Abstract
Glycosaminoglycans (GAGS) are anionic, linear, polysaccharides involved in cell signaling. The GAG content, composition and structure of human tissue have been suggested to play a role in cancer and might provide useful diagnostic or prognostic markers. The current study examines 17 stomach tissue biopsy samples taken from normal individuals and from patients with gastric cancers. An ultrasensitive liquid chromatography (LC) – mass spectrometry assay was applied to individual biopsy samples as small 250 µg providing GAG content and disaccharide composition. The results of these analyses show a significant increase in non-sulfated chondroitin/dermatan sulfate concentration in all cancer samples when compared to normal tissues. In addition in advanced gastric cancer, a significant decrease is observed in hyaluronan.
Keywords: Stomach cancer, Glycosaminoglycan, Chondroitin sulfate, Hyaluronan, Disaccharide analysis
Introduction
While mortality rates of gastric cancer have fallen in the United States over the past few decades, gastric cancer is still the fourth leading cause of cancer in the world and the second most common cause of cancer related deaths worldwide [1,2]. Although the majority of cases occur in developing nations, the incidence of gastric cancer varies widely from nation to nation, with China, Japan and Korea have been found to have the highest rates of occurrence [1,2]. Gastric cancer is associated with a wide range of risk factors, including obesity, tobacco use, and infection with gram-negative Helicobacter pylori [1].
Over the years many characteristics of gastric tumors have been studied, especially those pertaining to carbohydrate expression levels, which are of particular interest here. Among other things, these studies have analyzed glycosaminoglycan (GAG) structural composition, special patterns of carbohydrate expression, hyaluronan (HA) expression levels, and the expression of carbohydrate receptors such as CD44 [3–7]. Since carbohydrates, and GAGs in particular, are important in cellular signaling processes, many of these studies are designed to uncover links between the metastatic ability or the lethality of gastric cancer and particular carbohydrate-based biomarkers. Especially relevant to this work are studies in the sulfation patterns of GAGs and in the HA expression levels.
Cancer has long been associated with changing carbohydrate structures [8]. However, there is often a lack of consensus in the literature over the levels present and the importance of various sulfation patterns in gastric cancer [4], or a lack of comprehensive data on sulfation patterns. For example, a study on sulfation patterns in gastric cancer combined several tissue samples together to amass enough material for analysis, obscuring information on natural variation of GAG sulfation levels. This study found a higher expression of 6-O-sulfo (6S) group and non-sulfated (0S) GAG disaccharides in gastric cancer [4], while a complementary study found that expression of a 6-O-sulfatase (which removes 6-O-sulfo groups GAGs lowering the 6S disaccharide content) suppresses a Wnt signaling pathway, restricting the tumor’s ability to proliferate and invade [9]. Other studies have focused on HA levels in gastric cancer. These studies have found increased HA levels in tumors [4] and increased HA levels in individual tumor cells [10,11], but subsequent work found no statistical correlation to lymphatic or blood vessel invasion [12], and even varying HA levels within different histological subtypes [13,14]. Many of these studies show over a 100-fold variation in the range of measured HA levels for any particular diagnostic grouping, making it difficult to use this assay (based on a radiolabel) as a diagnostic method [11,12].
In this paper, a simple, direct and ultra-sensitive method of disaccharide analysis was used to measure the heparan sulfate (HS), chondroitin sulfate (CS) and HA levels (Figure 1). This analysis relied on the fluorescent labeling of disaccharides followed by their analysis using liquid chromotography (LC)-mass spectrometry (MS). This provided a limit of detection (LOD) of 0.1 ng of GAG allowing for the analysis of as little as 250 µg of biopsy sample. In this study, 17 stomach cancer samples were analyzed representing four different types of cancer or pre-cancerous tissues of varying histology. These four different types are normal tissue, gastric adenoma, early gastric cancer (EGC) and advanced gastric cancer (AGC). The sensitivity and reproducibility of this method make it a good candidate for the development of future diagnostic and prognostic methods. Differences in the disaccharide composition between the different tissue types may provide signature disaccharide patterns useful for diagnosis.
Figure 1.
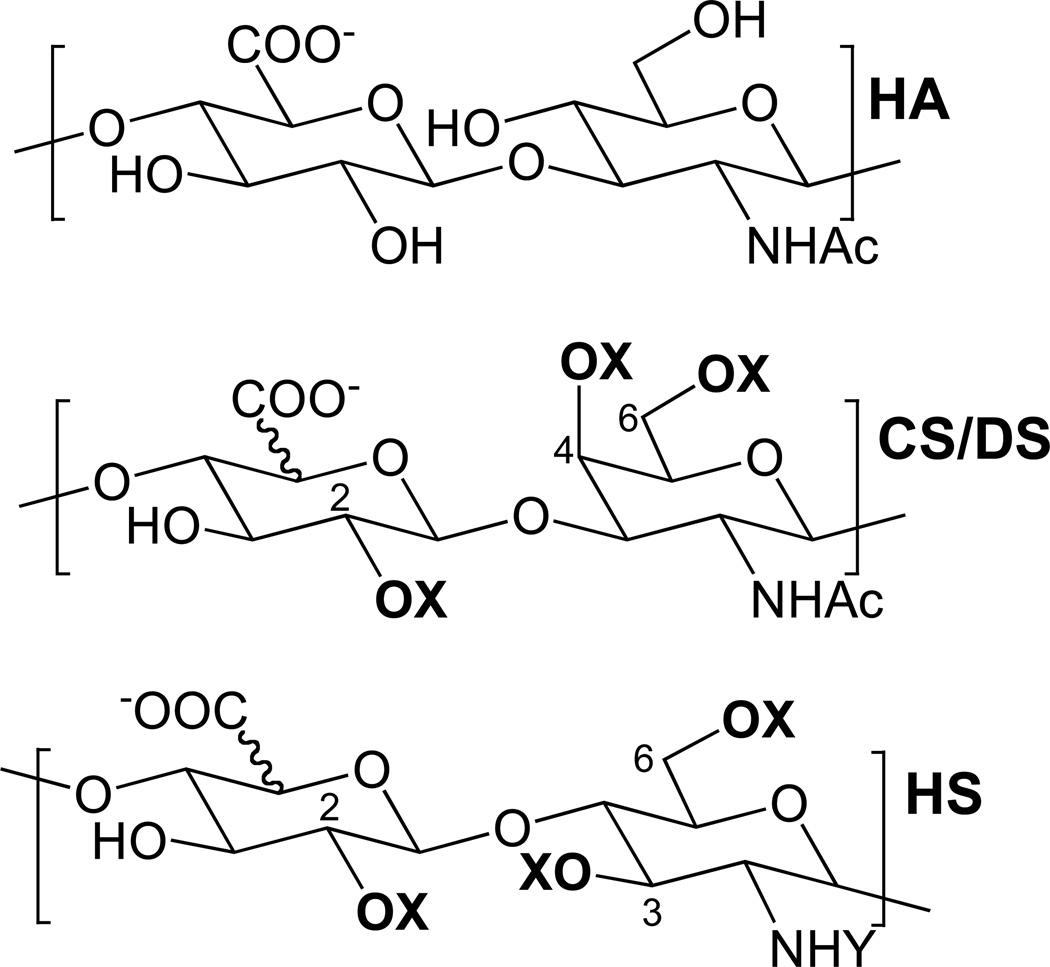
Structures of the GAGs analyzed in this study. Glycosaminoglycans are long, highly charged, linear polysaccharides comprised of repeating disaccharide units. HA, hyaluronan [→4)GlcA(1→3)GlcNAc(1→]; CS, chondroitin sulfate [→4)GlcA2S/OH(1→3)GalNAc4S/OH6S/OH(1→]; DS, dermatan sulfate [→4)IdoA2S/OH(1→3)GalNAc4S/OH6S/OH(1→]; and HS, heparan sulfate [→4)GlcA/IdoA2S/OH(1→4)GlclNAc/S/3S/OH6S/OH(1→]. Potential sulfo group locations are highlighted in bold; X = H or SO3−; Y = COCH3 or SO3−, or H. The numbers on the HS and CS/DS structures indicate carbons where O-sulfo groups can be found.
Materials and methods
Tissue samples
Stomach tissue samples collected from each of 14 different patients were obtained at Konyang University Hospital Daejeon, South Korea. Biopsy samples were taken from separate patients, except for two patients who gave both normal and advanced gastric cancer (AGC) samples (samples 4 & 16, 5 & 17, respectively). Gastric adenoma tissue samples were obtained from endoscopic submucosal dissection and all other malignant tissue samples were obtained surgically from gastrectomy. Normal tissue samples were obtained as distal as possible from tumor mass at gastrectomy. Samples were stored in liquid nitrogen before use. The clinicopathologic data were collected by reviewing medical charts and pathologic records. All patients provided written informed consent before the collection of gastric tissues samples. The collection and use of the samples were approved by the Institutional Review Board of the Konyang University Daejeon Hospital.
Reagents
Amicon Ultra centrifugal filters (10 kDa and 30 kDa) were from Millipore (Billerica, MA). Actinase E was from Kaken Biochemicals (Tokyo, Japan). Heparin was from Celsus Laboratories (Cincinnati, OH). Vivapure Mini Q-H colums were from Sartorius Stedium Biotech (Goettingen, Germany). Hexylamine and hexafluoroisopropanol were from Sigma Chemical Company (St. Louis, MO). CS and DS unsaturated disaccharides standards, including C-0S, ΔUA(1→3)GalNAc; C-4S, ΔUA(1→3)GalNAc4S; C-6S, ΔUA(1→3)GalNAc6S; C-2S, ΔUA2S(1→3)GalNAc; C-2S4S or C-SB, ΔUA2S(1→3)GalNAc4S; C-2S6S or C-SD, ΔUA2S(1→3)GalNAc6S; C-4S6S or C-SE, ΔUA(1→3)GalNAc4S6S; and C-2S4S6S or C-TriS, ΔUA2S(1→3)GalNAc4S6S, and chondroitin lyases ABC and ACII were purchased from Associates of Cape Cod, Inc. (East Falmouth, MA). HS unsaturated disaccharides standards, H-0S, ΔUA(1→4)GlcNAc; H-NS, ΔUA(1→4)GlcNS; H-6S, ΔUA(1→4)GlcNAc6S; H-2S, ΔUA2S(1→4)GlcNAc; H-2SNS, ΔUA2S(1→4)GlcNS; H-NS6S, ΔUA(1→4)GlcNS6S; H-2S6S, ΔUA2S(1→4)GlcNAc6S; and H-TriS, ΔUA2S(1→4)GlcNS6S, and HA unsaturated disaccharide standard, HA-OS, ΔUA(1→3)GlcNAc) were from Iduron (Manchester, UK). Cloning, E. coli expression, and purification of the recombinant heparin lyase I (EC 4.2.2.7), heparin lyase II (no EC assigned), and heparin lyase III (EC 4.2.2.8) from F. heparinum were performed in our laboratory as described [15–17]. All other chemicals were of reagent grade.
Carbohydrate isolation
Two years after biopsy, the tissues were freeze-dried and transferred from Konyang Hospital in Korea to Rensselaer for glycan analysis. Lyophilized tissue samples were weighed and individually proteolyzed using Actinase E (55 °C, 800 µL of 2 mg/mL aqueous solution) for two days.
GAGs were next isolated using Vivapure Mini Q-H spin columns. Each spin column was first equilibrated with a urea 3-[(3-drolamidopropyl) dimethylammonio]-1-propane sulfate (CHAPS) solution (2 wt.% CHAPS, 8 M urea). CHAPS and urea were added to each tissue isolate solution to afford a final concentration of 2 wt.% CHAPS and 8 M urea. Undigested particulates were removed from these solutions by centrifugation (5000 × g, 30 min), and the resulting tissue solutions were then loaded on the Vivapure spin columns (500 × g). Spin columns were washed with three column volumes of 200 mM NaCl to remove impurities. GAGs were released from the spin columns using two washes of 16% NaCl (0.5 column volumes). Methanol (80 % total volume) was added to the released GAG solutions, precipitating the GAGs overnight at 4 °C. Precipitated GAGs were recovered by centrifugation (5000 × g, 30 min) and the isolated GAG precipitate was then dissolved in doubly distilled water and analyzed.
LC-MS disaccharide analysis
The isolated GAGs were subject to enzymatic depolymerization. GAGs (20 µg) were digested with chondrotinase ABC (10 mU) and ACII (5 mU) at 37 °C for 10 h and the resulting dissacharide products were isolated by centrifugal filtration (30 kDa molecular weight cut-off (MWCO)), freeze-dried, and were ready for LC-MS analysis. Centrifugal filters having various MWCO values were tested for disaccharide recovery without loss of GAG chains. The use of a 30 kDa MWCO membrane resulted in the nearly quantitative recovery od disaccharide products of polysaccharide lyase-treatment of GAGs obtained from cultered cells without the loss of GAG chains of MW≥1,200 [18,19]. The remaining undigested GAGs were then incubated with heparinases I, II and III at 37 °C for 10 h. The resulting disaccharide products were similarly isolated (30 kDa filter), freeze-dried, and subject to LC-MS analysis [20]. Fluoresecent labeling of disaccharide was next undertaken to ensure highly sensitive detection and to eliminate potential complications associated with UV absorbing impurities [18] coming from the biopsy tissues being analyzed. The freeze-dried sample containing the GAG-derived disaccharides (~2 µg) was added 10 µL a 0.1 M 2-aminoacridone (AMAC) solution in acetic acid dimethyl sulfoxide (3:17, v/v) and mixed by vortexing for 5 min. Next, 10 µL of 1 M NaBH3CN was added in the reaction mixture and incubated at 45°C for 4 h [21]. LC-MS analyses were performed on an Agilent 1200 LC/MSD instrument (Agilent Technologies, Inc. Wilmington, DE) equipped with a 6300 ion-trap and a binary pump followed by a UV detector equipped with a high-pressure cell. The column used was a poroshell120 C18 column (2.1 × 150 mm, 2.6 µm, Agilent) at 45°C. Solution A was 80 mM ammonium acetate solution and Solution B was methanol. Solution A and 15% solution B was flowed (150 µL/min) through the column for 5 min followed by a linear gradient of 15–30% solution B from 5 to 30 min.
The column effluent entered the electrospray ionization (ESI)-MS source for continuous detection by MS. The electrospray interface was set in negative ionization mode with a skimmer potential of −40.0 V, a capillary exit of −40.0 V, and a source temperature of 350 °C, to obtain the maximum abundance of the ions in a full-scan spectrum (150–1200 Da). Nitrogen (8 L/min, 40 psi) was used as a drying and nebulizing gas [22].
Statistical analysis of data
All statistical calculations were done using IBM SPSS Statistics 20. Comparisons of disaccharide content amongst the four tissue types (normal, gastric adenoma, early gastric cancer, advanced gastric cancer), were done using a one-way Welch ANOVA (analysis of variance) followed by the post-hoc Dunnett’s T3 multiple pairwise test for unequal variance.
Results and discussion
The CS, HS, and HA content of the stomach cancer samples were analyzed using LC-MS to quantify the disaccharide composition and content of each type of GAG. The total quantity of each disaccharide type was then calculated based on a standard curve for each disaccharide [22] and the mole percentage of each were compared across the different biopsy samples.
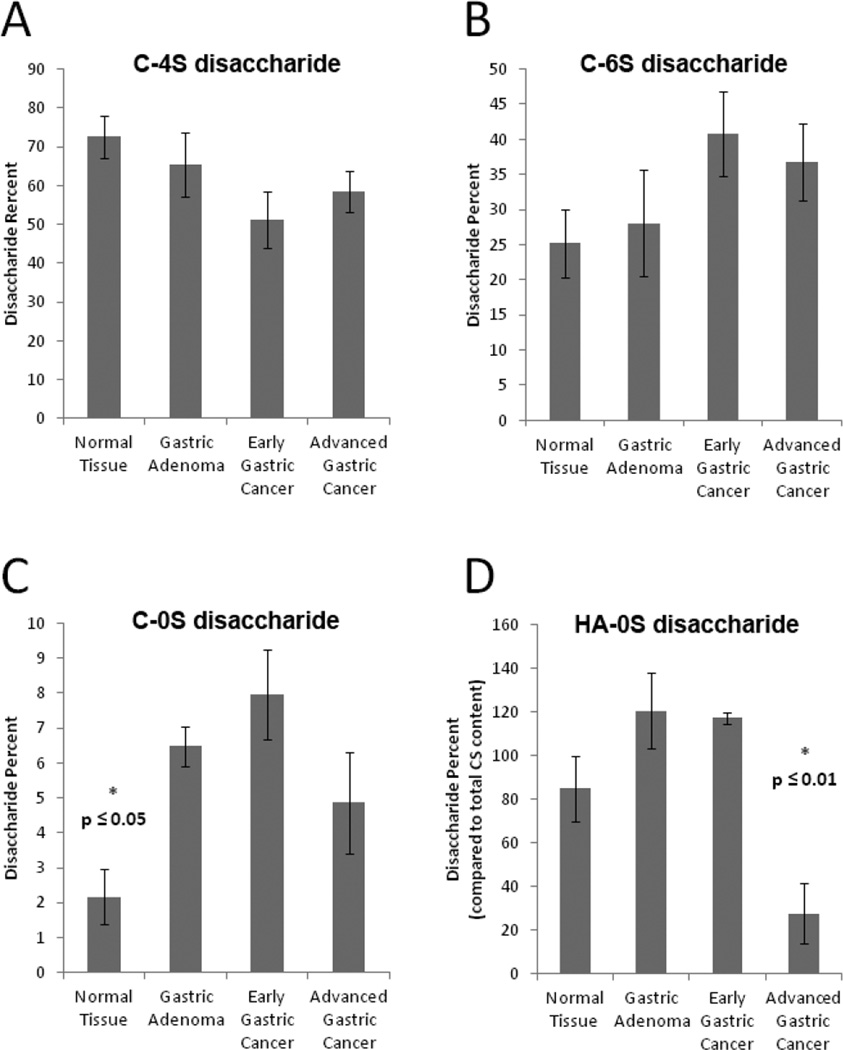
There was no notable difference between the different tissue types in the average content of CS, HS and HA. The average total content of CS, HS and HA was 2-µg mg−1 of freeze-dried tissue sample. Next, the HS disaccharide composition was compared and no apparent trends or major differences were observed across the four tissue types (Figures 2 and 3). In contrast there were several significant differences in the CS disaccharide composition of the four tissue types (Figure 4A–C). There were decreases in the average level of 4-sulfo group substitution of the tissue samples, and elevations in the average level 6-sulfo group substitution in the three cancer samples, particularly the two most developed cancer tissue types, EGC and AGC. However, these differences were not significant. There were, however, differences in the levels of HA-OS and C-OS disaccharides found in the tissue samples analyzed (Figure 4D). Specifically, the HA levels of the most developed cancer tissue type, AGC showed a significantly lower amount of HA than all other biopsy samples. Normal, non-cancerous tissue showed a significantly lower content of non-sulfated disaccharide than the cancerous tissue samples.
Figure 2.
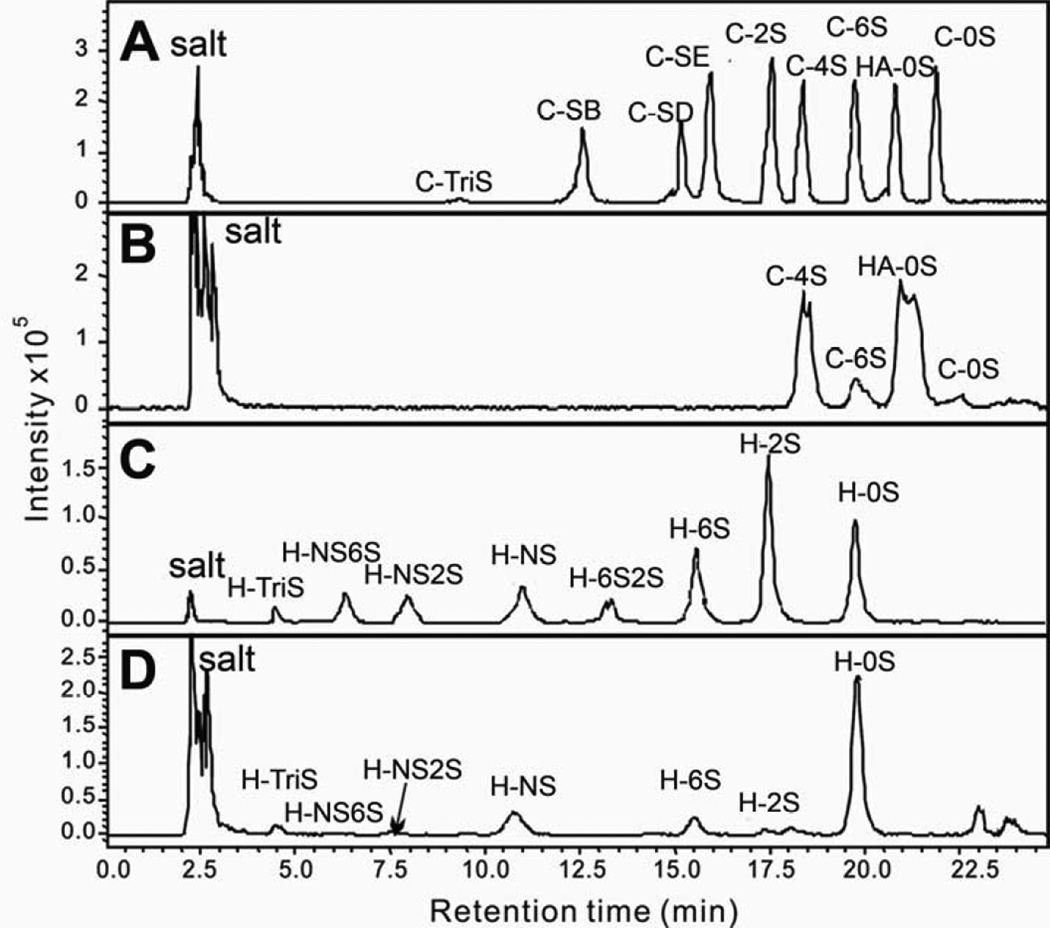
LCMS separation and quantification of HA, CS and HS disaccharides. Shown are (A) CS/DS and HA disaccharide standards; (B) CS/DS and HA disaccharides isolated from sample #6, a typical gastric adenoma tissue sample; (C) HS disaccharide standards; (D) HS disaccharides isolated from sample #6, a typical gastric adenoma tissue sample.
Figure 3.
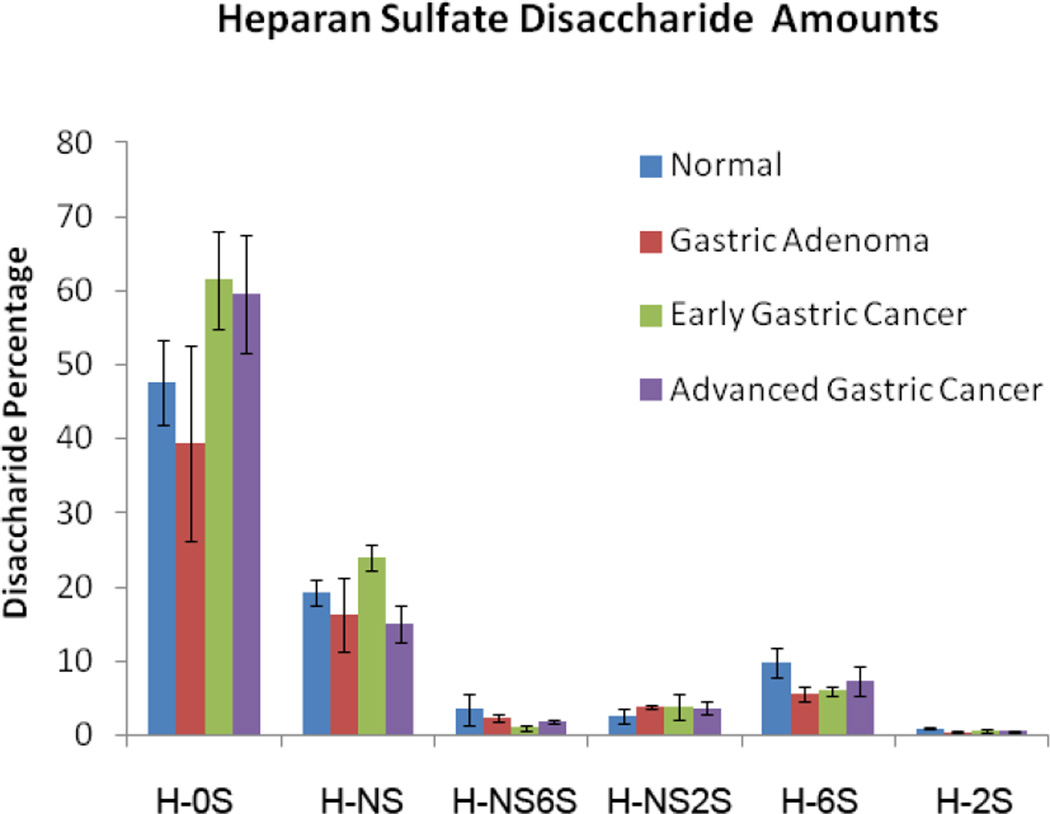
Measured levels of HS disaccharides in GAGs present in stomach tissues. The HS disaccharide amounts, calculated by comparison to HS disaccharide standards, were normalized to the dry tissue weight of each sample. No significant differences were seen between the different stomach tissue types.
Figure 4.
Measured levels of CS disaccharides in GAGs present in stomach tissues. (A) C-4S disaccharide obtained from the different tissue samples; (B) C-6S disaccharide obtained from the different tissue samples; (C) C-0S disaccharide obtained from the different tissue samples; (D) HA-0S disaccharide obtained from the different tissue samples The CS disaccharide amounts, calculated by comparison to CS disaccharide standards, were also normalized to the dry tissue weight of each sample. No significant differences were observed in different stomach tissue types for content of 4S and 6S disaccharides. Note that the C-4S + C-6S + C-0S = 100 for each tissue sample. The HA-0S disaccharide is presented relative to the total CS found in each sample. Significant differences were observed in both non-sulfated chondroitin (C-0S) and hyaluronan (HA-0S) disaccharides.
The impact of GAG content, disaccharide content sulfation patterns, and GAG sequence on signaling in cancerous tissue is still an open question. The current study on stomach cancer tissue shows that HA content is higher in AGC. HA has been suggested to play an important role in the metastasis of cancerous cells based on its linkage to cell mobility in embryonic tissue [14]. Similarly, in cancer HA may support cell migration signaling and provide the cells with a coating of a GAG with low protein binding affinity, a role played by HA in wound healing [14, 23].
Decreases in HA levels in the most severe type of gastric cancer tissue measured, AGC, must be interpreted with caution. While many studies have found links between HA expression and high metastasis, other studies have shown that HA levels are relatively specific to the tissue of origin, and are spatially dependent (different levels at different places on the tumor) [13,14]. Specifically, Wang and coworkers found little to no HA expression within the body of the tumor in a set of 10 stomach cancer samples, finding that HA was limited to the outer stromal layer [14]. The differences observed here may in some ways be a reflection of the tumor morphology – in the more developed, cohesive AGC tumors, the overall HA content decreases as a larger percentage of the tissue mass is the HA deficient tumor body.
The significantly decreased levels of undersulfated CS, rich in C-OS disaccharide, observed in the stomach cancer samples in the current study, is entirely consistent with other observations of lowered sulfation levels in cancerous tissues [24–26], including gastric cancers [4]. The shift in cancerous tissues to a lower sulfation pattern may reflect a larger metabolic shift in enzyme expression levels and biosynthetic pathways that have yet to be determined. Of note here is the increase in undersulfated CS tissue in all pre-cancerous and early cancerous tissue, indicating that these tissues already share significant metabolic patterns with advanced tumors.
Although the results of this study do not provide disaccharide composition patterns that could provide significant diagnostic or prognostic matrices, they do shed light on the changing metabolism and GAG expression of cancerous and pre-cancerous tissue. In addition the current study provides a sensitive analytical method with an LOD of 0.1 ng GAG capable of providing GAG composition on very small (250 µg) biopsy samples.
Table 1.
Stomach tissue sample descriptions.
| Case | Tissue Type |
Tissue Dry Weight (mg) |
Sex | Age | Tumor Size |
Lymph Nodes |
Metastasis | Prognosisa | Histologyb |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Normal | 0.694 | M | 65 | |||||
| 2 | Normal | 0.693 | F | 63 | |||||
| 3 | Normal | 1.53 | M | 72 | |||||
| 4 | Normal | 45.0 | M | 35 | |||||
| 5 | Normal | 59.0 | M | 43 | |||||
| 6 | Gastric adenoma | 1.56 | F | 72 | Tubular adenoma, Low grade | ||||
| 7 | Gastric adenoma | 1.48 | M | 59 | Tubular adenoma, Low grade | ||||
| 8 | Gastric adenoma | 0.541 | F | 68 | Tubular adenoma, Low grade | ||||
| 9 | EGC | 2.97 | M | 71 | T1b | Negative | Negative | Alive | Adenocarcinoma (MD) |
| 10 | EGC | 17.9 | F | 57 | T1a | Negative | Negative | Alive | Adenocarcinoma (WD) |
| 11 | EGC | 3.00 | F | 75 | T1a | Negative | Negative | Alive | Adenocarcinoma (MD) |
| 12 | AGC | 0.263 | M | 72 | T4a | Positive | Negative | Alive | Adenocarcinoma (MD) |
| 13 | AGC | 26.3 | M | 75 | T4a | Positive | Negative | Dead | Signet ring cell carcinoma |
| 14 | AGC | 88.3 | M | 59 | T4 | Positive | Negative | Alive | Adenocaricnoma (PD) |
| 15 | AGC | 4.29 | F | 72 | T2 | Positive | Negative | Alive | Adenocaricnoma (MD) |
| 16 | AGC | 44.5 | M | 35 | T3 | Positive | Negative | Alive | Adenocaricnoma (PD) |
| 17 | AGC | 43.4 | M | 43 | T1 | Negative | Negative | Alive | Adenocaricnoma (PD) |
State of patient 2 years or longer after biopsy was taken
PD, MD, WD = poorly, moderately and well defined tumors
Acknowledgments
The authors are grateful to the US National Institutes of Health (grant GM38060) and by (Grant No. 2011-0002726) for Basic Research in Science and Engineering of the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology of Korea (MEST).
Abbreviations
- AGC
advanced gastric cancer
- AMAC
2-aminoacridone
- CHAPS
3-[(3-cholamidopropyl) dimethylammonio]-1-propane sulfonate
- CS
chondroitin sulfate
- DS
dermatan sulfate
- EGC
early gastric cancer
- ESI
electrospray ionization
- GAG
glycosaminoglycan
- HA
hyaluronan
- HS
heparan sulfate
- LOD
limit of detection
- MWCO
molecular weight cut-off
References
- 1.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J. Gastroenterol. 2006;12:354–362. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartgrink HH, Jansen EPM, Van Grieken NCT, Van de Velde CJH. Gastric cancer. Lancet. 2009;374:477–490. doi: 10.1016/S0140-6736(09)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machado G, Clamp JR, Read aE. Carbohydrate content of endoscopic gastric biopsies in carcinoma of the stomach. Gut. 1977;18:670–672. doi: 10.1136/gut.18.8.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Theocharis AD, Vynios DH, Papageorgakopoulou N, Skandalis SS, Theocharis DA. Altered content composition and structure of glycosaminoglycans and proteoglycans in gastric carcinoma. Int. J. Biochem. Cell Biol. 2003;35:376–390. doi: 10.1016/s1357-2725(02)00264-9. [DOI] [PubMed] [Google Scholar]
- 5.Li H, Guo L, Li J, Liu N, Qi R, Liu J. Expression of hyaluronan receptors CD44 and RHAMM in stomach cancers: relevance with tumor progression. International Journal of Oncology. 2000;17:927–932. [PubMed] [Google Scholar]
- 6.Maury C. Carbohydrate patterns of endoscopic mucosal biopsies in cancer of the stomach and chronic gastritis. Clin. Chim. Acta. 1982;126:155–159. doi: 10.1016/0009-8981(82)90031-6. [DOI] [PubMed] [Google Scholar]
- 7.Geocze S, Nader H, Mincis M, Novo N, Paiva E. Sulfated glycosaminoglycan composition of human gastric mucosa: effect of aging, chronic superficial gastritis and adenocarcinoma. Brazilian. J. Med. Biol. Res. 1985;18:487–492. [PubMed] [Google Scholar]
- 8.Afratis N, Gialeli C, Nikitovic D, Tsegenidis T, Karousou E, Theocharis AD, Pavão MS, Tzanakakis GN, Karamanos NK. Glycosaminoglycans: key players in cancer cell biology and treatment. FEBS J. 2012;279:1177–1197. doi: 10.1111/j.1742-4658.2012.08529.x. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Mo M-L, Chen Z, Yang J, Li Q-S, Wang D-J, Zhang H, Ye Y-J, Li H-L, Zhang F, Zhou H-M. HSulf-1 inhibits cell proliferation and invasion in human gastric cancer. Cancer Sci. 2011;102:1815–1821. doi: 10.1111/j.1349-7006.2011.02024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Setälä L, Lipponen P, Tammi R, Tammi M, Eskelinen M, Alhava E, Kosma VM. Expression of CD44 and its variant isoform v3 has no prognostic value in gastric cancer. Histopathol. 2001;38:13–20. doi: 10.1046/j.1365-2559.2001.01038.x. [DOI] [PubMed] [Google Scholar]
- 11.Vizoso FJ, Del Casar J, Corte MGD, Del Casar JM, García I, Alvarez A, García-Muñiz JL. Significance of cytosolic hyaluronan levels in gastric cancer. Eur. J. Surg. Oncol. 2004;30:318–324. doi: 10.1016/j.ejso.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Del Casar JM, Corte MD, Alvarez A, García I, Bongera M, González LO, García-Muñiz JL, Allende MT, Astudillo A, Vizoso FJ. Lymphatic and/or blood vessel invasion in gastric cancer: relationship with clinicopathological parameters, biological factors and prognostic significance. J. Cancer Res. Clin. Oncol. 2008;134:153–161. doi: 10.1007/s00432-007-0264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Setälä LP, Tammi MI, Tammi RH, Eskelinen MJ, Lipponen PK, Agren UM, Parkkinen J, Alhava EM, Kosma VM. Hyaluronan expression in gastric cancer cells is associated with local and nodal spread and reduced survival rate. Brit. J. Cancer. 1999;79:1133–1138. doi: 10.1038/sj.bjc.6690180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang C, Tammi M, Guo H, Tammi R. Hyaluronan distribution in the normal epithelium of esophagus, stomach, and colon and their cancers. Am. J. Pathol. 1996;148:1861–1869. [PMC free article] [PubMed] [Google Scholar]
- 15.Godavarti R, Davis M, Venkataraman G, Cooney C, Langer R, Sasisekharan R. Sas.: Heparinase III from Flavobacterium heparinum: cloning and recombinant expression in Escherichia coli. Biochem. Biophys. Res. Commun. 1996;225:751–758. doi: 10.1006/bbrc.1996.1246. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida E, Arakawa S, Matsunaga T, Toriumi S, Tokuyama S, Morikawa K, Tahara Y. Cloning, sequencing, and expression of the gene from Bacillus circulans that codes for a heparinase that degrades both heparin and heparan sulfate. Biosci. Biotechnol. Biochem. 2002;66:1873–1879. doi: 10.1271/bbb.66.1873. [DOI] [PubMed] [Google Scholar]
- 17.Shaya D, Tocilj A, Li Y, Myette J, Venkataraman G, Sasisekharan R, Cygler M. Crystal structure of heparinase II from Pedobacter heparinus and its complex with a disaccharide product. J. Biol. Chem. 2006;281:15525–15535. doi: 10.1074/jbc.M512055200. [DOI] [PubMed] [Google Scholar]
- 18.Zhao X, Yang B, Dutta P, Gasimli L, Zhang F, Linhardt RJ. Cell-based microscale isolation of glycoaminoglycans for glycomics study. J. Carbohydr. Chem. 2012;31:420–435. doi: 10.1080/07328303.2012.658126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao X, Yang B, Linkens K, Datta P, Onishi A, Zhang F, Linhardt RJ. Microscale separation of heparosan, heparan sulfate and heparin. Anal. Biochem. 2013;434:215–217. doi: 10.1016/j.ab.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang Y, Yang B, Zhao X, Linhardt RJ. Analysis of glycosaminoglycan-derived disaccharides by capillary electrophoresis using laser-induced fluorescence detection. Anal. Biochem. 2012;427:91–98. doi: 10.1016/j.ab.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Militsopoulou M, Lamari FN, Hjerpe A, Karamanos NK. Determination of twelve heparin- and heparan sulfate-derived disaccharides as 2-aminoacridone derivatives by capillary zone electrophoresis using ultraviolet and laser-induced fluorescence detection. Electrophoresis. 2002;23:1104–1109. doi: 10.1002/1522-2683(200204)23:7/8<1104::AID-ELPS1104>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 22.Yang B, Weyers A, Baik JY, Sterner E, Sharfstein S, Mousa SA, Zhang F, Dordick JS, Linhardt RJ. Ultra-performance ion-pairing liquid chromatography with online electrospray ion trap mass spectrometry for heparin disaccharide analysis. Anal. Biochem. 2011;415:59–66. doi: 10.1016/j.ab.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knudson W, Biswas C, Li X, Nemec R, Toole B. The Role and Regulation of Tumor Associated Hyaluronan. CIBA Found. Symp. 1989;143:150–159. doi: 10.1002/9780470513774.ch10. [DOI] [PubMed] [Google Scholar]
- 24.Teng YH-F, Tan P-H, Chia S-J, Zam NABM, Lau WK-O, Cheng CW-S, Bay B-H, Yip GW-C. Increased expression of non-sulfated chondroitin correlates with adverse clinicopathological parameters in prostate cancer. Modern Pathol. 2008;21:893–901. doi: 10.1038/modpathol.2008.70. [DOI] [PubMed] [Google Scholar]
- 25.Sakko AJ, Butler MS, Byers S, Reinboth BJ, Stahl J, Kench JG, Horvath LG, Sutherland RL, Stricker PD, Henshall SM, Marshall VR, Tilley WD, Horsfall DJ, Ricciardelli C. Immunohistochemical level of unsulfated chondroitin disaccharides in the cancer stroma is an independent predictor of prostate cancer relapse. Cancer Epidemiol. Biomark. Prevent. 2008;17:2488–2497. doi: 10.1158/1055-9965.EPI-08-0204. [DOI] [PubMed] [Google Scholar]
- 26.Theocharis AD, Tsara ME, Papageorgacopoulou N, Karavias DD, Theocharis DA. Pancreatic carcinoma is characterized by elevated content of hyaluronan and chondroitin sulfate with altered disaccharide composition. Biochim. Biophys. Acta. 2000;1502:150–159. doi: 10.1016/s0925-4439(00)00051-x. [DOI] [PubMed] [Google Scholar]