Abstract
Bleomycin causes acute lung injury through production of reactive species and initiation of inflammation. Previous work has shown alteration to the production of reactive oxygen species results in attenuation of injury. Vitamin E, in particular, γ-tocopherol, isoform, has the potential to scavenge reactive oxygen and nitrogen species. This study examines the utility of dietary supplementation with tocopherols in reducing bleomycin-mediated acute lung injury. Male C57BL6/J mice were intratracheally instilled with PBS or 2 units/kg bleomycin. Animals were analyzed 3 and 8 days post instillation at the cellular, tissue, and organ levels. Results showed successful delivery of tocopherols to the lung via dietary supplementation. Also, increases in reactive oxygen and nitrogen species due to bleomycin are normalized in those mice fed tocopherol diet. Injury was not prevented but inflammation progression was altered, in particular macrophage activation and function. Inflammatory scores based on histology demonstrate limited progression of inflammation in those mice treated with bleomycin and fed tocopherol diet compared to control diet. Upregulation of enzymes and cytokines involved in pro-inflammation were limited by tocopherol supplementation. Day 3 functional changes in elastance in response to bleomycin are prevented, however, 8 days post injury the effect of the tocopherol diet is lost. The effect of tocopherol supplementation upon the inflammatory process is demonstrated by a shift in the phenotype of macrophage activation. The effect of these changes on resolution and the progression of pulmonary fibrosis has yet to be elucidated.
Keywords: Lung Mechanics, Macrophage Phenotype, Macrophage Activation, Reactive Oxygen, Nitrogen Species
1. Introduction
Bleomycin is currently used clinically as a chemotherapeutic drug, but its use is limited by development of pulmonary fibrosis. As a model of lung injury, intratracheal bleomycin (ITB) causes inflammation and consequent pulmonary fibrosis. Activated bleomycin acts as a redox-cycler causing damage to proteins, lipids and DNA through the production of reactive oxygen species (ROS) [1]. The inflammatory process that is initiated by ITB also generates ROS. Furthermore, pulmonary inflammation and fibrosis in response to ITB is associated with decreases in antioxidant enzyme expression and activity, in particular superoxide dismutase, catalase and glutathione peroxidase [2; 3]. In this regard, it has been shown that increasing antioxidant defenses by supplementation of lecithinized superoxide dismutase, or increasing host injury defenses by over expressing Hsp70, abrogates ITB acute injury and inflammation [4; 5].
Vitamin E is a powerful antioxidant capable of terminating propagation of radical reactions. Due to its antioxidant potential, vitamin E has been studied in many models of inflammation. In particular, α-tocopherol, the most well recognized form of vitamin E, has been extensively studied as an antioxidant. α-tocopherol levels in the lungs are reduced by bleomycin administration suggesting α-tocopherol is consumed in times of oxidative stress [6]. Intratracheal liposomal α-tocopherol administered to rats prior to ITB reveals that α-tocopherol successfully normalizes hydroxyproline concentrations 21 days after instillation [7].
Unlike α-tocopherol, γ-tocopherol is uniquely capable of scavenging reactive nitrogen species in addition to its antioxidant potential. Due to an unsubstituted carbon at the carbon-5-position, γ-tocopherol interacts with reactive nitrogen species resulting in the formation of 5-nitro-γ-tocopherol. γ -tocopherol also inhibits lipid peroxidation, in particular by sequestering peroxynitrite. [8; 9]
Nitric oxide plays a significant role in the initiation and mediation of inflammatory signaling. Inducible nitric oxide synthase (iNOS) produces NO at a high flux rate in inflammatory and epithelial cells in times of inflammation. After ITB, loss of functional inducible nitric oxide synthase (iNOS) as demonstrated through inhibition via GW274150 or NOS2−/− knockout mouse, prevents nitrosative stress. Loss of functional iNOS also abrogates the development of fibrosis in CD mice measured 15 days post ITB by hydroxyproline levels and trichrome stain. [10]
Additional models of lung injury and inflammation demonstrate a role of NOS2 in inflammation progression. Intratracheal lipopolysaccharide (LPS) also causes acute lung injury and inflammation with evidence of nitrosative stress. NOS2−/− knockout mice, treated with LPS, experience less nitrosative stress as evidenced by nitrotyrosine stain. NOS2−/− knockout mice also experience less cell infiltrate and edema as seen on histology [11]. We have also previously shown the role of iNOS in the initiation and progression of chronic inflammation. Surfactant protein-D knock out mice experience chronic inflammation that can be inhibited with 1400W, an iNOS selective inhibitor, administered early in disease progression or during active inflammation [12].
Nitric oxide is known to play a role in the promotion and progression of inflammation in response to ITB [10]. By using a mixture of tocopherols, with a particularly high concentration of γ-tocopherol, within the diet there is the potential to increase the scavenging of reactive oxygen and nitrogen species and hence reduce inflammation. The ITB model is a progressive model of inflammation allowing one to evaluate both early and late responses. Pulmonary injury and inflammation are evident 3 days after instillation of bleomycin and peak after 8 days. Therefore, we examined the effects of tocopherol supplementation at both of these time points. We hypothesized that tocopherols will act early in the inflammatory process by sequestering reactive oxygen and nitrogen species (RONS) thus reduce NO driven inflammation.
2. Methods
2.1 Animals and Diet
48 10-week-old male C57BL6/J mice were obtained from Jackson Laboratory (Bar Harbor, ME). Animals weighed between 20–32 grams. Animals were fed ad libitum either control diet (AIN93M) [13] or treatment diet (0.3%γTmT diet) prepared by Research Diets, Inc. (New Brunswick, NJ). γTmT is a tocopherol-rich mixture of tocopherols consisting of (per gram) 130mg α-tocopherol, 15mg β-tocopherol, 568mg γ-tocopherol and 243mg δ-tocopherol (Cognis Corporation Fairfield, NJ). The tocopherol content of the two diets is shown in Table 1. Animal protocols were reviewed and approved by Animal Care and Use Committee, Rutgers University.
Table 1.
Composition of diets. Tocopherols are an essential nutrient and therefore a component of control diet, AIN93M. The tocopherol enriched diet, 0.3% γ-TmT, is supplemented with additional tocopherols in particular γ-tocopherol. Diet was initiated 2 weeks prior to instillation of saline or bleomycin. Animals ate ad libitum through out the course of the study. Tocopherol composition of both diets is listed as mg tocopherol/kg fed.
| Diet | α-T | β-T | γ-T | δ-T |
|---|---|---|---|---|
| AIN93M | 79.8 | 0.4 | 24.4 | 7.6 |
|
AIN93M +0.3% γ-TmT |
439.8 | 45.4 | 1764.4 | 637.6 |
2.2 Intratracheal Instillation
Animals were anesthetized by intraperitoneal injection of ketamine (300mg/kg) and xylazine (15mg/kg) (Butler Schein). Each mouse was then laid on a tilting rodent work stand (Hallowell EMC Pittsfield, MA) in the supine position. The larynx was visualized using a hemi-sectioned 3mm diameter speculum with otoscope (Welch Allyn Skaneateles Falls NY). Either LPS-negative sterile phosphate buffered saline (PBS) or LPS-negative sterile bleomycin sulfate (ITB) (Sigma Aldrich St. Louis, MO) was instilled at 2units/kg. No animals died during instillation.
2.3 Tocopherol Measurement
Tocopherol concentrations were measured in the serum and lung by high performance liquid chromatography as previously described[14]. Measurements were made from in 30mg lung tissue and 20µL plasma collected from mice 8 days post instillation.
2.4 Cell Count and Protein Measurement
Bronchoalveolar lavage (BALF) was performed with 5 washes of 1mL PBS via an intratracheally-inserted cannula. BALF was centriguged at 30,000 g for 10 mins and the cells was resuspended in PBS and counted via Multisizer Coutler Counter (Beckman Coulter). Supernatant was analyzed for protein content with Bradford protein assay kit (BioRad, Hercules, CA) reading at 595nm using MAXline Kinetic microplate reader (Molecular Devices, Sunnyvale, CA) with bovine serum albumin as standard.
2.5 mRNA Measurement
BALF cells were analyzed by RT-PCR for expression of inflammatory genes using High Capacity cDNA Reverse Transcriptase Kit (Applied Biosystems Foster City, CA). cDNA samples were analyzed on the 7300 Real Time PCR system using Taqman polymerase and primary probes obtained from Applied Biosystems. Samples ran for 40 cycles of 2 minutes at 50°C, 10 minutes at 95°C, and 15 seconds at 95°C. Fold expression was calculated using the 2−ΔΔCt method using β-actin as the control gene and the PBS/AIN93M samples as the control group. Samples from each treatment group were pooled into 2 sets of 4 animals for analysis.
2.6 Histochemistry
Lung tissue was inflation fixed with 1mL 3% paraformadehyde, 2% sucrose and 4% sodium cacodylate 0.1M (pH 7.3). Lungs were embedded in paraffin and stained with hematoxylin and eosin. Inflammation scores were generated using a 5-point scale by two blinded and independent observers as previously published [15]. Tissue sections were analyzed for protein expression by immunohistochemistry. Tissue sections were deparrafinized and rehydrated with xylene and ethanol. Antigen retrieval was achieved with sodium citrate followed by endogenous peroxidase quench. Tissue was blocked with 10% goat serum in PBS for 1 hour followed by primary antibody overnight in 1% serum in PBS. Sections were washed with 0.1 to 0.3% tween20 in PBS and incubated with secondary antibody (Vector Laboratories ABC Kit Peroxidase Rabbit IgG Burlingame, CA) in 1% serum in PBS. Following further washing sections were visualized with DAB (Vector Laboratories DAB Peroxidase Substrate Kit Burlingame, CA). Antibody concentrations for the different epitopes are given in the legends of the figures. Hematoxylin was used for counterstain.
Aliquots of BALF cells (30,000 cells) were spun onto slides with Thermo Shandon Cytospin-4 at 800rpm for 3 minutes and stained for identification with KWIK-DIFF (Thermo). Cell differentials were obtained by counting 5 different fields at an optical zoom of 400x. Cytospin slides were also used to quantify the number of cells greater than 25 microns.
2.7 Chemotaxis Measurement
100,000 RAW264.7 cells (American Type Culture Collection, TIB-71 Manassas, VA) (suspended at 1×106 cells/mL) in Dulbecco’s Modified Eagle Medium were placed in the upper chamber of a 96 well plate microchemotaxis chamber (Neuro Probe Gaithersburg, MD). 30µL of sample or control solution was placed in the lower chamber of the apparatus.. A filter, with a pore size of 5µm was used. The chamber was incubated for 4 hours at 37° C with 5% CO2. Migrating cells were collected on the membrane and stained with KWIK-DIFF (Thermo), visualized at an optical zoom of 400x and counted in 5 fields on the BX51 (Olympus).
2.8 Nitric Oxide Metabolite Analysis
Using the first aliquot of BALF supernatant, samples were analyzed with Ionics/Sievers nitric oxide analyzer 280 (NOA 280 Ionics Instruments). Vanadium chloride (Sigma Aldrich) (1M HCl at 95°C) was used as the reducing agent and sodium nitrate as the standard.
2.9 Lung Function Testing
Lung function was measured 3 or 8 days after instillation of PBS or bleomycin. Using SciReq flexiVent (Montreal, Canada) baseline function and mechanics were measured. Measurements were taken via Forced Oscillation Technique at increasing Positive End-Expiratory Pressures (0−9 cmH2O). Matlab software was used for analysis of parameters to calculate overall elastance and resistance profiles. Results are shown at a PEEP of 3 cm H2O, physiological PEEP.
2.10 Statistics
Data was analyzed in 2-way ANOVA and unpaired t-test or Mann-Whitney. Pulmonary inflammation scores were analyzed by Wilcoxon-Rank Test. Cell size was analyzed by Tukey’s test and chemotactic potential by Dunn’s test. A p-value of <0.05 was considered statistically significant.
3. Results
3.1 Diet increases tocopherol content within the lung
Due to their ability to scavenge ROS, and reactive nitrogen species, we proposed that increasing the tocopherol content within the lung would provide protection against bleomycin-mediated injury. In this study, we manipulated tocopherol content by means of diet. Mice were fed either a control AIN93M diet or one enriched in tocopherols, especially γ-tocopherol. At the conclusion of the study, both serum and lung tissue were analyzed for tocopherol content (Table 2). The tocopherol enriched diet led to an increase in the content within both the serum and the lung with γ-tocopherol increasing 4 fold in the lung. Lung concentrations of α and δ tocopherol were also increased in those fed tocopherol enriched diet. Notably, in the absence of supplementation, ITB reduced γ-tocopherol levels two fold in the serum.
Table 2.
Tocopherol concentration in the lung and serum of mice 8 days after instillation of saline or bleomycin. Tocopherol content was assessed by HPLC as per the methods section. Tocopherols were successfully absorbed and distributed to the lung and concentrations (expressed as mean ± standard error) increased in those fed 0.3%γTmT diet accordingly.
| α-T | β-T | γ-T | |
|---|---|---|---|
| Treatment Group | Serum (µM) | ||
| PBS/AIN93M | 0.13 ± 0.02 | 7.72 ± 0.51 | 0.07 ± 0 |
| ITB/AIN93M | 0.06 ± 0.01* | 7.62 ± 0.81 | 0.14 ± 0.01* |
| ITB/0.3%γTmT | 0.55 ± 0.04*,+ | 10.60 ± 0.67*,+ | 0.08 ± 0+ |
| Lung Tocopherols (µmol/kg) | |||
| PBS/AIN93M | 0.41 ± 0.03 | 14.83 ± 3.40 | 0.09 ± 0 |
| ITB/AIN93M | 0.72 ± 0.14* | 16.47 ± 2.92 | 0.22 ± 0.06* |
| ITB/0.3%γTmT | 2.9 ± 0.56*,+ | 28.45 ± 5.80*,+ | 1.55 ± 0.29*,+ |
statistically different from PBS/AIN93M and
statistically different from ITB/AIN93M (p<0.05).
3.2 Tocopherol supplementation reduces lung injury and inflammation
Hematoxylin and eosin staining of lung tissue 3 days and 8 days after ITB shows that there is extensive consolidation and peribronchial and perivascular infiltration (Figure 1). As early as 3 days post administration there is evidence of leukocytic invasion within the lung as well as thickening of the pulmonary epithelium (Figure 1B). However, by day 8 these markers are increased in severity as well as increasing tissue destruction (Figure 1E). In the presence of tocopherol, there is still evidence of both injury and inflammation at day 3; however, there is a minimal increase in these markers over the following 5 days. Non-parametric inflammatory scoring [15] demonstrates that inflammation resulting from ITB is progressive from day 3 to day 8 and appears to be inhibited by the tocopherol-rich diet (Figure 1G). The progressive nature of this injury is reflected in the protein content of the BAL, which increases at both day 3 and, to a greater extent, at day 8; but is largely unaffected by tocopherol supplementation (Table 3).
Figure 1.
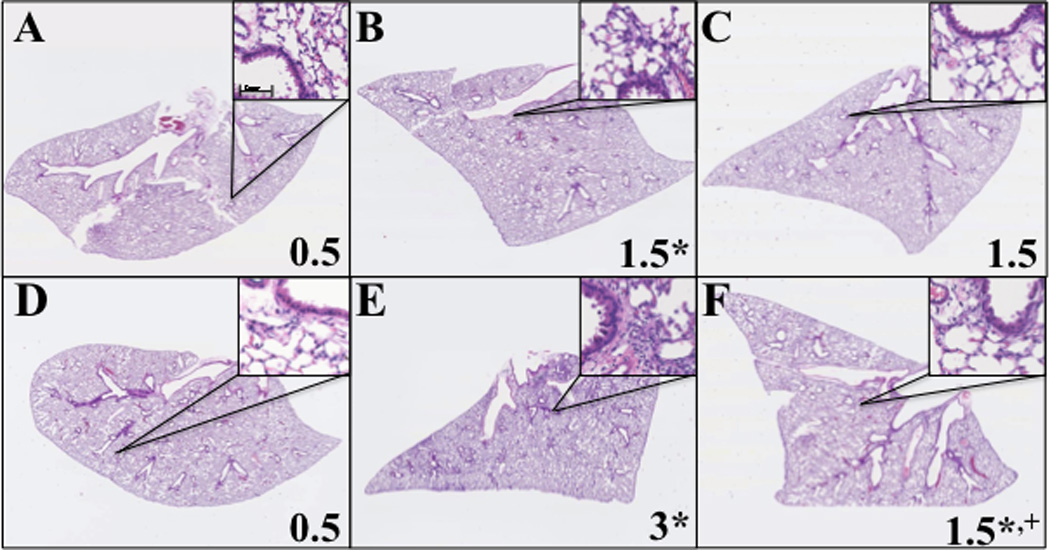
Effect of tocopherol diet on progression of inflammation. Lungs were analyzed from mice fed control diet AIN93M (A,B,D,E) or tocopherol enriched 0.3%γTmT (C,F). The right lungs of mice were harvested 3 days (A,B,C) and 8 days (D,E,F) after instillation of PBS (A,D) or ITB (B,C,E,F) and inflation fixed for histology. Representative pictures were taken with Nanozoomer (Olympus) at a magnification of 6.5x and inserts at 100x. * P<0.05 compared to PBS/AIN93M and +<0.05 compared to ITB/AIN93M. (n=8)
Table 3.
Protein concentration and cell yield in the BALF. Cells retrieved in the BALF were counted via Multisizer, while protein concentration in the supernatant was measured by the BCA assay.
| Treatment Group |
Days Post Instillation |
Protein (mg/mL) |
Total Cells |
|---|---|---|---|
| PBS/AIN93M | 3 | 0.177 ± 0.007 | 94,269 ± 8,923 |
| ITB/AIN93M | 3 | 0.519 ± 0.052* | 168,719 ± 15,343 * |
| ITB/0.3%γTmT | 3 | 0.619 ± 0.086* | 127,969 ± 20,720 |
| PBS/AIN93M | 8 | 0.185 ± 0.004 | 120,375 ± 9,446 |
| ITB/AIN93M | 8 | 1.301 ± 0.157* | 306,807 ± 289,070* |
| ITB/0.3%γTmT | 8 | 1.098 ± 0.140* | 329,121 ± 128,784* |
P<0.05 compared to PBS/AIN93M at corresponding duration of exposure.
3.3 Effect of tocopherol supplementation on inflammatory protein expression
The production of ROS from ITB-initiated inflammation is thought to contribute to the progress of the pathology. Cyclooxygenase-2 (COX2) and inducible Nitric Oxide Synthase (iNOS) are both key enzymes in the production of reactive oxygen and nitrogen species in the progress of inflammation. Therefore, we examined their production in response to ITB via immunohistochemistry (Figure 2 & 3). COX2 expression was evident in epithelial and invading inflammatory cells as early as 3 days post ITB (Figure 2B). However, the intensity of COX2 staining increased considerably by day 8, especially within the pulmonary epithelium (Figure 2E). In tocopherol-supplemented mice, ITB was still capable of increasing COX2 staining at day 3, however, there was minimal increase in COX2 expression by day 8 when compared to day 3 (Figure 2 C & F).
Figure 2.
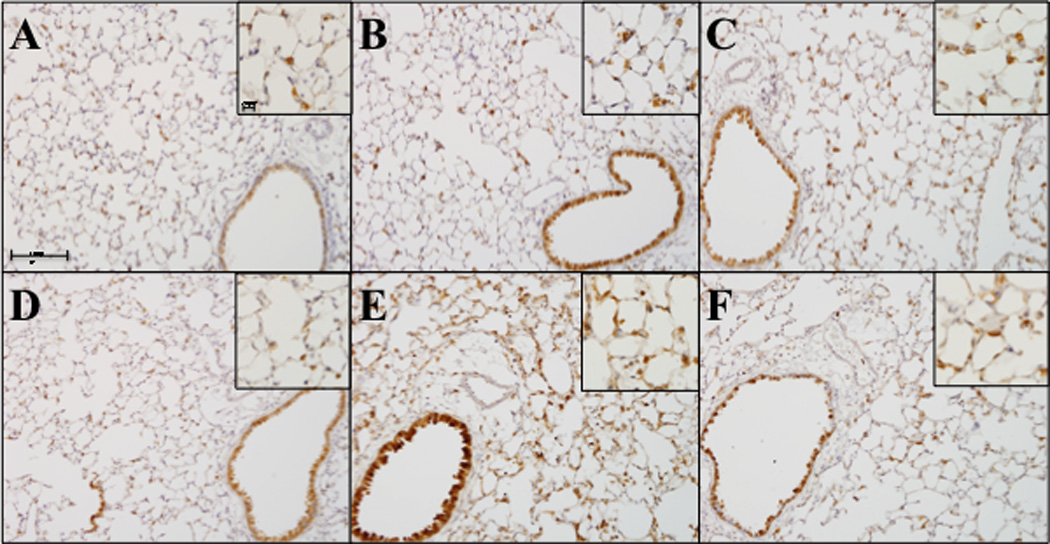
COX-2 expression following ITB. Lungs were stained to evaluate cyclo-oxygenase-2 protein expression by immunohistochemistry Lungs were harvested and sectioned at 3 days (A,B,C) and 8 days (D,E,F) after PBS (A, D) and ITB (B,C,E,F) instillation. Mice were fed control diet AIN93M (A,B,D,E) or tocopherol enriched 0.3%γTmT diet (C,F). Tissue was stained for COX-2; primary antibody dilution 1:2000 overnight (Abcam ab15191 Cambridge, MA) and secondary antibody at 1:2000 for 30 minutes. Representative pictures were taken via the VS120 (Olympus) at an optical zoom 100x and inserts 200x. (n=8)
Figure 3.
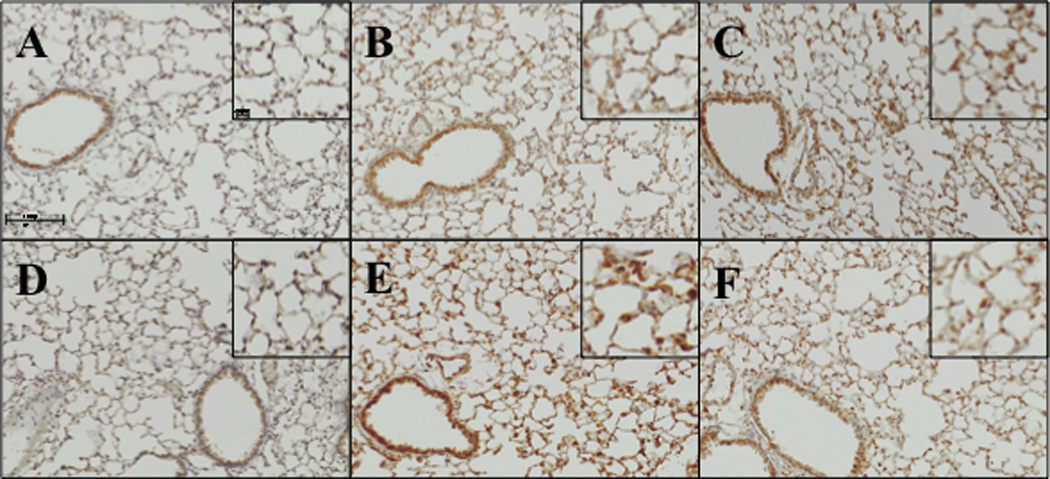
Effect of tocopherol diet on increased iNOS expression in response to ITB. iNOS expression was evaluated by immunohistochemistry. Tissues were collected 3 days (A,B,C) and 8 days (D,E,F) after instillation of PBS (A, D) or ITB (B,C,E,F). Mice were fed control diet AIN93M (A,B,D,E) and tocopherol enriched 0.3%γTmT diet (C,F). Tissues were incubated with primary antibody (Abcam, ab15323 Cambridge, MA) at 1:100 dilution overnight followed by secondary antibody at 1:200 for 30 minutes. Representative pictures were taken via the BX51 (Olympus) at an optical zoom 200x and 400x. (n=8)
The time course of changes in iNOS expression, in response to ITB, mirror those observed for COX2 (Figure 3). At day 3 post ITB there is a small increase in iNOS expression throughout the lung parenchyma. Eight days post injury there is a focal increase within invading inflammatory cells and a less pronounced increase in the epithelium than seen for COX2. Similar to the observations with COX2, feeding with the tocopherol-supplemented diet reduced iNOS staining, although this difference was only apparent at 8 days post ITB.
Ym1 is synthesized and secreted by macrophages and is typically associated with alternative activation. In saline treated mice a few Ym1 positive macrophages can be observed (Figure 4 A & D). Following ITB treatment there is an increase in both the frequency of Ym1 positive cells observed and the intensity of their staining. This is more pronounced at 8 days post ITB than at 3 days (Figure 4 B & E). Tocopherol supplementation reduces the frequency of Ym1 positive cells at both time points (Figure 4 C & F). However, it does not reduce the intensity with which these cells are stained. Indeed at 3 days post ITB the intensity of stain appears greater than in mice fed the control diet (Figure 4C).
Figure 4.
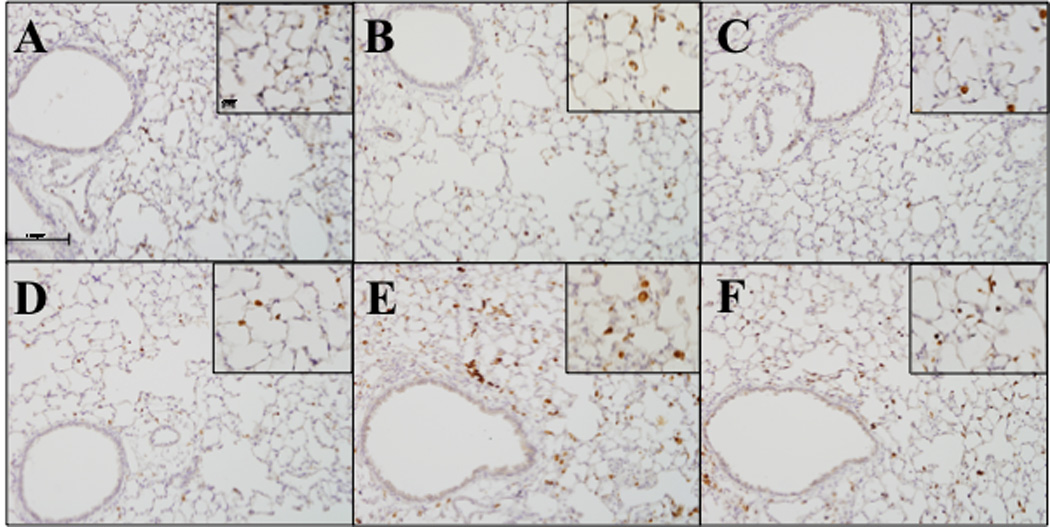
Ym1 expression in response to ITB. Lung sections were stained to evaluate Ym1 protein expression by immunohistochemistry Lungs were harvested 3 days (A,B,C) and 8 days (D,E,F) after PBS (A, D) and ITB (B,C,E,F) instillation. Mice were fed control diet AIN93M (A,B,D,E) and tocopherol enriched 0.3%γTmT diet (C,F). Tissues were incubated with primary antibody at 1:500 overnight (StemCell Technologies, 01404 Vancouver Canada) and secondary antibody at 1:2000 for 30 minutes. Representative pictures were taken via the VS120 (Olympus) at an optical zoom 100x and inserts 200x. (n=8)
3.4 Cells in the Bronchoaveolar Lavage Fluid
In parallel with the histological evidence of inflammation, there is an increase in total cell count within the BAL following ITB (Table 3). In this context, tocopherol supplementation appears to alter the kinetics of cellular accumulation, as the increase in cell number was not significant at 3 days post ITB. Examination of cell differentiation showed that ITB leads to early neutrophilia at day 3, which is not altered by the tocopherol-rich diet, with consequent increases in all inflammatory cell types 8 days post injury (Figure 5). Tocopherol supplementation alters the kinetics of the response, as both lymphocytes and eosinophils accumulate later in this condition. Macrophage size, an indicator of activation status, was examined. The number of cells greater than 25 microns is statistically higher in mice treated with ITB; however, ITB treated mice fed control diet have statistically more large cells than those fed tocopherol diet (Figure 6A).
Figure 5.
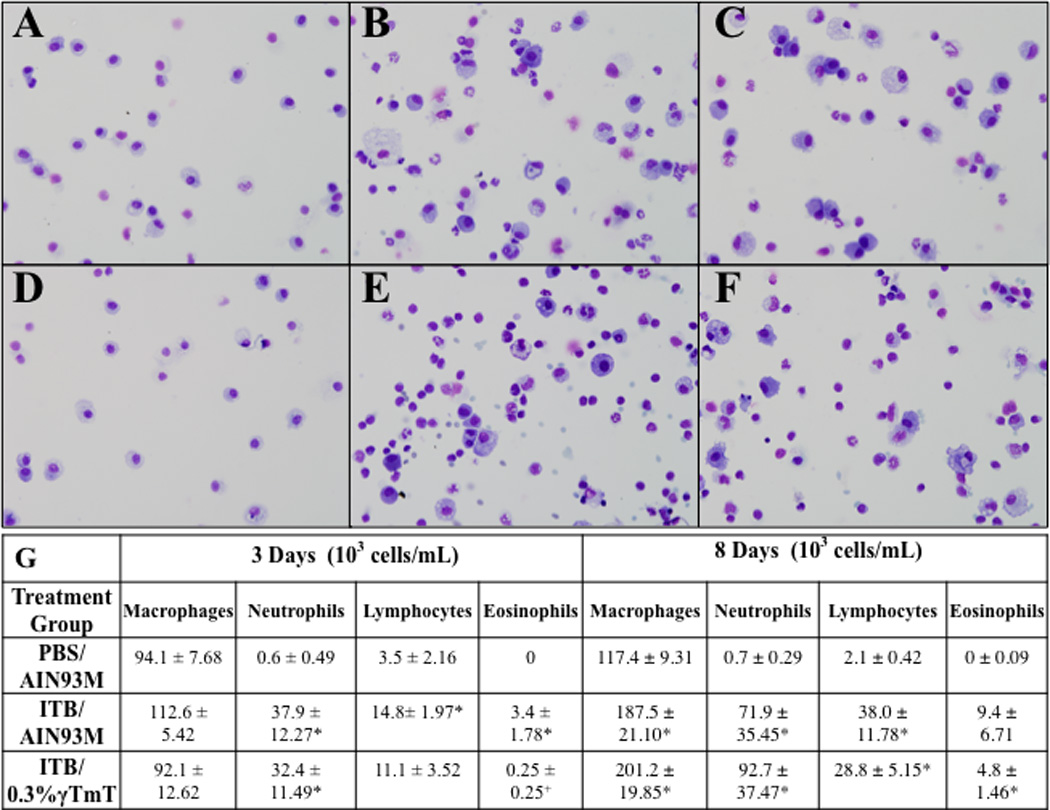
Effect of tocopherol diet on recruitment of leukocytes. BAL cells were collected from mice 3 days (A,B,C) and 8 days (D,E,F) after PBS (A, D) and ITB (B,C,E,F) instillatn. Mice were fed control diet AIN93M (A,D) and tocopherol enriched 0.3%γTmT diet (B,C,E,F). * P<0.05 compared to PBS/AIN93M and P<0.05 compared to ITB/AIN93M at corresponding duration of exposure. Representative pictures were taken with the BX51 (Olympus) at an optical zoom of 400x. (n=8)
Figure 6.
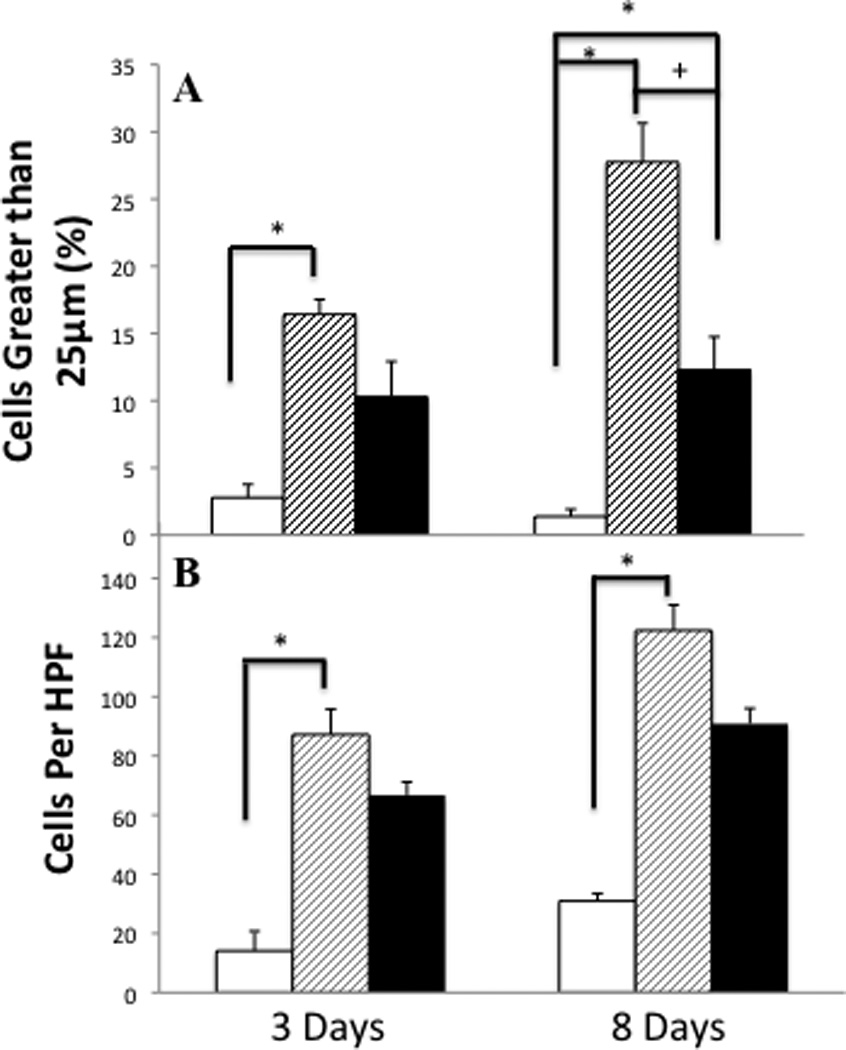
Activity of leukocytes post ITB and the effect of tocopherol diet. BALF was collected in mice from the PBS/AIN93M group (white bars), ITB/AIN93M group (dashed bars) and ITB/0.3%γTmT (closed bars). Identification of cells in the BALF greater than 25 microns reveals a population of activated cells (A). Measured in 5 different fields, at an optical zoom of 400x, cells were grouped based on diameter size. The number of cells >25µm is expressed as a percent of total cells counted in the BAL. The chemotactic potential (B) of the BALF is measured by the migration of RAW 264.7 cells in response to BALF. The chemotactic potential is expressed as the mean number of migrated cells per high power field (HPF).
Chemotactic activity of the BALF can be assessed as a means of measuring the pro-inflammatory nature. BALF from ITB treated mice displayed a significantly greater chemotactic potential than that from saline treated animals (Figure 6B). The bleomycin effect was greater in mice fed the control diet than in those fed tocopherol diet. The BAL chemotactic potential was increased in bleomycin treated mice both 3 and 8 days post injury. At both time points, bleomycin increased the chemotactic potential in tocopherol fed mice, however, this increase was significantly less than that seen in mice fed control diet.
To measure the effect of bleomycin injury on the cells of the lung lining we examined the BAL cell pellet for expression of certain key cytokines/enzymes by rt-PCR (Table 4). As one would predict, bleomycin treatment increased expression of a range of inflammation related mRNAs 3 days post injury. In general, expression was lower in mice fed a tocopherol diet. NOS2 expression is not detectable within mice treated with saline, however, it reaches significant levels post ITB. The ratio of ARG1 to NOS2 expression is a marker of classical vs alternative activation [16]. The ARG1:NOS2 ratio in mice fed the control diet was 500 while in those fed tocopherol it was 1000, indicating a bias towards alternative activation. At 8 days post injury all markers, with the exception of YM1, remain higher in bleomycin treated vs saline control mice. However, there is a shift in expression with the tocopherol diet. NOS2, PTGS2, IL1B, which are all markers associated with classical activation [17; 18] are more strongly induced in mice fed the control rather than the tocopherol diet. However, ARG1, YM1, RELM-a, and CCL2 are all more strongly induced in tocopherol fed mice, indicating a bias towards alternative activation. Indeed the ARG1:NOS2 ratio is ten fold higher in tocopherol fed mice compared to control diet following bleomycin treatment (125 vs 13).
Table 4.
Inflammatory gene expression within BALF cells. Results were normalized to β-actin and expressed as fold increase compared to PBS/AIN93M at respective time point by the 2−ΔΔCt method. NOS2 is undetectable at 3 days in PBS/AIN93M therefore data is expressed as fold increase compared to ITB/AIN93M.
| Treatment Group |
Days Post Instillation |
NOS2 | PTGS2 | IL1B | CCL2 | ARG1 | Ym1 | RELM-α |
|---|---|---|---|---|---|---|---|---|
| ITB/ AIN93M | 3 | 1 | 17.95 | 31.35 | 66.83 | 465.74 | 1.06 | 14.53 |
| ITB/ 0.3%γTmT | 3 | 0.30 | 10.56 | 14.41 | 40.45 | 275.03 | 0.44 | 12.49 |
| ITB/ AIN93M | 8 | 1 | 12.35 | 12.57 | 5.22 | 170.91 | 0.39 | 122.93 |
| ITB/ 0.3%γTmT | 8 | 0.12 | 5.41 | 5.06 | 9.29 | 192.9 | 0.53 | 290.77 |
As the tocopherol diet resulted in a shift in the ratio of ARG1 to NOS2 expression, and as tocopherol can act as a scavenger of NO metabolites, we examined the BAL for NO metabolite content (Figure 7 A & B). Bleomycin instillation caused a significant increase in both nitrite and nitrate levels within the BAL at 3 and 8 days post injury. In mice fed the tocopherol diet there was no significant increase in either metabolite post bleomycin injury at either day 3 or day 8, indicating either minimal NO production or complete scavenging.
Figure 7.
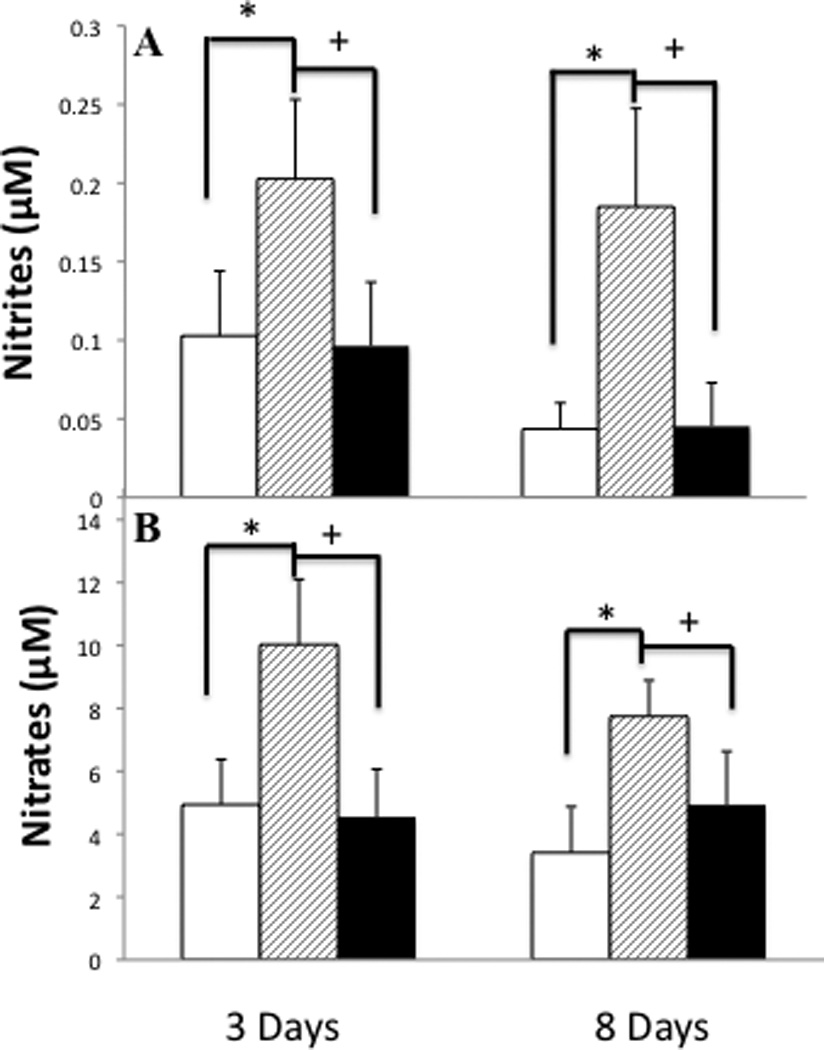
Increases in NO metabolites in response to ITB and the effect of tocopherol diet. Nitrites (A) and nitrates (B), metabolites of nitric oxide, were measured in the BALF supernatant at 3 and 8 days post-ITB. Measurements were made in mice from the PBS/AIN93M group (white bars), ITB/AIN93M group (dashed bars) and ITB/0.3%γTmT (closed bars). * P<0.05 compared to PBS/AIN93M, +<0.05 compared to ITB/AIN93M (p<0.05). (n=8)
3.5 Analysis of Pulmonary-Mechanics
Pulmonary function was assessed using a Forced Oscillation Technique and the resulting elastance and resistance spectra fit to an empirical model. At 3 and 8 days post injury ITB results in a significant increase in the elastance spectra of mice fed the control diet (Figure 8). Component analysis reveals that the change in spectrum at day 3 results from a significant increase in ΔE, the frequency dependent change in elastance (Figure 8D). At 8 days post injury there is an increase both in ΔE and the inherent tissue elastance (E0) (Figure 8C). In mice fed the tocopherol diet there was no change in pulmonary elastance 3 days post bleomycin administration, however, at 8 days post there was a significant increase in both ΔE and E0 that was similar to that seen in animals fed the control diet.
Figure 8.
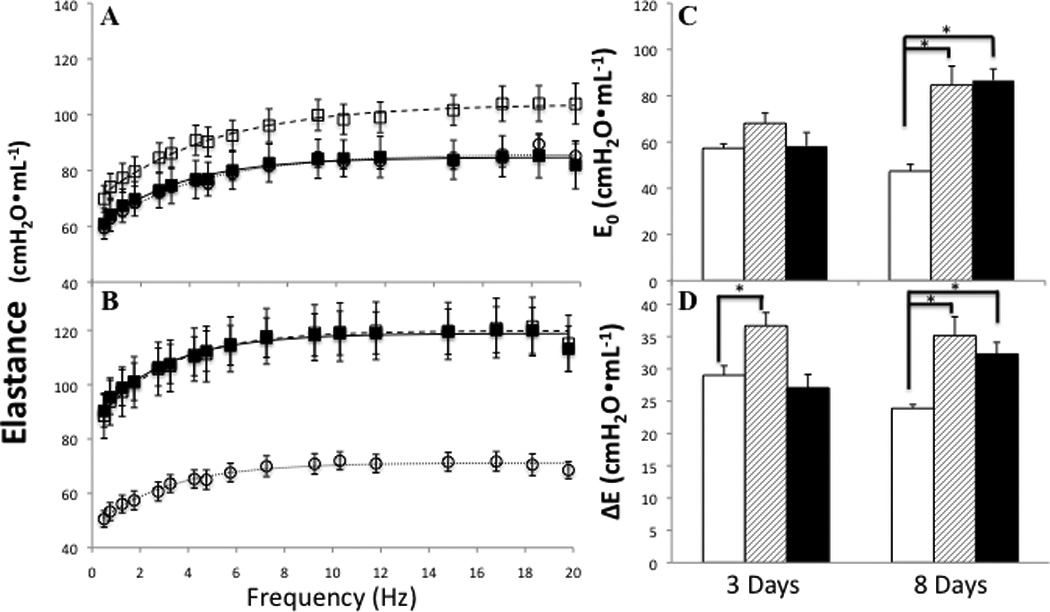
Tocopherol effect on pulmonary elastance changes in response to ITB. Elastance spectra 3 days (A) and 8 days (B) post instillation in PBS/AIN93M (○), ITB/AIN93M (□), and ITB/0.3%γTmT (■). Parameters of elastance highlight changes in tissue and airway function; E0 elastance at the static limit (C) and ΔE the change in elastance with change in frequency ΔE (D); PBS/AIN93M (open bars), ITB/AIN93M (dashed bars) and ITB/0.3%γTmT (closed bars). * P<0.05 compared to PBS/AIN93M.
Despite the significant increase in elastance spectra with bleomycin administration, there was no significant change observed in the resistance spectra at either 3 or 8 days post injury (Figure 9 A & B). However, component analysis demonstrates that, while there is no change in high frequency resistance (b, Figure 9D), there is a significant increase in low frequency resistance 8 days post injury (a/c, Figure 9C). This increase in low frequency, or tissue, resistance is not abrogated by the tocopherol diet.
Figure 9.
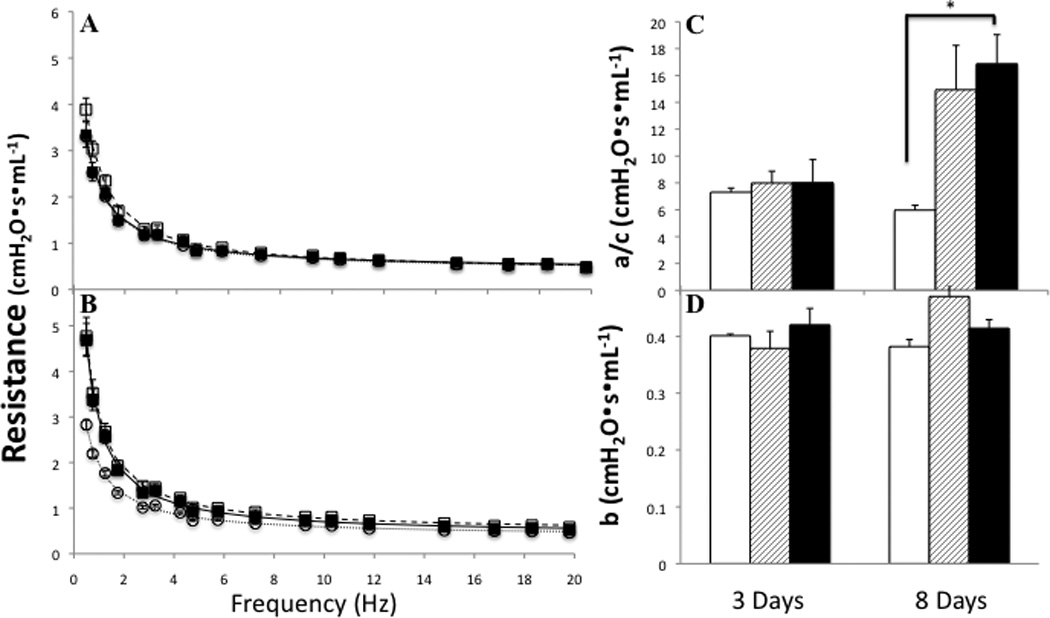
Effect of ITB on pulmonary resistance. Resistance spectra was obtained in conjunction with elastance spectra. Resistance is also shown at a PEEP of 3cm H2O is shown at 3 (A) and 8 (B) days post treatment. PBS/AIN93M (○), ITB/AIN93M (□), and ITB/0.3%γTmT (■). a/c low frequency resistance (C) and b high frequency asymptote (D); PBS/AIN93M (open bars), ITB/AIN93M (dashed bars) and ITB/0.3%γTmT (closed bars). * statistically different from PBS/AIN93M (p<0.05).
4. Discussion
The goal of this study was to examine whether dietary supplementation with tocopherols was capable of reducing bleomycin-mediated lung injury and inflammation. Here we have shown that α, δ and γ-tocopherol levels are increased in both the lung and serum by dietary supplementation. ITB administration produces a significant pulmonary injury with accompanying early neutrophilia and later inflammatory changes including macrophage infiltration and activation. These changes are accompanied by a significant increase in NO metabolites and reduced lung function, demonstrated by an increase in inherent and frequency dependent elastance. Tocopherol supplementation, while not appearing to reduce the direct ITB-mediated injury, completely abrogated the increase in NO metabolites, altered the kinetics of inflammation and macrophage activation, while significantly reducing the acute loss of lung function.
Bleomycin is a redox cycling compound that intercalates into DNA, thus it is capable of generating high concentrations of ROS within the nucleus of cells, producing injury and death [19]. As tocopherols can act as ROS scavengers, it is conceivable that by increasing the tissue concentration of these compounds one may reduce ITB mediated lung injury. This appears not to be the case in the present study, as the early accumulation of protein within the BALF (Table 3) and the injury/inflammation score at day 3 (Figure 1) are not altered by tocopherol supplementation. That the injury process itself is not altered by tocopherol is further confirmed by the observation that neutrophilic invasion, a direct correlate of injury, is not altered (Figure 5). As only 10% of intracellular tocopherol is found in the nucleus [20] it is not surprising that supplementation is unable to abrogate ITB-mediated injury. However, tocopherols do operate as efficient scavengers of RONS [8; 9] generated within ITB as there is no significant increase in either nitrite or nitrate in the presence of tocopherol. It is possible that this reduction in NO metabolites results from reduced iNOS activity, as its increase in expression is inhibited by tocopherol supplementation (Figure 3, Table 4). However, there is still increased expression over saline administered mice and there is no increase in metabolites, which does suggest that the tocopherols have acted as efficient scavengers.
From the present data it is clear that tocopherol supplementation has altered both the time course and the pathway of inflammatory activation following ITB. Despite the fact that injury scores at day 3 are unaltered by tocopherol, it is clear that there is a reduction in inflammation/injury at day 8 (Figure 1). While total cellular invasion of the lung is not significantly reduced 8 days post ITB (Table 3, Figure 5); there is a significant reduction in the lymphocytic and eosinophilic content of these recruited cells (Figure 5). Also at this later time point there is a significant reduction in the size of the macrophages within the BAL, indicating an altered activation state (Figure 6A).
This potential change in activation is further confirmed by examination of RNA expression within BAL cells. Three days post ITB, a range of macrophage activation markers are increased in expression. Tocopherol supplementation reduces this ITB-mediated increase (Table 4). However, this reduction seems to be greatest in the classical activation pathway as reflected by the higher ARG1:NOS2 ratio [16] within tocopherol fed as opposed to control diet mice. At 8 days post ITB, while classical M1 activation markers such as NOS2, PTGS2 & IL1B remain reduced with tocopherol treatment, a number of alternative M2 markers, namely ARG1, YM1, RELM-α & CCL2, are expressed at higher level with supplementation. These data imply that the inflammatory response is altered such that the acute phase is reduced with tocopherol supplementation, but that the later repair signaling pathways may be enhanced. Such an observation raises the possibility that the fibrotic response may actually be increased by tocopherol supplementation. Further studies at later time points, such as 21 days post ITB, would need to be examined to address this possibility.
This shift in inflammatory signaling is further emphasized by the immunohistochemistry. Both iNOS and COX2 are significantly increased in their expression in response to ITB (Figure 2 & 3); however, this increase is modulated by tocopherol supplementation. This difference is most obvious at days post administration, evidenced by increases in iNOS and COX2 expression increase between day 3 and day 8 in control fed ITB treated mice. Such an increase is not seen with tocopherol supplementation. In contrast, Ym-1, which is only expressed in inflammatory cells in the lung [21] and is often associated with alternative activation [22], remains elevated in these cells in both tocopherol supplemented and control diet mice (Figure 4).
Using forced oscillation measurements we examined how the observed inflammatory changes relate to both tissue and airway components of lung function. These data are often analyzed using a constant phase model [23], however this model can sometimes fail to adequately fit in heterogeneous lung injury [24; 25]. ITB is inherently patchy as an injury mechanism as seen in the whole lung sections (Figure 1). Furthermore, that there was significant heterogeneity of response was confirmed by significant alterations in the ERS spectra with minimal change in RRS at three days post ITB (Figure 8A & 9A) [26]. At three days post ITB there is no significant alteration in the resistance spectra either with or without tocopherol supplementation. However, there is a clear increase in lung elastance with ITB-treated control diet mice (Figure 8A). Although, the E0 value appears higher with ITB-treatment this change did not reach statistical significance (Figure 8C). These data indicate that despite the increase in cell number and protein content in the BAL observed with ITB-treatment there was not a significant change in the tissue stiffness. Of note, tocopherol supplementation did not alter cell number, protein content, or injury score at 3 days post ITB and showed no change in E0 when compared to saline treated animals. The parameter ΔE, which represents the frequency dependent change in elastance, was significantly raised in only ITB-treated animals fed the control diet (Figure 8D). In conjunction with the lack of change in resistance and tissue elastance, this observation can best be explained by an increase in airway stiffness in these mice. Observation of the histology does show significant airway thickening (Figure 1), which may explain the change in ΔE.
Eight days post-ITB there is a considerable shift in the lung function response. ITB produces a significant increase in E0 and the low frequency component of the resistance spectrum, a/c (Figure 9D). There is no further increase in ΔE, although it is still significantly higher than in saline-treated mice (Figure 8D). These data imply that the bulk of the functional change that occurs with ITB as the inflammatory process progresses from day 3 to day 8 occurs at the tissue level. Indeed, the protein content within the BAL doubles over this time period as does the number of cells within the lung lining (Table 3). It is reasonable to suppose that the wetter lung with more observable consolidation (Figure 1) 8 days post ITB is what leads to this increased parenchymal stiffness and resistance. In this regard, it is interesting that tocopherol supplementation does not reduce the protein content or the cell number within the BAL, when compared to control diet fed mice (Table 3); and that a/c and E0 are significantly increased in these mice. Thus although tocopherol diet alters the inflammatory signaling resulting from ITB, it does not reduce the functional changes that occur within the parenchyma 8 days post injury. It will be interesting to observe how these parameters change at later time points in this injury response.
In conclusion, we have demonstrated that the lung tocopherol content can be successfully increased by diet, and that this supplementation can efficiently scavenge RONS generated by the ITB-mediated inflammatory process. This scavenging appears to reduce acute inflammation and to induce a shift towards alternative macrophage activation. These studies show that dietary supplementation with tocopherols can effectively reduce oxidative stress in pulmonary inflammation. Although, we have demonstrated that acutely tocopherol supplementation reduces the functional deficit that occurs as a result of ITB, we do not know how the resulting signaling change affects resolution and long-term fibrosis in this model. Finally, through the use of an empirical lung functional model we have been able to relate observable inflammatory changes with alterations in airway and tissue function.
Highlights.
Dietary supplementation with tocopherol increases the concentration in the lung.
Tocopherol blocks the bleomycin-dependent increase of NO metabolites.
Tocopherol reduces bleomycin-stimulated inflammation.
Bleomycin-dependent loss of lung mechanical function due is reduced by tocopherol.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hay J, Shahzeidi S, Laurent G. Mechanisms of bleomycin-induced lung damage. Arch Toxicol. 1991;65:81–94. doi: 10.1007/BF02034932. [DOI] [PubMed] [Google Scholar]
- 2.Santos-Silva MA, Pires KM, Trajano ET, Martins V, Nesi RT, Benjamin CF, Caetano MS, Sternberg C, Machado MN, Zin WA, Valenca SS, Porto LC. Redox Imbalance and Pulmonary Function in Bleomycin-Induced Fibrosis in C57BL/6, DBA/2, and BALB/c Mice. Toxicol Pathol. 2012 doi: 10.1177/0192623312441404. [DOI] [PubMed] [Google Scholar]
- 3.Togeiro SM, Carneiro G, Filho FF, Zanella MT, Santos-Silva R, Taddei JA, Bittencourt LR, Tufik S. Consequences of Obstructive Sleep Apnea on metabolic profile: a population-based survey. Obesity (Silver Spring) 2012 doi: 10.1002/oby.20288. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka K, Ishihara T, Azuma A, Kudoh S, Ebina M, Nukiwa T, Sugiyama Y, Tasaka Y, Namba T, Sato K, Mizushima Y, Mizushima T. Therapeutic effect of lecithinized superoxide dismutase on bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2010;298:L348–L360. doi: 10.1152/ajplung.00289.2009. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka K, Tanaka Y, Namba T, Azuma A, Mizushima T. Heat shock protein 70 protects against bleomycin-induced pulmonary fibrosis in mice. Biochem Pharmacol. 2010;80:920–931. doi: 10.1016/j.bcp.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 6.Mert H, Yoruk I, Ertekin A, Dede S, Deger Y, Yur F, Mert N. Vitamin levels in lung tissue of rats with bleomycin induced pulmonary fibrosis. J Nutr Sci Vitaminol (Tokyo) 2009;55:186–190. doi: 10.3177/jnsv.55.186. [DOI] [PubMed] [Google Scholar]
- 7.Suntres ZE, Shek PN. Protective effect of liposomal alpha-tocopherol against bleomycin-induced lung injury. Biomed Environ Sci. 1997;10:47–59. [PubMed] [Google Scholar]
- 8.Campbell S, Stone W, Whaley S, Krishnan K. Development of gamma (gamma)-tocopherol as a colorectal cancer chemopreventive agent. Crit Rev Oncol Hematol. 2003;47:249–259. doi: 10.1016/s1040-8428(03)00042-8. [DOI] [PubMed] [Google Scholar]
- 9.Ju J, Picinich SC, Yang Y, Zhao Z, Suh N, Kong AN, Yang CS. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis. 2010;31:533–542. doi: 10.1093/carcin/bgp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genovese T, Cuzzocrea S, Di Paola R, Failla M, Mazzon E, Sortino MA, Frasca G, Gili E, Crimi N, Caputi AP, Vancheri C. Inhibition or knock out of inducible nitric oxide synthase result in resistance to bleomycin-induced lung injury. Respir Res. 2005;6:58. doi: 10.1186/1465-9921-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kristof AS, Goldberg V, Laubach P, Hussain SN. Role of inducible nitric oxide synthase in endotoxin-induced acute lung injury. Am J Respir Crit Care Med. 1998;158:1883–1889. doi: 10.1164/ajrccm.158.6.9802100. [DOI] [PubMed] [Google Scholar]
- 12.Atochina-Vasserman EN, Beers MF, Kadire H, Tomer Y, Inch A, Scott P, Guo CJ, Gow AJ. Selective inhibition of inducible NO synthase activity in vivo reverses inflammatory abnormalities in surfactant protein D-deficient mice. J Immunol. 2007;179:8090–8097. doi: 10.4049/jimmunol.179.12.8090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reeves PG, Nielsen FH, Fahey GC., Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 14.Guan F, Li G, Liu AB, Lee MJ, Yang Z, Chen YK, Lin Y, Shih W, Yang CS. delta- and gamma-tocopherols, but not alpha-tocopherol, inhibit colon carcinogenesis in azoxymethane-treated F344 rats. Cancer Prev Res (Phila) 2012;5:644–654. doi: 10.1158/1940-6207.CAPR-11-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudmann DG, Preston AM, Moore MW, Beck JM. Susceptibility to Pneumocystis carinii in mice is dependent on simultaneous deletion of IFN-gamma and type 1 and 2 TNF receptor genes. J Immunol. 1998;161:360–366. [PubMed] [Google Scholar]
- 16.Menzies FM, Henriquez FL, Alexander J, Roberts CW. Sequential expression of macrophage anti-microbial/inflammatory and wound healing markers following innate, alternative and classical activation. Clin Exp Immunol. 2010;160:369–379. doi: 10.1111/j.1365-2249.2009.04086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 19.Hecht SM. Bleomycin: new perspectives on the mechanism of action. J Nat Prod. 2000;63:158–168. doi: 10.1021/np990549f. [DOI] [PubMed] [Google Scholar]
- 20.McVean M, Liebler DC. Prevention of DNA photodamage by vitamin E compounds and sunscreens: roles of ultraviolet absorbance and cellular uptake. Mol Carcinog. 1999;24:169–176. doi: 10.1002/(sici)1098-2744(199903)24:3<169::aid-mc3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 21.Hung SI, Chang AC, Kato I, Chang NC. Transient expression of Ym1, a heparin-binding lectin, during developmental hematopoiesis and inflammation. J Leukoc Biol. 2002;72:72–82. [PubMed] [Google Scholar]
- 22.Raes G, De Baetselier P, Noel W, Beschin A, Brombacher F, Hassanzadeh Gh G. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J Leukoc Biol. 2002;71:597–602. [PubMed] [Google Scholar]
- 23.Hantos Z, Daroczy B, Csendes T, Suki B, Nagy S. Modeling of low-frequency pulmonary impedance in dogs. J Appl Physiol. 1990;68:849–860. doi: 10.1152/jappl.1990.68.3.849. [DOI] [PubMed] [Google Scholar]
- 24.Kaczka DW, Hager DN, Hawley ML, Simon BA. Quantifying mechanical heterogeneity in canine acute lung injury: impact of mean airway pressure. Anesthesiology. 2005;103:306–317. doi: 10.1097/00000542-200508000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Ito S, Ingenito EP, Arold SP, Parameswaran H, Tgavalekos NT, Lutchen KR, Suki B. Tissue heterogeneity in the mouse lung: effects of elastase treatment. J Appl Physiol. 2004;97:204–212. doi: 10.1152/japplphysiol.01246.2003. [DOI] [PubMed] [Google Scholar]
- 26.Bates JHT. Lung Mechanics: An Inverse Modeling Approach. United Kingdom: Cambridge University Press; 2009. [Google Scholar]


