scott LC, Bench CJ, Dolan RJ. Cognitive function in depression: its relationship to the presence and severity of intellectual decline. Psychological Medicine. 1994;24(4):829–847. doi: 10.1017/s0033291700028932. [DOI] [PubMed] [Google Scholar]
- Butters MA, Young JB, Lopez O, Aizenstein HJ, Mulsant BH, Reynolds CF, DeKosky ST, Becker JT. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues in Clinical Neuroscience. 2008;10(3):345–357. doi: 10.31887/DCNS.2008.10.3/mabutters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. Social relationships and health. American Psychologist. 2004;59(8):676–684. doi: 10.1037/0003-066X.59.8.676. [DOI] [PubMed] [Google Scholar]
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychological Bulletin. 1985;98(2):310–357. [PubMed] [Google Scholar]
- Comijs HC, van den Kommer TN, Minnaar RW, Penninx BW, Deeg DJ. Accumulated and differential effects of life events on cognitive decline in older persons: depending on depression, baseline cognition, or ApoE epsilon4 status? Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2011;66(Suppl 1):i111–120. doi: 10.1093/geronb/gbr019. [DOI] [PubMed] [Google Scholar]
- Dean A, Kolody B, Wood P. Effects of Social Support from Various Sources on Depression in Elderly Persons. Journal of Health and Social Behavior. 1990;31(2):148–161. [PubMed] [Google Scholar]
- Dickinson WJ, Potter GG, Hybels CF, McQuoid DR, Steffens DC. Change in stress and social support as predictors of cognitive decline in older adults with and without depression. International Journal of Geriatric Psychiatry. 2011;26(12):1267–1274. doi: 10.1002/gps.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling NM, Hermann B, La Rue A, Sager MA. Latent structure and factorial invariance of a neuropsychological test battery for the study of preclinical Alzheimer’s disease. Neuropsychology. 2010;24(6):742–756. doi: 10.1037/a0020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillit HM, Butler RN, O’Connell AW, Albert MS, Birren JE, Cotman CW, Greenough WT, Gold PE, Kramer AF, Kuller LH, Perls TT, Sahagan BG, Tully T. Achieving and maintaining cognitive vitality with aging. Mayo Clinic Proceedings. 2002;77(7):681–696. doi: 10.4065/77.7.681. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Wang HX, Ericsson K, Maytan M, Winblad B. Influence of social network on occurrence of dementia: a community-based longitudinal study. Lancet. 2000;355(9212):1315–1319. doi: 10.1016/S0140-6736(00)02113-9. [DOI] [PubMed] [Google Scholar]
- Grice JW. Computing and evaluating factor scores. Psychological Methods. 2001;6(4):430–450. [PubMed] [Google Scholar]
- Gualtieri CT, Johnson LG, Benedict KB. Neurocognition in Depression: Patients on and Off Medication Versus Healthy Comparison Subjects. Journal of Neuropsychiatry and Clinical Neuroscience. 2006;18(2):217–225. doi: 10.1176/jnp.2006.18.2.217. [DOI] [PubMed] [Google Scholar]
- Johansson L, Guo X, Waern M, Ostling S, Gustafson D, Bengtsson C, Skoog I. Midlife psychological stress and risk of dementia: a 35-year longitudinal population study. Brain. 2010;133(Pt 8):2217–2224. doi: 10.1093/brain/awq116. [DOI] [PubMed] [Google Scholar]
- Johnson SC, La Rue A, Hermann BP, Xu G, Koscik RL, Jonaitis EM, Bendlin BB, Hogan KJ, Roses AD, Saunders AM, Lutz MW, Asthana S, Green RC, Sager MA. The effect of TOMM40 poly-T length on gray matter volume and cognition in middle-aged persons with APOE epsilon3/epsilon3 genotype. Alzheimers & Dementia. 2011;7(4):456–465. doi: 10.1016/j.jalz.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rue A, Hermann BP, Jones JE, Johnson SC, Asthana S, Sager MA. Effect of parental family history of Alzheimer’s disease on serial position profiles. Alzheimers & Dementia. 2008;4(4):285–290. doi: 10.1016/j.jalz.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rue A, Matsuyama SS, McPherson S, Sherman J, Jarvik LF. Cognitive performance in relatives of patients with probable Alzheimer disease: an age at onset effect? Journal of Clinical and Experimental Neuropsychology. 1992;14(4):533–538. doi: 10.1080/01688639208402842. [DOI] [PubMed] [Google Scholar]
- La Rue A, O’Hara R, Matsuyama SS, Jarvik LF. Cognitive changes in young-old adults: effect of family history of dementia. Journal of Clinical and Experimental Neuropsychology. 1995;17(1):65–70. doi: 10.1080/13803399508406582. [DOI] [PubMed] [Google Scholar]
- Lakey B, Tardiff TA, Drew JB. Negative social interactions: Assessment and relations to social support, cognition, and psychological distress. Journal of Social & Clinical Psychology. 1994;13(1):42–62. [Google Scholar]
- Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological Assessment. Oxford University Press; USA: 2004. [Google Scholar]
- Lupien SJ, Schwartz G, Ng YK, Fiocco A, Wan N, Pruessner JC, Meaney MJ, Nair NP. The Douglas Hospital Longitudinal Study of Normal and Pathological Aging: summary of findings. Journal of Psychiatry and Neuroscience. 2005;30(5):328–334. [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Petersen KL, Sathanoori R, Muldoon MF, Neumann SA, Ryan C, Flory JD, Manuck SB. Interleukin-6 covaries inversely with cognitive performance among middle-aged community volunteers. Psychosomatic Medicine. 2006;68(6):895–903. doi: 10.1097/01.psy.0000238451.22174.92. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Shumaker SA, Bowen DJ, Langer RD, Hunt JR, Kaplan RM, Klesges RC, Ritenbaugh C. Women’s health initiative. Why now? What is it? What’s new? American Psychologist. 1997;52(2):101–116. doi: 10.1037//0003-066x.52.2.101. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Sapolsky RM. Stress and cognitive function. Current Opinion in Neurobiology. 1995;5(2):205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Butters MA, Mulsant BH, Pollock BG, Zmuda MD, Houck PR, Reynolds CF. Decreased working memory and processing speed mediate cognitive impairment in geriatric depression. Psychological Medicine. 2000;30(3):679–691. doi: 10.1017/s0033291799001968. [DOI] [PubMed] [Google Scholar]
- Noble JM, Manly JJ, Schupf N, Tang MX, Mayeux R, Luchsinger JA. Association of C-reactive protein with cognitive impairment. Archives of Neurology. 2010;67(1):87–92. doi: 10.1001/archneurol.2009.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskind ER, Wilkinson CW, Petrie EC, Schellenberg GD, Raskind MA. Increased CSF cortisol in AD is a function of APOE genotype. Neurology. 2001;56(8):1094–1098. doi: 10.1212/wnl.56.8.1094. [DOI] [PubMed] [Google Scholar]
- Rosnick CB, Small BJ, McEvoy CL, Borenstein AR, Mortimer JA. Negative life events and cognitive performance in a population of older adults. Journal of Aging and Health. 2007;19(4):612–629. doi: 10.1177/0898264307300975. [DOI] [PubMed] [Google Scholar]
- Saczynski JS, Beiser A, Seshadri S, Auerbach S, Wolf PA, Au R. Depressive symptoms and risk of dementia: the Framingham Heart Study. Neurology. 2010;75(1):35–41. doi: 10.1212/WNL.0b013e3181e62138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager MA, Hermann B, La Rue A. Middle-aged children of persons with Alzheimer’s disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer’s Prevention. Journal of Geriatric Psychiatry and Neurology. 2005;18(4):245–249. doi: 10.1177/0891988705281882. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Lusignolo TM, Albert M, Berkman L. Social Relationships, Social Support, and Patterns of Cognitive Aging in Healthy, High-Functioning Older Adults: MacArthur Studies of Successful Aging. Health Psychology. 2001;20(4):243–255. doi: 10.1037//0278-6133.20.4.243. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Miller-Martinez DM, Stein Merkin S, Lachman ME, Tun PA, Karlamangla AS. Histories of social engagement and adult cognition: Midlife in the U.S. Study. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2011;66(Suppl 1):i141–152. doi: 10.1093/geronb/gbq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherbourne CD, Stewart AL. The MOS social support survey. Social Science & Medicine. 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- Stawski RS, Sliwinski MJ, Smyth JM. Stress-related cognitive interference predicts cognitive function in old age. Psychology and Aging. 2006;21(3):535–544. doi: 10.1037/0882-7974.21.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AJ, Gallagher P, Robinson LJ, Porter RJ, Young AH, Ferrier IN, O’Brien JT. A comparison of neurocognitive impairment in younger and older adults with major depression. Psychological Medicine. 2009;39(05):725–733. doi: 10.1017/S0033291708004042. [DOI] [PubMed] [Google Scholar]
- Twamley EW, Ropacki SA, Bondi MW. Neuropsychological and neuroimaging changes in preclinical Alzheimer’s disease. Journal of the International Neuropsychological Society. 2006;12(5):707–735. doi: 10.1017/S1355617706060863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: A review with emphasis on underlying mechanisms and implications for health. Psychological Bulletin. 1996;119(3):488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]