Abstract
Many studies have described potent effects of BDNF, 17β-estradiol or androgen on hippocampal synapses and their plasticity. Far less information is available about the interactions between 17β-estradiol and BDNF in hippocampus, or interactions between androgen and BDNF in hippocampus. Here we review the regulation of BDNF in the mossy fiber pathway, a critical part of hippocampal circuitry. We discuss the emerging view that 17β-estradiol upregulates mossy fiber BDNF synthesis in the adult female rat, while testosterone exerts a tonic suppression of mossy fiber BDNF levels in the adult male rat. The consequences are interesting to consider: in females, increased excitability associated with high levels of BDNF in mossy fibers could - on the one hand - improve normal functions of area CA3, such as the ability to perform pattern completion. On the other hand, memory retrieval may lead to anxiety if stressful events are recalled. Therefore, the actions of 17β-estradiol on the mossy fiber pathway in females may provide a potential explanation for the greater incidence of anxiety-related disorders and post-traumatic stress syndrome (PTSD) in women relative to men. In males, suppression of BDNF-dependent plasticity in the mossy fibers may be protective, but at the `price' of reduced synaptic plasticity in CA3.
Keywords: neurotrophin, area CA3, hippocampus, mossy fiber sprouting, testosterone, estradiol
I. BDNF, the hippocampus and the mossy fiber pathway
BDNF is one of the members of the neurotrophin family, which includes nerve growth factor (NGF), neurotrophin-3 (NT-3) and neurotrophin 4/5 (NT-4/5). All neurotrophins bind to p75NTR, a so-called `death' receptor because of its critical role in programmed cell death during development; neurotrophins also bind to tyrosine kinase receptors (trk) with NGF binding specifically to trkA, BDNF to trkB, and NT-3 to trkC. NT-4/5 can bind to trkB (Figure 1; Chao, 2003; Reichardt, 2006; Zampieri and Chao, 2006; Teng et al., 2010).
Figure 1.
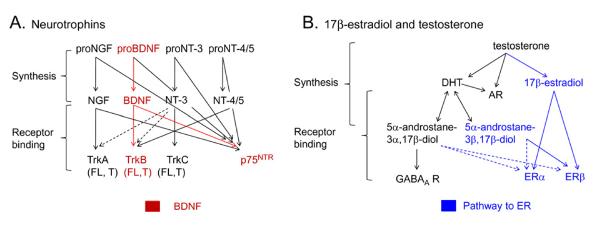
Synthesis and receptors for BDNF, 17β-estradiol, testosterone and their metabolites.
A. The neurotrophin family includes nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4/5 (NT-4/5). Neurotrophins are produced from precursors (proneurotrophins) which can bind to p75NTR receptors. Mature neurotrophins bind to trk (tropomyosin-related kinase) receptors, which exist in a full-length or truncated form. The truncated receptors lack the intracellular kinase domain of full-length trk. For trkB, there are two truncated forms, trkB.T1 and trkB.T2. Mature neurotrophins can also bind to p75NTR. NGF binds selectively to trkA, BDNF to trkB, NT-3 to trkC, and NT-4/5 is a ligand for trkB. Dotted lines indicate relatively weak binding to receptors.
B. Testosterone binds to androgen receptors (AR) and is the precursor to metabolites acting at estrogen receptors (ER) and androgen receptors (AR). The pathways that lead to ER activation are in blue. DHT= dihydrotestosterone.
In hippocampus, BDNF mRNA is expressed in all principal cells: the granule cells of the dentate gyrus, pyramidal cells of area CA3, and pyramidal cells of area CA1. However, the granule cells synthesize the most BDNF protein in the normal adult rat (Conner et al., 1997; Yan et al., 1997), in the monkey (Zhang et al., 2007; Nagahara et al., 2009) and human hippocampus (Mathern et al., 1997; Murray et al., 2000; Murer et al., 2001). Granule cells primarily transport BDNF to their axons, the mossy fibers, where BDNF is packaged in dense core vesicles like neuropeptides (Conner et al., 1997; Dieni et al., 2012). The precursor of BDNF, proBDNF, is also present in mossy fibers (Dieni et al., 2012) and binds primarly to p75NTR (Lee et al., 2001; Ibanez, 2002). It seems unlikely that proBDNF would play a major role in adult hippocampus because p75NTR levels are low in the adult hippocampus, whereas trkB levels are relatively high (for review, see Harte-Hargrove et al., 2013). TrkB receptors exist in a full length form as well as a truncated form without the internal kinase domain of full-length trkB. Truncated trkB receptors are expressed in many locations (Fryer et al., 1996; Kryl et al., 1999), and have many potential functions, including actions as a dominant negative receptor that inhibits effects of BDNF at trkB, and actions independent of trkB (Fenner, 2012). In general, the effects of truncated trkB include 1) effects during neurodevelopment (Fryer et al., 1997; Yacoubian and Lo, 2000; Luikart et al., 2003; Tervonen et al., 2006; Liu et al., 2012), 2) effects mediated by astrocytes (Rose et al., 2003), 3) neuroprotection (Saarelainen et al., 2000; Haapasalo et al., 2001; De Wit et al., 2006; Dorsey et al., 2006; Yanpallewar et al., 2012) and 4) the control of BDNF actions at trkB (Fryer et al., 1997; Haapasalo et al., 2002; Carim-Todd et al., 2009).
The mossy fiber boutons containing BDNF are complex compared to typical glutamatergic synapses (Figure 2). There are several types of boutons, large complex boutons sometimes termed “giant” or “massive” because of their size, and smaller boutons, similar in size to other nerve terminals in hippocampus (Acsady et al., 1998). A single mossy fiber from a granule cell collateralizes in the hilus, making both large and small boutons, and travels into CA3 where it forms a fiber that parallels the cell layer in stratum lucidum, ending in CA3a at the junction with CA2 (Claiborne et al., 1986; Henze et al., 2000; Blaabjerg and Zimmer, 2007). The part of the mossy fiber that is located in stratum ludicum gives rise to giant boutons at regularly-spaced intervals. Each of the massive boutons innervates the complex spines of pyramidal cell proximal dendrites called thorny excrescences (Chicurel and Harris, 1992). These giant boutons also have filamentous extensions, which make synapses onto local GABAergic neurons (Figure 2; (Acsady et al., 1998)). The remarkable presynaptic giant boutons and postsynaptic thorny excrescences make the mossy fiber pathway the most complex of hippocampal afferent systems (Henze et al., 2000; Blaabjerg and Zimmer, 2007). Mossy fibers are also complex because they are able to alter their neurochemical content rapidly and in diverse ways (Jaffe and Gutierrez, 2007). Increased neuronal activity is one stimulus that induces multiple changes in the neurotransmitter and peptide content of mossy fibers. For example, BDNF mRNA increases rapidly in granule cells in response to stimulation (Isackson et al., 1991), even a brief stimulus such as a high frequency burst of electrical pulses to the perforant path, used to elicit LTP (Bramham et al., 1996). Strong neuronal activity, such as seizures, is an even more potent stimulus: diverse peptides change expression in mossy fibers in response to seizures. Neuropeptide Y, normally absent in mossy fibers, is expressed throughout the mossy fiber pathway after repetitive spontaneous seizures (Sperk et al., 1992; Winawer et al., 2007; Minkeviciene et al., 2009) where it is considered potentially `anticonvulsant' because neuropeptide Y decreases glutamate release (Sperk et al., 2007). Seizures also increase the expression of GABA in mossy fibers (Gutierrez, 2005). Together these effects would seem likely to decrease network activity, potentially a homeostatic response to seizures. However, mossy fibers also express the opiate peptide dynorphin, which is generally inhibitory and reduced by seizures; mossy fibers also increase synthesis of the opiate peptide enkephalin after seizures and enkephalin is typically excitatory (White et al., 1987; Hong et al., 1988; Gall et al., 1990).
Figure 2.
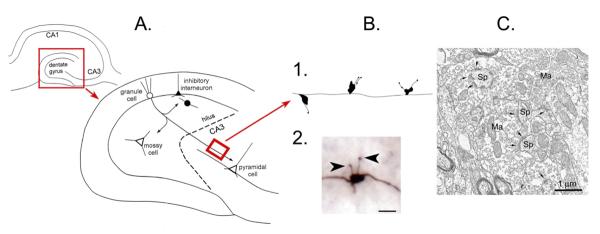
The mossy fiber pathway.
A.
Top left: A schematic or the rodent hippocampus illustrates the location of the major subfields. The area in the red box is expanded to the lower right.
Lower right: The dentate gyrus and area CA3 is shown to illustrate the pathway of the axons of the dentate gyrus granule cells to area CA3 pyramidal cells, the mossy fiber pathway. The mossy fibers collateralize in the hilus where they innervate hilar neurons (mossy cells or inhibitory interneurons) or the hilar dendrites of inhibitory interneurons with cell bodies in the granule cell layer. The main mossy fiber axon innervates pyramidal cells in area CA3 with periodic en passant synapses along the axon, which travels parallel to the CA3 cell layer. Glutamatergic neurons have white cell bodies; inhibitory interneurons are black. The area in the red box is a section from the main mossy fiber axon, expanded in B. From (Scharfman, 2002).
B. 1. A drawing of the periodic massive boutons in stratum lucidum, showing the diversity of irregular shapes of the giant boutons and the diversity in filamentous extensions. 2. A photomicrograph of a mossy fiber bouton from a biocytin-filled granule cell shows the filamentous extensions (arrowheads) that extend from the main bouton. Calibration= 1 μm.
C. An example of a mossy fiber bouton (Ma= massive bouton) contacting spines (Sp) illustrates the complex morphology of the bouton. Kindly provided by Dr. Csaba Leranth.
Another distinguishing feature of the mossy fiber pathway is its continuous regeneration in adulthood because of the addition of new granule cells, a consequence of postnatal neurogenesis (Kempermann, 2006). The new granule cells are thought to contribute to the mossy fiber pathway in the same way as granule cells born in early life (Toni et al., 2008; Gu et al., 2012) although this issue is still under investigation by many laboratories.
Increasing the dynamic range of mossy fiber transmission: a role for BDNF?
What reason might there be for the remarkable structural complexity and neurochemical diversity of mossy fibers? One possibility is that this pathway needs to have a very large dynamic range to adequately serve its purpose, which involves a nonlinear transformation of input from the cortex, through the dentate gyrus granule cells, to area CA3. Most pathways in the CNS that use glutamate as their primary neurotransmitter have a modest dynamic range, ranging from weak to strong excitation. In contrast, mossy fibers may exert a primarily inhibitory effect on pyramidal cells, an excitatory effect, and an extremely large excitatory effect that has been referred to as `detonation” (Figure 2; Henze et al., 2000). Moreover, the effects of mossy fibers will vary based on the history of granule cell activity, because granule cell activity will change the neurochemical content of mossy fibers as described above. For example, after a stimulus to induce LTP, granule cells will increase BDNF expression (Bramham et al., 1996). Because repetitive presynaptic discharge is typically necessary to release neuropeptides (Scalettar, 2006; Bergquist and Ludvig, 2009), repetitive granule cell firing after LTP induction would be expected to have a distinct, and possibly more powerful effect than repetitive firing before LTP induction.
How the mossy fibers could exert a primarily inhibitory effect under normal conditions has been explained by quantitative anatomical studies which show that mossy fiber boutons on GABAergic neurons greatly outnumber those on pyramidal cells (Acsady et al., 1998). In addition, GABA can be released from mossy fibers, although it is not clear if GABA release occurs normally; most effects are detected only when glutamatergic transmission is blocked, or experimental manipulations are used that increase the concentration of GABA in mossy fibers, such as seizure induction (Walker et al., 2001; Gutierrez, 2005). One reason to be cautious in concluding that mossy fibers are normally inhibitory is based on recordings of CA3 pyramidal cells in hippocampal slices, showing that the time for feedforward inhibitory transmission is slow compared to excitation of pyramidal cells by mossy fibers, and this difference weakens feedforward inhibition (Torberg et al. 2010).
With increased frequency of presynaptic activity, any normal disynaptic inhibitory effect on pyramidal cells which is produced by GABAergic interneurons would shift to an excitatory effect because of strong frequency facilitation of glutamate release from the large mossy fiber boutons that innervate pyramidal cells (Salin et al., 1996). These large mossy fiber boutons are less common than the smaller boutons that innervate GABAergic cells, but they are packed with a larger concentration of glutamatergic vesicles than the synapses onto GABAergic neurons (Figure 3).
Figure 3.
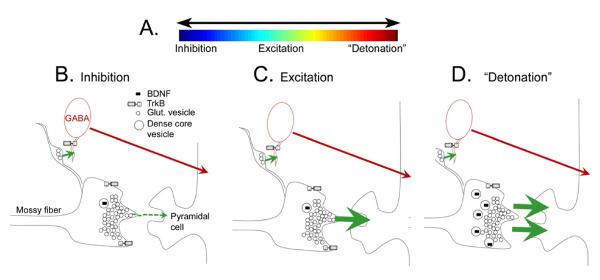
The dynamic range of mossy fibers.
A. A schematic illustrates the idea that mossy fibers can exert a large range of effects on CA3 pyramidal cells, including a net inhibitory effect, excitatory, or extremely excitatory effect, because of the high concentration of glutamate released from the large boutons (“detonation”).
B. A schematic illustrates the local circuitry of the massive boutons in stratum lucidum that innervate pyramidal cell thorny excrescences. The large boutons are packed densely with glutamatergic vesicles (small circles) and a much lower concentration of dense core vesicles (larger circles, containing BDNF, represented by a black symbol, as well as other neuropeptides). Filamentous extensions from the massive boutons make synapses on GABAergic neurons (red). Under normal conditions GABAergic neurons are innervated more than thorny excrescences of pyramidal cells, suggesting that the net effect of mossy fibers is pyramidal cell inhibition (Acsady, 1998).
C. A schematic illustrates the effect of repetitive mossy fiber stimulation, such as 2–3 stimuli 40 msec apart, which leads to large frequency facilitation of EPSPs of pyramidal cells, and a greater net excitatory effect on pyramidal cells than in B.
D. A schematic of the effects of BDNF, which is increased by 17β-estradiol in females or gonadectomy in males, as discussed in the text. Increased BDNF increases the excitatory effects of mossy fibers on pyramidal cells, which could be due to a preferential increase in BDNF in the massive boutons that innvervate pyramidal cells preferentially, because that is known to occur after activity-dependent increases in BDNF (Danzer and McNamara, 2004). BDNF could trigger `detonation' by facilitating release of glutamate from the mossy fiber bouton; this idea is consistent with the ability to induce spreading depression episodes by mossy fiber stimulation under conditions where BDNF levels are high (Scharfman, 1997; Croll et al., 1999; Scharfman et al., 2003; Scharfman et al., 2007; Skucas et al., 2013).
With experimental manipulations that increase BDNF protein content of the mossy fibers, it has been shown that BDNF content increases preferentially in the large boutons that innervate pyramidal cells rather than the filamentous extensions innervating GABAergic neurons (Danzer and McNamara, 2004). Therefore, BDNF could increase the excitatory effects of the large boutons that innervate pyramidal cells, and extend the dynamic range of mossy fiber effects on pyramidal cells in the excitatory direction (Figure 3). For this and other reasons, BDNF has been suggested to play a pro-convulsant role in epilepsy (McNamara and Scharfman, 2012).
II. BDNF and the mossy fiber pathway in female rats
Estrogen in the female rat
There are three primary natural estrogens: estrone (E1), 17β-estradiol (E2), and estriol (E3). The main site of estradiol synthesis is the ovary, although this steroid can also be synthesized in other locations, including the brain. The primary form of estrogen that exerts actions in hippocampus is 17β-estradiol. Throughout the body and brain, 17β - estradiol acts on estrogen receptor α (ERα) and estrogen receptor β (ERβ; Figure 1). Once activated, these receptors couple to co-activator and co-repressor proteins to regulate gene transcription. In addition, some ERs are located in the plasma membrane where they initiate actions on a faster timescale. In hippocampus, ERα and ERβ are located in diverse areas within the dentate gyrus and CA3, in both neurons and glia. ERs also exist in the vasculature. In the mossy fibers, ERα and β have been localized to many areas of the pathway (Milner et al., 2001; Milner et al., 2005). Therefore, ER, BDNF and trkB are widely distributed in the hippocampus, in all subfields and many subcellular locations of both neurons and glia.
In the periphery, estrogen exerts diverse actions at both nuclear and membrane ER, and some of these effects appear to be mediated by neurotrophins, either NGF, BDNF, or NT-3, and their respective trk receptors. In the ovary, as in the brain, induction of BDNF appears to contribute to the effects of estrogen (Dissen et al., 2009; Dorfman et al., 2011). For example, removal of trkB receptors slows follicular growth, but does not stop it completely (Paredes et al., 2004). Therefore, it is not that surprising that the same relationship exists in the brain, with BDNF contributing to actions of estrogen.
Estrogens and BDNF
The first suggestion that estrogen exerts actions in the brain mediated by neurotrophins was made after it was observed that sites of action of 17β-estradiol were also locations of neurotrophin expression (Toran-Allerand et al., 1992; Miranda et al., 1993a,b). Later, an estrogen response-like element was identified on the BDNF gene (Sohrabji et al., 1995), suggesting a potential mechanism by which 17β-estradiol could exert effects mediated by BDNF. That idea was supported by the findings that ovariectomy of adult female rats reduced BDNF protein, and estrogen replacement restored it (Singh et al., 1995; Sohrabji et al., 1995; Berchtold et al., 2001; Liu et al., 2001) although not in all areas of the brain (Jezierski and Sohrabji, 2000; Zhou et al., 2005). The increase in BDNF protein in hippocampus after estrogen replacement is robust, having been replicated again recently (Kiss et al., 2012), it is notable that all published findings do not all completely agree, presumably because of the importance of technical details, such as the amount of time that has passed since ovariectomy, the dose of estrogen, and the technique used to measure BDNF. Moreover, there may be more than one mechanism to upregulate BDNF in response to 17β-estradiol. It has been suggested that 17β-estradiol inhibits GABAergic neurons, disinhibiting hippocampal principal cells, and increasing BDNF synthesis by an activity-dependent mechanism (Blurton-Jones and Tuszynski, 2006).
Estrogen increases BDNF protein content of the mossy fibers
One of the questions raised by these initial findings was the physiological vs. pharmacological actions of 17β-estradiol. Most studies examined the effects of estrogen replacement to ovariectomized female rats, but this approach does not necessarily simulate the effects of endogenous estrogen: indeed, estradiol replacement injection paradigms may result in serum estradiol levels that are well above the normal physiological range (MacLusky et al., 2005). Therefore, a question was raised: did the changes in BDNF protein expression observed after estradiol injection represent physiological responses, or were only observed after pharmacological levels of 17β-estradiol? To address this issue, intact female rats were studied at times when endogenous levels of circulating17β-estradiol were high or low.
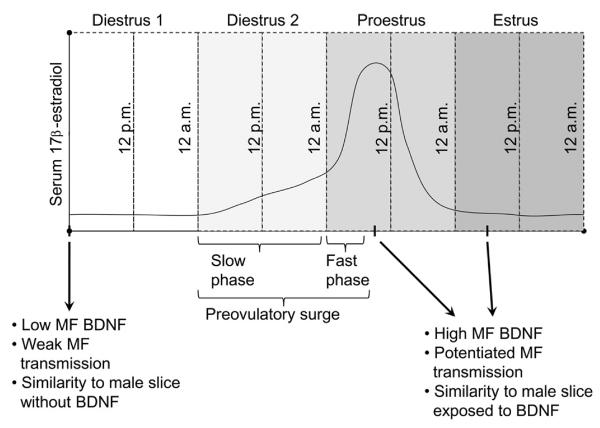
For this purpose, female rats were examined daily using vaginal cytology to determine the stage of their ovarian cycle (the estrous cycle; (Scharfman et al., 2003). Because serum levels of estradiol peak during the mid-morning of proestrous day (Figure 4; Freeman, 1984), a cohort of females was perfusion-fixed at that time, and compared to females that were perfused on other mornings of the estrous cycle. The results showed that there was greater immunoreactivity for BDNF protein on proestrous morning (Scharfman et al., 2003); Figure 4). Interestingly, BDNF immunoreactivity in the mossy fibers was even greater the following morning, estrous morning, probably because of accumulation of anterogradely-transported BDNF (Scharfman et al., 2003). These data are consistent with the idea that BDNF was increased when serum levels of 17β -estradiol rose, and the elevation in BDNF lasted for at least 24 hrs before declining. In other studies, phosphorylated trkB was found to have a similar temporal relationship to the estrous cycle as BDNF, increasing on proestrous day (Spencer-Segal et al., 2011). However, BDNF mRNA in the granule cells peaks on the day before proestrous, diestrous 2 (Gibbs, 1998). The mRNA data suggest that there is a rapid rise in BDNF mRNA early during the preovulatory surge, leads to BDNF protein synthesis by the following morning, and anterograde transport along mossy fiber axons continues for another 24 hrs (Figure 4).
Figure 4.
Estrous cycle-dependent changes in mossy fiber BDNF and excitability evoked by mossy fiber stimulation. A schematic is shown that illustrates the four days of the estrous cycle in the adult female rat. Serum levels of 17β-estradiol are illustrated to show the slow and fast phases of the preovulatory surge in serum levels of 17β-estradiol. BDNF protein levels and mossy fiber-driven effects in area CA3 are weak when serum levels are low, and have been low for at least 24 hrs, which occurs on diestrous 1 morning (arrow; this day is also called `metestrus'). In contrast, at the peak of the preovulatory surge, which occurs mid-morning of proestrus, BDNF protein levels are higher in mossy fibers and responses to mossy fiber stimulation are increased. High BDNF levels and increased excitability appear to persist for 24 hrs, because they also are evident on estrous morning (Scharfman et al., 2003).
Effects of estradiol in intact female rats on mossy fiber BDNF levels
To determine whether the increase in BDNF protein in mossy fibers on proestrous and estrous mornings had a physiological effect, hippocampal slices were prepared at these times and compared to other stages of the estrous cycle. It had already been shown that adding recombinant BDNF to hippocampal slices led to a potentiation of MF transmission in the male rat, and a similar phenomenon was observed in BDNF overexpressing male mice (without adding recombinant BDNF at all). Therefore one would predict a similar effect would occur mid-morning of proestrous and on estrous morning, because these are the times when BDNF protein is elevated in the mossy fibers. Indeed, the results confirmed the prediction: on proestrous and estrous mornings, the responses to mossy fiber stimulation simulated the effects of adding recombinant BDNF to slices of male rats (Scharfman et al., 2003). Notably, there were some slices that did not exhibit a detectable increase in mossy fiber transmission; strangely this also occurs when recombinant BDNF is used in male rats (Scharfman, 1997). One possible explanation for variability in the effects of BDNF in male rats is the penetration of BDNF into the slices, which can vary depending on the rate of perfusion of the slices (Kang et al., 1996; Xu et al., 2004). Another factor is slice preparation, because some methods to prepare slices lead to changes in BDNF protein afterwards (Danzer et al., 2004).
In slices from proestrous and estrous rats, an additional effect of BDNF was also identified, besides the effects on the response to a single mossy fiber stimulus: in response to a series of mossy fiber stimuli at 1 Hz, repetitive synchronous firing occurred in CA3 pyramidal cells. The repetitive synchronous discharges were also observed in the rat slices from males that were exposed to recombinant BDNF and slices of BDNF overexpressing mice (Scharfman, 1997; Croll et al., 1999). Interestingly, this type of repetitive firing was distinct from epileptiform or `interictal' bursts of pyramidal cell action potentials that occur during acute disinhibition of the slice by the GABAA receptor antagonist bicuculline (Scharfman, 2005). The unique pattern of repetitive firing that was observed across the female and male rats, when BDNF levels were high, suggested that BDNF increased excitability in area CA3 by a unique mechanism. Another effect that distinguished slices from animals with elevated BDNF levels was spreading depression episodes after repetitive firing (Scharfman, 1997; Croll et al., 1999; Scharfman et al., 2003). Repetitive firing and spreading depression only occurred after mossy fiber stimulation; remarkably, stimulation of other pathways in the same slices could not elicit repetitive firing or spreading depression (Scharfman, 1997; Scharfman et al., 2003).
The data from intact female rats was followed by a series of studies in ovariectomized rats which proved that 17β-estradiol was specifically the cause of elevated BDNF in intact rats. For these experiments, an attempt was made to simulate the complex rise in serum 17β-estradiol that occurs in the adult female rat during the preovulatory period, which begins on diestrus 2 and continues until the mid-morning of the next day, proestrus (Scharfman et al., 2007). With that procedure as a tool, female rats were ovariectomized and approximately 2 weeks later they were injected with 3 doses of estradiol: the first two injections used 17β-estradiol benzoate, a slow-acting form, and the last injection used 17β-estradiol to rapidly increase serum levels. At the time of the simulated peak of 17β-estradiol in the circulation, animals were euthanized and BDNF-ir showed greater immunoreactivity in the mossy fibers compared to vehicle-treated controls (Scharfman et al., 2007). When slices were prepared at the time that would correspond to the mid-morning of proestrus, the effects of mossy fiber transmission were comparable to the intact rat at proestrous morning (Scharfman et al., 2003).
These experiments showed that BDNF was elevated in the mossy fibers by 17β-estradiol but did not prove that the effects on physiology were dependent on BDNF or trk. Therefore, additional experiments were conducted to prove that trk antagonism blocked the physiological effects attributed to increased BDNF protein in the mossy fibers. Trk antagonism blocked the repetitive firing and spreading depression episodes, but did not block basal transmission; i.e. the response that immediately follows a mossy fiber stimulus (Scharfman et al., 2003; 2007). In addition, an α7 nicotinic receptor antagonist had a similar effect as trk antagonism (Scharfman et al., 2003). From these data, we suggested that increased mossy fiber BDNF content led to a trkB receptor- and α7 nicotinic receptor- sensitive increase in mossy fiber transmission. Based on the fact that mossy fiber-evoked responses were affected but not other pathways, the site of action appeared to be presynaptic, presumably at trkB and α7 nicotinic receptors on mossy fiber boutons (Scharfman et al., 2003; Grybko et al., 2009).
Notably, the mossy fibers may not be the only pathway that changes in response to 17β–estradiol because it has been shown that there are potent effects of 17β-estradiol on other pathways, and particularly the recurrent collateral pathway (Kim et al., 2006). Area CA3 is definitely not the only subfield where there were changes in physiology either. Area CA1 was affected by 17β–estradiol as well as area CA3 (Scharfman et al., 2003; Scharfman et al., 2007). Both the field EPSP and population spike evoked by stimulation of the area CA3 input to CA1, the Schaffer collaterals, were increased in slices from rats that were treated with 17β–estradiol, when compared to vehicle treatment (Scharfman et al., 2003; 2007). The increase in Schaffer collateral -evoked responses in area CA1 may have been caused by increased pyramidal cell BDNF protein in the Schaffer collateral boutons, because there was an increase in BDNF immunoreactivity in stratum radiatum where these boutons are located (Scharfman et al., 2007). More support for this idea has emerged recently in a study which showed that acute exposure to BDNF in hippocampal slices reversed deficits in synaptic plasticity in ovariectomized rats in area CA1 (Kramar et al., 2010).
III. BDNF and the mossy fibers in male rats
Androgen
The primary androgen in mammals is testosterone, which is aromatized to 17β-estradiol or reduced to dihydrotestosterone (DHT; Figure 1). DHT is a ligand for androgen receptors (AR), which are responsible for secondary sex characteristics in males. DHT is reversibly converted to 5α-androstane-3α, 17β-diol, a neurosteroid that is a weak ligand for ERα and ERβ and also facilitates the actions of GABA at GABAA receptors. The 3β isomer, 5α-androstan-3β, 17β-diol, is a ligand for ERβ ((Handa et al., 2011); Figure 1). It is not always appreciated that testosterone is present in the circulation of both sexes, not only males. Like ER, AR include a nuclear and membrane receptor, with nuclear receptors acting at target genes in the nucleus and membrane AR mediating fast actions of androgens. In hippocampus, ARs have been located to multiple sites, similar to ERs. Like ERs and trkB, ARs are found in the axons and terminals of the mossy fibers (Tabori et al., 2005).
Androgen and BDNF
There are many studies in systems besides hippocampus that have documented interactions between androgen and BDNF. In the songbird, seasonal plasticity is marked by effects of both androgen and estrogens in the HVC, a critical center in the pathway involved in song learning. The effects of androgen on BDNF in the HVC are indirect: androgen increases levels of the angiogenic factor vascular endothelial growth factor (VEGF), leading to an increase in the HVC vascular network and endothelial cell synthesis of BDNF (Goldman and Chen, 2012). BDNF release from the HVC stimulates growth of two other areas that are essential, RA and X (Ottem et al., 2012). Therefore, in this system, androgen appears to increase BDNF synthesis and release, and is critical to seasonal plasticity (Brenowitz, 2012).
Another system where androgen appears to influence BDNF levels is the spinal nucleus of the bulbocavernosus (SNB) in the lumbar spinal cord. The SNB motoneurons innervate the bulbocavernosus, levator ani, and external anal sphincter muscles, which are muscles that are required for male copulatory behavior. In the adult, androgens play an important role in the maintenance of SNB motorneurons because castration leads to atrophy of the SNB motorneuron somata and dendrites (for reviews, see Ottem et al., 2012; Verhovshek and Sengelaub et al., 2012). BDNF has been implicated in the supportive role of androgen on SNB motorneurons, but its interaction with androgen is complex. Regarding the atrophy of motorneuron somata after castration, BDNF can protect against atrophy independent of testosterone replacement; regarding androgen receptor expression after castration, the restorative effects of BDNF are only observed if BDNF is administered with androgen (Yang and Arnold 2000). Furthermore, when the atrophy of motorneuron dendrites is examined after gonadectomy, administration of both testosterone and BDNF is restorative, but independently neither testosterone or BDNF exerted an effect (Yang et al., 2004). It was suggested that testosterone exerts its protective effects by maintaining BDNF levels in dendrites, because testosterone treatment after gonadectomy restored BDNF levels in the motorneuron dendrites and prevented dendritic atrophy (Ottem et al 2007). On the other hand, trkB-IgG, a scavenger of BDNF, led to protection from the deleterious effects of castration on motorneurons; the authors suggested that testosterone normally suppresses BDNF levels (Verhovshek et al., 2010).
In areas of the rodent CNS, diverse relationships have been reported for androgen and BDNF. In the striatum and frontal cortex of C57bl/6 mice, the rise in serum androgen levels during adolescence were positively correlated with BDNF mRNA and protein levels (by ELISA), although BDNF expression (mRNA and protein) in hippocampus was not (Hill, 2012; Wu et al., 2012) (discussed further below). Similarly, in the pelvic ganglia, androgen levels were correlated with BDNF protein expression, because castration decreased the number of BDNF-immunoreactive neurons (Squillacioti et al., 2008). However, in the prostate gland, castration increases BDNF (Mirabella et al., 2006).
Mossy fibers increase BDNF protein after gonadectomy in the male rat
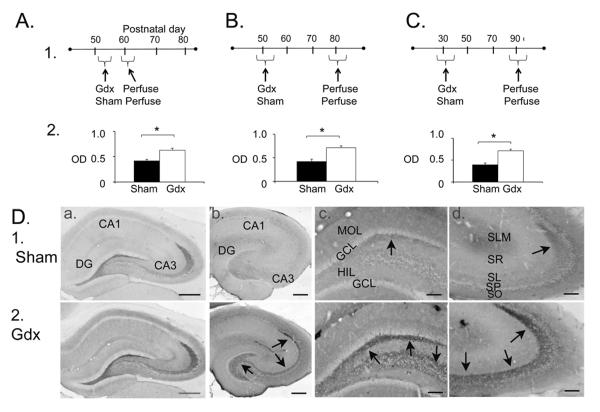
Our studies of BDNF in adult male rat hippocampus suggest a similar effect as castration in the prostate (Mirabella et al., 2006), because castration increased BDNF immunoreactivity in the mossy fiber pathway (Figure 5; note that castration and gonadectomy are used synonymously). The effect was similar whether animals were examined 2 weeks or 2 months after gonadectomy (Figure 5), suggesting that the effects of gonadectomy on mossy fiber BDNF protein were long-lasting. The effects of gonadectomy also appeared to be independent of pubertal status, because young male rats, gonadectomized at about the time when puberty begins (30 days old), exhibited a robust increase in BDNF expression when they were examined 2 months later (Figure 5). This result was similar to the adult male rats who were gonadectomized after puberty and examined 2 months later (Figure 5). They are also consistent with studies of the male rat during early postnatal life, because castration at postnatal day 0 led to a reduction in hippocampal BDNF protein (by Western blot; measured at postnatal days 4, 7 and 10; (Solum and Handa, 2002)). As one might expect from the female rat, 17β-estradiol replacement at postnatal day 0 restored BDNF protein levels. Analogous to the findings of Gibbs (1998) discussed above, where hippocampal BDNF mRNA levels were high when BDNF protein was low, Solum and Handa (Solum and Handa, 2002) also found that BDNF mRNA levels followed the opposite pattern as BDNF protein levels. Interestingly, another area of the brain, medial basal hypothalamus, did not show effects of castration on BDNF levels (Solum and Handa, 2002) supporting the view that each brain area is not the same.
Figure 5.
Gonadectomized male rats exhibit increased BDNF protein in mossy fibers.
A–C. 1) Experimental timelines and 2) results for male rats that were gonadectomized or subjected to sham surgery (Sham) and perfused approximately 2 weeks later to quantify immunoreactivity for BDNF in stratum lucidum of CA3a. Three groups were examined: A) Surgery at an adult age followed by perfusion approximately 2 weeks later, B) Surgery as adults and perfusion approximately 2 months later, or C) surgery prior to puberty and perfusion approximately 2 months later. The results showed greater immunoreactivity in all gonadectomized rats. Asterisks here and in other figures indicate p<0.05.
D. Comparison of sham (1) and gonadectomized (2) rats that were perfused and processed for immunocytochemistry using a rabbit polyclonal BDNF antibody (A; antibody from Amgen Regeneron Partners) or a mouse monoclonal antibody to BDNF (B–D; antibody from Sigma). Arrows point to mossy fiber staining. Calibrations: A–B, 250; C–D, 100 μm. Parts A, B and D are from (Skucas et al., 2013).
These results are also are interesting to consider when compared to studies of adolescence. Hill and colleagues (Hill et al., 2012) studied serum androgen levels, BDNF mRNA (by Western blot), and BDNF protein (by ELISA) in hippocampus from 3–12 weeks of age and found that androgen levels increased with age, as expected, but there was not a change in BDNF mRNA or BDNF protein. In contrast, both BDNF mRNA and protein increased in striatum and frontal cortex. The results are consistent with the idea that effects of androgens on BDNF expression in hippocampus may be different from other brain regions: in extrahippocampal sites, serum androgen levels correlate with BDNF expression, but in hippocampus a negative (Figure 5) or no correlation (Hill et al., 2012) exists. The differences between results shown in Figure 5 and the results from Hill et al. (2012) may be related to the greater ability to detect changes occurring primarily in the mossy fibers with immunoreactivity, but not ELISA.
One interpretation of the findings in the adult male rat that BDNF protein increased in the mossy fibers is that BDNF is increased to compensate for the loss of testosterone, which in other studies and other systems, has been demonstrated to have neurotrophic effects (discussed above). Another interpretation is that depletion of the neurosteroid metabolite of testosterone, 5α-androstane-3α,17β-diol, which normally enhances actions of GABA at GABAA receptors (Figure 1) led to increased neuronal activity in hippocampus and activity-dependent synthesis of BDNF. One argument against this idea is that females that were ovariectomized had reduced mossy fiber BDNF immunoreactivity (Scharfman et al., 2003) - in the female, gonadectomy reduces the neurosteroid metabolite of progesterone that is analogous in its actions at GABAA receptors to 5α-androstane-3α,17β-diol.
Another possibility is that androgen many tonically suppress BDNF synthesis in the mossy fiber pathway, suggested by others who studied the prostate (Mirabella et al., 2006). This idea would explain the relatively low levels of BDNF in the mossy fibers of adult male rats when compared with intact adult female rats (Scharfman et al., 2003). The suppression of BDNF synthesis by circulating androgen could explain this result. It could be advantageous to the species if testosterone exerted a tonic suppression of BDNF synthesis in the mossy fiber pathway, because excess BDNF in mossy fibers, in female rats, causes hyperexcitability (e.g., repetitive firing and spreading depression).
In early life, it is not clear that the same logic applies. In neonatal life, it appears that estradiol plays a critical role in maintaining BDNF levels in male rats, because levels of BDNF fall after gonadectomy of male rate pups on postnatal day 0, but are restored by estradiol injection at the time of gonadectomy (Solum and Handa 2002). The effects of estradiol at this time of life are considered critical to the `organizational' stage of development, when the brain becomes sexually differentiated (Wallen, 2009); it is intriguing that these effects may be partly mediated by BDNF (Lenz et al., 2012).
Mossy fiber transmission and synaptic plasticity are increased after gonadectomy of male rats
Based on the studies of BDNF in male and female rats (Scharfman, 1997; Scharfman et al., 2003; 2007) or overexpressing male mice (Croll et al., 1999) one would predict that an increase in BDNF in the mossy fibers would also cause an increase in mossy fiber transmission. Therefore, gonadectomized male rats were compared to age-matched sham controls, and the mossy fiber pathway was examined after serum testosterone levels were depleted and the animals had recovered from surgery. The results were consistent with the prediction: mossy fiber transmission was enhanced in the gonadectomized rats compared to sham controls (Figure 6). In addition, short term and long term plasticity were enhanced: paired pulse facilitation was increased in gonadectomized rats, and both PTP and LTP of the mossy fibers was increased (Figure 6).
Figure 6.
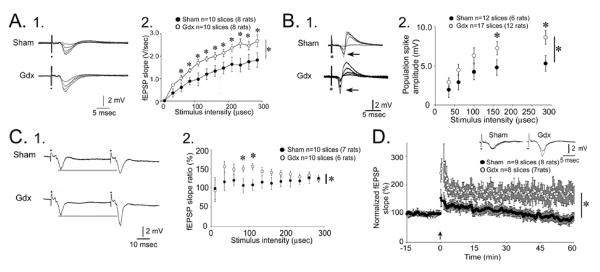
Male gonadectomized rats exhibit increased mossy fiber transmission, paired pulse facilitation and LTP.
A. 1. Responses to a series of stimuli to the mossy fibers, recorded in stratum lucidum, are superimposed. Increasing stimulus strength produced field EPSPs (fEPSPs) with increasing slope and amplitude. Representative examples from a slice of a sham and gonadectomized rat are shown. 2. The responses to all stimuli are plotted for all sham (black circles) and gonadectomized (white) rats. Repeated measures ANOVA showed that there was a significant effect of gonadectomized (asterisk) and post-hoc tests showed that most individual comparisons were significantly different (asterisks above the symbols).
B. 1. Analogous to A1, representative responses are shown for a sham and gonadectomized rat with the recording electrode positioned in the pyramidal cell layer to examine the population spike (arrow). 2. Analogous to A2, population spike amplitude was significantly larger in gonadectomized rats.
C. 1. Representative responses are shown for a sham and gonadectomized rat, where recording electrodes were placed in stratum lucidum to examine paired pulse facilitation, the response to two identical stimuli with a 40 msec interstimulus interval. 2. Analysis of paired pulse facilitation with many interstimulus intervals from 20–200 msec showed that there was a significant effect of gonadectomized; post-hoc tests showed greater paired pulse facilitation in gonadectomized rats than sham controls at intermediate intervals.
D. A comparison of LTP in gonadectomized (white circles) and sham rats (black circles) illustrates greater LTP in gonadectomized rats. Insets: representative examples of fEPSPs before and after LTP induction, with LTP evident only in the slice from the gonadectomized rat. From (Skucas et al., 2013).
Notably, sham controls failed to exhibit LTP (Figure 6). At first this may seem surprising, because most LTP studies are conducted in male rodent slices and LTP in CA3 is robust. However, most of those studies that are published have been conducted in prepubertal rats (less than 30 days old; (Salin et al., 1996; Kwon and Castillo, 2008)). Puberty in the male rat (defined for example using preputial separation as a landmark) commences between 38 and 40 days of age (Noriega et al., 2009). Our animals were all studied after postnatal day 50. Importantly, the same methods used in the postpubertal sham controls that failed to evoke LTP were able to do so in 30 day old rats (Skucas et al., 2013). Therefore, the results suggest that circulating androgen in the adult male rat suppresses mossy fiber plasticity by reducing BDNF protein levels.
Why would the adult male rat have evolved to exhibit suppression of plasticity after puberty in a structure where plasticity is important to normal cognitive function, such as hippocampus? One reason why this effect might be important teleologically is based on the evidence that male rats have a robust social hierarchy in the wild, with one male exerting a dominant role. In laboratory animals, male mice that are housed together also show a hierarchy, and the male that is dominant has the higher level of circulating androgen (Raab and Haedenkamp, 1981; Blanchard et al., 1993). If the subordinate male, with lower levels of testosterone, had increased levels of BDNF in hippocampus, the increased plasticity that develops as a consequence could help the subordinate male develop new strategies to escape attack by the dominant male, and strategies to find a female to mate. It is also relevant to note that male mice which are bred to increase aggressive behavior have a more restricted mossy fiber plexus (Guillot et al., 1994; Sluyter et al., 1994). In these mice, androgen levels may be high, reducing the extent of the mossy fiber pathway. Consistent with that idea, gonadectomized rats exhibited mossy fiber sprouting (discussed further below).
Additional data from gonadectomized male rats shed light on the mechanisms underlying increased mossy fiber transmission in gonadectomized rats. The increase in mossy fiber transmission and the increase in LTP could be blocked by trk antagonism (Figure 6). Experiments that examined LTP also showed that trkB-IgG, a scavenger of BDNF, normalized the LTP in gonadectomized rats - in other words, exposing slices to trkB-IgG reduced mossy fiber LTP in gonadectomized rats so that the graph of potentiation vs. time was similar to sham controls (Skucas et al., 2013). These data suggest that gonadectomizy led to an increase in the BDNF-dependence of mossy fiber transmission and plasticity. That conclusion is consistent with the idea described above, that increased expression of BDNF in mossy fibers leads to an increase in the excitatory effect of MF transmission on pyramidal cells.
We also found that there was an increased sensitivity of mossy fiber transmission and plasticity to androgens after gonadectomized. By comparing the effects of testosterone metabolites on hippocampal slices from gonadectomized and sham rats, surprising results were obtained. There was no detectable sensitivity of mossy fiber transmission to DHT or 5α-androstane-3α17β-diol in sham controls, but in gonadectomized rats, dihydrotestosterone (DHT) normalized mossy fiber transmission. 5α-androstane-3α,17β-diol was ineffective in slices from gonadectomized rats. Therefore, mossy fibers appear to develop a novel BDNF/trk- and DHT-sensitivity after gonadectomy.
Mossy fiber sprouting after gonadectomy of adult male rats
After recording from many slices of gonadectomized rats, we noticed that it was harder to pinpoint the site of the maximal fEPSP in gonadectomized rats compared to sham controls. Typically this procedure is straightforward: one compares several recording locations in stratum lucidum to find the site where the largest field EPSP with the largest fiber volley is located. A range of sites are tested to be sure the optimal site is found. One assumes that the pyramidal cell layer is the location where the highest density of single units are detected, and that is usually not an area where the largest fEPSP is recorded. In gonadectomized rats, however, there were diverse locations in stratum lucidum, stratum pyramidale and stratum oriens where mossy fiber-evoked fEPSPs could be elicited (Figure 7).
Figure 7.
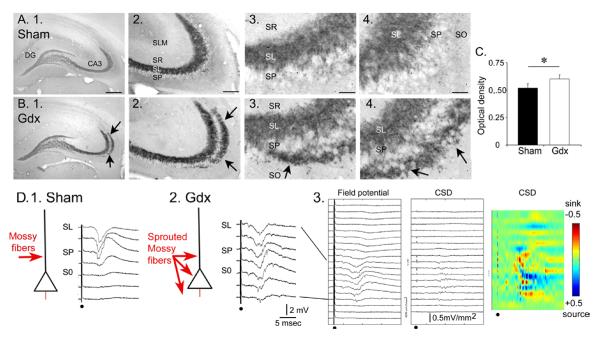
Gonadectomized male rats exhibit mossy fiber sprouting.
A–B. Examples of dynorphin immunocytochemistry in sham and gonadectomized rats illustrates a novel band of immunoreactivity in stratum oriens (arrows) in gonadectomized rats. SF= stratum radiatum. SL= stratum lucidum; SP= stratum pyramidale; SO= stratum oriens. Calibration = 500 μm (1); 100 μm (2) and 25 μm (3–4).
C. Quantification of the dynorphin immunoreactivity by optical density measurements of a region outlining SL and SO of CA3a illustrates a significantly greater optical density in gonadectomized rats (white bar) compared to sham (black). There was no significant difference in the intensity of dynorphin immunoreactivity in stratum lucidum.
D. 1–2. An illustration of mossy fiber (red) innervation of pyramidal cells in sham and gonadectomized rats with representative responses to stimuli recorded throughout the layers of CA3b. Note that fEPSPs could be evoked outside of SL in the gonadectomized rat, but only in SL in the sham rat. 3. Current source density (CSD) for all responses recorded from the slice used in (2) illustrate a lack of specificity of current sinks (red) for one layer. Calibration: ±0.5 mV/mm2. From (Skucas et al., 2013).
One explanation was mossy fiber sprouting in response to increased BDNF levels in the mossy fiber pathway. There is now a great deal of evidence showing that diverse experimental manipulations can induce sprouting into stratum oriens, ranging from electrical stimulation of the mossy fibers (Escobar et al., 1997), spatial learning (Ramirez-Amaya et al., 1999; for review, see Routtenberg, 2010), hyperthyroidism (Represa et al 1987), weaning (Holahan et al., 2007) and exercise (Ramirez-Amaya et al., 1999; Toscano-Silva et al., 2010). In culture, it has also been shown that BDNF exposure to explants containing mossy fibers leads to sprouting of the mossy fibers (Lowenstein and Arsenault, 1996). However, whether increased mossy fiber BDNF levels are the cause of mossy fiber sprouting is not clear. For example, transgenic mice with impaired BDNF signalling suggest mossy fiber sprouting does not require BDNF(Qiao et al., 2001). On the other hand, BDNF infusion to the hippocampus in vivo led to mossy fiber sprouting (Scharfman et al., 2002; Gomez-Palacio-Schjetnam and Escobar, 2008). Therefore, BDNF may be only one factor, and there could be many mechanisms besides those that involve BDNF. One of the problems in clarifying the effects of BDNF is that BDNF usually increases neuronal activity, and therefore any effect of BDNF application could be related to activity-dependent expression of target genes - related only indirectly to BDNF. Indeed, BDNF infusion induced seizure activity (Scharfman et al., 2002) and lidocaine infusion into area CA3 blocked learning-induced mossy fiber sprouting in stratum oriens (Holahan and Routtenberg, 2011).
To determine if sprouting in stratum oriens occurred in gonadectomized rats, dynorphin b was used as a marker of mossy fibers (Pierce et al., 1999) and comparisons were made to sham controls (Figure 7). Sprouting was significantly increased in the gonadectomized rats compared to sham controls (Figure 7). The implication of the results is that structural plasticity is induced after gonadectomy, presumably because of the increase in BDNF protein in mossy fibers, and this leads to a loss of the normal afferent specificity of the mossy fiber pathway (Figure 8).
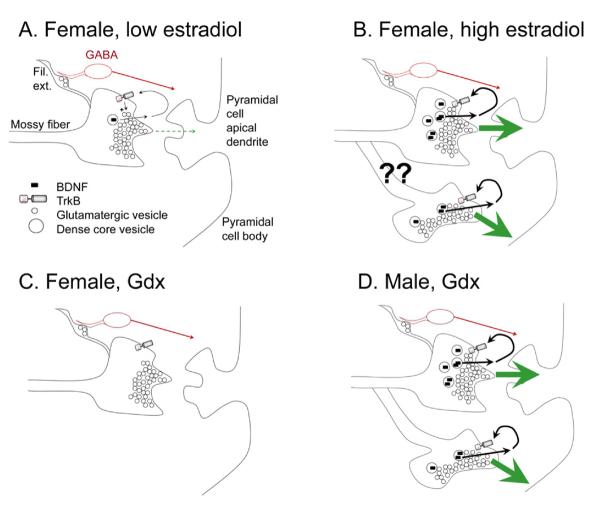
Figure 8.
Comparisons of mossy fiber structural and functional changes in female and male rats.
A. A schematic illustrates the effects of mossy fiber transmission in an intact female rat when serum levels of estradiol are low, e.g., during diestrous 1 morning. The predominant effect at rest is inhibition of pyramidal cells by GABAergic neurons innervated by the filamentous extensions (Fil. Ext.) of mossy fiber axons. BDNF is low so it would have little effect under these conditions. Higher levels of BDNF (in B) would enhance glutamate release (+ sign) after release from the mossy fiber bouton and activation of presynaptic trkB receptors.
B. When serum levels of estradiol have peaked during the estrous cycle, i.e., mid-morning of proestrus, increased BDNF is present in the large boutons innervating pyramidal cells, leading to a greater excitatory effect of mossy fiber transmission on pyramidal cells. Sprouting may also occur (arrows), increasing the excitation of the pyramidal cells.
C. After ovariectomy, BDNF content decreases and glutamatergic transmission is relatively weak.
D. The gonadectomized male rat is similar to the intact female rat because BDNF levels have increased. In this condition, sprouting is known to occur, leading to increased excitatory effects of mossy fiber transmission on pyramidal cells of area CA3.
IV. Implications
Comparing the female and male hippocampus
The results suggest some aspects of mossy fiber function are surprising. In the gonadectomized (ovariectomized) female rat, BDNF protein and mossy fiber synaptic function decreased. In the male, gonadectomy (orchidectomy) led to what would seem to be the opposite effect: an increase in mossy fiber BDNF protein and mossy fiber synaptic transmission. Synaptic plasticity was increased, and mossy fiber sprouting occurred.
The results support the view that inherent organization of the hippocampus in terms of hormonal responses is programmed early in life, so that even when the major source of gonadal steroids are removed, the response is not the same in females and males. Presumably the neonatal surge in androgen which accompanies the differentiation of male and female brain and behavior (Wallen, 2009) leads to a circuitry upon which adult hormone levels exert a modifying influence. However, removal of that influence leads to a very different response.
Why would females and males regulate BDNF differently in the mossy fiber pathway?
The potent effects of estrogen and androgen at the mossy fiber synapse suggest that this is a critical pathway to the effects of these gonadal steroids. We suggest that by modulating BDNF protein levels, estrogen and androgen allow the mossy fiber pathway to exhibit a larger range of effects on its targets, preferentially increasing the extent that mossy fibers excite pyramidal cells and initiate synaptic and structural plasticity. In the female, estrogen can exert a positive effect on this process by stimulating BDNF synthesis, whether it does so by a genomic action or by disinhibition and activity-dependent upregulation of BDNF. In the male, testosterone appears to exert a negative effect on BDNF protein in the mossy fibers, as well as mossy fiber transmission and plasticity. Androgen receptor and trkB receptor are suppressed, which would appear to be an adverse effect, but could be advantageous by maintaining afferent specificity of the mossy fibers for its targets and reducing the risk of hyperexcitability. We suggest that these differences could be relevant to the differences in cognitive function in men and women. The differences also could help explain the bias of several diseases to affect one sex or the other.
Differences in cognitive function
Of the many proposed functions of area CA3, one that has perhaps gained the most attention is the likely role it plays in performing pattern completion (Kesner, 2007; Leutgeb and Leutgeb, 2007). Due to the recurrent collateral axons of area CA3 pyramidal cells, the area CA3 circuitry has been called an autoassociative network. Thus, after area CA3 encodes a pattern of afferent input, it can retrieve the complete pattern even if presented with only a part of the pattern. There are two main steps to this process: encoding and retrieval. The encoding of the pattern requires synaptic plasticity at several synapses onto and between pyramidal cells and probably other neurons also. The mossy fibers, as a potentially strong excitatory input, may act as a `teaching input' that greatly increases pyramidal cell depolarization so that synaptic plasticity occurring at another synapses at about the same time as mossy fiber activation is more likely to exhibit robust long-term plasticity. Increased BDNF in the mossy fiber pathway may enhance the ability of the mossy fibers to act as a teaching input. Another view of pattern completion is that it requires polysynaptic recurrent circuitry between the dentate gyrus and area CA3 mediated by the backprojecting axon collaterals of CA3 pyramidal cells to the dentate gyrus, and mossy fibers to area CA3. Here BDNF in the mossy fibers could also enhance the excitatory actions within these circuits, potentially.
As a result, one would expect that cognitive performance on tasks that require area CA3 would be improved in women when they experience the preovulatory surge in the menstrual cycle. Although there have been many studies that have provided support for that idea, CA3-dependent behavior does not appear to have ever been tested.
One might expect that cognitive performance in men might improve with aging, as testosterone levels fall dramatically compared to the levels in young adulthood, and one would predict rising BDNF levels in the mossy fiber pathway. Also, one might expect cognitive performance to improve during androgen ablation therapy for prostate cancer, as the suppression of mossy fiber BDNF levels is removed. However, whether the results of our work in rats also pertains to humans is unclear, and it also is not clear that a slow decline in androgen levels, like what would occur in aging, would cause the same effects on mossy fibers as gonadectomy. This may be one reason that tests of cognitive function in men after androgen ablation therapy have provided mixed results.
Differences in the incidence of disorders that involve the hippocampus in men and women
One of the surprising aspects of epidemiological studies in neurological and psychiatric disorders is the fact that females are more vulnerable than men in some cases, but in other instances, men are afflicted more than women. The results of our studies of BDNF in area CA3 may shed light onto some of the reasons for the sex differences. One example is anxiety-related disorders and post-traumatic stress disorder (PTSD), which are much more common in women than men (Altemus and Epstein, 2008; Breslau, 2002; Olff et al., 2007). One reason could be related to the increased levels of BDNF in mossy fibers of female rodents compared to males. If the data from rat can be generalized, one would predict greater synaptic plasticity at the mossy fiber synapses onto pyramidal cells in women, particularly at the time when their estrogen levels are relatively high. The consequence could be greater retrieval of a memory even if the pattern of input is partial - related to the function of CA3 in pattern completion. A situation that only modestly resembles one that is fearful may retrieve the fearful memory very easily, leading to anxiety. An environment that is only partly similar to the one that occurred during a traumatic experience might be recalled too easily, leading to a PTSD-like syndrome. In some respects, retrieval may work `too well.'
Conclusions
Since the seminal findings that form the basis of the experiments reviewed here, ranging from the discovery of BDNF in the 1980's (Barde et al., 1982, 1987; Turner et al., 1982), the first identification of the potential role of neurotrophins in hormone action in the 1990's (Toran-Allerand et al., 1992; Miranda et al., 1993a,b) and the discovery of estrogen-mediated neuroplasticity in hippocampus at about the same time (Woolley et al., 1990; Woolley and McEwen, 1992), many laboratories have contributed to the rapid expansion in our knowledge about the ways estrogen and androgen regulate the brain and behavior. Surprisingly, there are still many areas that require further elucidation, and the actions of estrogen and androgen in the CA3 region is clearly one of them. One of the pressing questions is more reductionist: what are the molecular mechanisms of estrogen and androgen action in CA3? Another is more comprehensive: what are the effects on interneurons, glia, and the vasculature? How well can we generalize from the rat to man? Many more questions remain to be answered. Ultimately the hope is that the answers will help us understand the regulation of brain and behavior in men and women, and develop more effective methods for treating illnesses that differentially affect men and women.
Highlights.
Estrogens and androgens have robust effects on the hippocampal mossy fiber pathway of the adult rat
17β-estradiol in females increase BDNF immunoreactivity and plasticity in the mossy fibers
Testosterone depletion in males increases BDNF immunoreactivity and plasticity in the mossy fibers
Effects on mossy fibers include immunoreactivity, synaptic transmission, LTP and sprouting
Acknowledgements
We thank Dr. Csaba Leranth for the micrograph in Figure 2C, and support from the NIH NS-37562, the New York State Office of Mental Health, and NSERC Discovery Grant 197293/2007.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acsady L, Kamondi A, Sik A, Freund T, Buzsaki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J Neurosci. 1998;18:3386–3403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altemus M, Epstein L. Sex differences in anxiety disorders. In: Becker JB, Berkley KJ, Feary N, Hampson E, Herman JP, Young EA, editors. Sex Differences in the Brain. Oxford University Press; Oxford, U.K.: 2008. pp. 397–397. [Google Scholar]
- Barde YA, Davies AM, Johnson JE, Lindsay RM, Thoenen H. Brain derived neurotrophic factor. Prog Brain Res. 1987;71:185–189. doi: 10.1016/s0079-6123(08)61823-3. [DOI] [PubMed] [Google Scholar]
- Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1:549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquist F, Ludvig M. Neuropeptide release. In: Malenka RC, editor. Intercellular communication in the nervous system. Academic Press; New York: 2009. pp. 519–519. [Google Scholar]
- Berchtold NC, Kesslak JP, Pike CJ, Adlard PA, Cotman CW. Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. Eur J Neurosci. 2001;14:1992–2002. doi: 10.1046/j.0953-816x.2001.01825.x. [DOI] [PubMed] [Google Scholar]
- Blaabjerg M, Zimmer J. The dentate mossy fibers: Structural organization, development and plasticity. Prog Brain Res. 2007;163:85–107. doi: 10.1016/S0079-6123(07)63005-2. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Sakai RR, McEwen B, Weiss SM, Blanchard RJ. Subordination stress: Behavioral, brain, and neuroendocrine correlates. Behav Brain Res. 1993;58:113–121. doi: 10.1016/0166-4328(93)90096-9. [DOI] [PubMed] [Google Scholar]
- Blurton-Jones M, Tuszynski MH. Estradiol-induced modulation of estrogen receptor-beta and GABA within the adult neocortex: A potential transsynaptic mechanism for estrogen modulation of BDNF. J Comp Neurol. 2006;499:603–612. doi: 10.1002/cne.21122. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Southard T, Sarvey JM, Herkenham M, Brady LS. Unilateral LTP triggers bilateral increases in hippocampal neurotrophin and trk receptor mRNA expression in behaving rats: Evidence for interhemispheric communication. J Comp Neurol. 1996;368:371–382. doi: 10.1002/(SICI)1096-9861(19960506)368:3<371::AID-CNE4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA. Neuroscience. 2012. Testosterone and brain-derived neurotrophic factor interactions in the avian song control system. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N. Epidemiologic studies of trauma, posttraumatic stress disorder, and other psychiatric disorders. Can J Psychiatry. 2002;47:923–923. doi: 10.1177/070674370204701003. [DOI] [PubMed] [Google Scholar]
- Carim-Todd L, Bath KG, Fulgenzi G, Yanpallewar S, Jing D, Barrick CA, Becker J, Buckley H, Dorsey SG, Lee FS, Tessarollo L. Endogenous truncated trkb.T1 receptor regulates neuronal complexity and trkb kinase receptor function in vivo. J Neurosci. 2009;29:678–685. doi: 10.1523/JNEUROSCI.5060-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Chicurel ME, Harris KM. Three-dimensional analysis of the structure and composition of CA3 branched dendritic spines and their synaptic relationships with mossy fiber boutons in the rat hippocampus. J Comp Neurol. 1992;325:169–182. doi: 10.1002/cne.903250204. [DOI] [PubMed] [Google Scholar]
- Claiborne BJ, Amaral DG, Cowan WM. A light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J Comp Neurol. 1986;246:435–458. doi: 10.1002/cne.902460403. [DOI] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: Evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croll SD, Suri C, Compton DL, Simmons MV, Yancopoulos GD, Lindsay RM, Wiegand SJ, Rudge JS, Scharfman HE. Brain-derived neurotrophic factor transgenic mice exhibit passive avoidance deficits, increased seizure severity and in vitro hyperexcitability in the hippocampus and entorhinal cortex. Neuroscience. 1999;93:1491–1506. doi: 10.1016/s0306-4522(99)00296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer SC, McNamara JO. Localization of brain-derived neurotrophic factor to distinct terminals of mossy fiber axons implies regulation of both excitation and feedforward inhibition of CA3 pyramidal cells. J Neurosci. 2004;24:11346–11355. doi: 10.1523/JNEUROSCI.3846-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer SC, Pan E, Nef S, Parada LF, McNamara JO. Altered regulation of brain-derived neurotrophic factor protein in hippocampus following slice preparation. Neuroscience. 2004;126:859–869. doi: 10.1016/j.neuroscience.2004.03.025. [DOI] [PubMed] [Google Scholar]
- De Wit J, Eggers R, Evers R, Castren E, Verhaagen J. Long-term adeno-associated viral vector-mediated expression of truncated trkb in the adult rat facial nucleus results in motor neuron degeneration. J Neurosci. 2006;26:1516–1530. doi: 10.1523/JNEUROSCI.4543-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieni S, Matsumoto T, Dekkers M, Rauskolb S, Ionescu MS, Deogracias R, Gundelfinger ED, Kojima M, Nestel S, Frotscher M, Barde YA. BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J Cell Biol. 2012;196:775–788. doi: 10.1083/jcb.201201038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissen GA, Garcia-Rudaz C, Ojeda SR. Role of neurotrophic factors in early ovarian development. Semin Reprod Med. 2009;27:24–31. doi: 10.1055/s-0028-1108007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman MD, Kerr B, Garcia-Rudaz C, Paredes AH, Dissen GA, Ojeda SR. Neurotrophins acting via trkb receptors activate the jagged1-notch2 cell-cell communication pathway to facilitate early ovarian development. Endocrinology. 2011;152:5005–5016. doi: 10.1210/en.2011-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsey SG, Renn CL, Carim-Todd L, Barrick CA, Bambrick L, Krueger BK, Ward CW, Tessarollo L. In vivo restoration of physiological levels of truncated trkb.T1 receptor rescues neuronal cell death in a trisomic mouse model. Neuron. 2006;51:21–28. doi: 10.1016/j.neuron.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Escobar ML, Barea-Rodríguez EJ, Derrick BE, Reyes JA, Martinez JL. Opioid receptor modulation of mossy fiber synaptogenesis: independence from long-term potentiation. Brain Res. 1997;751:330–330. doi: 10.1016/s0006-8993(96)01373-x. [DOI] [PubMed] [Google Scholar]
- Fenner BM. Truncated trkb: Beyond a dominant negative receptor. Cytokine Growth Factor Rev. 2012;23:15–24. doi: 10.1016/j.cytogfr.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Freeman M, E. Neuroendocrine control of the ovarian cycle in the rat. In: Neill J, D., editors. Knobil and Neill's Physiology of Reproduction. St. Elsevier Academic Press; Louis, MO: 1984. pp. 2327–2327. [Google Scholar]
- Fryer RH, Kaplan DR, Feinstein SC, Radeke MJ, Grayson DR, Kromer LF. Developmental and mature expression of full-length and truncated trkb receptors in the rat forebrain. J Comp Neurol. 1996;374:21–40. doi: 10.1002/(SICI)1096-9861(19961007)374:1<21::AID-CNE2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Fryer RH, Kaplan DR, Kromer LF. Truncated trkb receptors on nonneuronal cells inhibit BDNF-induced neurite outgrowth in vitro. Exp Neurol. 1997;148:616–627. doi: 10.1006/exnr.1997.6699. [DOI] [PubMed] [Google Scholar]
- Gall C, Lauterborn J, Isackson P, White J. Seizures, neuropeptide regulation, and mRNA expression in the hippocampus. Prog Brain Res. 1990. 1990;83:371–371. doi: 10.1016/s0079-6123(08)61263-7. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Levels of trka and BDNF mRNA, but not NGF mRNA, fluctuate across the estrous cycle and increase in response to acute hormone replacement. Brain Res. 1998;787:259–268. doi: 10.1016/s0006-8993(97)01511-4. [DOI] [PubMed] [Google Scholar]
- Goldman SA, Chen Z. Testosterone modulation of angiogenesis and neurogenesis in the adult songbird brain Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.12.043. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Palacio-Schjetnan A, Escobar ML. In vivo BDNF modulation of adult functional and morphological synaptic plasticity at hippocampal mossy fibers. Neurosci Lett. 2008;445:62–62. doi: 10.1016/j.neulet.2008.08.069. [DOI] [PubMed] [Google Scholar]
- Grybko M, Sharma G, Vijayaraghavan S. Functional distribution of nicotinic receptors in CA3 region of the hippocampus. J Mol Neurosci. 2009;40:114–120. doi: 10.1007/s12031-009-9266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Arruda-Carvalho M, Wang J, Janoschka SR, Josselyn SA, Frankland PW, Ge S. Optical controlling reveals time-dependent roles for adult-born dentate granule cells. Nat Neurosci. 2012;15:1700–1706. doi: 10.1038/nn.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot PV, Roubertoux PL, Crusio WE. Hippocampal mossy fiber distributions and intermale aggression in seven inbred mouse strains. Brain Res. 1994;660:167–169. doi: 10.1016/0006-8993(94)90852-4. [DOI] [PubMed] [Google Scholar]
- Gutierrez R. The dual glutamatergic-GABAergic phenotype of hippocampal granule cells. Trends Neurosci. 2005;28:297–303. doi: 10.1016/j.tins.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Haapasalo A, Koponen E, Hoppe E, Wong G, Castren E. Truncated trkb.T1 is dominant negative inhibitor of trkb.Tk+-mediated cell survival. Biochem Biophys Res Commun. 2001;280:1352–1358. doi: 10.1006/bbrc.2001.4296. [DOI] [PubMed] [Google Scholar]
- Haapasalo A, Sipola I, Larsson K, Akerman KE, Stoilov P, Stamm S, Wong G, Castren E. Regulation of trkb surface expression by brain-derived neurotrophic factor and truncated trkb isoforms. J Biol Chem. 2002;277:43160–43167. doi: 10.1074/jbc.M205202200. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Ogawa S, Wang JM, Herbison AE. Roles for oestrogen receptor β in adult brain function. J Neuroendocrinol. 2011;24:160–173. doi: 10.1111/j.1365-2826.2011.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte-Hargrove LC, MacLusky NJ, Scharfman HE. BDNF-estrogen interactions in the hippocampal mossy fiber pathway: Implications for normal brain function and disease. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2012.12.029. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze DA, Urban NN, Barrionuevo G. The multifarious hippocampal mossy fiber pathway: A review. Neuroscience. 2000;98:407–427. doi: 10.1016/s0306-4522(00)00146-9. [DOI] [PubMed] [Google Scholar]
- Hill RA. Interaction of sex steroid hormones and brain-derived neurotrophic factor-tyrosine kinase b signalling: Relevance to schizophrenia and depression. J Neuroendocrinol. 2012;24:1553–1561. doi: 10.1111/j.1365-2826.2012.02365.x. [DOI] [PubMed] [Google Scholar]
- Holahan MR, Honegger KS, Routtenberg A. Expansion and retraction of hippocampal mossy fibers during postweaning development: strain-specific effects of NMDA receptor blockade. Hippocampus. 2007;17:58–58. doi: 10.1002/hipo.20242. [DOI] [PubMed] [Google Scholar]
- Holahan MR, Routtenberg A. Lidocaine injections targeting CA3 hippocampus impair long-term spatial memory and prevent learning-induced mossy fiber remodeling. Hippocampus. 2011;21:532–532. doi: 10.1002/hipo.20786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JS, McGinty JF, Grimes L, Kanamatsu T, Obie J, Mitchell CL. Seizure-induced alterations in the metabolism of hippocampal opioid peptides suggest opioid modulation of seizure-related behaviors. NIDA Res Monogr. 1988;82:48–48. [PubMed] [Google Scholar]
- Ibanez CF. Jekyll-hyde neurotrophins: The story of proNGF. Trends Neurosci. 2002;25:284–286. doi: 10.1016/s0166-2236(02)02169-0. [DOI] [PubMed] [Google Scholar]
- Isackson PJ, Huntsman MM, Murray KD, Gall CM. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: Temporal patterns of induction distinct from NGF. Neuron. 1991;6:937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- Jaffe DB, Gutierrez R. Mossy fiber synaptic transmission: Communication from the dentate gyrus to area CA3. Prog Brain Res. 2007;163:109–132. doi: 10.1016/S0079-6123(07)63006-4. [DOI] [PubMed] [Google Scholar]
- Jezierski MK, Sohrabji F. Region- and peptide-specific regulation of the neurotrophins by estrogen. Brain Res Mol Brain Res. 2000;85:77–84. doi: 10.1016/s0169-328x(00)00244-8. [DOI] [PubMed] [Google Scholar]
- Kang H, Jia LZ, Suh KY, Tang L, Schuman EM. Determinants of BDNF-induced hippocampal synaptic plasticity: Role of the trk b receptor and the kinetics of neurotrophin delivery. Learn Mem. 1996;3:188–196. doi: 10.1101/lm.3.2-3.188. [DOI] [PubMed] [Google Scholar]
- Kempermann G. Adult neurogenesis. Oxford; Oxford, U.K.: 2006. [Google Scholar]
- Kesner R. A behavioural analysis of dentate gyrus function. Prog Brain Res. 2007;163:567–576. doi: 10.1016/S0079-6123(07)63030-1. [DOI] [PubMed] [Google Scholar]
- Kim MT, Soussou W, Gholmieh G, Ahuja A, Tanguay A, Berger TW, Brinton RD. 17β-estradiol potentiates field excitatory postsynaptic potentials within each subfield of the hippocampus with greatest potentiation of the associational/commissural afferents of CA3. Neuroscience. 2006;141:391–406. doi: 10.1016/j.neuroscience.2006.03.075. [DOI] [PubMed] [Google Scholar]
- Kiss A, Delattre AM, Pereira SI, Carolino RG, Szawka RE, Anselmo-Franci JA, Zanata SM, Ferraz AC. 17β-estradiol replacement in young, adult and middle-aged female ovariectomized rats promotes improvement of spatial reference memory and an antidepressant effect and alters monoamines and BDNF levels in memory- and depression-related brain areas. Behav Brain Res. 2012;227:100–108. doi: 10.1016/j.bbr.2011.10.047. [DOI] [PubMed] [Google Scholar]
- Kramar EA, Chen LY, Lauterborn JC, Simmons DA, Gall CM, Lynch G. BDNF upregulation rescues synaptic plasticity in middle-aged ovariectomized rats. Neurobiol Aging. 2010;33:708–719. doi: 10.1016/j.neurobiolaging.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryl D, Yacoubian T, Haapasalo A, Castren E, Lo D, Barker PA. Subcellular localization of full-length and truncated trk receptor isoforms in polarized neurons and epithelial cells. J Neurosci. 1999;19:5823–5833. doi: 10.1523/JNEUROSCI.19-14-05823.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HB, Castillo PE. Long-term potentiation selectively expressed by NMDA receptors at hippocampal mossy fiber synapses. Neuron. 2008;57:108–120. doi: 10.1016/j.neuron.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Lenz KM, Nugent BM, McCarthy MM. Sexual differentiation of the rodent brain: Dogma and beyond. Front Neurosci. 2012;6:26. doi: 10.3389/fnins.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK. Pattern separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learn Mem. 2007;14:745–745. doi: 10.1101/lm.703907. [DOI] [PubMed] [Google Scholar]
- Liu Y, Fowler CD, Young LJ, Yan Q, Insel TR, Wang Z. Expression and estrogen regulation of brain-derived neurotrophic factor gene and protein in the forebrain of female prairie voles. J Comp Neurol. 2001;433:499–514. doi: 10.1002/cne.1156. [DOI] [PubMed] [Google Scholar]
- Liu Y, Rutlin M, Huang S, Barrick CA, Wang F, Jones KR, Tessarollo L, Ginty DD. Sexually dimorphic BDNF signaling directs sensory innervation of the mammary gland. Science. 2012;338:1357–1360. doi: 10.1126/science.1228258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein DH, Arsenault L. Dentate granule cell layer collagen explant cultures: Spontaneous axonal growth and induction by brain-derived neurotrophic factor or basic fibroblast growth factor. Neuroscience. 1996;74:1197–1208. doi: 10.1016/0306-4522(96)00226-6. [DOI] [PubMed] [Google Scholar]
- Luikart BW, Nef S, Shipman T, Parada LF. In vivo role of truncated trkb receptors during sensory ganglion neurogenesis. Neuroscience. 2003;117:847–858. doi: 10.1016/s0306-4522(02)00719-4. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17α and 17β isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287–293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Babb TL, Micevych PE, Blanco CE, Pretorius JK. Granule cell mRNA levels for BDNF, NGF, and NT-3 correlate with neuron losses or supragranular mossy fiber sprouting in the chronically damaged and epileptic human hippocampus. Mol Chem Neuropathol. 1997;30:53–76. doi: 10.1007/BF02815150. [DOI] [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal α estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- Minkeviciene R, Rheims S, Dobszay MB, Zilberter M, Hartikainen J, Fülöp L, Penke B, Zilberter Y, Harkany T, Pitkänen A, Tanila H. Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. J Neurosci. 2009;29:3453–3462. doi: 10.1523/JNEUROSCI.5215-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabella N, Squillacioti C, Paone I, Ciarcia R, Russo M, Paino G. Effects of castration on the expression of brain-derived neurotrophic factor (BDNF) in the vas deferens and male accessory genital glands of the rat. Cell Tissue Res. 2006;323:513–522. doi: 10.1007/s00441-005-0084-1. [DOI] [PubMed] [Google Scholar]
- Miranda RC, Sohrabji F, Toran-Allerand CD. Neuronal colocalization of mRNAs for neurotrophins and their receptors in the developing central nervous system suggests a potential for autocrine interactions. Proc Natl Acad Sci U S A. 1993a;90:6439–6443. doi: 10.1073/pnas.90.14.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda RC, Sohrabji F, Toran-Allerand CD. Presumptive estrogen target neurons express mrnas for both the neurotrophins and neurotrophin receptors: A basis for potential developmental interactions of estrogen with the neurotrophins. Mol Cell Neurosci. 1993b;4:510–525. doi: 10.1006/mcne.1993.1063. [DOI] [PubMed] [Google Scholar]
- Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer's disease and parkinson's disease. Prog Neurobiol. 2001;63:71–124. doi: 10.1016/s0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]
- Murray KD, Isackson PJ, Eskin TA, King MA, Montesinos SP, Abraham LA, Roper SN. Altered mRNA expression for brain-derived neurotrophic factor and type II calcium/calmodulin-dependent protein kinase in the hippocampus of patients with intractable temporal lobe epilepsy. J Comp Neurol. 2000;418:411–422. doi: 10.1002/(sici)1096-9861(20000320)418:4<411::aid-cne4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM, Rockenstein E, Chao MV, Koo EH, Geschwind D, Masliah E, Chiba AA, Tuszynski MH. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med. 2009;15:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriega NC, Howdeshell KL, Furr J, Lambright CR, Wilson VS, Gray LE. Pubertal administration of dehp delays puberty, suppresses testosterone production, and inhibits reproductive tract development in male sprague-dawley and long-evans rats. Toxicol Sci. 2009;111:163–178. doi: 10.1093/toxsci/kfp129. [DOI] [PubMed] [Google Scholar]
- Olff M, Langeland W, Draijer N, Gersons BP. Gender differences in posttraumatic stress disorder. Psychol Bull. 2007;133:183–183. doi: 10.1037/0033-2909.133.2.183. [DOI] [PubMed] [Google Scholar]
- Ottem EN, Beck LA, Jordan CL, Breedlove SM. Androgen-dependent regulation of brain-derived neurotrophic factor and tyrosine kinase B in the sexually dimorphic spinal nucleus of the bulbocavernosus. Endocrinology. 2007;148:3655–3665. doi: 10.1210/en.2007-0308. [DOI] [PubMed] [Google Scholar]
- Ottem EN, Bailey DJ, Jordan CL, Marc Breedlove S. With a little help from my friends: Androgens tap BDNF signaling pathways to alter neural circuits. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.12.019. in press. [DOI] [PubMed] [Google Scholar]
- Paredes A, Romero C, Dissen GA, DeChiara TM, Reichardt L, Cornea A, Ojeda SR, Xu B. Trkb receptors are required for follicular growth and oocyte survival in the mammalian ovary. Dev Biol. 2004;267:430–449. doi: 10.1016/j.ydbio.2003.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JP, Kurucz OS, Milner TA. Morphometry of a peptidergic transmitter system: Dynorphin b-like immunoreactivity in the rat hippocampal mossy fiber pathway before and after seizures. Hippocampus. 1999;9:255–276. doi: 10.1002/(SICI)1098-1063(1999)9:3<255::AID-HIPO6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Qiao X, Suri C, Knusel B, Noebels JL. Absence of hippocampal mossy fiber sprouting in transgenic mice overexpressing brain-derived neurotrophic factor. J Neurosci Res. 2001;64:268–276. doi: 10.1002/jnr.1075. [DOI] [PubMed] [Google Scholar]
- Raab A, Haedenkamp G. Impact of social conflict between mice on testosterone binding in the central nervous system. Neuroendocrinology. 1981;32:272–277. doi: 10.1159/000123172. [DOI] [PubMed] [Google Scholar]
- Ramírez-Amaya V, Escobar ML, Chao V, Bermúdez-Rattoni F. Hippocampus. 1999;9:631–631. doi: 10.1002/(SICI)1098-1063(1999)9:6<631::AID-HIPO3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Represa A, Tremblay E, Ben-Ari Y. Aberrant growth of mossy fibers and enhanced kainic acid binding sites induced in rats by early hyperthyroidism. Brain Res. 1987;423:325–325. doi: 10.1016/0006-8993(87)90856-0. [DOI] [PubMed] [Google Scholar]
- Rose CR, Blum R, Pichler B, Lepier A, Kafitz KW, Konnerth A. Truncated trkb-t1 mediates neurotrophin-evoked calcium signalling in glia cells. Nature. 2003;426:74–78. doi: 10.1038/nature01983. [DOI] [PubMed] [Google Scholar]
- Routtenberg A. Adult learning and remodeling of hippocampal mossy fibers: unheralded participant in circuitry for long-lasting spatial memory. Hippocampus. 2010;20:44–45. doi: 10.1002/hipo.20664. [DOI] [PubMed] [Google Scholar]
- Saarelainen T, Lukkarinen JA, Koponen S, Grohn OH, Jolkkonen J, Koponen E, Haapasalo A, Alhonen L, Wong G, Koistinaho J, Kauppinen RA, Castren E. Transgenic mice overexpressing truncated trkb neurotrophin receptors in neurons show increased susceptibility to cortical injury after focal cerebral ischemia. Mol Cell Neurosci. 2000;16:87–96. doi: 10.1006/mcne.2000.0863. [DOI] [PubMed] [Google Scholar]
- Salin PA, Scanziani M, Malenka RC, Nicoll RA. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc Natl Acad Sci U S A. 1996;93:13304–13309. doi: 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalettar BA. How neurosecretory vesicles release their cargo. Neuroscientist. 2006;12:164–76. doi: 10.1177/1073858405284258. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. Hyperexcitability in combined entorhinal/hippocampal slices of adult rat after exposure to brain-derived neurotrophic factor. J Neurophysiol. 1997;78:1082–1095. doi: 10.1152/jn.1997.78.2.1082. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. Epilepsy as an example of neural plasticity. The Neuroscientist. 2002;8:154–173. doi: 10.1177/107385840200800211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. BDNF and the mossy fibers: Implications for epilepsy. In: Stanton PK, Bramham C, Scharfman HE, editors. The Transynaptic Dialogue in Synaptic Plasticity. Springer Science and Business Media; New York: 2005. [Google Scholar]
- Scharfman HE, Hintz TM, Gomez J, Stormes KA, Barouk S, Malthankar-Phatak GH, McCloskey DP, Luine VN, Maclusky NJ. Changes in hippocampal function of ovariectomized rats after sequential low doses of estradiol to simulate the preovulatory estrogen surge. Eur J Neurosci. 2007;26:2595–2612. doi: 10.1111/j.1460-9568.2007.05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: A potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23:11641–11652. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Sollas AL, Croll SD. Spontaneous limbic seizures after intrahippocampal infusion of brain-derived neurotrophic factor. Exp Neurol. 2002;174:201–201. doi: 10.1006/exnr.2002.7869. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Pierce JP. New insights into the role of hilar ectopic granule cells in the dentate gyrus based on quantitative anatomic analysis and three-dimensional reconstruction. Epilepsia. 2012;53(Suppl 1):109–115. doi: 10.1111/j.1528-1167.2012.03480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Simpkins JW. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female sprague-dawley rats. Endocrinology. 1995;136:2320–2324. doi: 10.1210/endo.136.5.7720680. [DOI] [PubMed] [Google Scholar]
- Skucas VA, Duffy AM, Harte-Hargrove LC, Magagna-Poveda A, Radman T, Schroeder CE, MacLusky NJ, Scharfman HE. Testosterone depletion increases mossy fiber transmission, synaptic plasticity and sprouting in adult male rats. J Neurosci. 2013 doi: 10.1523/JNEUROSCI.3857-12.2013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluyter F, Jamot L, van Oortmerssen GA, Crusio WE. Hippocampal mossy fiber distributions in mice selected for aggression. Brain Res. 1994;646:145–148. doi: 10.1016/0006-8993(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solum DT, Handa RJ. Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. J Neurosci. 2002;22:2650–2659. doi: 10.1523/JNEUROSCI.22-07-02650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer-Segal JL, Waters EM, Bath KG, Chao MV, McEwen BS, Milner TA. Distribution of phosphorylated trkb receptor in the mouse hippocampal formation depends on sex and estrous cycle stage. J Neurosci. 2011;31:6780–6790. doi: 10.1523/JNEUROSCI.0910-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperk G, Hamilton T, Colmers WF. Neuropeptide Y in the dentate gyrus. Prog Br Res. 2007;163:285–285. doi: 10.1016/S0079-6123(07)63017-9. [DOI] [PubMed] [Google Scholar]
- Sperk G, Marksteiner J, Gruber B, Bellmann R, Mahata M, Ortler M. Functional changes in neuropeptide y-and somatostatin-containing neurons induced by limbic seizures in the rat. Neuroscience. 1992;50:831–846. doi: 10.1016/0306-4522(92)90207-i. [DOI] [PubMed] [Google Scholar]
- Squillacioti C, De Luca A, Paino G, Mirabella N. Effects of castration on the immunoreactivity to NGF, BDNF and their receptors in the pelvic ganglia of the male rat. Eur J Histochem. 2008;52:101–106. doi: 10.4081/1194. [DOI] [PubMed] [Google Scholar]
- Tabori NE, Stewart LS, Znamensky V, Romeo RD, Alves SE, McEwen BS, Milner TA. Ultrastructural evidence that androgen receptors are located at extranuclear sites in the rat hippocampal formation. Neuroscience. 2005;130:151–163. doi: 10.1016/j.neuroscience.2004.08.048. [DOI] [PubMed] [Google Scholar]
- Teng KK, Felice S, Kim T, Hempstead BL. Understanding proneurotrophin actions: Recent advances and challenges. Dev Neurobiol. 2010;70:350–359. doi: 10.1002/dneu.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervonen TA, Ajamian F, De Wit J, Verhaagen J, Castren E, Castren M. Overexpression of a truncated trkb isoform increases the proliferation of neural progenitors. Eur J Neurosci. 2006;24:1277–1285. doi: 10.1111/j.1460-9568.2006.05010.x. [DOI] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toran-Allerand CD, Miranda RC, Bentham WD, Sohrabji F, Brown TJ, Hochberg RB, MacLusky NJ. Estrogen receptors colocalize with low-affinity nerve growth factor receptors in cholinergic neurons of the basal forebrain. Proc Natl Acad Sci U S A. 1992;89:4668–4672. doi: 10.1073/pnas.89.10.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torborg CL, Nakashiba T, Tonegawa S, McBain CJ. Control of CA3 output by feedforward inhibition despite developmental changes in the excitation-inhibition balance. J Neurosci. 2010;30:15628–15628. doi: 10.1523/JNEUROSCI.3099-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano-Silva M, Gomes da Silva S, Scorza FA, Bonvent JJ, Cavalheiro EA, Arida RM. Hippocampal mossy fiber sprouting induced by forced and voluntary physical exercise. Physiol Behav. 2010;101:302–308. doi: 10.1016/j.physbeh.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Turner JE, Barde YA, Schwab ME, Thoenen H. Extract from brain stimulates neurite outgrowth from fetal rat retinal explants. Brain Res. 1982;282:77–83. doi: 10.1016/0165-3806(82)90176-6. [DOI] [PubMed] [Google Scholar]
- Verhovshek T, Sengelaub DR. Trophic effects of brain-derived neurotrophic factor blockade in an androgen-sensitive neuromuscular system. Endocrinology. 2010;151:5337–5348. doi: 10.1210/en.2010-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhovshek T, Sengelaub D. BDNF and androgen interactions in spinal neuromuscular systems. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.10.028. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MC, Ruiz A, Kullmann DM. Monosynaptic GABAergic signaling from dentate to CA3 with a pharmacological and physiological profile typical of mossy fiber synapses. Neuron. 2001;29:703–715. doi: 10.1016/s0896-6273(01)00245-8. [DOI] [PubMed] [Google Scholar]
- Wallen K, editor. 50th anniversary of the publication of Phoenix, Goy, Gerall & Young 1959: Organizational effects of hormones. Horm behav. 2009;55(5):561–561. doi: 10.1016/j.yhbeh.2009.03.009. [DOI] [PubMed] [Google Scholar]
- White JD, Gall CM, McKelvy JF. Enkephalin biosynthesis and enkephalin gene expression are increased in hippocampal mossy fibers following a unilateral lesion of the hilus. J Neurosci. 1987;7:753–759. doi: 10.1523/JNEUROSCI.07-03-00753.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winawer MR, Makarenko N, McCloskey DP, Hintz TM, Nair N, Palme r A.A., Scharfman HE. Acute and chronic responses to the convulsant pilocarpine in DBA/2J and A/J mice. Neuroscience. 2007;149:465–475. doi: 10.1016/j.neuroscience.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YC, Hill RA, Gogos A, van den Buuse M. Sex differences and the role of estrogen in animal models of schizophrenia: Interaction with BDNF. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.10.024. [DOI] [PubMed] [Google Scholar]
- Xu B, Michalski B, Racine RJ, Fahnestock M. The effects of brain-derived neurotrophic factor (BDNF) administration on kindling induction, trk expression and seizure-related morphological changes. Neuroscience. 2004;126:521–531. doi: 10.1016/j.neuroscience.2004.03.044. [DOI] [PubMed] [Google Scholar]
- Yacoubian TA, Lo DC. Truncated and full-length trkb receptors regulate distinct modes of dendritic growth. Nat Neurosci. 2000;3:342–349. doi: 10.1038/73911. [DOI] [PubMed] [Google Scholar]
- Yan Q, Rosenfeld RD, Matheson CR, Hawkins N, Lopez OT, Bennett L, Welcher AA. Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience. 1997;78:431–448. doi: 10.1016/s0306-4522(96)00613-6. [DOI] [PubMed] [Google Scholar]
- Yang LY, Arnold AP. Interaction of BDNF and testosterone in the regulation of adult perineal motoneurons. J Neurobiol. 2000;44:308–319. doi: 10.1002/1097-4695(20000905)44:3<308::aid-neu2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Yang LY, Verhovshek T, Sengelaub DR. Brain-derived neurotrophic factor and androgen interact in the maintenance of dendritic morphology in a sexually dimorphic rat spinal nucleus. Endocrinology. 2004;145:161–168. doi: 10.1210/en.2003-0853. [DOI] [PubMed] [Google Scholar]
- Yanpallewar SU, Barrick CA, Buckley H, Becker J, Tessarollo L. Deletion of the BDNF truncated receptor trkb.T1 delays disease onset in a mouse model of amyotrophic lateral sclerosis. PLoS One. 2012;7:e39946. doi: 10.1371/journal.pone.0039946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampieri N, Chao MV. Mechanisms of neurotrophin receptor signalling. Biochem Soc Trans. 2006;34:607–611. doi: 10.1042/BST0340607. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Li LY, Zou XL, Song XB, Hu YL, Feng ZT, Wang TT. Immunohistochemical distribution of NGF, BDNF, NT-3, and NT-4 in adult rhesus monkey brains. J Histochem Cytochem. 2007;55:1–19. doi: 10.1369/jhc.6A6952.2006. [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhang H, Cohen RS, Pandey SC. Effects of estrogen treatment on expression of brain-derived neurotrophic factor and camp response element-binding protein expression and phosphorylation in rat amygdaloid and hippocampal structures. Neuroendocrinology. 2005;81:294–310. doi: 10.1159/000088448. [DOI] [PMC free article] [PubMed] [Google Scholar]