Abstract
Chemotherapy regimens for early stage breast cancer have been tested by randomized clinical trials, and specified by evidence-based practice guidelines. However, little is known about the translation of trial results and guidelines to clinical practice. We extracted individual-level data on chemotherapy administration from the electronic medical records of Kaiser Permanente Northern California (KPNC), a pre-paid integrated healthcare system serving 29% of the local population. We linked data to the California Cancer Registry, incorporating socio-demographic and tumor factors, and performed multivariable logistic regression analyses on the receipt of specific chemotherapy regimens. We identified 6,004 women diagnosed with Stage I–III breast cancer at KPNC during 2004–2007; 2,669 (44.5 %) received at least one chemotherapy infusion at KPNC within 12 months of diagnosis. Factors associated with receiving chemotherapy included age <50 years [odds ratio (OR) 2.27, 95 % confidence interval (CI) 1.81–2.86], tumor >2 cm (OR 2.14, 95 % CI 1.75-2.61), involved lymph nodes (OR 11.3, 95 % CI 9.29–13.6), hormone receptor-negative (OR 6.94, 95% CI 4.89–9.86), Her2/neu-positive (OR 2.71, 95% CI 2.10–3.51), or high grade (OR 3.53, 95 % CI 2.77-4.49) tumors; comorbidities associated inversely with chemotherapy use [heart disease for anthracyclines (OR 0.24, 95 % CI 0.14–0.41), neuropathy for taxanes (OR 0.45, 95 % CI 0.22–0.89)]. Relative to high-socioeconomic status (SES) non-Hispanic Whites, we observed less anthracycline and taxane use by SES non-Hispanic Whites (OR 0.63, 95 % CI 0.49-0.82) and American Indians (OR 0.23, 95 % CI 0.06–0.93), and more anthracycline use by high-SES Asians/Pacific Islanders (OR 1.72, 95 % CI 1.02–2.90). In this equal-access healthcare system, chemotherapy use followed practice guidelines, but varied by race and socio-demographic factors. These findings may inform efforts to optimize quality in breast cancer care.
Keywords: Breast cancer, Chemotherapy, Patterns of care, Electronic medical record, Disparities, Outcomes research, Quality of care
Introduction
Breast cancer is the most common non-cutaneous malignancy of women in the United States (U.S.), and survivors comprise approximately 2% of the population [1]. The high incidence of breast cancer has facilitated large randomized clinical trials, generations of which have defined effective adjuvant chemotherapy regimens for Stage I–III disease [2–6]. Evidence-based practice guidelines and quality measures have translated clinical trial results into recommendations about chemotherapy agents, doses and schedules, based on patient and tumor characteristics [7, 8]. However, considerable uncertainty remains as to how research findings and guidelines are applied outside of clinical trials, the setting in which the great majority of patients are treated [9–12].
Barriers to population-based studies of chemotherapy use include lack of treatment detail and under-reporting. Population-based cancer registries such as the Surveillance, Epidemiology and End Results (SEER) program record chemotherapy as part of the first course of treatment, but details such as drug combinations, schedules and number of cycles—all of which have been demonstrated to impact cancer recurrence and survival in clinical trials [5, 6, 13, 14]—are unavailable. Moreover, administration of chemotherapy appears under-reported in registry data [15, 16]. Studies using linkage to Medicare claims are limited to adults aged 65 years or older, and based on claims data rather than actual drug administrations. To overcome these limitations, we linked chemotherapy administration data from the electronic medical records (EMR) of Kaiser Permanente Northern California (KPNC, a participant in the National Cancer Institute's Cancer Research Network) [17] with the California Cancer Registry (CCR) database; members comprise nearly one-third of the local population, and are representative in terms of race/ethnicity and socioeconomic status (SES). To determine how evidence-based guidelines disseminate into cancer care across the population, we analyzed factors associated with adjuvant chemotherapy use in Stage I–III breast cancer patients diagnosed from 2004 to 2007.
Methods
Data sources and linkage
The KPNC coverage area includes 23 counties in the San Francisco Bay Area and the Central Valley of California, from Sacramento to Fresno. We used two chemotherapy administration data repositories from KPNC: the Case Management for Medical Oncology with Laboratory and Outcome Tracking (CAMMOLOT) database, initiated in 1998, and the Clinical Oncology Pharmacy System (COPS) database, initiated in 1999. KPNC pharmacy records were used to identify filled prescriptions for endocrine therapy of breast cancer, including tamoxifen and aromatase inhibitors.[18] We used KPNC data on diagnoses associated with inpatient and outpatient encounters to identify specific comorbidities present from 12 months before to one month after diagnosis, and likely to influence chemotherapy selection, including heart disease (ICD-9 codes 410.0–410.9, 428.0–428.9, 411.0–411.9, 413.0–413.9, 394.0–396.9, 424.0–424.9, 425.0–425.9), diabetes (ICD-9 codes 250.0–250.9), and neuropathy (ICD-9 codes 356.0–357.9, 250.6, 249.6, 337.0). In addition, we used a modified Charlson Comorbidity Index to measure the burden of other serious comorbities; this weighted score includes conditions found in the original Charlson Index including liver disease, cerebrovascular disease, and acquired immune deficiency syndrome, among others [19–22].
Comprising three registries (Greater Bay Area, Los Angeles, and Greater California) within the SEER program, the CCR is a population-based registry which has collected data about all primary cancers diagnosed among California residents since 1988. Demographic and tumor information is abstracted from medical records according to standard protocols [23]. CCR data have been described [24], and include age and marital status at diagnosis, race/ethnicity, tumor size, presence of lymph node involvement, cancer stage according to the American Joint Committee on Cancer [25], tumor grade, histology, laterality, focality, expression of estrogen receptor (ER), progesterone receptor (PR) and Her2/neu (HER2), other cancer treatments including surgery and radiation, and vital status at the time of last contact or vital status record linkage. For this cohort, most clinical information is derived from the KPNC cancer registry; however, CCR data may incorporate additional reports from facilities outside KPNC. We included information on distance between a patient's address and the KPNC facility reporting her diagnosis [26], and on neighborhood (block group-level) SES, using a previously developed index that combines Census 2000 measures of education, income, occupation and housing characteristics [27].
KPNC records over 1999–2007 were extracted for linkage to CCR tumor-level data. KPNC chemotherapy infusion databases became fully implemented in 2004; therefore, we restricted analyses to 2004–2007. Infusion data were limited to 12 months following diagnosis to maximize capture of chemotherapy used in the post-surgical adjuvant setting, while minimizing capture of chemotherapy administered for cancer recurrence. Analyses were approved by the Institutional Review Boards of the state of California, the Cancer Prevention Institute of California, and KPNC Division of Research.
Chemotherapy data extraction and coding
We extracted data on drug names, infusion dates, and number of infusions. We focused on two of the most active drug classes for breast cancer, anthracyclines and taxanes [5, 8, 13, 14, 28]; for comparison, we evaluated the older cyclophosphamide, methotrexate, and 5-flurouracil (CMF) regimen [29, 30]. We also assessed use of the monoclonal antibody trastuzumab (Herceptin), which was FDA-approved for adjuvant treatment of Stage I–III HER2-positive breast cancer in 2006 [31, 32]. We defined cycles according to the number of drug infusions, with cycle length defined according to practice guidelines [5, 8, 13, 14, 28].
Statistical analysis
We used exploratory multivariable logistic regression to model the association of clinical and socio-demographic factors with treatment. We included age, race/ethnicity, neighborhood SES, and ER/PR in all models because these variables were of interest a priori, and then used stepwise selection to select other significant covariates among tumor size, histology, grade, lymph node involvement, laterality, HER2 status, marital status, distance to reporting KPNC facility, diabetes, heart disease, neuropathy, modified Charlson Comorbidity Index, multiple breast tumors, endocrine therapy, and surgery type for inclusion in the final model. Because of high correlations among variables, two combined variables were modeled: receipt of endocrine therapy and ER/PR status, and race/ethnicity and neighborhood SES. To control for changes in guidelines over time, year of diagnosis was included as a continuous variable. Analyses were conducted using SAS, Version 9.3 (SAS Institute, Cary, NC).
Outcomes of multivariable analyses included receipt of (1) any chemotherapy, (2) at least four cycles (defined as infusions at least 1-week apart) of an anthracycline (doxorubicin or epirubicin), (3) at least four cycles of a taxane (docetaxel or paclitaxel), (4) at least four cycles of an anthracycline plus at least four cycles of a taxane, (5) at least four cycles of an anthracycline and taxane compared to at least six cycles of CMF. For analyses 1–4, each outcome was compared to receiving any other chemotherapy (fewer cycles of the drug/s of interest, or any other drugs), a comparison we chose to estimate the odds of receiving particular chemotherapy drugs, compared to other drugs. We selected a threshold of four cycles because it was the minimum number for all regimens recommended by practice guidelines [8]. We also examined two measures of the Quality Oncology Practice Initiative (QOPI) of the American Society of Clinical Oncology (ASCO) [7], which we chose for their feasibility with available data: receipt of at least one cycle of combination chemotherapy within 4 months of diagnosis by women age <70 years with ER/PR-negative tumors of size ≥1 cm (cm), and receipt of trastuzumab by women with HER2-positive tumors, compared in each analysis to eligible women who did not receive the specified regimen. We restricted the trastuzumab analysis to 2006–2007, because adjuvant trastuzumab was not FDA-approved until 2006 [31, 32].
Results
Patient characteristics
We identified 6,004 women who were residents of California and diagnosed with a first primary, Stage I–III breast cancer at KPNC from 2004 to 2007 (Table 1; Fig. 1). Twenty-one percent was younger than 50 years of age; 52.9 % were age 50–69, and 25.9 % were age ≥70. The majority (68.3 %) was non-Hispanic (NH) White, in the top two state-wide SES quintiles (64 %), and married (59.1 %). The majority had tumors <2 cm (58.2 %), without lymph node involvement (68.9 %) and Stage I (52.8 %). Most tumors expressed ER and/or PR (81.3 %); HER2 amplification or over-expression was present in 11.3 %, unknown or borderline in 23.5 %; HER2 status was based on SEER data, with missing rates as previously reported [24, 33, 34]. Diabetes was present in 11.3 %, neuropathy in 3.1 %, and heart disease in 7.2 %. KPNC recorded endocrine therapy in 85.5 % of patients with ER/PR-positive cancer; 38.9 % of all patients underwent mastectomy, 59.5 % breast conserving surgery, and 50.4 % radiation therapy. In total, 2,669 women (44.5 %) received at least one infusion of chemotherapy at a KPNC facility. There was 96.5 % agreement between CCR and KPNC on chemotherapy records: KPNC missed 2.62 % of patients who received chemotherapy, and CCR missed 4.01 %.
Table 1. Patient characteristics, according to receipt of chemotherapy, Stage I-III breast cancer patients, Kaiser Permanente Northern California, 2004-2007.
| Received chemotherapyb | Total | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Yes | No | |||||||
|
|
|
|||||||
| N | Column (%) | Row (%) | N | Column (%) | Row (%) | N | Column (%) | |
| Age at diagnosis (years)a | ||||||||
| 0–39 | 213 | 8.0 | 86.9 | 32 | 1.0 | 13.1 | 245 | 4.1 |
| 40–49 | 763 | 28.6 | 74.4 | 263 | 7.9 | 25.6 | 1026 | 17.1 |
| 50–59 | 927 | 34.7 | 55.6 | 739 | 22.2 | 44.4 | 1666 | 27.7 |
| 60–69 | 556 | 20.8 | 36.8 | 955 | 28.6 | 63.2 | 1511 | 25.2 |
| ≥70 | 210 | 7.9 | 13.5 | 1346 | 40.4 | 86.5 | 1556 | 25.9 |
| Race/Ethnicitya | ||||||||
| Non–Hispanic (NH) White | 1670 | 62.6 | 40.8 | 2428 | 72.8 | 59.2 | 4098 | 68.3 |
| NH Black | 228 | 8.5 | 53.0 | 202 | 6.1 | 47.0 | 430 | 7.2 |
| Hispanic | 345 | 12.9 | 54.2 | 291 | 8.7 | 45.8 | 636 | 10.6 |
| NH Asian/Pacific Islander | 400 | 15.0 | 51.9 | 370 | 11.1 | 48.1 | 770 | 12.8 |
| NH American Indian/Alaskan Native, other, or unknown | 26 | 1.0 | 37.1 | 44 | 1.3 | 62.9 | 70 | 1.2 |
| Socio-economic status, quintilesa | ||||||||
| Lowest quintile | 122 | 4.6 | 43.3 | 160 | 4.8 | 56.7 | 282 | 4.7 |
| Second quintile | 325 | 12.2 | 44.9 | 399 | 12.0 | 55.1 | 724 | 12.1 |
| Third quintile | 499 | 18.7 | 43.5 | 647 | 19.4 | 56.5 | 1146 | 19.1 |
| Fourth quintile | 780 | 29.2 | 44.4 | 977 | 29.3 | 55.6 | 1757 | 29.3 |
| Highest quintile | 943 | 35.3 | 45.0 | 1152 | 34.5 | 55.0 | 2095 | 34.9 |
| Marital statusa | ||||||||
| Single, separated, divorced, widowed | 903 | 33.8 | 37.6 | 1501 | 45.0 | 62.4 | 2404 | 40.0 |
| Married | 1745 | 65.4 | 49.2 | 1804 | 54.1 | 50.8 | 3549 | 59.1 |
| Unknown | 21 | 0.8 | 41.2 | 30 | 0.9 | 58.8 | 51 | 0.8 |
| Tumor Size (cm)a | ||||||||
| <1 | 156 | 5.8 | 13.1 | 1034 | 31.0 | 86.9 | 1190 | 19.8 |
| 1 to <2 | 888 | 33.3 | 38.6 | 1415 | 42.4 | 61.4 | 2303 | 38.4 |
| 2 to <3 | 810 | 30.3 | 61.3 | 512 | 15.4 | 38.7 | 1322 | 22.0 |
| 3 to <4 | 391 | 14.6 | 71.0 | 160 | 4.8 | 29.0 | 551 | 9.2 |
| 4 to <5 | 219 | 8.2 | 77.9 | 62 | 1.9 | 22.1 | 281 | 4.7 |
| ≥5 | 165 | 6.2 | 74.7 | 56 | 1.7 | 25.3 | 221 | 3.7 |
| Microscopic foci, diffuse, or not stated | 40 | 1.5 | 29.4 | 96 | 2.9 | 70.6 | 136 | 2.3 |
| Lymph node involvementa | ||||||||
| No nodes | 1202 | 45.0 | 29.1 | 2935 | 88.0 | 70.9 | 4137 | 68.9 |
| Positive nodes | 1464 | 54.9 | 78.7 | 397 | 11.9 | 21.3 | 1861 | 31.0 |
| Unknown | 3 | 0.1 | 50.0 | 3 | 0.1 | 50.0 | 6 | 0.1 |
| Stagea | ||||||||
| Stage I | 667 | 25.0 | 21.1 | 2501 | 75.0 | 78.9 | 3168 | 52.8 |
| Stage II | 1451 | 54.4 | 66.5 | 732 | 21.9 | 33.5 | 2183 | 36.4 |
| Stage III | 551 | 20.6 | 84.4 | 102 | 3.1 | 15.6 | 653 | 10.9 |
| Gradea | ||||||||
| Grade I (well differentiated) | 267 | 10.0 | 19.8 | 1081 | 32.4 | 80.2 | 1348 | 22.5 |
| Grade II (moderately well differentiated) | 1028 | 38.5 | 42.0 | 1419 | 42.5 | 58.0 | 2447 | 40.8 |
| Grade III (poorly or undifferentiated) | 1219 | 45.7 | 69.4 | 537 | 16.1 | 30.6 | 1756 | 29.2 |
| Grade and differentiation not stated | 155 | 5.8 | 34.2 | 298 | 8.9 | 65.8 | 453 | 7.5 |
| Histologya | ||||||||
| Ductal | 2002 | 75.0 | 46.0 | 2347 | 70.4 | 54.0 | 4349 | 72.4 |
| Lobular | 510 | 19.1 | 42.0 | 703 | 21.1 | 58.0 | 1213 | 20.2 |
| Both | 157 | 5.9 | 35.5 | 285 | 8.5 | 64.5 | 442 | 7.4 |
| Estrogen and progesterone receptorsa | ||||||||
| Positive (either or both) | 1878 | 70.4 | 38.5 | 2997 | 89.9 | 61.5 | 4875 | 81.2 |
| Negative (both) | 778 | 29.1 | 71.6 | 309 | 9.3 | 28.4 | 1087 | 18.1 |
| Unknown (both) | 13 | 0.5 | 31.0 | 29 | 0.9 | 69.0 | 42 | 0.7 |
| HER2/Neu (HER2)a | ||||||||
| Positive | 482 | 18.1 | 71.3 | 194 | 5.8 | 28.7 | 676 | 11.3 |
| Negative | 1539 | 57.7 | 39.3 | 2381 | 71.4 | 60.7 | 3920 | 65.3 |
| Unknown or borderline | 648 | 24.3 | 46.0 | 760 | 22.8 | 54.0 | 1408 | 23.5 |
| Charlson Comorbidity Indexb | ||||||||
| 0 | 2232 | 83.6 | 48.2 | 2403 | 72.1 | 51.8 | 4635 | 77.2 |
| 1 | 338 | 12.7 | 35.7 | 608 | 18.2 | 64.3 | 946 | 15.8 |
| 2 | 72 | 2.7 | 28.0 | 185 | 5.5 | 72.0 | 257 | 4.3 |
| ≥3 | 27 | 1.0 | 16.3 | 139 | 4.2 | 83.7 | 166 | 2.8 |
| Diabetesb | ||||||||
| Yes | 205 | 7.7 | 30.1 | 475 | 14.2 | 69.9 | 680 | 11.3 |
| No | 2464 | 92.3 | 46.3 | 2860 | 85.8 | 53.7 | 5324 | 88.7 |
| Neuropathyb | ||||||||
| Yes | 44 | 1.6 | 23.5 | 143 | 4.3 | 76.5 | 187 | 3.1 |
| No | 2625 | 98.4 | 45.1 | 3192 | 95.7 | 54.9 | 5817 | 96.9 |
| Heart Diseaseb | ||||||||
| Yes | 82 | 3.1 | 18.9 | 353 | 10.6 | 81.1 | 435 | 7.2 |
| No | 2587 | 96.9 | 46.5 | 2982 | 89.4 | 53.5 | 5569 | 92.8 |
| Modified Charlson Comorbidity Indexc | ||||||||
| 0 | 2403 | 90.0 | 46.1 | 2813 | 84.3 | 53.9 | 5216 | 86.9 |
| 1 | 239 | 9.0 | 36.5 | 416 | 12.5 | 63.5 | 655 | 10.9 |
| 2 | 24 | 0.9 | 24.7 | 73 | 2.2 | 75.3 | 97 | 1.6 |
| ≥3 | 3 | 0.1 | 8.3 | 33 | 1.0 | 91.7 | 36 | 0.6 |
| Multiple primary tumors diagnosed within 60 daysa | ||||||||
| Yes | 44 | 1.6 | 44.9 | 54 | 1.6 | 55.1 | 98 | 1.6 |
| No | 2625 | 98.4 | 44.4 | 3281 | 98.4 | 55.6 | 5906 | 98.4 |
| Endocrine therapyb, d | ||||||||
| Yes | 1750 | 93.2 | 42.0 | 2419 | 80.7 | 58.0 | 4169 | 85.5 |
| No | 128 | 6.8 | 18.1 | 578 | 19.3 | 81.9 | 706 | 14.5 |
| Radiation therapya | ||||||||
| Yes | 1137 | 42.6 | 37.6 | 1887 | 56.6 | 62.4 | 3024 | 50.4 |
| No | 1532 | 57.4 | 51.4 | 1448 | 43.4 | 48.6 | 2980 | 49.6 |
| Surgerya | ||||||||
| Mastectomy | 1326 | 49.7 | 56.8 | 1008 | 30.2 | 43.2 | 2334 | 38.9 |
| Breast conserving surgery | 1300 | 48.7 | 36.4 | 2271 | 68.1 | 63.6 | 3571 | 59.5 |
| Other or no surgery | 43 | 1.6 | 43.4 | 56 | 1.7 | 56.6 | 99 | 1.6 |
| Anthracycline (doxorubixcin, epirubicin)e | ||||||||
| Yes | 2477 | 92.8 | 100.0 | 2477 | 41.3 | |||
| No | 192 | 7.2 | 5.4 | 3335 | 100.0 | 94.6 | 3527 | 58.7 |
| Taxane (docetaxel, paclitaxel)e | ||||||||
| Yes | 1659 | 62.2 | 100.0 | 1659 | 27.6 | |||
| No | 1010 | 37.8 | 23.2 | 3335 | 100.0 | 76.8 | 4345 | 72.4 |
| Trastuzumabe | ||||||||
| Yes | 491 | 18.4 | 100.0 | 491 | 8.2 | |||
| No | 2178 | 81.6 | 39.5 | 3335 | 100.0 | 60.5 | 5513 | 91.8 |
| Combination chemotherapy within 4 months, among women with ER/PR-negative tumors ≥1 cm in size and age <70 yearsf | ||||||||
| Yes | 595 | 92.5 | 100.0 | 595 | 81.3 | |||
| No | 48 | 7.5 | 35.0 | 89 | 100.0 | 65.0 | 137 | 18.7 |
| Trastuzumab, among women diagnosed in 2006–2007 with HER2-positive tumorsf | ||||||||
| Yes | 199 | 88.8 | 100.0 | 199 | 68.4 | |||
| No | 25 | 11.2 | 27.2 | 67 | 100.0 | 72.8 | 92 | 31.6 |
| Total | 2669 | 100.0 | 44.5 | 3335 | 100.0 | 55.5 | 6004 | 100.0 |
Data from the California Cancer Registry
Data from Kaiser Permanente Northern California
This modified Charlson Comorbidity Index excluded heart disease, diabetes and neuropathy, which were evaluated separately
Limited to women with estrogen receptor and/or progesterone receptor-positive breast cancers (N = 4875)
Among women who received chemotherapy
Measure of the American Society of Clinical Oncology Quality Oncology Practice Initiative (ASCO QOPI)
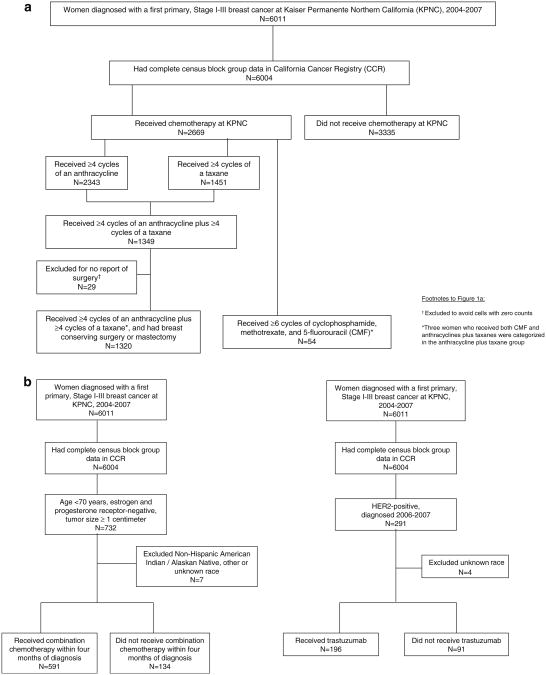
Fig 1.
Flow chart of case inclusion for multivariable analysis of a receipt of any chemotherapy, of anthracyclines plus taxanes, and of cyclophosphamide, methotrexate, and 5-flurouracil (CMF) and b American Society of Clinical Oncology Quality Oncology Practice Initiative measures: receipt of combination chemotherapy within 4 months of diagnosis by women aged <70 years having estrogen and progesterone receptor-negative tumors ≥1 centimeter, and receipt of trastuzumab by women having HER2-positive tumors
Any chemotherapy
Odds of receiving chemotherapy were greater among women who were younger [age <40, odds ratio (OR) 4.45, 95 % confidence interval (CI) 2.73–7.26], had tumors ≥2 cm (OR ≥ 2.14, 95 % CI 1.75–2.61), involved lymph nodes (OR 11.3, 95 % CI 9.29–13.6), high grade (OR 3.53, 95 % CI 2.77–4.49), ER/PR-negative (OR 6.94, 95 % CI 4.89–9.86) or HER2-positive (OR 2.71, 95 % CI 2.10–3.51) tumors, or received mastectomy (OR 1.41, 95 % CI 1.19–1.67). Chemotherapy was less used by women who were ≥70 years of age (OR 0.05, 95 % CI 0.04–0.07), unmarried (OR 0.8, 95 % CI 0.68–0.95), or had diabetes (0.71, 95% CI 0.54–0.94), neuropathy (OR 0.53, 95 % CI 0.31–0.89), heart disease (OR 0.38, 95 % CI 0.26–0.56), or a comorbidity index ≥3 (OR 0.03, 95 % CI 0.01–0.18, Table 2).
Table 2. Multivariable analysis of receiving any chemotherapy versus no chemotherapy, Stage I–III breast cancer patients, Kaiser Permanente Northern California (KPNC), 2004–2007 (N = 6004).
| Odds ratio | 95 % CI | |
|---|---|---|
| Age (years) | ||
| ≤39 | 4.45 | 2.73–7.26 |
| 40–49 | 2.27 | 1.81–2.86 |
| 50–59 | 1.0 | |
| 60–69 | 0.39 | 0.32–0.48 |
| ≥70 | 0.05 | 0.04–0.07 |
| Race, socio-economic status (SES) | ||
| High SES Non-Hispanic (NH) White | 1.0 | |
| High SES Hispanic | 1.25 | 0.89–1.75 |
| High SES NH American Indian, Alaskan Native, other, or unknown (AIAN | 0.48 | 0.16–1.47 |
| High SES NH Asian or Pacific Islander (API | 0.92 | 0.70–1.21 |
| High SES NH Black | 1.08 | 0.67–1.74 |
| Low SES NH White | 0.84 | 0.68–1.03 |
| Low SES Hispanic | 1.06 | 0.73–1.52 |
| Low SES NH AIAN | 0.65 | 0.23–1.86 |
| Low SES API | 0.98 | 0.64–1.48 |
| Low SES NH Black | 0.98 | 0.67–1.44 |
| Marital status | ||
| Married | 1.0 | |
| Unmarried (single, separated, divorced, widowed | 0.80 | 0.68–0.95 |
| Unknown | 0.51 | 0.22–1.23 |
| Tumor size (cm) | ||
| <1 cm | 0.24 | 0.19–0.31 |
| 1 to <2 | 1.0 | |
| 2 to <3 | 2.14 | 1.75–2.61 |
| 3 to <4 | 3.02 | 2.26–4.05 |
| 4 to <5 | 4.27 | 2.74–6.64 |
| ≥5 | 2.89 | 1.77–4.72 |
| Microscopic foci, diffuse, or unknown | 0.17 | 0.10–0.28 |
| Lymph node involvement | ||
| Negative or unknown | 1.0 | |
| Positive | 11.3 | 9.29–13.6 |
| Grade | ||
| Grade I (well differentiated) | 1.0 | |
| Grade II (moderately differentiated) | 2.06 | 1.66–2.55 |
| Grade III (poorly or undifferentiated | 3.53 | 2.77–4.49 |
| Unknown | 1.49 | 1.07–2.09 |
| Estrogen, progesterone receptors (ER/PR) and endocrine therapya | ||
| ER/PR-positive, no endocrine therapy | 1.0 | |
| ER/PR-negative, no endocrine therapy | 6.94 | 4.89–9.86 |
| ER/PR-unknown, no endocrine therapy | 0.20 | 0.06–0.75 |
| ER/PR-positive, received endocrine therapy | 2.31 | 1.71–3.13 |
| HER2 | ||
| Negative | 1.0 | |
| Positive | 2.71 | 2.10–3.51 |
| Unknown or borderline | 1.38 | 1.14–1.67 |
| Diabetes | ||
| No | 1.0 | |
| Yes | 0.71 | 0.54–0.94 |
| Neuropathy | ||
| No | 1.0 | |
| Yes | 0.53 | 0.31–0.89 |
| Heart disease | ||
| No | 1.0 | |
| Yes | 0.38 | 0.26–0.56 |
| Modified Charlson Comorbidity Indexb | ||
| 0 | 1.0 | |
| 1 | 1.12 | 0.86–1.45 |
| 2 | 1.10 | 0.55–2.20 |
| ≥3 | 0.03 | 0.01–0.18 |
| Distance to reporting KPNC facility (miles) | ||
| <5 | 1.0 | |
| 5 to <10 | 1.05 | 0.87–1.27 |
| 10 to <15 | 0.93 | 0.73–1.20 |
| 15 to <20 | 1.31 | 0.99–1.74 |
| 20 to <30 | 0.89 | 0.65–1.22 |
| 30 to <40 | 0.57 | 0.33–0.97 |
| ≥40 | 1.15 | 0.63–2.09 |
| Surgery type | ||
| Breast conserving surgery | 1.0 | |
| Mastectomy | 1.41 | 1.19–1.67 |
| None or other | 0.72 | 0.37–1.39 |
| Year of diagnosis, per year | 1.03 | 0.96–1.12 |
Excluded ER/PR-negative or unknown, received endocrine therapy, given the high probability that these groups reflect errors in data collection, since endocrine therapy is not clinically indicated in this setting
This Charlson Comorbidity Index excluded heart disease, diabetes and neuropathy, which were evaluated separately
Anthracyclines and taxanes
Table 3 presents receipt of anthracyclines and taxanes. Use of at least four cycles of an anthracycline, compared to any other chemotherapy, was more frequent among women who were 40–49 years of age (OR 1.89, 95 % CI 1.30–2.75), high-SES Asians/Pacific Islanders (OR 1.72, 95 % CI 1.02–2.90), or had involved lymph nodes (OR 2.14, 95 % CI 1.64–2.79). Use of four or more cycles of an anthracycline was less frequent among women who were age ≥70 (OR 0.18, 95 % CI 0.12–0.25), low-SES NH White (OR 0.65, 95 % CI 0.48–0.89), had tumors <1 cm (OR 0.46, 95 % CI 0.29–0.73), positive HER2 (OR 0.58, 95 % CI 0.43–0.82), or heart disease (OR 0.24, 95 % CI 0.14–0.41).
Table 3. Multivariable analyses of anthracyclines and taxanes, Stage I–III breast cancer patients, Kaiser Permanente Northern California, 2004-2007.
| At least 4 cycles of an anthracyline (A), vs. any other chemotherapy (N = 2669) | At least 4 cycles of a taxane (T), vs. any other chemotherapy (N = 2669) | At least 4 cycles of A and at least 4 cycles of T, vs. any other chemotherapy (N = 2669) | At least 6 cycles of cyclophosphamide, methotrexate, and 5-fluorouracil (CMF), vs. at least 4 cycles of A and Ta (N = 1374) | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| OR | 95 % CI | OR | 95 % CI | OR | 95 % CI | OR | 95 % CI | |
| Age (years) | ||||||||
| ≤39 | 1.19 | 0.70 –2.03 | 2.14 | 1.45 –3.17 | 1.91 | 1.30 –2.81 | 0.21 | 0.03 –1.77 |
| 40–49 | 1.89 | 1.30 –2.75 | 1.12 | 0.88 –1.42 | 1.21 | 0.95 –1.53 | 0.46 | 0.16 –1.35 |
| 50–59 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 60–69 | 0.73 | 0.54 –1.01 | 0.78 | 0.60 –1.01 | 0.78 | 0.60 –1.01 | 1.23 | 0.46 –3.27 |
| ≥70 | 0.18 | 0.12 –0.25 | 0.24 | 0.16 –0.34 | 0.25 | 0.17 –0.36 | 22.8 | 8.45 –61.4 |
| Race, socio-economic status (SES) | ||||||||
| High SES Non-Hispanic (NH) White | 1.00 | 1.00 | 1.00 | 1.0 | ||||
| High SES Hispanic | 1.16 | 0.67–2.02 | 1.07 | 0.72–1.58 | 1.15 | 0.78–1.69 | 0.91a | 0.33–2.50 |
| High SES NH AIAN b, other, unknown | Not interpretable (0) | 1.84 | 0.35–6.48 | 2.25 | 0.41–12.4 | |||
| High SES NH APIb | 1.72 | 1.02–2.90 | 1.03 | 0.75–1.42 | 1.15 | 0.84–1.58 | ||
| High SES NH Black | 1.00 | 0.50–2.03 | 1.24 | 0.73–2.14 | 0.89 | 0.52–1.53 | ||
| Low SES NH White | 0.65 | 0.48–0.89 | 0.66 | 0.51–0.85 | 0. | 0.49–0.82 | 2.79a | 1.25–6.23 |
| Low SES Hispanic | 0.89 | 0.52–1.52 | 0.82 | 0.54–1.24 | 0.85 | 0.57–1.27 | 0.61a | 0.18–2.05 |
| Low SES NH AIANb,other, unknown | 0.55 | 0.14–2.17 | 0.26 | 0.07–1.00 | 0.23 | 0.06–0.93 | ||
| Low SES NH APIb | 1.46 | 0.70–3.08 | 0.83 | 0.51–1.34 | 0.89 | 0.55–1.44 | ||
| Low SES NH Black | 0.82 | 0.47–1.41 | 0.67 | 0.44–1.03 | 0.66 | 0.43–1.01 | ||
| Tumor size (cm) | ||||||||
| <1 cm | 0.46 | 0.29–0.73 | 1.12 | 0.75–1.70 | 0.97 | 0.64–1.46 | 1.00 | |
| 1 to <2 | 1.00 | 1.00 | 1.00 | |||||
| 2 to <3 | 1.07 | 0.78–1.47 | 1.29 | 1.03–1.63 | 1.39 | 1.10–1.76 | 0.34a | 0.17–0.69 |
| 3 to <4 | 1.41 | 0.93–2.14 | 1.87 | 1.38–2.52 | 2.30 | 1.71–3.10 | ||
| 4 to <5 | 1.15 | 0.69–1.92 | 2.61 | 1.74–3.89 | 2.72 | 1.86–4.00 | ||
| ≥5 | 0.66 | 0.41–1.14 | 1.91 | 1.20–3.04 | 1.73 | 1.13–2.67 | ||
| Microscopic foci, diffuse, unknown | 0.57 | 0.22–1.49 | 1.65 | 0.70 –3.88 | 1.56 | 0.71 –3.43 | ||
| Lymph node involvement | ||||||||
| Negative or unknown | 1.00 | 1.00 | 1.00 | 27.5 | 11.6–65.2 | |||
| Positive | 2.14 | 1.64–2.79 | 13.5 | 10.9–16.6 | 1 | 10.5–15.8 | 1.0 | |
| Histology | ||||||||
| Ductal | NAc | 1.00 | 1.00 | NAc | ||||
| Lobular | 0.78 | 0.60–1.01 | 0. | 0.58– 0.98 | ||||
| Both ductal and lobular | 0. 74 | 0.48–1.13 | 0.73 | 0.48–1.12 | ||||
| Grade | ||||||||
| Grade I | NAc | 1.00 | 1.00 | NAc | ||||
| Grade II | 1.17 | 0.84–1.64 | 1.42 | 1.02–1.99 | ||||
| Grade III | 1.56 | 1.10–2.22 | 1.71 | 1.20–2.42 | ||||
| Unknown | 0.96 | 0.58–1.60 | 1.17 | 0.71–1.92 | ||||
| Estrogen, progesterone receptors | ||||||||
| Positive either or both | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Unknown both | 0.54 | 0.13–2.21 | 1.39 | 0.34–5.69 | 0.88 | 0.21–3.77 | ||
| Negative both | 1.08 | 0.82– 1.42 | 1.44 | 1.13–1.84 | 1.29 | 1.01–1.64 | 0.54a | 0.25–1.16 |
| HER2 | ||||||||
| Negative | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Positive | 0.58 | 0.43–0.82 | 1.82 | 1.39–2.37 | 1.37 | 1.06–1.78 | 0.16 | 0.04 –0.69 |
| Unknown or borderline | 0.73 | 0.54–0.99 | 1.07 | 0.85–1.35 | 1.04 | 0.82–1.31 | 1.75 | 0.84–3.67 |
| Diabetes | ||||||||
| No | 1.00 | NAc | 1.00 | 1.00 | ||||
| Yes | 0.70 | 0.46–1.05 | 0.66 | 0.46–0.94 | 4.09 | 1.59–10.5 | ||
| Heart Disease | ||||||||
| No | 1.00 | NAc | NAc | 1.00 | ||||
| Yes | 0.24 | 0.14–0.41 | 10.2 | 3.64–28.6 | ||||
| Neuropathy | ||||||||
| None | NAc | 1.00 | NAc | NAc | ||||
| Yes | 0.45 | 0.22–0.89 | ||||||
| Modified Charlson Comorbidity Indexd | ||||||||
| 0 | NAc | NAc | 1.00 | NAc | ||||
| 1 | 0.65 | 0.47–0.90 | ||||||
| 2 | 0.44 | 0.16–1.19 | ||||||
| ≥3 | 0.55 | 0.04–7.09 | ||||||
| Multiple tumors within 60 days | ||||||||
| No | NAc | 1.00 | 1.00 | NAc | ||||
| Yes | 2.33 | 1.08–5.05 | 2.54 | 1.19–5.45 | ||||
| Surgery type | ||||||||
| Breast conserving surgery | NAc | 1.00 | NAc | NAc | ||||
| Mastectomy | 1.02 | 0.84–1.25 | ||||||
| None or other | 4.32 | 1.67–11.2 | ||||||
| Endocrine therapy | ||||||||
| No | NAc | 1.00 | NAc | NAc | ||||
| Yes | ||||||||
| Year of diagnosis (per year) | 0. 90 | 0.80–1.02 | 1.10 | 1.01–1.21 | 1.21 | 0.95–1.15 | 0.61 | 0.41 –0.92 |
Due to small sample size for this analysis, variable categories were collapsed as follows: race and SES high SES NH White, high SES non-White, low SES NH White, Low SES non-White, tumor size (<2 cm, >2 cm/microscopic foci/diffuse/unknown) and estrogen receptor status (positive/unknown, negative)
AIAN, American Indian/Alaskan Native; API, Asian/Pacific Islander
Not applicable; this variable was not included in the final multivariable model
This modified Charlson Comorbidity Index excludes heart disease, diabetes and neuropathy, since these variables are measured separately
Receipt of at least four cycles of a taxane was more common among women who were age <40 (OR 2.14, 95 % CI 1.45–3.17), had tumors ≥2 cm (OR 1.29, 95 % CI 1.03–1.63), involved lymph nodes (OR 13.5, 95 % CI 10.9–16.6), high grade (OR 1.56, 95 % CI 1.10–2.22), ER/PR-negative (OR 1.44, 95 % CI 1.13–1.84) or HER2-positive (OR 1.82, 95 % CI 1.39–2.37) tumors, multiple primary tumors (OR 2.33, 95 % CI 1.08–5.05), no or other breast surgery (OR 4.32, 95 % CI 1.67–11.2) or later year of diagnosis (OR 1.10, 95 % CI 1.01–1.21). Taxanes were less used by women who were ≥70 years of age (OR 0.24, 95 % CI 0.16–0.34), low-SES NH White (OR 0.66, 95 % CI 0.51–0.85), or had neuropathy (OR 0.45, 95% CI 0.22–0.89).
At least four cycles of anthracycline plus taxane was more likely among women who were age <40 (OR 1.91, 95% CI 1.30–2.81), had tumors ≥2 cm (OR ≥1.39, 95 % CI 1.10–1.76), involved lymph nodes (OR 12.9, 95 % CI 10.5–15.8), high grade (OR 1.71, 95% CI 1.20-2.42), ER/PR-negative (OR 1.29, 95 % CI 1.01–1.64), HER2-positive (OR 1.37, 95 % CI 1.06–1.78), or multiple primary tumors (OR 2.54, 95% CI 1.19–5.45). Anthracyclines plus taxanes were less likely among women who were ≥70 years of age (OR 0.25, 95% CI 0.17–0.36), low-SES NH Whites (OR 0.63, 95 % CI 0.49–0.82), low-SES AIAN, other, or unknown ethnicity (OR 0.23, 95 % CI 0.06–0.93) had diabetes (OR 0.66, 95% CI 0.46–0.94), or a comorbidity index of 1 (OR 0.65, 95 % CI 0.47–0.90).
Women were more likely to receive CMF, compared to an anthracycline plus taxane, if they were ≥70 years of age (OR 22.8, 95 % CI 8.45–61.4), low-SES NH White (OR 2.79, 95 % CI 1.25–6.23), had uninvolved lymph nodes (OR 27.5, 95 % CI 11.6–65.2), diabetes (OR 4.09, 95 % CI 1.59–10.5), or heart disease (OR 10.2, 95 % CI 3.64–28.6); they were less likely to receive CMF, compared to an anthracycline plus taxane, if they had a tumor ≥2 cm (OR 0.34, 95 % CI 0.17–0.69), positive HER2 (OR 0.16, 95 % CI 0.04–0.69), or a later diagnosis year (OR 0.61, 95 % CI 0.41–0.92).
Consistency with ASCO QOPI guidelines
Women age <70 with ER/PR-negative tumors of ≥1 cm were more likely to receive combination chemotherapy within four months of diagnosis if they were 40–49 years of age (OR 2.05, 95 % CI 1.16–3.62), had involved lymph nodes (OR 2.90, 95 % CI 1.77–4.57), tumors ≥2 cm (OR 2.32, 95 % CI 1.38–3.90) or high grade (OR 4.26, 95 % CI 1.16–15.7); this was less likely if they were 60–69 years of age (OR 0.61, 95 % CI 0.38–0.98), high SES Asian or Black (OR 0.47, 95 % CI 0.23–0.97 and 0.37, 95 % CI 0.16–0.86, respectively), had neuropathy (OR 0.35, 95 % CI 0.13–0.98), or a comorbidity index of ≥3 (OR 0.05, 95 % CI 0.00–0.58). Women with HER2-positive tumors were more likely to receive trastuzumab if they were <40 years of age (OR 10.9, 95 % CI 1.51–79.3), had involved lymph nodes (OR 3.67, 95 % CI 1.66–8.12), high grade (OR 6.46, 95% CI 1.72–24.2) or ER/PR-negative (OR 2.99, 95 % CI 1.36–6.57) tumors. Trastuzumab was less used by women who were ≥70 years of age (OR 0.03, 95 % CI 0.01–0.10) or had tumors <1 cm (OR 0.17, 95 % CI 0.06–0.47) (Table 4).
Table 4. Multivariable analysis of American Society of Clinical Oncology Quality Oncology Practice Initiative measures, Stage I–III breast cancer patients, Kaiser Permanente Northern California, 2004–2007.
| Combination chemotherapy within 4 months of diagnosis, if estrogen and progesterone receptor-negative, size ≥ 1 cm and age <70 years (N = 725) | Trastuzumab if HER2-positive, 2006–2007a (N = 287) | |||
|---|---|---|---|---|
| OR | 95 % CI | OR | 95 % CI | |
| Age years | ||||
| ≤39 | 2.62 | 0.88–7.85 | 10.9 | 1.51–79.3 |
| 40–49 | 2.05 | 1.16–3.62 | 0.63 | 0.24–1.65 |
| 50–59 | 1.0 | 1.0 | ||
| 60–69 | 0.61 | 0.38–0.98 | 0.41 | 0.16–1.04 |
| ≥70 | NA b | 0.03 | 0.01–0.10 | |
| Race, socio-economic status (SES) | ||||
| High SES NH White | 1.0 | 1.0 | ||
| High SES Hispanic | 1.09 | 0.41–2.87 | 1.32 | 0.34–5.13 |
| High SES NH Asian or Pacific Islander (API) | 0.47 | 0.23–0.97 | 0.79 | 0.27–2.31 |
| High SES NH Black | 0.37 | 0.16–0.86 | 2.54 | 0.45–14.5 |
| Low SES NH White | 0.63 | 0.35–1.11 | 1.47 | 0.53–4.09 |
| Low SES Hispanic | 0.69 | 0.30–1.59 | 0.87 | 0.21–3.63 |
| Low SES API | 0.54 | 0.20–1.48 | 0.49 | 0.12–2.05 |
| Low SES NH Black | 0.50 | 0.25–1.03 | 1.89 | 0.38–9.33 |
| Tumor size (cm) | ||||
| <1 | NA b | 0.17 | 0.06–0.47 | |
| 1 to <2 | 1.0 | 1.0 | ||
| 2 to <3 | 2.32 | 1.38–3.90 | 1.27 | 0.52–3.10 |
| 3 to <4 | 1.46 | 0.81–2.63 | 2.60 | 0.79–8.62 |
| 4 to <5 | 2.18 | 0.86–5.51 | 2.55 | 0.30–22.0 |
| ≥5 | 1.11 | 0.41–3.00 | 2.91 | 0.44–19.5 |
| Microscopic foci, diffuse, or unknown | NA b | 0.24 | 0.01–4.05 | |
| Lymph node involvement | ||||
| Negative or unknown | 1.0 | 1.0 | ||
| Positive | 2.90 | 1.77–4.57 | 3.67 | 1.66–8.12 |
| Grade | ||||
| Grade I | 1.0 | 1.0 | ||
| Grade II | 4.13 | 1.06–16.1 | 5.05 | 1.38–18.5 |
| Grade III | 4.26 | 1.16–15.7 | 6.46 | 1.72–24.2 |
| Unknown | 1.91 | 0.24–15.3 | ||
| Estrogen, Progesterone Receptors | ||||
| Positive (either or both) | NA b | 1.0 | ||
| Negative (both) | 2.99 | 1.36–6.57 | ||
| Neuropathy | ||||
| No | 1.0 | NA b | ||
| Yes | 0.35 | 0.13–0.98 | ||
| Modified Charlson Comorbidity Indexc | ||||
| 0 | 1.0 | NA b | ||
| 1 | 1.32 | 0.61–2.85 | ||
| 2 | 1.35 | 0.13–13.9 | ||
| ≤3 | 0.05 | 0.00–0.58 | ||
| Year of diagnosis (per year) | 1.01 | 0.83–1.23 | 1.86 | 0.90–3.86 |
This analysis was limited to the years 2006–2007, because trastuzumab was not recommended for Stage I–III breast cancer prior to that time
Not applicable; this variable was not included in the final multivariable model
This Charlson Comorbidity Index excluded heart disease, diabetes and neuropathy, since they are separate variables
Discussion
To our knowledge, this is the first use of linked electronic drug administration records and SEER registry data for a detailed analysis of breast cancer chemotherapy; this linkage provides a more complete picture than either source alone. Leveraging high-quality EMR data available through a large, integrated health care system covering a diverse yet representative population, we observed more chemotherapy use by women with adverse prognostic factors (including young age, large tumor size, and involved lymph nodes), and less chemotherapy use by women at higher risk for drug-specific toxicities, given their comorbidities (including heart disease for anthracyclines, and neuropathy for taxanes); these patterns follow clinical trial results and practice guidelines. However, we found variations in specific drug use according to race/ethnicity and SES; compared to high-SES NH Whites, there was less receipt of highly active anthracycline- and taxane-based regimens by low-SES NH Whites and American Indian/Alaskan Natives (AIAN), and more receipt of anthracyclines by high-SES Asians/Pacific Islanders (API). High-SES Blacks and API were less likely than high-SES NH Whites to receive timely combination chemotherapy for ER/PR-negative cancer. This variability occurred despite the equal-access care setting, and warrants further study of cultural and socio-demographic influences on cancer care.
Given the coverage structure, KPNC patients are unlikely to seek out-of-network care, which reduces the chance that we missed records of chemotherapy administered at other institutions; only 2.6 % of patients were coded by CCR, but not by KPNC, as having had chemotherapy. Moreover, all KPNC patients have health insurance, reducing variability in access. This study thus offers a nearly complete picture of chemotherapy use in a large, diverse healthcare system [17, 35, 36]. Predictably, most care followed results of clinical trials, particularly those reporting that women with younger age, larger tumor size, involved lymph nodes, higher grade, positive HER2 and negative ER/PR status derive greater benefit from chemotherapy [2–5, 31, 32]. Relevant practice guidelines include those of the National Comprehensive Cancer Network and St. Gallen Consensus Conference [8, 37]; these lengthy documents incorporate nuances of decision making, including patient preferences, about which we lacked information. Thus, we focused on the ASCO QOPI measures, which establish common ground for quality care [7]. Even though QOPI guidelines were not formalized until 2010 [7], we observed high concordance (88.8–92.5%) with two QOPI measures which we could assess using available data, for women with ER/PR-negative and HER2-positive tumors. We found evidence of a switch from the older CMF regimen to newer “third generation” anthracycline and taxane-based combinations over time, consistent with emerging randomized trials and evidence summaries during this period [5, 13, 38–41]. Our finding of less anthracycline use by women with heart disease, less taxane use by women with neuropathy, and less combination chemotherapy among women with diabetes or more comorbidities, likely reflects appropriate patient-level tailoring of care.
KPNC members are representative of the general population, allowing us to evaluate chemotherapy use by socio-demographic characteristics [42, 43]. Our findings of less chemotherapy use among unmarried women, and less anthracycline and taxane use by low-SES NH Whites and NH AIANs, may result from limited social or financial resources with which to offset the burden of chemotherapy. Prior studies have investigated breast cancer treatment across diverse populations [44–50], with recent work reporting an interplay of race and marital status [51], interactions between race and tumor subtype within clinical trials [52], and a lower probability of guideline-consistent care among patients with less insurance coverage [53]. A novel contribution of our work is its consideration of specific chemotherapy agents and combinations, which may shed light on observed patterns of care; for example, our finding of more anthracycline use among high-SES APIs compared to high-SES NH Whites might reflect differences in education, body mass index, or lifestyle, which could mitigate concerns about the cardiac side effects of this drug class. Conversely, we observed lower odds of receiving timely combination chemotherapy for ER/PR-negative cancers (an ASCO QOPI measure) among high-SES APIs and Blacks compared to high-SES NH Whites, a finding which warrants investigation of potential barriers to treatment initiation.
Although these associations control for temporal trends, tumor prognostic factors, age, and comorbidities, they cannot unravel the complexity of applying trial results and practice guidelines to the care of an individual, a therapeutic process that is enmeshed with the goals, fears, and experiences of both physician and patient. Further study of the factors that guide decisions about cancer treatment might clarify the socio-demographic use patterns that we observed.
Our study has some limitations. We extracted individual drug names and infusion patterns from KPNC administrative data, an approach validated by a recent study of the Cancer Research Network [54]; however, we did not evaluate treatment delays, deviations from standard dosing by body surface area or other parameters, use of ancillary medications (such as hematopoietic growth factors and bisphosphonates) or novel diagnostics which may be used to target chemotherapy (such as tumor genomic profiling for recurrence risk). These questions are key priorities for future research. We were particularly interested in examining racial/ethnic and SES interactions; however, our significant findings should be interpreted with caution given the number of race/ethnicity by SES combinations, small numbers in some groups, and potential for chance findings.
Many of the above questions are not amenable to investigation by currently available databases. However, KPNC has recently implemented the Beacon infusion medication module for its Epic™ (Verona, WI)-based electronic medical record that replaces the stand-alone CAMMOLOT and COPS databases. This database will facilitate future investigation of these questions as it provides substantially greater detail on infusion medication planning and administration.
We used EMRs to study patterns of chemotherapy treatment for breast cancer in a large, diverse medical care program from 2004 to 2007. We observed care patterns consistent with practice guidelines, including more chemotherapy use by women having the most to gain from it, given their adverse prognostic factors, and less chemotherapy use by women with comorbidities that increased risk for drug-specific toxicities. However, we also observed significant variability according to race and socio-demographic factors, despite the equal-access setting. These results may inform efforts to optimize treatment for all patients, and guide future studies of quality in breast cancer care.
Acknowledgments
This research was supported by the National Cancer Institute's Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C and a SEER Rapid Response Surveillance Study under contract N01-PC-35136 awarded to the Cancer Prevention Institute of California, and under a subcontract to Kaiser Permanente Northern California Division of Research. This research was also supported by grants from the National Cancer Institute (R01 CA105274 to L.H.K. and R01 CA098838 to L.A.H.) and from the American Cancer Society (RSGT-08-009-01-CPHPS to D.L.H.). The collection of cancer incidence data used in this study was supported by the California Department of Health Services as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology, and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement #1U58 DP000807-01 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the authors, and endorsement by the State of California, the California Department of Health Services, the National Cancer Institute, or the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred. We thank Dr. Lou Fehrenbacher for his helpful comments on the manuscript.
Footnotes
Conflict of interest The authors declare that they have no conflicts of interest.
Ethical Standards All reported work in this study complies with the current laws of the United States.
Contributor Information
Allison W. Kurian, Department of Medicine, Stanford University School of Medicine, Stanford, CA, USA; Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA, USA
Daphne Y. Lichtensztajn, Cancer Prevention Institute of California, 2201 Walnut Avenue, Suite 300, Fremont, CA 94538, USA
Theresa H. M. Keegan, Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA, USA; Cancer Prevention Institute of California, 2201 Walnut Avenue, Suite 300, Fremont, CA 94538, USA
Rita W. Leung, Cancer Prevention Institute of California, 2201 Walnut Avenue, Suite 300, Fremont, CA 94538, USA
Sarah J. Shema, Cancer Prevention Institute of California, 2201 Walnut Avenue, Suite 300, Fremont, CA 94538, USA
Dawn L. Hershman, Columbia University Medical Center, New York, NY, USA
Lawrence H. Kushi, Division of Research, Kaiser Permanente, Oakland, CA, USA
Laurel A. Habel, Division of Research, Kaiser Permanente, Oakland, CA, USA
Tatjana Kolevska, Kaiser Permanente, Vallejo, CA, USA.
Bette J. Caan, Division of Research, Kaiser Permanente, Oakland, CA, USA
Scarlett L. Gomez, Email: scarlett@cpic.org, Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA, USA; Cancer Prevention Institute of California, 2201 Walnut Avenue, Suite 300, Fremont, CA 94538, USA.
References
- 1.De Angelis R, Tavilla A, Verdecchia A, et al. Breast cancer survivors in the United States: geographic variability and time trends, 2005-2015. Cancer. 2009;115:1954–1966. doi: 10.1002/cncr.24217. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists' Collaborative Group. Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer. An overview of 61 randomized trials among 28,896 women. N Engl J Med. 1988;319:1681–1692. doi: 10.1056/NEJM198812293192601. [DOI] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists' Collaborative Group. Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Lancet. 1992;339:71–85. [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists' Collaborative Group. Polychemotherapy for early breast cancer: an overview of the randomised trials. Lancet. 1998;352:930–942. [PubMed] [Google Scholar]
- 5.Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006;295:1658–1667. doi: 10.1001/jama.295.14.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke M. Meta-analyses of adjuvant therapies for women with early breast cancer: the Early Breast Cancer Trialists' Collaborative Group overview. Ann Oncol. 2006;17(Suppl 10):x59–x62. doi: 10.1093/annonc/mdl238. [DOI] [PubMed] [Google Scholar]
- 7.Blayney DW, McNiff K, Hanauer D, Miela G, Markstrom D, Neuss M. Implementation of the Quality Oncology Practice Initiative at a university comprehensive cancer center. J Clin Oncol. 2009;27:3802–3807. doi: 10.1200/JCO.2008.21.6770. [DOI] [PubMed] [Google Scholar]
- 8.Carlson RW, Allred DC, Anderson BO, et al. Invasive breast cancer. J Natl Compr Canc Netw. 2011;9:136–222. doi: 10.6004/jnccn.2011.0016. [DOI] [PubMed] [Google Scholar]
- 9.Du W, Mood D, Gadgeel S, Simon MS. An educational video to increase clinical trials enrollment among breast cancer patients. Breast Cancer Res Treat. 2009;117:339–347. doi: 10.1007/s10549-009-0311-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimmick GG, Peterson BL, Kornblith AB, et al. Improving accrual of older persons to cancer treatment trials: a randomized trial comparing an educational intervention with standard information: CALGB 360001. J Clin Oncol. 2005;23:2201–2207. doi: 10.1200/JCO.2005.01.222. [DOI] [PubMed] [Google Scholar]
- 11.Simon MS, Du W, Flaherty L, et al. Factors associated with breast cancer clinical trials participation and enrollment at a large academic medical center. J Clin Oncol. 2004;22:2046–2052. doi: 10.1200/JCO.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Winn RJ. Obstacles to the accrual of patients to clinical trials in the community setting. Semin Oncol. 1994;21:112–117. [PubMed] [Google Scholar]
- 13.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 14.Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–1671. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bickell NA, McAlearney AS, Wellner J, Fei K, Franco R. Understanding the challenges of adjuvant treatment measurement and reporting in breast Cancer: cancer treatment measuring and reporting. Med Care. 2011 Dec 30; doi: 10.1097/MLR.0b013e3182422f7b. electronic publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lodrigues W, Dumas J, Rao M, Lilley L, Rao R. Compliance with the commission on cancer quality of breast cancer care measures: self-evaluation advised. Breast J. 2011;17:167–171. doi: 10.1111/j.1524-4741.2010.01047.x. [DOI] [PubMed] [Google Scholar]
- 17.Wagner EH, Greene SM, Hart G, et al. Building a research consortium of large health systems: the Cancer Research Network. J Natl Cancer Inst Monogr. 2005;2005:3–11. doi: 10.1093/jncimonographs/lgi032. [DOI] [PubMed] [Google Scholar]
- 18.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 21.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 22.Shavers VL, Brown M, Klabunde CN, et al. Race/ethnicity and the intensity of medical monitoring under ‘watchful waiting’ for prostate cancer. Med Care. 2004;42:239–250. doi: 10.1097/01.mlr.0000117361.61444.71. [DOI] [PubMed] [Google Scholar]
- 23.California cancer reporting system standards. tenth. I-IV. California Department of Public Health, Cancer Surveillance and Research Branch; Sacramento, CA: 2009-2011. [Accessed May 30, 2012]. Cancer reporting in California: abstracting and coding procedures for hospitals. http://www.ccrcal.org/Cancer_Reporting/Registrar_Resources/Reporting_Cancer_Cal.shtml. [Google Scholar]
- 24.California Cancer Registry. [Accessed May 30, 2012]; http://www.ccrcal.org.
- 25.American Joint Committee on Cancer Staging. [Accessed May 30, 2012]; http://www.cancerstaging.org.
- 26.North American Association of Central Cancer Registries. [Accessed May 30, 2012];Great Circle Distance Calculator. http://www.naaccr.org/Research/DataAnalysisTools.aspx.
- 27.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12:703–711. doi: 10.1023/a:1011240019516. [DOI] [PubMed] [Google Scholar]
- 28.Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 29.Bonadonna G, Moliterni A, Zambetti M, et al. 30 years' follow up of randomised studies of adjuvant CMF in operable breast cancer: cohort study. BMJ. 2005;330:217. doi: 10.1136/bmj.38314.622095.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonadonna G, Rossi A, Valagussa P, Banfi A, Veronesi U. The CMF program for operable breast cancer with positive axillary nodes. Updated analysis on the disease-free interval, site of relapse and drug tolerance. Cancer. 1977;39:2904–2915. doi: 10.1002/1097-0142(197706)39:6<2904::aid-cncr2820390677>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 31.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 32.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 33.Telli ML, Chang ET, Kurian AW, et al. Asian ethnicity and breast cancer subtypes: a study from the California Cancer Registry. Breast Cancer Res Treat. 2011;127:471–478. doi: 10.1007/s10549-010-1173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma H, Wang Y, Sullivan-Halley J, et al. Breast cancer receptor status: do results from a centralized pathology laboratory agree with SEER registry reports? Cancer Epidemiol Biomarkers Prev. 2009;18:2214–2220. doi: 10.1158/1055-9965.EPI-09-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caan B, Sternfeld B, Gunderson E, Coates A, Quesenberry C, Slattery ML. Life After Cancer Epidemiology (LACE) Study: a cohort of early stage breast cancer survivors (United States) Cancer Causes Control. 2005;16:545–556. doi: 10.1007/s10552-004-8340-3. [DOI] [PubMed] [Google Scholar]
- 36.Kwan ML, Ambrosone CB, Lee MM, et al. The Pathways Study: a prospective study of breast cancer survivorship within Kaiser Permanente Northern California. Cancer Causes Control. 2008;19:1065–1076. doi: 10.1007/s10552-008-9170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gnant M, Harbeck N, Thomssen C. St. Gallen 2011: Summary of the Consensus Discussion. Breast Care (Basel) 2011;6:136–141. doi: 10.1159/000328054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Early Breast Cancer Trialists' Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 39.Carlson RW, Anderson BO, Burstein HJ, et al. Invasive breast cancer. J Natl Compr Canc Netw. 2007;5:246–312. doi: 10.6004/jnccn.2007.0025. [DOI] [PubMed] [Google Scholar]
- 40.Olivotto IA, Bajdik CD, Ravdin PM, et al. Population-based validation of the prognostic model ADJUVANT! For early breast cancer. J Clin Oncol. 2005;23:2716–2725. doi: 10.1200/JCO.2005.06.178. [DOI] [PubMed] [Google Scholar]
- 41.Ravdin PM, Siminoff LA, Davis GJ, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001;19:980–991. doi: 10.1200/JCO.2001.19.4.980. [DOI] [PubMed] [Google Scholar]
- 42.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gordon NP. Internal Division of Research Report. Oakland, CA: Kaiser Permanente Division of Research; 2012. [Accessed May 30, 2012]. Similarity of the Adult Kaiser Permanente Membership in Northern California to the Insured and General Population in Northern California: Statistics from the 2009 California Health Interview Survey. http://www.dor.kaiser.org/external/chis_non_kp_2009. [Google Scholar]
- 44.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 45.Curtis E, Quale C, Haggstrom D, Smith-Bindman R. Racial and ethnic differences in breast cancer survival: how much is explained by screening, tumor severity, biology, treatment, comorbidities, and demographics? Cancer. 2008;112:171–180. doi: 10.1002/cncr.23131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li CI. Racial and ethnic disparities in breast cancer stage, treatment, and survival in the United States. Ethn Dis. 2005;15(2 Suppl 2):S5–S9. [PubMed] [Google Scholar]
- 47.West DW, Satariano WA, Ragland DR, Hiatt RA. Comorbidity and breast cancer survival: a comparison between black and white women. Ann Epidemiol. 1996;6:413–419. doi: 10.1016/s1047-2797(96)00096-8. [DOI] [PubMed] [Google Scholar]
- 48.Giordano SH, Lin YL, Kuo YF, Hortobagyi GN, Goodwin JS. Decline in the use of anthracyclines for breast cancer. J Clin Oncol. 2012;30:2232–2239. doi: 10.1200/JCO.2011.40.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Griggs JJ, Culakova E, Sorbero ME, et al. Social and racial differences in selection of breast cancer adjuvant chemotherapy regimens. J Clin Oncol. 2007;25:2522–2527. doi: 10.1200/JCO.2006.10.2749. [DOI] [PubMed] [Google Scholar]
- 50.Griggs JJ, Culakova E, Sorbero ME, et al. Effect of patient socioeconomic status and body mass index on the quality of breast cancer adjuvant chemotherapy. J Clin Oncol. 2007;25:277–284. doi: 10.1200/JCO.2006.08.3063. [DOI] [PubMed] [Google Scholar]
- 51.Lipscomb J, Gillespie TW, Goodman M, et al. Black–white differences in receipt and completion of adjuvant chemotherapy among breast cancer patients in a rural region of the US. Breast Cancer Res Treat. 2012;133:285–296. doi: 10.1007/s10549-011-1916-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sparano JA, Wang M, Zhao F, et al. Race and hormone receptor-positive breast cancer outcomes in a randomized chemotherapy trial. J Natl Cancer Inst. 2012;104:406–414. doi: 10.1093/jnci/djr543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu XC, Lund MJ, Kimmick GG, et al. Influence of race, insurance, socioeconomic status, and hospital type on receipt of guideline-concordant adjuvant systemic therapy for locoregional breast cancers. J Clin Oncol. 2012;30:142–150. doi: 10.1200/JCO.2011.36.8399. [DOI] [PubMed] [Google Scholar]
- 54.Delate T, Bowles EJ, Pardee R, et al. Validity of eight integrated healthcare delivery organizations' administrative clinical data to capture breast cancer chemotherapy exposure. Cancer Epidemiol Biomarkers Prev. 2012;21:673–680. doi: 10.1158/1055-9965.EPI-11-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]