Abstract
A burgeoning literature suggests that exercise has a therapeutic benefit in persons with Parkinson disease (PD) and in animal models of PD, especially when animals exercise at high intensity. If exercise is to be prescribed as “first-line” or “add-on” therapy in patients with PD, we must demonstrate its efficacy and dose-response effects through testing phases similar to those used in the testing of pharmacologic agents. The SPARX Trial is a multicenter, randomized, controlled, single-blinded, Phase II study that we designed to test the feasibility of using high-intensity exercise to modify symptoms of PD and to simultaneously test the nonfutility of achieving a prespecified change in patients’ motor scores on the Unified Parkinson Disease Rating Scale (UPDRS). The trial began in May 2102 and is in the process of screening, enrolling, and randomly assigning 126 patients with early-stage PD to 1 of 3 groups: usual care (wait-listed controls), moderate-intensity exercise (4 days/week at 60%–65% maximal heart rate [HRmax]), or high-intensity exercise (4 days/week at 80%–85% HRmax). At 6-month follow-up, the trial is randomly reassigning usual care participants to a moderate-intensity or high-intensity exercise group for the remaining 6 months. The goals of the Phase II trial are to determine if participants can exercise at moderate and high intensities; to determine if either exercise yields benefits consistent with meaningful clinical change (nonfutility); and to document safety and attrition. The advantage of using a non-futility approach allows us to efficiently determine if moderate- or high-intensity exercise warrants further large-scale investigation in PD.
Keywords: Parkinson disease, exercise, futility, Phase II, randomized controlled trial, Unified Parkinson Disease Rating Scale
1. Introduction
Numerous studies have provided preliminary evidence that different types of exercise have positive effects on outcomes such as strength, gait, range of motion, balance, and cardiovascular fitness in patients with early and middle stages of Parkinson disease (PD) [1–12]. In addition, there is growing evidence that exercise, particularly when vigorous, has a neuroprotective effect in animals with PD [1, 13]. Although translating high-intensity exercise regimens from animals to humans remains a critical next step [14–15], this will require knowledge about the optimal intensity of exercise [16–17] and the feasiblity of implementing high-intensity exercise in the human population.
Large, well-designed, randomized controlled trials are needed to establish the impact of endurance exercise to remediate long-term deficits in individuals with PD [17–18]. Phase III multicenter trials are typically large in sample size and costly with respect to resources. Exercise trials can be particularly costly due to the personnel needed for training and supervision of participants to control exposure and ensure safety. As an alternative to launching into a Phase III trial, Schwid and Cutter have indicated, Phase II futility trials appear to be “a clever method of dealing with the trade-off between investment risk and clinical promise” [21, p. 626]. For PD and for other conditions, Phase II futility trials have been used to identify pharmacologic agents that are least likely to warrant further testing in resource-intense Phase III trials [19–23]. The use of a Phase II trial with a futility design allows for testing of an intervention over a shorter period of time and in a smaller number of subjects than does a Phase III trial.
Before we embark on a Phase III trial, we are conducting a rigorous Phase II futility trial to simultaneously establish if either moderate- or high-intensity exercise is feasible and warrants further investigation as a clinically promising intervention for PD. The Study in Parkinson Disease of Exercise SPARX Trial is a multicenter, randomized, controlled, single-blinded study of 2 intensities of endurance exercise. The SPARX trial was dually designed to (1) determine the feasibility of moderate- versus high-intensity endurance exercise in individuals with PD who have not initiated drug therapy and (2) inform the “go, no-go” decision for proceeding to a larger, more resource-intensive trial to determine the efficacy of endurance exercise on the symptomatic improvement of PD. For the Phase II trial, we elected to focus on individuals with de novo PD, defined as patients who are in the earliest stages of PD [24–26] and are naive to therapy or have been receiving therapy for a short period. We made the choice to focus on these individuals not only to minimize the confounding effects of medication and dosage changes on exercise intervention but also to minimize the likelihood that they would have functional limitations that would preclude exercise.
2. Primary research goals
The primary research goals of this exploratory study are to:
test whether individuals with de novo PD can adhere to an exercise protocol 4 days a week for 6 months to achieve one of the following randomly assigned levels of exercise intensity: a moderate level with a 60–65% average maximum heart rate (HRmax) or a high level with 80–85% average HRmax;
determine if either moderate- or high-intensity endurance exercise warrants further investigation as a therapeutic intervention for motor symptoms in the treatment of de novo PD by conducting a futility trial[21]. The alternate hypothesis is that endurance exercise does not sufficiently differ from usual care to warrant further investigation and is therefore futile;
assess the safety of the exercise intervention and the attrition to assist with planning larger exercise trials in persons with de novo PD.
3. Study design
3.1 Overview
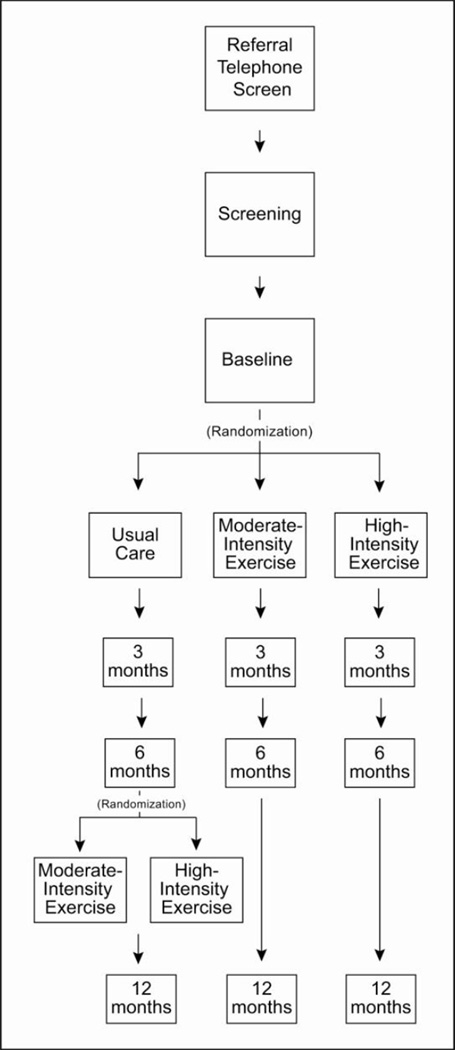
One hundred and twenty six patients with de novo PD will be randomly assigned to 3 groups: 1) high-intensity (80–85% HRmax 4 days/week); 2) moderate intensity (60–65% HRmax 4 days/week); 3) usual care wait-list controls (42 patients per group; Figure 1). After 6 months, eligible participants in the usual care group will be randomized to one of the exercise arms for 26 weeks. The rationale for using usual care wait-list control is that: 1) we have extensive data showing that moderate interventions for PD can lead to short term improvement that can compromise our ability to detect differences,[12] 2) we need to know the “natural” rate of progression over six months rather than progression while on placebo drug[27] or intervention for the futility component of our trial[28], 3) the investigative team hypothesized a wait-list control would enhance recruitment because everyone enrolled would eventually be prescribed exercise, and 4) the use of a usual care control group allowed participants to continue prior levels of physical activity. Since there is now compelling evidence of the benefits of exercise, we were not comfortable proposing a no exercise control group (see section 3.7). With respect to point 2 above, we do use the natural progression of the disease in our sample size estimates. This does mean that significant differences can arise partially from the treatment effect and partially from the natural progression, a point we return to in the discussion.
Figure 1.
Design of the Study in Parkinson Disease of Exercise (SPARX) Trial. The moderate-intensity exercise group was assigned to exercise 4 days a week at 60%–65% maximal heart rate (HRmax), and the high-intensity exercise group was assigned to exercise 4 days a week at 80%– 85% HRmax. The usual care group was assigned to begin an exercise regimen only after the first 6 months of the trial.
Immediately after baseline testing, eligible participants are randomized and followed for 12 months with follow-up assessments for disease symptoms at 3, 6, and 12 months. The primary outcomes for the trial, at 6 months, are the achieved intensity and frequency of exercise and the Unified Parkinson Disease Rating Scale Motor Score (UPDRS) [29].
The study is being conducted at 3 clinical sites: the University of Colorado–Anschutz Medical Campus; the University of Illinois at Chicago, in collaboration with Rush University Medical Center; and the University of Pittsburgh. All 3 sites are affiliated with academic medical centers with neurology practices specializing in movement disorders. The study has been approved by the institutional review boards of all participating institutions, and the University of Pittsburgh Center for Research on Health Care is serving as the data coordinating center. The SPARX trial is registered with clinicaltrials.gov, Clinical Trials.gov Identifier NCT01506479.
3.2 Eligibility
Our target population is persons with early stage PD [24–26] naïve to therapy or on it for a very short time period (de novo). Eligibility is established through telephone screening and in-person assessments.
Inclusion criteria
Exclusion criteria
Currently treated with dopaminergic therapies, including levodopa, dopamine agonists, and amantadine
Expected to require treatment with symptomatic medication in the next 6 months
Used any PD medication within 60 days prior to baseline visit including levodopa, dopamine agonists, amantadine, rasagiline, selegiline, trihexyphenidyl, and/or mucuna pruriens
Used PD medication more than 90 days
Regularly use neuroleptics/dopamine receptor blockers
Mild cognitive impairment as indicated by Montreal Cognitive Assessment (MoCA)[32]<26
Disorders that interfere with the ability to perform high-intensity endurance exercise
Any clinically significant medical conditions, psychiatric condition, drug or alcohol abuse, or laboratory abnormality that might interfere with ability to participate in the study
Regular participation in moderate to vigorous endurance exercise (>2 days/week for at least 4 months)
Temporary exclusion criteria which can be re-evaluated
Poorly controlled or unstable cardiovascular disease
Uncontrolled hypertension
Indication of depression with Beck’s Depression Inventory Score[33] >16
Recent use of psychotropic medications
Hypo- or hyperthyroidism (TSH < 0.5 or >5.0 mU/L)
Abnormal liver function (AST or ALT > 2 times the upper limit)
Abnormal renal function (serum creatinine > 2mg/dL)
Complete blood count out of range and clinically significant
Evidence of serious arrhythmias or ischemic heart disease during a maximal graded exercise stress test
Serious illness requiring systemic treatment and/or hospitalization within the last 4 weeks.
3.3 Recruitment
The goal of the study is to randomize 126 individuals with de novo PD across an approximately 36-month period. Participants are recruited from outpatient neurology practices. All three sites are affiliated with academic medical centers with neurology practices specializing in movement disorders. In addition, flyers are posted and brochures distributed across the medical centers, in offices of local neurologists and in life care communities.
3.4 Screening and baseline assessment
Eligibility for the study is established through telephone screening and in-person assessments. In person assessments include 1) medical screening by a study neurologist, 2) laboratory tests for metabolic panel, complete blood count, and thyroid stimulating hormone, and 3) a maximal graded exercise test (GXT) including measurement of maximal aerobic power by indirect calorimetry (VO2 max; Figure 1, Table 1) and maximal heart rate (HRmax). The medical screen confirms idiopathic PD and rules out any medical conditions that preclude endurance exercise. Participants who pass the medical screen have blood drawn to identify conditions that require follow-up evaluation or would preclude exercising up to 85% HRmax. Next, the maximal graded exercise test (GXT) is performed to determine presence of serious arrhythmias or evidence of ischemia and, increases of diastolic blood pressure above 110 mmHg or increases of systolic blood pressure above 220 mmHg. A positive results in the GXT requires follow-up by a cardiologist to determine whether high-intensity exercise can be performed safely.
Table 1.
Schedule of assessments for the Study in Parkinson Disease of Exercise (SPARX) Trial
| Type | Time | ||||
|---|---|---|---|---|---|
| Screening | Baseline | 3 Months |
6 Months |
12 Months |
|
| Demographics | X | ||||
| Medical history and physical exam | X | ||||
| Montreal Cognitive Assessment [32] | X | X | X | ||
| Beck’s Depression Inventory [33] | X | X | X | ||
| Laboratory measures | X | Xa | |||
| Exercise stress test | X | Xa | |||
| VO2 max (maximal aerobic power) | X | X | X | ||
| Parkinson Disease Questionnaire-39[34] (quality of life) | X | X | X | ||
| Veterans RAND 36-item Health Survey[35–36] (quality of life) |
X | X | X | ||
| Parkinson Disease Sleep Scale-2[37] | X | X | X | ||
| Epworth Sleepiness Scale[38] | X | X | X | ||
| Modified Fatigue Impact Scale[39] | X | X | X | ||
| Unified Parkinson Disease Rating Scale (UPDRS) [29] and Movement Disorder Society UPDRS (MDS-UPDRS) [40] |
X | X | X | X | |
| Health status update | X | Xb | Xb | Xb | |
| Activity level | X | Xb | Xb | Xb | |
| Exercise heart rate and adherence to the exercise regimen |
Weekly data collection | ||||
For usual care wait-list controls randomized at 6 months
Conducted monthly between study visits by phone or in-person
At baseline, participants complete the Parkinson Disease Questionnaire-39 (PDQ-39)[34], the Veterans RAND 36-Item Health Survey (RAND)[35–36], the Parkinson Disease Sleep Scale (PDSS-2)[37], the Epworth Sleepiness Scale (ESS)[38], the Modified Fatigue Impact Scale (MFIS)[39], and a health status update questionnaire (Table 1). The Unified Parkinson Disease Rating Scale (UPDRS)[29] and the Movement Disorder Society UPDRS (MDS-UPDRS)[40] are administered by a neurologist who is a movement disorders specialist. In addition, they undergo monitoring with an accelerator-based activity monitor 1 week before they are randomly assigned to a group.
3.5 Randomization and blinding
Within 8 weeks of consent and upon completion of baseline assessments, each participant is randomly assigned to 1 of 3 groups, as described earlier (Section 3.1). The procedure involves the use of a Web-based data entry system and randomization lists with permuted blocks stratified by study site. These lists were generated by the study biostatistician, using SAS software version 9.2 (SAS Institute, Cary, North Carolina).
The participants, study research coordinators, and exercise trainers are not blinded to group assignment. However, the principal investigators of the study, the neurologists responsible for assessments, and the lead biostatistician all remain blinded to individual assignments and to the ongoing results of exercise feasibility. This ensures that the primary outcome of feasibility will remain unknown until the study is complete and that there will be no undue influence over study outcomes.
An unblinded statistician is responsible for providing all exercise data to the Safety Monitoring Committee for its review of protocol implementation. This committee in turn reports its finding to the Steering Committee. This ensures that all data about protocol deviations related to the exercise interventions at the study sites are available to the Steering Committee without revealing the identification of participants.
3.6 Intervention for the 2 exercise groups
Participants exercise in the 2 exercise groups use treadmill procedures that study team members have used over the past 2 decades [4, 41–42]. The exercise regimen consists of 5–10 minutes of warm up, 30 minutes of exercise at the target HR and 5–10 minutes of cool down. For the moderate-intensity exercise group and the high-intensity exercise group, the target HRs are 60%–65% and 80%–85%, respectively, of the HRmax measured during the GXT.
During all exercise sessions, including exercise training sessions, participants must wear HR monitors that capture and store HR data. Study team members extract data from these monitors for upload into the study database which then converts the raw data into summary statistics number of exercise sessions, length of exercise sessions, average heart rate, total time in target heart rate zone, and percentage of time in target heart rate zone.
During the first 2 weeks, participants must exercise at the main study sites under supervision of the exercise training research assistants. These assistants assures exercise fidelity for each participant by downloading data from the HR monitor, comparing the data on a weekly basis, and working with the participant to make necessary adjustments. If cleared by the research assistant, the participant can later exercise at off-site facilities but is expected to exercise at least twice a month at the main site. Off-site facilities must be approved by the research assistant and site principal investigator.
During the first 8 weeks of training, exercise duration and intensity are gradually increased to the target levels. Participants are instructed to monitor their HR and are taught how to adjust the treadmill speed or treadmill incline to remain in the target HR range.
Participants randomized to one of the exercise arms are encouraged to continue to exercise after the completion of the 6-month period. Adherence is assessed after the first six months (primary end point) and again after an additional 6 months (12 months).
3.7 Usual care and subsequent intervention for the wait-listed control group
Participants randomized to the wait-list control group are instructed not to change their exercise habits for 6 months. If they request information on exercise, the study coordinators provide a copy of the Fitness Counts, a booklet available from the National Parkinson Disease Foundation[43]. After the participants complete the 6-month usual care period, those who meet eligibility requirements are randomly assigned to the moderate-intensity group or high-intensity group for the remaining 6 months of follow-up.
3.8 Primary and secondary outcomes measures
The primary feasibility outcome for achieving levels of exercise intensity is derived from the average HR during an exercise session (HRex) and expressed as a percentage of the HRmax for the individual: %HRmax=(HRex/HRmax) * 100. Because exercise intensity is gradually increased over weeks 1 to 8 to the target intensity, the daily session data from weeks 9 to 26 are used to calculate an average %HRmax for each week and for the entire period. Adherence to frequency of exercise is determined by the number of days per week that a participant exercises at the assigned intensity (moderate or high).
The primary clinical outcome for the futility component of the trial is the change in the UPDRS Motor score [29] from baseline to 6 months or from the last study visit before PD drug therapy is begun. Both the UPDRS motor score, which measures the severity of PD symptoms, and the onset of the need for dopaminergic therapy [22] have been recognized as an outcome for futility studies. The study protocol calls for UPDRS and MDS-UPDRS scores to be assessed at baseline, 3, 6, and 12 months. If a participant is to begin medication for PD symptoms, we will schedule an additional visit to administer the UPDRS and MDS-UPDRS before medication is begun, and the scores from the additional visit will be used as the primary clinical outcome. If drug therapy has already begun, we considered using UPDRS scores from the participants’ “off-medication” state; however, these scores improve with therapy over their true trajectory if no therapy had been initiated and the amount of improvement is positively correlated with the duration of treatment [44–45]. This means that the use of scores from the “off-medication” state could significantly impact our intervention effect estimates if one study group were to have a higher rate of drug initiation than another.
Safety outcomes are monitored during all exercise sessions and on a monthly basis through a health status update. During the monthly update, the participant is queried for medication changes, health care utilization, any event of falling, overall health, and upcoming visits with their neurologist. All adverse events (AEs) and serious adverse events (SAEs) are captured using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 [46].
Additional outcomes of the trial include measures of quality of life (PDQ-39, Veterans RAND 36-item Health Survey), sleep (PD Sleep Scale, Epworth Sleepiness Scale), fatigue (Modified Fatigue Impact Scale), cognitive function (MoCA), physical activity and VO2 max, a measure of maximal aerobic power (Table 1). Participants in all 3 groups wear an activity monitor 1 week each month for 12 months to allow longitudinal investigation of the activity levels outside of the structured exercise sessions.
3.9 Statistical considerations
The analyses for the first objective of feasibility are two-fold: (1) within- group comparisons to test for differences in achieving the specified exercise intensity (either 60–65% HRmax or 80–85% HRmax) and (2) between-group comparisons to test for differences in achieved levels. We will estimate the overall average %HRmax and its corresponding 95% confidence interval for each exercise group for weeks 9 to 26. We will then use a one-sample t test with a two-sided α of 0.05 to compare the average to the intended target intensity (62.5% or 82.5%). We will use linear mixed models to test for trends over time in weekly %HRmax stratified by group. For the second part of the analysis, we will test if the groups exercised at different levels of intensity even if they did not reach the intensity specified. We will use a two-sample t-test to compare the overall average %HRmax between the two groups over weeks 9 to 26 and combine the linear mixed models to test for any differential changes in performance over time.
For each group, we will analyze adherence to the treadmill exercise specified in the protocol and test the hypothesis that participants in each exercise group demonstrate adherence more than 3 days a week. Using descriptive statistics, we will calculate the average number of days per week exercised and the duration of time exercised at the specified intensity range. We will determine if the 95% confidence interval for the average number of exercise days per week falls above 3 days.
In addition to feasibility, we are using a futility design strategy to guide our decisions about larger trials involving intensive exercise in PD. In futility trials, the null hypothesis is that the intervention should be studied further (non-futility) and the alternative hypothesis is that no more investigation is warranted (futility). Our outcome is defined as the short-term change in the UPDRS Motor Scores (6 months measurement – baseline). An increase from baseline (positive change) in the UPDRS motor scores infers worsening of symptoms and a decrease infers improvement of symptoms.
We will analyze these data using intention-to-treat principle. For the primary outcome of 6 month change, the UPDRS from the last “off-medication” study visit will be used for participants who initiate dopaminergic therapy prior to 6 months. We will compare the rate of change in usual care group to each of the exercise groups (ΔUPDRSusual care - ΔUPDRSexercise). We will use a two-sample t test with one-sided α of 0.10 for the efficacy analysis[22] and a futility threshold of θ=3.5 points on the UPDRS motor scale. We will also calculate the null-adjusted (Δ −3.5) difference in the rates and a 90% confidence interval (upper bound for the null-adjusted difference in the rates).
In secondary analyses, we will adjust for the study site and any baseline variables that differ statistically among groups. We will explore the impact that the initiationg of medication has on intervention effects when we use the “off” medication state for the 6-month assessment of UPDRS scores. These results of secondary analyses will be informative for planning the larger confirmatory study and will also contribute methodologic information for future studies in individuals with de novo PD.
To estimate the 6-month incidence of specific safety outcomes (AEs and SAEs) in each group, we will use percentages and exact confidence intervals.
We define attrition as incomplete 6-month data for primary disease outcomes and incomplete monthly data for secondary outcomes. We will use proportions and 95% confidence intervals to estimate the 6-month attrition. In secondary analysis, we will combine attrition data from the original exercise groups with data from the usual care group after exercise participation.
3.10 Justification of sample size
Our trial was powered for feasibility of moderate- and high-intensity exercise and for futility based on 6-month change in symptom severity via the UPDRS motor score. We first discuss sample size jusitification for the feasibility objective then discuss the complexities of sample size justification for the futility component of the trial.
The primary outcome for feasibility is the average %HR max during exercise in weeks 9–26. The sample size analysis was based on within-group precision and comparisons to the targeted exercise intensity. Preliminary data were taken from a study of the effects of gender, age, and fitness level on response to training in 60–71 year olds where within-group standard deviations (SD) of %HRmax ranged from 5 to 6 at 6 months of training. [47] The number of participants needed per exercise arm to provide good precision (±2.5%) for the average %HRmax was n=36 (95% confidence interval half width of 2.4%, σ=7.0 80% upper bound for the SD). This provides 83% power to detect a difference of 3.5% from the specified intensity in each group (σ=7, effect size=0.5, α=0.05, two-sided test). Very few participants would be needed to establish a 20% point difference between the two exercise groups given the standard deviations (effect size>2.0). We decided a priori that we want to be able to detect differences in relative exercise intensity as small as 5% between the two groups. We will have 85% power to detect a difference of 5% or greater between the two groups (n=36,σ=7). A sample size of n=42 is required per exercise group if we assume an attrition rate of 15% at 6 months. With respect to adherence (average days exercised), with n=36 per group we will be able to estimate the average days exercised per week with ±0.24 precision (standard deviation=0.7) [47].
Power analyses for the futility component of our trial required estimating the expected 6-month change in the UPDRS motor score and the standard deviation of the change for the usual care (wait-list controls). This presented challenges since data on natural progression in de novo PD are scarce. Published and preliminary data for the UPDRS Motor score in placebo groups from landmark trials show an average increase ranging from 0.88 [20] to ~3.47 [48] points at 6 months (SDs ranging from 4.43 to 6.68 [9, 22]). Another study from the 1990s suggested the natural progression of motor impairment measured by the UPDRS to be a 2–3 unit increase per year [49]. Concurrent placebo controls in a very recent de novo PD study for creatine and minocycline [19] showed less increase on the UPDRS Total and Motor scores compared to the older DATATOP trial. [48] Given these studies, we assumed a small increase (worsening) at 6 months (change=+1) on the UPDRS motor for our control group and standard deviations between 5.5 and 6.5.
The final parameter of the power analyses for the futility component of our trial was the futility threshold, θ, which is the minimum clinically meaningful effect size that should be chosen as the effect used in designing a Phase III[50]. Futility trials in PD have primarily been single-armed studies powered using change on the UPDRS Total Score at 12 months in historical placebo controls. For example, in the DATATOP trial, the participants in the placebo group showed an average increase of 10.1 points [22]. The futility threshold,θ, has been set to a lessening of the increase on the UPDRS Total score at 12 months, for example 30% less, resulting in 70% of the historical control change. In contrast to the change in the UPDRS Total Score, studies show minimal change in UPDRS Motor symptoms at 6 months ranging from 0 (no worsening) to 3.5 in placebo groups [48].
Basing power analyses on the percentage lessening increase (less worsening) would result in unattainable sample sizes for null hypotheses that are not clinically meaningful [22]. Therefore, we looked at the literature to determine meaningful improvement in motor scores based on pharmacologic interventions and minimal clinically important change (MCIC). With respect to treatment groups, pharmacological dopaminergic agents showed 30%–35% improvement in motor scores at 6 months relative to baseline, translating to ≈7 points absolute change [51–52]. The MCIC in the UPDRS motor score at 6 months has been shown to be −5, indicating a 5 point improvement [53] for patients with Hoehn and Yahr Stages I-III deemed to have at least minimally improved based on clinician evaluation using the Clinical Global Impression-Global Improvement (CGI) scale. Further analyses by the authors showed lower MCIC for patients HY I/I.5 and HY II with average values of −3.6 and −4.8, respectively, for those minimally improved based on the CGI. These MCICs apply to within-patient change, not difference in change between two intervention arms. Given these levels of improvement, we used an absolute difference of θ =3.5 in the 6-month UPDRS motor change between usual care and intervention as the lower boundary for what would be considered clinically important and worth further investigation. Of note, several large Phase III clinical trials for pharmacologic agents have been powered to detect differences ranging from 3–4 on the short term change in UPDRS motor scores [54–56]. Therefore, our study’s futility threshold is similar to a meaningful difference in early stage PD and to differences used in larger confirmatory trials as suggested for futility designs [50, 57].
The power analyses for the futility objective has a null hypothesis (non-futility) that the difference in the rates of change is greater than θ=3.5 points (6 month change usual care group = 1, worsening; 6-month change exercise group = −2.5, improvement; θ=ΔUPDRSusual care -ΔUPDRSexercise). The alternative hypothesis (futility) is that the difference in the rates is less than θ=3.5. An n=36 per group completing the study provides over 84% power to reject the null hypothesis of further testing if there is truly no difference in the rates of change in the UPDRS motor score between the usual care and exercise groups (SD=5.5 to 6.5, a one-sided α =0.1[22]).
4. Discussion
The SPARX Trial is a multicenter exploratory trial that is designed to test the feasibility of translating high-intensity exercise from animals to humans by determining if patients with de novo PD can exercise 4 days a week at moderate and high intensities. The trial is also designed to test whether these intensities of exercise are associated with sufficient symptom alleviation compared with the wait listed controls to warrant further investigation before launching a larger, more resource-intensive, Phase III trial of exercise in patients with PD. It should be noted that it is possible that the UPDRS of the exercise group will improve compared with the wait-listed control group or alternatively that the UPDRS of the wait-listed control group will decline while the UPDRS of the exercise group stays stable. In people who have a progressive neurological condition such as PD, preserving function or preventing change is of paramount importance, whether or not improvement occurs. To our knowledge, the use of a Phase II design for feasibility and simultaneous testing of futility represents a novel contribution to the exercise field. To date, this approach has not been used for “staging” the evidence for exercise interventions representing a “progressive” step for taking non-pharmacologic interventions to Phase III trials [58].
The full-scale Phase III trial would determine whether endurance exercise has a long-term impact on symptom severity in patients with early-stage PD. For the larger-scale trial, we have developed several possible approaches and will choose the most appropriate one on the basis of the feasibility and nonfutility findings in the moderate-intensity and high-intensity exercise arms. Both exercise arms could be declared nonfutile, but the feasibility data are critical in the event that HR intensities are not distinct and adherence to the 4-days-per-week exercise schedule is not attained. In the simplest design, a Phase III trial would have 2 arms, with exercise increasing from 6 months to 12 months (in both cases with 2 months of acclimation). The primary outcome would be a 12-month change in the UPDRS motor score and would require from 204 to 274 participants if we assume 90% power, a difference in means of 3–3.5 (an effect size of 0.43–0.5, SD=7), and 15% attrition.
The dose-response intervention in the SPARX Trial is predicated on the principle that exercise regulates brain function[14, 59–61] and modifies the symptoms of PD[62]. There is mounting evidence that it also protects against neurological damage in animal models [17, 63]. Both the symptom-modifying and disease-modifying effects of exercise are important to understand. However, in our exploratory trial, we are focusing on symptom-modifying effects because this is the necessary first step in understanding the dose-response effects of endurance exercise. Once the dose-response effects on PD symptoms are known, further studies can investigate protection against neurological damage.
A recent study in patients with PD found that lower-intensity treadmill training lead to the most increase in gait speed compared to higher-intensity treadmill exercise and stretching and resistance exercises [8]. Moreover, the 2008 Physical Activity Guidelines Advisory Committee Report of the U.S. Department of Health and Human Services[64] provided strong evidence for the multiple health benefits of moderate-intensity physical activity (i.e., 65% HRmax). However, according to the executive summary of this committee report, some health outcomes showed greater improvement with high-intensity exercise (i.e., 80% HRmax) than with moderate-intensity exercise [64].
From a study of neuronal plasticity following brain damage [65], the following principles for modifying the symptoms of neurologic insult have emerged: specificity of training is important, repetition is critical, and exercise intensity matters. Investigators have applied these principles to animal models of PD in an attempt to reduce parkinsonian symptoms resulting from neurochemical damage [66–68]. In these studies, they have used 2 approaches: an emphasis on skill development [67] and an emphasis on gait [66, 68–69]. The mechanism by which exercise modifies brain function is not well understood, but one possibility is increased cortical vascularity [60]. It will be difficult to determine the mechanisms by which exercise mitigates symptoms of PD in humans until the optimal intensity of exercise is established and beneficial effects of exercise are confirmed in a Phase III clinical trial.
Movement disorders specialists currently have no clear guidelines for prescribing exercise to mitigate symptoms of PD in their patients. One important result of our exploratory study in patients with de novo PD is that it will show what percentage of these patients who are assigned to an exercise group will actually complete the exercise that they are “prescribed.” The results of 2 recent studies of patients with more advanced PD are not encouraging in terms of the number of patients who were willing to exercise. In one of these studies, Shulman and colleagues [8] found that of 945 patients assessed for eligibility, 169 (18%) did not meet inclusion criteria, 696 (74%) declined to participate in the exercise trial, and among 80 randomized, 12 (15%) discontinued their exercise intervention. In the other study, Schenkman and colleagues [9] found that of 325 patients assessed, 113 (35%) were ineligible and 65% were uninterested in participation (data not shown, Pamela Mettler, personal communication), and among 121 randomized, 16 (13%) discontinued their exercise intervention at 4 months. This underscores the importance of providing clear evidence of exercise efficacy to help motivate patients.
Our exploratory study is designed to demonstrate whether endurance exercise is futile or nonfutile. It may demonstrate that moderate-intensity exercise is best, that high-intensity exercise is best, or that both intensities are nonfutile and have similar effects. If it is able to show significant differences in the dose-related effects, this would provide clear, objective, class 1 evidence concerning what intensity of exercise is optimal. There are preclinical data suggesting that exercise has a neuroprotective effect on PD, but because a large sample size needed to conduct neuroprotective studies, it is desirable to use only one level of exercise intensity in these studies. If our exploratory study indicates that endurance exercise is futile, this would call into question whether it is feasible to design a clinical trial to look at the effects of endurance exercise as a neuroprotective agent.
5. Conclusions
In a recent review of studies of vigorous exercise in patients with PD, Ahlskog argued that a prospective clinical trial in which patients with PD are randomly assigned to regular aerobic exercise or to a passive intervention would be warranted, despite the numerous practical challenges that it would encounter [1]. These challenges include the need to encourage participants to comply with PD drug therapy and exercise, the need to follow participants for a long time because of the slow progression of PD, and the lack of a reliable biomarker for PD progression.
We are directly responding to the call for a prospective clinical trial by launching the SPARX Trial, an exploratory Phase II trial of patients who have early stage (de novo) PD. The study is designed to test the feasibility of conducting exercise interventions across multiple sites, to determine the ability of patients to comply with a moderate-intensity or high-intensity exercise regimen, and to directly inform the planning of a larger Phase III trial to assess the impact of exercise on longer-term outcomes. The design of the Phase III trial will depend not only on the outcome of the futility trial but also on the %HRmax that participants attain and the average number of days that they exercise.
Acknowledgements
This work was supported by a grant from the National Institute of Neurological Disease and Stroke R01 NS074343. Clinical trials.gov identifier: NCT 01506479.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahlskog JE. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology. 2011;77(3):288–294. doi: 10.1212/WNL.0b013e318225ab66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyai I, et al. Treadmill training with body weight support: its effect on Parkinson's disease. Arch Phys Med Rehabil. 2000;81(7):849–852. doi: 10.1053/apmr.2000.4439. [DOI] [PubMed] [Google Scholar]
- 3.Fisher BE, et al. The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson's disease. Arch Phys Med Rehabil. 2008;89(7):1221–1229. doi: 10.1016/j.apmr.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schenkman M, et al. Endurance exercise training to improve economy of movement of people with Parkinson disease: three case reports. Phys Ther. 2008;88(1):63–76. doi: 10.2522/ptj.20060351. [DOI] [PubMed] [Google Scholar]
- 5.Ridgel AL, Vitek JL, Alberts JL. Forced, not voluntary, exercise improves motor function in Parkinson's disease patients. Neurorehabil Neural Repair. 2009;23(6):600–608. doi: 10.1177/1545968308328726. [DOI] [PubMed] [Google Scholar]
- 6.Burini D, et al. A randomised controlled cross-over trial of aerobic training versus Qigong in advanced Parkinson's disease. Eura Medicophys. 2006;42(3):231–238. [PubMed] [Google Scholar]
- 7.Herman T, et al. Six weeks of intensive treadmill training improves gait and quality of life in patients with Parkinson's disease: a pilot study. Arch Phys Med Rehabil. 2007;88(9):1154–1158. doi: 10.1016/j.apmr.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Shulman LM, et al. Randomized Clinical Trial of 3 Types of Physical Exercise for Patients With Parkinson Disease. Arch Neurol. 2012:1–8. doi: 10.1001/jamaneurol.2013.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schenkman M, et al. Exercise for People in Early- or Mid-Stage Parkinson Disease: A 16-Month Randomized Controlled Trial. Phys Ther. 2012 doi: 10.2522/ptj.20110472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyai I, et al. Long-term effect of body weight-supported treadmill training in Parkinson's disease: a randomized controlled trial. Arch Phys Med Rehabil. 2002;83(10):1370–1373. doi: 10.1053/apmr.2002.34603. [DOI] [PubMed] [Google Scholar]
- 11.Toole T, et al. The effects of loading and unloading treadmill walking on balance, gait, fall risk, and daily function in Parkinsonism. NeuroRehabilitation. 2005;20(4):307–322. [PubMed] [Google Scholar]
- 12.Corcos D, et al. A two year randomized controlled trial of progressive resistance exercise for Parkinson's disease. Movement Disorders, 0000. doi: 10.1002/mds.25380. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerecke KM, et al. Exercise protects against MPTP-induced neurotoxicity in mice. Brain Res. 2010;1341:72–83. doi: 10.1016/j.brainres.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petzinger GM, et al. Enhancing neuroplasticity in the basal ganglia: the role of exercise in Parkinson's disease. Mov Disord. 2010;25(Suppl 1):S141–S145. doi: 10.1002/mds.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zigmond MJ, et al. Triggering endogenous neuroprotective processes through exercise in models of dopamine deficiency. Parkinsonism Relat Disord. 2009;15(Suppl 3):S42–S45. doi: 10.1016/S1353-8020(09)70778-3. [DOI] [PubMed] [Google Scholar]
- 16.Goodwin VA, et al. The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta-analysis. Mov Disord. 2008;23(5):631–640. doi: 10.1002/mds.21922. [DOI] [PubMed] [Google Scholar]
- 17.Zigmond MJ, et al. Neurorestoration by physical exercise: moving forward. Parkinsonism Relat Disord. 2012;18(Suppl 1):S147–S150. doi: 10.1016/S1353-8020(11)70046-3. [DOI] [PubMed] [Google Scholar]
- 18.Tomlinson CL, et al. Physiotherapy intervention in Parkinson's disease: systematic review and meta-analysis. BMJ. 2012;345:e5004. doi: 10.1136/bmj.e5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The NINDS NET-PD Investigators. A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology. 2006;66(5):664–671. doi: 10.1212/01.wnl.0000201252.57661.e1. [DOI] [PubMed] [Google Scholar]
- 20.The NINDS NET-PD Investigators. A randomized clinical trial of coenzyme Q10 and GPI-1485 in early Parkinson disease. Neurology. 2007;68(1):20–28. doi: 10.1212/01.wnl.0000250355.28474.8e. [DOI] [PubMed] [Google Scholar]
- 21.Schwid SR, Cutter GR. Futility studies: spending a little to save a lot. Neurology. 2006;66(5):626–627. doi: 10.1212/01.wnl.0000204644.81956.65. [DOI] [PubMed] [Google Scholar]
- 22.Elm JJ, et al. A responsive outcome for Parkinson's disease neuroprotection futility studies. Ann Neurol. 2005;57(2):197–203. doi: 10.1002/ana.20361. [DOI] [PubMed] [Google Scholar]
- 23.Tilley BC, et al. Optimizing the ongoing search for new treatments for Parkinson disease: using futility designs. Neurology. 2006;66(5):628–633. doi: 10.1212/01.wnl.0000201251.33253.fb. [DOI] [PubMed] [Google Scholar]
- 24.Hughes AJ, et al. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. Neurology. 1992;426:1142–1146. doi: 10.1212/wnl.42.6.1142. [DOI] [PubMed] [Google Scholar]
- 25.Hughes AJ, et al. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes AJ, et al. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. 1992. Neurology. 2001;57(10 Suppl 3):S34–S38. [PubMed] [Google Scholar]
- 27.Goetz CG, et al. Placebo response in Parkinson's disease: comparisons among 11 trials covering medical and surgical interventions. Mov Disord. 2008;23(5):690–699. doi: 10.1002/mds.21894. [DOI] [PubMed] [Google Scholar]
- 28.Levin B. The utility of futility. Stroke. 2005;36(11):2331–2332. doi: 10.1161/01.STR.0000185722.99167.56. [DOI] [PubMed] [Google Scholar]
- 29.Fahn S, Elton RL, Committee UD. Unified Parkinson's Disease Rating Scale. In: Fahn S, et al., editors. Recent Developments in Parkinson's Disease. Florham Park, NJ: Macmillan Healthcare Information; 1987. pp. 153–163. [Google Scholar]
- 30.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 31.Goetz CG, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord. 2004;19(9):1020–1028. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- 32.Chou KL, et al. A recommended scale for cognitive screening in clinical trials of Parkinson's disease. Mov Disord. 2010;25(15):2501–2507. doi: 10.1002/mds.23362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beck AT, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 34.Jenkinson C, et al. New user manual for the PDQ-39, PDQ-8 and PDQ index. University of Oxford: Oxford: Health Services Research Unit; 2008. [Google Scholar]
- 35.Kazis LE, et al. Dissemination of methods and results from the veterans health study: final comments and implications for future monitoring strategies within and outside the veterans healthcare system. J Ambul Care Manage. 2006;29(4):310–319. doi: 10.1097/00004479-200610000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Kazis LE, et al. Applications of methodologies of the Veterans Health Study in the VA healthcare system: conclusions and summary. J Ambul Care Manage. 2006;29(2):182–188. doi: 10.1097/00004479-200604000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Trenkwalder C, et al. Parkinson's disease sleep scale--validation of the revised version PDSS-2. Mov Disord. 2011;26(4):644–652. doi: 10.1002/mds.23476. [DOI] [PubMed] [Google Scholar]
- 38.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 39.Ritvo PG, et al. Multiple Sclerosis Quality of Life Inventory: A User's Manual. New York, NY: National Multiple Sclerosis Society; 1997. [Google Scholar]
- 40.Goetz CG, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 41.Kirwan JP, et al. Effects of treadmill exercise to exhaustion on the insulin response to hyperglycemia in untrained men. J Appl Physiol. 1991;70(1):246–250. doi: 10.1152/jappl.1991.70.1.246. [DOI] [PubMed] [Google Scholar]
- 42.Christiansen CL, et al. Walking economy in people with Parkinson's disease. Mov Disord. 2009;24(10):1481–1487. doi: 10.1002/mds.22621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cianci H. Parkinson's Disease: Fitness Counts. 2 ed. National Parkinson Foundation; 2004. [Google Scholar]
- 44.Kang UJ, Auinger P. Activity enhances dopaminergic long-duration response in Parkinson disease. Neurology. 2012;78(15):1146–1149. doi: 10.1212/WNL.0b013e31824f8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fahn S, et al. Levodopa and the progression of Parkinson's disease. N Engl J Med. 2004;351(24):2498–2508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- 46.CTCAE. Common Terminology Criteria for Adverse Events Version 4.0. National Cancer Institute; 2010. [Google Scholar]
- 47.Kohrt WM, et al. Effects of gender, age, and fitness level on response of VO2max to training in 60–71 yr olds. J Appl Physiol. 1991;71(5):2004–2011. doi: 10.1152/jappl.1991.71.5.2004. [DOI] [PubMed] [Google Scholar]
- 48.Jankovic J, et al. Variable expression of Parkinson's disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology. 1990;40(10):1529–1534. doi: 10.1212/wnl.40.10.1529. [DOI] [PubMed] [Google Scholar]
- 49.Poewe WH, Wenning GK. The natural history of Parkinson's disease. Neurology. 1996;47(6 Suppl 3):S146–S152. doi: 10.1212/wnl.47.6_suppl_3.146s. [DOI] [PubMed] [Google Scholar]
- 50.Palesch YY, et al. Applying a phase II futility study design to therapeutic stroke trials. Stroke. 2005;36(11):2410–2414. doi: 10.1161/01.STR.0000185718.26377.07. [DOI] [PubMed] [Google Scholar]
- 51.Korczyn AD, et al. Ropinirole versus bromocriptine in the treatment of early Parkinson's disease: a 6-month interim report of a 3-year study. 053 Study Group. Mov Disord. 1998;13(1):46–51. doi: 10.1002/mds.870130112. [DOI] [PubMed] [Google Scholar]
- 52.Rascol O, et al. Ropinirole in the treatment of early Parkinson's disease: a 6-month interim report of a 5-year levodopa-controlled study. 056 Study Group. Mov Disord. 1998;13(1):39–45. doi: 10.1002/mds.870130111. [DOI] [PubMed] [Google Scholar]
- 53.Schrag A, et al. Minimal clinically important change on the unified Parkinson's disease rating scale. Mov Disord. 2006;21(8):1200–1207. doi: 10.1002/mds.20914. [DOI] [PubMed] [Google Scholar]
- 54.Jankovic J, et al. Transdermal rotigotine: double-blind, placebo-controlled trial in Parkinson disease. Arch Neurol. 2007;64(5):676–682. doi: 10.1001/archneur.64.5.676. [DOI] [PubMed] [Google Scholar]
- 55.Rascol O, et al. Early piribedil monotherapy of Parkinson's disease: A planned seven-month report of the REGAIN study. Mov Disord. 2006;21(12):2110–2115. doi: 10.1002/mds.21122. [DOI] [PubMed] [Google Scholar]
- 56.Trenkwalder C, et al. Rotigotine effects on early morning motor function and sleep in Parkinson's disease: a double-blind, randomized, placebo-controlled study (RECOVER) Mov Disord. 2011;26(1):90–99. doi: 10.1002/mds.23441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ravina B, Palesch Y. The Phase II futility clinical trial design. Progress in Neurotherapeutics and Neuropsychopharmacology. 2007;2(1):27–38. [Google Scholar]
- 58.Dobkin BH. Progressive Staging of Pilot Studies to Improve Phase III Trials for Motor Interventions. Neurorehabilitation and Neural Repair. 2009;23(3):197–206. doi: 10.1177/1545968309331863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sutoo D, Akiyama K. Regulation of brain function by exercise. Neurobiol Dis. 2003;13(1):1–14. doi: 10.1016/s0969-9961(03)00030-5. [DOI] [PubMed] [Google Scholar]
- 60.Rhyu IJ, et al. Effects of aerobic exercise training on cognitive function and cortical vascularity in monkeys. Neuroscience. 2010;167(4):1239–1248. doi: 10.1016/j.neuroscience.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vucckovic MG, et al. Exercise elevates dopamine D2 receptor in a mouse model of Parkinson's disease: in vivo imaging with [(1)F]fallypride. Mov Disord. 2010;25(16):2777–2784. doi: 10.1002/mds.23407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robichaud JA, Corcos DM. Motor deficts, exercise and Parkinson's disease. Quest. 2005;57:85–107. [Google Scholar]
- 63.Zigmond M, Smeyne RJ. Foreword: exercise and the brain. Brain Res. 2010;1341:1–2. doi: 10.1016/j.brainres.2010.05.053. [DOI] [PubMed] [Google Scholar]
- 64.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report. Washington, DC: U.S.D.o.H.a.H. Services; 2008. [Google Scholar]
- 65.Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51(1):S225–S239. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- 66.Tillerson JL, et al. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson's disease. Neuroscience. 2003;119(3):899–911. doi: 10.1016/s0306-4522(03)00096-4. [DOI] [PubMed] [Google Scholar]
- 67.Tillerson JL, et al. Forced limb-use effects on the behavioral and neurochemical effects of 6-hydroxydopamine. J Neurosci. 2001;21(12):4427–4435. doi: 10.1523/JNEUROSCI.21-12-04427.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fisher BE, et al. Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse basal ganglia. J Neurosci Res. 2004;77(3):378–390. doi: 10.1002/jnr.20162. [DOI] [PubMed] [Google Scholar]
- 69.Cameron JL, et al. Exercise protects the striatum against MPTP damage in nonhuman primates in Society for Neuroscience. Chicago, IL: 2009. [Google Scholar]