Abstract
Purpose
Nausea and vomiting are among the most feared complications of chemotherapy reported by patients. The objective of this study was to establish the overall complete response (CR; no emesis or use of rescue medication 0–120 h after chemotherapy) with either ondansetron- or palonosetron-containing antiemetic regimens in patients receiving highly emetogenic chemotherapy (HEC).
Methods
This was a prospective, open-label, randomized, single-center, pilot study that enrolled patients receiving their first cycle of HEC. Patients were randomized to receive either palonosetron 0.25 mg IV (PAD) or ondansetron 24 mg orally (OAD) on day 1 prior to HEC. All patients received oral aprepitant 125 mg on day 1, then 80 mg on days 2 and 3, and oral dexamethasone 12 mg on day 1, then 8 mg on days 2, 3, and 4. Descriptive statistics were used to summarize the data.
Results
A total of 40 patients were enrolled, 20 in each arm. All patients were female, and 39 received doxorubicin/cyclophosphamide chemotherapy for breast cancer. For the primary endpoint, 65 % (95 % CI, 40.8–84.6 %) of patients in the PAD arm and 40 % (95 % CI, 19.1–63.9 %) of patients in the OAD arm achieved an overall CR.
Conclusions
While CR rates for aprepitant and dexamethasone plus palonosetron or ondansetron-containing regimens have been published previously, this is the first documentation of CR rates with these regimens in the same patient population. These results may be used to design a larger, adequately powered, prospective study comparing these regimens.
Keywords: Ondansetron, Palonosetron, Aprepitant, Dexamethasone, Highly emetogenic chemotherapy
Introduction
Nausea and vomiting are among the most feared complications of chemotherapy reported by patients. Nausea and vomiting can adversely affect patients' quality of life, cause electrolyte imbalances and dehydration, increase healthcare costs (increased medication use, hospital admissions, and longer hospital stays), and could lead to delays in treatment or patient refusal of further treatment [1, 2].
Chemotherapy-induced nausea and vomiting (CINV) is often classified by its temporal relationship to chemotherapy administration: acute, delayed, or anticipatory. Acute CINV is defined as nausea and vomiting occurring between 0 and 24 h after chemotherapy. Delayed CINV is defined as nausea and vomiting occurring 24 to 120 h after chemotherapy. Anticipatory CINVoccurs in the absence of chemotherapy, as a conditioned response in patients that have experienced nausea and/or vomiting with previous cycles of chemotherapy. Acute and delayed CINV can overlap; therefore, the evaluation of “overall” nausea and vomiting (0 to 120 h) may be a more appropriate measure of CINV control [3].
The introduction of the 5-hydroxytryptophan receptor type-3 (5HT3) antagonists, ondansetron, granisetron, and dolasetron, in the 1990s dramatically changed the management of CINV for patients receiving chemotherapy with high or moderate emetogenic potential [4]. These agents are used primarily for the prevention of acute nausea and vomiting and demonstrate less activity in the delayed setting. The addition of dexamethasone to 5HT3 antagonists provides further protection against acute and delayed CINV [3, 5].
In 2003, two agents were Food and Drug Administration (FDA)-approved for the prevention of CINV, aprepitant, a novel neurokinin-1 (NK1) receptor antagonist, and palonosetron, a second-generation 5HT3 antagonist [4, 6]. Aprepitant demonstrated further reduction in acute and delayed CINV associated with HEC when combined with 5HT3 antagonists and dexamethasone compared with 5HT3 antagonists and dexamethasone alone. It is FDA-approved in this setting [6].
Palonosetron differs from first-generation 5HT3 antagonists [2, 6]. It has a longer half-life (around 40 h compared with 3–9 h with other 5HT3 antagonists) and a 100-fold greater binding affinity to the 5HT3 receptor [7–10]. Palonosetron has a unique mechanism of action in which it induces 5HT3 receptor internalization, causing a prolonged reduction in 5HT3 receptor density on the cell surface. It also uniquely inhibits cross-talk between serotonin and NK1 receptor pathways [11]. Due to its unique pharmacokinetic parameters, a single dose of palonosetron provides protection against delayed CINV, unlike the currently available 5HT3 antagonists. Palonosetron is FDA-approved for the prevention of acute and delayed CINV associated with moderately emetogenic chemotherapy and acute CINV associated with HEC [2, 6].
The 2012 National Comprehensive Cancer Network (NCCN) antiemetic guidelines (v1.2012) and the 2011 American Society of Clinical Oncology antiemetic guidelines recommend the use of a 5HT3 antagonist in combination with dexamethasone and an NK1 receptor antagonist for the prevention of acute and delayed CINV in patients receiving HEC. ASCO does not designate a preferred 5HT3 antagonist for patients receiving HEC, however, NCCN lists palonosetron as the preferred 5HT3 antagonist, based upon two studies performed in patients receiving two drug regimens for HEC [3, 12–14].
Designation of palonosetron as the preferred 5HT3 antagonist has waxed and waned in recent years due to concerns with studies conducted by Saito and Aapro. A Phase III trial conducted by Saito et al. analyzed the efficacy of granisetron 40 mcg/kg IV versus palonosetron 0.75 mg IV in combination with dexamethasone was analyzed in 1,114 patients receiving single-day HEC. For the primary endpoints, non-inferiority was achieved for achievement of acute CR (75.3 % versus 73.3 %), and superiority was achieved for delayed CR (56.8 % versus 44.5 %, p=<0.0001) in the palonosetron and granisetron groups, respectively. One of the major concerns of this study was that an NK1 receptor antagonist was not used in the regimen. Also, the palonosetron dose was higher than the FDA-approved dose in the US (0.25 mg is the only available dose in the US) [10, 13]. In 2006, Aapro et al. conducted a Phase III trial investigating the efficacy of ondansetron 32 mg IV, palonosetron 0.25 mg IV, and palonosetron 0.75 mg IV in 667 patients receiving single-day HEC. Their primary endpoint of achievement of CR in the acute setting (0 to 24 h) was attained in 57 %, 59.2 %, and 65.5 % (p=NS) of patients, respectively. The secondary endpoints of achievement of CR in the delayed setting was attained in 38.9 %, 45.3 %, and 48 % (p=NS) of patients, while achievement of overall CR was seen in 33 %, 40.8 %, and 42.2 % (p=NS) of patients, respectively. In a post hoc analysis, the authors found that there was a statistically significant improvement in delayed and overall CR with palonosetron 0.25 mg over ondansetron when combined with dexamethasone (delayed CR, 42 % versus 28.6 %, p=0.005, overall CR, 40.7 % versus 25.2 %, p=0.005). Some of the concerns of this study were the absence of an NK1 receptor antagonist and scheduled steroids. Dexamethasone use was left up to the physicians' discretion and was only utilized in two thirds of patients [14].
To date, there have been no studies directly comparing the effectiveness of a first-generation 5HT3 antagonist and palonosetron in combination with dexamethasone and an NK1 receptor antagonist for the prevention of CINV associated with HEC. The goal of this randomized pilot study is to determine the rate of overall CR in single-day palonosetronor ondansetron-containing regimen in combination with aprepitant and dexamethasone. Findings from this study could provide much needed data for the development of an adequately powered prospective study comparing the efficacy of these regimens in a head-to-head fashion.
Patients and methods
Design
This prospective, open-label, randomized, single-center, pilot study was conducted at The Arthur G. James Cancer Hospital and Richard J. Solove Research Institute at The Ohio State University and associated outpatient oncology clinics. Investigational review board approval was obtained. All patients provided written informed consent prior to study enrollment.
Patients
Patients with a confirmed malignancy, between the ages of 18 and 89 years, scheduled to receive the first dose of their first cycle of HEC were included. In patients receiving multi-day chemotherapy, the HEC portion had to be on day 1, and the remaining days of chemotherapy could be minimally emetogenic. Patients were required to be chemotherapy-naïve or treated with only low or minimally emetogenic chemotherapy in the past, as defined by the NCCN v.2.2010 Antiemetic Guidelines [15]. Patients were required to have an Eastern Cooperative Group (ECOG) performance status of grade 0–2 and capable of taking oral medications. Patients were allowed to participate in other clinical trials if the other trials did not mandate an antiemetic regimen that interfered with the study, allowed antiemetic administration at the physician's discretion, and did not prohibit the patient from participating in this study.
Exclusion criteria included any vomiting or retching within 24 h before administration of the study medications; administration of an antiemetic within 24 h before study medication administration, excluding the use of benzodiazepines; grade 2 nausea or greater, according to the Common Terminology Criteria for Adverse Events (CTCAE) v4.0, within 24 h before administration of chemotherapy; administration of strong CYP450 3A4 inducers and/or inhibitors known to cause clinically relevant drug interactions within 1 week prior to study treatment and continuing through day 5; alanine aminotranferease and/or aspartate aminotransferase>2.5 times upper limit of normal or total bilirubin>1.5 times upper limit of normal; and a known hypersensitivity to ondansetron, palonosetron, aprepitant, or dexamethasone [16].
Procedures
Patients deemed eligible for this study were stratified by HEC (cisplatin versus non-cisplatin-containing) and then randomized to one of two treatments according to a permuted block-design with block sizes of 2, 4, or 6. Patients randomized to the ondansetron group received ondansetron 24 mg once orally on day 1, 30 min prior to chemotherapy. Patients randomized to the palonosetron group received palonosetron 0.25 mg IV once on day 1, 30 min prior to chemotherapy. All patients received aprepitant 125 mg orally once on day 1, 60 min prior to chemotherapy, then 80 mg once daily on days 2 and 3, and dexamethasone 12 mg orally once on day 1, 30 min prior to chemotherapy, then 8 mg once daily on days 2 through 4. Rescue agents were prescribed for all patients at the start of cycle 1 of HEC. The choice of rescue agent was at the discretion of the ordering physician, although oral prochlorperazine 10 mg scheduled every 6 h at onset of nausea was encouraged. Patients were given a medication calendar and were counseled by the research pharmacist regarding the appropriate home medication administration schedule of aprepitant and dexamethasone and use of rescue agents (at the onset of grade 1 nausea or grade 1 vomiting). Patients were allowed to use benzodiazepines for anxiety or sleep as needed but were discouraged from using them as an initial nausea rescue medication. If the initial rescue agent failed, the prescriber was permitted to order additional rescue agents as needed.
Assessments
Patients were given a diary on the day of treatment to record symptoms experienced on days 1 through 6. Although symptoms were documented on days 1 through 6, they were only evaluated based upon days 1 through 5. The additional day of recording was instituted to ensure that the investigators captured the entire days 1 through 5 time period. Patients were instructed to document day and time of vomiting, retching, nausea, and use of rescue antiemetics in real time. They were also instructed to rate their nausea based upon the CTCAE v4.0 criteria at the end of each day. The investigators made follow-up phone calls to assess medication and diary adherence. If patients stated they did not fill out the diary, the investigator asked the patient a series of questions to evaluate their nausea, vomiting, retching, and rescue antiemetic use. These phone calls were conducted on days 2 or 3 to capture the “acute” time period and days 4, 5, or 6 to capture the “delayed” time period.
Statistical analysis
Descriptive statistics were used to describe the two study arms. Means and standard deviations (SD) were used to describe continuous variables while frequencies and proportions described categorical variables. This pilot study was not powered to test if the primary endpoint of overall CR differed across groups. Overall CR proportions were descriptively compared between study arms after the first cycle of HEC. A potential difference between therapies could be detected if the 95 % confidence intervals (determined by exact binomial methods) for the palonosetron- and ondansetron-containing groups did not overlap. Descriptive statistics were used for other outcomes, such as acute (0–24 h) and delayed (24–120 h) CR, grade of nausea and vomiting, and use of rescue medication for each treatment group as well as sub-groups of the population. An intention-to-treat approach was used to evaluate patients. All analyses were run using Stata 10.1, Stata Corporation, College Station, Texas.
Results
Patients
The patient characteristics are summarized in Table 1. Forty patients receiving single-day HEC were enrolled and treated on this study from January 2011 to July 2011. All but one patient returned their diary to the investigators for evaluation. The patient who did not return her diary communicated the documentation to the investigators via telephone. Baseline characteristics were similar in both groups. All patients were female, and nearly all (39 of 40 patients) had breast cancer and received a doxorubicin and cyclophosphamide (AC)-containing chemotherapy regimen. One patient received bevacizumab 10 mg/kg in addition to AC. One patient had Hodgkin lymphoma and received doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) for her chemotherapy treatment.
Table 1.
Baseline characteristics
| Demographic | Ondansetron (n=20) | Palonosetron (n=20) |
|---|---|---|
| Age, years, mean (SD) | 52.9 (12.7) | 50.9 (9.2) |
| Gender, females, n (%) | 20 (100) | 20 (100) |
| ECOG of 0, n (%) | 16 (80) | 18 (90) |
| Motion sickness, n (%) | 4 (20) | 9 (45) |
| Morning sickness, n (%) | 6 (30) | 6 (30) |
| Alcohol use (>10 drinks/week), n (%) | 1 (5) | 0 (0) |
| Cancer type | ||
| Breast cancer, n (%) | 19 (95) | 20 (100) |
| Lymphoma, n (%) | 1 (5) | 0 (0) |
| Chemotherapy regimen | ||
| AC, n (%) | 19 (95) | 19 (95) |
| AC plus bevacizumab, n (%) | 0 (0) | 1 (5) |
| ABVD, n (%) | 1 (5) | 0 (0) |
ECOG Eastern Cooperative Group, AC doxorubicin and cyclophosphamide, ABVD doxorubicin, bleomycin, vinblastine, and dacarbazine
The doses of doxorubicin and cyclophosphamide in the AC regimen were 60 mg/m2 and 600 mg/m2, respectively. The doses of ABVD were as follows: doxorubicin 25 mg/m2, bleomycin 10 mg/m2, vinblastine 6 mg/m2, and dacarbazine 375 mg/m2. Patients in the ondansetron and palonosetron groups were a mean age of 52.9 (SD, 12.7) and 50.9 (SD, 9.2)years, respectively. There were four patients in the ondansetron group and nine in the palonosetron group that reported a history of motion sickness. Six patients in each group reported a history of morning sickness. One patient in the ondansetron group reported a history of significant alcohol use (defined as greater than ten drinks per week). Most patients had an ECOG performance status of 0 (16 patients in the ondansetron group and 18 in the palonosetron).
Efficacy
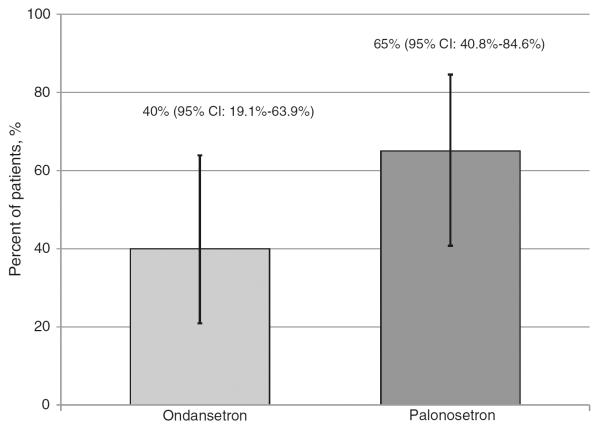
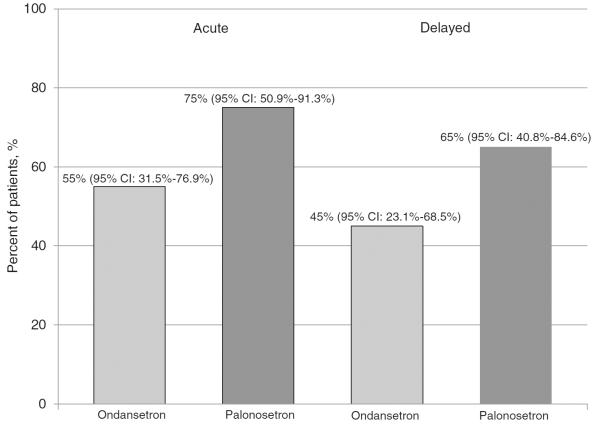
Overall CR was achieved in eight patients (40 %) in the ondansetron group (95 % CI, 19.1–63.9 %) and 13 patients (65 %) in the palonosetron group (95 % CI, 40.8–84.6 %, Fig. 1). The 95 % confidence intervals for overall CR were overlapping for the groups, thus a statistically significant difference could not be determined. Eleven patients (55 %) in the ondansetron group and 15 (75 %) in the palonosetron group achieved a CR in the acute setting, whereas nine patients (45 %) in the ondansetron group and 13 (65 %) in the palonosetron group achieved a CR in the delayed setting (Fig. 2).
Fig. 1.
Patients achieving an overall CR
Fig. 2.
Patients achieving an acute and delayed CR
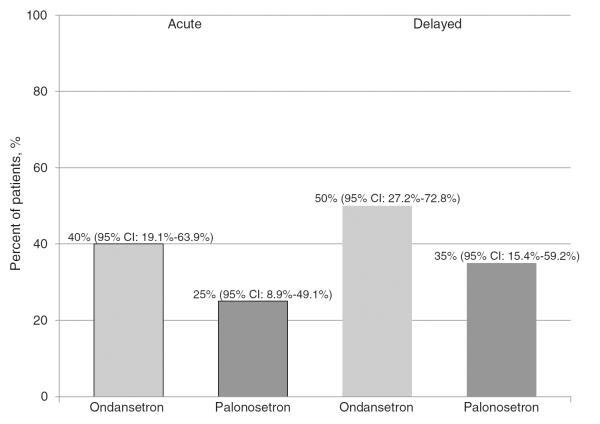
In most patients, failure to achieve a CR was due to use of rescue antiemetics. Overall, 11 patients in the ondansetron and seven in the palonosetron group reported using a rescue antiemetic during the overall study period. The use of rescue antiemetics occurred in both the acute and delayed time periods. Evaluation of the time period in which patients used rescue antiemetics, eight patients in the ondansetron group and five in the palonosetron group reported use during the acute setting, whereas ten patients in the ondansetron group and seven in the palonosetron group reported use in the delayed setting (Fig. 3).
Fig. 3.
Patient reported rescue antiemetic use
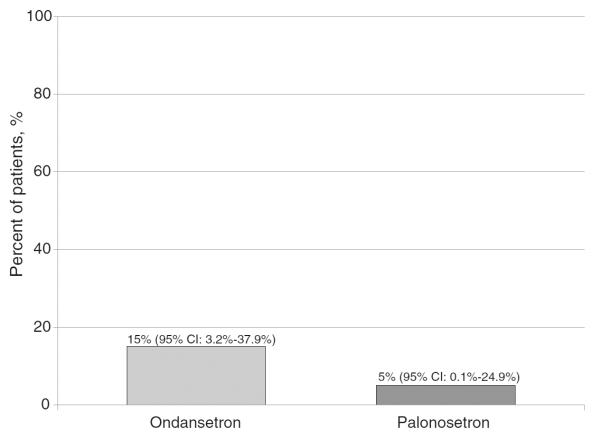
Few patients experienced episodes of retching and/or vomiting. Three patients in the ondansetron group and one in the palonosetron group reported vomiting and/or retching during this study (Fig. 4). In the ondansetron group, one patient had a retching event in the acute phase followed by six retching events in the delayed phase. Another patient had one retching event in the acute phase and one vomiting event in the delayed phase. The final patient had one retching event in the delayed phase only. In the palonosetron group, one patient experienced retching that occurred only in the delayed setting.
Fig. 4.
Patient reported vomiting and/or retching
Nausea was reported in many patients (Table 2). In the acute setting, seven patients in the ondansetron group and eight in the palonosetron group reported nausea, with grade 3 nausea occurring in only one patient who received palonosetron. Eleven patients in the ondansetron group and 12 in the palonosetron group reported delayed nausea. Of these, two patients who received palonosetron experienced grade 3 nausea.
Table 2.
Patient-reported nausea
| All grades, n (%) |
Grade 3, n (%) |
|||
|---|---|---|---|---|
| Ondansetron | Palonosetron | Ondansetron | Palonosetron | |
| Acute | 7 (35) | 8 (40) | 0 | 1 (5) |
| Delayed | 11 (55) | 12 (60) | 0 | 2 (10) |
Discussion
Many patients are fearful of the risk of nausea and vomiting associated with chemotherapy. Historically, prior to the advent of 5HT3 receptor antagonists, patients were often unable to complete chemotherapy regimens due to profound nausea or vomiting. Therefore, the goal for prevention of CINV is defined as no vomiting or retching and no use of rescue antiemetics during the overall (0 to 120 h) time period. In order to achieve this in all patients, the optimal prophylactic antiemetic regimen must still be identified.
Currently, the NCCN and ASCO guidelines recommend the use of a triple-antiemetic regimen for prevention of CINV associated with HEC. This antiemetic regimen includes a 5-HT3 receptor antagonist in combination with an NK1 receptor antagonist and dexamethasone [3, 13]. Although NCCN recommends palonosetron as the preferred 5HT3 receptor antagonist for HEC, sufficient data are lacking to support this recommendation when used in combination with aprepitant [3].
This pilot study provides data on two triple-antiemetic regimens for HEC utilizing two different 5HT3 receptor antagonists used in the same setting. Although no statistically significant differences were detected, this study provides long-awaited data on the incidence of CR rates for these two triple-antiemetic regimens for HEC in the same patient population. These data can be used to design an appropriately powered prospective study that evaluates the difference in efficacy of these two regimens.
There are some limitations to this study. The biggest limitation is the use of patient-reported diaries. Data regarding the use of rescue antiemetics, scheduled antiemetics, vomiting, retching, and daily nausea were dependent upon the accurate and appropriate use of the diaries. Patients were counseled on how to document in the diaries and when to document the information (i.e., at the time of antiemetic use, vomiting, or retching and nausea recorded each evening). Phone calls on days 2 or 3 and days 5, 6, or 7 were also made to ensure patient compliance with the diaries and scheduled antiemetics. Despite these measures, one patient failed to complete all items within the diary, and it is possible that others may have omitted or incorrectly documented information. The two phone calls served as a reminder for patients to take their scheduled antiemetics and to verbally assess patient compliance. Although the questions listed in the diaries were based on the CTCAE v4.0 nausea grading scale, they were not formally validated. Despite efforts to counsel patients on when to take rescue antiemetics (at the onset of grade 1 nausea), there were patients who took a rescue agent when they did not report nausea and others who did not take a rescue antiemetic when they did report nausea. Another potential limitation is the rather homogenous group of patients in this study. Although male and female patients receiving cisplatin- and non-cisplatin-containing chemotherapy regimens were eligible, only female patients receiving noncisplatin containing regimens were enrolled in this study. Alternatively, having a homogenous group of patients could have allowed for a more accurate assessment of this particular patient population.
The overall CR rates of 40 % in the ondansetron group and 65 % in the palonosetron group are in line with previously published data. Palonosetron does have pharmacologic and pharmacokinetic advantages over first-generation 5HT3-receptor antagonists; however, these have not been appropriately compared in combination with aprepitant and dexamethasone for HEC in a prospective fashion [7–11]. Further research should be conducted to indentify the superior 5HT3-receptor antagonist-containing regimen.
Conclusion
To our knowledge, this is the first study to investigate the overall CR rates of palonosetron- and ondansetron-containing antiemetic regimens in combination with aprepitant and dexamethasone for HEC in the same patient population. Although a statistically significant difference was not shown, these data may demonstrate consistent numerically higher rates of CR and lower rates of vomiting and retching in the palonosetron-containing group. These data may be used to design a larger, adequately powered, prospective study comparing these regimens
Acknowledgments
Funding There has been no funding provided to this research study.
Footnotes
Conflict of interest The authors have no conflicts of interest to disclose.
References
- 1.de Boer-Dennert M, de Wit R, Schmitz PI, Djontono J, v.Beurden V, Stoter G, et al. Patient perceptions of the side-effects of chemotherapy: the influence of 5HT3 antagonists. Br J Cancer. 1997;76:1055–1061. doi: 10.1038/bjc.1997.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Q, Roddy JVF, Berger M. Palonosetron hydrochloride in the treatment of chemotherapy-induced nausea and vomiting. Clin Med Ther. 2009;1:1145–1158. [Google Scholar]
- 3.National Comprehensive Cancer Network [Accessed 1 July 2012];Clinical practice guidelines in oncology: antiemesis; V.1.2012. 2012 http://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf.
- 4.Ruhlmann C, Herrstedt J. Palonosetron hydrochloride for the prevention of chemotherapy-induced nausea and vomiting. Expert Rev Anticancer Ther. 2010;10:137–148. doi: 10.1586/era.09.175. [DOI] [PubMed] [Google Scholar]
- 5.Basch E, Prestrud AA, Hesketh PJ, Kris MG, Feyer PC, Somerfield MR, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29:4189–4198. doi: 10.1200/JCO.2010.34.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358:2482–2494. doi: 10.1056/NEJMra0706547. [DOI] [PubMed] [Google Scholar]
- 7.Zofran® tablets (package insert) GlaxoSmithKline; Research Triangle Park, NC: May, 2010. [Google Scholar]
- 8.Kytril® tablets and oral solution (package insert) Roche Laboratories, Inc; Nutley, NJ: Mar, 2010. [Google Scholar]
- 9.Anzemet® tablets (package insert) Sanofi-Aventis U.S. LLC; Bridgewater, NJ: Oct, 2009. [Google Scholar]
- 10.Aloxi® injection for intravenous use (package insert) Catalent Pharma Solutions; Albuquerque, NM: Feb, 2008. [Google Scholar]
- 11.Rojas C, Li Y, Zhang J, Stathis M, Alt J, Thomas AG, et al. The antiemetic 5-HT3 receptor antagonist palonosetron inhibits substance P-mediated responses in vitro and in vivo. J Pharmacol Exp Ther. 2010;335:362–368. doi: 10.1124/jpet.110.166181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basch E, Prestrud AA, Hesketh PJ, Kris MG, Feyer PC, Somerfield MR, et al. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2011:4189–4198. doi: 10.1200/JCO.2010.34.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saito M, Aogi K, Sekine I, Yoshizawa H, Yanagita Y, Sakai H, et al. Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol. 2009;10:115–124. doi: 10.1016/S1470-2045(08)70313-9. [DOI] [PubMed] [Google Scholar]
- 14.Aapro MS, Grunberg SM, Manikhas GM, Olivares G, Suarez T, Tjulandin SA, et al. A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol. 2006;17:1441–1449. doi: 10.1093/annonc/mdl137. [DOI] [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network [Accessed 1 September 2010];Clinical practice guidelines in oncology: antiemesis; V.1.2010. 2010 http://www.nccn.org.
- 16.Oken MM, Creech H, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]