Abstract
Erythropoietin (EPO) has protective effects in neurodegenerative and neuroinflammatory diseases, including in animal models of multiple sclerosis, where EPO decreases disease severity. EPO also promotes neurogenesis and is protective in models of toxic demyelination. In this study, we asked whether EPO could promote neurorepair by also inducing remyelination. In addition, we investigated whether the effect of EPO could be mediated by the classical erythropoietic EPO receptor (EPOR), since it is still questioned if EPOR is functional in nonhematopoietic cells. Using CG4 cells, a line of rat oligodendrocyte precursor cells, we found that EPO increases the expression of myelin genes (myelin oligodendrocyte glycoprotein [MOG] and myelin basic protein [MBP]). EPO had no effect in wild-type CG4 cells, which do not express EPOR, whereas it increased MOG and MBP expression in cells engineered to overexpress EPOR (CG4-EPOR). This was reflected in a marked increase in MOG protein levels, as detected by Western blot. In these cells, EPO induced by 10-fold the early growth response gene 2 (Egr2), which is required for peripheral myelination. However, Egr2 silencing with a siRNA did not reverse the effect of EPO, indicating that EPO acts through other pathways. In conclusion, EPO induces the expression of myelin genes in oligodendrocytes and this effect requires the presence of EPOR. This study demonstrates that EPOR can mediate neuroreparative effects.
INTRODUCTION
Erythropoietin (EPO) has protective effects and decreases neuroinflammation in various models of neurological diseases, including traumatic and ischemic injury of the brain and the spinal cord and multiple sclerosis (MS) (1,2). Inhibition of neuronal death and neuroinflammation are important for the protective effects (3). However, many studies have pointed out that EPO also promotes neurorepair, in terms of neurogenesis, angiogenesis and promotion of synaptic plasticity (4–6).
In the context of MS, EPO has antiinflammatory (7,8) and immunoregulatory properties (9,10). In addition, it inhibits demyelination and axonal damage (11,12), but it is unclear whether this effect is secondary to its antiinflammatory and immunoregulatory action. However, there are evidences that EPO is effective also in nonimmune models of demyelination. EPO is protective in vivo in a model of chemically induced demyelination (13) and induces myelin repair in an ex vivo model of demyelination induced by lysolecithin (14). Interestingly, EPO increases the number of myelin basic protein (MBP)-positive cells in primary oligodendrocytes (15).
The role of the EPO receptor (EPOR) in the neuroprotective actions of EPO is a debated issue (16). EPO mediates erythropoiesis by homodimerizing EPOR (17) but derivatives of EPO that do not bind the homodimeric EPOR, and are therefore not erythropoietic, are still neuroprotective (18,19), and EPO can reduce brain damage in mice lacking neural EPOR (20). On the other hand, EPOR is required for normal brain development (21) and for inhibition of apoptosis in neuronal cells (22). Also, the observation that brain EPOR expression is increased during pathological conditions in humans, including ischemic infarcts and hypoxic brain damage, suggests a potential protective role of the classical receptor (23). Recent studies have indicated that the spectrum of actions of EPOR can go beyond those induced by its homodimerization, and the tissue-protective activities of EPO might be due, at least in part, to heterodimerization of EPOR with the common β chain (bc) of interleukin (IL)-3/IL-5 and granulocyte-macrophage colony-stimulating factor (GM-CSF). EPO variants (for example, carbamylated EPO, CEPO) that can bind the heterodimeric EPOR/bc but not the EPOR dimer have tissue-protective effects equivalent to EPO in multiple animal models of disease (24).
Here, we studied the effect of EPO on myelination, specifically investigating the role of EPOR. For this purpose, we measured the expression of two major myelin genes, myelin oligodendrocyte glycoprotein (MOG) and myelin basic protein (MBP), in the differentiated CG4 oligodendrocytic cell line, with or without EPOR expression. CG4 cells are considered a good tool to study myelination in vitro(25,26). We also investigated the role of early growth response gene 2 (Egr2), a gene required for myelination in the peripheral nervous system (27), whose expression is induced by EPO in the brain (6). The results of this study clearly indicate that EPOR is required for EPO-induced myelination.
MATERIALS AND METHODS
Cell Culture and Generation of CG4 Cells Expressing EPOR (CG4-EPOR)
The oligodendrocyte progenitor cell line CG4 was cultured in poly-l-ornithine–coated tissue culture plates (Sigma-Aldrich, St. Louis, MO, USA). Cells were maintained at the precursor stage using growth medium (GM) consisting of Dulbecco’s modified Eagle medium (DMEM) (PAA Laboratories, Yevil, Sommerset, UK) supplemented with biotin (10 ng/mL), bFGF (5 ng/mL), PDGF (1 ng/mL), N1 supplement (all from Sigma-Aldrich) and 30% B104- conditioned medium. Rat neuroblastoma B104 cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) (Invitrogen/Life Technologies, Carlsbad, CA, USA). For the preparation of the B104-conditioned medium, B104 confluent cells were cultured for 4 d in DMEM, without FBS, but with the addition of N1 supplement. CG4 cells overexpressing EPO receptor (CG4-EPOR) were obtained by transduction of CG4 cells with the mouse EPOR gene in a constitutive lentiviral vector (28), modified to include the V5 epitope, the mouse encephalomyocarditis internal ribosome entry site (IRES) and the enhanced green fluorescent protein (EGFP) reporter (6,25). Clones were isolated by limiting dilution, by plating transduced cells at the concentration of 0.3 cells/well in 96 well plates in GM, and then screened for EPOR expression by quantitative polymerase chain reaction (qPCR), as described below. Control CG4 cells (CG4-EGFP) were obtained by transduction of CG4 cells with a lentiviral vector containing EGFP only.
CG4 cells were induced to differentiate to oligodendrocytes by switching to differentiation medium (DM) consisting of DMEM-F12 (PAA) supplemented with progesterone (3 ng/mL), putrescine (5 μg/mL), sodium selenite (4 ng/mL), insulin (12.5 μg/mL), transferrin (50 μg/mL), biotin (10 ng/mL), thyroxine (0.4 μg/mL) and glucose (3 g/L) (all from Sigma-Aldrich). Cells were treated with recombinant human erythropoietin (rhEPO) (Creative Dynamics, New York, NY, USA) at the doses indicated. Carbamylated EPO (CEPO), prepared as described (18), was kindly supplied by Warren Pharmaceuticals, Ossining, NY, USA.
EPOR Expression in CG4-EPOR Cells
The expression of recombinant V5-tagged EPOR in transduced CG4 cells was verified by measuring by flow cytometry the EGFP reporter expression, as well as by immunoblotting with the anti-V5-tag mouse monoclonal antibody (Invitrogen/Life Technologies), as described (6,25). EGFP, whose translation is linked to EPOR via the internal ribosome entry site (IRES), is a very reliable marker of the gene of interest expression when bicistronic vectors are used (25).
Quantitative PCR
Total RNA was extracted from cultured cells using TRIzol (Invitrogen/Life Technologies). RNA quality and concentration were determined using a NanoDrop ND-1000 (NanoDrop Technologies/Thermo Fisher Scientific, Wilmington, DE, USA). Reverse transcription and real-time qPCR were carried out as reported (6), using TaqMan gene expression assays for rat MOG, rat MBP, rat Egr2, mouse EPOR and rat hypoxanthine phosphoribosyltransferase 1 (HPRT1, housekeeping gene), commercially available from Applied Biosystems/Life Technologies. For quantification, we used the comparative threshold cycle (ΔΔCt) method, following Applied Biosystems/Life Technologies guidelines. Results were normalized to HPRT1 expression and expressed as arbitrary units, using as a calibrator one of the control samples, as specified in the figure legends. Gene expression was considered undetectable when the threshold cycle for fluorescence detection was >38. Statistical significance was determined using the unpaired two-tailed Student t test or, for multiple comparisons, the Dunnett or Tukey-Kramer test.
MOG Western Blot
CG4 and CG4-EPOR cells were plated in poly-l-ornithine–coated petri dishes in GM, differentiated by switching to DM and treated with or without EPO. Cells were collected and centrifuged, supernatants were discarded and pellets lysed in 50 μL of lysis buffer without β-mercaptoethanol, as described (25). Protein concentration was measured using the BCA reagent kit (Pierce Biotechnology, Rockford, IL, USA), and 100 μg of cellular proteins were analyzed by polyacrylamide gel electrophoresis. Separated proteins were blotted using Trans-Blot Turbo Blotting System (Bio-Rad, Hemel Hempstead, Hertfordshire, UK) onto a polyvinylidenefluoride (PVDF) membrane. Membranes were blocked overnight with 2% casein (Fisher Scientific, Loughborough, Leicestershire, UK) in PBS, as reported (25). Antibodies used were: mouse monoclonal anti-MOG Z12 (kindly donated by Gareth Pryce, ICMS, Queen Mary University of London, UK), mouse anti-β-actin (clone AC-15, #A54541) (Sigma-Aldrich), goat anti-mouse IgG-HRP (#3697) (Santa Cruz Biotechnology, Heidelberg, Germany). Protein bands were visualized by exposing membranes developed with the ECL reagent (Amersham/GE Healthcare Life Sciences, Little Chalfont, Buckinghamshire, UK) to chemiluminescence film (Hyperfilm ECL) (Amersham/GE Healthcare Life Sciences).
siRNA Transfection
CG4-EPOR cells were plated in GM without penicillin and streptomycin. The next day cells were switched to DM and transfected with a Silencer Select Pre-designed Egr2-siRNA (s137448) (Ambion/Life Technologies) or control siRNA (Ambion/Life Technologies) using lipofectamine RNAiMAX (Invitrogen/Life Technologies), according to the manufacturer’s instructions. After a further 24 h, the cells were treated with or without EPO as indicated.
RESULTS
EPO Does Not Induce Myelin Gene Expression in Wild-type CG4 Cells Not Expressing EPOR
We first studied the effect of EPO on MOG and MBP gene expression in wild-type CG4 cells. Cells were differentiated for 5 d in differentiation medium (DM) with or without 80 ng/mL EPO. Differentiation alone induced both MOG and MBP gene expression by about nine-fold compared with undifferentiated cells (Figure 1). The addition of 80 ng/mL EPO during the differentiation period did not increase MOG or MBP gene expression compared with untreated cells (see Figure 1). However, EPO receptor (EPOR) was not detected in these cells by qPCR analysis (fluorescence threshold cycle for EPOR amplification was >38).
Figure 1.
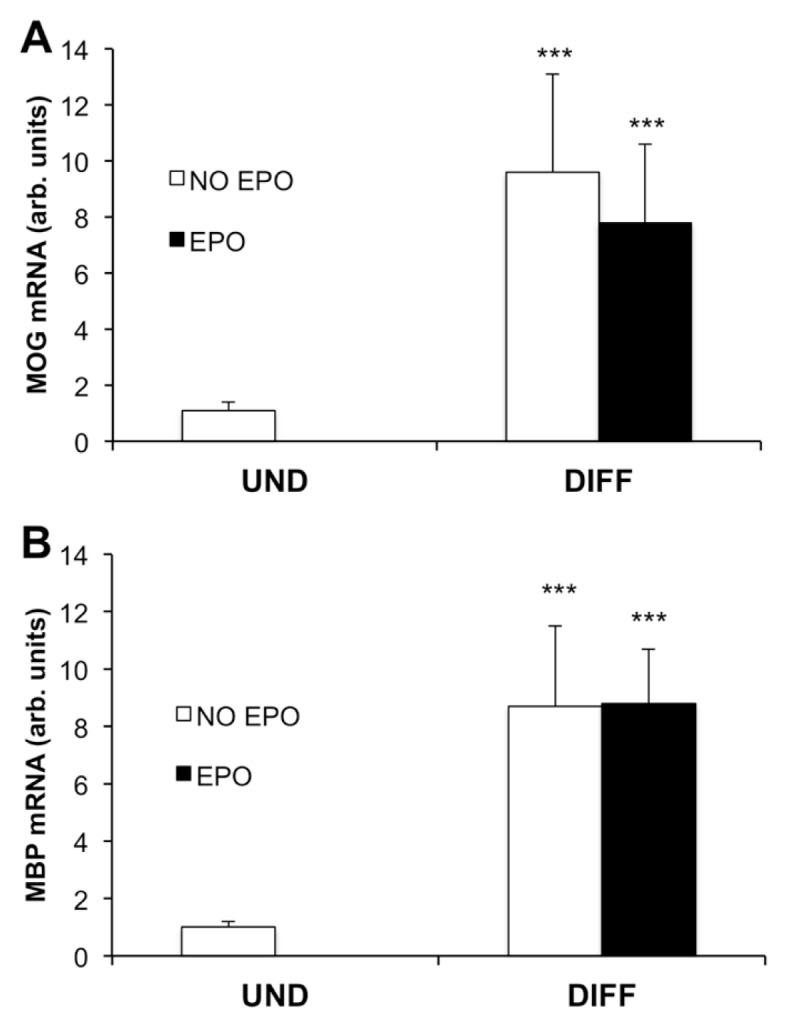
EPO does not increase myelin gene expression in wild-type CG4 cells. CG4 cells were plated in poly-l-ornithine–coated 24 well plates in growth medium (GM). After 2 d, cells were switched to differentiation medium (DM) and cultured in the absence or in the presence of EPO 80 ng/mL. At d 5 of differentiation MOG (A) and MBP (B) gene expression were analyzed by quantitative PCR (qPCR). Results are expressed as arbitrary units versus undifferentiated (UND) cells and are the mean ± standard deviation (SD) of six samples from two independent experiments analyzed in duplicate. ***P < 0.001 versus UND cells by two-tailed unpaired Student t test.
EPO Induces Myelin Gene Expression in EPOR-Expressing CG4 Cells
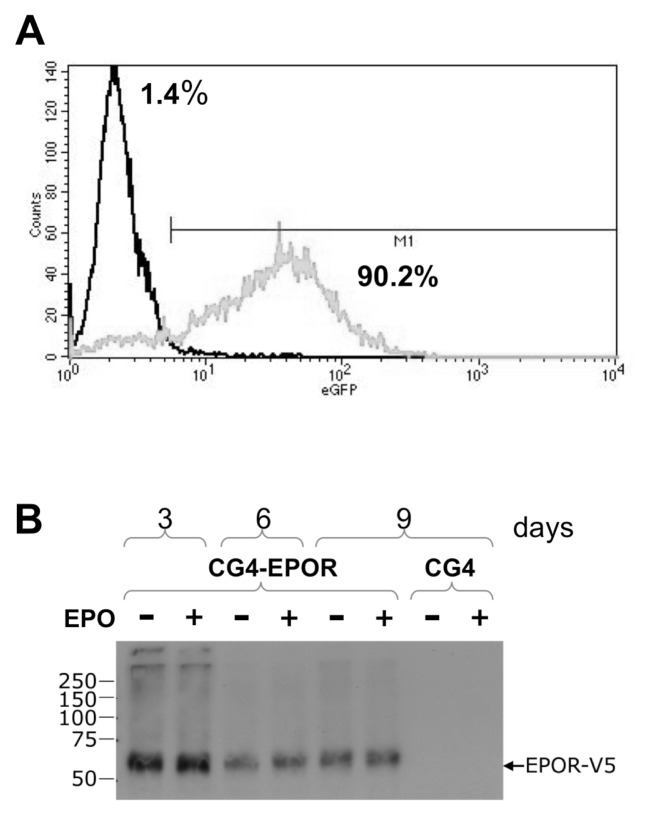
EPOR expression in genetically modified CG4-EPOR cells was verified by measuring the EGFP reporter expression by flow cytometry, which showed that 90.2% of cells expressed the transgene (Figure 2A). We then characterized the expression of EPOR in CG4-EPOR cells differentiated for 3, 6 and 9 d with or without EPO by Western blot, using anti-V5-tag antibodies. EPOR was detected in CG4-EPOR cells at all time points (Figure 2B), and its expression level was not affected by EPO.
Figure 2.
EPOR expression in CG4-EPOR cells. (A) CG4 cells were transduced with a bicistronic lentiviral vector expressing EPOR and EGFP and cultured in GM. The efficiency of transfection in genetically modified (CG4-EPOR) cells was verified by measuring by flow cytometry the EGFP reporter expression, whose translation is linked to EPOR via the internal ribosome entry site (IRES). About 90% of the CG4-EPOR cells expressed EGFP, as opposed to 1.4% of background fluorescence in wild-type CG4 cells. The experiment is representative of three experiments. EGFP expression in single experiments was 83%, 90.2% and 87.9%. (B) CG4-EPOR were switched to DM and cultured for 3, 6 and 9 d with or without EPO. EPOR expression during cell differentiation was confirmed by immunoblotting with anti-V5-tag mouse antibody. As a negative control, wild-type CG4 cells at d 9 of differentiation also were analyzed for transduced EPOR expression. The numbers on the left of the panel indicate the positions of molecular weight markers of the indicated sizes in kDa.
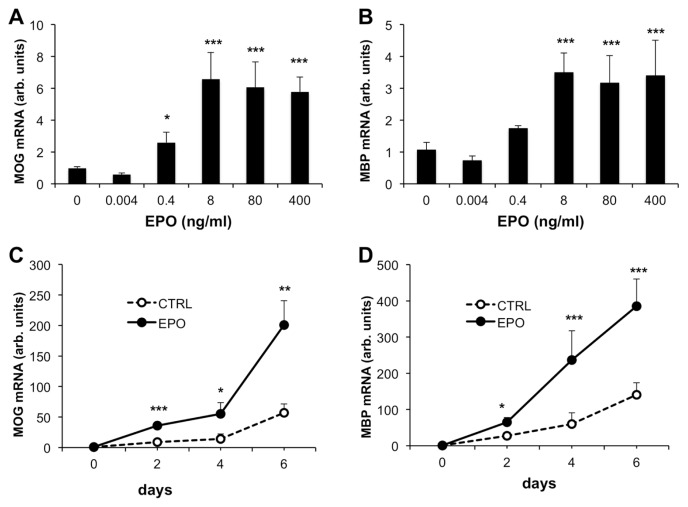
Having established that EPOR was expressed, we then treated CG4-EPOR cells with different concentrations of EPO for 6 d during cell differentiation and analyzed MOG and MBP mRNA. As shown in Figure 3A–B, EPO, at concentrations of 8 ng/mL and higher, increased MOG and MBP gene expression by six- and three-fold respectively, compared with untreated cells. Although no further induction was found with doses of 80 and 400 ng/mL, the intermediate dose of 80 ng/mL was chosen for further experiments since this dose is the most used in neuroprotection studies (6). A time-course experiment investigating the effect of EPO at 80 ng/mL on MOG and MBP mRNA expression in CG4-EPOR cells is shown in Figure 3C–D. EPO increased MOG (see Figure 3C) and MBP (see Figure 3D) expression in differentiated cells by two- to four-fold at all time points analyzed.
Figure 3.
Dose-response and time-course of myelin gene expression in CG4-EPOR cells upon EPO treatment. (A–B) CG4-EPOR cells were differentiated and treated with different concentrations of EPO. MOG (A) and MBP (B) gene expression were analyzed by qPCR at d 6 of differentiation. Results are expressed as arbitrary units versus untreated (no EPO) cells and are the mean ± SD of six to nine samples from three independent experiments analyzed in duplicate (panel A, MOG: no EPO, N = 9; EPO doses of 0.004 and 0.4 ng/mL, N = 6; EPO doses of 8, 80 and 400 ng/mL, N = 9; panel B, MBP: no EPO, N = 6; EPO doses of 0.004 and 0.4 ng/mL, N = 6; EPO doses of 8, 80 and 400 ng/mL, N = 6). *P < 0.05; ***P < 0.001 versus no EPO by Dunnett’s method. (C–D) CG4-EPOR cells were differentiated and treated with or without EPO 80 ng/mL. MOG (C) and MBP (D) gene expression were analyzed by qPCR at 2, 4 and 6 d of differentiation. The results are expressed as arbitrary units versus undifferentiated (time 0) cells and are the mean ± SD of four to six samples from two independent experiments analyzed in duplicate. *P < 0.05; **P < 0.01; ***P < 0.001 versus no EPO at the same time point by Student t test.
Induction of MOG Protein Expression by EPO
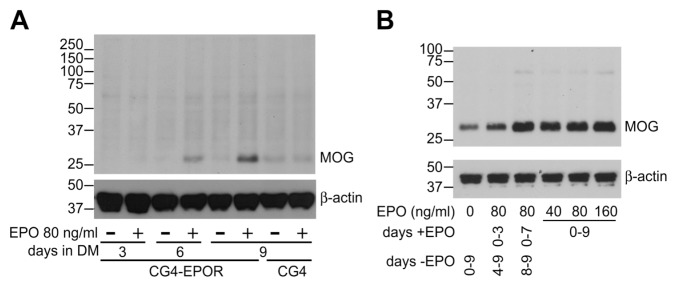
As all the above experiments were performed analyzing the mRNA expression for myelin genes, we sought confirmation of the effect of EPO at the protein level. In preliminary experiments, we set up a reliable detection of MOG protein by Western blot. In the experiments shown in Figure 4A, CG4-EPOR and wild-type cells were differentiated with DM and treated with or without 80 ng/mL EPO. MOG protein expression was analyzed by Western blot at 3, 6 and 9 d of differentiation in CG4-EPOR cells and at d 9 in wild-type CG4 cells. Western blot for β-actin was used as a loading control (Figure 4).
Figure 4.
EPO increases MOG protein expression. CG4-EPOR (A and B) and wild-type CG4 cells (A) were differentiated with or without EPO. MOG protein expression (with β-actin as loading control) was analyzed by Western blot at the indicated days of differentiation (A) or at d 9 (B). The set of numbers along the left side of the images represents the position of molecular weight markers of the indicated sizes in kDa.
The inducing effect of EPO on MOG protein was very clear at 6 and 9 d in CG4-EPOR cells. No induction was observed in wild-type CG4 cells, confirming the results obtained by qPCR. We then confirmed EPO-induced MOG protein increase in CG4-EPOR cells in an independent experiment (Figure 4B). MOG protein was detectable at d 9 of differentiation in the absence of EPO, but was induced at higher levels upon incubation with EPO for the whole differentiation period (d 0–9), confirming the results of the experiment shown in Figure 4A. This effect also was observed when the cells where incubated with EPO on d 0–7 of differentiation, whereas treatment with EPO only on d 0–3 was less effective (see Figure 4B). Therefore, EPO increased MOG expression also at the protein level in CG4-EPOR cells.
EPOR is Specifically Required by EPO to Induce Myelin Genes
Because in the experiments reported above we compared EPOR-expressing CG4 cells obtained using a lentiviral vector with wild-type cells, we wanted to ascertain that the observed differences were actually due to expression of EPOR and not to the vector itself.
We thus transduced CG4 cells with a vector containing only EGFP (CG4-EGFP), used as negative control. Cells were differentiated for 5 d with DM and treated with or without 80 ng/mL EPO exactly as in the experiments described above. Then, MOG expression was measured by qPCR. As shown in Figure 5A, differentiation induced expression of MOG but EPO was ineffective, in accord with the results obtained in wild-type CG4 cells (see Figure 1A). Thus, EPOR expression is responsible for the effects of EPO observed in CG4-EPOR cells.
Figure 5.
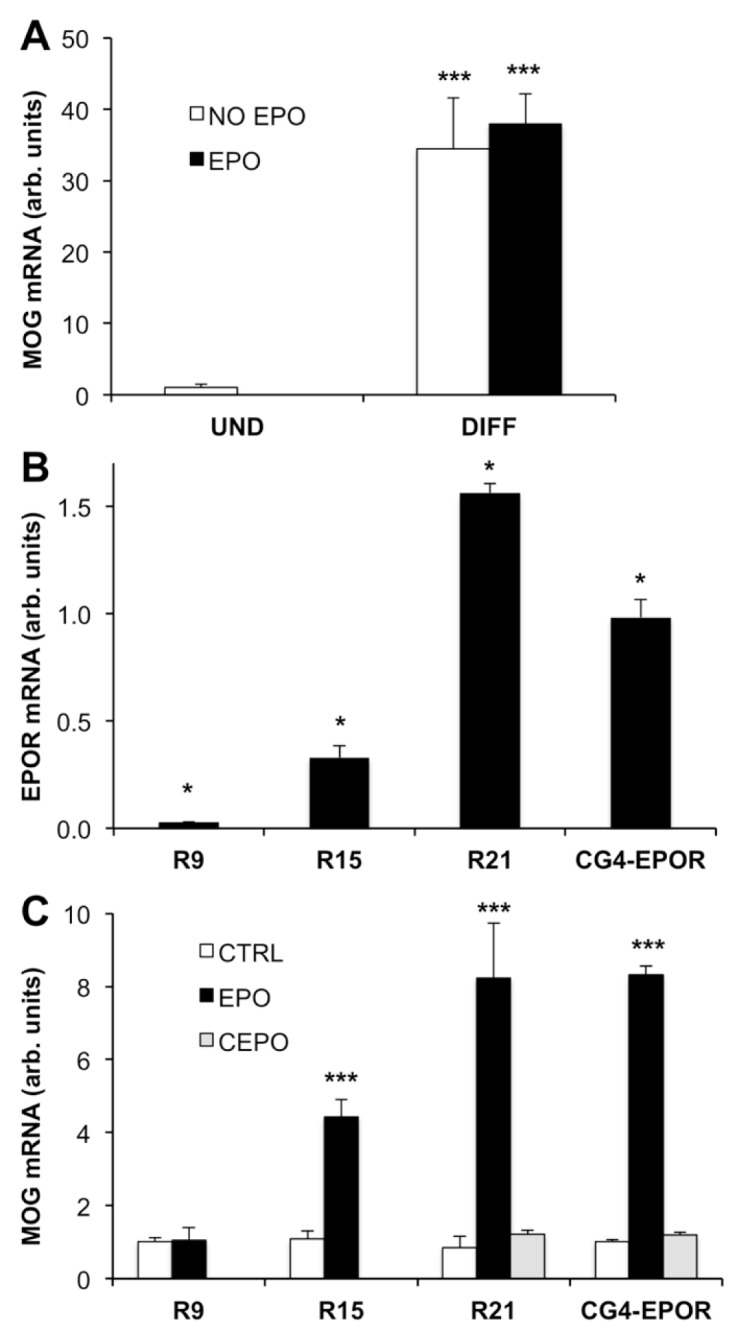
EPO effect is dependent on EPOR expression. (A) EPO has no effect in CG4 cells transduced with the EGFP vector. CG4 cells transduced with EGFP (CG4-EGFP) were differentiated and treated with or without EPO 80 ng/mL. MOG gene expression was analyzed by qPCR at d 5 of differentiation. Results are expressed as arbitrary units versus undifferentiated (UND) cells and are the mean ± SD of four samples analyzed in duplicate. **P < 0.05 versus UND cells by Student t test. (B-C) EPO increases MOG expression in a receptor-dependent manner. (B) Expression of EPOR in three CG4-EPOR clones (R9, R15 and R21) isolated by plating the cells at clonal densities. EPOR expression was measured by qPCR in quadruplicate samples analyzed in duplicate and expressed as arbitrary units versus EPOR expression in the CG4-EPOR cell line. *P < 0.05 by Tukey-Kramer’s test. (C) CG4-EPOR cells and R9, R15 and R21 cell clones were differentiated in the absence or in the presence of EPO 80 ng/mL; CG4-EPOR and R21 were also differentiated in the presence of carbamylated EPO (CEPO) at 80 ng/mL. MOG gene expression was analyzed by qPCR at d 5 of differentiation. Results are expressed as arbitrary units versus no EPO and are the mean ± SD of four samples analyzed in duplicate. ***P < 0.001 versus no EPO by Student t test.
To further confirm the requirement of EPOR for EPO induction of myelin genes, CG4-EPOR cells were cloned by limiting dilution as described in the Materials and Methods section. Three clones expressing differential levels of EPOR (R9, R15 and R21; Figure 5B) were used to study the effect of EPO on myelin gene induction. Cells were differentiated for 5 d with and without EPO and MOG gene expression was measured by qPCR. EPO did not have any effect in the low-EPOR clone R9, but induced MOG mRNA by 4.4-fold in the intermediate-EPOR clone R15 and 8-fold in the high-EPOR clone R21 and in the parental CG4-EPOR line (Figure 5C). Therefore, the MOG response to EPO was increased as the level of EPOR expression increased. Moreover, carbamylated EPO (CEPO), that does not bind to the homodimeric EPOR but is still cytoprotective and neuroprotective (18), did not have any effect on MOG gene expression in CG4-EPOR cells and in clone R21, showing that in this model induction of MOG likely occurs through the classical EPOR (see Figure 5C). In conclusion, MOG was not induced by EPO when EPOR expression was low (in CG4-EGFP cells [see Figure 5A] and in clone R9 [see Figure 5C]), and was induced by EPO, but not by CEPO (that does not bind the EPOR dimer), when EPOR expression was high (in CG4-EPOR cells and in clone R21 [see Figure 5C]). Therefore, in our in vitro system high EPOR expression was necessary and sufficient to induce MOG gene expression.
Egr2 is Induced by EPO but Does Not Mediate EPO-Induced Increase of Myelin Genes
We first investigated whether EPO induces Egr2 gene expression in CG4-EPOR cells. Based on our similar experiments where Egr2 expression was studied in EPOR-expressing neuronal B104 cells (6), we exposed differentiated CG4-EPOR cells to EPO for 1 h and Egr2 expression was measured by qPCR. EPO induced Egr2 by about 20-fold compared with untreated cells (Figure 6A).
Figure 6.
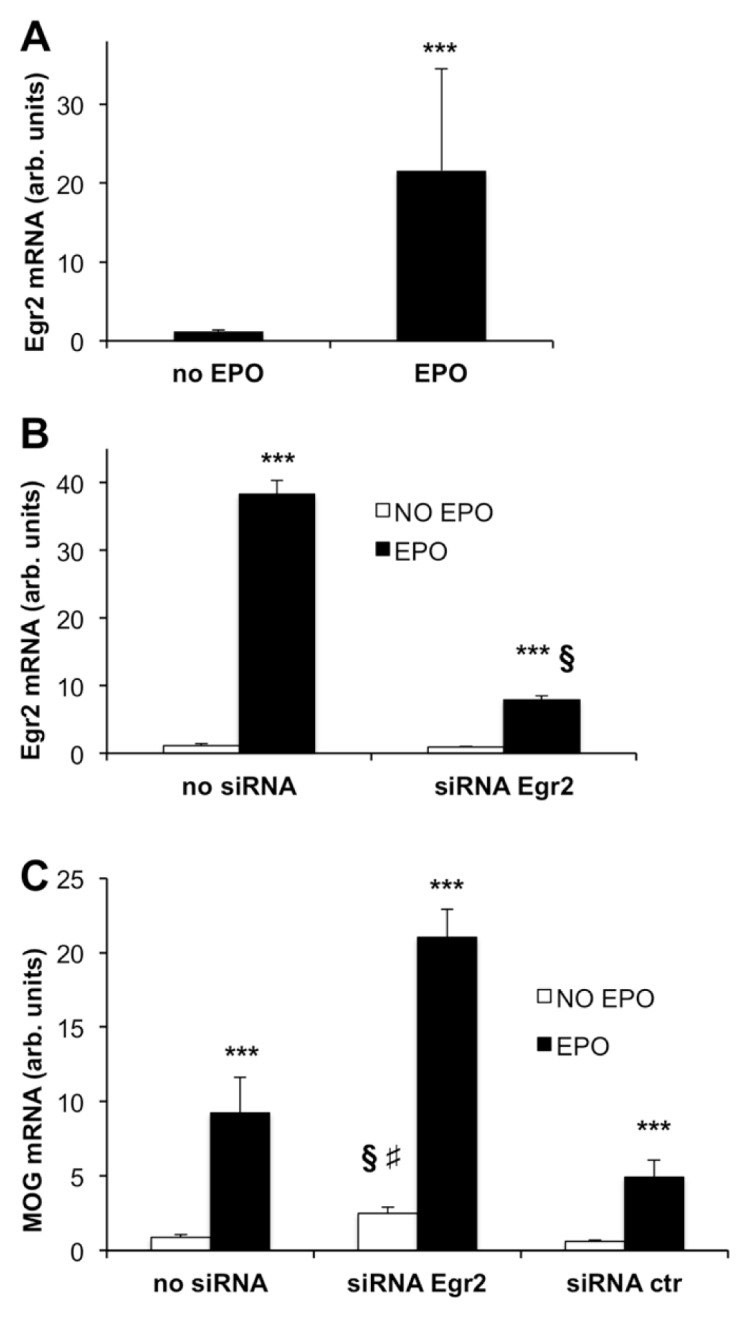
EPO does not act through Egr2 to increase myelin gene expression. (A) EPO induces Egr2 gene expression in CG4-EPOR cells. CG4-EPOR cells were plated in GM, then switched to DM and after 24 h treated with or without EPO 80 ng/mL for 1 h. Egr2 expression was measured by qPCR. Results are expressed as arbitrary units versus no EPO and are the mean ± SD of nine to twelve samples analyzed in duplicate (no EPO, N = 9; EPO, N = 12). ***P < 0.001 versus no EPO by Student t test. (B–C) EPO effect on MOG expression is not blocked by Egr2 silencing. CG4-EPOR cells were differentiated and transfected with siRNA targeting Egr2 or with a negative control siRNA. After 24 h cells were treated with or without EPO 80 ng/mL. Egr2 gene expression was measured 1 h after EPO stimulation (B) and MOG was measured at d 4 of differentiation (C). Results are expressed as arbitrary units versus control cells (no siRNA, no EPO) and are the mean ± SD of four to six samples analyzed in duplicate (panel B, N = 4; panel C, N = 6). ***P < 0.001 versus no EPO; §P < 0.001 versus no siRNA; #P < 0.001 versus control siRNA; Student t test.
To establish whether the observed up-regulation of Egr2 has a role in the induction of myelin genes by EPO, Egr2 was silenced with an Egr2-siRNA, upon switching the cells to DM. After 1 d, when EPO-induced Egr2 expression was inhibited by 80% in Egr2-siRNA transfected cells (Figure 6B), cells were treated with or without EPO and MOG gene expression was measured at d 4 of differentiation. EPO significantly increased MOG mRNA in CG4-EPOR cells nontransfected (no siRNA) or transfected with a control siRNA (by 11-fold and 10-fold, respectively), as expected, and the EPO effect was not blocked by Egr2 silencing. In fact, in the presence of Egr2-siRNA, EPO still increased MOG expression by 8-fold (Figure 6C).
Of note, Egr2 silencing induced MOG by 2.7-fold as compared with nontransfected cells; no induction was observed using a control siRNA (see Figure 6C).
Therefore, EPO increased myelin gene expression in CG4-EPOR cells by a signaling pathway independent of Egr2. Moreover, knocking down Egr2 expression promoted expression of MOG with and without EPO stimulation, suggesting that Egr2 might be a negative regulator of MOG whose effect is overridden by EPOR activation.
DISCUSSION
This study shows that EPO increases the expression of myelin genes (MOG and MBP) in differentiated CG4-EPOR cells, and that this effect requires EPOR. The induction of both myelin genes was observed starting from an EPO concentration of 8 ng/mL, was already detectable at d 2 and still present at d 6 of differentiation The requirement for EPOR was demonstrated by the lack of effect of EPO in non-EPOR-expressing wild-type cells or in cells transduced with EGFP only in a lentiviral vector, and by experiments showing that the effect of EPO is smaller in clones expressing lower EPOR levels. Interestingly, primary oligodendrocytes express low levels of EPOR under physiological conditions (15); however, EPOR is increased in the central nervous system in pathologies where EPO shows a protective effect (23,29).
To identify transcription factors implicated in the effect of EPO on myelination, we focused on Egr2. Egr2 is induced alongside with myelin genes in dorsal root ganglia cells treated with IL-6, which also stimulates myelination in vivo(30,31). Since EPO shares structural similarities with IL-6 (both are 4-α helical cytokines), and we observed that Egr2 is upregulated by EPO in models of cerebral ischemia (6), we asked whether Egr2 is involved in the induction of MOG and MBP by EPO in CG4 cells. However, silencing Egr2 gene expression in CG4-EPOR cells by an Egr2-siRNA did not inhibit the effect of EPO on myelin gene expression, indicating that Egr2 is not implicated in the action of EPO on oligodendrocytes. In fact, Egr2 silencing unexpectedly induced expression of myelin genes per se. Therefore, it may well be that Egr2 has an essential role in myelination only in the peripheral nervous system, where indeed its deficiency results in severe demyelination (27).
It is possible that other signaling pathways are implicated in EPO/EPOR- induced myelination. For instance, the survival kinase Akt has a key role in the neuroprotective action of EPO (32) and it has also been reported to promote myelination in the CNS (33).
CONCLUSION
The direct effect of EPO on myelination in oligodendrocytes reported here is further evidence that EPO, in addition to cytoprotective and antiinflammatory actions, can promote myelin repair. In terms of repair, increased expression of myelin genes might contribute to the overall effect of EPO, along with prevention of oligodendrocyte cell death (34,35), stimulation of neurogenesis and angiogenesis (4), and induction of neuronal plasticity (6). This might be relevant to demyelinating diseases, and it is interesting to note that EPO is effective not only in animal models of MS but also in a pilot clinical trial (36). Furthermore, induction of myelin genes, together with promotion of oligodendrogenesis, might play a role in EPO-induced neurological recovery in stroke as well as in neonatal hypoxic-ischemic brain injury, where oligodendrocyte damage is an important pathogenic component (5,37–39).
Our experiments using CG4 cells engineered to express EPOR provide a clear demonstration that EPOR can mediate at least some of the reparative effects of EPO in nonerythroid cells and that EPO-EPOR interaction can induce effects other than the erythropoietic ones.
ACKNOWLEDGMENTS
PG is supported by the RM Phillips Charitable Trust and the European Regional Development Fund, TC2N. YC and AA are supported by NMSS-USA (Promise 2010).
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Sargin D, Friedrichs H, El-Kordi A, Ehrenreich H. Erythropoietin as neuroprotective and neuroregenerative treatment strategy: comprehensive overview of 12 years of preclinical and clinical research. Best Pract Res Clin Anaesthesiol. 2010;24:573–94. doi: 10.1016/j.bpa.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Bartels C, Spate K, Krampe H, Ehrenreich H. Recombinant human erythropoietin: novel strategies for neuroprotective/neuro-regenerative treatment of multiple sclerosis. Ther Adv Neurol Disord. 2008;1:193–206. doi: 10.1177/1756285608098422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghezzi P, Brines M. Erythropoietin as an antiapoptotic, tissue-protective cytokine. Cell Death Differ. 2004;11(Suppl 1):S37–44. doi: 10.1038/sj.cdd.4401450. [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–7. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, et al. Erythropoietin amplifies stroke-induced oligodendrogenesis in the rat. PLoS One. 2010;5:e11016. doi: 10.1371/journal.pone.0011016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mengozzi M, et al. Erythropoietin-induced changes in brain gene expression reveal induction of synaptic plasticity genes in experimental stroke. Proc Natl Acad Sci U S A. 2012;109:9617–22. doi: 10.1073/pnas.1200554109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agnello D, et al. Erythropoietin exerts an anti-inflammatory effect on the CNS in a model of experimental autoimmune encephalomyelitis. Brain Res. 2002;952:128–34. doi: 10.1016/s0006-8993(02)03239-0. [DOI] [PubMed] [Google Scholar]
- 8.Savino C, et al. Delayed administration of erythropoietin and its non-erythropoietic derivatives ameliorates chronic murine autoimmune encephalomyelitis. J Neuroimmunol. 2006;172:27–37. doi: 10.1016/j.jneuroim.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Yuan R, et al. Erythropoietin: a potent inducer of peripheral immuno/inflammatory modulation in autoimmune EAE. PLoS ONE. 2008;3:e1924. doi: 10.1371/journal.pone.0001924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen SJ, et al. Erythropoietin enhances endogenous haem oxygenase-1 and represses immune responses to ameliorate experimental auto-immune encephalomyelitis. Clin Exp Immunol. 2010;162:210–23. doi: 10.1111/j.1365-2249.2010.04238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W, et al. Beneficial effect of erythropoietin on experimental allergic encephalomyelitis. Ann Neurol. 2004;56:767–77. doi: 10.1002/ana.20274. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, et al. Erythropoietin treatment improves neurological functional recovery in EAE mice. Brain Res. 2005;1034:34–9. doi: 10.1016/j.brainres.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 13.Hagemeyer N, et al. Erythropoietin attenuates neurological and histological consequences of toxic demyelination in mice. Mol Med. 2012;18:628–35. doi: 10.2119/molmed.2011.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho YK, et al. Erythropoietin promotes oligodendrogenesis and myelin repair following lysolecithin-induced injury in spinal cord slice culture. Biochem Biophys Res Commun. 2012;417:753–9. doi: 10.1016/j.bbrc.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 15.Sugawa M, Sakurai Y, Ishikawa-Ieda Y, Suzuki H, Asou H. Effects of erythropoietin on glial cell development; oligodendrocyte maturation and astrocyte proliferation. Neurosci Res. 2002;44:391–403. doi: 10.1016/s0168-0102(02)00161-x. [DOI] [PubMed] [Google Scholar]
- 16.Ghezzi P, et al. Erythropoietin: not just about erythropoiesis. Lancet. 2010;375:2142. doi: 10.1016/S0140-6736(10)60992-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watowich SS, et al. Homodimerization and constitutive activation of the erythropoietin receptor. Proc Natl Acad Sci U S A. 1992;89:2140–4. doi: 10.1073/pnas.89.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leist M, et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–42. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- 19.Brines M, et al. Nonerythropoietic, tissue-protective peptides derived from the tertiary structure of erythropoietin. Proc Natl Acad Sci U S A. 2008;105:10925–30. doi: 10.1073/pnas.0805594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiong Y, et al. Erythropoietin improves histological and functional outcomes after traumatic brain injury in mice in the absence of the neural erythropoietin receptor. J Neurotrauma. 2010;27:205–15. doi: 10.1089/neu.2009.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu X, et al. Erythropoietin receptor signalling is required for normal brain development. Development. 2002;129:505–16. doi: 10.1242/dev.129.2.505. [DOI] [PubMed] [Google Scholar]
- 22.Um M, Gross AW, Lodish HF. A “classical” homodimeric erythropoietin receptor is essential for the antiapoptotic effects of erythropoietin on differentiated neuroblastoma SH-SY5Y and pheochromocytoma PC-12 cells. Cell Signal. 2007;19:634–45. doi: 10.1016/j.cellsig.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Siren AL, et al. Erythropoietin and erythropoietin receptor in human ischemic/hypoxic brain. Acta Neuropathol. 2001;101:271–6. doi: 10.1007/s004010000297. [DOI] [PubMed] [Google Scholar]
- 24.Brines M, Cerami A. The receptor that tames the innate immune response. Mol Med. 2012;18:486–96. doi: 10.2119/molmed.2011.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Annenkov A, et al. A chimeric receptor of the insulin-like growth factor receptor type 1 (IGFR1) and a single chain antibody specific to myelin oligodendrocyte glycoprotein activates the IGF1R signalling cascade in CG4 oligodendrocyte progenitors. Biochim Biophys Acta. 20111813:1428–37. doi: 10.1016/j.bbamcr.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Wang M, Doucette JR, Nazarali AJ. Conditional Tet-regulated over-expression of Hoxa2 in CG4 cells increases their proliferation and delays their differentiation into oligodendrocyte-like cells expressing myelin basic protein. Cell Mol Neurobiol. 2011;31:875–86. doi: 10.1007/s10571-011-9685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Topilko P, et al. Krox-20 controls myelination in the peripheral nervous system. Nature. 1994;371:796–9. doi: 10.1038/371796a0. [DOI] [PubMed] [Google Scholar]
- 28.Demaison C, et al. High-level transduction and gene expression in hematopoietic repopulating cells using a human imunodeficiency [immunodeficiency] virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum Gene Ther. 2002;13:803–13. doi: 10.1089/10430340252898984. [DOI] [PubMed] [Google Scholar]
- 29.Chen ZY, Wang L, Asavaritkrai P, Noguchi CT. Up-regulation of erythropoietin receptor by nitric oxide mediates hypoxia preconditioning. J. Neurosci. Res. 2010;88:3180–8. doi: 10.1002/jnr.22473. [DOI] [PubMed] [Google Scholar]
- 30.Zhang PL, et al. Increased myelinating capacity of embryonic stem cell derived oligodendrocyte precursors after treatment by interleukin-6/soluble interleukin-6 receptor fusion protein. Mol Cell Neurosci. 2006;31:387–98. doi: 10.1016/j.mcn.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 31.Zhang PL, et al. Induction of neuronal and myelin-related gene expression by IL-6-receptor/IL-6: a study on embryonic dorsal root ganglia cells and isolated Schwann cells. Exp Neurol. 2007;208:285–96. doi: 10.1016/j.expneurol.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 32.Siren AL, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A. 2001;98:4044–9. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flores AI, et al. Constitutively active Akt induces enhanced myelination in the CNS. J Neurosci. 2008;28:7174–83. doi: 10.1523/JNEUROSCI.0150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Genc K, Genc S, Baskin H, Semin I. Erythropoietin decreases cytotoxicity and nitric oxide formation induced by inflammatory stimuli in rat oligodendrocytes. Physiol Res. 2006;55:33–8. doi: 10.33549/physiolres.930749. [DOI] [PubMed] [Google Scholar]
- 35.Kato S, et al. Endogenous erythropoietin from astrocyte protects the oligodendrocyte precursor cell against hypoxic and reoxygenation injury. J. Neurosci. Res. 2011;89:1566–74. doi: 10.1002/jnr.22702. [DOI] [PubMed] [Google Scholar]
- 36.Ehrenreich H, et al. Exploring recombinant human erythropoietin in chronic progressive multiple sclerosis. Brain. 2007;130:2577–88. doi: 10.1093/brain/awm203. [DOI] [PubMed] [Google Scholar]
- 37.Iwai M, et al. Enhanced oligodendrogenesis and recovery of neurological function by erythropoietin after neonatal hypoxic/ischemic brain injury. Stroke. 2010;41:1032–7. doi: 10.1161/STROKEAHA.109.570325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamada M, Burke C, Colditz P, Johnson DW, Gobe GC. Erythropoietin protects against apoptosis and increases expression of non-neuronal cell markers in the hypoxia-injured developing brain. J Pathol. 2011;224:101–9. doi: 10.1002/path.2862. [DOI] [PubMed] [Google Scholar]
- 39.McIver SR, et al. Oligodendrocyte degeneration and recovery after focal cerebral ischemia. Neuroscience. 2010;169:1364–75. doi: 10.1016/j.neuroscience.2010.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]