Abstract
Vertebrate hosts actively sequester iron, and fungal and other pathogens must therefore adapt to a severe limitation in iron availability to cause disease. Recent studies reveal that the pathogenic fungus Cryptococcus neoformans overcomes iron limitation by multiple mechanisms that target transferrin and heme. The regulation of iron uptake is mediated by an interconnected set of transcription factors that include the master iron regulator Cir1 and the pH-responsive factor Rim 101. These factors integrate iron homeostasis with a myriad of other functions including pH sensing, nutrient and stress signaling pathways, virulence factor elaboration and cell wall biogenesis.
Keywords: Heme, capsule, melanin, virulence, fungal pathogenesis, pH, cell wall, signaling
Iron is a critical nutrient and a signal during fungal pathogenesis
Cryptococcus neoformans causes cryptococcosis (see Glossary), the most common type of fungal meningitis, and this disease is a tremendous hazard for the HIV/AIDS population. The severity of the threat is illustrated by recent estimates that there are over 1 million cases of cryptococcal meningitis per year resulting in ∼625,000 deaths, with the greatest occurrence in sub-Saharan Africa [1]. The majority of these cases are caused by C. neoformans. However, a sibling species, Cryptococcus gattii, has recently emerged as a pathogen of immunocompetent people, as demonstrated by an increasing number of cases and deaths in British Columbia and the western U.S. [2–4]. As with other fungal diseases (e.g., aspergillosis, candidiasis, and mucormycosis), cryptococcosis is particularly difficult to treat because of challenges with diagnosis and the limited number of efficacious antifungal drugs [5]. In fact cryptococcosis is often fatal in the absence of timely therapy, and there is an acute need to understand cryptococcal disease mechanisms and to develop new drugs. In this review, we discuss recent studies that illuminate the mechanisms of iron acquisition and sensing for C. neoformans, and that highlight iron homeostasis as a promising area for therapeutic intervention (Box 1).
Box 1. Iron and antifungal therapy.
Iron uptake functions are important targets for new antimicrobial drugs, candidates for vaccine development, and potential conduits for drug delivery into pathogens [38]. Iron chelation may also be an effective strategy to interfere with pathogen growth, although caution is needed because fungi can obtain iron from the siderophores that are used as chelators (e.g., deferoxamine) [38]. Iron acquisition is actively being pursued as a drug target and, for example, inhibitors of siderophore biosynthesis have been developed for bacterial pathogens [38]. This approach may also be useful for those fungal pathogens that rely on siderophores (e.g., Aspergillus fumigatus) [11]. Drugs that influence iron access by other means also show promise for fungal diseases. For example, the antimalarial drug chloroquine accumulates in the phagolysosome of macrophages and inhibits the growth of the intracellular fungal pathogens C. neoformans. Paracoccidioides brasiliensis and Histoplasma capsulatum [46–51]. Chloroquine limits access to iron in a pH-dependent manner and is therapeutic in a mouse model of histoplasmosis; the drug also protects mice against crypotococcosis but may not act via an iron-related mechanism [48–51]. The utility of iron acquisition functions as vaccine targets has been demonstrated in bacteria, Candida albicans, and the fungus Rhizopus oryzae, the cause of mucormycosis in patients with diabetic ketoacidosis (DKA) [52–54]. Similarly, a monoclonal antibody against the ferritin-binding protein Als3 interferes with iron acquisition in C. albicans and is fungicidal [55]. Drugs that exploit iron uptake mechanism in pathogens also show great promise for therapy. For example, siderophores conjugated to antibiotics and other drugs could exploit uptake systems for entry and this Trojan horse’ approach has been investigated for bacterial, fungal and parasitic diseases [39, 56, 57]. Similarly, toxic analogs of heme, such as non-iron metalloprotoporphyhns depend on heme uptake systems for their inhibitory activity towards bacterial pathogens and show promise against C. neoformans [26, 35, 38]. Finally, chelation with synthetic iron chelators that are not exploited as iron sources by pathogens shows therapeutic promise [58–60]. For example, patients with DKA or receiving dialysis treatment are at risk for mucormycosis upon chelation with the bacterial siderophore deferoxamine [61]. However, chelators that are not used by the fungus (deferiprone and deferasirox) are protective in mice with diabetic ketoacidosis and have been used in clinical trials [59–62].
As with bacterial pathogens, parasites and other fungal pathogens, iron is both a nutrient and a signal for C. neoformans, and the fungus must compete with iron-withholding strategies of vertebrate hosts [6–11]. The importance of iron for cryptococcosis is illustrated by the fact that experimental overload with the metal exacerbates the disease in mice [12]. Additionally, altered host iron homeostasis may be a factor in the susceptibility of liver transplant patients to cryptococcosis [13, 14]. Iron also regulates the elaboration of key virulence factors in C. neoformans including the pigment melanin and the polysaccharide capsule [6, 15–17]. Notably, the cell wall is a key component of virulence factor deployment because it is the site of melanin deposition and capsule polysaccharide attachment via α-1,3 glucan [17, 18]. Melanin is thought to contribute to virulence by protecting the fungus from oxidative killing by host immune effector cells while the capsule plays multiple roles in virulence including mediating interactions with phagocytic cells [17, 19]. Interestingly, the capsule is a useful indicator of iron levels because depletion results in cells with a large capsule and repletion causes a small capsule phenotype [20]. A thorough review of the many other environmental factors that influence capsule size has recently appeared [21]. Below we examine recent studies to address the questions of how C. neoformans acquires iron in the (host) environment and how the fungus integrates iron sensing and cyclic AMP (cAMP) signaling (Box 2) to influence the cell wall and capsule. Importantly, these studies illustrate the tight association between iron homeostasis and the elaboration of virulence factors.
Box 2. cAMP and stress signaling are integrated with iron homeostasis.
The cAMP signal transduction pathway and cAMP-dependent protein kinase A (PKA) participate in iron homeostasis and virulence in C. neoformans by regulating transcription factors and downstream iron acquisition functions. This pathway is known to influence the virulence of C. neoformans and, in particular, to regulate both melanin formation and the size of the polysaccharide capsule [21, 63]. At the transcriptional level, PKA both activates the pH-responsive transcription factor Rim101 and regulates its localization [37]. In particular, Rim 101 is found in the nucleus in C. neoformans and is partially mislocalized to the cytoplasm in a pkal mutant lacking PKA, or in a rim20 mutant lacking the protease that cleaves Rim101 [37]. In other fungi, such as C. albicans, the signaling pathway to recruit Rim20 for activation of Rim 101 involves the ESCRT complexes that participate in endocytosis and sorting of internalized membrane proteins to the multivesicular body and the vacuole [41, 64]. The Rim20-mediated processing of Rim 101 in C. neoformans is consistent with the involvement of ESCRT functions in heme uptake, capsule attachment and virulence [35]. That is, defects in ESCRT functions could have an indirect influence because of their role in activating Rim101. A role for the ESCRT-II complex protein Vps25 has also been uncovered for copper uptake, capsule formation and growth on low iron medium [65]. PKA also controls the expression and localization of iron uptake functions in C. neoformans and in other fungi [24, 66, 67]. For example, transcriptome analysis of a mutant lacking PKA revealed decreased expression of the Cft1 and CfO1 components of the high affinity uptake system [23, 68]. In contrast, targeted analysis of the highly iron-responsive siderophore transporter Sit1 indicated negative regulation by PKA [25, 68]. The pattern of regulation is therefore complex, perhaps because of PKA regulation of transcription factors in addition to Rim101. Part of the regulation by PKA involves an influence on trafficking of materials to the cell surface [63, 68]. For example, a defect in PKA also caused mislocalization of a CfO1-GFP fusion from the plasma membrane to a perinuclear location that may be the endoplasmic reticulum [24]. Finally, it should be noted that additional signaling pathways influence the expression of iron uptake and virulence functions [21]. Notably, the stress-activated HOG signaling pathway regulates the expression of CFO1, CFO2, CFT1 and SIT1 in C. neoformans, along with virulence related factors such as the capsule and melanin [69]. These findings further illustrate the complexity of the network for sensing the environment and regulating key virulence functions.
Multiple iron uptake mechanisms contribute to virulence in C. neoformans
The molecular mechanisms of iron uptake in C. neoformans have been reviewed recently along with earlier work on the physiology of iron uptake, and interconnections between iron, copper and hypoxia [15, 22]. In brief, early work defined high and low affinity uptake systems for iron, as well as roles for melanin, reductases and the secreted reductant 3-hydroxyanthranilic acid in the reduction of ferric to ferrous iron. Additionally, C. neoformans lacks the ability to produce siderophores but efficiently uses these iron chelators from other microbes. These mechanisms of iron acquisition are generally conserved among fungal pathogens although there is variability in the dependence on siderophores and the ability to use heme as an iron source [9–11]. Figure 1 summarizes the current view of iron uptake mechanisms for C. neoformans. As discussed below, recent work has focused on dissecting the virulence contributions of the high affinity reductive uptake system and the mannoprotein Cig1 that participates in heme uptake [23–26].
Figure 1.
Mechanisms of iron acquisition for C. neoformans. The best-characterized mechanism for iron acquisition is the reductive, high affinity uptake pathway that depends on ferric reductase activity, the iron permease Cft1 and the ferroxidase CfO1 [15, 23, 24]. A related iron permease (Cft2) and a ferroxidase (CfO2) are hypothesized to function in vacuolar iron transport but their locations remain to be determined. There are eight ferric or metallo-reductases (Fre1-8) predicted from the genome sequence and reducing activities have also been reported for cell wall melanin (produced by the laccases Lad and Lac2) and the secreted reductant 3-hydroxyanthranilic acid (3-HAA) [15, 70, 71]. At least six siderophore transporters are predicted from the genome although only the Sit1 transporter has been genetically characterized [25]. Existing evidence indicates that C. neoformans does not produce its own siderophores [72]. A heme uptake system involves the mannoprotein Cig1, which may be a hemophore, and trafficking functions that include the ESCRT protein Vps23 [26, 35]. This pathway is hypothesized to deliver heme to the vacuole via a pathway that may include the homotypic fusion and vacuole protein sorting (HOPS) complex [73, 74].
The high affinity iron uptake system contributes to virulence and promotes brain colonization
Reductive, high affinity iron transport at the plasma membrane in fungi generally involves reduction of ferric iron to ferrous iron by cell surface reductases, with subsequent oxidation by a ferroxidase to generate ferric iron for transport into the cell via an iron permease [27]. The iron permease Cft1 and the ferroxidase (multicopper oxidase) CfO1 are the main components of the high affinity iron uptake system in C. neoformans [23, 24]. The CFT1 and CFO1 genes are adjacent to one another on chromosome 12 and divergently transcribed. An orthologous gene pair, CFT2 and CFO2, that encode an iron permease and a ferroxidase, respectively, are present on chromosome 3. The relevance of these genes to disease is supported by the elevated expression of the CFT1 gene in C. neoformans cells collected from infected mouse lungs and the observation that the CFT2 and CFO2 transcripts are elevated in fungal cells after phagocytosis [28, 29]. Single gene deletion mutants have been constructed for all four of the genes along with the double mutants cftl cft2 and cfOl cfO2. These mutants have been employed to determine the role of high affinity uptake in the use of inorganic and host-related iron sources in vitro and for virulence in a mouse model of cryptococcosis.
The CFT1 and CFO1 genes are required for growth on iron chloride and transferrin as iron sources in vitro, but not for growth on heme or the siderophore feroxamine [23, 24]. Cft1 is also necessary for iron uptake from 55Fe-loaded transferrin or ferric chloride. In contrast, CFT2 and CFO2 make little or no contribution to iron acquisition from any source in culture. For CFT2, this may be due to the much lower expression of the gene compared to CFT1 because overexpression of CFT2 compensates for loss of CFT1 [23, 30]. The function of a recently discovered third iron permease gene, CFT3, is not clear because it does not compensate for deletion of CFT1 [30]. It is likely that Cft1 and CfO1 are present at the plasma membrane to mediate high affinity transport, and it is possible that Cft2 and CfO2 may be partially redundant under some conditions and/or contribute to iron transport from stores in the vacuole (Figure 1).
Mutants lacking Cft1 or CfO1 have additional phenotypes that likely reflect reduced intracellular iron levels, including an interesting hyper-susceptibility to the azole antifungal drugs that block the ergosterol biosynthetic pathway [23, 24, 31]. This pathway contains iron-dependent enzymes including Erg11, the heme-containing lanosterol 14-α-demethylase that is the target of azoles. For the cfO1 mutant, exogenous heme or the siderophore feroxamine, but not ferric chloride, restored growth to the wild type level thus supporting the idea that iron deficiency underlies the increased susceptibility. Reduced iron levels in the cfO1 mutant were also confirmed by direct measurement [31]. The lack of rescue by ferric chloride suggests that low affinity uptake mechanisms do not provide sufficient iron for heme biosynthesis [24].
The connection between iron deficiency and anti-fungal drug susceptibility raises the interesting possibility of synergistic combination therapy. Therefore, a more detailed examination of the interaction between azoles and iron homeostasis was performed with the cfO1 mutant [31]. In particular, it was found that the deletion of CFO1 resulted in a reduced tolerance to the fungistatic activity of fluconazole and a switch to a fungicidal effect for the drug. Transcriptional profiling by RNA-Seq of wild-type cells and the cfO1 mutant with and without exposure to fluconazole demonstrated elevated expression of the ERG genes for ergosterol biosynthesis in both strains. The mutant also showed differential expression of genes for iron-related processes, as well as mitochondrial functions such as Fe-S cluster synthesis and respiration. These results indicate a more extensive impact of iron depletion on processes that could influence drug susceptibility. This idea was supported by the observation that treatment of the wild-type strain with the respiration inhibitor diphenyleneiodonium increased susceptibility to fluconazole. Interestingly, a comparison of transcriptome data for the cfPO1 mutant with previous data for mutants lacking the Hap3 or HapX transcription factors revealed reciprocal patterns of regulation for respiration functions [32]. Consistent with this observation, hap3 and hapX mutants displayed reduced susceptibility to fluconazole [31]. Overall, these analyses reinforce the idea that inhibitors of iron uptake or availability may be useful in combination therapy with azoles.
The cft1 and cfO1 mutants with defects in high affinity iron uptake were also examined for virulence in a mouse inhalation model of cryptococcosis. The prediction was that the mutants would be attenuated for virulence because of their defect in iron acquisition from transferrin [23, 24]. This was indeed the case because mice infected with the mutants survived 10–20 days longer than mice inoculated with the wild-type strain, although the mice eventually succumbed to cryptococcosis. In contrast, the virulence of cft2 and cfO2 single mutants was not attenuated, although a cft1 cft2 double mutant was further attenuated compared with the cft1 mutant alone. This result indicates that Cft2 makes a contribution in the absence of Cft1, and this is consistent with the finding mentioned above that overexpression of Cft2 can compensate for loss of Cft1 [30]. Interestingly, a cfO1 cfO2 mutant had similar virulence to a cfO1 mutant and this may reflect greater redundancy for ferroxidase activity in C. neoformans.
A key aspect of cryptococcosis is the propensity of the fungus to disseminate from the initial site of infection in the lungs to cause disease in the central nervous system. Remarkably, the cft1 and cfO1 mutants were unable to colonize the brains of mice infected by inhalation to any appreciable level, compared to robust growth in the lungs. A more detailed examination of dissemination by tail vein inoculation of mice also revealed that the cft1 mutant failed to appear in the brain in appreciable numbers over a three-day time course of infection [23]. Given that these mutants have defects in iron use from transferrin, these results may reflect a particular dependency on this iron source for transport across the blood brain barrier and/or proliferation in the brain. One could imagine for example that transferrin may be a particularly important iron source for C. neoformans when it is passing through the microvascular endothelial cells of blood brain barrier, as previously discussed [23]. In general, the virulence studies reveal an important contribution of high affinity uptake for virulence and dissemination to the brain. However, this uptake system only makes a partial contribution to disease, given that the mice still succumbed to infection with the mutants. Therefore, other iron sources besides transferrin and other uptake systems must support proliferation of C. neoformans in the host.
The extracellular mannoprotein Cig1 and the ESCRT pathway contribute to heme uptake and virulence
A series of transcriptomic, proteomic and genetic approaches have been used to identify and characterize iron uptake systems in C. neoformans and C. gattii [6, 31–35]. In particular, initial transcriptional profiling of cells grown in low-iron medium (LIM) versus iron-replete medium identified differentially expressed genes for many functions including iron acquisition and use, capsule regulation, cell wall biosynthesis (e.g., glucan synthase) and extracellular mannoproteins [33]. Although few capsule-related genes (CAP genes) were iron-regulated, the transcript level for CAP60 was elevated in cells from the low-iron condition along with the expected transcripts for iron uptake functions (e.g., iron permeases, ferroxidase, ferric reductases and siderophore transporters). Interestingly, the most abundant transcript in LIM-grown cells encoded a cytokine-inducing glycoprotein (Cig1) that was originally identified in a group of extracellular mannoproteins associated with the capsule [33, 36]. The Cig1 transcript was 10-fold higher in cells from LIM compared with the iron-replete condition and an insertion mutation in the gene resulted in poor growth in LIM as well as ablation of the iron influence on capsule size [33]. These experiments were performed with a strain of capsular serotype D (see Glossary) because the genome sequence was first available for this strain. Subsequent deletion of the gene in the serotype A strain H99 that is widely used for virulence assays did not reveal a growth phenotype on LIM, perhaps because of differences in strain/serotype background or the type of mutation in each strain (i.e., disruption versus deletion) [26].
A subsequent broader survey of the growth of a cig1 deletion mutant on different iron sources revealed delayed growth on heme [26]. The involvement of Cig1 in heme use is interesting because heme is the most abundant source of iron in vertebrate hosts and, as mentioned above, high affinity uptake does not completely account for iron acquisition and virulence during cryptococcosis [23, 24]. Although the cig1 deletion mutant had an extended lag phase on heme, the strain eventually grew to the same density as wild type suggesting that C. neoformans has at least one additional mechanism for growth on heme. This idea was reinforced by the observations that the growth defect of the cig1 mutant on heme was only seen at physiological pH (7.2) and not at acidic pH (5.6), and that CIG1 transcript levels were greatly reduced in cells grown at acidic pH [26]. These observations are interesting in light of the known influence of pH on the oxidation state and bioavailability of iron [27].
The influence of pH and the report by O’Meara et al. that the pH-responsive transcription factor Rim 101 positively regulates expression of the Cig1 gene led to the discovery that the rim101 deletion mutant also shows delayed but eventual growth on heme [26, 37]. Expression of Cig1 from a glucose-repressed promoter in the background of a rim101 deletion revealed an even longer lag phase on medium with heme and glucose, compared with either the rim101 or cig1 mutants alone. This additive growth defect indicated that Rim101 also plays a role in heme use that is independent of its regulation of Cig1 Additionally, these experiments revealed that growth in galactose reduces the expression of Cigl and impairs the growth of a wild-type strain on heme [26].
It is possible that iron could be extracted from heme at the cell surface and subsequently taken up into cells and/or that iron could be released intracellular^ after heme import. The contribution of Cig1 and Rim 101 to the latter process (heme uptake prior to iron release) was examined by testing mutants for susceptibility to non-iron metalloporphyrins [26]. These porphyrin derivatives contain other metals such as gallium or manganese and are dependent on heme uptake into cells to exert toxicity, as demonstrated for bacterial pathogens [38]. Toxicity is thought to result from the formation of reactive oxygen species and/or displacement of heme in heme-dependent processes [38]. Both the rim101 and cig1 deletion mutants were less susceptible to gallium or manganese protoporphyrin IX compared with the wild-type strain or a cfO1 mutant lacking the high affinity uptake system. This result suggests that Rim101 and Cig1 participate in a mechanism to take up heme before iron release, rather than extraction of iron from heme prior to import. Additional work is needed to determine whether C. neoformans can also release iron from heme in the extracellular environment. Non-iron metalloporphyrins have been proposed as antibacterial agents and the results for C. neoformans indicate that these and other porphyrins may also be useful antifungal drugs [26, 38, 39].
The cig1 mutant provided an opportunity to examine the role of heme uptake in virulence and it was found that the mutant behaved like the wild-type parental strain in the mouse inhalation model of cryptococcosis [26]. This result suggested that Cig1 had no role in virulence or that its contribution was masked by other iron acquisition systems during infection. The latter conclusion appears to be correct because deletion of CIG1 in the background of a strain lacking CfO1 resulted in attenuated virulence relative to the cfO1 mutant alone [26]. Complementation of the cig1 cfO1 double mutant with the CIG1 gene restored virulence to nearly the level of the cfO1 mutant alone thus confirming a contribution of Cig1 [26]. The double mutant also did not accumulate in the brains of infected mice, although this phenotype was likely due to the previously documented lack of dissemination observed for the cfO1 mutant [26]. Notably, the mice inoculated with the double mutant eventually succumbed to the infection thus reinforcing the idea that multiple iron acquisition systems are employed by C. neoformans to cause disease. It is likely that a mutant lacking multiple systems would have to be constructed to fully ablate virulence as illustrated in Figure 2.
Figure 2.
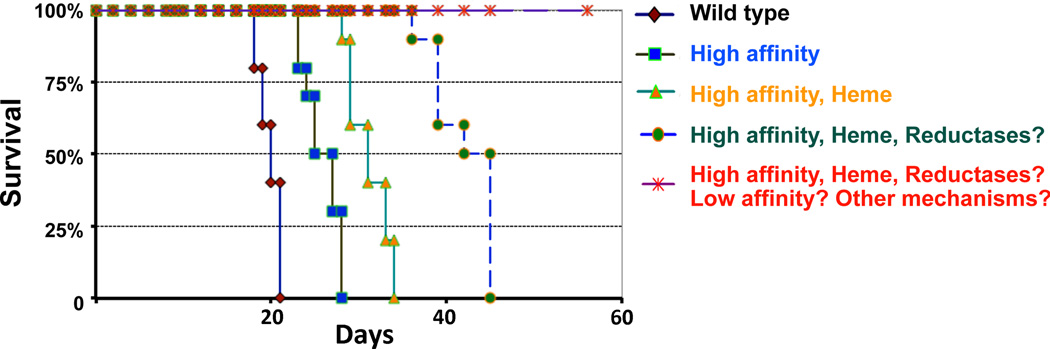
Multiple characterized and uncharacterized iron acquisition systems may contribute to the virulence of C. neoformans. A hypothetical virulence assay is depicted in which 100% of the mice infected with a wild-type strain succumb to cryptococcosis in a relatively short period of time. Mutants defective in high affinity iron uptake functions (designated high affinity) such as Cft1 or CfO1 have reduced virulence resulting in longer survival of infected mice [23, 24]. A mutant lacking both the high affinity system and the ability to take up heme (e.g., a cfO1 cig1 mutant designated high affinity, heme) is further attenuated for virulence [26]. It is possible that the addition of further mutations to block reductase activity (reductases?) will also partially attenuate virulence. Finally, defects in characterized and uncharacterized (low affinity?) systems, and mutations in yet to be discovered iron uptake functions (other mechanisms?) may be required to completely eliminate disease. The time for a complete assay in the mouse inhalation model of cryptococcosis (as shown) is typically ∼60 days. The survival patterns associated with the wild-type strain or mutants with defects in characterized iron acquisition systems are indicated with solid lines while the hypothesized patterns for mutants lacking combinations of characterized and uncharacterized systems are shown with dashed lines.
Cig1 may be a hemophore because a Cig1-glutathione-S-transferase (GST) fusion protein produced in Escherichia coli had characteristics of a heme-binding protein. Titration of the Cig1-GST fusion protein with heme identified an absorption spectrum with a maximum Soret peak at 407 nm and α and β peaks at 600 nm and 570 nm, respectively [26]. However, additional work is needed to confirm that the native protein from C. neoformans binds heme, given that the protein produced in E. coli would lack the predicted glycosylation of the protein from the fungus. If Cig1 is indeed a heme-binding protein, then it may act as a hemophore to sequester heme at the cell surface for internalization via a receptor. Therefore, a screen for receptor and transport functions was performed by testing a library of Agrobacterium tumefaciens transfer DNA (T-DNA) insertion mutants for growth defects on heme [35]. One such mutant identified Vps23, a component of the ESCRT-I complex required for endosomal sorting of membrane proteins [35]. In addition to a defect in growth on heme, a vps23 deletion mutant was defective in capsule attachment, melanin formation, growth at alkaline pH, and virulence (likely because of the myriad of defects). As with cig1 and rim101 mutants, a similar reduction in susceptibility to non-iron metalloporphyrins was observed for the vps23 mutant thus suggesting a function in heme uptake, perhaps by endocytosis. A similar role for ESCRT proteins and endocytosis was found for internalization of the heme/hemoglobin receptor Rbt51 in Candida albicans [40]. Interestingly, ESCRT functions are involved in processing and activation of Rim 101 in other fungi, and loss of Vps23 in C. neoformans may therefore also impact regulation of iron acquisition functions via Rim 101 [35, 37, 41]. More information on the fungal response to pH in the context of Rim 101 and iron acquisition can be found in a recent review [41].
A network of transcription factors and signaling pathways connect iron homeostasis and virulence
Several transcription factors regulate iron homeostasis in C. neoformans and other aspects of adaptation to the host environment [42]. Of particular note, the GATA-type transcription factor Cir1 plays a central role in the transcriptional response to iron limitation through both positive and negative regulatory influences [6]. Cir1 is interesting because it regulates the expression of genes involved in iron homeostasis and a wide variety of other functions including transcription factors, cell wall-related enzymes, and components of the cAMP, Ca2+/Calmodulin, and PKC MAP kinase signaling pathways, some of which impinge on iron homeostasis (Box 2). Additionally, deletion of CIR1 results in de-repression of laccase expression for melanin formation, loss of capsule formation, a defect in growth at 37°C and complete avirulence. Thus, Cir1 is a master regulator of both iron homeostasis and virulence factor expression. A recent metabolic analysis indicates that Cir1 also influences processes that require iron-dependent enzymes including glycolysis, ergosterol biosynthesis and inositol metabolism [43].
Additional transcription factors such as Nrg1, HapX and Rim101 regulate overlapping sets of iron uptake functions with Cir1 (Figure 3) [15, 16, 25, 32, 42, 44]. As described above, Rim 101 is important because its expression is positively regulated by Cir1, it positively regulates the expression of Cig1 and other iron uptake functions, and it is required for robust growth on heme [26, 32, 37]. It is curious, however, that a rim101 mutant has equal or perhaps slightly greater virulence compared to the wild-type strain given that failure to induce the expression of iron uptake functions might be expected to reduce growth in the host. Additionally, rim101 mutants have a small capsule and this is due to a requirement for Rim 101 in capsule attachment rather than polysaccharide production [37].
Figure 3.
Regulatory connections between some of the transcription factors that regulate iron uptake functions in C. neoformans. Transcriptome analysis of mutants defective in the Cir1 and HapX transcription factors revealed that HapX positively influences the transcript level of CIR1 and that Cir1 positively regulates RIM101 mRNA levels [32]. These experiments were performed with cells grown in low iron medium and the level of up regulation is indicated [32]. This transcriptome analysis and an earlier study identified regulatory targets of Cir1 and HapX that are involved in iron acquisition from host source (e.g., the iron permease Cft1 and ferroxidase CfO1 for transferrin use) and from environmental sources (e.g., the siderophore transporter Sit1) [6, 23–25]. Whether the regulatory relationships involve direct transcription factor binding to the promoters of target genes or indirect mechanisms remains to be determined. The influence of Rim101 on heme uptake has been established by mutant analysis [26]. In addition, O’Meara et al. performed transcriptome analyses with the rim101 mutant grow under different conditions (Dulbecco’s modified Eagle's medium, DMEM and 5% C02) and found that Rim101 up regulates the level of HAPX mRNA [37]. More detailed studies are needed to determine the extent to which each of the transcription factors contribute to iron use in vertebrate hosts and the environment and whether there are distinct regulatory schemes for each of these conditions.
A recent detailed investigation of mouse infection with a rim101 mutant revealed that the strain triggered more severe pulmonary inflammation than a wild-type strain [45]. This inflammatory response led to predominantly respiratory symptoms for the mutant in contrast to the neurological symptoms observed with wild-type strains. This marked change in the immune response likely overrides the apparent reduced growth of the mutant in lung tissue that could result from issues with iron acquisition. A proliferation defect was evident for the rim101 mutant in that mice infected with the wild-type strain had ∼5 fold higher fungal burden in whole lung homogenates compared with mice infected with the mutant at day 9. The change in the immune response was attributed to an altered fungal cell surface and detailed transcriptional profiling identified a role for Rim101 in regulating cell wall processes. Additionally, electron microscopy revealed that the mutant had a thicker cell wall and an excess of chitin oligomers compared with wild type. An important discovery from this work, therefore, is that Rim101 appears to direct the remodeling of the cell wall in response to the host environment such that capsule attachment is maintained to mask the cells from recognition by the immune system [45]. Therefore, the analyses of Rim 101 and Cir1 at least partially address a key question about the regulation of capsule size by iron availability. That is, these factors respond directly or indirectly to host-relevant signals such as iron levels and pH to regulate processes that are critical for capsule elaboration, including organization of the cell wall.
Concluding remarks
Iron is a prized commodity during pathogen colonization of vertebrate hosts and its poor availability triggers an adaptive shift for C. neoformans that includes deployment of specific iron acquisition systems and virulence determinants such as the polysaccharide capsule. The mechanisms underlying these adaptations to the host environment prompt many questions, some of which are listed in Box 3, but recent work provides glimpses into the roles of multiple iron acquisition systems and their regulation by a network of transcriptional regulators and signaling pathways. Many challenges remain, including assessing the potentially redundant contributions of multiple iron acquisition mechanisms to proliferation in the host and disease (Figure 2). In this regard, the goal is to obtain a comprehensive list of uptake mechanisms and to genetically test the contribution of each to iron acquisition from specific iron sources in culture and in the host. Additionally, it will be challenging to determine the overlapping and distinct roles of iron regulators such as Cir1 and Rim101 in response to signals and signaling pathways, and to translate that information into an understanding of major phenotypic outcomes such as remodeling of the cell wall in response to iron availability. The way forward will undoubtedly involve systematic genetic screens and systems approaches to define key components, examine connections between them and evaluate their roles in the host environment.
Box 3. Outstanding questions.
What are the components of the complete set of iron acquisition functions in C. neoformans, and what are their contributions to virulence? In particular, what are the contributions to iron uptake of cell-surface ferric reductases, the secreted reductant 3-hydroxyanthranilic acid and the reducing activity of melanin?
Are there preferred and niche-specific iron sources for C. neoformans proliferation in vertebrate hosts, as suggested by the reduced appearance of cftl and cfO1 mutants in the central nervous system? If there are preferred iron sources, which specific acquisition systems are needed to exploit them? Are there iron acquisition mechanisms that are needed only during growth in the environment versus the host?
Which specific functions are the most promising for therapeutic intervention via the development of inhibitors of iron uptake, by drug delivery with iron transporters or for vaccine development?
Given that C. neoformans has at least six siderophore transporters, are there sources of siderophores available to the fungus in the vertebrate host or are these transporters used to compete for iron in the environment? More specifically, can C. neoformans take advantage of human siderophores or xenosiderophores produced by commensal microbes in lung tissue?
How does C. neoformans access heme in the host? What are the iron sources available to the fungus during intracellular proliferation in phagocytic cells and extracellular growth? Given that macrophages play an important role in iron sequestration and recycling, is heme an important intracellular iron source?
-Does native Cig1 from C. neoformans bind heme and, if so, how does it facilitate heme uptake? Are there specific heme receptors that interact with Cig1 or independently to promote uptake?
How do components of the ESCRT machinery contribute to heme uptake and capsule attachment? Is the contribution strictly regulatory through activation of Rim 101 or does the pathway also influence trafficking functions for heme and/or capsule polysaccharide?
What is the pathway for heme internalization and how is heme processed in C. neoformans? Is there a role for a heme oxygenase and the vacuole in removing iron from heme, and how much exogenous heme is recycled?
What are all of the regulatory factors that control the expression of iron uptake functions? How are these factors controlled by specific signaling pathways and what are the direct and indirect targets of each transcription factor?
Highlights.
Multiple mechanisms of iron acquisition from host sources contribute to virulence.
The mannoprotein Cig1 and its regulator Rim 101 are required for growth on heme.
A regulatory and signaling network links environmental sensing and virulence.
Rim 101 regulates cell wall synthesis and evasion of the immune response.
Acknowledgements
Work in the Kronstad group is supported by the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada and the National Institute of Allergy and Infectious Diseases (RO1 AI053721). The Jung group is supported by the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2012-0004062). J.W.K. gratefully acknowledges a Scholar Award in Molecular Pathogenic Mycology from the Burroughs Wellcome Fund. We apologize to those authors whose work could not be cited due to space limitations.
Glossary
- Capsule serotypes
a classification system for Cryptococcus neoformans and Cryptococcus gattll isolates based on antigenic differences in the polysaccharides that make up the capsule. The capsule is a major virulence determinant and it is composed of glucuronoxylomannan (GXM, ∼90%) and glucuronoxylomannogalactan (GXMGal, ∼10%). The DNA sequence divergence between the genomes of serotype A and D strains is estimated at 10–15%
- Cell wall
a surface organelle that is composed of a- and (3-linked glucans, chitin and its deacetylated derivative chitosan, and mannoproteins in C. neoformans. The wall is critical for establishing cellular morphology, maintaining viability in response to environmental stresses and mediating interactions with the host
- Cir1
the Cryptococcus iron regulator is a GATA-type zinc finger transcription factor that regulates the expression of iron uptake functions and virulence factors
- Cryptococcosis
a fungal disease typically initiated by inhalation of yeast-like cells or spore of C. neoformans or C. gattii. The resulting pulmonary infection is poorly contained in people with a defective immune system (e.g. AIDS patients) and the fungal cells disseminate to the brain to cause disease
- Ergosterol
a sterol found in fungal membranes with functional similarity to cholesterol found in animal cells. Ergosterol biosynthetic enzymes are encoded by ERG genes. Drugs that target ergosterol biosynthesis (e.g., fluconazole) or that bind ergosterol (e.g., amphotericin B) are commonly used to treat patients with cryptococcosis
- ESCRT machinery
the highly conserved endosomal sorting complex required for transport machinery functions in several processes including the endosomal sorting of monoubiquitinated membrane proteins for degradation and the formation of multivesicular bodies
- Heme
a prosthetic group comprised of iron bound to a porphyrin molecule. Heme is abundant in vertebrates, particularly as a component of the abundant oxygen transport protein hemoglobin
- Hemophore
a heme-binding protein generally found in bacterial pathogens
- High affinity iron uptake
an iron uptake system comprised of an iron permease and a ferroxidase (also called a multicopper oxidase). In C. neoformans, these components are encoded by the CFT1 and CFO1 genes, respectively
- Iron homeostasis
maintenance of a steady state for intracellular iron levels that balances intake and efflux, and that includes cycles of oxidation and reduction to ensure iron bioavailability and limit toxicity
- Rim101
a transcription factor that functions in fungal adaptation to changes in extracellular pH. The protein has been characterized in several yeasts and it is designated PacC in filamentous fungi
- Siderophores
low molecular weight chelators with high affinity for ferric ions
- Signaling pathways
protein networks to sense environmental conditions and transmit the information to intracellular response machinery to allow adaptation. For C. neoformans, key pathways related to iron include the high osmolarity glycerol (HOG) pathway that responds to environmental stresses (e.g., osmotic shock) and the cAMP pathway that senses nutrients (e.g., glucose). The key kinase activated by the HOG pathway is a mitogen activated kinase designated Hog1. The key kinase for the cAMP pathway is the cAMP-dependent protein kinase called protein kinase A (PKA)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Park BJ, et al. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett KH, et al. The emergence of Cryptococcus gattll in British Columbia and the Pacific Northwest. Curr. Fungal Infect. Rep. 2007;1:108–115. [Google Scholar]
- 3.Byrnes EJ3rd, et al. Emergence and pathogenicity of highly virulent Cryptococcus gattll genotypes in the northwest United States. PLoS Pathog. 2010;6:e1000850. doi: 10.1371/journal.ppat.1000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris JR, et al. Cryptococcus gattll in the United States: clinical aspects of infection with an emerging pathogen. Clin. Infect. Dis. 2011;53:1188–1195. doi: 10.1093/cid/cir723. [DOI] [PubMed] [Google Scholar]
- 5.Brown GD, et al. Hidden killers: human fungal infections. Sci. Transl. Med. 2012;4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 6.Jung WH, et al. Iron regulation of the major virulence factors in the AIDS-associated pathogen Cryptococcus neoformans . PLoS Biol. 2006;4:e410. doi: 10.1371/journal.pbio.0040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skaar EP. The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog. 2010;6:e1000949. doi: 10.1371/journal.ppat.1000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutak R, et al. Crusade for iron: iron uptake in unicellular eukaryotes and its significance for virulence. Trends Microbiol. 2008;16:261–268. doi: 10.1016/j.tim.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Almeida RS, et al. Candida albicans iron acquisition within the host. FEMS Yeast Res. 2009;9:1000–1012. doi: 10.1111/j.1567-1364.2009.00570.x. [DOI] [PubMed] [Google Scholar]
- 10.Kornitzer D. Fungal mechanisms for host iron acquisition. Curr. Opin. Microbiol. 2009;12:377–383. doi: 10.1016/j.mib.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Haas H. Iron - A key nexus in the virulence of Aspergillus fumigatus Front. Microbiol. 2012;3:28. doi: 10.3389/fmicb.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barluzzi R, et al. Iron overload exacerbates experimental meningoencephalitis by Cryptococcus neoformans . J Neuroimmunol. 2002;132:140–146. doi: 10.1016/s0165-5728(02)00324-7. [DOI] [PubMed] [Google Scholar]
- 13.Singh N, Sun HY. Iron overload and unique susceptibility of liver transplant recipients to disseminated disease due to opportunistic pathogens. Liver Transpl. 2008;14:1249–1255. doi: 10.1002/lt.21587. [DOI] [PubMed] [Google Scholar]
- 14.Sifri CD, et al. Pretransplant cryptococcosis and outcome after liver transplantation. Liver Transpl. 2010;16:499–502. doi: 10.1002/lt.22024. [DOI] [PubMed] [Google Scholar]
- 15.Jung WH, Kronstad JW. Iron and fungal pathogenesis: A case study with Cryptococcus neoformans . Cell. Microbiol. 2008;10:277–284. doi: 10.1111/j.1462-5822.2007.01077.x. [DOI] [PubMed] [Google Scholar]
- 16.Kumar P, et al. Emerging themes in cryptococcal capsule synthesis. Curr. Opin. Struct. Biol. 2011;21:597–602. doi: 10.1016/j.sbi.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu GY, Nizet V. Color me bad: microbial pigments as virulence factors. Trends Microbiol. 2009;17:406–413. doi: 10.1016/j.tim.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reese AJ, Doering TL. Cell wall alpha-1,3-glucan is required to anchor the Cryptococcus neoformans capsule. Mol. Microbiol. 2003;50:1401–1409. doi: 10.1046/j.1365-2958.2003.03780.x. [DOI] [PubMed] [Google Scholar]
- 19.Kronstad JW, et al. Expanding fungal pathogenesis: Cryptococcus species break out of the opportunistic box. Nat. Rev. Microbiol. 2011;9:193–203. doi: 10.1038/nrmicro2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vartivarian SE, et al. Regulation of cryptococcal capsular polysaccharide by iron. J. Infect. Dis. 1993;167:186–190. doi: 10.1093/infdis/167.1.186. [DOI] [PubMed] [Google Scholar]
- 21.O'Meara TR, Alspaugh JA. The Cryptococcus neoformans capsule: a sword and a shield. Clin. Microbiol. Rev. 2012;25:387–408. doi: 10.1128/CMR.00001-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva MG, et al. The homeostasis of iron, copper, and zinc in Paracoccidioides brasiliensis, Cryptococcus neoformans var. grubii, and Cryptococcus gattii: a comparative analysis. Front Microbiol. 2011;2:49. doi: 10.3389/fmicb.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung WH, et al. Iron source preference and regulation of iron uptake in the AIDS-associated pathogen Cryptococcus neoformans . PLoS Pathog. 2008;4:e45. doi: 10.1371/journal.ppat.0040045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung WH, et al. The role of ferroxidases in iron uptake and virulence of Cryptococcus neoformans . Eukaryot. Cell. 2009;8:1511–1520. doi: 10.1128/EC.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tangen KT, et al. The iron and cAMP regulated gene SIT1 influences siderophore utilization, melanization and cell wall structure in Cryptococcus neoformans . Microbiol. 2007;153:29–41. doi: 10.1099/mic.0.2006/000927-0. [DOI] [PubMed] [Google Scholar]
- 26.Cadieux B, et al. The mannoprotein Cig1 supports iron acquisition from heme and virulence in the pathogenic fungus Cryptococcus neoformans . J. Infect Dis. 2013;207:1339–1347. doi: 10.1093/infdis/jit029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kosman DJ. Iron metabolism in aerobes: managing ferric iron hydrolysis and ferrous iron autoxidation. Coord. Chem. Rev. 2013;257:210–217. doi: 10.1016/j.ccr.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu G, et al. Metabolic adaptation in Cryptococcus neoformans during early murine pulmonary infection. Mol. Microbiol. 2008;69:1456–1475. doi: 10.1111/j.1365-2958.2008.06374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan W, et al. Cryptococcus neoformans gene expression during murine macrophage infection. Eukaryot. Cell. 2005;4:1420–1433. doi: 10.1128/EC.4.8.1420-1433.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han K, et al. A human fungal pathogen Cryptococcus neoformans expresses three distinct iron permease homologs. J. Microbiol. Biotechnol. 2012;22:1644–1652. doi: 10.4014/jmb.1209.09019. [DOI] [PubMed] [Google Scholar]
- 31.Kim J, et al. A defect in iron uptake enhances the susceptibility of Cryptococcus neoformans to azole antifungal drugs. Fung. Genet. Biol. 2012;49:955–966. doi: 10.1016/j.fgb.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jung WH, et al. HapX positively and negatively regulates the transcriptional response to iron deprivation in Cryptococcus neoformans. PloS Pathog. 2010;6:e1001209. doi: 10.1371/journal.ppat.1001209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lian TS, et al. Iron-regulated transcription and capsule formation in the fungal pathogen Cryptococcus neoformans. Mol Microbiol. 2005;55:1452–1472. doi: 10.1111/j.1365-2958.2004.04474.x. [DOI] [PubMed] [Google Scholar]
- 34.Crestani J, et al. Proteomic profiling of the influence of iron availability on Cryptococcus gattii. J. Proteome. Res. 2012;11:189–205. doi: 10.1021/pr2005296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu G, et al. Cryptococcus neoformans requires the ESCRT protein Vps23 for iron acquisition from heme, capsule formation and virulence. Infect. Immun. 2013;81:292–302. doi: 10.1128/IAI.01037-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biondo C, et al. Identification of major proteins secreted by Cryptococcus neoformans . FEMS Yeast Res. 2006;6:645–651. doi: 10.1111/j.1567-1364.2006.00043.x. [DOI] [PubMed] [Google Scholar]
- 37.O'Meara TR, et al. Interaction of Cryptococcus neoformans Rim 101 and protein kinase A regulates capsule. PLoS Pathog. 2010;6:e1000776. doi: 10.1371/journal.ppat.1000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stojiljkovic I, et al. Antimicrobial properties of porphyrins. Expert. Opin. Investig. Drugs. 2001;10:309–320. doi: 10.1517/13543784.10.2.309. [DOI] [PubMed] [Google Scholar]
- 39.Foley TL, Simeonov A. Targeting iron assimilation to develop new antibacterials. Expert Opin. Drug Discov. 2012;7:831–847. doi: 10.1517/17460441.2012.708335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weissman Z, et al. An endocytic mechanism for haemoglobin-iron acquisition in Candida albicans . Mol. Microbiol. 2008;69:201–217. doi: 10.1111/j.1365-2958.2008.06277.x. [DOI] [PubMed] [Google Scholar]
- 41.Davis DA. How human pathogenic fungi sense and adapt to pH: the link to virulence. Curr. Opin. Microbiol. 2009;12:365–370. doi: 10.1016/j.mib.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 42.Kronstad JW, et al. Adaptation of Cryptococcus neoformans to mammalian hosts: Integrated regulation of metabolism and virulence. Eukaryot. Cell. 2012;11:109–118. doi: 10.1128/EC.05273-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi JN, et al. Influence of iron regulation on the metabolome of Cryptococcus neoformans . PLoS One. 2012;7:e41654. doi: 10.1371/journal.pone.0041654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cramer KL, et al. Transcription factor Nrg1 mediates capsule formation, stress response, and pathogenesis in Cryptococcus neoformans . Eukaryot Cell. 2006;5:1147–1156. doi: 10.1128/EC.00145-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Meara TR, et al. Cryptococcus neoformans Rim 101 is associated with cell wall remodeling and evasion of the host immune responses. mBio. 2013;4:e00522–e00512. doi: 10.1128/mBio.00522-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dias-Melicio LA, et al. Chloroquine inhibits Paracoccidioides brasiliensis survival within human monocytes by limiting the availability of intracellular iron. Microbiol. Immunol. 2006;50:307–314. doi: 10.1111/j.1348-0421.2006.tb03798.x. [DOI] [PubMed] [Google Scholar]
- 47.Dias-Melicio LA, et al. Chloroquine is therapeutic in murine experimental model of paracoccidioidomycosis. FEMS Immunol. Med. Microbiol. 2007;50:133–143. doi: 10.1111/j.1574-695X.2007.00243.x. [DOI] [PubMed] [Google Scholar]
- 48.Mazzolla R, et al. Enhanced resistance to Cryptococcus neoformans infection induced by chloroquine in a murine model of meningoencephalitis. Antimicrob. Agents Chemother. 1997;41:802–807. doi: 10.1128/aac.41.4.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Newman SL, et al. Chloroquine induces human macrophage killing of Histoplasma capsulatum by limiting the availability of intracellular iron and is therapeutic in a murine model of histoplasmosis. J. Clin. Invest. 1994;93:1422–1429. doi: 10.1172/JCI117119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harrison TS, et al. Conditional lethality of the diprotic weak bases chloroquine and quinacrine against Cryptococcus neoformans . J. Infect. Dis. 2000;182:283–289. doi: 10.1086/315649. [DOI] [PubMed] [Google Scholar]
- 51.Weber SM, et al. Chloroquine and the fungal phagosome. Curr. Opln. Microbiol. 2000;3:349–353. doi: 10.1016/s1369-5274(00)00102-8. [DOI] [PubMed] [Google Scholar]
- 52.Alteri CJ, et al. Mucosal immunization with iron receptor antigens protects against urinary tract infection. PLoS Pathog. 2009;5:e1000586. doi: 10.1371/journal.ppat.1000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ibrahim AS, et al. The high affinity iron permease is a key virulence factor required for Rhlzopus oryzae pathogenesis. Mol. Microbiol. 2010;77:587–604. doi: 10.1111/j.1365-2958.2010.07234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brena S, et al. Fungicidal monoclonal antibody C7 interferes with iron acquisition in Candida albicans . Antimicrob Agents Chemother. 2011;55:3156–3163. doi: 10.1128/AAC.00892-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Y, Filler SG. Candida albicans Als3, a multifunctional adhesin and invasin. Eukaryot. Cell. 2011;10:168–173. doi: 10.1128/EC.00279-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Möllmann U, et al. Siderophores as drug delivery agents: application of the "Trojan Horse" strategy. Blometals. 2009;22:615–624. doi: 10.1007/s10534-009-9219-2. [DOI] [PubMed] [Google Scholar]
- 57.Bernier G, et al. Desketoneoenactin-siderophore conjugates for Candida: evidence of iron transport-dependent species selectivity. Antimicrob. Agents Chemother. 2005;49:241–248. doi: 10.1128/AAC.49.1.241-248.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zarember KA, et al. Antifungal activities of natural and synthetic iron chelators alone and in combination with azole and polyene antibiotics against Aspergillus fumigatus . Antimicrob. Agents Chemother. 2009;53:2654–2656. doi: 10.1128/AAC.01547-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ibrahim AS, et al. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. J. Clin. Invest. 2007;117:2649–2657. doi: 10.1172/JCI32338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ibrahim AS, et al. The iron chelator deferasirox enhances liposomal amphotericin B efficacy in treating murine invasive pulmonary aspergillosis. J. Antimicrob. Chemother. 2010;65:289–292. doi: 10.1093/jac/dkp426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ibrahim AS, et al. Pathogenesis of mucormycosis. Clin. Infect. Dis. 2012;(54 Suppl 1):S16–S22. doi: 10.1093/cid/cir865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spellberg B, et al. The Deferasirox-AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J. Antimicrob. Chemother. 2012;67:715–722. doi: 10.1093/jac/dkr375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi J, et al. Regulated expression of cyclic AMP-dependent protein kinase A reveals an influence on cell size and the secretion of virulence factors in Cryptococcus neoformans . Mol Microbiol. 2012;85:700–715. doi: 10.1111/j.1365-2958.2012.08134.x. [DOI] [PubMed] [Google Scholar]
- 64.Selvig K, Alspaugh JA. pH response pathways in fungi: adapting to host-derived and environmental signals. Mycobiol. 2011;39:249–256. doi: 10.5941/MYCO.2011.39.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chun CD, Madhani HD. Ctr2 links copper homeostasis to polysaccharide capsule formation and phagocytosis inhibition in the human fungal pathogen Cryptococcus neoformans . PLoS One. 2010;5:e12503. doi: 10.1371/journal.pone.0012503. doi:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robertson LS, et al. The yeast A kinases differentially regulate iron uptake and respiratory function. Proc. Natl. Acad. Sci. U. S. A. 2000;97:5984–5988. doi: 10.1073/pnas.100113397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eichhorn H, et al. Aferroxidation/permeation iron uptake system is required for virulence in Ustilago maydis. Plant Cell. 2006;18:3332–3345. doi: 10.1105/tpc.106.043588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu G, et al. Transcriptional regulation by protein kinase A in Cryptococcus neoformans . PLoS Pathog. 2007;3:e42. doi: 10.1371/journal.ppat.0030042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ko YJ, et al. Remodeling of global transcription patterns of Cryptococcus neoformans genes mediated by the stress-activated HOG signaling pathways. Eukaryot. Cell. 2009;8:1197–1217. doi: 10.1128/EC.00120-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nyhus KJ, et al. Ferric iron reduction by Cryptococcus neoformans . Infect. Immun. 1997;65:434–438. doi: 10.1128/iai.65.2.434-438.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jacobson ES, et al. Ferrous iron uptake in Cryptococcus neoformans . Infect. Immun. 1998;66:4169–4175. doi: 10.1128/iai.66.9.4169-4175.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jacobson ES, Petro MJ. Extracellular iron chelation in Cryptococcus neoformans . J. Med. Vet. Mycol. 1987;25:415–418. [PubMed] [Google Scholar]
- 73.Liu X, et al. Role of a VPS41 homologue in starvation response, intracellular survival and virulence of Cryptococcus neoformans . Mol. Microbiol. 2006;61:1132–1146. doi: 10.1111/j.1365-2958.2006.05299.x. [DOI] [PubMed] [Google Scholar]
- 74.Solinger JA, Spang A. Tethering complexes in the endocytic pathway: CORVET and HOPS. FEBS J. 2013 doi: 10.1111/febs.12151. [DOI] [PubMed] [Google Scholar]




