Abstract
Background & Aims
15-hydroxprostaglandin dehydrogenase (15-PGDH) mediates a colon neoplasia suppressor pathway, acting through metabolic antagonism of cyclooxygenase (COX)-mediated colon carcinogenesis. To determine whether the colon tumor prevention activity of 15-PGDH acts as a constant or variable effect among individuals, we determined whether 15-PGDH levels remains stable over subsite and time in the human colon, determined the extent of differences in 15-PGDH levels between different individuals, and determined whether or not 15-PGDH modulation mediates any part of the anti-colon tumor effect of aspirin.
Methods
Using real-time PCR, we measured 15-PGDH mRNA, determining the correlation of 15-PGDH level in replicate colon biopsies, in biopsies from throughout the length of the colon, in repeat biopsies taken 4 months apart, and in paired biopsies of individuals taken before and after aspirin treatment, and by western for 15-PGDH protein in mice.
Results
Colonic 15-PGDH levels varied 4.4-fold across the human population. Within individuals, 15-PGDH levels proved highly reproducible (r=0.81 in duplicate biopsies) and stable along the length of the colon, with average 15-PGDH levels deviating by only 17% from rectum to cecum. An individual’s 15-PGDH levels are also highly stable over time, with a median coefficient of variation over a 4-month interval of only 12%. Last, colonic 15-PGDH levels proved resistant to alteration by aspirin, with only a 10% difference in 15-PGDH levels measured before and after aspirin treatment.
Conclusions
15-PGDH levels vary across the population in a stable and reproducible manner, and are resistant to alteration by aspirin. 15-PGDH represents an independent target for modulation by candidate colon tumor chemopreventive agents.
Keywords: Colon Cancer, 15-PGDH, NSAIDs, Biomarker
INTRODUCTION
Colorectal cancer is the end result of a multistep process of neoplasia that occurs over the course of multiple years [1]. During this process, multiple genetic and epigenetic changes occur, resulting in the activation of oncogenic pathways as well as inactivation of tumor suppressor pathways. Key events include upregulation of expression of the cyclooxygenase-2 (COX-2) oncogene and mutations that inactivate the TGF-β tumor suppressor pathway [2, 3].
We have previously reported the findings that: 15-hydroxprostaglandin dehydrogenase (15-PGDH) is a TGF-β-induced tumor suppressor gene; is highly expressed in normal colonic epithelial cells; and is ubiquitously lost in human colon cancers [4–6]. 15-PGDH is a prostaglandin degrading enzyme and hence is positioned to directly antagonize the oncogenic activity of the prostaglandin synthetic enzyme COX-2 [5, 6]. We have confirmed that 15-PGDH mediates potent colon tumor suppressor activity by showing, first, that restoring 15-PGDH expression in human colon cancer cells blocks their ability to form tumors in nude mice hosts, and, second, that knockout of murine 15-PGDH genes markedly sensitizes mice to development of colon tumors [5, 6]. Knockout of the 15-PGDH gene also elevates levels of the oncogenic prostaglandin PGE2 in the mouse colon, similar to the increases in PGE2 found in human colonic neoplasias [5, 6]. Additionally, 15-PGDH-gene-knockout mice exhibit resistance to the anti-tumor effect of the COX-2 inhibitor, celecoxib [7].
Medicinal antagonists of COX activity have shown remarkable activity as chemopreventive agents that can decrease risk of developing colon adenomas and cancers [8–11]. In particular, aspirin use is associated with decreased development of colon cancer in both observational and intervention studies [12–14].
We and others have hypothesized that colonic 15-PGDH may provide an independent target for the development of colon tumor chemopreventive agents. To determine whether 15-PGDH modulation could provide a potential strategy for colon tumor prevention we have addressed several key questions. First, we determined whether human colonic 15-PGDH mRNA levels are reproducible when measured in replicate biopsies. Second, we determined whether human colonic 15-PGDH mRNA levels are stable along the length of the colon. Third, we determined whether human colonic 15-PGDH mRNA levels are stable over time. Fourth, we determined whether 15-PGDH mRNA levels are similar among different individuals, or whether some individuals are characterized by having low levels of colonic 15-PGDH. Last, we have determined whether aspirin itself modulates colonic 15-PGDH, both at the mRNA level in patients and at the protein level in mice, with a view to ultimately asking whether targeting of 15-PGDH would offer an independent method for regulating colonic prostaglandin levels that would provide an additional and novel strategy for colon tumor chemoprevention.
MATERIALS AND METHODS
Human colon mucosal biopsies
For studies measuring 15-PGDH assay reproducibility and levels across the colon from rectum to cecum, human colon mucosal tissues were obtained by forcep biopsy during screening colonoscopy or colonoscopic polypectomy. At least two specimens were taken from each subsite of colon (ascending, transverse, descending, sigmoid colon and rectum) from 22 patients. At the time of biopsy, samples were flash frozen under liquid nitrogen with subsequent transfer to long-term storage at −70°C. All procedures were performed under patient consent and approved by the institutional review board of Asan Medical Center. Human colon mucosal tissues from a second, independent set of 45 healthy individuals participating in a randomized cross-over trial were used to assess 15-PGDH expression levels over time. From each participant, duplicate sigmoid mucosal biopsies were obtained approximately 4 months apart at the end of each of two 60-day intervention periods, during one of which subjects received aspirin (325 mg/day for 60 days), and during the other of which subjects received placebo, with the order being randomized. Study procedures were approved by the Fred Hutchinson Cancer Research Center institutional review board and participants provided informed, written consent.
Reverse Transcription Quantitative Real-time PCR (RT-qPCR) measurement of 15-PGDH, COX-1, COX-2, and TGFBR2 transcripts in colon
RNA was isolated from colon mucosal biopsies (~5–15 mg) using an RNAqueous® kit (Ambion, Austin, TX) following the manufacturer’s recommended protocol with minor alterations. Briefly, approximately 10 mg of frozen tissue was ground to a fine powder using a liquid-nitrogen-cooled mortar. The samples were then transferred to microcentrifuge tubes containing 400 µl of Lysis/Binding solution. Each sample was then passed through a 26-gauge needle several times until no longer viscous and then loaded onto a filter cartridge for the subsequent washing steps. RNA from each sample was eluted from the filter cartridge using 40 µl of Elution Solution followed by another elution with 20 µl of Elution Solution, for a pooled elution volume of 60 µl. Concentration and quality of the RNA samples were determined using a ND-1000 Spectrophotometer (NanoDrop, Wilmington, DE) and all samples used had an A260/280 ratio value greater than 1.70. All RT-qPCR assays were performed following the MIQE guidelines [15]. cDNA was synthesized from 500 ng of input RNA using AMV Reverse Transcriptase (Roche, Indianapolis, IN) following the manufactures recommended protocol and used for subsequent qPCR assays. Real-time PCR measurement of 15-PGDH, COX-1, COX-2, and TGFBR2 was performed using the human hydrolysis Probe/Primer sets Hs00168359_m1 (15-PGDH (HPGD), NM_000860), Hs00924808_m1 (COX-1 (PTGS1), NM_080591), Hs01573477_g1 (COX-2 (PTGS2), NM_000963), and Hs00559661_m1 (TGFBR2, NM_001024847) from Applied Biosystems (Foster City, CA). A 25ul reaction mix contained 1 µl of cDNA template and a 1:20 dilution of an individual primer/probe set in 1X Supermix (Bio-Rad, CA) and was run on a CFX96 optical module (Biorad, Hercules, CA). Thermal cycling conditions for all assays were 95°C for 4 min, followed by 50 cycles of 95°C for 15 sec and 60°C for 1 min. Cytokeratin 20 (KRT20) was used as the reference gene for normalization and was amplified using the human KRT20 (NM_019010) hydrolysis probe/primer kit Hs00300643_m1 from Applied Biosystems following the same reaction conditions above. KRT20 was selected because it is a specific marker for colonic epithelial mass [7, 16, 17], as well as having uniform expression by microarray analysis across 16 normal colon tissue biopsies and colonic crypt epithelial cells isolated from an additional 5 normal biopsy samples (Markowitz, unpublished data). The level of gene of interest (GOI) RNA was determined as the ratio of GOI:KRT20 = 2 exp (CqGOI – CqKRT20). For each reverse transcription reaction, CqGOI and CqKRT20 values were determined as the average values obtained from three independent real-time PCR reactions. RNA that had not undergone the reverse transcriptase step, as well as a water sample that was carried through the reverse transcriptase step, were used as negative controls. Both controls were negative for all assays performed. Specificity of all PCR primers was confirmed by observing a single band on a gel as well as sequencing amplified product. PCR efficiency, R2, slope, and y intercept for the calibration curve for each assay was as follows 15-PGDH (97.5, 0.998, −3.38, 24.59), KRT20 (97.7, 1.0, −3.38, 23.17), TGFBR2 (97.3, 0.998, −3.39, 26.00), COX1 (93.1, 0.999, −3.49, 28.68), and COX2 (90.9, 0.999, −3.56, 25.70).
Statistical analysis
Average 15-PGDH gene expression and standard errors were calculated using duplicate biopsy measurements throughout the colon (i.e., rectum, sigmoid, descending colon, transverse colon, and ascending colon). Correlation in duplicate biopsies for measurement of 15-PGDH gene expression throughout the colon was determined using the Pearson correlation coefficient (r). Variation in 15-PGDH gene expression throughout the length of the colon was measured using Spearman correlation coefficients (ρ) generating two-sided p-values. Additionally an exploratory comparison of the association between rectal 15-PGDH gene expression and history of adenomas (yes/no) or sex was performed using a Wilcoxon test generating two-sided p-values. Correlation in duplicate biopsies for measurements of COX-1, COX-2, and TGFBR2 gene expression was determined using the Pearson correlation coefficient (r). Differences in 15-PGDH expression in duplicate biopsies taken 4 months apart in aspirin and placebo treated patients as well as control and aspirin treated mice were compared using a Wilcoxon signed rank sum paired test.
Western blots of colonic 15-PGDH from aspirin-treated mice
FVB/NJ mice 8–12 weeks of age were fed for two weeks with either a control AIN-76A diet (Harlan Teklad, Madison, WI) (n=5 mice), or diet supplemented with 300 ppm aspirin (30 mg/kg/day) (LKT laboratories, St. Paul, MN) (n=6 mice) as determined from the literature [18]. Mice were subsequently euthanized and distal colonic mucosal scrapes were collected, snap-frozen in liquid nitrogen, then stored at −80°C. Protein lysates were prepared using T-PER tissue protein extraction reagent (Thermo Scientific, Rockford, IL) supplemented with Roche Complete protease inhibitor cocktail (Roche, Indianapolis, IN), Sigma phosphatase inhibitor Cocktail I, and Sigma phosphatase inhibitor Cocktail II (Sigma, St. Louis, MO). Western blotting for mouse 15-PGDH was performed as previously described [7].
RESULTS
Human colonic mucosal 15-PGDH levels are highly reproducible
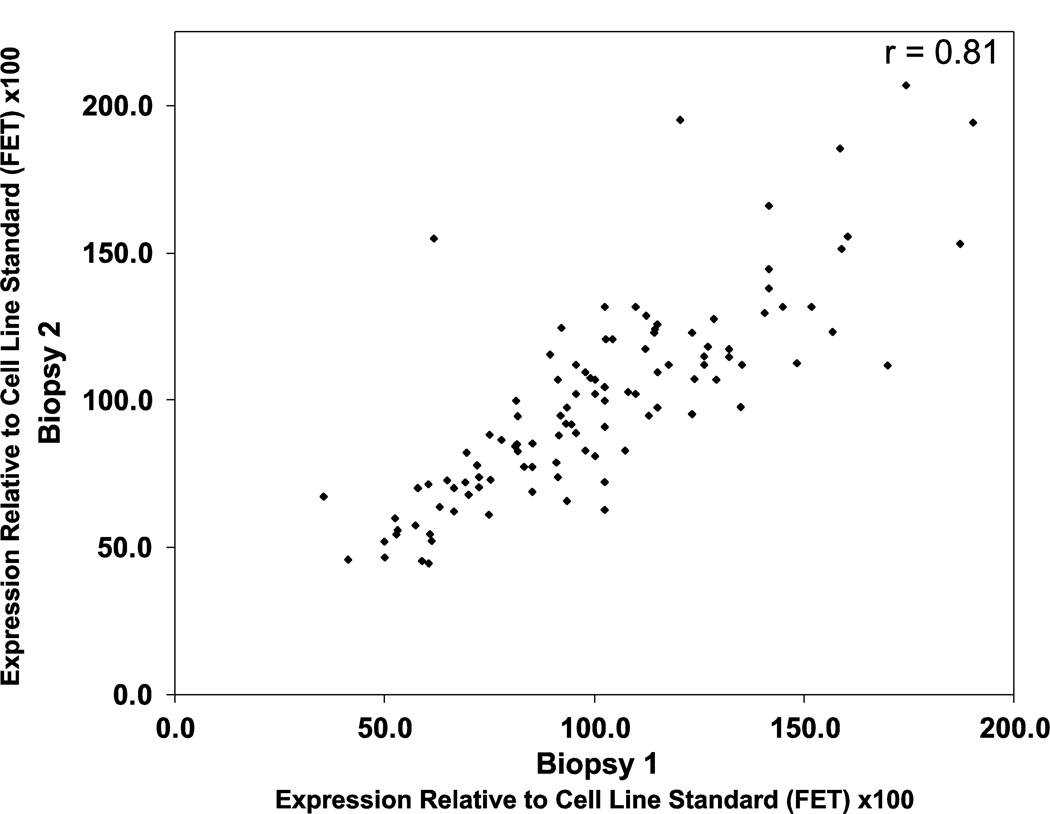
To determine if colonic 15-PGDH levels could be reliably measured in patients, we examined mRNA isolated from 107 duplicate pairs of frozen normal colon mucosal biopsy specimens collected from 22 patients. The duplicate biopsies were collected from the ascending, transverse, descending, sigmoid, and rectal regions of the colon. 15-PGDH transcript levels were measured by RT-qPCR. As shown in Figure 1, measuring the level of 15-PGDH transcript in these duplicate paired biopsies proved highly reproducible and yielded a Pearson correlation coefficient of r=0.81.
Figure 1.
15-PGDH mRNA levels in paired duplicate biopsies of normal colorectal mucosa, as assayed by real-time PCR. Shown is a scatter plot for 15-PGDH assayed in replicate biopsies (biopsy 1, x-axis; biopsy 2, y-axis). 15-PGDH mRNA values are normalized against expression of the house-keeping gene Cytokeratin 20 (KRT20). Expression levels are relative to a cell line internal standard (FET) x100.
Rectal 15-PGDH mRNA levels vary by 4-fold among the population
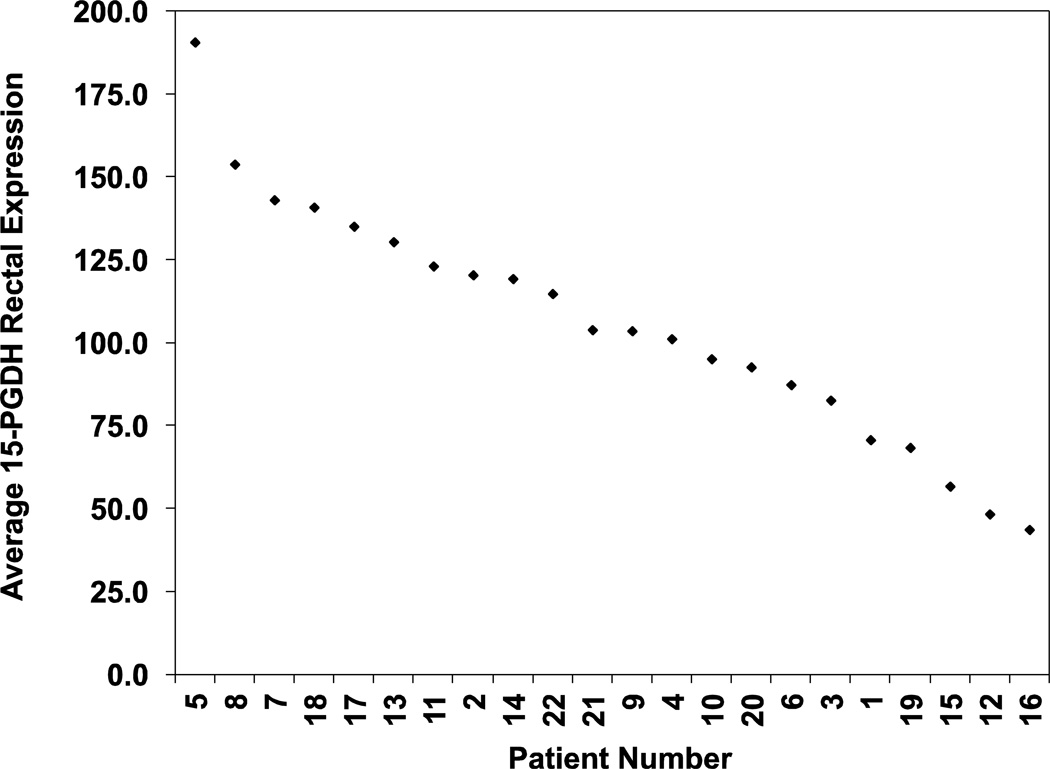
Among these 22 individuals studied, we observed a 4.4-fold range of inter-individual variation in rectal 15-PGDH expression levels, from a low of 43.7 to a high of 190.6 (units of relative expression compared to a reference cell line standard (FET) x100), with 15-PGDH levels being distributed relatively uniformly across this range (Figure 2). Thus rectal 15-PGDH demonstrates pronounced variability among different individuals in the population.
Figure 2.
Rectal 15-PGDH expression levels in 22 patients. Plotted is the average 15-PGDH expression level for duplicate rectal biopsies obtained from 22 patients. Expression levels are relative to a cell line internal standard (FET) x100.
Average 15-PGDH mRNA levels are flat along the human colon
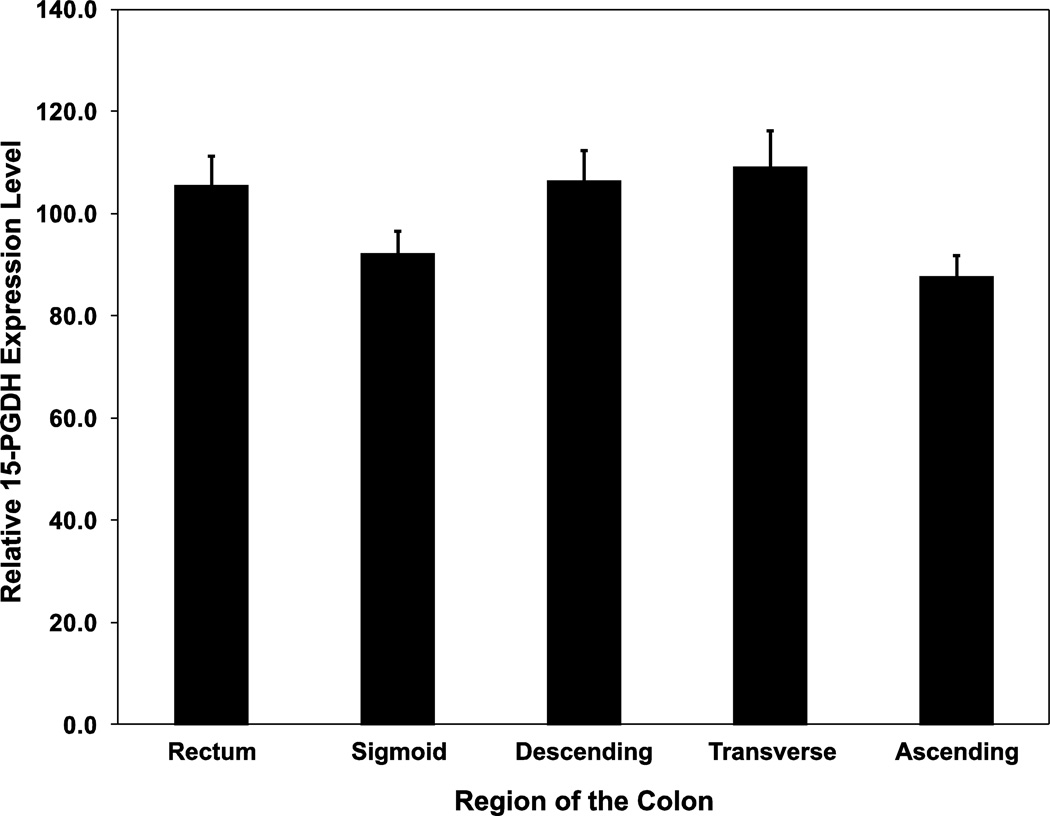
We next determined whether 15-PGDH levels showed any systematic variation along the length of the colon, moving from rectum to ascending colon. We found that within-individual 15-PGDH levels are highly uniform along the length of the colon, with average 15-PGDH levels in the sigmoid, descending colon, transverse colon, and ascending colon all being within 17% of the average rectal 15-PGDH level (Figure 3). Taken together, these data demonstrate that 15-PGDH mRNA levels are consistent across the colon and that a given individual’s colonic 15-PGDH level can be reliably determined.
Figure 3.
15-PGDH expression levels across the colon. Plotted is the average 15-PGDH expression level for each region of the colon as determined in duplicate biopsies from 22 patients. Bars indicate standard error. Expression levels are relative to a cell line internal standard (FET) x100.
The finding that average 15-PGDH levels remained uniform along the length of the colon suggested that rectal 15-PGDH levels could serve as a surrogate for an individual’s pan-colonic 15-PGDH expression level. To test this hypothesis, we determined the within-individual correlation of rectal 15-PGDH level with 15-PGDH levels throughout the other regions of the colon. Spearman correlation coefficient values (ρ) were high, with correlations to rectal 15-PGDH ranging from 0.8 in the sigmoid (P<0.0001), to 0.59 in the transverse colon (P=0.006), and still maintained at 0.47 in the ascending colon (P=0.029) Table 1. Stated otherwise, if individuals are ranked by highest to lowest 15-PGDH levels in their rectum, these ranking are significantly correlated to individuals’ relative 15-PGDH levels at all other locations throughout their colons (dSupplementary Figure 1).
Table 1.
Spearman’s correlation coefficient (ρ) of 15-PGDH expression across the colon.
| Pair-Wise Comparison | ρ Correlation Coefficient | p-value |
|---|---|---|
| Rectum-Sigmoid | 0.80 | p<0.0001 |
| Rectum-Descending | 0.64 | p=0.002 |
| Rectum-Transverse | 0.59 | p=0.006 |
| Rectum-Ascending | 0.47 | p=0.029 |
Pair-wise comparison was performed using the Spearman’s rank correlation test.
15-PGDH mRNA levels are stable over time
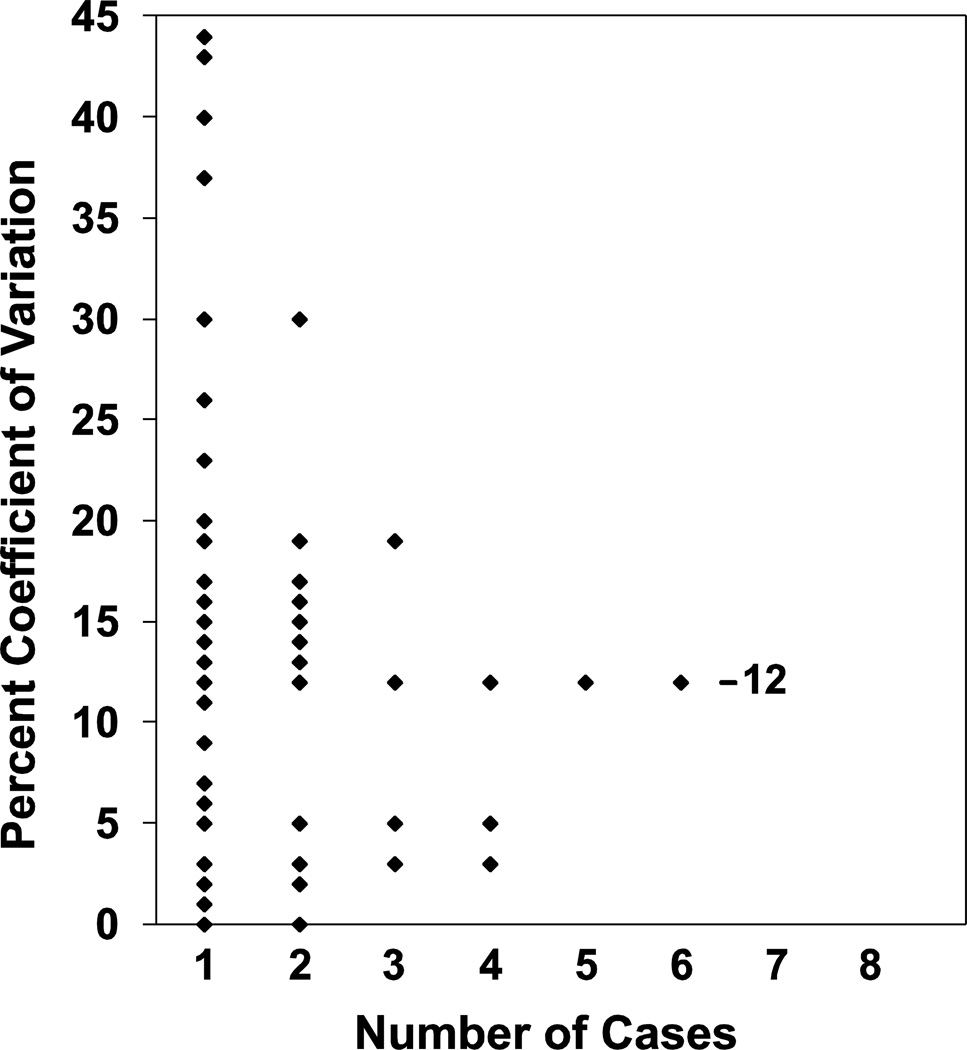
To examine the stability of 15-PGDH expression over time, we studied a cohort of 45 individuals in whom paired sigmoid colon research biopsies had been obtained four months apart. 15-PGDH transcript levels were again measured by RT-qPCR. As shown in Figure 4, we found that 15-PGDH levels are highly stable across time, with a median coefficient of variation between individual’s two 15-PGDH measurements of only 12%.
Figure 4.
Coefficient of variation in rectal 15-PGDH expression level over time. Shown is a histogram that for each of the 45 individuals studied plots the percent coefficient of variation between biopsies taken 4 months apart (Y-axis). The value on the X-axis indicates the number of cases that have the same identical value for a given coefficient of variation. Bar indicates the median coefficient of variation of the population at value of 12%.
15-PGDH mRNA and protein levels are not perturbed by aspirin
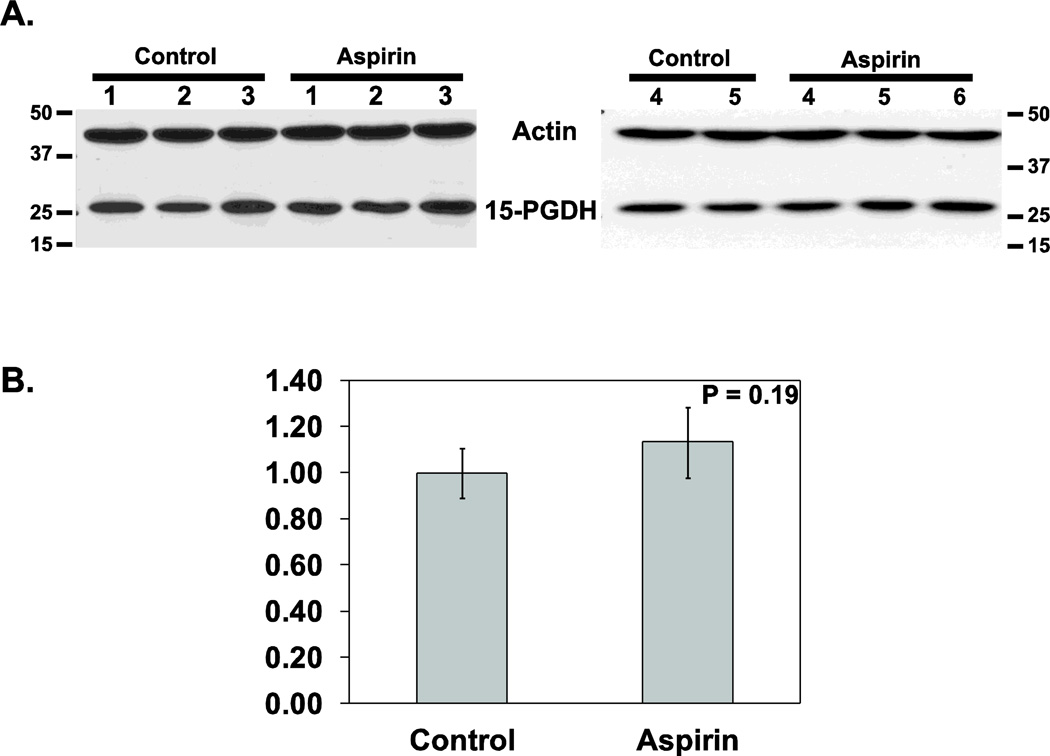
We initially investigated whether 15-PGDH modulation contributes to the antitumor effects of aspirin by study of mouse models. Aspirin was administered for 2 weeks in the diet to 6 test mice, as compared to 5 control mice, with 15-PGDH protein levels then determined by Western blot analysis. Aspirin administration induced a statistically insignificant 13% increase in 15-PGDH protein level (P=0.19 (Wilcoxon signed rank sum paired test); Figure 5A&B).
Figure 5.
15-PGDH protein levels in murine colonic mucosa. (A) Western analysis of 15-PGDH protein expression determined in colonic mucosa from mice receiving 2 weeks of a control diet (n=5) or 2 weeks of an aspirin-containing diet (n=6). Actin protein levels serve as a loading control. (B) Graph of 15-PGDH protein expression levels in the above mice cohorts after normalizing 15-PGDH expression to actin loading control. Bars indicate standard deviation.
Conveniently, it was also the case that the 45 individuals whose paired sigmoid biopsies we had assayed had been participants in a study in which each subject had been treated with 60 days of aspirin prior to one sigmoid biopsy, and with 60 days of placebo prior to the other biopsy, with the order of aspirin and placebo administration randomly assigned for each subject. As is implicit in the finding of stability of 15-PGDH transcript levels over time in this cohort, breaking the code of whether a biopsy was taken following aspirin or placebo confirmed that aspirin administration had little effect on colonic 15-PGDH levels among these subjects. When separately compared, biopsies taken after 60 days of aspirin administration showed a small but statistically non-significant increase in 15-PGDH levels compared to biopsies taken following placebo (aspirin versus placebo mean difference = 10.0%, P=0.12 (Wilcoxon signed rank sum paired test)).
Comparison of rectal 15-PGDH mRNA levels with clinical variables of interest
Although this study was not powered to examine secondary hypotheses, we nonetheless conducted an exploratory comparison of the relationship of rectal 15-PGDH to clinical and demographic variables of interest. Provocatively, we noted that rectal 15-PGDH was lower among individuals with adenomas (mean 100 ± 40 (units of relative expression compared to an internal cell line standard (FET) x100)) than without adenomas (mean 120 ± 20) (P=0.2), and that rectal 15-PGDH was also lower among males (mean 98 ± 39) than among females (mean 121 ± 24) (P=0.12) (Table 2). Although, in this small sample, the findings fall short of statistical significance (p>0.05), they generate hypotheses that will certainly be of interest to test in larger studies.
Table 2.
Association of 15-PGDH expression levels with Clinical variables of interest.
| Clinical Variable | Status | N= | Mean | p-value |
|---|---|---|---|---|
| Adenoma (Y/N) | No | 6 | 119.9 | 0.20 |
| Yes | 16 | 100.4 | ||
| Sex | Male | 15 | 98.4 | 0.12 |
| Female | 7 | 121.5 | ||
P values were determined using the non-parametric Wilcoxon test. Mean values are expressed as units of relative expression compared to an internal cell line standard (FET) ×100)
Colonic 15-PGDH mRNA measurements are more clinically reproducible than are COX-1, COX-2, or TGFBR2
15-PGDH is a TGF-β pathway regulated gene and an antagonist to the COX-mediated prostaglandin production pathway [6, 19]. Both are pathways that play an important role in inflammation and progression of colon cancer. As such, 15-PGDH is uniquely positioned at the nexus of TGF-β signaling and prostaglandin-mediated inflammation. To test whether 15-PGDH expression would be the more reliable clinical marker for measuring the activity of these pathways in normal colonic mucosa, we compared the Pearson’s r value for measuring 15-PGDH mRNA in duplicate colonic mucosal biopsies with the Pearson’s r values obtained from real-time PCR assays for the prostaglandin pathway markers, COX-1 and COX-2, as well as for the TGF-β signaling pathway marker, TGFBR2, performing each of these assays on the same set of duplicate colonic biopsies described earlier. Upon analysis, the clinical reproducibility of the 15-PGDH assay was highly robust (r=0.81) and was superior to that for COX-1 (r=0.67), COX-2 (r=0.69), or TGFBR2 (r=0.75).
DISCUSSION
In this study, we first demonstrated that colonic 15-PGDH mRNA levels show substantial variability among people, with a distinct set of individuals characterized by having lower colonic 15-PGDH expression compared to the population as a whole. We have further shown that the determination of these differences between individuals is highly reliable, demonstrating first that measurement of 15-PGDH mRNA is robust and highly reproducible in duplicate colonic mucosal biopsies (r=0.81). 15-PGDH levels proved reproducible in duplicate biopsies taken at the same time, as well as in duplicate biopsies taken 4 months apart. Moreover, 15-PGDH mRNA expression proved uniform along the length of the bowel from ascending colon to rectum, with rectal 15-PGDH levels statistically significantly correlated with 15-PGDH levels throughout the colon. Rectal 15-PGDH mRNA measurements are thus a reliable assay for classifying individuals as having high versus low pan-colonic 15-PGDH levels. Last, we find that colonic 15-PGDH mRNA and protein levels are resistant to alteration by aspirin administration. Thus, modulation of 15-PGDH represents a potential independent target for other chemopreventive agents.
Our finding that colonic 15-PGDH mRNA levels are highly variable is consistent with our earlier observation in a smaller cohort of subjects [7]. However, in that study we lacked replicate biopsies to determine whether the variation observed was truly due to differences between individuals, or to variability in measurement of 15-PGDH [7]. Taken together, the two studies suggest that a >4-fold variation in 15-PGDH is the norm among the general population. In our initial pilot study [7], lower levels of 15-PGDH mRNA were associated with development of colon adenomas. In our current study, individuals with adenomas had lower average 15-PGDH level than individuals who were adenoma free, although with the small number of individuals with adenomas in the current cohort, this difference falls well short of statistical significance. However, taken cumulatively, the available data from human subjects and from mouse models clearly supports the value of testing the association between low 15-PGDH and increased adenoma risk in an adequately powered and substantially larger human cohort.
Aspirin and other non-steroidal anti-inflammatory agents are currently the best validated agents for reducing risk of developing colon adenomas and colon cancers. However, aspirin is associated with risk of gastrointestinal bleeding and COX-2 inhibitors such as celecoxib and rofecoxib have been associated with cardiovascular toxicity [20–22]. Moreover, these agents are not uniformly effective. In murine models and in a pilot human study, we have suggested that low levels of colonic 15-PGDH may confer resistance to celecoxib as a colon tumor chemopreventive agent [7]. The data from this study shows that the chemopreventive effects of aspirin do not involve any significant increase of colonic 15-PGDH levels. Thus, development of agents that could increase colonic 15-PGDH levels would provide an independent strategy for colon tumor chemoprevention, and may be particularly applicable to individuals, such as some of those described in this study, who are relatively low expressers of colonic 15-PGDH.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank James Lutterbaugh for skillful technical assistance.
Grant Support: Supported by PHS award 1U01 CA152756-01 (SDM), 1P50CA150964 (SDM), by NCI award P30-CA043703 (JBS), R01-CA94954 (JDP), and by the National Research Foundation of Korea (2011-0019632), Korean Health Technology R&D Project (A062254), and KRIBB Research Initiative Program (KGM2210911) (SJM)
Abbreviations
- 15-PGDH
15-hydroxyprostaglandin dehydrogenase
- COX-1
Cyclooxygenase-1
- COX-2
Cyclooxygenase-2
- TGFBR2
TGF-Beta Type II Receptor
Footnotes
Disclosure of potential conflicts of interest: No potential conflicts of interest are present.
References
- 1.Markowitz SD, Dawson DM, Willis J, et al. Focus on colon cancer. Cancer Cell. 2002;1(3):233–236. doi: 10.1016/s1535-6108(02)00053-3. [DOI] [PubMed] [Google Scholar]
- 2.Grady WM, Markowitz SD. Genetic and epigenetic alterations in colon cancer. Annu Rev Genomics Hum Genet. 2002;3:101–128. doi: 10.1146/annurev.genom.3.022502.103043. [DOI] [PubMed] [Google Scholar]
- 3.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361(25):2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Backlund MG, Mann JR, Holla VR, et al. 15-Hydroxyprostaglandin dehydrogenase is down-regulated in colorectal cancer. J Biol Chem. 2005;280(5):3217–3223. doi: 10.1074/jbc.M411221200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myung SJ, Rerko RM, Yan M, et al. 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proc Natl Acad Sci U S A. 2006;103(32):12098–12102. doi: 10.1073/pnas.0603235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan M, Rerko RM, Platzer P, et al. 15-Hydroxyprostaglandin dehydrogenase, a COX-2 oncogene antagonist, is a TGF-beta-induced suppressor of human gastrointestinal cancers. Proc Natl Acad Sci U S A. 2004;101(50):17468–17473. doi: 10.1073/pnas.0406142101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan M, Myung SJ, Fink SP, et al. 15-Hydroxyprostaglandin dehydrogenase inactivation as a mechanism of resistance to celecoxib chemoprevention of colon tumors. Proc Natl Acad Sci U S A. 2009;106(23):9409–9413. doi: 10.1073/pnas.0902367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arber N, Eagle CJ, Spicak J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355(9):885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 9.Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348(10):891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 10.Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355(9):873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 11.Sandler RS, Halabi S, Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348(10):883–890. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 12.Bastiaannet E, Sampieri K, Dekkers OM, et al. Use of aspirin postdiagnosis improves survival for colon cancer patients. Br J Cancer. 2012;106(9):1564–1570. doi: 10.1038/bjc.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burn J, Gerdes AM, Macrae F, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378(9809):2081–2087. doi: 10.1016/S0140-6736(11)61049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan AT, Arber N, Burn J, et al. Aspirin in the chemoprevention of colorectal neoplasia: an overview. Cancer Prev Res (Phila) 2012;5(2):164–178. doi: 10.1158/1940-6207.CAPR-11-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bustin SA, Benes V, Garson JA, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55(4):611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 16.Chan CW, Wong NA, Liu Y, et al. Gastrointestinal differentiation marker Cytokeratin 20 is regulated by homeobox gene CDX1. Proc Natl Acad Sci U S A. 2009;106(6):1936–1941. doi: 10.1073/pnas.0812904106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moll R, Schiller DL, Franke WW. Identification of protein IT of the intestinal cytoskeleton as a novel type I cytokeratin with unusual properties and expression patterns. J Cell Biol. 1990;111(2):567–580. doi: 10.1083/jcb.111.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williamson SL, Kartheuser A, Coaker J, et al. Intestinal tumorigenesis in the Apc1638N mouse treated with aspirin and resistant starch for up to 5 months. Carcinogenesis. 1999;20(5):805–810. doi: 10.1093/carcin/20.5.805. [DOI] [PubMed] [Google Scholar]
- 19.Markowitz SD. Aspirin and colon cancer--targeting prevention? N Engl J Med. 2007;356(21):2195–2198. doi: 10.1056/NEJMe078044. [DOI] [PubMed] [Google Scholar]
- 20.Baron JA, Sandler RS, Bresalier RS, et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology. 2006;131(6):1674–1682. doi: 10.1053/j.gastro.2006.08.079. [DOI] [PubMed] [Google Scholar]
- 21.Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352(11):1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 22.Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352(11):1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.