Abstract
In this study, we explored the growth dynamics of Rickettsia sp. phylotype G021 during transovarial transmission and transstadial passage by Ixodes pacificus using real-time quantitative PCR. Four parental engorged I. pacificus females were allowed to complete their developmental stages until the F2-generation eggs yielded unfed larvae. All eggs, larvae, nymphs, and adults tested through 2 generations were found to be infected with phylotype G021. Hence, we conclude that the efficiency of transovarial transmission and transstadial passage of this phylotype in I. pacificus was 100%. Acquisition of a blood meal by all 3 parasitic stages (larva, nymph, adult) significantly increased the rickettsial burden as fed larvae, nymphs, and adults had respective 19-, 12-, and 313-fold increases of rickettsiae compared with unfed ticks representing each developmental stage. I. pacificus eggs contained high rickettsial burdens at the time of oviposition. While I. pacificus egg cells underwent rapid proliferation during early embryonic development, the rickettsiae remained relatively quiescent, which resulted in depressed numbers of phylotype G021 per tick cell. However, the rickettsial burden remained constant over a period of 56 days, as the rate of I. pacificus cell division slowed during later embryonic development.
Keywords: Rickettsia, Ixodes pacificus, Transovarial transmission, Transstadial passage, Growth dynamics
Introduction
Long-lasting interactions between arthropods and bacteria are commonly found in nature (Baumann, 2005). Although the maintenance of bacterial infections in arthropods is driven by several transmission routes, vertical transmission is crucial for the stable maintenance of some bacterial species from one generation to the next (Bright and Bulgheresi, 2010; Buchner, 1965). Many bacteria transmitted by ticks are transmitted vertically via transovarial transmission and transstadially from stage to stage, such as certain Rickettsia species in ticks (Horta et al., 2006; Socolovschi et al., 2009), cat fleas (Azad et al., 1992) or mites (Takahashi et al., 1988), and Borrelia species (Lane and Burgdorfer, 1987; Scoles et al., 2001), as well as Francisella-like endosymbionts and Anaplasma species in ticks (Baldridge et al., 2009). Compared with other transmission routes, transovarial transmission is a highly efficient mechanism for perpetuating certain bacteria (Matsumoto et al., 2005).
The western black-legged tick, Ixodes pacificus Cooley & Kohls, which is broadly distributed in the far-western United States, is the primary vector of the bacteria causing Lyme borreliosis and anaplasmosis (Burgdorfer et al., 1985; Clover and Lane, 1995; Foley et al., 2008; Piesman et al., 1999). Its life cycle requires 3 years to complete and includes the egg and 3 parasitic stages, the larva, nymph, and adult. The larva and nymph require a blood meal in order to develop into the next stage, whereas the adult female needs blood to mature a batch of about 900–1,000 eggs (Padgett and Lane, 2001). During the blood meal, ticks undergo profound expansion in mass and regulation of internal cellular and molecular pathways. In response, the quantity and location of bacteria in ticks also undergo dramatic changes (Liu et al., 2011; Radolf et al., 2012).
Spotted fever group rickettsiae are Gram-negative, intracellular bacteria commonly found in association with ixodid ticks (Boretti et al., 2009; Dalton et al., 1995). Unlike pathogenic rickettsiae that cause human and animal diseases, some nonpathogenic rickettsiae in the spotted fever group are bacterial endosymbionts (Douglas, 2007). Endosymbiotic rickettsial species are nonpathogenic and intracellular, are transmitted transovarially from female arthropods to their offspring, and have an intimate and persistent relationship with the hosts (Douglas, 2007; Perlman et al., 2006; Sakurai et al., 2005). The rickettsial endosymbionts have been identified in many tick hosts, such as I. scapularis, I. ricinus, and Amblyomma americanum, to name but a few (Jasinskas et al., 2007; Weller et al., 1998).
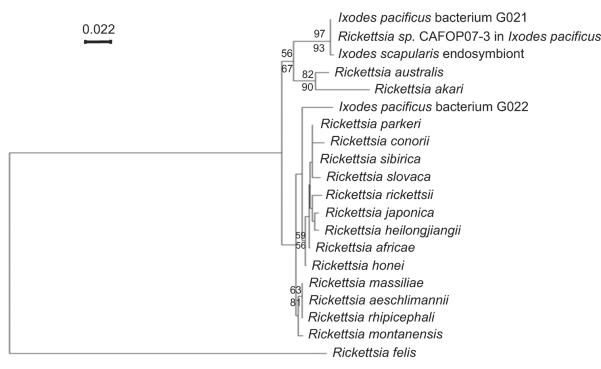
Recently, 2 rickettsial phylotypes, G021 and G022, were detected in host-seeking I. pacificus collected in Napa, California. Phylogenetic analysis suggested that phylotype G021 is closely related to a rickettsial endosymbiont infecting I. scapularis, whereas phylotype G022 is a novel, deeply-branched spotted fever group Rickettsia (Fig. 1) (Phan et al., 2011). The prevalence of phylotype G021 in I. pacificus collected from 7 counties in California was 100%, which suggested that it is an endosymbiotic rickettsia that is transmitted transovarially and passed transstadially (Cheng et al., in press).
Fig. 1.
Phylogenetic tree of the ompA genes of Rickettsia phylotypes G021 and G022 in Ixodes pacificus. Other selected sequences on the phylogenetic tree of the ompA gene are Rickettsia sp. CAFOP07-3 (EU544297.1), I. scapularis endosymbiont (AB002268.1), R. aeschlimannii (DQ379981.1), R. massiliae (DQ212707.1), R. rhipicephali (EU109177.1), R. australis (AF149108.1), R. montanensis (AF045223.1), R. sibirica (AABW01000001.1), R. rickettsii (AY319293.1), R. slovaca (EU622810.1), R. honei (AF018075.1), R. africae (EU622980.1), R. parkeri (EU715288.1), R. heilongjiangii (AF179362.2), R. conorii (U43794.1), R. felis (AY727036.1), R. japonica (U83442.1), and R. akari (L01461.1). The tree is inferred from the comparison of ompA sequences by the neighbor-joining method using PHYLO_WIN. The numbers at nodes are the bootstrap values obtained from 1000 replicates. Bootstrap values >50 are shown at the nodes. Bootstrap values determined by the neighbor-joining method and maximum-parsimony are shown above the line and below the line, respectively. Bar, nucleotide distance. The phylogenetic tree of rickettsial ompA genes was provided with permission of use by the journal of “Vector-borne and Zoonotic Diseases” (Phan et al., 2011).
The objective of the current study was to experimentally evaluate the rate of transovarial transmission and transstadial passage of G021 in I. pacificus through the first (F1) and part of the second (F2) generation by real-time quantitative PCR. In addition, the temporal pattern of bacterial growth in all developmental stages of I. pacificus, as determined by real-time quantitative PCR, was determined to further study the interaction of phylotype G021 with its tick host.
Materials and methods
Ticks
Host-seeking I. pacificus adults were collected by dragging a white flannel cloth, 1×1.25 m, over low vegetation in October 2009 at the University of California Hopland Research and Extension Center in Mendocino County, California (Universal Transverse Mercator coordinates: 10N 492815 4316885). Additionally, 18 engorged female ticks were collected from dogs brought into the Mendocino County Animal Care Services in Ukiah, California, in May, June, and July 2010. All I. pacificus were maintained in desiccators (Fisher Scientific, Houston, TX) at 25°C and 90% relative humidity. The day/night cycle was set at 12 hours light:12 hours dark.
Laboratory rearing and experimental design
All procedures followed protocols approved by the Institutional Animal Care and Use Committee at Humboldt State University. To study the transmission routes of phylotype G021 by I. pacificus, 16 ten-week-old male New Zealand white rabbits (Oryctolagus cuniculus) (Western Oregon Rabbit Co., Philomath, OR) were used for feeding ticks. Four rabbits were used for each of the parasitic developmental stages, including the parental female, F1-generation larvae, F1-generation nymphs, and F1-generation females. The ticks were placed in tin capsules (1.5×1 inches) previously glued onto pre-shaven skin on the rabbits' dorsal thoracic and abdominal regions. Engorged ticks were removed from the capsules following detachment (Eisen et al., 2003).
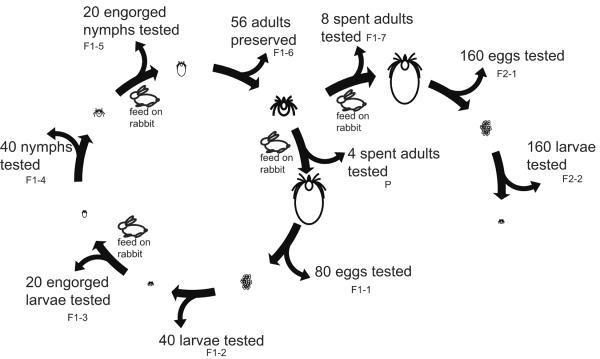
Initially, 30 female and 30 male ticks were fed on each rabbit. Next, 4 engorged I. pacificus parental females were allowed to complete their developmental cycles. Offspring from the 4 lineages in the F1 generation including 80 eggs, 40 larvae, 20 engorged larvae, 40 nymphs, 20 engorged nymphs, and 56 adults, were preserved in 95% ethanol at 4°C for further studies. In the F2 generation, 2 engorged adults were used in each lineage to construct 2 sublineages. In total, 8 sublineages including 8 engorged adults, 160 eggs, and 160 larvae, were saved in 95% ethanol at 4°C for further studies (Fig. 2).
Fig. 2.
Diagram depicting the experimental design used to study the transmission routes of the Rickettsia sp. phylotype G021 in Ixodes pacificus. Two generations of I. pacificus were fed to repletion on New Zealand white rabbits. The procedure for feeding ticks and preserving samples are shown on the diagram.
To study the growth dynamics of phylotype G021 in eggs of I. pacificus, 50 eggs from a laboratory-reared engorged female were collected 1, 4, 10, 16, 32, 45, and 60 days after tick oviposition and stored in 95% ethanol at 4°C, prior to DNA extraction. For field-derived engorged I. pacificus females, 5 eggs were collected from each of the 18 engorged females. Spent females and their eggs were preserved in 95% ethanol at 4°C.
DNA extraction
The DNA extraction method was described previously (Zhong et al., 2007). Briefly, ticks representing all stages were extracted individually. Eggs, flat and engorged larvae, flat and engorged nymphs, and flat adults were surface-sterilized in 70% ethanol 3 times and pulverized in liquid nitrogen by applying DNA/DNase-free plastic pestles in microcentrifuge tubes. The larger engorged female adults were cut through the sagittal plane into 2 parts with sterilized surgical blades prior to pulverization. Genomic DNA from each tick sample was extracted using DNeasy Blood & Tissue Kits (QIAGEN, Valencia, CA).
Cloning and sequencing
The gene encoding the outer membrane protein A (ompA) of rickettsial phylotypes G021 (GenBank accession number: GQ375161) and G022 (GQ375162) and the actin gene (GU556973) of I. pacificus were cloned into the pSC-A-amp/Kan plasmid (Stratagene, La Jolla, CA) in order to set up DNA standards for quantification in real-time quantitative PCR. Both ompA and actin genes are single-copy genes. The cloning of the genes was performed as instructed by the manufacturer (Stratagene, La Jolla, CA). DNA plasmids were purified by PureYield™ Plasmid Miniprep System (Promega, Madison, WI), and concentrations were determined with a NanoDrop™ 1000 Spectrophotometer (Thermo Scientific, Wilmington, DE). The sequences of clones were confirmed by DNA sequencing (Elim Biopharm, Inc., Hayward, CA).
Real-time quantitative PCR (qPCR)
qPCR was used to assess the quantity of bacteria per tick cell (Zhong et al., 2007). Specifically, the copy number of the rickettsial ompA and tick actin genes was used to detect the bacteria (phylotypes GO21 and G022) and correlate their numbers with the host, I. pacificus. Quantification of gene copy numbers was carried out using a Taqman qPCR assay run on an Applied Biosystems 7300 Real-Time PCR System (Life Technologies Corporation, Carlsbad, CA). PCR conditions consisted of an initial denaturation step at 95°C for 10 min and 40 cycles of denaturation at 95°C for 30 s and annealing-elongation at 60°C for 60 s. Reagent per reaction consisted of qPCR MasterMix containing ROX passive reference (AnaSpec, Fremont, CA), 0.25 μM of each primer and probe, and 0.04% final concentration of bovine serum albumin. The lowest detectable DNA copy numbers for each gene were determined earlier to be 3.1 for phylotype G021, 3.6 for phylotype G022, and 2.8 for the actin gene of I. pacificus (Cheng et al., in press).
Statistical analysis
The SPSS statistical software package (version 17) (IBM SPSS North American Headquarters, Chicago, IL) was utilized to carry out all statistical analyses. One-way ANOVA and Tukey's Post-hoc tests were used to determine the presence of statistically significant differences between rickettsial burdens in the developmental stages of I. pacificus. A P value less than 0.05 was considered statistically significant.
Results
Transmission routes of the rickettsial phylotype G021 by Ixodes pacificus
All 4 parental females tested positive for phylotype G021, but none of them tested positive for phylotype G022. To investigate the transovarial transmission and transstadial passage of phylotype G021 in I. pacificus, laboratory-reared engorged females were allowed to oviposit. A total of 588 tick samples was tested for infection with phylotype G021, including 4 engorged adults, 80 eggs, 40 larvae, 20 engorged larvae, 40 nymphs, 20 engorged nymphs, and 56 adults in the F1 generation and 8 engorged adults, 160 eggs, and 160 larvae in the F2 generation. Of these, all eggs and larvae tested positive for the phylotype; thus the transovarial transmission rate was 100% in each generation of I. pacificus. Likewise, G021 was transstadially passed among tick developmental stages at a rate of 100% because all 40 nymphs and 56 adults tested in the F1-generation were found to contain this rickettsia (Table 1).
Table 1.
Transovarial transmission and transstadial passage of Rickettsia sp. phylotype G021 in Ixodes pacificus ticks that had fed on New Zealand white rabbits.
| Generational cohort | ||||||||
|---|---|---|---|---|---|---|---|---|
| Tick | Cohort #1 |
Cohort #2 |
Cohort #3 |
Cohort #4 |
||||
| stages | total | positive (% of transmission) | total | positive (% of transmission) | total | positive (% of transmission) | total | positive (% of transmission) |
| Parental adult | 1 | 1(100%) | 1 | 1(100%) | 1 | 1(100%) | 1 | 1(100%) |
| F1 egg | 20 | 20(100%) | 20 | 20(100%) | 20 | 20(100%) | 20 | 20(100%) |
| F1 larva | 10 | 10(100%) | 10 | 10(100%) | 10 | 10(100%) | 10 | 10(100%) |
| F1 nymph | 10 | 10(100%) | 10 | 10(100%) | 10 | 10(100%) | 10 | 10(100%) |
| F1 adult | 12 | 12(100%) | 20 | 20(100%) | 15 | 15(100%) | 9 | 9(100%) |
| F1 spent adult | 2 | 2(100%) | 2 | 2(100%) | 2 | 2(100%) | 2 | 2(100%) |
| F2 egg | 40 | 40(100%) | 40 | 40(100%) | 40 | 40(100%) | 40 | 40(100%) |
| F2 larva | 40 | 40(100%) | 40 | 40(100%) | 40 | 40(100%) | 40 | 40(100%) |
The rate of transovarial transmission by G021 by I. pacificus also was determined using field-derived engorged females. Eighteen engorged I. pacificus females were collected from domestic dogs. Five eggs harvested from each engorged female, and all 90 eggs tested, were positive for G021, which reconfirmed that the rate of transovarial transmission of phylotype G021 by I. pacificus is 100%.
Burden of phylotype G021 in the developmental stages of I. pacificus
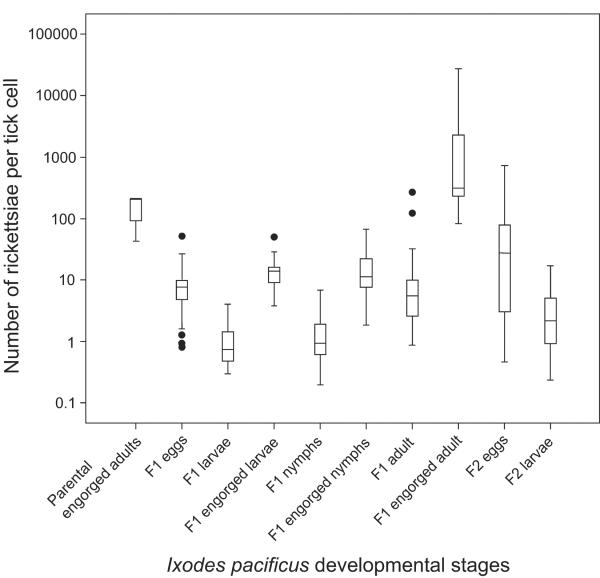
The burden of the Rickettsia sp. phylotype G021 varied in different developmental stages of I. pacificus (Fig. 3). A one-way ANOVA demonstrated significant differences in the burden between developmental stages (F=13.80, p<0.001). Tukey's post-hoc analysis revealed that unfed larvae and unfed nymphs had the lowest burden, with median burdens of 0.73 and 0.94 per tick cell, respectively (p<0.01). The rickettsial burdens of engorged larvae and engorged nymphs were significantly higher than those in unfed larvae (p<0.001) and nymphs in the F1 generation (p<0.001), respectively. There was an 18.8-fold increase in the median rickettsial burden in larvae after a blood meal (i.e., from 0.73 per tick cell in unfed larvae to 13.73 per tick cell in engorged larvae). Similarly, a 12.0-fold increase in rickettsial burden was observed from unfed nymphs (0.94 per tick cell) to engorged nymphs (11.26 per tick cell). The significant increase observed in the burden of rickettsiae from unfed ticks to engorged ticks was even more dramatic for the adult stage. Specifically, the median burden was 5.44 per tick cell in unfed adults, whereas it underwent a 57.5-fold increase and reached 312.7 per tick cell in engorged adults (p<0.01). In contrast, Tukey's post-hoc analysis demonstrated that there was no significant difference in rickettsial burden between any of the 4 engorged stages (i.e., parental engorged adult, F1-generation engorged larva, F1-generation engorged nymph, and F1-generation engorged adult) (p>0.05).
Fig. 3.
Box-whisker plot of the ratio of the ompA gene of Rickettsia sp. phylotype G021 and the β-actin gene of Ixodes pacificus in the developmental stages of this tick.
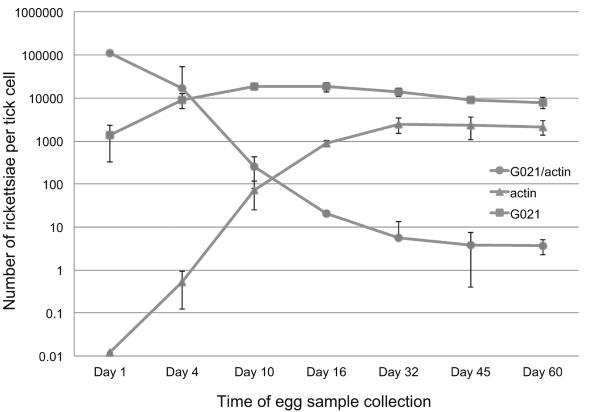
Although there was no significant difference in rickettsial burden in larvae between the F1 and F2 generations (p>0.05), the burden in eggs between the 2 tick generations differed significantly (p<0.001) (Fig. 3). Further, the rickettsial burden in eggs was found to be significantly different between any of the 2 sublineages of 4 engorged ticks in the F2 generation (p<0.05). Because the eggs from the engorged females were collected and preserved at different days after oviposition (days of eggs collected after laid was day 14 for cohort #1, day 20 for cohort #2, day 30 for cohort #3, and day 11 for cohort #4 in the F1 generation), we investigated if the differences in the rickettsial burden in eggs from the 2 generations were due to differences in their ages. To that end, eggs were collected from one engorged female of I. pacificus on days 1, 4, 10, 16, 32, 45, and 60 after tick oviposition. On day 1 post-oviposition, the actin gene was at a relatively low average copy of 4.03E-2 per egg, as determined by qPCR. Between day 1 and day 10 after the eggs were laid, however, an exponential increase in the copy number of the actin gene was observed; the average copy of the actin gene on day 10 was 71.53. Beginning on day 10, the curve began to flatten out and reached a plateau by day 32, which had an average of 2474.9 copies of the actin gene per tick (Fig. 4).
Fig. 4.
Growth dynamics of rickettsial phylotype G021 in the developing egg of Ixodes pacificus. Lines with triangular dots indicate the copy number of the β-actin gene. Lines with square dots indicate the copy number of the ompA gene of phylotype G021. Lines with round dots indicate the ratio of rickettsial ompA gene over the β-actin gene of I. pacificus.
It should be considered that rickettsial phylotype G021 began at a relatively high quantity on day 1, with an average of 1345.93 copies per egg. The quantity kept growing until it reached a plateau on day 10, with an average of 18,430 copies per egg. The increase in copy numbers of phylotype G021 was slower than that of the I. pacificus actin gene. The average ratio of phylotype G021 versus the actin gene started from as high as 110,213 rickettsiae per cell at day 1, then declined throughout the development of the egg until it reached a plateau on day 45 and day 60 with 3.89 and 3.71 rickettsiae per tick egg cell, respectively (Fig. 4).
Discussion
In total, 588 individual I. pacificus samples from 4 lineages embracing all developmental stages and 2 generations of I. pacificus were used to investigate the transmission routes of the rickettsial phylotype G021 in ticks. We discovered that phylotype G021 is a bacterium maintained with 100% efficiency in populations of I. pacificus by transovarial transmission and transstadial passage (Table 1). We also found that phylotype G021 undergoes massive proliferation in ticks following a blood meal.
A growing body of literature indicates that many Rickettsia species are maintained in their tick hosts by transovarial transmission and transstadial passage, but only a few Rickettsia species are transovarially and transstadially transmitted by individual ticks at an efficiency of 100%. Rickettsia bellii manifested 100% transovarial transmission and transstadial passage rates through 2 generations in Ixodes loricatus (Horta et al., 2006). The transovarial transmission and transstadial passage rates for R. africae in both Amblyomma variegatum and Amblyomma aureolatum were observed to be 100% (Labruna et al., 2011; Socolovschi et al., 2009). Besides Ixodes and Amblyomma species ticks, Rhipicephalus turanicus females transmit R. massiliae to their offspring through transovarial transmission (100% efficiency) and transstadial passage (98.5% efficiency) (Matsumoto et al., 2005). Unlike the stable maintenance of phylotype G021 in I. pacificus, however, some rickettsial species lose their ability to be transovarially transmitted by the second generation of ticks (Macaluso et al., 2002). Our finding of 100% efficiency of the transmission of phylotype G021 by I. pacificus suggests that this spotted fever group rickettsia is an endosymbiont of the western black-legged tick. Although phylotype G021 theoretically could be transmitted by horizontal transmission as well, its efficient transovarial transmission and transstadial passage are sufficient in and of themselves to maintain the infection within populations of I. pacificus.
Ticks and other blood-sucking arthropods take blood meals to nourish themselves, to complete their developmental cycle, and to produce eggs (Beaty and Marquardt, 1996). The blood meal, which is enriched with hemoglobin and other nutrients, stimulates replication of microbes and changes microbial population structure within the arthropods (Wang et al., 2011). However, digestion of the hemoglobin in the midgut of the arthropods results in the release of heme, a toxic molecule that is considered to be a pro-oxidant that exerts cytolytic activity on cellular phospholipid membranes of microbes (Graca-Souza et al., 2006). Despite the pro-oxidant environment after the blood meal, the burden of phylotype G021 increased by roughly 10 to 100-fold following ingestion of the blood meal in all 3 parasitic stages of I. pacificus (Fig. 3). Similarly, the cellular growth of several other bacterial species in ticks reportedly proliferates after a blood meal. The burden of Candidatus Midichloria mitochondrii, a ubiquitous intramitochondrial bacterium in I. ricinus ticks, also multiplies after a tick blood meal (Sassera et al., 2006, 2008). Rapid multiplication of the agent of Lyme borreliosis, B. burgdorferi, occurs during and after the blood meal in I. scapularis (De Silva and Fikrig, 1995; Piesman et al., 1990). In Aedes aegypti, microbiota in its midgut and ovary increased dramatically during digestion of the blood meal (Oliveira et al., 2011). Our finding that phylotype G021 proliferates after a blood meal suggests that this rickettsial symbiont has metabolic adaptations against the pro-oxidant environment. However, the exact molecular mechanisms responsible for the multiplication of Rickettsia species in I. pacificus await discovery.
Interestingly, eggs in the F2 generation had significantly higher rickettsial burdens than those in the F1 generation, though there was no significant difference in rickettsial burdens between larvae from the F1 and F2 generations (Fig. 3). One explanation of these observations is that co-feeding transmission occurred as ticks fed on the rabbits. Co-feeding transmission is a phenomenon in which tick-borne pathogens or symbionts are amplified among infected and noninfected ticks as they feed side-by-side on the same animal (Randolph et al., 1996). Compared with the F1 eggs, the higher rickettsial burden in the F2 eggs may have been caused by greater number of co-feeding ticks in the capsules.
We conclude that transovarial transmission and transstadial passage of phylotype G021 by I. pacificus occurs in nature and in the laboratory with 100% efficiency. Future molecular studies are needed to identify the specific bacterial genes driving these transmission routes. Once identified, they should be evaluated further as potential targets for decreasing populations of this important tick vector.
Acknowledgements
We are grateful to Esther Omi-Olsen from the University of California, Berkeley, for assistance with the laboratory animal aspects of this study. We thank Bliss Fisher of Mendocino County Animal Care Services for helping with tick collections. This work was funded by National Institute of Health R15 grant 1 R15 AI 82515-01, by the American Society for Microbiology (ASM) Undergraduate Research Fellowship, and by the California State University Program for Education and Research in Biotechnology (CSUPERB) Howell Research Scholar Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interest The authors declare that they have no competing interests.
References
- Azad AF, Sacci JB, Jr., Nelson WM, Dasch GA, Schmidtmann ET, Carl M. Genetic characterization and transovarial transmission of a typhus-like rickettsia found in cat fleas. Proc. Natl. Acad. Sci. U.S.A. 1992;89:43–46. doi: 10.1073/pnas.89.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldridge GD, Scoles GA, Burkhardt NY, Schloeder B, Kurtti TJ, Munderloh UG. Transovarial transmission of Francisella-like endosymbionts and Anaplasma phagocytophilum variants in Dermacentor albipictus (Acari: Ixodidae) J. Med. Entomol. 2009;46:625–632. doi: 10.1603/033.046.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P. Biology bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu. Rev. Microbiol. 2005;59:155–189. doi: 10.1146/annurev.micro.59.030804.121041. [DOI] [PubMed] [Google Scholar]
- Beaty BJ, Marquardt WC. The Biology of Disease Vectors. Niwot; Colorado: 1996. [Google Scholar]
- Boretti FS, Perreten A, Meli ML, Cattori V, Willi B, Wengi N, Hornok S, Honegger H, Hegglin D, Woelfel R, Reusch CE, Lutz H, Hofmann-Lehmann R. Molecular investigations of Rickettsia helvetica infection in dogs, foxes, humans, and Ixodes ticks. Appl. Environ. Microbiol. 2009;75:3230–3237. doi: 10.1128/AEM.00220-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright M, Bulgheresi S. A complex journey: transmission of microbial symbionts. Nat. Rev. Microbiol. 2010;8:218–230. doi: 10.1038/nrmicro2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner P. Endosymbiosis of animals with plant microorganisms. John Wiley & Sons, Inc.; New York: 1965. [Google Scholar]
- Burgdorfer W, Lane RS, Barbour AG, Gresbrink RA, Anderson JR. The western black-legged tick, Ixodes pacificus: a vector of Borrelia burgdorferi. Am. J. Trop. Med. Hyg. 1985;34:925–930. doi: 10.4269/ajtmh.1985.34.925. [DOI] [PubMed] [Google Scholar]
- Cheng D, Vigil K, Schanes P, Brown R, Zhong J. Prevalence and burden of two rickettsial phylotypes (G021 and G022) in Ixodes pacificus from California by real-time quantitative PCR. Ticks Tick Borne Dis. 2013 doi: 10.1016/j.ttbdis.2012.12.005. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clover JR, Lane RS. Evidence implicating nymphal Ixodes pacificus (Acari: Ixodidae) in the epidemiology of Lyme disease in California. Am. J. Trop. Med. Hyg. 1995;53:237–240. doi: 10.4269/ajtmh.1995.53.237. [DOI] [PubMed] [Google Scholar]
- Dalton MJ, Clarke MJ, Holman RC, Krebs JW, Fishbein DB, Olson JG, Childs JE. National surveillance for Rocky Mountain spotted fever, 1981–1992: epidemiologic summary and evaluation of risk factors for fatal outcome. Am. J. Trop. Med. Hyg. 1995;52:405–413. doi: 10.4269/ajtmh.1995.52.405. [DOI] [PubMed] [Google Scholar]
- De Silva AM, Fikrig E. Growth and migration of Borrelia burgdorferi in Ixodes ticks during blood feeding. Am. J. Trop. Med. Hyg. 1995;53:397–404. doi: 10.4269/ajtmh.1995.53.397. [DOI] [PubMed] [Google Scholar]
- Douglas AE. Symbiotic microorganisms: untapped resources for insect pest control. Trends. Biotechnol. 2007;25:338–342. doi: 10.1016/j.tibtech.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Eisen L, Dolan MC, Piesman J, Lane RS. Vector competence of Ixodes pacificus and I. spinipalpis (Acari: Ixodidae), and reservoir competence of the dusky-footed woodrat (Neotoma fuscipes) and the deer mouse (Peromyscus maniculatus), for Borrelia bissettii. J. Med. Entomol. 2003;40:311–320. doi: 10.1603/0022-2585-40.3.311. [DOI] [PubMed] [Google Scholar]
- Foley JE, Nieto NC, Adjemian J, Dabritz H, Brown RN. Anaplasma phagocytophilum infection in small mammal hosts of Ixodes ticks, western United States. Emerg. Infect. Dis. 2008;14:1147–1150. doi: 10.3201/eid1407.071599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graca-Souza AV, Maya-Monteiro C, Paiva-Silva GO, Braz GR, Paes MC, Sorgine MH, Oliveira MF, Oliveira PL. Adaptations against heme toxicity in blood-feeding arthropods. Insect Biochem. Molec. Biol. 2006;36:322–335. doi: 10.1016/j.ibmb.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Horta MC, Pinter A, Schumaker TT, Labruna MB. Natural infection, transovarial transmission, and transstadial survival of Rickettsia bellii in the tick Ixodes loricatus (Acari: Ixodidae) from Brazil. Ann. N. Y. Acad. Sci. 2006;1078:285–290. doi: 10.1196/annals.1374.053. [DOI] [PubMed] [Google Scholar]
- Jasinskas A, Zhong J, Barbour AG. Highly prevalent Coxiella sp. bacterium in the tick vector Amblyomma americanum. Appl. Environ. Microbiol. 2007;73:334–336. doi: 10.1128/AEM.02009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labruna MB, Ogrzewalska M, Soares JF, Martins TF, Soares HS, Moraes-Filho J, Nieri-Bastos FA, Almeida AP, Pinter A. Experimental infection of Amblyomma aureolatum ticks with Rickettsia rickettsii. Emerg. Infect. Dis. 2011;17:829–834. doi: 10.3201/eid1705.101524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RS, Burgdorfer W. Transovarial and transstadial passage of Borrelia burgdorferi in the western black-legged tick, Ixodes pacificus (Acari: Ixodidae) Am. J. Trop. Med. Hyg. 1987;37:188–192. doi: 10.4269/ajtmh.1987.37.188. [DOI] [PubMed] [Google Scholar]
- Liu L, Narasimhan S, Dai J, Zhang L, Cheng G, Fikrig E. Ixodes scapularis salivary gland protein P11 facilitates migration of Anaplasma phagocytophilum from the tick gut to salivary glands. EMBO Rep. 2011;12:1196–1203. doi: 10.1038/embor.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso KR, Sonenshine DE, Ceraul SM, Azad AF. Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second Rickettsia. J. Med. Entomol. 2002;39:809–813. doi: 10.1603/0022-2585-39.6.809. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Ogawa M, Brouqui P, Raoult D, Parola P. Transmission of Rickettsia massiliae in the tick, Rhipicephalus turanicus. Med. Vet. Entomol. 2005;19:263–270. doi: 10.1111/j.1365-2915.2005.00569.x. [DOI] [PubMed] [Google Scholar]
- Oliveira JH, Goncalves RL, Lara FA, Dias FA, Gandara AC, Menna-Barreto RF, Edwards MC, Laurindo FR, Silva-Neto MA, Sorgine MH, Oliveira PL. Blood meal-derived heme decreases ROS levels in the midgut of Aedes aegypti and allows proliferation of intestinal microbiota. PLoS Pathog. 2011;7:e1001320. doi: 10.1371/journal.ppat.1001320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett KA, Lane RS. Life cycle of Ixodes pacificus (Acari: Ixodidae): timing of developmental processes under field and laboratory conditions. J. Med. Entomol. 2001;38:684–693. doi: 10.1603/0022-2585-38.5.684. [DOI] [PubMed] [Google Scholar]
- Perlman SJ, Hunter MS, Zchori-Fein E. The emerging diversity of Rickettsia. Proc. Biol. Sci. 2006;273:2097–2106. doi: 10.1098/rspb.2006.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan JN, Lu CR, Bender WG, Smoak RM, Zhong J. Molecular detection and identification of Rickettsia species in Ixodes pacificus in California. Vector Borne Zoonotic Dis. 2011;11:957–961. doi: 10.1089/vbz.2010.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piesman J, Clark KL, Dolan MC, Happ CM, Burkot TR. Geographic survey of vector ticks (Ixodes scapularis and Ixodes pacificus) for infection with the Lyme disease spirochete, Borrelia burgdorferi. J. Vector Ecol. 1999;24:91–98. [PubMed] [Google Scholar]
- Piesman J, Oliver JR, Sinsky RJ. Growth kinetics of the Lyme disease spirochete (Borrelia burgdorferi) in vector ticks (Ixodes dammini) Am. J. Trop. Med. Hyg. 1990;42:352–357. doi: 10.4269/ajtmh.1990.42.352. [DOI] [PubMed] [Google Scholar]
- Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat. Rev. Microbiol. 2012;10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph SE, Gern L, Nuttall PA. Co-feeding ticks: Epidemiological significance for tick-borne pathogen transmission. Parasitol. 1996;12:472–479. doi: 10.1016/s0169-4758(96)10072-7. Today. [DOI] [PubMed] [Google Scholar]
- Sakurai M, Koga R, Tsuchida T, Meng XY, Fukatsu T. Rickettsia symbiont in the pea aphid Acyrthosiphon pisum: novel cellular tropism, effect on host fitness, and interaction with the essential symbiont Buchnera. Appl. Environ. Microbiol. 2005;71:4069–4075. doi: 10.1128/AEM.71.7.4069-4075.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassera D, Beninati T, Bandi C, Bouman EA, Sacchi L, Fabbi M, Lo N. `Candidatus Midichloria mitochondrii', an endosymbiont of the tick Ixodes ricinus with a unique intramitochondrial lifestyle. Int. J. Syst. Evol. Micr. 2006;56:2535–2540. doi: 10.1099/ijs.0.64386-0. [DOI] [PubMed] [Google Scholar]
- Sassera D, Lo N, Bouman EA, Epis S, Mortarino M, Bandi C. “Candidatus Midichloria” endosymbionts bloom after the blood meal of the host, the hard tick Ixodes ricinus. Appl. Environ. Microbiol. 2008;74:6138–6140. doi: 10.1128/AEM.00248-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoles GA, Papero M, Beati L, Fish D. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 2001;1:21–34. doi: 10.1089/153036601750137624. [DOI] [PubMed] [Google Scholar]
- Socolovschi C, Huynh TP, Davoust B, Gomez J, Raoult D, Parola P. Transovarial and trans-stadial transmission of Rickettsiae africae in Amblyomma variegatum ticks. Clin. Microbiol. Infect. 2009;15(Suppl 2):317–318. doi: 10.1111/j.1469-0691.2008.02278.x. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Murata M, Nogami S, Hori E, Kawamura A, Jr., Tanaka H. Transovarial transmission of Rickettsia tsutsugamushi in Leptotrombidium pallidum successively reared in the laboratory. Jpn. J. Exp. Med. 1988;58:213–218. [PubMed] [Google Scholar]
- Wang Y, Gilbreath TM, 3rd, Kukutla P, Yan G, Xu J. Dynamic gut microbiome across life history of the malaria mosquito Anopheles gambiae in Kenya. PLoS ONE. 2011;6:e24767. doi: 10.1371/journal.pone.0024767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller SJ, Baldridge GD, Munderloh UG, Noda H, Simser J, Kurtti TJ. Phylogenetic placement of rickettsiae from the ticks Amblyomma americanum and Ixodes scapularis. J. Clin. Microbiol. 1998;36:1305–1317. doi: 10.1128/jcm.36.5.1305-1317.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Jasinskas A, Barbour AG. Antibiotic treatment of the tick vector Amblyomma americanum reduced reproductive fitness. PLoS ONE. 2007;2:e405. doi: 10.1371/journal.pone.0000405. [DOI] [PMC free article] [PubMed] [Google Scholar]